Abstract
The aryl-hydrocarbon receptor repressor (AhRR) negatively regulates aryl-hydrocarbon receptor (AhR) signaling via its inhibitory transactivation. AhR is well known to suppress adipocyte differentiation, but the function of AhRR during adipogenesis is unclear. The purpose of this study was to investigate the role of AhRR in adipocyte differentiation using 3T3-L1 cells. During the early phase of differentiation, AhRR expression was transiently induced, but throughout the entire differentiation process, low levels of AhR expression were maintained. AhRR knockdown significantly increased not only glycerol-3-phosphate dehydrogenase (GPDH) activity but also lipid accumulation inside the cells. AhRR overexpression clearly reduced GPDH activity and lipid accumulation, indicating that AhRR upregulation during the early stage of adipogenesis suppresses adipocyte differentiation. Since AhRR knockdown increases the expression and activity of peroxisome proliferator-activated receptor γ(PPARγ), AhRR negatively regulates PPARγ during adipogenesis. In summary, similar to AhR, AhRR acts as an inhibitor of adipocyte differentiation. In addition to controlling the negative feedback loop of AhR, AhRR might be involved in other functions, especially in adipocyte differentiation processes.
1. Introduction
The aryl-hydrocarbon receptor (AhR) belongs to the superfamily of basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) domain-containing proteins that is activated by various low molecular weight compounds such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benzo(a) pyrene (B(a)P). AhR is a transcription factor located in the cytoplasm that is retained in complex with a variety of chaperone proteins including HSP90. Following ligand binding, the AhR complex translocates into the nucleus where it forms a heterodimer with the AhR nuclear translocator (ARNT) and binds to Xenobiotic/Dioxin response element (XRE/DRE) sequences within regulatory regions of a wide variety of target genes to activate mRNA transcription. Classical AhR target genes encode ligand-metabolizing enzymes such as CYP1A1 and UGT1A1, which promote the elimination of the original activating ligands. Recently, an increasing number of studies have shown that AhR has a wide variety of physiological roles ranging from ligand metabolism to the regulation of cellular functions such as cell proliferation, differentiation and apoptosis [1].
AhR is expressed in a broad range of various cell types and tissues with different functions [2]. Several lines of evidence suggest that AhR can negatively regulate adipocyte differentiation. We and others have previously reported that a potent AhR agonist, TCDD, suppressed adipocyte differentiation in the 3T3-L1 preadipocyte cell line [3,4]. TCDD has also been reported to inhibit adipocyte differentiation in mouse embryonic fibroblasts (MEFs) [5]. AhR can suppress the expression of transcription factors that regulate adipocyte differentiation, such as CCAAT-enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) [3]. The tyrosine kinase c-Src may be involved in TCDD-induced differentiation suppression since c-Src-deficient MEFs are less sensitive to TCDD-induced differentiation suppression and have lower induced expression levels of C/EBPβ and C/EBPδ [6]. The overexpression of AhR reportedly inhibits adipocyte differentiation in 3T3-L1 cells, suggesting that this inhibitory effect is independent of any AhR ligand [7]. AhR null mice exhibited fatty metamorphosis in the liver during their first 2 weeks of life [8]. These results suggest that AhR inhibits early adipogenesis via multiple pathways.
The AhR repressor (AhRR) was discovered during a screening of the mouse genomic library using AhR cDNA as the hybridization probe [9], and this repressor is known as a negative feedback regulator of AhR signaling [10]. AhRR is upregulated by AhR signaling because the murine and human Ahrr genes contain one or more XREs in its promoter region [10]. AhRR interacts with ARNT in the same manner as AhR to form an AhRR–ARNT complex, which down-regulates AhR signaling since AhRR lacks an AhR ligand binding domain (PAS-B domain) and transactivation domain making AhRR transcriptionally inactive. Recently, studies in AhRR knockout mice revealed that CYP1A1 induction was enhanced in the heart and spleen by the AhR ligand 3-methyl-cholanthrene, but there was no altered mRNA expression in the lung or liver despite AhRR being secreted from these tissues after treatment with 3-methylcholanthrene [11]. Thus, AhRR might function in an organ- and/or cell type-specific manner.
Several studies reported the repressive function of AhR in adipocyte differentiation, but how AhRR expression is regulated in adipocyte differentiation and whether AhRR can compete with AhR-induced differentiation suppression is unclear. Here, we examined the role of AhRR in adipocyte differentiation using 3T3-L1 preadipocyte cells.
2. Materials and methods
2.1. Culture and differentiation of 3T3-L1 cells
3T3-L1 cells were cultured and differentiated according to our previous method [12]. The 3T3-L1 cells, obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 1 mg/mL D-glucose (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA). Over the course of 48 h, confluent 3T3-L1 cells were converted into adipocytes in DMEM containing 1 mg/mL D-glucose supplemented with 1 μM dexamethasone (Dex), 0.2 mM 3-isobutyl-1-methylxanthine (IBMX), 10 μg/ mL insulin (Ins) and 10% FBS in the presence or absence of 25 mM D-allulose or D-fructose. After 2 days of culture, the cells were cultured in medium containing 10 μg/mL Ins and 10% FBS with 25 mM D-allulose or D-fructose.
2.2. Total RNA extraction and real-time PCR
mRNA levels were determined according to our previous report [13]. The primer sequences are shown in Table 1. The mRNA amount was normalized to the housekeeping gene β-actin, and the values for the treated samples were divided by those for the untreated samples to calculate the relative mRNA levels.
Table 1.
List of real-time PCR primers used in this study.
| Forward (5′–3′) | Reverse (5′–3′) | |
|---|---|---|
| C/EBPα | TAACTCCCCCATGGAGTCGG | CTGGAGGTGACTGCTCATCG |
| C/EBPβ | TGCAGAAGAAGGTGGAGCAG | GCTTGAACAAGTTCCGCAGG |
| PPARγ | GGTGTGATCTTAACTGCCGGA | GCCCAAACCTGATGGCATTG |
| AhR | TGATGCCAAAGGGCAGCTTA | TGAACTGGTACCCCGATCCT |
| AhRR | TATGGTAGAGGCCAGGAACC | GCTGCCTTTTTGTCCCTAAG |
| CYP1A1 | GGCCACTTTGACCCTTACAA | CAGGTAACGGAGGACAGGAA |
| β-Actin | CTAGGCACCAGGGTGTGATG | GGGGTACTTCAGGGTCAGGA |
2.3. Immunoblotting
3T3-L1 cells were collected and lysed with radio-immunoprecipitation assay buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% SDS). Equal amounts of protein were loaded, separated via SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The blocked membranes were incubated with the following primary antibodies: anti-AhRR [14], anti-C/EBPα (Santa Cruz Biotechnology, Dallas, TX, USA), anti-C/EBPβ (Santa Cruz Biotechnology), anti-PPARγ (Santa Cruz Biotechnology) and anti-α-tubulin (Sigma-Aldrich). Then, the membrane was incubated in solutions with peroxide-conjugated secondary antibodies (Thermo Fisher Scientific) and was visualized using peroxide substrates (SuperSignal West Dura, Thermo Fisher Scientific). The band intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
2.4. Transfection with siRNA or expression vectors
Three selected AhRR siRNA constructs against mouse AhRR were provided by the Invitrogen Stealth Select RNAi library; the catalog numbers of the constructs are MSS201854, MSS201855 and MSS201856 (Invitrogen, Waltham, MA, USA). Two control siRNAs (12935–300 and 12935–200, Invitrogen) were used as the control. The cloning and generation of the mouse AhRR expression vector (pAhRR) has been described recently [14]. A mixture of 3 AhRR siRNAs or 2 control siRNAs, pAhRR and the empty vector were transfected into 3T3-L1 cells using Lipofectamine 2000 reagent (Invitrogen) as recommended by the manufacturer. At 24 h after transfection, the cells were used for further experiments.
2.5. Oil red O (ORO) staining
Cells were fixed with 10% formalin for 1 h and then treated with 0.18% ORO solution (6: 4 isopropanol: dH2O) for 2 h. After washing the cells, the ORO dye inside the cells was extracted by gently pipetting with isopropanol. The absorbance was measured at 510 nm, and the ORO concentration was determined using a molar extinction coefficient of 3.4 × 104 cm−1 M−1.
2.6. Measurement of GPDH activity
To determine the degree of 3T3-L1 cell differentiation, glycerol-3-phosphate dehydrogenase (GPDH) activity was measured according to our previous report [12]. 3T3-L1 cells were suspended in extraction buffer (50 mM Tris-HCl (pH 7.5) containing 1 mM EDTA and 1 mM β-mercaptoethanol) and subsequently were sonicated to obtain the cell lysate. The amount of NADH consumed by dihydroxyacetone phosphate metabolism at room temperature was monitored based on the change in absorbance at 340 nm. One unit of enzyme activity corresponds to the oxidation of 1 nmol of NADH per minute.
2.7. Luciferase assay
Either a PPAR luciferase reporter plasmid (kindly gift from Dr. Haarmann-Stemmann, IUF-Leibniz-Research Institute for Environmental Medicine, Düsseldorf, Germany) or an empty vector was transfected into 3T3-L1 cells according to the manufacturer’s instructions. Twenty-four hours after transfection, the cells were used in the experiments. Luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI, USA) with a Lumat LB9501 Luminometer (Berthold, Bad Wildbad, Germany).
2.8. Statistical analyses
All data are expressed as the means ± S.E. The statistical analyses were performed using a one-way analysis of variance (ANOVA), followed by Student’s t-test or Dunnett’s test. P values of < 0.05 were considered to be statistically significant.
3. Results
3.1. Increased AhRR expression during the early stage of adipocyte differentiation
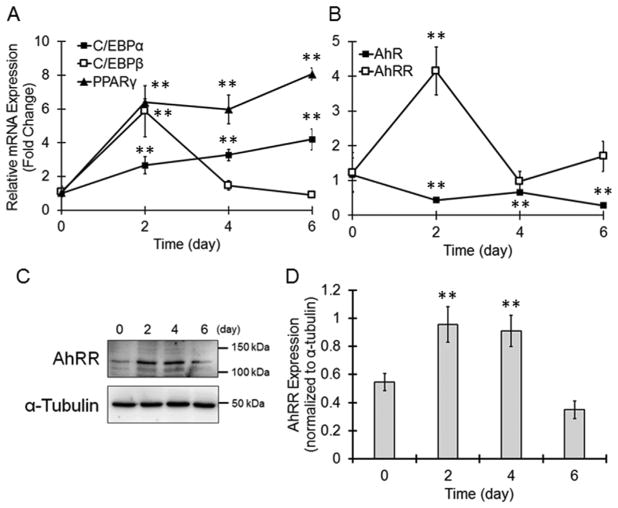
When 3T3-L1 cells were treated with Dex, IBMX and Ins, C/EBPβ expression transiently increased after 2 days, and the expression of C/ EBPα and PPARγ was upregulated in a time-dependent manner (Fig. 1A). According to previous reports, these changes are typical of adipocyte differentiation [15]. The AhR mRNA level in differentiating cells was decreased at day 2 and was maintained at a lower level than that in undifferentiated cells (Fig. 1B). Interestingly, AhRR mRNA expression was transiently increased at day 2 and reduced to basal level at day 4 (Fig. 1B). Between day 0 and 4, AhRR protein level increased in differentiating 3T3-L1 cells compared to untreated cells (Fig. 1C and D), indicating that AhRR is upregulated during the early stage of adipocyte differentiation.
Fig. 1. Increased AhRR expression during the early stage of adipogenesis in 3T3-L1 cells.
(A and B) Cells were collected at the indicated time, and the mRNA expression of (A) C/EBPα, C/EBPβ and PPARγ and (B) AhR and AhRR was evaluated by real-time PCR. Target mRNA levels were normalized to the β-actin mRNA level. The values represent the means ± SE of 3 separate experiments. The data were analyzed using one-way ANOVA, followed by Dunnett’s test. **P < 0.01 vs. day 0 group. (C and D) Cells were collected and lysed. Cellular AhRR proteins were determined by immunoblotting. (C) Representative immunoblot images. (D) The bands were quantified by ImageJ software. The protein levels of AhRR were normalized to the α-tubulin protein level. The values represent the means ± SE of 3 separate experiments. The data were analyzed using one-way ANOVA, followed by Dunnett’s test. **P < 0.01 vs. day 0 group.
3.2. Suppression of adipocyte differentiation by AhRR
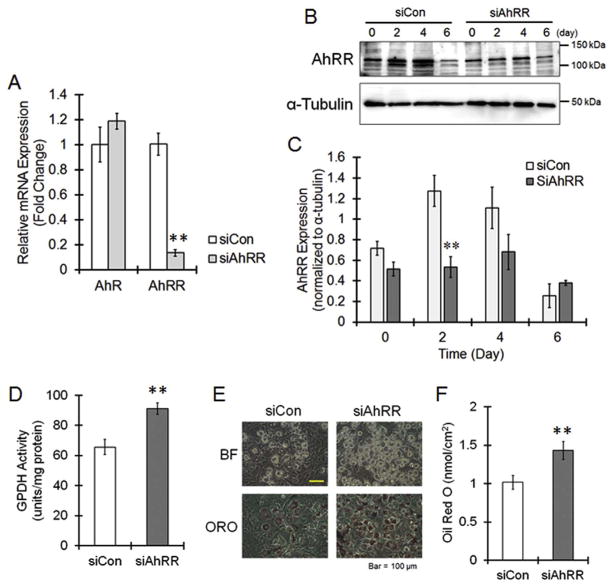
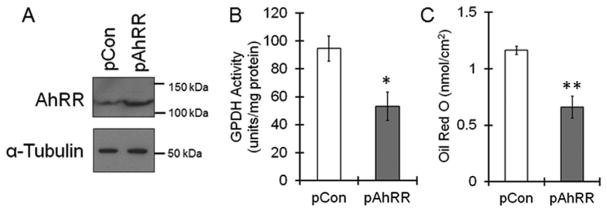
To investigate AhRR function during adipogenesis, we knocked down AhRR using siRNAs. When the 3 different siRNAs were transfected into 3T3-L1 cells, AhRR mRNA was reduced to approximately 10% of that of the control siRNA-transfected cells (Fig. 2A). The transient increase in the AhRR protein level at day 2 was significantly suppressed by siRNA treatment (Fig. 2B). AhR expression was not affected by AhRR siRNA transfection (Fig. 2A). Compared with un-transfected cells, 3T3-L1 cells transfected with AhRR siRNAs had significantly higher GPDH activity (Fig. 2D). The lipid accumulation inside cells, as assayed by Oil Red O staining, was clearly enhanced by AhRR knockdown (Fig. 2E and F). These results suggest that AhRR suppresses adipocyte differentiation. To confirm this hypothesis, we used AhRR-overexpressing 3T3-L1 cells. Increases in AhRR protein levels were observed 24 h after the AhRR expression plasmid was transfected into 3T3-L1 cells (Fig. 3A). The GPDH activity and lipid accumulation was significantly decreased in pAhRR-transfected cells compared with un-transfected cells (Fig. 3B and C). In summary, AhRR, which is upregulated during the early stage of adipogenesis, suppresses the differentiation of 3T3-L1 cells.
Fig. 2. Suppressive action of AhRR on adipocyte differentiation.
Control siRNA (siCon) or AhRR siRNA (siAhRR) complexes were added to the culture plate with Lipofectamine 2000, and the cells were cultured for 24 h, followed by stimulation with Ins, Dex and IBMX. (A) Cells were collected at day 2, and the mRNA level of AhR and AhRR was determined. The values represent the means ± SE of 3 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. siCon group. (B, C) Cells were collected in a time-dependent manner, and AhRR expression was evaluated by immunoblotting. The values represent the means ± SE of 3 separate experiments. The data were analyzed using Student’s t-test. *P < 0.05 vs. siCon group. (D) Cells were collected at day 6, and GPDH activity was measured. The values represent the means ± SE of 4 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. siCon group. (E and F) At day 6, the cells were stained with oil red O (E), and the dye was extracted for quantification (F). The values represent the means ± SE of 4 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. siCon group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3. Inhibition of adipocyte differentiation by AhRR over-expression.
Mouse AhRR expression plasmid (pAhRR) or empty vector (pCon) was transfected into the cells. Cells were cultured for 24 h, followed by stimulation with Ins, Dex and IBMX. (A) AhRR protein expression was measured by immunoblotting 24 h after transfection. (B) Cells were collected at day 6, and GPDH activity was measured. The values represent the means ± SE of 4 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. pCon group. (C) At day 6, the cells were stained with oil red O, and the dye was extracted for quantification. The values represent the means ± SE of 4 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. pCon group.
3.3. Potentiation of PPARγ expression and activity by AhRR knockdown in 3T3-L1 cells
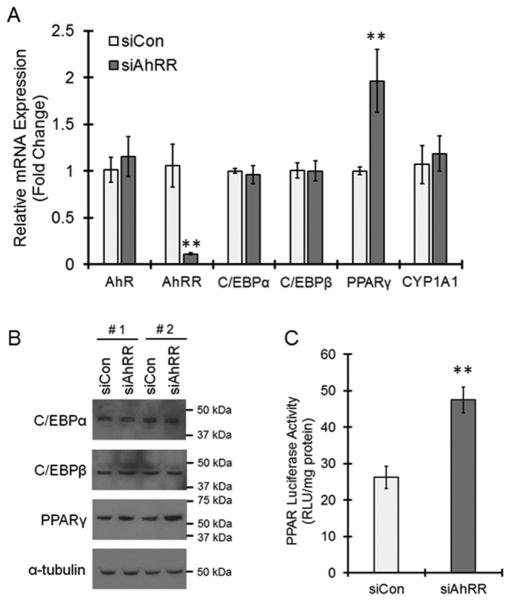
We next examined the mechanism of AhRR action in adipogenesis inhibition via focusing on the expression of transcription factors responsible for adipocyte differentiation. Although AhRR knockdown did not affect the mRNA and protein expressions of C/EBPα and C/EBPβ, PPARγ mRNA and protein expressions were induced by AhRR transfection (Fig. 4A and B). The luciferase assay results suggested that PPARγ transcriptional activity was also significantly increased by AhRR knockdown (Fig. 4C). Therefore, PPARγ is downregulated by AhRR during adipogenesis. In this experimental condition, AhRR knockdown showed no change in the mRNA expressions of CYP1A1, a major target gene of AhR, as well as AhR itself (Fig. 4A), suggesting that AhR signaling is not involved in AhRR-mediated PPARγ regulation.
Fig. 4. Inhibitory action of AhRR on PPARγ expression and activity.
(A and B) Cells were transfected with control (siCon) or AhRR (siAhRR) siRNAs and then were treated with Ins, Dex and IBMX. (A) Cells were collected at day 1, and real-time PCR was performed. The values represent the means ± SE of 4 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. siCon group. (B) Cells were collected at day 1 and lysed. C/EBPα, C/EBPβ and PPARγ proteins were determined by immunoblotting. The results of two independent experiments (#1 and #2) are shown. (C) Cells were co-transfected with the PPAR luciferase vector in the presence of control siRNA or AhRR siRNA. After 24 h of culture, the cells were stimulated by Ins, Dex and IBMX for 24 h. Cells were collected and lysed, and luciferase activity was measured. The values represent the means ± SE of 6 separate experiments. The data were analyzed using Student’s t-test. **P < 0.01 vs. siCon group.
4. Discussion
AhR signaling is well known to negatively regulate adipocyte differentiation. AhRR, a negative regulator of AhR transactivation, is transiently induced during the early stage of adipocyte differentiation and suppresses differentiation. Therefore, AhRR has been suggested to have the same role as AhR in adipogenesis.
AhRR was identified as an AhR binding protein, and this repressor reportedly suppresses AhR signaling by competing with ARNT for binding with AhR. On the other hand, CYP1A1 induction by AhR activation is not always enhanced in AhRR KO mice [11], and thus, the function of AhRR has remained a subject of dispute. Interestingly, in AhRR transgenic mice, which we produced previously, the basal expression of CYP1A1 in the kidney was increased compared to wild-type mice [14], suggesting that AhRR may have a positive regulatory function in murine kidneys. AhRR has been reported not to suppress CYP1A1 expression in human skin fibroblasts [16]. Compared with wild-type mice, novel AhRR KO mice exhibited a low sensitivity to lipopolysaccharide (LPS) shock but a high sensitivity to intestinal inflammation induced by dextran sodium sulfate [17]. In addition, like AhR, AhRR has been suggested to activate NF-kB [18], indicating a novel function for AhRR beyond the mere suppression of AhR. AhRR has been reported to be sumoylated, which recruits co-repressors to inhibit the transcription mediated by AhR-ARNT heterodimers [19]. We previously showed that RelB, a subunit of NF-kB, is a binding partner of AhR that enhances cytokine/chemokine transcription [20], but whether AhRR acts on complexes other than that of AhR-ARNT, such as an AhR-RelB complex, is unclear. To gain a more detailed mechanistic insight into AhRR’s action, future analyses of posttranslational modifications and/or the identification of binding partners are needed.
AhRR has several XREs in its promoter region. AhR signaling up-regulates AhRR and then negatively regulates its own (AhR) function; AhRR is a major component of the negative feedback loop of AhR signaling. However, because AhR expression decreases during the early stage of 3T3-L1 differentiation, AhR is believed to not contribute to the transient increase in AhRR. In addition to binding sites for AhR, murine AhRR has binding motifs for NF-kB and SP-1/3 in its promoter region [10]. NF-kB activity has been reported to be inversely correlated with adipocyte differentiation [21], and thus, the function of AhRR and NF-kB may have a similar effects on adipocyte differentiation. In addition, AhRR expression is reportedly promoted by a PKC activator, phorbol 12-myristate 13-acetate [9]. It is well known that several MAP kinases such as ERK and p38 are activated downstream of PKC activation. Although there is still debate about whether ERK is necessary for adipocyte differentiation, it is accepted that ERK is activated during the early stage of adipogenesis [22,23]. Furthermore, p38 is also activated during the early stage of adipogenesis [24]. Therefore, the upregulation of AhRR in differentiating 3T3-L1 cells might occur downstream of MAPK signaling.
In adipose tissues, PPARγ, a master regulator of adipogenesis, is induced by C/EBPβ, and then C/EBPα plays a role in maintaining the expression of PPARγ during adipogenesis [25]. In this study, AhRR suppressed PPARγ expression in the early stage of adipogenesis, while AhRR did not affect the expressions of C/EBPα and C/EBPβ, suggesting PPARγ suppression by AhRR is independent of C/EBP signaling that generally observed in adipocyte differentiation. AhRR is reported to attenuate estrogen receptor α-mediated transcription via direct interaction of AhRR with estrogen receptor α on an estrogen response element [26]. Estradiol is known to induce PPARγ expression in various types of tissues [27–29]. Therefore, suppressive effect of AhRR in estrogen signaling might be involved in the attenuation of PPARγ expression during adipogenesis.
Compared with wild-type mice, AhRR KO mice show no change in body weight [11]. The body weight of AhRR transgenic mice was nearly identical to that of wild-type mice (data not shown). The upregulation of AhRR in adipogenesis was transient and the increased AhRR levels returned to basal levels at day 4. Thus, these results are consistent with the finding that the body weight of the adult, AhRR genetically-modified mice was unchanged. Meanwhile, mature adipocyte produces several humoral factors such as adiponectin to not only maintain homeostasis but also prevent disorders such as arteriosclerosis, type 2 diabetes and carcinogenesis [30]. Cells with AhRR knockdown presented with increased adiponectin expression (data not shown). Therefore, the transient expression of AhRR during early adipogenesis might be involved in pathological processes beyond the transient pause in adipocyte differentiation. Further study is needed to reveal the (patho)physiological role of the transient AhRR expression in differ-entiating adipocytes.
In conclusion, we have shown that AhRR inhibits adipocyte differentiation associated with the suppression of PPARγ expression, indicating that AhRR, similar to AhR, can act as an inhibitor of adipocyte differentiation. In addition to controlling the negative feedback loop of AhR, AhRR may be involved in other cellular functions, especially in adipocyte differentiation processes.
Acknowledgments
Funding
This work was partially supported by Grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan, KAKENHI to YI (No. 15KK0024) and the National Institute of Environmental Health Sciences of the National Institutes of Health to CV (No. R01 ES019898).
This manuscript has been reviewed by a professional language editing service (American Journal Experts).
Footnotes
Author contribution
YI and CV designed this research, performed the experiments and wrote the paper. MT helped with critical advices and discussion. All authors reviewed the manuscript.
Competing financial interests
The authors declare no conflicts of interest in association with the present study.
This article is dedicated to the memory of Professor Fumio Matsumura, who passed away in December 2012.
References
- 1.Bock KW, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem. 2009;390(12):1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 3.Liu PC, Phillips MA, Matsumura F. Alteration by 2,3,7,8-Tetrachlorodibenzo-p-dioxin of CCAAT/enhancer binding protein correlates with suppression of adipocyte differentiation in 3T3-L1 cells. Mol Pharmacol. 1996;49(6):989–997. [PubMed] [Google Scholar]
- 4.Wu-Wang CY, Xu XR, Wang SL. Benzo[a]pyrene and nicotine alter prostaglandin E2 receptor and its functions in hamster buccal mucosa. Toxicol Lett. 1996;84(2):81–88. doi: 10.1016/0378-4274(95)03619-9. [DOI] [PubMed] [Google Scholar]
- 5.Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CF, Matsumura F. Interaction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with induced adipocyte differentiation in mouse embryonic fibroblasts (MEFs) involves tyrosine kinase c-Src. Biochem Pharmacol. 2003;66(7):1231–1244. doi: 10.1016/s0006-2952(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 7.Shimba S, Wada T, Tezuka M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J Cell Sci. 2001;114(Pt 15):2809–2817. doi: 10.1242/jcs.114.15.2809. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77(4):485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosoya T, Harada N, Mimura J, Motohashi H, Takahashi S, Nakajima O, Morita M, Kawauchi S, Yamamoto M, Fujii-Kuriyama Y. Inducibility of cytochrome P450 1A1 and chemical carcinogenesis by benzo[a]pyrene in AhR repressor-deficient mice. Biochem Biophys Res Commun. 2008;365(3):562–567. doi: 10.1016/j.bbrc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Itoh K, Mizuno S, Hama S, Oshima W, Kawamata M, Hossain A, Ishihara Y, Tokuda M. Beneficial effects of supplementation of the rare sugar D-allulose against hepatic steatosis and severe obesity in lep(ob)/lep(ob) mice. J Food Sci. 2015;80(7):H1619–H1626. doi: 10.1111/1750-3841.12908. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara Y, Takemoto T, Itoh K, Ishida A, Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J Biol Chem. 2015;290(37):22805–22817. doi: 10.1074/jbc.M115.659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel CF, Chang WL, Kado S, McCulloh K, Vogel H, Wu D, Haarmann-Stemmann T, Yang G, Leung PS, Matsumura F, Gershwin ME. Transgenic over-expression of aryl hydrocarbon receptor repressor (AhRR) and AhR-mediated induction of CYP1A1, cytokines, and acute toxicity. Environ Health Perspect. 2016;124(7):1071–1083. doi: 10.1289/ehp.1510194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamm JK, Park BH, Farmer SR. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 pre-adipocytes. J Biol Chem. 2001;276(21):18464–18471. doi: 10.1074/jbc.M100797200. [DOI] [PubMed] [Google Scholar]
- 16.Tigges J, Weighardt H, Wolff S, Gotz C, Forster I, Kohne Z, Huebenthal U, Merk HF, Abel J, Haarmann-Stemmann T, Krutmann J, Fritsche E. Aryl hydrocarbon receptor repressor (AhRR) function revisited: repression of CYP1 activity in human skin fibroblasts is not related to AhRR expression. J Invest Dermatol. 2013;133(1):87–96. doi: 10.1038/jid.2012.259. [DOI] [PubMed] [Google Scholar]
- 17.Brandstatter O, Schanz O, Vorac J, Konig J, Mori T, Maruyama T, Korkowski M, Haarmann-Stemmann T, von Smolinski D, Schultze JL, Abel J, Esser C, Takeyama H, Weighardt H, Forster I. Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor. Sci Rep. 2016;6:26091. doi: 10.1038/srep26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang DD, Wang WT, Xiong J, Xie XM, Cui SS, Zhao ZG, Li MJ, Zhang ZQ, Hao DL, Zhao X, Li YJ, Wang J, Chen HZ, Lv X, Liu DP. Long noncoding RNA LINC00305 promotes inflammation by activating the AHRR-NF-kappaB pathway in human monocytes. Sci Rep. 2017;7:46204. doi: 10.1038/srep46204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima M, Mimura J, Sekine H, Okawa H, Fujii-Kuriyama Y. SUMO modification regulates the transcriptional repressor function of aryl hydrocarbon receptor repressor. J Biol Chem. 2009;284(17):11017–11026. doi: 10.1074/jbc.M808694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21(12):2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Li Y, Liang W, Wong S, Apovian C, Kirkland JL, Corkey BE. Beta-mecaptoethanol suppresses inflammation and induces adipogenic differentiation in 3T3-F442A murine preadipocytes. PLoS One. 2012;7(7):e40958. doi: 10.1371/journal.pone.0040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sale EM, Atkinson PG, Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14(4):674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Font de Mora J, Porras A, Ahn N, Santos E. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3-L1 adipocytic differentiation. Mol Cell Biol. 1997;17(10):6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273(48):32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16(1):22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanno Y, Takane Y, Takizawa Y, Inouye Y. Suppressive effect of aryl hydrocarbon receptor repressor on transcriptional activity of estrogen receptor alpha by protein-protein interaction in stably and transiently expressing cell lines. Mol Cell Endocrinol. 2008;291(1–2):87–94. doi: 10.1016/j.mce.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Ueki S, Oguma M, Usami A, Kamada Y, Kato H, Kamada R, Takeda M, Ito W, Tanigai T, Kayaba H, Chihara J. Regulation of peroxisome proliferator-activated receptor-gamma expression in human eosinophils by estradiol. Int Arch Allergy Immunol. 2009;149(1):51–56. doi: 10.1159/000210654. [DOI] [PubMed] [Google Scholar]
- 28.Jeong YJ, Noh EM, Lee YR, Yu HN, Jang KY, Lee SJ, Kim J, Kim JS. 17beta-estradiol induces up-regulation of PTEN and PPARgamma in leiomyoma cells, but not in normal cells. Int J Oncol. 2010;36(4):921–927. doi: 10.3892/ijo_00000571. [DOI] [PubMed] [Google Scholar]
- 29.Tiyerili V, Muller CF, Fung S, Panek D, Nickenig G, Becher UM. Estrogen improves vascular function via peroxisome-proliferator-activated-receptor-gamma. J Mol Cell Cardiol. 2012;53(2):268–276. doi: 10.1016/j.yjmcc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]