Abstract
Background
Cannabis use disorder (CUD) is a common condition with few treatments. Several studies in other substance use disorders have found that applying repetitive transcranial magnetic stimulation (rTMS) to the dorsolateral prefrontal cortex (DLPFC) decreases cue-elicited craving and possibly decreases use. To date, there have been no studies attempting to use rTMS in CUD.
Objectives
This study was conducted to determine if rTMS could be feasibly delivered to a group of non-treatment seeking CUD participants. Secondarily, the study aimed to estimate the effect of rTMS on craving.
Methods
In a double-blind, sham-controlled, crossover design, a single session of active or sham rTMS (Left DLPFC, 10 Hz, 110% rMT, 4000 pulses) was delivered during a validated cannabis cue paradigm. Participants crossed over to complete the other condition one week later. The feasibility and tolerability were measured by the rate of retention, and the percentage of participants able to tolerate full dose rTMS, respectively. Craving was measured using the Marijuana Craving Questionnaire (MCQ).
Results
Eighteen non-treatment seeking CUD participants were recruited from the community; 16 (three women) completed the trial (89% retained for the three study visits). All of the treatment completers tolerated rTMS at full dose without adverse effects. There was not a significant reduction in the total MCQ when participants received active rTMS as compared to sham rTMS.
Conclusion
rTMS can be safely and feasibly delivered to CUD participants, and treatment is well tolerated. A single session of rTMS applied to the DLPFC may not reduce cue-elicited craving in heavy cannabis users.
Keywords: Cannabis, marijuana, craving, transcranial magnetic stimulation, TMS
Introduction
Cannabis Use Disorder (CUD) is a common and escalating problem in the United States (1). Coinciding with the high prevalence of CUD, there has been a high demand for treatment. According to SAMHSA, in 2014 over one million individuals sought treatment for CUD (2). In recent years, it has become increasingly clear that those desiring to quit cannabis are rarely able to do so on their own and suffer from a clear withdrawal syndrome (3). Currently, the available treatments for CUD have low long-term success rates (4–7). There has subsequently been significant interest in the development of new treatment options for those individuals with CUD who desire to stop using.
In substance use disorders, it is theorized that there is an imbalance of executive control and reward networks. This theory posits that addiction results when normal inhibitory processes mediated by prefrontal regions, including the dorsolateral prefrontal cortex (DLPFC), are unable to control reward processes mediated by the limbic system (8–10). The DLPFC, in particular, has been implicated in the modulation of craving across substance use disorders including cocaine use disorder (11), nicotine use disorder (12), and cannabis use disorder (13).
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique that is able to alter cortical excitability and is an established, FDA-cleared, treatment for major depressive disorder. Magnetic fields pass unimpeded through the scalp, skull, and meninges and can directly excite cortical areas. High frequency rTMS (greater than 5 pulses per second) increases cortical excitability (14), and, when multiple sessions of rTMS are delivered, there are long lasting behavioral alterations (best characterized by the long lasting antidepressant effect of rTMS (15)).
If, in fact, an imbalance of executive control and reward networks results in craving, then it would follow that either the application of excitatory rTMS to the executive control network or inhibitory rTMS to the reward network would result in decreased craving. Multiple studies have confirmed this relationship [see reviews (16–19)]. The majority of these studies applied single sessions of excitatory stimulation to the DLPFC with the idea that this type of stimulation can result in enhanced top-down modulation of the reward network, resulting in less reactivity to drug cues. Of note, another study demonstrated that inhibitory rTMS applied to the DLPFC resulted in increased craving (20), providing further strength to this relationship. Sparked by the promising literature suggesting that single sessions of pre-frontal rTMS reduce craving, two recent clinical trials demonstrated that multiple sessions of rTMS may have a more durable effect on craving and reduce drug use in nicotine use disorder (21), and cocaine use disorder (22).
If excitatory rTMS of the DLPFC has an anti-craving effect in cannabis users, then it could represent a novel treatment option for those attempting to diminish use or become abstinent from cannabis. TMS is typically a well tolerated intervention with few adverse effects (23). The major limiting factor in the initial delivery of rTMS is site discomfort (the magnetic field excites nociceptive neurons on the way through the skin and skull). Additionally, the sham condition must match the site discomfort of active rTMS in order to be an effective blind, and is subsequently also mildly uncomfortable. Given the ambivalence common in CUD, this site discomfort may result in either dropout, or intolerance of full dose rTMS (resulting in being treated at a sub-therapeutic dose, or discontinuing treatment).
Prior to engaging in a large scale clinical trial utilizing multiple sessions of rTMS, we thought it was necessary to determine simply if rTMS can be feasibly delivered to CUD participants. Additionally, given that single sessions of rTMS have had small effects on craving in other substance use disorders, we sought to preliminarily determine the effect of rTMS on cannabis craving. We subsequently took the first step in developing this technique by determining the safety, feasibility, and preliminary effect size of a single session of rTMS in a CUD population.
Methods
This was a randomized, double-blinded, cross-over study in which participants with CUD were exposed to a validated cannabis cue reactivity paradigm during a single session of either active or sham rTMS. Each participant then crossed over and underwent the same procedure with the opposite stimulation intervention approximately one week later (see Figure 1). Potential participants were recruited via media advertisements from the community and were compensated a total of $140 for participation in the study. All study procedures were approved by the Medical University of South Carolina’s Institutional Review Board, and all procedures were performed in accordance with the Declaration of Helsinki. All participants gave written informed consent prior to any study related procedures. Potential participants were included if they were between the ages of 18 and 65, met criteria for cannabis use disorder, and reported cannabis use on at least 20 out of the 30 days prior to enrollment. Potential participants were excluded if they had any active neurologic, psychiatric, or sleep disorder; met criteria for any other substance use disorder with the exception of nicotine use disorder; had a positive drug screen for any drug of abuse besides cannabis; were taking any medications with central nervous system activity; had a history of seizures; had metal implants above the waist; or had a history of any brain lesions.
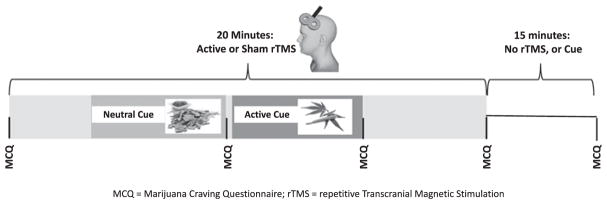
Figure 1.
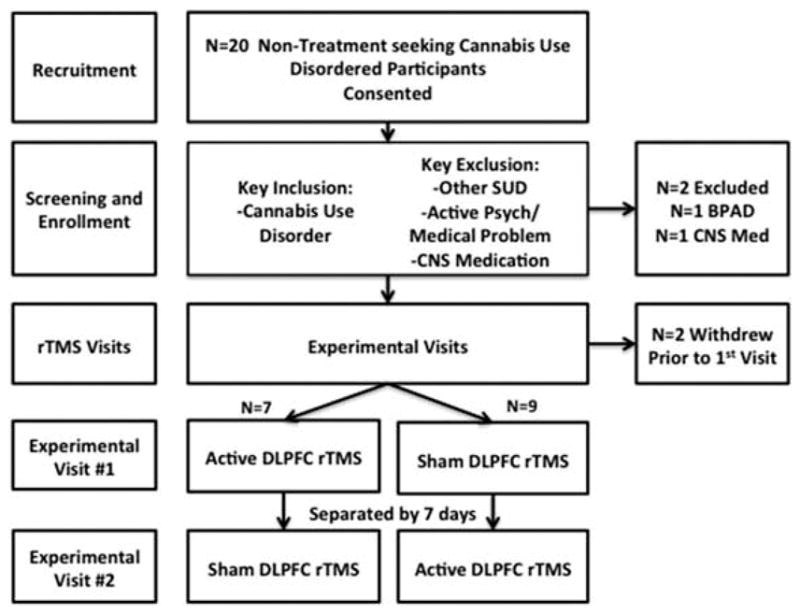
Enrollment flow chart and study design.
Each participant completed three visits. On the initial visit, participants underwent a standardized evaluation that included a Mini International Neuropsychiatric Interview (MINI) (24), a Time Line Follow-Back (TLFB)(25), the Cannabis Use Disorder Identification Test-R (CUDIT-R) (26), the Inventory of Depressive Symptoms (IDS) (27), and the Pittsburgh Sleep Quality Index (PSQI)(28). Participants additionally underwent both a urine (Alere Toxicology, testing for amphetamines, benzodiazepines, cannabis, cocaine, and opiates), and saliva drug test (Confirm Biosciences, testing for amphetamines, benzodiazepines, cocaine, cannabis, and opiates) to detect the presence of cannabis and other drugs of abuse. Both the urine and saliva drug test was performed on all three visits. The urine drug screening was performed in order to confirm the recent use of cannabis, and to ensure participants had not recently used any other drugs of abuse. The saliva drug test confirmed that participants had not used any drugs for the previous 14 hours.
Over each of the next two visits, participants underwent a validated cannabis cue reactivity paradigm during a single session of either active or sham rTMS (see Figure 2, and description below). On the day of each experimental visit, participants were instructed not to use alcohol, cannabis, or any other drugs. Abstinence was verified by self-report, urine, and salivary drug testing.
Figure 2.
Treatment visit protocol. This figure represents the time course of each of the experimental visits.
Repetitive Transcranial Magnetic Stimulation (rTMS) was delivered using a Magventure Magpro ×100 TMS machine. Treatments were delivered at the EEG coordinate for F3, which was found using the Beam F3 method (29) (which closely approximates the Left DLPFC). Each rTMS treatment consisted of a total of 4000 pulses of 10 Hz stimulation (5s-on, 10s-off), at a standardized intensity of 110% of the individual’s resting motor threshold (rMT), using a figure of eight coil. For tolerability, intensity was ramped up 10% of rMT every train from 60% rMT (200 pulses delivered sub 100%). Sham treatments were delivered by an electronic sham system that has been used extensively in our laboratory (30). The sham system consists of a coil that mimics the appearance and sound of rTMS, combined with a transcutaneous electrical nerve stimulator (TENS) device which produces a small electrical stimulus delivered to the scalp just below the hairline, mimicking the feeling of active rTMS (30). Both the participant and the treater were blind to the stimulation condition.
During rTMS, participants were presented both a neutral cue paradigm as well as a cannabis cue paradigm. The cue paradigm was presented during rTMS (as apposed to before or after rTMS), given that a recent study showed that rTMS had a greater antismoking effect if delivered during a smoking cue (21). The cannabis cue paradigm consisted of an auditory script and a tray of cannabis related items, and is closely adapted from (31). The auditory script consisted of an imaginal recall of a recent pleasurable cannabis experience. The physical cues consisted of a number of items associated with cannabis use such as blunt wraps, rolling papers, pipes, and a small bag containing marijuana. The small bag of cannabis also acted as an olfactory cue. The neutral cue paradigm consisted of a tray of items unassociated with drug use, such as a note pad, pencil, tea-bags, and wood chips (as an olfactory cue). The neutral auditory script asked participants to imagine a day at the beach. We elected to deliver a neutral cue paradigm in addition to an active cue paradigm as a negative control (to ensure items in general did not increase craving). We elected to deliver the neutral cue prior to the active cue on all experimental visits to reduce the variability of the protocol. The Marijuana Craving Questionnaire (MCQ) (32) was used to assess craving, and results in a total score as well as four sub-scores. In three separate studies validating two forms of the MCQ, four independent clusters of craving type were observed (32–34). The observed sub-types of craving include; 1: compulsivity, with higher levels of compulsivity indicating less ability to modulate cannabis use, 2: emotionality, with higher levels of emotionality indicating a greater desire to use cannabis to reduce withdrawal symptoms, 3: expectancy, with higher levels indicating anticipation of positive outcomes from using cannabis, and 4: purposefulness, with higher levels indicating more planning and anticipation of using cannabis for positive effects (Figure 3). Higher scores on the MCQ indicate higher levels of craving. The MCQ was administered prior to the application of rTMS, during rTMS following the neutral cue, during rTMS following the active cue, directly following rTMS, and 15 minutes after the completion of rTMS. We elected to collect craving data at each of these time points given that this was an exploratory study, and it was unclear if and when rTMS would have an anti-craving effect. We extended craving analysis out to 15 minutes past treatment to see if rTMS has a delayed craving effect, and to ensure that rTMS does not result in increased craving following treatment.
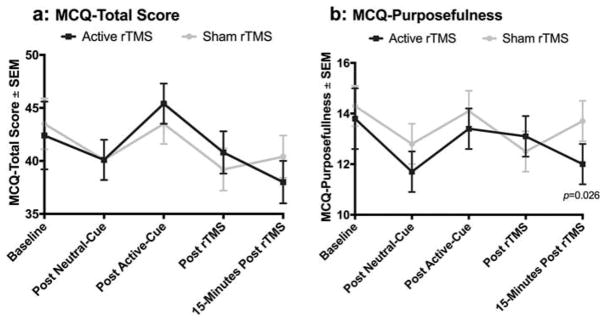
Figure 3.
3a) Marijuana Craving Questionnaire (MCQ) total score; and 3b) Marijuana Craving Questionnaire (MCQ) purposefulness score. Error bars denote S.E.M. This figure denotes the course of cue induced craving. Baseline refers to craving measured prior to any cue presentation, or rTMS. Post neutral-cue refers to craving following a neutral cue presentation during rTMS, post active-cue refers to craving following an active cannabis cue presentation during rTMS, post rTMS refers to craving following the cessation of rTMS, and 15-minutes post rTMS refers to craving 15 minutes following the cessation of rTMS.
Data analysis
Standard descriptive statistics were used to summarize the general demographic and clinical data for the entire sample and across randomized order of treatment. A Wilcoxon rank sum test statistic assessed baseline differences in continuous characteristics, and differences in categorical characteristics were assessed using Fisher’s exact test statistic.
Feasibility, safety, and tolerability were examined as the primary outcomes of the study. Primarily, the retention rate (feasibility), and the number of participants who reached the allotted treatment dose of 110% of motor threshold (tolerability) were assessed. Additionally, had any treatment emergent adverse events occurred, we would have compared these across the treatment condition upon which they had occurred (tolerability). Had any participants dropped out following a treatment visit, or failed to reach the allotted treatment dose, we would have additionally compared the rate of drop out and percent motor threshold dose between those receiving active or sham stimulation.
Secondarily, estimates of treatment effects on marijuana craving following the rTMS cue sessions were of interest to determine effect sizes and power estimates for larger clinical trials. To estimate treatment differences in craving after receiving active rTMS, linear mixed effects models that assessed all serially measured post-baseline time points were developed. The models tested the efficacy of active rTMS on craving responses to both the neutral and active cue (during rTMS) as well as durable effects following the rTMS procedure (immediately following, and 15 minutes post rTMS). Model-based estimates were used to construct group level pair-wise comparisons across treatment conditions at all post-baseline time points; comparisons were taken at the neutral cue, active cue, post rTMS, and at rTMS +15 minutes time points. Overall treatment effect sizes for the main effect of rTMS treatment, time, their interaction as well as the time-varying baseline craving measures taken prior to each condition were initially assessed as covariates in the model. Models additionally adjusted for active rTMS/sham rTMS order and study day. Estimated group differences and their associated 95% confidence intervals are presented. Effect sizes are calculated at each time point and presented as Cohen’s d values (35). Potential baseline confounders (gender, age, depression levels, and CUDIT scores) were independently assessed in adjusted models for evidence of effect modification using appropriate interaction terms.
As this was an exploratory study, no pre-study power calculations were performed. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, US). No correction for multiple comparisons was applied to the reported results.
Results
Demographics and clinical characteristics
Eighteen total participants were enrolled in the study, and were randomized to receive either active or sham rTMS in the first experimental visit. The mean age of study participants was 26 years (SD = 17.9), 13 were men (81.3%), and 10 were Caucasian (62.5%). All participants met criteria for cannabis use disorder (MINI), and did not meet criteria for any other axis I condition, or substance use disorder. Self-reported marijuana use at study entry showed high levels of use. Participants used cannabis 23.5 (SD = 4.3) days out of the last 30 with an average of 1.3 (SD = 1.3) grams used per day (Table 1). Additionally, participants drank alcohol on average 7.4 (SD = 7.8) days out of the last 30 days [1.2 (SD = 1.3) drinks per day]. Seven participants smoked with an average of 11.7 (SD = 9.8) cigarettes per day.
Table 1.
Demographics and clinical characteristics at study baseline.
| Overall | First randomized treatment | P-Value | ||
|---|---|---|---|---|
|
|
|
|||
| Sham first N = 7 |
rTMS first N = 9 |
|||
| Age | 26.0 ± 7.9 | 24.6 ± 3.7 | 27.2 ± 10.1 | 0.952 |
| Male gender | 81.3 (13) | 85.7 (6) | 77.9 (7) | 0.999 |
| Caucasian | 62.5 (10) | 42.8 (3) | 77.8 (7) | 0.302 |
| Age at regular MJ use | 18.1 ± 2.6 | 18.7 ± 3.1 | 17.6 ± 2.1 | 0.301 |
| MJ smoked per day (grams) | 1.3 ± 1.3 | 1.4 ± 1.5 | 1.3 ± 1.1 | 0.835 |
| MJ days smoked (past 30) | 23.5 ± 4.3 | 22.0 ± 3.7 | 24.7 ± 4.5 | 0.094 |
| CUDIT score | 17.1 ± 6.1 | 14.9 ± 5.4 | 18.9 ± 6.4 | 0.279 |
| CUDIT score > 12 | 68.8 (11) | 57.1 (4) | 77.8 (7) | 0.596 |
| IDS-SR | 10.3 ± 10.0 | 12.9 ± 10.8 | 8.3 ± 9.6 | 0.204 |
Feasibility and safety
The primary aim of the current study was to assess the feasibility and tolerability of a single session of rTMS in non-treatment seeking CUD participants. The study retention rate was high with 14 of 16 (89%) participants completing the study protocol as designed (Figure 1). There were no treatment related adverse events reported in this trial. All recruited participants tolerated treatment at the fully intended dose of rTMS and had treatment related complaints. Of the two participants who withdrew from the study, one was lost to follow-up prior to the first experimental visit, and one withdrew due to a headache occurring on the day after the enrollment visit (where their motor threshold was found). The described headache was considered not likely to be related to TMS, as motor thresholds are determined using single pulse TMS only, and the event occurred on the day following the session (headaches associated with TMS typically occur directly after treatment, and are more commonly associated with rTMS).
Craving response
Craving to cannabis was measured using the MCQ total and subscale scores and was assessed prior to active or sham rTMS (time-varying baseline), in response to a neutral and active marijuana cue during rTMS, immediately following rTMS, and 15 minutes following the conclusion of rTMS. There were no statistically significant differences in MCQ total score between the active sham rTMS condition at baseline (F1,56 = 0.09; = 0.769). Although there was a statistically significant increase in craving to the active cue as compared to the neutral cue under both conditions (Δ = 4.4 ± 1.5 SEM; = 0.005), there was no differential increase between the two treatment conditions (Sham Cue Response: = 3.4 ± 1.7 vs. rTMS Cue Response: Δ = 5.4 ± 1.7; = 0.227). There was no difference in MCQ total score between treatment conditions either during or following rTMS (Table 2). Additionally, post rTMS craving subscale measures of the emotionality, compulsion, and expectancy subscales scores were not significantly different across the two conditions (Table 2). At 15-minutes following completion of the rTMS condition, participants reported moderately lower purposefulness subscale scores following active treatment as compared sham treatment (Sham Response: Δ = 13.7 ± 10.8 vs. rTMS Cue Response: Δ = 12.0 ± 0.8).
Table 2.
Craving as measured by the Marijuana Craving Questionnaire (MCQ).
| Outcome | Treatment condition | Treatment Difference | Effect Size† | |
|---|---|---|---|---|
|
| ||||
| Sham rTMS | Active rTMS | |||
| Baseline craving prior to rTMS* | ||||
| MCQ Total Score | 43.5 (37.4,49.6) | 41.9 (35.8,47.9) | −1.7 (−6.6,3.3) | 0.15 |
| Emotionality | 10.4 (8.0,12.7) | 10.1 (7.7,12.4) | −0.3 (−2.1,1.5) | 0.08 |
| Purposefulness | 14.3 (12.1,16.6) | 13.6 (11.4,15.9) | −0.7 (−3.2,1.8) | 0.17 |
| Compulsion | 5.0 (3.8,6.3) | 4.7 (3.4,5.9) | −0.4 (−1.5,0.7) | 0.16 |
| Expectancy | 13.7 (11.6,15.9) | 13.5 (11.4,15.6) | −0.2 (−2.0,1.6) | 0.05 |
| Analytic model results** | ||||
| Neutral cue during rTMS | ||||
| MCQ Total Score | 40.1 (36.2,44.0) | 40.1 (36.2,43.9) | 0.0 (−3.2,3.2) | 0.00 |
| Emotionality | 9.7 (8.2,11.2) | 10.2 (8.7,11.7) | 0.5 (−1.0,2.0) | 0.16 |
| Purposefulness | 12.8 (11.2,14.4) | 11.7 (10.2,13.3) | −1.1 (−2.5,0.4) | 0.38 |
| Compulsion | 5.0 (4.0,6.0) | 5.5 (4.5,6.5) | 0.5 (−0.3,1.4) | 0.31 |
| Expectancy | 12.7 (11.4,13.9) | 12.4 (11.2,13.7) | −0.2 (−1.6,1.1) | 0.08 |
| Active cue during rTMS | ||||
| MCQ Total Score | 43.5 (39.6,47.3) | 45.4 (41.6,49.3) | 2.0 (−1.8,5.7) | 0.27 |
| Emotionality | 10.5 (9.0,12.0) | 11.3 (9.8,12.8) | 0.8 (−0.7,2.2) | 0.27 |
| Purposefulness | 14.1 (12.5,15.7) | 13.4 (11.9,15.0) | −0.6 (−2.1,0.8) | 0.22 |
| Compulsion | 6.0 (5.0,7.0) | 6.9 (5.9,7.9) | 0.9 (0.0,1.7) | 0.49 |
| Expectancy | 13.0 (11.7,14.2) | 13.7 (12.4,14.9) | 0.7 (−0.6,2.2) | 0.26 |
| Immediately following rTMS | ||||
| MCQ Total Score | 39.2 (35.3,43.1) | 40.9 (37.0,44.7) | 1.7 (−2.1,5.4) | 0.22 |
| Emotionality | 9.2 (7.7,10.7) | 9.6 (8.1,11.1) | 0.4 (−1.2,1.9) | 0.12 |
| Purposefulness | 12.5 (10.9,14.1) | 13.1 (11.5,14.6) | 0.6 (−0.9,2.0) | 0.19 |
| Compulsion | 5.4 (4.4,6.4) | 5.4 (4.4,6.4) | 0.0 (−0.8,0.9) | 0.03 |
| Expectancy | 12.2 (10.9,13.4) | 12.6 (11.4,14.0) | 0.5 (−0.9,1.8) | 0.17 |
| 15 Minutes following rTMS | ||||
| MCQ total score | 40.4 (36.5,44.3) | 38.6 (34.7,42.5) | −1.8 (−5.6,2.1) | 0.23 |
| Emotionality | 9.5 (8.0,11.0) | 9.0 (7.5,10.5) | −0.5 (−2.1,1.0) | 0.17 |
| Purposefulness | 13.7 (12.1,15.3) | 12.0 (10.4,13.6) | −1.7 (−3.2, −0.2) | 0.63 |
| Compulsion | 5.4 (4.3,6.4) | 4.8 (3.8,5.8) | −0.6 (−1.5,0.4) | 0.31 |
| Expectancy | 11.9 (10.6,13.2) | 12.7 (11.4,14.0) | 0.8 (−0.6,2.2) | 0.28 |
Note.
Baseline results are adjusted for rTMS treatment, study day, and treatment order.
Results obtained from the analytic models are adjusted for the main effect of rTMS treatment, time, their interaction, study day, treatment order, as well as the time-varying baseline craving measures taken prior to each condition.
Although not specifically powered to assess treatment age, gender, and severity of cannabis use (CUDIT) on the relationship between rTMS conditions and cannabis craving were investigated for potentially clinically relevant signals. No significant moderating effect on the relationship between treatment conditions and cannabis craving (MCQ total) were found in these analyses.
Efficacy of the blind
Following each experimental visit, participants were asked whether they believed they received active or sham rTMS. They were also asked how confident they were of their guess on a 5 point Likert scale with 0 = “Not confident at all”, and 4 = “Almost certain”. Overall 23 of 31 (74%) of the queries correctly guessed their stimulation condition with a median confidence of 1 (“somewhat confident”). Eleven out of sixteen participants (69%) correctly guessed that they received active stimulation with a median confidence of 1.5 (between “somewhat confident”, and “confident”). Twelve out of fifteen (80%) correctly guessed that they received sham stimulation with a median confidence of 1 (“Somewhat confident”).
Discussion
This is the first study to demonstrate that figure of eight coil rTMS can be safely and feasibly delivered to the dorsolateral prefrontal cortex of heavy cannabis users. Though the primary purpose of this study was to determine if rTMS could be feasibly delivered in this population, it also demonstrated that the application of a single session of rTMS may result in a reduction of the purposefulness aspect of cue elicited cannabis craving. These two findings taken together open the door for further study in the application of rTMS as a potential treatment for CUD.
The primary purpose of this study was to determine if rTMS was well tolerated by CUD patients. We managed to retain 89% of our enrolled participants. Furthermore, all of our participants tolerated rTMS at the full intended dose (110% of rMT), without deviation from the planned ramp of intensity, and without adverse events. As described previously, studies in participants with nicotine, alcohol, cocaine, and methamphetamine use disorders (20,22,36–45) have demonstrated that rTMS is safe and tolerable in these groups. The retention rate in other single session studies using non-treatment seekers in other addictions range from 79%–100%. Our only dropouts occurred before any treatment visit suggesting that both drop outs were non-specific to the delivery of rTMS.
With regard to cannabis craving, the observed effect size between groups was 0.23 for the MCQ-total, and 0.64 for the MCQ-Purposefulness which falls into the small to moderate categories, respectively. Our effect sizes were smaller than some recent single session investigations (37,44,45), and larger than another (42). The two most likely explanations for our smaller than anticipated effect for cue elicited craving include that there is a smaller effect of treatment in cannabis users, or that our small sample size was insufficient to detect a real change. Of interest, a statistically significant effect was seen in the Purposefulness subscale of the MCQ, which measures an individual’s intention and planning to use cannabis for positive outcomes, implying that rTMS may reduce intention and planning to use cannabis. It is unclear neurobiologically why rTMS applied to the DLPFC would have a larger effect on this aspect of craving as compared to the others. Purposefulness is related to planning and intent to use cannabis for its positive effects. Given that the DLPFC is involved in cognition and planning, DLPFC stimulation may enhance cognitive control in this area.
It should be noted that this study was designed to determine feasibility and safety of rTMS rather than to determine clinical efficacy. In order to observe a clinical effect using rTMS in the treatment of major depressive disorder, multiple sessions of treatment are required (commonly 20 or more) (46). A similar phenomenon has been observed in alcohol use disorder, where one study failed to find an anti-craving effect after delivering a single session of rTMS (42), but other studies have found a robust, stable anti-craving effect after delivering multiple sessions (38,47). Studies delivering more than one session of rTMS in larger groups of CUD participants will be needed in order to definitively determine if rTMS has an anti-craving effect in this population.
The results of this pilot study should be interpreted in light of several limitations. First, our sample size was relatively small, though within the range of sample sizes found in other single session investigations. Despite our relatively small sample, we managed to retain nearly 90% of those who enrolled, and each enrolled participant was able to tolerate 110% of motor threshold stimulation without any adverse events. Our high retention rate, and the fact that our participants tolerated a therapeutic dose of stimulation, thus demonstrates that it is possible to recruit and retain CUD participants. It is subsequently likely that it is possible to implement larger, more definitive trials using rTMS. Second, participants managed to correctly guess their condition nearly 69% of the time. This high rate of correctly guessing the treatment condition may suggest that our sham system was ineffective in this study. Though inadequate blinding may have contributed to our craving results, the primary aim of our study (to determine feasibility and tolerability) would not be influenced by an ineffective blind. In future studies, using a parallel design may result in a more effective blind, as there would be less of a direct comparison of active and sham sensations. The blinding system used in this study is more effective when patients are not crossed over and just receive one form of either active or sham stimulation. In order to achieve full blinding in crossover studies, one must titrate and match the painfulness of the sham stimulation (48), and should be considered in future crossover trials.
Our findings taken in composite suggest that DLPFC rTMS can be feasibly delivered to CUD participants. Additionally we found that the anti-craving effects found in other substance use disorders may be replicated in CUD. Though our findings are not definitive, they support future studies in this population.
Acknowledgments
We would like to thank all of the many contributors to this work including, Bashar Badran, Annabel Franz, Lisa Nunn, Jessica Lydiard, Amanda Wagner, and Margaret Caruso. We would also like to acknowledge the following grants which supported this research: K24DA038240 (PI: McRae-Clark, NIH/NIDA), K12DA031794 (Co-PIs: Brady and Malcolm, NIH/NIDA), and K23DA043628 (PI: Sahlem, NIH/NIDA).
References
- 1.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015:1. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SAMHSA. Behavioral health trends in the United States: results from the 2014 national survey on drug use and health. Substance Abuse and Mental Health Services Administration; 2015. [accessed 1 Mar 2017]. http://www.samhsa.gov/data/ [Google Scholar]
- 3.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–68. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, Pringle B. Services research on adolescent drug treatment. commentary on “The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials”. J Subst Abuse Treat. 2004;27:195–96. doi: 10.1016/j.jsat.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–53. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16:331–42. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- 7.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–36. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 10.Bickel WK, Quisenberry AJ, Moody L, Wilson AG. Therapeutic opportunities for self-control repair in addiction and related disorders: change and the limits of change in trans-disease processes. Clin Psychol Sci. 2015;3:140–53. doi: 10.1177/2167702614541260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14811–16. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology: Official Publication Am Coll Neuropsychopharmacol. 2014;39:2288–98. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol: Official Publication American Electroencephalographic Society. 1998;15:333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, Brock DG, Bonneh-Barkay D, Cook IA, Lanocha K, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014:1394–401. doi: 10.4088/JCP.13m08977. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Intl Rev Psychiatry. 2011;23:454–66. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- 18.Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol. 2014;2014:815215. doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–30. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT, George MS, See RE. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol Depend. 2013b;133:641–46. doi: 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–49. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Terraneoa ALL, Saladinie M, Ermanie M, Boncib A, Gallimbertia L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2015;1:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janicak P, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015:1549–1560. doi: 10.2147/NDT.S67477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I. N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB. Timeline follow-back. measuring alcohol consumption. Center for Psychological Studies, Nova Southeastern University; 1992. pp. 41–72. [Google Scholar]
- 26.Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, Sellman JD. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R) Drug Alcohol Depend. 2010;110:137–43. doi: 10.1016/j.drugalcdep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borckardt JJ, Walker J, Branham RK, Rydin-Gray S, Hunter C, Beeson H, Reeves ST, Madan A, Sackeim H, George MS. Development and evaluation of a portable sham transcranial magnetic stimulation system. Brain Stimul. 2008;1:52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology. 2011;218:49–58. doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the marijuana craving questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the marijuana craving questionnaire using imagery scripts. Exp Clin Psychopharmacol. 2002;10:47–53. doi: 10.1037/1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- 34.Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–34. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical Power analysis for the behavioral sciences. New York: Erlbaum; 1988. [Google Scholar]
- 36.Bolloni C, Panella R, Pedetti M, Frascella AG, Gambelunghe C, Piccoli T, Maniaci G, Brancato A, Cannizzaro C, Diana M. Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: a pilot study. Front Psychiatry. 2016;7:133. doi: 10.3389/fpsyt.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–33. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/add.2010.105.issue-1. [DOI] [PubMed] [Google Scholar]
- 39.Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–60. doi: 10.1111/add.2009.104.issue-4. [DOI] [PubMed] [Google Scholar]
- 40.Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS. What goes up, can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herremans SC, Van Schuerbeek P, De Raedt R, Matthys F, Buyl R, De Mey J, Baeken C. The impact of accelerated right prefrontal high-frequency Repetitive Transcranial Magnetic Stimulation (rTMS) on cue-reactivity: an fmri study on craving in recently detoxified alcohol-dependent patients. PloS One. 2015;10:e0136182. doi: 10.1371/journal.pone.0136182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120:209–13. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64:951–53. doi: 10.4088/JCP.v64n0815. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, Brady KT, George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013a;73:714–20. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 46.McDonald WM, Durkalski V, Ball ER, Holtzheimer PE, Pavlicova M, Lisanby SH, Avery D, Anderson BS, Nahas Z, Zarkowski P, et al. Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety. 2011;28:973–80. doi: 10.1002/da.v28.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, Nizamie SH. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J Neuropsychiatry Clin Neurosci. 2015;27:e54–59. doi: 10.1176/appi.neuropsych.13010013. [DOI] [PubMed] [Google Scholar]
- 48.Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, Long J, Sackeim HA, George MS. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul. 2008;1:44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]