Abstract
Objective
Abdominal obesity has been associated with erosive oesophagitis (EO) and Barrett’s oesophagus (BO). As gluteofemoral obesity protects against diabetes mellitus and cardiovascular disease, we hypothesised that gluteofemoral obesity would be inversely associated with EO and BO.
Design
We conducted a cross-sectional study on 822 male colorectal cancer screenees who were recruited to also undergo upper endoscopy. An additional 80 patients with BO clinically detected by upper endoscopy referred for clinical indications were recruited shortly after their diagnoses of BO. Logistic regression was used to estimate the effects of abdominal obesity (waist circumference), gluteofemoral obesity (hip circumference) and waist-to-hip ratio (WHR) on EO and BO (vs neither condition).
Results
There were 225 cases of either BO or EO and 675 controls. After adjustment for potential confounders, a positive association was observed between waist circumference and BO and/or EO, which became stronger with further adjustment for hip circumference. In contrast, hip circumference was inversely associated with BO and/or EO. Compared with the lowest quartile of WHR, the adjusted ORs were 1.32 (95% CI 0.747 to 2.33) for the 2nd quartile, 1.54 (95% CI 0.898 to 2.63) for the 3rd quartile, and 2.68 (95% CI 1.57 to 4.55) for the highest quartile. Similar results were obtained for BO and EO treated as separate outcomes.
Conclusions
In a population of older, mostly overweight men, the distribution of obesity is associated with the presence of EO and BO. Abdominal obesity appears to increase the risk of these outcomes, whereas gluteofemoral obesity may be protective.
INTRODUCTION
Multiple studies have demonstrated an association of obesity with gastro-oesophageal reflux disease (GORD) and its sequelae of erosive oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma.1–12 A few studies have also demonstrated a specific role for obesity localised to the abdomen with these sequelae.4,9 Thus, obesity may promote GORD and its sequelae through a mechanical effect, and, indeed, abdominal obesity has been found to be associated with differences in intraoesophageal and intragastric pressures which can promote GORD.5,13 However, abdominal obesity is also associated with a number of health outcomes that do not have a mechanical explanation, including cardiovascular disease, diabetes mellitus and cancers. In contrast with the adverse effects of abdominal obesity, adipose located in the hips and thighs (‘gluteofemoral obesity’) is inversely associated with cardiovascular disease and diabetes, once adjustments are made for abdominal obesity.14 Gluteofemoral adipose is less active than visceral adipose in terms of secretion of inflammatory adipokines.15 Therefore, gluteofemoral adipose may serve as a sink for storing adipose in a manner that avoids the inflammatory and other humoral effects of adipose stored in the visceral compartment.15 We sought to clarify the differential effects of abdominal and gluteofemoral obesity on the outcomes of GORD and its sequelae. We hypothesised that abdominal obesity (measured by waist circumference) would be positively associated with acid reflux, erosive oesophagitis and Barrett’s oesophagus due to its mechanical effects, but that gluteofemoral obesity would be inversely associated with erosive oesophagitis and Barrett’s oesophagus (after adjustment for abdominal obesity) due to humoral effects.
METHODS
Study design
We conducted a cross-sectional study of male colorectal cancer (CRC) screenees, aged 50–79, presenting for colonoscopy at two medical centres and recruited to undergo upper endoscopy (consent rate 71%) and ambulatory oesophageal pH monitoring. We enrolled the CRC screenees regardless of symptoms of GORD, and prospectively classified them on the basis of erosive oesophagitis or Barrett’s oesophagus, and a subcohort of them on the basis of oesophageal acid exposure time (detailed below). In addition, we recruited men aged 50–79 who had recently been diagnosed for the first time with Barrett’s oesophagus by a clinically indicated upper endoscopy at either of the two clinical centres. The details of the inclusion and exclusion criteria and enrolment and questionnaires have been reported separately.16 The study was approved by the institutional review boards of the University of Michigan and the Ann Arbor Veterans Affairs Medical Center.
After informed consent had been obtained, patients changed into a hospital gown or pyjamas and had their weight, height, waist circumference and hip circumference measured in duplicate by previously described techniques.17–19 CRC screenees answered questions administered by the research staff regarding GORD symptoms and medication use before undergoing endoscopy. Patients were classified as having symptomatic GORD if they reported heartburn or regurgitation at least once a week while not taking proton pump inhibitors or histamine-2 receptor antagonists. CRC screenees first underwent colonoscopy, followed by the upper endoscopy. The distal oesophagus and gastro-oesophageal junction were inspected using narrow band imaging. If Barrett’s oesophagus was suspected by the endoscopist, biopsy specimens were obtained in four quadrants every 2 cm in addition to specimens of any visible irregularities for review by an expert pathologist (HDA). Barrett’s oesophagus was defined as endoscopic suspicion of columnar mucosa proximal to the gastric folds with a pathology finding of specialised intestinal metaplasia. Patients with Barrett’s oesophagus identified on a clinically indicated upper endoscopy fulfilled the same criteria for diagnosis. Patients were classified as having oesophagitis if at least Los Angeles Class B oesophagitis was found.20 If Los Angeles Class C or D oesophagitis were found, CRC screenees were instructed to repeat the endoscopy while taking a proton pump inhibitor, and Barrett’s oesophagus status was determined from the repeat endoscopy. Patients self-administered the Block Brief Food Frequency Questionnaire,21 the Morgenstern Physical Activity Questionnaire22 and a questionnaire including queries regarding tobacco use—typically completed after the endoscopy.
The CRC screenees who underwent upper endoscopy were also recruited to undergo ambulatory oesophageal pH monitoring while off proton pump inhibitors for at least 10 days and histamine-2 receptor antagonists for at least 7 days. Sixty-four patients underwent this procedure (consent rate 7.8%). After the patient had completed an overnight fast, the pH catheter was placed transnasally, with the sensor located 5 cm above the lower oesophageal sphincter. Patients returned after 18–24 h of recording for removal of the catheter. Acid reflux while in the upright position was considered abnormal if the oesophageal exposure time to pH <4 was at least 6.3%, and in the supine position if at least 1.2%.
Statistical analysis
Data were manually entered into Microsoft Access (Microsoft, Bellevue, Washington, USA), then imported into SAS V.9.1. Waist circumference and hip circumference were dichotomised at their medians and categorised into tertiles based on the observed frequencies among the CRC screenees. Waist-to-hip ratio (WHR) was calculated, and dichotomised at the median, and categorised into tertiles and quartiles based on the observed frequencies among the CRC screenees.
Cases of Barrett’s oesophagus identified among CRC screenees were compared with cases identified among patients undergoing clinically indicated upper endoscopies using t tests. We used logistic regression to estimate the effects of waist circumference, hip circumference and WHR on each of three outcome variables: erosive oesophagitis or Barrett’s oesophagus (vs neither condition), abnormal reflux while upright, and abnormal reflux while supine, after adjustment for the following potential confounders: age, pack-years of cigarette use, daily energy intake, proportion of energy from fat, and physical activity score. The effects of waist and hip circumferences were estimated with and without adjustment for each other; the effect of WHR was estimated with and without adjustment for waist circumference. Because of the similarity in directions of the estimated effects of waist and hip circumferences on oesophagitis and Barrett’s oesophagus (see online supplementary tables S1 and S2), the strong association between waist and hip circumferences (table 1) and the relatively small number of cases with either outcome, these effects were estimated on the combined outcome of either oesophagitis or Barrett’s oesophagus versus neither condition in order to minimise error resulting from sparse data.
Table 1.
Pearson correlation coefficients between measures of obesity
| Obesity measure | BMI | Waist circumference | Hip circumference | WHR |
|---|---|---|---|---|
| BMI | n/a | 0.93 | 0.91 | 0.48 |
| Waist circumference | 0.93 | n/a | 0.90 | 0.64 |
| Hip circumference | 0.91 | 0.90 | n/a | 0.26 |
| WHR | 0.48 | 0.64 | 0.26 | n/a |
BMI, body mass index; WHR, waist-to-hip ratio.
To further understand the possible effects of abdominal and gluteofemoral obesity on oesophagitis and Barrett’s oesophagus, we also estimated the combined effects (interaction) of waist and hip circumferences. Waist and hip circumferences were categorised into tertiles, and subjects were cross-classified into nine groups, with the reference group being those in both the 1st tertile of waist and 1st tertile of hip. For estimates of their combined effects on the separate outcomes of erosive oesophagitis and Barrett’s oesophagus, we created four groups of waist and hip cross-classified from their medians.
RESULTS
A total of 822 CRC screenees underwent upper endoscopy. The median age was 59 years (IQR=53, 63), mean body mass index (BMI) was 30 kg/m2 (SD=5.5), mean waist circumference was 107.7 cm (SD=13.6), mean hip circumference was 107.3 cm (SD=10.8), and mean WHR was 1.000 (SD=0.056). There were very strong correlations between BMI, waist circumference and hip circumference (table 1). WHR correlated only moderately with BMI and waist circumference and weakly positively with hip circumference. Weekly GORD symptoms were reported by 155 (19.3%) of the CRC screenees. Erosive oesophagitis was found in 93 CRC screenees (11.3%), and Barrett’s oesophagus was found in 70 (8.5%). A minority of patients with Barrett’s oesophagus or with erosive oesophagitis reported weekly GORD symptoms (figure 1). A minority of patients with Barrett’s oesophagus also had erosive oesophagitis, and vice versa (figure 1).
Figure 1.
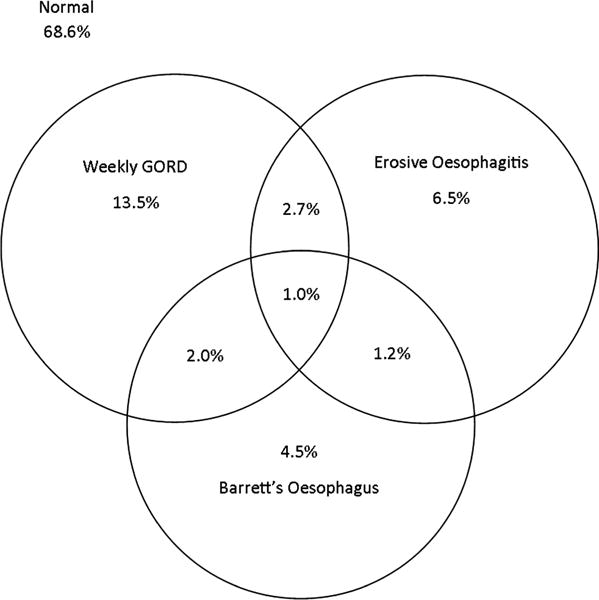
Ven diagram of gastro-oesophageal reflux disease (GORD)-related findings among colorectal cancer screenees. Results do not precisely match proportions reported in the text because the figure only reports data from the 805 subjects without any missing data for the three outcomes of GORD frequency, erosive oesophagitis or Barrett’s oesophagus.
In addition, 80 men diagnosed with Barrett’s oesophagus by a clinically indicated upper endoscopy were also studied. The leading indications for the upper endoscopy were symptoms of GORD in 41 (51.3%), dysphagia in 12 (15%), abdominal pain in 11 (13.8%), blood loss in eight (10%), and nausea or vomiting in four (5%). Compared with CRC screenees without either erosive oesophagitis or Barrett’s oesophagus, patients with these conditions had greater BMI, waist circumference and WHR (table 2). Patients with Barrett’s oesophagus were also older, smoked more and had a higher energy intake and a greater proportion of energy obtained from fat than CRC screenees without either condition (table 2). Compared with the cases of Barrett’s oesophagus identified among the CRC screenees, the clinically diagnosed cases of Barrett’s oesophagus had similar BMI (means 31.0 vs 30.3 kg/m2, p=0.35), waist circumference (means 110.4 vs 109.7 cm, p=0.77), hip circumference (means 107.9 vs 107.6 cm, p=0.73) and WHR (means 1.021 vs 1.020, p=0.93).
Table 2.
Characteristics of patients with Barrett’s oesophagus, erosive oesophagitis and neither
| Characteristic | CRC screenees without EO or BO (n=676)* | CRC screenees with EO (n=93)* | BO (n=150)* |
|---|---|---|---|
| Age (years)† | 59 (52, 63) | 59 (53, 62) | 61 (56, 66) |
| Body mass index (kg/m2)‡ | 29.8 (5.5) | 31.1 (5.5) | 30.7 (5.0) |
| Waist circumference (cm)‡ | 107.0 (13.8) | 111.5 (12.9) | 110.1 (12.9) |
| Hip circumference (cm)‡ | 107.1 (11.0) | 108.7 (10.7) | 107.7 (9.5) |
| Waist-to-hip ratio‡ | 0.998 (0.055) | 1.025 (0.056) | 1.021 (0.053) |
| Pack-years smoking† | 7 (0, 41) | 2 (0, 36) | 32 (1, 68) |
| Energy intake (kcal/day)† | 1618 (1230, 2120) | 1779 (1244, 2161) | 1758 (1253, 2241) |
| Proportion of energy intake from fat (%)‡ | 37.9 (7.4) | 38.7 (6.8) | 39.7 (6.6) |
| Physical activity score (kcal/kg/week)† | 148 (95, 217) | 152 (87, 209) | 149 (88, 218) |
Numbers do not sum to 902 because 18 CRC screenees had both EO and BO, and are included in two columns, and one CRC could not be classified because BO was suspected endoscopically, but biopsy samples were not obtained because of coexisting oesophageal varices.
Data presented as median (IQR).
Data presented as mean (SD).
BO, Barrett’s oesophagus; CRC, colorectal cancer; EO, erosive oesophagitis.
Waist circumference was positively associated with erosive oesophagitis or Barrett’s oesophagus (vs neither condition) (table 3). After adjustment for hip circumference, the magnitude of the association with waist circumference was strengthened. In contrast, after adjustment for waist circumference, the association with hip circumference became inverse, albeit with an imprecise estimate (table 3). Similar effect estimates were found if we excluded cases of Barrett’s oesophagus diagnosed by a clinically indicated upper endoscopy (data not shown).
Table 3.
Associations of waist and hip circumferences with erosive oesophagitis (EO) or Barrett’s oesophagus (BO)
| No with no EO or BO/No with EO or BO | Crude OR (95% CI) | Adjusted OR (95% CI)* | OR (95% CI) further adjusted for each other† | |
|---|---|---|---|---|
| Waist circumference | ||||
| 1st tertile (<100.8 cm) | 242/51 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2nd tertile (100.8–111.9 cm) | 216/85 | 1.89 (1.26 to 2.77) | 1.61 (1.03 to 2.52) | 1.73 (0.964 to 3.09) |
| 3rd tertile (≥ 111.9 cm) | 217/89 | 1.95 (1.32 to 2.87) | 1.54 (0.979 to 2.43) | 1.96 (0.926 to 4.15) |
| Per 5 cm increments in waist | 1.09 (1.04 to 1.15) | 1.06 (0.990 to 1.13) | 1.09 (0.985 to 1.22) | |
| p Value for trend | 0.002 | 0.10 | 0.09 | |
| Hip circumference | ||||
| 1st tertile (<102.0 cm) | 233/60 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2nd tertile (102.0–110.1 cm) | 221/82 | 1.44 (0.985 to 2.11) | 1.39 (0.901 to 2.14) | 0.959 (0.541 to 1.70) |
| 3rd tertile (≥110.1 cm) | 221/83 | 1.46 (0.998 to 2.13) | 1.24 (0.801 to 1.92) | 0.743 (0.361 to 1.53) |
| Per 5 cm increments in hip | 1.04 (0.970 to 1.11) | 0.995 (0.918 to 1.08) | 0.872 (0.766 to 0.995) | |
| p Value for trend | 0.07 | 0.42 | 0.38 |
Adjusted for age, pack-years of cigarette use, daily energy intake, proportion of energy from fat, and physical activity.
Adjusted for the same variables as above plus waist or hip circumference treated as categorical variables (tertiles).
The estimated effects of combinations of waist and hip circumferences on erosive oesophagitis or Barrett’s oesophagus are displayed in table 4. This provides further evidence for the opposite associations of waist and hip circumference with the combined outcome of Barrett’s oesophagus or erosive oesophagitis. Specifically, among men in the middle tertile of hip circumference, there was a positive, monotonically increasing association with larger waist circumference. In contrast, among men in the middle tertile of waist circumference, there was a negative monotonic association with larger hip circumference: men in the lowest tertile of hip and middle tertile of waist were approximately twice as likely to have Barrett’s oesophagus or erosive oesophagitis than those in the lowest tertile of both, but men in the highest tertile of hip and middle tertile of waist had little or no excess prevalence of these outcomes. Similar patterns of a negative association with hip circumference among those with larger waists were observed for the outcomes of Barrett’s oesophagus as for erosive oesophagitis (see online supplementary tables S3 and S4). The results in table 4 provide little information about the effect modification between waist and hip because there was a very strong association between waist and hip, resulting in small numbers of subjects in the off-diagonal cells.
Table 4.
Associations of combinations of waist and hip circumferences with either erosive oesophagitis (EO) or Barrett’s oesophagus (BO)
| Hip circumference
|
||||
|---|---|---|---|---|
| 1st tertile (<102.0 cm) | 2nd tertile (102.0–110.1 cm) | 3rd tertile (≥110.1 cm) | ||
| Waist circumference | 1st tertile (<100.8 cm) | 195/43 | 46/7 | 1/1 |
| 1 (reference) | 0.690 (0.292 to 1.63) | n/a* | ||
| 1 (reference) | 1.08 (0.435 to 2.70) | n/a* | ||
| 2nd tertile (100.8–111.9 cm) | 36/17 | 136/56 | 44/12 | |
| 2.14 (1.10 to 4.16) | 1.87 (1.19 to 2.94) | 1.24 (0.603 to 2.54) | ||
| 2.14 (1.01 to 4.50) | 1.70 (1.01 to 2.86) | 1.15 (0.502 to 2.63) | ||
| 3rd tertile (≥111.9 cm) | 2/0 | 39/19 | 176/70 | |
| n/a* | 2.21 (1.17 to 4.19) | 1.80 (1.17 to 2.78) | ||
| n/a* | 1.97 (0.929 to 4.16) | 1.54 (0.935 to 2.54) | ||
The 1st line of each cell is the number of patients without BO or EO/number with BO or EO. The 2nd line of each cell is the crude OR (95% CI). The 3rd line of each cell is the OR (95% CI) adjusted for age, pack-years of cigarette use, daily energy intake, proportion of energy from fat, and physical activity.
The effects could not be validly estimated.
Since erosive oesophagitis and Barrett’s oesophagus, as well as the combined outcome, were positively associated with waist circumference and inversely associated with hip circumference, the effects of both obesity measures might be more efficiently estimated by modelling the outcome as a function of WHR. Indeed, WHR was strongly positively associated with erosive oesophagitis or Barrett’s oesophagus (table 5), after adjustment for potential confounders. Compared with the lowest quartile of WHR, the adjusted ORs were 1.32 (95% CI 0.747 to 2.33) for the second quartile, 1.54 (95% CI 0.898 to 2.63) for the third quartile, and 2.68 (95% CI 1.57 to 4.55) for the highest quartile (p for trend <0.001). These results were similar when also adjusted for waist circumference (table 5). Similar effect estimates were found for Barrett’s oesophagus and erosive oesophagitis treated as separate outcomes (see online supplementary tables S5 and S6) and when cases of Barrett’s oesophagus diagnosed by a clinically indicated upper endoscopy were excluded (results not shown). Given the small sample size and the strong correlation between waist and hip circumference, the mutually adjusted estimates of waist and hip circumferences on acid exposure could not be validly estimated (see online supplementary materials and supplementary tables S7 and S8).
Table 5.
Association of waist-to-hip ratio (WHR) with erosive oesophagitis (EO) or Barrett’s oesophagus (BO)
| WHR | No with no BO or EO/No with BO or EO | Crude OR (95% CI) | Adjusted OR (95% CI)* | OR (95% CI) further adjusted for waist† |
|---|---|---|---|---|
| 1st quartile (<0.964) | 185/30 | 1 (reference) | 1 (reference) | 1 (reference) |
| 2nd quartile (0.964–1.000) | 164/48 | 1.81 (1.09 to 2.98) | 1.32 (0.747 to 2.33) | 0.803 (0.369 to 1.74) |
| 3rd quartile (1.000–1.039) | 177/63 | 2.20 (1.36 to 3.55) | 1.54 (0.898 to 2.63) | 1.46 (0.703 to 3.01) |
| 4th quartile (>1.039) | 149/84 | 3.48 (2.17 to 5.56) | 2.68 (1.57 to 4.55) | 2.87 (1.31 to 6.33) |
| Per 1 SD increment in WHR (0.056) | 1.57 (1.33 to 1.83) | 1.49 (1.23 to 1.79) | 1.66 (1.25 to 2.20) | |
| p Value for trend | <0.001 | <0.001 | <0.001 |
Adjusted for age, pack-years of cigarette use, daily energy intake, proportion of energy from fat, and physical activity.
Adjusted for the same variables as above plus waist circumference (treated as categorical in tertiles).
DISCUSSION
To our knowledge, previous studies have not explored the possible protective role of gluteofemoral obesity in GORD or its sequelae. Multiple studies have demonstrated an association between total body obesity and GORD or its sequelae. For instance, BMI has been associated with acid reflux among patients referred for clinical investigation.3–5 One of these studies also identified an association of acid reflux with waist circumference, but it did not report the associations with hip circumference or WHR.4 BMI and waist circumference are associated with increased intra-abdominal pressure, an increased pressure gradient across the gastro-oesophageal junction, and an increased resting lower oesophageal sphincter pressure.5,13 The increase in the pressure gradient is larger than the increase in the lower oesophageal sphincter pressure. Abdominal obesity is also associated with migration of the lower oesophageal sphincter along the continuum toward a hiatal hernia such that a smaller proportion of the sphincter is located intra-abdominally.13 Therefore abdominal obesity probably promotes GORD through a mechanical effect. In addition, obesity has been associated with an increased frequency of transient relaxations of the lower oesophageal sphincter, a major causative factor in most individuals with GORD symptoms.23 Abdominal obesity has previously been associated with erosive oesophagitis, including in cross-sectional studies of Asians undergoing upper endoscopy for average risk screening for foregut cancers.6–8 Abdominal obesity has been associated with Barrett’s oesophagus, dysplastic Barrett’s oesophagus and oesophageal adenocarcinoma.9–12 WHR has been shown to be associated with Barrett’s oesophagus after adjustment for BMI, but that study did not present data distinguishing whether the association was due to the effect of abdominal obesity, gluteofemoral obesity or their combined effect, and it did not examine the associations with erosive oesophagitis or acid reflux.9
In this study, we found that both abdominal obesity (as measured by waist circumference) and gluteofermoral obesity (as measured by hip circumference) were positively associated with erosive oesophagitis or Barrett’s oesophagus; however, after adjustment for abdominal obesity, gluteofemoral obesity was inversely associated. Our findings suggest that having relatively large waists and small hips may increase the risk of erosive oesophagitis and Barrett’s oesophagus in men. We infer from this study that abdominal obesity may promote acid reflux while upright, and gluteofemoral obesity may protect against erosive oesophagitis and Barrett’s oesophagus. Put another way, abdominal obesity promotes acid reflux while upright, but for two men with the same waist circumference, the one with the larger hip circumference may be less likely to have erosive oesophagitis or Barrett’s oesophagus. Given the small numbers of subjects with abnormal acid reflux receiving ambulatory oesophageal pH monitoring and the resulting wide CIs, we cannot exclude a protective role for gluteofemoral obesity in acid reflux. It would be difficult to explain such a protective role of gluteofemoral obesity on a direct mechanical basis. Therefore, it seems more likely that gluteofemoral adipose has no role in acid reflux, but it may have a protective role in reflux’s sequelae of erosive oesophagitis and Barrett’s oesophagus. Gluteofemoral obesity has been found to have similar inverse associations (once adjusted for abdominal obesity or BMI) with diabetes mellitus and cardiovascular disease.14 Gluteofemoral adipose may represent a metabolic ‘sink’ where excess energy can be safely stored without the metabolic consequences associated with visceral adipose.15 For instance, gluteofemoral obesity is inversely associated with low-density lipoprotein cholesterol and trigylcerides and positively associated with high-density lipoprotein cholesterol.15 In contrast with abdominal obesity, which is positively associated with insulin resistance and inversely associated with adiponectin levels, gluteofemoral obesity is positively associated with insulin sensitivity and adiponectin levels.24,25 Barrett’s oesophagus has been positively associated with insulin resistance and with altered circulating levels of adiponectin.26–29 Visceral obesity is also positively associated with elevated circulating levels of the proinflammatory cytokine, interleukin 6, but thigh subcutaneous adipose is inversely associated.30
Our study had a few important limitations. First, as in any observational study, we cannot be sure that we controlled completely for confounding; therefore, we cannot be certain that the distribution of fat caused the erosive oesophagitis or Barrett’s oesophagus. Furthermore, because of the cross-sectional design, our effect estimates may have been influenced by selection bias, particularly for the outcomes of oesophageal pH monitoring where the consent rate was very low. In addition, there was a strong correlation between waist and hip circumferences, which results in imprecise effect estimates when one effect is estimated after adjustment for the other, especially in those analyses with few cases or patients. Given the small numbers of subjects with abnormal acid reflux, the estimates of the effects of waist and hip circumferences for these outcomes were particularly imprecise. Finally, we used BMI and waist and hip circumference rather than more direct measures of the adipose compartments by CT or dual-energy x-ray absorptiometry, as we found that the latter were impractical to perform for the relatively large number of subjects enrolled. Estimates of the associations with the direct measures of the adipose compartments might have yielded different results.
Our study also had a number of notable strengths. We minimised the risk of detection bias by enrolling the CRC screenees regardless of symptoms of GORD, and prospectively classified them on the basis of erosive oesophagitis or Barrett’s oesophagus and a subcohort on the basis of oesophageal acid exposure time. We thereby identified cases and non-cases for each outcome within the same screened population. In order to increase the precision of the effect estimates, we also included men diagnosed with Barrett’s oesophagus by a clinically indicated upper endoscopy, finding similar associations whether or not these cases were included in the analyses. Finally, we were able to adjust for important potential confounders, including dietary habits and physical activity.
In summary, we found that the distribution of obesity is associated with the presence of erosive oesophagitis or Barrett’s oesophagus. Abdominal obesity may promote acid reflux while upright, erosive oesophagitis and Barrett’s oesophagus. However, in the setting of abdominal obesity, gluteofemoral obesity may protect against the sequelae of erosive oesophagitis and Barrett’s oesophagus. We infer that abdominal obesity may promote acid reflux through a mechanical effect, and erosive oesophagitis and Barrett’s oesophagus additionally through a humoral effect, but that gluteofemoral obesity may protect against the humoral effect on erosive oesophagitis and Barrett’s oesophagus. Additional studies are needed to determine which, if any, circulating mediators account for the apparently discordant effects of abdominal and gluteofemoral obesity on erosive oesophagitis and Barrett’s oesophagus.
Significance of this study.
What is already known about this subject
-
▸
Abdominal obesity is associated with erosive oesophagitis and Barrett’s oesophagus.
-
▸
The association is believed to be due to a mechanical effect promoting gastro-oesophageal reflux.
-
▸
Abdominal obesity is associated with other health outcomes (eg, diabetes mellitus, colorectal cancer), for which there is no known mechanical effect.
-
▸
In contrast, gluteofemoral obesity may protect against diabetes and cardiovascular disease.
What are the new findings
-
▸
Compared with men with small waists and small hips, men with large waists and small hips are more likely to have erosive oesophagitis or Barrett’s oesophagus.
-
▸
However, men with both large waist and large hips are not as likely, suggesting that gluteofemoral obesity may protect against erosive oesophagitis and Barrett’s oesophagus.
How might it impact on clinical practice in the foreseeable future?
-
▸Understanding which if any circulating adipokines might explain the protective role of gluteofemoral obesity might:
-
–improve our ability to predict who is at risk of oesophageal adenocarcinoma, and
-
–lead to novel strategies for preventing oesophageal adenocarcinoma.
-
–
Acknowledgments
Brenda Vibbart assisted with administration of the grants and clerical work. Donald May, Jeffrey Cole and Jeffrey Holden assisted with administration of the grants. We greatly appreciate the assistance of the endoscopists and staff at the University of Michigan and the Ann Arbor Veterans Affairs Medical Center for performing and assisting with the research upper endoscopies and biopsies, and Mary Pegler and Marc McDermott for performing pH studies.
Funding Research and salary funding was provided for JHR by the National Institutes of Health (K23DK079291). JMS was supported by a Senior Mentoring Grant from the American Society for Gastrointestinal Endoscopy. JMI was supported by the National Institutes of Health (K24DK 080941). None of the sponsors had any role in the study design, data collection, analysis, or interpretation of data.
Footnotes
Competing interests None.
Contributors JHR: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; obtained funding; study supervision. HM, WDC, LM and JMI: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. JM, JMS, PS and HDA: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. VM, JK, TK and JB: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. .
Ethics approval University of Michigan and the Ann Arbor Veterans Affairs Medical Center Institutional Review Boards.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jacobson BC, Chan AT, Giovannucci EL, et al. Body mass index and Barrett’s oesophagus in women. Gut. 2009;58:1460–6. doi: 10.1136/gut.2008.174508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–8. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowell MD, Bradley A, Hansel S, et al. Obesity is associated with increased 48-h esophageal acid exposure in patients with symptomatic gastroesophageal reflux. Am J Gastroenterol. 2009;104:553–9. doi: 10.1038/ajg.2009.5. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Ergun GA, Pandolfino J, et al. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–55. doi: 10.1136/gut.2006.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derakhshan MH, Robertson EV, Fletcher J, et al. Mechanism of association between BMI and dysfunction of the gastro-oesophageal barrier in patients with normal endoscopy. Gut. 2012;61:337–43. doi: 10.1136/gutjnl-2011-300633. [DOI] [PubMed] [Google Scholar]
- 6.Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–5. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 7.Yasuhara H, Miyake Y, Toyokawa T, et al. Large waist circumference is a risk factor for reflux esophagitis in Japanese males. Digestion. 2010;81:181–7. doi: 10.1159/000235919. [DOI] [PubMed] [Google Scholar]
- 8.Nam SY, Choi IJ, Ryu KH, et al. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology. 2010;139:1902–11.e2. doi: 10.1053/j.gastro.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–11. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 11.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–8. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan TL, Kristal AR, Blount PL, et al. Nonsteroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2002;11:745–52. [PubMed] [Google Scholar]
- 13.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Heitmann BL, Lissner L. Hip Hip Hurrah! Hip size inversely related to heart disease and total mortality. Obes Rev. 2011;12:478–81. doi: 10.1111/j.1467-789X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 15.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34:949–59. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein JH, Morgenstern H, Appelman HD, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterole2. 2013 doi: 10.1038/ajg.2012.446. Published Online First: 15 January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health and Nutrition Examination Survey III: Body Measurements (Anthropometry) Centers for Disease Control; 1998. [Google Scholar]
- 18.National Health and Nutrition Examination Survey: Anthropometry Procedures Manual. Centers for Disease Control; 2004. [Google Scholar]
- 19.The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health; 2000. [Google Scholar]
- 20.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein JH, Morgenstern H, Kellenberg J, et al. Validation of a new physical activity questionnaire for a sedentary population. Dig Dis Sci. 2011;56:2678–87. doi: 10.1007/s10620-011-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JC-Y, Mui L-M, Cheung CM-Y, et al. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology. 2007;132:883–9. doi: 10.1053/j.gastro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Snijder M, Dekker J, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 25.Buemann B, Sorensen TIA, Pedersen O, et al. Lower-body fat mass as an independent marker of insulin sensitivity—the role of adiponectin. Int J Obes Relat Metab Disord. 2005;29:624–31. doi: 10.1038/sj.ijo.0802929. [DOI] [PubMed] [Google Scholar]
- 26.Greer KB, Thompson CL, Brenner L, et al. Association of insulin and insulin-like growth factors with Barrett’s oesophagus. Gut. 2012;61:665–72. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenstein JH, Dahlkemper A, Kao JY, et al. A pilot study of the association of low plasma adiponectin and Barrett’s esophagus. Am J Gastroenterol. 2008;103:1358–64. doi: 10.1111/j.1572-0241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s esophagus. Gut. 2009;58:1583–9. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson OM, Beresford SAA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18:2204–11. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beasley LE, Koster A, Newman AB, et al. Inflammation and Race and Gender Differences in Computerized Tomography-measured Adipose Depots. Obesity (Silver Spring) 2009;17:1062–9. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]


