Abstract
Potential solid-organ transplant recipients broadly sensitized to HLA have long wait times, low transplant rates and poor outcomes. The new kidney allocation system has improved access to the most highly sensitized recipients; however, their long-term outcomes are unknown. Emerging data suggest that memory B cell repertoire is broader than the plasma cell repertoire, therefore, despite refinements in anti-HLA antibody detection technology, donor-specific HLA- specific memory B cells may in fact be present in some, if not most, highly sensitized recipients with no detectable donor-specific antibodies. In addition, new findings have underscored the heterogeneity in memory B cell generation, and in the signals that determine memory versus plasma cell fate during primary antigen encounter, as well as memory B cell differentiation upon antigen reencounter into plasma cells or reentry into germinal centers to subsequently emerge as higher affinity and class-switched plasma cells. Thus, heterogeneity memory B cells generation may affect the efficacy of specific immunomodulation during the recall response. We propose that the ability to quantify donor-specific B cell in transplant recipients is urgently required to provide insights into the mechanisms of sensitization and recall, and for the early detection of acute and chronic AMR.
Introduction
One of the goals of the new kidney allocation system implemented in December 2014, is to increase transplant opportunities for difficult-to-match patients. Indeed, transplantation rates significantly increased for patients with calculated panel reactive antibody (cPRA) 99-100%, suggesting that more broadly-sensitized recipients are receiving kidney transplants (1). While early 6-month graft survival appears unchanged in these highly-sensitized recipients receiving permissive donor allografts, Hart et al. (1) cautioned that the long-term graft survival requires close monitoring as the potential impact of these high cPRA on long term grafts outcomes is unknown. Indeed, previous studies show that PRA at the time of transplant was associated with a step-wise graded association with death-censored graft failure, death with function, and the combined outcome (2). On the other hand, with the refinements in the anti-HLA antibody detection technology, cPRA per se may not imply an increased immunological risk when modern DSA assignment is used. In a recent study, donor specificity but not broadness of sensitization was noted to be associated with antibody mediated rejection and graft loss (3). Amidst this uncertainty, little is currently known about the pathophysiologic mechanisms that lead to the development of these extremely high cPRA antibodies, especially in the individuals who may have not been exposed to the breadth of HLA antigens. Emerging data suggest that the memory B cell (memB) repertoire is broader than the plasma cell repertoire (4), so that serological memory may not be equivalent to the memB repertoire. Interestingly, a relatively large retrospective study demonstrated that past high-sensitization status defined by either a cPRA (>50%) or peak-PRA (pPRA) (>50%) correlates with inferior graft outcomes, including increased incidence of delayed graft function, increased rejection rates and decreased graft survival (5). Furthermore, graft outcomes were inferior even in the low-sensitized group (PRA 5-50%) and in those that “converted” from a high-sensitized to low-sensitized group over time prior to transplantation. These observations raise the possibility that donor-specific memB may in fact be present in some, if not most, highly sensitized recipients of permissive donor allografts.
The diversity of the B cell repertoire supports the hypothesis that high cPRA is product of a broad repertoire of plasma cells producing antibodies that recognize specific HLA alleles or shared eplets (6, 7). The universality of this notion has however been challenged by the series of publications by Zorn and colleagues (8-11), (12) that anti-HLA serum reactivity may comprise, at least in part, polyreactive antibodies. Thus, it is possible that high cPRA may be explained by polyreactive antibodies produced by a limited repertoire of plasma cells. These antibodies may bind to antigens exposed on apoptotic cells or to denatured antigens on single HLA antigen beads used to detect HLA-specific antibodies (8, 10). Furthermore, these polyreactive antibodies, similar to HLA-specific antibodies, can activate complement to cause cell injury (10), and potentially, promote the generation of opsonins that enhance antigen uptake and presentation to donor-specific T and B cells (13, 14) or mediate the recruitment Fc-expressing cells that elicit graft injury (15-18). The potential role of polyreactive antibodies in solid organ transplantation has recently been reviewed (11) and will not be discussed further; instead we focus on discussing the latest findings on the heterogeneity in memB cells mediating the recall humoral response and potential implications to solid organ transplantation.
How naïve B cells differentiate into antibody secreting cells and memory B cells
The progression of a naïve B cell into a memory and plasma cells upon soluble antigen encounter has been extensively studied in mouse models, where the fate of antigen-specific B cells in secondary lymphoid organs can be examined in detail. When the B cell receptor (BCR) on naïve B cells engages antigen in the draining lymph node, the activated B cells upregulate CCR7 and migrate to the T-B interface. At the same time, naïve CD4+ T cells recognizing antigen processed and presented by dendritic cells downregulate CCR7 and upregulate CXCR5 and EBi2 that collectively direct the T cell to move to the T-B cell border. There, these differentiated extrafollicular T follicular helper (Tfh) cells engage in cognate interactions with B cells and specialized IL-2 quenching CD4+ICOSL+CD25+ dendritic cells (19, 20). This interaction results in B cell proliferation and differentiation into extrafollicular memB cells, short-lived plasmablasts (PB), or B cells that upregulate CXCR5 to migrate back into the center of the B cell follicle where they initiate a germinal center (GC) response.
The GC is divided into two compartments; the light zone comprising follicular dendritic cells (FDCs) and Tfh cells and is proximal to the marginal zone in the spleen or to the capsule in the LN, and the dark zone that is closest to the T cell zone. Within the light zone, B cells encounter antigen presented on specialized follicular dendritic cells (FDCs), and also receive survival signals from GC-Tfh cells (reviewed in (21, 22)). Survival signals include those provided by the interaction between costimulatory molecules, CD28-B7, CD40-CD154 and ICOS-ICOSL, and by cytokines such as IL-21 and BAFF. In the absence of these signals, B cells undergo apoptosis, while positively selected B cells migrate into the dark zone to undergo cell proliferation and BCR diversification through Ig somatic hypermutation (SMH) mediated by activation-induced cytidine deaminase (AID) (23, 24), (25, 26). One consequence of AID activity is damage of BCR genes and apoptosis of these B cells, while those with intact BCR exit the dark zone and reenter the light zone where their newly generated BCR are tested for binding to antigen and access to T cell help (25). Following repeated rounds of entry and exit from the dark zone (reviewed in (22)), post-GC B cells emerge as plasma cells (PC) that are ultimately responsible for persistent circulating antibodies, or as memB cells that are responsible for the recall antibody response upon antigen reencounter.
Central to the generation of protective humoral immunity is the controlled differentiation of antigen-activated B cells into short-lived effector antibody-secreting PB, long-lived PC and quiescent memB cells. The early generated PB play critical roles in containing infection, the later post-GC PC are responsible for preventing re-infection, while the quiescent memB cells generate the recall antibody response upon re-infection. Factors that determine these cell fates during primary antigen encounter have been extensively investigated, and critical roles for the affinity of the BCR for antigen, and factors derived from Tfh have been demonstrated (reviewed by (27)). The acquisition of a plasma cell over GC or memB cell phenotypes is facilitated by high BCR affinity for antigens, both in the early T:B border response and within ongoing GCs (28-30). The downstream signals of BCR engagement that determine cell fate that promote plasma cell differentiation have also been characterized. Specifically, Sciammas and colleagues (31-33) reported that a measure of BCR signaling intensity is graded expression of interferon regulatory factor-4 (IRF4). High levels of IRF4 dial up the Blimp-1 expression and promotes the plasma cell program, while shutting down the expression of Bach2 controlling germinal center B cell fate (33, 34). In contrast, more modest levels of IRF4 are required for differentiation into GC B cells (31, 33). In the late GC-dependent phase, the generation of plasma cells is also dependent on their affinity for antigen presented on FDCs, and cognate interactions with Tfh (30) (24). Kräutler et al. (35) recently provided new mechanistic details whereby PCs differentiation was shown to be induced in a discrete subset of high-affinity B cells residing within the light zone of the GC. Differentiation into PC is initiated upon engagement with intact antigen presented on FDC, while cognate interaction with Tfh cells provided signals essential for completing the PC differentiation process and for driving the migration of maturing PCs through the dark zone and out of the GC.
Memory B cells had classically been defined as class-switched B cells with non-GC phenotype; however, recent publications within the past decade have provided evidence for heterogeneity in memB cells generation. Using a new mouse model of memB cell-labeling based on cytidine deaminase expression, Dogan et al. (36) reported that following mouse immunization with sheep red blood cells, IgM+ quiescent memB cells were present at 4-times the frequency of IgG1+ memB cells. Subsequently, memory IgM+ and IgG+ B cells have been reported following immunization with other model antigens or malaria-infected erythrocytes (37, 38) (39) (40). As with antibody secreting cells, memB cells are generated in two distinct phases: the early phase that produce memB cells in a pre-GC or GC-independent manner, and a late post-GC phase. Pre-GC memB cells tend to be of low affinity and enriched for IgM+ whereas the post-GC memB cells are of higher affinity and comprised both IgM+ and IgG+ subsets (37, 38). Similar to pre-GC B cells, lower-affinity B cells with lower mutation load in the GC are preferentially recruited into the memB cell pool while higher affinity GC B cells are PC-prone (41). Memory-prone B cells have higher expression of the transcriptional repressor Bach2, which is induced by lower levels of T cell help. Interestingly, GC B cells that lacked CXCR4 and therefore failed to enter the DZ were more likely to enter the memory compartment, suggesting that T cell help may be sufficient to drive memory differentiation without further proliferation or SMH that is necessary for PC differentiation (42). Despite these new insights, the full spectrum of signals that control the post-GC memB cell fate decision and their exit from the GC remains unclear, and their full definition is hampered by the lack of a “master” transcription factor that can be used to trace these cells in vivo (reviewed in(43)).
Memory B cell subsets exhibit different longevity, with the IgM+ memory surviving significantly longer than the IgG+ memB cells (37). Because IgG+ memory cells tend to be more mutated than their IgM counterparts, whether BCR affinity or isotype switching determined memB cell persistence was recently ascertained using a novel mouse model where class-switching and SMH could be independently controlled. Gitlin et al. (44) reported that class-switching to IgG1 biased the fate choice to PC over memory fate, whereas SHM reduced the longevity of memory cells. The authors speculated that SHM reduced the longevity of memB cells by creating polyreactive specificities that were autoreactive and selected against over time, resulting in the preservation of a lower affinity, nonautoreactive memB cells.
A consequence of BCR affinity affecting B cell fate is manifest in an altered propensity to differentiate into memory or effector antibody secreting cells over the course of an immune response. Elegant pulse-chase experiments by Weisel et al. (45) showed that memB cells are generated mostly in the pre-GC and early GC period after immunization, whereas long-lived PC emerge late post-immunization. These findings of differences in affinity and kinetics of memory versus PC generation have important implications for transplant recipients. Firstly, treatment to reverse ongoing rejection process may successfully prevent the generation of long-lived plasma cells, but may not be able to prevent the earlier generation of memory donor-specific B cells. Second, the antigen-specificity of memB cells will not be identical to that of long-lived PC and the circulating antibodies they produce. Indeed, Lavinder et al. (4) used high-resolution liquid chromatography tandem mass spectrometry proteomic analyses of serum antibodies coupled with next-generation sequencing of the V gene repertoire of peripheral B cells following tetanus toxoid booster vaccination. They showed that ~100 antibody clonotypes and that just 3 clonotyptes accounting for >40% of the entire serological response. This antibody repertoire represented <5% of the peripheral memB cells that were anti-tetanus specific. Given that antibodies are used to measure B cell sensitization in pre- and post-transplant recipients, it is likely that the donor specific antibody (DSA) assays under identifies pre-sensitized patients that have developed donor-reactive memB cells but have undetectable circulating DSA. Assays that quantify the frequency of memory HLA-specific B cells (46) independently of serum anti-HLA IgG will be able to test this hypothesis. Finally, the differential longevity of memB cells suggest the possibility that higher affinity and class-switched IgG memB cells may be more quickly lost compared to lower affinity and IgM+ B cells. If true, then time after sensitization may affect the quality of the recall B cell response, with those recently sensitized having the highest affinity recall response compared to those who had been sensitized a long time ago.
Heterogeneity of the memory B cell recall response
A faster, higher-titer and class-switched antibody response are hallmarks of a recall response upon reencounter with antigen. Heterogeneity in memB cell generation suggests that these B cells will have different fates upon antigen re-encounter, with some favoring plasmablast differentiation, while others developing into GC B cells that can undergo rounds of proliferation and SMH to generate new, higher-affinity and class-switched memory cells and antibody-secreting cells. The heterogeneity in cellular response during antigen recall is well documented experimentally, although the rules that predict their cell fates in the recall response remain to be clarified. Seminal studies by Dogan et al. (36) demonstrated that the adoptive transfer of memIgM+ B cells and boosted with SRBC differentiated into mGC cells, whereas the IgG1+ memB cells gave rise primarily to mPCs. Likewise, Jenkins and colleagues (37) reported that the IgG+ memB cells predominated over IgM+ memB cells in the recall response when challenged soon (d320) after primary immunization. These IgG+ memB cells preferentially differentiated into antibody-secreting cells. The lack of a memIgM+ B cell response was due to the presence of circulating antibodies that outcompeted for access to antigen, the low affinity BCR expressed on IgM+ memB cells. Upon adoptive transfer, the IgM+ memB responded to antigen by differentiating into GC B cells that underwent additional rounds of class-switching and affinity maturation to generate a high- affinity IgG response. In contrast, when recall was performed at d450 post-primary immunization, at a time when circulating antigen-specific IgG titers and the frequency of IgG+ memB cells had significantly declined, IgM+ B cells were the primary cells responding by differentiating into GC cells.
The phenotype memB that predicts PC or GC fate has been further refined by recent studies. Shlomchik and colleagues (39, 45, 47, 48) reported that irrespective of IgM or IgG expression, memB cells that express CD80, PD-L2 and CD73 were mostly likely to produce PC, while those that lack these markers were more likely to produce GC cells. Since most of the IgM+ memB cells lack CD80 and CD73, while IgG+ memory cells expressed CD80 and CD73, these observations are not inconsistent with those of Jenkins and colleagues. More recently, Krishnamurthy et al. (40) investigated the memB cell and recall response in mice infected with malaria. Three sets of memB cells were detected; somatically hypermutated IgM+ and IgG+, and an unmutated IgD+ subsets, with their longevity corresponding to their levels of somatic hypermutation. Challenge with malaria-infected erythrocytes, resulted in the early (d3) proliferation of high-affinity IgM+ memB cells and differentiation into PC in both a T cell-dependent and independent manner. Notably the more highly mutated IgM+ memory that responded to secondary challenge expressed CD80 and CD73 expression. Finally, the sequestration of malaria antigens within the infected cells may have contributed to the ability of the higher affinity IgM+ memB cells to respond even in the presence of IgG+ memB cells and circulating IgG.
Teleologically, the ability of memB cells to generate GC progeny that further diversify their BCR would be essential for protective immunity towards rapidly evolving pathogens. Supporting this notion, McHeyzer-Williams and colleagues (49, 50) have investigated the memB cell response following prime and boost with NP conjugated with keyhole limpet hemocyanin adjuvanted with monophosphoryl lipid A. They showed that the boost resulted in IgM+ memory cells that favored differentiation into PC, whereas the IgG+ memB cells preferentially differentiated into GC B cells that underwent BCR diversification and acquired a GC transcription program. Taken collectively, it appears that both IgM and IgG memory can participate in the recall response, with different propensities to PC or GC differentiation depending on their BCR isotype, affinity for antigen and differentiation state. More recently, Asrir et al. (51) reported that the location and access to the type of Tfh can also determine memB cell fate. Circulating memory Tfh promoted more secondary germinal centers, whereas the resident memory Tfh cells that localized close to memB cells in the B cell follicle promoted plasma cell differentiation. Those variables in turn dictated the kinetics of antibody production in the recall, and also the functional properties of the secreted antibodies.
Concluding remarks
How do these basic findings on heterogeneity of memB cells impact our understanding of the antibody response in sensitized patients after transplantation? When incompatible allografts are transplanted into recipients with pre-existing DSA, it is reasonable to assume that they will also harbor donor-specific memB and memory T cells. These individuals are more likely to subsequently develop a recall DSA response post-transplantation that mediates acute or chronic antibody-mediated rejection, or mixed AMR plus TCMR rejection (52-54). Whether the de novo DSA response is the result of memB cells differentiating directly into PCs or reentering a GC response to subsequently generate PCs may affect the efficacy to immunosuppression. Our recent studies show that the donor-MHC B response following cardiac transplantation in sensitized mouse recipients is T cell-dependent and skewed towards a PC response (55, 56). Thus, co-stimulation blockade with CTLA-4Ig was effective at preventing the development of a recall DSA response, a finding consistent with the clinical observations of significantly low rates of DSA development in transplant recipients receiving belatacept, despite high rates of acute rejection (57). In contrast, reversing an established recall DSA response that is mediated predominantly by PC may be more amenable to drugs that deplete PC, such as bortezomib or carfilzomib (58, 59), whereas a recall response that is dependent on GC output may additionally be responsive to drugs that inhibit the GC response. In addition, the notion that memB cells have different longevity depending on the mutation load suggests that recently sensitized recipients may mount a recall DSA response that has a more rapid kinetics and higher affinity, whereas when the sensitizing event occurred many decades ago, a functional recall response may require reentry into a GC response and thus be more sensitive to immunosuppression that prevents the reactivation of Tfh and lower-affinity memB cells. Donor specific memory B cells that reenter a germinal center may also switch from non-C1q binding to C1q-binding IgG producing cells, and thus produce IgG antibodies that not only mediate pathology via Fc-receptor binding but also through complement activation. Finally, the observation that circulating antibodies may shape the quality of the recall response, raises the possibility that plasmapheresis used to remove DSA in preparation of transplantation in sensitized recipients, may inadvertently promote a more vigorous recall response by allowing antigen to access memB cells with low affinity for alloantigen.
Testing the behavior of alloreactive B cells, with either naïve or memory phenotype, in transplant recipients or those waiting to receive a transplant are hampered by the need to study developing B cell responses in the secondary lymphoid structures, concerns that the blood does not fully capture the full repertoire of memB cells, and a lack of an assay to quantify the frequency of donor-specific B cells. Reports that memB cells specific for vaccine antigens can be detected in the blood, and that their frequency tracks with time post-antigen exposure (60, 61), suggest that an assay that accurately quantifies circulating donor-specific memB cells is feasible (46, 62). Such an assay will likely complement our ability to quantify DSA by providing an earlier diagnosis of acute or chronic AMR, as well as in identifying successful desensitization protocols and treatments of AMR.
Figure 1.
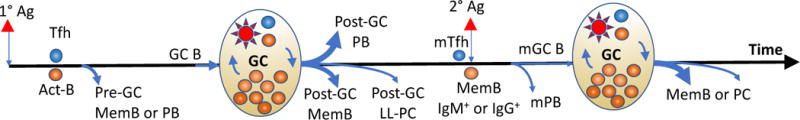
Summary of B cell differentiation after primary (1°) or secondary (2°) antigen (Ag) encounter. Primary antigen encounter generates two waves of memory B cells (MemB) and antibody secreting cells. The early wave is prior to and independent of the germinal center (GC) response, and generates pre-GC MemB cells as well as short-lived plasmablasts (PB). The later wave generates a post-GC MemB and long-lived plasma cells (LL-PC). Upon a secondary Ag encounter, IgM+ and IgG+ memory B cells have distinct abilities to differentiate directly into secondary PB (mPB) or GC cells that undergo further B cell receptor diversification to ultimately emerge as secondary MemB or PCs. Width of blue lines indicates relative quantities of cell output.
Acknowledgments
This work was supported in part by grants (R01 AI072630) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health.
Abbreviations
- AID
activation-induced cytidine deaminase
- BCR
B cell receptor
- BAF
B cell activation factor
- CPRA
calculated panel reactive antibody
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- DSA
donor-specific antibodies
- FDC
follicular dendritic cells
- GC
germinal center
- HLA
human leukocyte antigen
- IRF-4
interferon regulatory factor-4
- MemB
memory B cells
- PB
plasmablasts
- PC
plasma cells
- SHM
somatic hypermutation
- Tfh
T follicular helper
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Hart A, Gustafson SK, Skeans MA, Stock P, Stewart D, Kasiske BL, et al. OPTN/SRTR 2015 Annual Data Report: Early effects of the new kidney allocation system. Am J Transplant. 2017;17(Suppl 1):543–564. doi: 10.1111/ajt.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim HN, Skeans MA, Li Q, Ishani A, Snyder JJ. Blood transfusions in kidney transplant candidates are common and associated with adverse outcomes. Clin Transplant. 2011;25(4):653–659. doi: 10.1111/j.1399-0012.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 3.Wehmeier C, Honger G, Cun H, Amico P, Hirt-Minkowski P, Georgalis A, et al. Donor Specificity but Not Broadness of Sensitization Is Associated With Antibody-Mediated Rejection and Graft Loss in Renal Allograft Recipients. Am J Transplant. 2017;17(8):2092–2102. doi: 10.1111/ajt.14247. [DOI] [PubMed] [Google Scholar]
- 4.Lavinder JJ, Horton AP, Georgiou G, Ippolito GC. Next-generation sequencing and protein mass spectrometry for the comprehensive analysis of human cellular and serum antibody repertoires. Curr Opin Chem Biol. 2015;24:112–120. doi: 10.1016/j.cbpa.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Huber L, Lachmann N, Niemann M, Naik M, Liefeldt L, Glander P, et al. Pretransplant virtual PRA and long-term outcomes of kidney transplant recipients. Transpl Int. 2015;28(6):710–719. doi: 10.1111/tri.12533. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky FM, Parham P, Barnstable CJ, Crumpton MJ, Bodmer WF. Monoclonal antibodies for analysis of the HLA system. Immunological reviews. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 7.Duquesnoy RJ. Human leukocyte antigen epitope antigenicity and immunogenicity. Curr Opin Organ Transplant. 2014;19(4):428–435. doi: 10.1097/MOT.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 8.Gao B, Rong C, Porcheray F, Moore C, Girouard TC, Saidman SL, et al. Evidence to Support a Contribution of Polyreactive Antibodies to HLA Serum Reactivity. Transplantation. 2016;100(1):217–226. doi: 10.1097/TP.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porcheray F, DeVito J, Helou Y, Dargon I, Fraser JW, Nobecourt P, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12(8):2088–2097. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porcheray F, Fraser JW, Gao B, McColl A, DeVito J, Dargon I, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13(10):2590–2600. doi: 10.1111/ajt.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorn E, See SB. Polyreactive natural antibodies in transplantation. Curr Opin Organ Transplant. 2017;22(1):8–13. doi: 10.1097/MOT.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee D, Moore C, Gao B, Clerkin KJ, See SB, Shaked D, et al. Prevalence of polyreactive innate clones among graft–infiltrating B cells in human cardiac allograft vasculopathy. J Heart Lung Transplant. 2017 doi: 10.1016/j.healun.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. Journal of immunology. 2011;186(1):214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. Journal of immunology. 2009;182(3):1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 15.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12(2):313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin D, Zeng H, Ma L, Shen J, Xu H, Byrne GW, et al. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. Journal of immunology. 2004;172(12):7235–7238. doi: 10.4049/jimmunol.172.12.7235. [DOI] [PubMed] [Google Scholar]
- 17.Kohei N, Tanaka T, Tanabe K, Masumori N, Dvorina N, Valujskikh A, et al. Natural killer cells play a critical role in mediating inflammation and graft failure during antibody-mediated rejection of kidney allografts. Kidney Int. 2016;89(6):1293–1306. doi: 10.1016/j.kint.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkes MD, Halloran PF, Hidalgo LG. Evidence for CD16a-Mediated NK Cell Stimulation in Antibody-Mediated Kidney Transplant Rejection. Transplantation. 2017;101(4):e102–e111. doi: 10.1097/TP.0000000000001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 2016;533(7601):110–114. doi: 10.1038/nature17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu E, Dang EV, McDonald JG, Cyster JG. Distinct oxysterol requirements for positioning naive and activated dendritic cells in the spleen. Sci Immunol. 2017;2(10) doi: 10.1126/sciimmunol.aal5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarlinton D, Victora G. Editorial overview: Germinal centers and memory B-cells: from here to eternity. Current opinion in immunology. 2017;45:v–viii. doi: 10.1016/j.coi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Mesin L, Ersching J, Victora GD. Germinal Center B Cell Dynamics. Immunity. 2016;45(3):471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victora GD, Mesin L. Clonal and cellular dynamics in germinal centers. Current opinion in immunology. 2014;28:90–96. doi: 10.1016/j.coi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer CT, Gazumyan A, Kara EE, Gitlin AD, Golijanin J, Viant C, et al. The microanatomic segregation of selection by apoptosis in the germinal center. Science. 2017;358(6360) doi: 10.1126/science.aao2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 2015;42(4):704–718. doi: 10.1016/j.immuni.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Shinnakasu R, Kurosaki T. Regulation of memory B and plasma cell differentiation. Current opinion in immunology. 2017;45:126–131. doi: 10.1016/j.coi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Phan TG, Tangye SG. Memory B cells: total recall. Current opinion in immunology. 2017;45:132–140. doi: 10.1016/j.coi.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of experimental medicine. 2006;203(4):1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. The Journal of experimental medicine. 2006;203(11):2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochiai K, Maienschein-Cline M, Mandal M, Triggs JR, Bertolino E, Sciammas R, et al. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nature immunology. 2012;13(3):300–307. doi: 10.1038/ni.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25(2):225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38(5):918–929. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto A, Ochiai K, Kimura Y, Itoh-Nakadai A, Calame KL, Ikebe D, et al. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J. 2010;29(23):4048–4061. doi: 10.1038/emboj.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. The Journal of experimental medicine. 2017;214(5):1259–1267. doi: 10.1084/jem.20161533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, et al. Multiple layers of B cell memory with different effector functions. Nature immunology. 2009;10(12):1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 37.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. The Journal of experimental medicine. 2012;209(3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nature immunology. 2014;15(7):631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, et al. Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity. 2016;45(2):402–414. doi: 10.1016/j.immuni.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nature immunology. 2016;17(7):861–869. doi: 10.1038/ni.3460. [DOI] [PubMed] [Google Scholar]
- 42.Bannard O, Horton RM, Allen CD, An J, Nagasawa T, Cyster JG. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39(5):912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. Current opinion in immunology. 2017;45:97–102. doi: 10.1016/j.coi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44(4):769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016;44(1):116–130. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(1):21–28. doi: 10.1097/TP.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. Journal of immunology. 2010;185(12):7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. The Journal of experimental medicine. 2007;204(9):2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHeyzer-Williams LJ, Dufaud C, McHeyzer-Williams MG. Do Memory B Cells Form Secondary Germinal Centers? Impact of Antibody Class and Quality of Memory T-Cell Help at Recall. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nature immunology. 2015;16(3):296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asrir A, Aloulou M, Gador M, Perals C, Fazilleau N. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun. 2017;8(1):847. doi: 10.1038/s41467-017-00843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aubert O, Loupy A, Hidalgo L, Duong van Huyen JP, Higgins S, Viglietti D, et al. Antibody-Mediated Rejection Due to Preexisting versus De Novo Donor-Specific Antibodies in Kidney Allograft Recipients. J Am Soc Nephrol. 2017;28(6):1912–1923. doi: 10.1681/ASN.2016070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15(11):2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 54.Vo AA, Sinha A, Haas M, Choi J, Mirocha J, Kahwaji J, et al. Factors Predicting Risk for Antibody-mediated Rejection and Graft Loss in Highly Human Leukocyte Antigen Sensitized Patients Transplanted After Desensitization. Transplantation. 2015;99(7):1423–1430. doi: 10.1097/TP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. Journal of immunology. 2015;195(9):4069–4073. [Google Scholar]
- 56.Yang J, Chen J, Young JS, Wang Q, Yin D, Sciammas R, et al. Tracing Donor-MHC Class II Reactive B cells in Mouse Cardiac Transplantation: Delayed CTLA4-Ig Treatment Prevents Memory Alloreactive B-Cell Generation. Transplantation. 2016;100(8):1683–1691. doi: 10.1097/TP.0000000000001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincenti F. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(26):2600–2601. doi: 10.1056/NEJMc1602859. [DOI] [PubMed] [Google Scholar]
- 58.Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15(1):101–118. doi: 10.1111/ajt.13050. [DOI] [PubMed] [Google Scholar]
- 59.Ensor CR, Yousem SA, Marrari M, Morrell MR, Mangiola M, Pilewski JM, et al. Proteasome Inhibitor Carfilzomib-Based Therapy for Antibody-Mediated Rejection of the Pulmonary Allograft: Use and Short-Term Findings. Am J Transplant. 2017;17(5):1380–1388. doi: 10.1111/ajt.14222. [DOI] [PubMed] [Google Scholar]
- 60.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. Journal of immunology. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 61.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. European journal of immunology. 2009;39(5):1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young JS, McIntosh C, Alegre ML, Chong AS. Evolving Approaches in the Identification of Allograft-Reactive T and B Cells in Mice and Humans. Transplantation. 2017;101(11):2671–2681. doi: 10.1097/TP.0000000000001847. [DOI] [PMC free article] [PubMed] [Google Scholar]


