Abstract
Excessive substance use and obesity are underpinned by a number of shared risk factors (e.g., reward dysfunction, impulsivity). Food and drugs of abuse engage similar reward-related neural circuitry and the food-drug competition hypothesis proposes that excess consumption of food may diminish desire for drugs of abuse by competing for neural receptors associated with reward and motivation. Adolescence is a high-risk period for both increased substance use and excessive weight gain. In the present study, we tested whether, consistent with the food-drug competition hypothesis, elevated BMI across adolescence predicted fewer substance use problems in young adulthood. In a multi-wave prospective study of a community sample of families enriched for high levels of substance use disorders, we first identified BMI trajectories across adolescence in 565 participants using latent class growth analysis. We then used maximum likelihood methods to compare the equality of mean alcohol-, drug-, and nicotine-related problems during early adulthood across adolescent BMI trajectories. Participants in the obese relative to the normal weight trajectory in adolescence had fewer drinking and illicit drug problems in early adulthood. Relative to the overweight trajectory, nicotine dependence was significantly higher among both the normal weight and obese trajectories. The current findings provide partial support for the food-drug competition hypothesis, which suggests that highly palatable foods may be rewarding enough to compete with drugs of abuse and that transdiagnostic approaches to reducing problematic substance use and overeating in adolescence may be useful. However, the relationship between nicotine and food requires further study.
Keywords: Substance Use, Alcohol, Tobacco, Obesity, Adolescence
Introduction
Excessive substance use and elevated body mass index (BMI) are leading contributors to preventable death in the United States (Mokdad, Marks, Stroup, & Gerberding, 2004). Adolescence is a developmental period associated with increased use of substances (e.g., alcohol, marijuana, nicotine) (Johnston, 2010), but also a high-risk period for excessive weight gain (Ogden et al., 2016). However, less is known about how elevated BMI across adolescence is related to future risk for problematic substance use. As substance use disorders (SUDs) and obesity share a number of risk factors (e.g., reward dysfunction, impulsivity) (Volkow, Wang, Tomasi, & Baler, 2013), adolescents with higher BMI may be at elevated risk for problematic substance use. Yet, in adults, elevated BMI is associated with lower risk of problematic alcohol, illicit drug, and tobacco use (Gearhardt & Corbin, 2009; Kleiner et al., 2004; Liu, Serdula, Williamson, Mokdad, & Byers, 1994; Petry, Barry, Pietrzak, & Wagner, 2008; Rohrer, Rohland, Denison, & Way, 2005). Developing a greater understanding of how BMI across adolescence is associated with future problematic substance use may enhance our understanding of the shared mechanisms contributing to obesity and SUDs, as well as inform intervention efforts to reduce their impact on public health. Thus, in the current study we investigated how BMI trajectories across adolescence predicted substance use problems in early adulthood.
Elevated BMI and Substance Use in Adults
In adults, BMI and substance use were initially hypothesized to be positively associated with one another (Gruchow, Sobocinski, Barboriak, & Scheller, 1985). Elevated BMI and high levels of substance use are associated with a number of similar factors (e.g., impulsivity, reward dysfunction, emotion dysregulation, reinforcement pathology) (Carr, Oluyomi, Lin, & Epstein, 2011; Volkow, Wang, Fowler, & Telang, 2008) and a family history of addiction is associated with an increased risk of both obesity and SUDs (Grucza et al., 2010). Given these shared risk factors, it is plausible that elevated BMI and problematic substance use would be more likely to co-occur. Further, substance intoxication may increase caloric intake by lowering inhibitions (as with alcohol), increasing appetite (as with cannabis) or may be a source of additional calories to the diet (as with alcohol) (Caton, Bate, & Hetherington, 2007; Williams, Rogers, & Kirkham, 1998).
Interestingly, cross-sectional studies have identified a negative association between BMI and alcohol use (Gearhardt & Corbin, 2009; Kleiner et al., 2004; Liu et al., 1994; Rohrer et al., 2005) and having obesity is associated with a reduced likelihood of developing an alcohol use or illicit drug use disorder in the future (Pickering et al., 2011). Adults who smoke frequently also generally weigh less than non-smokers (Klesges, Klesges, & Meyers, 1991; Klesges, Meyers, Klesges, & LaVasque, 1989; Petry et al., 2008; Potter, Pederson, Chan, Aubut, & Koval, 2004). Although this inverse association between drugs of abuse and BMI might be expected for drugs that have appetite suppressant properties (e.g., stimulants, cigarettes) (Gold & Jacobs, 1997), it has also been demonstrated with drugs that increase appetite (i.e., cannabis) (Hayatbakhsh et al., 2010; Le Strat & Le Foll, 2011; Warren, Frost-Pineda, & Gold, 2005). Thus, elevated BMI may counterintuitively protect against the development of future substance use problems.
This negative association is consistent with the food-drug competition hypothesis (Kleiner et al., 2004). Consumption of highly palatable foods effectively activate neural systems (e.g., mesolimbic dopaminergic system, endogenous opioid system) implicated in the reinforcing nature of drugs of abuse (Avena, Rada, & Hoebel, 2008; Berridge, Ho, Richard, & DiFeliceantonio, 2010; Colantuoni et al., 2012). The food-drug competition hypothesis proposes that because food and drugs of abuse engage similar reward-related neural circuitry, they compete for shared brain reward sites (Kleiner et al., 2004). In other words, if the shared pathway is occupied by excess consumption of one substance (i.e., food or drugs of abuse), it may reduce or block desire for the other substance. Therefore, participants with elevated BMIs may be less likely to engage in problematic substance use. If excess food consumption is capable of competing with drugs of abuse, this also may be relevant to the current debate surrounding “food addiction” (Avena, Gearhardt, Gold, Wang, & Potenza, 2012; Ziauddeen & Fletcher, 2013). There is increasing scientific interest in whether certain foods (i.e., foods high in sugar, fat, and salt) are capable of triggering an addictive-like response in at least some individuals (Lindgren et al., 2018). If foods are rewarding enough to compete with drugs of abuse, this may provide further evidence for the addictive potential of certain highly rewarding foods (Cummings, Ray, & Tomiyama, 2015) and highlight the importance of considering interventions that target addictive mechanisms (e.g., cue-reactivity, inhibitory control) to reduce problematic overeating (Schulte, Grilo, & Gearhardt, 2016).
Elevated BMI and Substance Use in Adolescents
Adolescence is a particularly high-risk period for both problematic substance use and excessive weight gain, potentially due to an imbalance between more developed reward-related, subcortical neural systems and less developed cognitive-control cortical systems (Casey & Jones, 2010). These shared risk factors suggest that elevated BMI and high-risk substance use may be more likely to co-occur in adolescents due to a shared etiology. However, the food-drug competition hypothesis predicts that adolescents with higher BMIs would be less motivated to consume substances (Kleiner et al., 2004). There are also social considerations during adolescence that may impact the association between BMI and substance use. Adolescents with obesity are more likely to be socially isolated (Puhl & Heuer, 2009), which may limit access to addictive substances provided by peers (e.g., at parties) and reduce the likelihood of future substance use problems. However, adolescents with obesity are at greater risk for psychosocial maladjustment and are more likely to affiliate with more deviant peer groups who exhibit higher use of cigarettes and cannabis, which may increase the likelihood of risky substance use (Lanza, Grella, & Chung, 2015).
The current research on the association of BMI with substance use in adolescents is mixed. Cross-sectional studies have generally found that adolescents with higher BMIs report greater alcohol (Farhat, Iannotti, & Simons-Morton, 2010; Fonseca, Matos, Guerra, & Gomes Pedro, 2009) and cannabis (Farhat et al., 2010) use, but others have failed to detect differences (Thatcher & Clark, 2006) or found an inverse association between BMI and substance use (Neumark-Sztainer et al., 1997). Cross-sectional studies have also found mixed associations between BMI and tobacco use in adolescents (Farhat et al., 2010; Neumark-Sztainer et al., 1997; Potter et al., 2004). Longitudinal studies have also produced inconsistent findings, with some studies reporting no association between BMI and future substance use (Pasch, Nelson, Lytle, Moe, & Perry, 2008; Pasch, Velazquez, Cance, Moe, & Lytle, 2012). Other studies have identified a positive association between elevated weight status and future tobacco use (but not high-risk alcohol or illicit drug use) (Lanza, Grella, & Chung, 2014) and alcohol use in adolescent girls only (Weichold, Wiesner, & Silbereisen, 2014).
One potential contributor to the inconsistent literature on BMI and substance use during adolescence is that most studies have examined BMI at fixed ages (i.e., one time point). Examining BMI at a fixed age or changes in BMI between fixed ages assumes that participants are part of a homogeneous group with shared developmental patterns, which is an inaccurate assumption for childhood and adolescent BMI (Wen, Kleinman, Gillman, Rifas-Shiman, & Taveras, 2012). In children, BMI may reflect differences in growth patterns in bone, muscle, and fat (Wen et al., 2012), which becomes problematic during adolescence when varying rates of pubertal change could differentially impact body composition and the contribution of body fat to BMI (Kaplowitz, Slora, Wasserman, Pedlow, & Herman-Giddens, 2001). Thus, the use of BMI at a single age, especially during adolescence, may obscure associations between body weight and substance use. An alternative approach is to investigate BMI trajectories based on repeated measures across development. Moreover, while expected age-contingent increases in BMI exist across development, individuals vary according to their starting levels and relative increase in BMI over time. Relative to examining BMI at a single age, modeling classes of individuals who have specific trajectories of BMI can provide a more accurate representation of their BMI across development, especially compared to simply examining their “rank” or mean score at any one time point. Moreover, these analyses may help to uncover stronger or more accurate associations with problematic substance use during adulthood that would be missed in cross-sectional designs (Wen et al., 2012).
Currently, there is limited research investigating the association between BMI trajectories and substance use. Consistent with the food-drug competition hypothesis, Huang and colleagues (2013) found that participants in chronically obese trajectories from ages 6-18 years of age were less like to initiate alcohol at age 14 and binge drink in adolescence relative to their non-obese peers. However, chronically obese participants were more likely to show increases in smoking over time (Huang et al., 2013). Further research is needed to evaluate associations between different BMI trajectories across adolescence, particularly during high-risk periods for substance use problems.
Elevated BMI and Substance Use in Early Adulthood
Early adulthood is an especially important time to assess how BMI trajectories during adolescence predict substance use problems, as this is the peak developmental period for problematic substance use (Lanza et al., 2015). Further, it may be especially important to investigate the presence of problematic substance use, as elevated BMI can impact the way substances of abuse are absorbed and experienced. For example, individuals with higher BMIs need to consume greater quantities of alcohol due to higher body weight to reach comparable blood alcohol concentrations as individuals with lower BMIs (Gearhardt & Corbin, 2009). The assessment of problems related to substance use (e.g., interpersonal problems, loss of control) provides a marker of high-risk substance use that is not directly impacted by BMI. Further, focusing on the quantity and frequency of substance intake may underestimate problematic use, as clinically significant substance use problems can emerge at lower levels of use in younger individuals (Chen, Kandel, & Davies, 1997). However, no prior research has investigated how BMI trajectories across adolescence predict substance use problems during early adulthood.
In the current study, we aimed to establish whether distinct trajectories of BMI across adolescence predicted future substance use problems in a sample enriched for substance use disorder risk. We first identified BMI trajectories across adolescence using latent class growth analysis. We then investigated whether BMI trajectories across adolescence differentially predicted alcohol-related problems, illicit drug-related problems, and tobacco dependence in early adulthood.
Methods
Design and Sample
The present work is part of an ongoing multi-wave prospective study (Zucker et al., 2000) of a community sample of families with high levels of alcohol use disorders and other drug use, particularly among fathers. Substance abusing men were initially identified through the courts when they were convicted of drunk driving with a high blood alcohol level (at least 0.15%, or at least 0.12% and a previous drinking-related legal problem). To be included in the study, the fathers were required a) to have a Feighner diagnosis for probable or definite alcoholism (Feighner et al., 1972), b) to have at least one son between three and five years of age and c) to be living with both the child and his biological mother at the time of recruitment. Later funding allowed the inclusion of female siblings from the same families. A contrast/control group of families with no substance us disorder history was recruited. These families were recruited using door-to-door canvassing in the same neighborhoods where the families who had substance use disorders were residing. In addition, an intermediate risk group was provided by recruiting all families with an alcohol abuse/dependence diagnosis who were found during the community canvass. The final community sample included 158 (51.3%) families with fathers with alcoholism, 90 (29.2%) families from the contrast/control group, and 60 (19.5%) families from the intermediate risk group. Although the non-white samples are low, the sample was ethnic/racially representative of the Midwest region in which these families lived at this time. For a more detailed description see (Zucker et al., 2000). Thus, this sample is ideal in that it is enriched for genetic and contextual risk factors for substance abuse, but still includes both low-and high-risk families. The current sample included data from 565 children who had BMI data available at two or more assessment points (32% female). The majority of the participants were White (92.2%) with 2.8% African American, 2.3% Hispanic, and 2.7% biracial participants. Written informed consent was provided by all study participants and the protocol was approved by the University Of Michigan School Of Medicine Institutional Review Board.
Procedures
Children and parents were assessed in their home following initial recruitment (Wave 1, child age 3–5) with assessments repeated every three years for seven assessment waves. Data collection points are identified by the age of the child; thus Wave 2 includes children age 6-8; Wave 3 includes children age 9-11, etc. At waves 3 and 4 (target age 9-14), parents reported the child’s height and weight. Beginning at wave 5 (target age 15-17), participants reported on their own height and weight.
Covariates assessed in early childhood (Waves 1–2, Age 3–8)
Demographic variables
Demographics information was obtained from parents, including race, child sex, and income. Income was estimated as the mean of mother and father income reported at Wave 2. If income information was missing for one parent, the income reported by the other parent was used.
BMI trajectories across adolescence (Waves 3–6, Age 8 to 21)
We used BMI data from waves 3–61, which we sorted into six age groups from which to construct BMI trajectories. In addition to adolescence, we included BMI data from pre-adolescence (ages 8 to 12). Biological changes typically associated with adolescence (e.g., onset of puberty) are more likely to occur during pre-adolescence in children with obesity (Kaplowitz et al., 2001) and the inclusion of pre-adolescent BMI in the trajectories takes into account BMI status during this developmental period. To account for the fact that participants ranged in age by up to six years within each assessment wave, and given the sensitivity of BMI to age throughout childhood and adolescence, we re-structured the data using age rather than assessment wave to derive trajectories across time (Age 1, 8–10; Age 2, 11–12; Age 3, 13–14; Age 4, 15–16; Age 5, 17–18; Age 6, 19–21). BMI was computed from parent- or self-reported height and weight (Kg/M2). While converting BMI to z-scores may be methodologically preferable for assessing adiposity cross-sectionally, given the nonlinear effects of age and sex on mean BMI, studies have shown that changes in adiposity in children and adolescents are best evaluated in BMI units (Berkey & Colditz, 2007). We winsorized data constraining outliers to be at a maximum of 3 SD from the mean for each age point. Winsorizing led to constraining the following numbers of participants at each age point: ages 8-10, n = 1 (BMI maximum = 30.12), 11–12, n = 4 (BMI maximum = 34.02), ages 13–14, n = 3 (BMI maximum = 33.87), 15–16, n = 3 (35.61), 17–18, n = 3 (BMI maximum = 38.60), and ages 19–21, n = 4 (BMI maximum = 38.07). No outliers were identified for 3 SD below the mean BMI at any age.
Outcome variables assessed in early adulthood (Wave 7, Age 21–23)
Alcohol and drug problems
Number of alcohol problems and number of illicit drug problems were assessed with the Drinking and Drug History questionnaire (DDHQ) (Zucker et al., 2000), which contains a series of 31 problem items for alcohol use and 13 problem items for drug use from the V. A. Medical Center Research Questionnaire (Schuckit, 1978), answered in a yes/no format. Problem items include negative consequences resulting from alcohol/illicit drug use (e.g., loss of friends, loss of job) and other diagnostic indicators of a substance use disorder (e.g., withdrawal, tolerance). These items have been extensively used in a variety of survey and clinical settings (Room, 2000).
Nicotine dependence
Tobacco problems were evaluated by the Fagerstrom test for nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), which asks about smoking first thing in the morning, a quantity question, difficulty refraining from smoking in forbidden places, and smoking if sick in bed.
Analytic Strategy
Aim 1: Identification of BMI trajectories from pre-adolescence across adolescence
We conducted a latent class growth analysis (Muthén, 2004; Nagin, 2005) in Mplus version 7.2 (B. Muthén, 2014) to identify BMI trajectories. As outlined, BMI data was binned from across waves 3–6 into six age categorizations that were roughly equal in size: ages 8–10 (n = 249), 11–12 (n = 270), 13–14 (n = 260), 15–16 (n = 250), 17–18 (n = 230), and 19–21 (n = 239). BMI values for each age group were used as indicators for latent intercept, linear, and quadratic growth factors (Nagin, 2005). We derived solutions from one to six classes using a large number of start values (5,000 with 100 optimizations) to avoid solutions at local maxima.
We considered multiple criteria to select the best model, including the Bayesian Information Criterion (BIC), the Lo–Mendell–Rubin adjusted Likelihood Ratio Test, and entropy values. We also examined entropy values for different class solutions, with higher entropy values suggesting cleaner membership classifications. In addition, we took into account group size (i.e., with a goal of no groups being smaller than 5%) and the substantive importance of each model by evaluating how solutions compared with theoretical accounts and previous findings relating to BMI change across pre-adolescence and adolescence (Brault, Aimé, Bégin, Valois, & Craig, 2015; Nonnemaker, Morgan‐Lopez, Pais, & Finkelstein, 2009; Wen et al., 2012). Taken together, these criteria allowed us to compare the heterogeneous class structure suggested by each model with past theoretical and empirical information concerning BMI change across development and also helped us to factor parsimony into the model selection process. Using full maximum likelihood estimation with robust standard errors (MLR), we accounted for missing BMI data within our sample at each age point and included all 565 participants in analyses. Owing to the number of sibling pairs included in the sample (n = 176), we utilized the CLUSTER command in Mplus to account for the data being nested.
Aim 2: Examine substance use outcomes associated with BMI trajectories
We next tested whether BMI class membership predicted specific substance use outcomes. Outcome variables (i.e., number of drinking problems, number of other illicit drug use problems, and nicotine dependence,) were assessed at wave 7 when participants were at a mean age of 22.61 (SD = 1.11) minimizing overlap with when BMI trajectories were assessed. (Supplementary Material, Figure S1). To test differences in substance use for those in different trajectory classes, we use the DU3STEP procedure in Mplus. The DU3STEP procedure is an implementation of Vermunt’s 3-step maximum likelihood method (Vermunt, 2010) where outcome variables are included within the broader structural model to enable Chi Square equality of means tests across classes (Asparouhov & Muthén, 2014; Vermunt, 2010). As the automatic DU3STEP procedure in Mplus does not enable covariates to be included, we first examined regression models accounting for the effects of sex, race, and income on outcome variables (Table 1). Males had significantly more drinking problems (β = .12, p = .02), had lower incomes (β = −.25, p <.02) and were more likely to be non-white (β = −.16, p <.02). To control for the effects of these covariates on the association between trajectory group and outcome, we used residuals from the regression models as outcome variables in models using the DU3STEP procedure.
Table 1.
Descriptive Statistics for Study Variables
| Covariates | N | N | ||
|---|---|---|---|---|
|
| ||||
| Gender | Race | |||
| Female | 181 | Caucasian | 520 | |
| Male | 384 | Non-Caucasian | 45 | |
|
| ||||
| Family income (wave 2) | ||||
|
| ||||
| under $4,000 | 7 | $16,001–$20,000 | 23 | |
| $4,000–$7,000 | 1 | $20,001–$30,000 | 72 | |
| $7,001–$10,000 | 6 | $30,001–$50,000 | 145 | |
| $10,001–$13,000 | 13 | $50,001–$75,000 | 102 | |
| $13,001–$16,000 | 25 | $75,001–$100,000 | 32 | |
| over $100,000 | 16 | |||
| N | Min | Max | Mean | SD | |
|---|---|---|---|---|---|
| BMI at different age points | |||||
|
| |||||
| Ages 8–10 | 249 | 10.81 | 30.12 | 18.54 | 3.84 |
| Ages 11–12 | 270 | 12.48 | 34.02 | 20.17 | 4.27 |
| Ages 13–14 | 260 | 15.11 | 33.87 | 21.24 | 4.08 |
| Ages 15–16 | 251 | 16.18 | 35.61 | 22.65 | 4.22 |
| Ages 17–18 | 230 | 17.38 | 38.60 | 24.12 | 4.74 |
| Ages 19–21 | 239 | 16.30 | 38.07 | 24.10 | 4.14 |
|
| |||||
| Substance use (age 21–23) | |||||
| Binge drinking days last year | N (%) | N (%) | ||
|---|---|---|---|---|
| None | 108 (32%) | 120–149 days | 7 (2%) | |
| 1–29 days | 84 (25%) | 150–179 days | 15 (5%) | |
| 30–59 days | 31 (12%) | 180–209 days | 10 (3%) | |
| 60–89 days | 32 (10%) | 210–239 days | 7 (2%) | |
| 90–119 days | 13 (4%) | 240–269 days | 7 (2%) | |
| 270+ days | 11(3%) | |||
|
| ||||
| Frequency of marijuana smoked last year | N (%) | N (%) | ||
|
| ||||
| Never | 212 (38%) | 20–39 times | 14 (3%) | |
| 1–2 times | 33 (6%) | 40–99 times | 15 (3%) | |
| 3–5 times | 11 (2%) | 100–249 times | 13 (2%) | |
| 6–9 times | 6 (1%) | 250–499 times | 21 (4%) | |
| 10–19 times | 11 (2%) | 500+ times | 13 (2%) | |
|
| ||||
| Frequency of cigarette smoking last year | N (%) | N (%) | ||
|
| ||||
| Never | 164 (29%) | Regularly for part of the year | 28 (5%) | |
| 1–2 times | 29 (5%) | Regularly | 85 (15%) | |
| Occasionally, not regularly | 43 (8%) | |||
| Outcome variables (age 21–23) | |||||
|---|---|---|---|---|---|
| Nicotine dependence | 329 | 0 | 5 | .93 | 1.12 |
| Number of drinking problems | 337 | .00 | 23.87 | 4.27 | 4.91 |
| Number of illicit drug problems | 337 | .00 | 11.85 | .25 | 1.12 |
Results
Aim 1: Identification of BMI trajectories across adolescence
We estimated BMI trajectories across pre-adolescence and adolescence (ages 8–21 years) as summarized in Table 2. There was an overall increase in BMI across the whole sample from pre-adolescence to early adulthood (i.e., expected age-related changes in BMI consistent with development and prior studies; intercept, B = 18.50, SE = .23, p < .001; slope, B = 1.73, SE = .18, p < .001; quadratic, B = −.11, SE = .03, p = .001). To identify specific BMI classes within this overall pattern, we derived solutions from one to six classes, after which there were no significant improvements in model fit. Based on the number of trajectories identified in prior research (Brault et al., 2015; Nonnemaker et al., 2009; Wen et al., 2012), a lower Bayesian Information Criterion, sufficient entropy, and the Lo–Mendell–Rubin (LMR) adjusted Likelihood Ratio test, average latent trajectory probabilities between .83 and .96, the model with three classes was determined as the best model for the BMI trajectories. Further, the entropy value for the four-class solution was 0.78 (relative to 0.84 for the three-class solution) and falls below the accepted standard of 0.80 that indicates good separation of classes (Celeux & Soromenho, 1996) and the LMR test moving from the three- to four-class solution was not significant, leading us to determine that the three-class solution was a more optimal fit (see Table 2 for more details). However, details for classes that emerged from the 4-class solution are presented in the Supplemental Materials (Figure S3).
Table 2.
Indicators of model fit in the Latent Class Growth Analysis
| Number of latent classes | BIC | Adj. LRT | Entropy |
|---|---|---|---|
| 1 | 8612.22 | – | – |
| 2 | 7975.46 | 636.97, p < .001 | .87 |
| 3 | 7812.46 | 181.20, p = .06 | .84 |
| 4 | 7708.47 | 124.43, p = .07 | .78 |
| 5 | 7617.60 | 111.81, p < .05 | .80 |
| 6 | 7604.87 | 36.63, p = .84 | .75 |
Note. BIC = Bayesian Information Criterion; lower values suggest better model fit; Adj LRT = Lo Mendell– Rubin adjusted Likelihood Ratio Test; the emergence of a non-significant LMR suggests that the preceding model with one fewer class may be preferred. The 3-class model was chosen as the final version of the trajectory model, and the corresponding classes were utilized in all subsequent analyses. Note that a 3-class model was preferred over the 4-class model because of its higher entropy value, indicating better separation of the results classes (but see Figure S3 for detailed results of the 4-class model). The 3-class model was also preferred over the 5-class model, even though there was a significant Adj. LRT test moving from 4 to 5 classes because the 3-class model had higher entropy than the 5-class model and one of the class sizes in the 5-class model was very small (n = 16). Moreover, the 3-class model was more consistent with prior theoretical and empirical conceptualizations of BMI trajectories across development (Brault et al., 2015; Nonnemaker et al., 2009; Wen et al., 2012).
Based on the most likely latent class membership, participants were classified in one of three classes (Figure 1). The first trajectory class comprised the majority of the sample (n = 388, 69%) and included children with a stable low BMI in pre-adolescence with age-appropriate increases across time and the majority of members (80.7%) ending in the normal weight category in early adulthood (BMI between 18.5–24.9, “Normal trajectory”)2. The second trajectory (n = 133, 24%) represents children with a higher BMI that showed steady increases across adolescence into early adulthood with the majority (54.2%) ending in the overweight range (BMI between 25.0–29.9, “Overweight trajectory”). The third trajectory (n = 44, 8%) represents individuals with the highest BMI in pre-adolescence whose BMI increased steadily across adolescence, with the majority (80.0%) ending in the obese weight category (BMI > 30.0, “Obese trajectory”).
Figure 1. Three BMI trajectory classes from pre-adolescence to early adulthood.
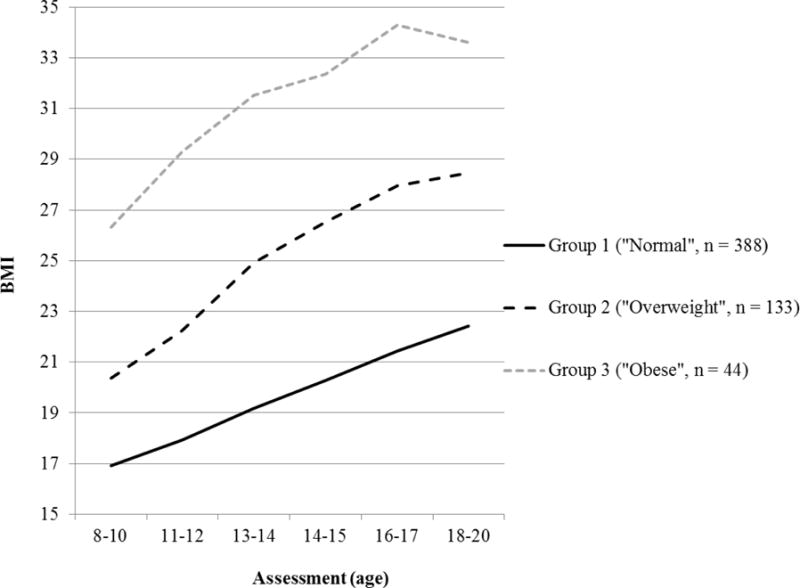
Note. Group 1, “normal”, n = 388 (69%); Group 2, “overweight”, n = 133 (24%); Group 3, “obese”, n = 44 (8%). Bayesian Information Criterion = 7812.46, entropy = .84, Lo–Mendell–Rubin adjusted Likelihood Ratio test = 181.20 (p = .06), and average latent trajectory probabilities for most likely latent class membership = .83–.96.
Aim 2: Examine substance use outcomes associated with BMI trajectories
To examine early adulthood outcomes related to each BMI class, we tested the equality of means across classes (Table 3; Figure 2). The “Normal” and “Overweight” classes exhibited significantly more drinking problems relative to the “Obese” class. Additionally, the “Normal” class displayed significantly more illicit drug problems relative to both the “Overweight” and “Obese” classes. There was no significant difference between the “Overweight” and “Obese” classes in number of illicit drug problems. Finally, compared to the “Overweight” class, nicotine dependence was significantly higher among both the “Normal” and “Obese” BMI classes. The “Obese” and “Normal” classes did not differ in their level of nicotine dependence3.
Table 3.
Results of equality tests of means for outcome variables assessed at wave 7 across BMI trajectory classes using the 3-step procedure
| Outcomes variables1 | ||||||
|---|---|---|---|---|---|---|
| Latent Trajectory Class | Number of drinking problems | Number of illicit drug problems | Nicotine dependence | |||
| M | SD | M | SD | M | SD | |
| “Normal” | .29 | .44 | .18 | .12 | .14 | .09 |
| “Overweight” | −.11 | .99 | −.21 | .01 | −.56 | .12 |
| “Obese” | −2.74 | .65 | −.31 | .05 | .51 | .38 |
| Equality tests of means | ||||||
| χ2 | χ2 | χ2 | ||||
| Overall test | 16.78*** | 13.07*** | 21.71*** | |||
| “Normal” vs. “Overweight” | n.s. | normal > overweight, 10.79** | normal > overweight, 18.52*** | |||
| “Normal” vs. “Obese” | normal > obese, 15.53*** | normal > obese, 13.07*** | n.s. | |||
| “Overweight” vs. “Obese” | overweight > obese, 4.41* | n.s. | obese > overweight, 7.01* | |||
Note.
p < .05,
p < .01,
p < .001;
Reported means are based on the residuals from regression models that account for covariates. Results of equality tests of means for outcome variables when a 4-class solution is chosen are presented in Table S1.
Figure 2. Summary of equality of means tests comparing outcomes in early adulthood for the three different BMI trajectory classes assessed across pre-adolescence and adolescence.
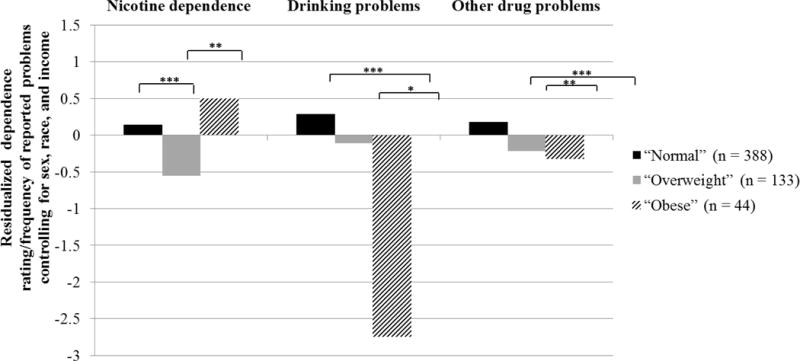
Note. * p < .05, ** p < .01, *** p < .001
Post-hoc analyses
In light of the intriguing finding that individuals in the “Normal” displayed more illicit drug and drinking problems, we examined a follow-up model taking advantage of existing longitudinal data available to test the temporal relationships of these variables earlier in adolescence. Specifically, we ran a series of reciprocal effects models examining cross-lagged relationships between BMI and either nicotine use, drug use problems, and drinking problems in early adolescence (M age = 13.49, SD = .93) and mid-late adolescence (M age = 16.57, SD = .93). We controlled for age, gender, and income as before (see Supplemental Methods for more details of the modeling approach). We found that higher drug use problems in early adolescence was related to lower BMI during mid-late adolescence, taking into account the stability in drug use problems and BMI over time (see Figure S2). Drinking problems and nicotine use were not reciprocally related to BMI across this period.
Discussion
The current study examined how BMI across adolescence was related to substance use problems during early adulthood in a sample enriched for increased risk for substance use disorders. BMI trajectories, which modeled developmental patterns of BMI rather than only examining a single measurement (Wen et al., 2012), were identified via repeated assessment of BMI from 8 to 21 years of age. Three distinct classes of BMI change were identified across pre-adolescence and adolescence, which is consistent with prior research on weight trajectories during development (Brault et al., 2015; Nonnemaker et al., 2009; Wen et al., 2012). The trajectory comprising the majority of the sample (69%) included children with a lower BMI in pre-adolescence and appropriate increases that resulted in a normal BMI by early adulthood. The second trajectory (24%) included participants who were mostly overweight by early adulthood. The final trajectory (8%) included participants with a steadily increasing BMI across adolescence, the majority of whom where obese by early adulthood. The latent class analysis identified three trajectories showing consistent increases in BMI with across adolescence in the sense that no evidence was found of changing BMI within groups (e.g., a group that started in a “normal” range for BMI but moved to an “obese” range). However, the use of this approach to categorize BMI enables participants to be classified based on repeated assessment of BMI, with greater probability of correct classification into the “Normal,” “Overweight,” or “Obese” groups, which helps to reduce measurement error and increase the likelihood of detecting significant associations with other outcomes of interest (i.e., substance use problems).
BMI Trajectories across Adolescence and Substance Use in Early Adulthood
Alcohol use problems during early adulthood differed by BMI trajectory. Participants in the “Obese” trajectory across adolescence were less likely to have alcohol-related problems during early adulthood than participants in the “Normal” or “Overweight” classes. Thus, despite shared risk factors (e.g., impulsivity, reward dysfunction) (Volkow et al., 2008), obesity across adolescence appears to be protective against the development of alcohol problems in early adulthood. These findings are consistent with prior research showing that chronically obese children exhibit less binge drinking during adolescence and a later age of initiating alcohol consumption (Huang et al., 2013). Regarding illicit drugs, participants in the “Normal” trajectory had more illicit drug-related problems in early adulthood than participants in either the “Overweight” or “Obese” trajectories. Similarly, Pickering and colleagues (2011) found that normal weight relative to overweight and obese adults were more likely to develop a SUD over a three-year follow up. The current study expands upon this prior research to suggest that elevated BMI across adolescence reduces the likelihood of alcohol and illicit drug-related problems during early adulthood. We also conducted post-hoc analyses to investigate the temporal relationships of BMI and substance use from early adolescence to mid-late adolescence. Higher drug use in early adolescence was related to lower BMI during middle adolescence, however no reciprocal relationships related to BMI or drinking problems and nicotine use were found. It is possible that relationships between BMI and substance use do not emerge unless trajectories of BMI are taken into consideration or high-risk periods for substance use problems (i.e., early adulthood) are assessed.
The lower level of alcohol and illicit drug problems during early adulthood for adolescents with higher BMIs across adolescence is consistent with the food-drug competition hypothesis. One interpretation of the current findings is that greater food intake (as indicated by a higher BMI) during adolescence occupies shared reward-related neural circuity, thus reducing the drive for drugs of abuse and decreasing the likelihood of developing alcohol or illicit drug-related problems. One implication of the food-drug competition hypothesis is that certain foods (particularly highly processed foods with high levels of sugar, fat, and salt) may mimic the effects of addictive drugs for at least some individuals (Cummings et al., 2015; Schulte, Avena, & Gearhardt, 2015). The ability of foods to compete with drugs of abuse appears to have become stronger over time, as the processed foods in the modern food environment have become progressively more rewarding, accessible, and cheaper (Grucza et al., 2010). Adolescence is a particularly high-risk period for excess weight gain and overconsumption of potentially addictive “junk” foods (Ogden et al., 2016; Story & Stang, 2005). If addictive processes are possibly contributing to the growing rates of obesity in adolescence, intervention approaches that have been effective in targeting problematic substance use during this developmental period, such as motivational interviewing or taxation of additive substances, might also be adapted to target problematic overeating (Jensen et al., 2011; Toumbourou et al., 2007).
Social factors may also contribute to the current findings. Adolescents with obesity are more likely to be socially marginalized (Puhl & Latner, 2007) or excluded from social functions that provide access to alcohol and illicit drugs during adolescence, which may reduce the likelihood of developing alcohol problems. Access to alcohol becomes less dependent on social functions and more widely available during young adulthood when participants reach the legal age of alcohol consumption in the United States (21 years old). As social barriers to accessing alcohol reduce, it will be important for future studies to assess whether obesity across adolescence is related to the development of substance use problems in adulthood.
In contrast to alcohol/illicit drugs, tobacco dependence during early adulthood was higher for participants in both the “Obese” and “Normal weight” classes relative to the “Overweight” trajectory. This is consistent with prior research that obesity is associated with a greater likelihood of tobacco use during adolescence (Huang et al., 2013; Lanza et al., 2015). However, the higher level of tobacco dependence in young adulthood for both the “Obese” and “Normal weight” trajectory is inconsistent with the food-drug competition hypothesis. One possibility is that tobacco and food share less neural overlap than substances like alcohol, thus leading to less direct competition. For example, the reinforcing effects of tobacco are driven in large part by nicotine binding to nicotinic cholinergic receptors (Benowitz, Hukkanen, & Jacob III, 2009), which is not a key neural system implicated in food intake (Berthoud, 2002). There are also important societal factors to consider. Unlike other more easily accessible drugs of abuse (e.g., alcohol, cannabis), cigarettes are perceived to aid in weight loss and prevent weight gain (White, McKee, & O’Malley, 2007), which may increase their appeal for adolescents with obesity. Dieting behaviors and body weight concerns (above and beyond actual BMI) are associated with a greater likelihood of smoking during adolescence (Potter et al., 2004). It is possible that dieting and body weight concerns may have increased the propensity to smoke to maintain a lower BMI for adolescents in the “Normal Weight” trajectory and to attempt to lose weight for adolescents in the “Obese” trajectories. Given that overweight adolescents would be expected to have higher levels of body weight concerns than normal weight adolescents (Paxton et al., 1991), further evaluation is needed to identify what contributed to the lower levels of tobacco dependence for participants in the “Overweight” relative to “Normal Weight” trajectory. However, these findings may have important public health implications. The prevalence of cigarette smoking in adolescents has been going down since the 1970s (Johnston, 2010), but the rising rates of adolescent obesity and body weight concerns may threaten these public health gains and be important intervention targets to reduce smoking initiation.
Although there were significant strengths of the current study, there are limitations to consider. First, the sample was recruited to oversample family history of alcoholism and may not be generalizable to the entire population. However, the sample was recruited from the community, not based on treatment, which helps to mitigate this limitation. Due to funding requirements at the beginning of recruitment, the sample contained over two-thirds males and thus provides less information about girls. In addition, the sample was largely Caucasian, which limits the generalizability. Second, BMI was computed based upon parent and participant self-report of height and weight, which is subject to reporting bias. Self-reported BMI is strongly associated with objectively measured BMI (for adolescent self-report r > 0.92 (Goodman, Hinden, & Khandelwal, 2000) and for parent-reported BMI, r > 0.90) However, future studies would benefit from the direct measurement of height and weight. Moreover, while BMI is strongly associated with caloric intake, other factors also contribute to obesity (e.g., physical inactivity). Future studies would benefit from the measurement of both caloric intake and physical activity. Fourth, the prevalence of problematic use of illicit drugs was relatively low in the current sample, which prevented us from having adequate power to separately examine classes of drugs (e.g., stimulants, cannabis) that may have different effects on appetite and obesity status. However, consistent with the current findings, prior research has found an inverse association between cannabis use (which enhances appetite) and obesity (Hayatbakhsh et al., 2010; Le Strat & Le Foll, 2011; Warren et al., 2005). Finally, certain types of foods (i.e., high fat-sweets) appear to be more effective in blocking desire for alcohol than others (Cummings et al., 2015). Thus, future studies would benefit from assessing types of food consumed.
Despite these limitations, the current study has important implications for our understanding of obesity and substance use problems. Obesity and substance use problems are the largest contributors to preventable deaths in the United States (Mokdad et al., 2004). The ability to prevent and treat these conditions early in development would result in major public health gains. Rates of obesity are rising more rapidly in adolescents relative to other developmental periods (Ogden et al., 2016). In the current study, higher BMI across adolescence was associated with fewer alcohol and illicit drug problems during early adulthood, consistent with the food-drug competition hypothesis. If foods, especially highly rewarding “junk foods, are able to compete with drugs of abuse this suggests that food and drugs of abuse may be engaging similar systems (e.g., reward-based systems). The use of empirically supported approaches to reduce problematic substance use in adolescence may also be effective in reducing problematic overeating during this developmental period, although more research is needed. However, inconsistent with the food-drug competition hypothesis, participants who were normal weight or obese across adolescence were more likely to be dependent on tobacco in early adulthood. Body image concerns are prevalent in adolescents across all weight classes (Carlson Jones, 2004) and have been associated with a greater likelihood to use cigarettes as a weight management strategy (White et al., 2007). Beliefs about the use of cigarettes to manage weight may be an important target for tobacco prevention efforts, particularly among teenagers.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute on Alcohol Abuse and Alcoholism: R37 AA07065 (Robert A. Zucker); R01 AA07065 (Robert A. Zucker); R01 DK102532 (Ashley N. Gearhardt)
Footnotes
The authors have no conflicts of interest to report.
Some of the data and ideas in this manuscript were presented in a talk at the 2017 Collaborative Perspectives on Addiction Meeting.
A small number of participants were outside the target age range for a given wave of data collection. For example, 10 participants were age 8 in Wave 3 (target age range 9–11) and 23 participants were age 21 in Wave 6 (target age range 18–20).
BMI estimates and percentiles based on Center for Disease Control and Prevention BMI-for-age growth charts (accessed from http://www.cdc.gov/growthcharts/clinical_charts.htm on January 8th 2016).
See Supplemental Table S1 for comparisons between classes identified based on the 4-class solution. Largely, these additional analyses confirmed significantly higher drinking and illicit drug problems, and nicotine dependence among a normal, low BMI trajectory group relative to a trajectory group with BMI values in the obese range (see Table S1 and Figure S3).
Contributor Information
Ashley N. Gearhardt, Department of Psychology, University of Michigan
Rebecca Waller, Department of Psychology and Department of Psychiatry, University of Michigan.
Jennifer M. Jester, Department of Psychiatry, University of Michigan
Luke W. Hyde, Department of Psychology, University of Michigan
Robert A. Zucker, Department of Psychiatry, University of Michigan
References
- Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using M plus. Structural Equation Modeling: A Multidisciplinary Journal. 2014;21(3):329–341. [Google Scholar]
- Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nature Reviews Neuroscience. 2012;13(7):514–514. doi: 10.1038/nrn3212-c1. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B Muthén LM. Mplus (Version 7.2) Los Angeles, CA: Muthén & Muthén; 2014. [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III . Nicotine psychopharmacology. Springer; 2009. Nicotine chemistry, metabolism, kinetics and biomarkers; pp. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Annals of epidemiology. 2007;17(1):44–50. doi: 10.1016/j.annepidem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neuroscience & Biobehavioral Reviews. 2002;26(4):393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Brault MC, Aimé A, Bégin C, Valois P, Craig W. Heterogeneity of sex-stratified BMI trajectories in children from 8 to 14years old. Physiology & behavior. 2015;142:111–120. doi: 10.1016/j.physbeh.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Carlson Jones D. Body image among adolescent girls and boys: a longitudinal study. Developmental Psychology. 2004;40(5):823. doi: 10.1037/0012-1649.40.5.823. [DOI] [PubMed] [Google Scholar]
- Carr KA, Oluyomi DT, Lin H, Epstein LH. Reinforcement pathology and obesity. Current drug abuse reviews. 2011;4(3):190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton S, Bate L, Hetherington M. Acute effects of an alcoholic drink on food intake: aperitif versus co-ingestion. Physiology & behavior. 2007;90(2):368–375. doi: 10.1016/j.physbeh.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of classification. 1996;13(2):195–212. [Google Scholar]
- Chen K, Kandel DB, Davies M. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug and alcohol dependence. 1997;46(1):53–67. doi: 10.1016/s0376-8716(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity research. 2012;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Ray LA, Tomiyama AJ. Food–alcohol competition: As young females eat more food, do they drink less alcohol? Journal of health psychology. 2015 doi: 10.1177/1359105315611955. 1359105315611955. [DOI] [PubMed] [Google Scholar]
- Farhat T, Iannotti RJ, Simons-Morton BG. Overweight, obesity, youth, and health-risk behaviors. American journal of preventive medicine. 2010;38(3):258–267. doi: 10.1016/j.amepre.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of general psychiatry. 1972;26(1):57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Fonseca H, Matos MG, Guerra A, Gomes Pedro J. Are overweight and obese adolescents different from their peers? International Journal of Pediatric Obesity. 2009;4(3):166–174. doi: 10.1080/17477160802464495. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychology of Addictive Behaviors. 2009;23(2):216. doi: 10.1037/a0015011. [DOI] [PubMed] [Google Scholar]
- Gold MS, Jacobs W. Cocaine (and crack): Clinical aspects. Substance Abuse: A Comprehensive Textbook. 1997:218–263. [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1):52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Gruchow HW, Sobocinski KA, Barboriak JJ, Scheller JG. Alcohol consumption, nutrient intake and relative body weight among US adults. The American journal of clinical nutrition. 1985;42(2):289–295. doi: 10.1093/ajcn/42.2.289. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Krueger RF, Racette SB, Norberg KE, Hipp PR, Bierut LJ. The emerging link between alcoholism risk and obesity in the United States. Archives of general psychiatry. 2010;67(12):1301–1308. doi: 10.1001/archgenpsychiatry.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, O’Callaghan MJ, Mamun AA, Williams GM, Clavarino A, Najman JM. Cannabis use and obesity and young adults. The American journal of drug and alcohol abuse. 2010;36(6):350–356. doi: 10.3109/00952990.2010.500438. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huang DY, Lanza HI, Wright-Volel K, Anglin MD. Developmental trajectories of childhood obesity and risk behaviors in adolescence. Journal of adolescence. 2013;36(1):139–148. doi: 10.1016/j.adolescence.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. American Psychological Association; 2011. [DOI] [PubMed] [Google Scholar]
- Johnston L. Monitoring the future: National results on adolescent drug use: Overview of key findings. DIANE Publishing; 2010. [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Kleiner KD, Gold MS, Frostpineda K, Lenzbrunsman B, Perri MG, Jacobs WS. Body mass index and alcohol use. Journal of addictive diseases. 2004;23(3):105–118. doi: 10.1300/J069v23n03_08. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Klesges LM, Meyers AW. Relationship of smoking status, energy balance, and body weight: analysis of the Second National Health and Nutrition Examination Survey. Journal of Consulting and Clinical Psychology. 1991;59(6):899. doi: 10.1037//0022-006x.59.6.899. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, LaVasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychological bulletin. 1989;106(2):204. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Lanza HI, Grella CE, Chung PJ. Does adolescent weight status predict problematic substance use patterns? American journal of health behavior. 2014;38(5):708–716. doi: 10.5993/AJHB.38.5.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza HI, Grella CE, Chung PJ. Adolescent obesity and future substance use: incorporating the psychosocial context. Journal of adolescence. 2015;45:20–30. doi: 10.1016/j.adolescence.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. American Journal of Epidemiology. 2011 doi: 10.1093/aje/kwr200. kwr200. [DOI] [PubMed] [Google Scholar]
- Lindgren E, Gray K, Miller G, Tyler R, Wiers C, Volkow N, Wang G. Food addiction: A common neurobiological mechanism with drug abuse. Frontiers in bioscience (Landmark edition) 2018;23:811. doi: 10.2741/4618. [DOI] [PubMed] [Google Scholar]
- Liu S, Serdula MK, Williamson DF, Mokdad AH, Byers T. A prospective study of alcohol intake and change in body weight among US adults. American Journal of Epidemiology. 1994;140(10):912–920. doi: 10.1093/oxfordjournals.aje.a117179. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA: the journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage; 2004. pp. 345–368. [Google Scholar]
- B Muthén LM. Mplus (Version 7.2) Los Angeles, CA: Muthén & Muthén; 2014. [Google Scholar]
- Nagin D. Group-based modeling of development. Harvard University Press; 2005. [Google Scholar]
- Neumark-Sztainer D, Story M, French SA, Hannan PJ, Resnick MD, Blum RW. Psychosocial Concerns and Health‐Compromising Behaviors among Overweight and Nonoverweight Adolescents. Obesity research. 1997;5(3):237–249. doi: 10.1002/j.1550-8528.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Nonnemaker JM, Morgan‐Lopez AA, Pais JM, Finkelstein EA. Youth BMI trajectories: evidence from the NLSY97. Obesity. 2009;17(6):1274–1280. doi: 10.1038/oby.2009.5. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch KE, Nelson MC, Lytle LA, Moe SG, Perry CL. Adoption of risk-related factors through early adolescence: associations with weight status and implications for causal mechanisms. Journal of Adolescent Health. 2008;43(4):387–393. doi: 10.1016/j.jadohealth.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch KE, Velazquez CE, Cance JD, Moe SG, Lytle LA. Youth substance use and body composition: does risk in one area predict risk in the other? Journal of youth and adolescence. 2012;41(1):14–26. doi: 10.1007/s10964-011-9706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton SJ, Wertheim EH, Gibbons K, Szmukler GI, Hillier L, Petrovich JL. Body image satisfaction, dieting beliefs, and weight loss behaviors in adolescent girls and boys. Journal of youth and adolescence. 1991;20(3):361–379. doi: 10.1007/BF01537402. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic medicine. 2008;70(3):288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Pickering RP, Goldstein RB, Hasin DS, Blanco C, Smith SM, Huang B, Stinson FS. Temporal Relationships Between Overweight and Obesity and< em> DSM-IV</em> Substance Use, Mood, and Anxiety Disorders: Results From a Prospective Study, the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2011;72(11):1494–1502. doi: 10.4088/JCP.10m06077gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter BK, Pederson LL, Chan SS, Aubut JAL, Koval JJ. Does a relationship exist between body weight, concerns about weight, and smoking among adolescents? An integration of the literature with an emphasis on gender. Nicotine & Tobacco Research. 2004;6(3):397–425. doi: 10.1080/14622200410001696529. [DOI] [PubMed] [Google Scholar]
- Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation’s children. Psychological bulletin. 2007;133(4):557. doi: 10.1037/0033-2909.133.4.557. [DOI] [PubMed] [Google Scholar]
- Rohrer JE, Rohland BM, Denison A, Way A. Frequency of alcohol use and obesity in community medicine patients. BMC family practice. 2005;6(1):1. doi: 10.1186/1471-2296-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room R. Measuring drinking patterns: the experience of the last half century. Journal of substance abuse. 2000;12(1):23–31. doi: 10.1016/s0899-3289(00)00038-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Research questionnaire San Diego, CA: Alcoholism Treatment Program. V. A. Medical Center, University of California; San Diego: 1978. [Google Scholar]
- Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS one. 2015;10(2):e0117959. doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clinical psychology review. 2016;44:125–139. doi: 10.1016/j.cpr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story M, Stang J. Understanding adolescent eating behaviors. Guidelines for adolescent nutrition services. 2005:9–19. [Google Scholar]
- Thatcher DL, Clark DB. Cardiovascular risk factors in adolescents with alcohol use disorders. International journal of adolescent medicine and health. 2006;18(1):151–158. doi: 10.1515/ijamh.2006.18.1.151. [DOI] [PubMed] [Google Scholar]
- Toumbourou JW, Stockwell T, Neighbors C, Marlatt G, Sturge J, Rehm J. Interventions to reduce harm associated with adolescent substance use. The Lancet. 2007;369(9570):1391–1401. doi: 10.1016/S0140-6736(07)60369-9. [DOI] [PubMed] [Google Scholar]
- Vermunt JK. Latent class modeling with covariates: Two improved three-step approaches. Political analysis. 2010;18(4):450–469. [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Reviews. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. Journal of addictive diseases. 2005;24(3):95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Weichold K, Wiesner MF, Silbereisen RK. Childhood predictors and mid-adolescent correlates of developmental trajectories of alcohol use among male and female youth. Journal of youth and adolescence. 2014;43(5):698–716. doi: 10.1007/s10964-013-0014-6. [DOI] [PubMed] [Google Scholar]
- Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC medical research methodology. 2012;12(1):1. doi: 10.1186/1471-2288-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MA, McKee SA, O’Malley SS. Smoke and mirrors: magnified beliefs that cigarette smoking suppresses weight. Addictive behaviors. 2007;32(10):2200–2210. doi: 10.1016/j.addbeh.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral Δ 9-THC. Physiology & behavior. 1998;65(2):343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obesity Reviews. 2013;14(1):19–28. doi: 10.1111/j.1467-789X.2012.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zuckerman BS, editors. Children of addiction: Research, health and policy issues. New York: Garland Press; 2000. pp. 174–222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


