Abstract
Regulatory T cells utilize a distinct TCR repertoire and are more self-reactive compared to conventional T cells. However, the extent to which TCR affinity regulates the function of self-reactive Tregs is largely unknown. In this study, we utilized a two-TCR model to assess the role of TCR affinity in Treg function during autoimmunity. We observed that both high and low affinity Tregs were recruited to the pancreas and contributed to protection from autoimmune diabetes. Interestingly, high affinity cells preferentially upregulated TCR-dependent Treg functional mediators IL-10, TIGIT, GITR and CTLA4, while low affinity cells displayed increased transcripts for Areg and Ebi3, suggesting distinct functional profiles. The results of this study suggest mechanistically distinct and potentially non-redundant roles for high and low affinity Tregs in controlling autoimmunity.
Keywords: T cell receptor, regulatory T cell, type 1 diabetes, autoimmunity
Introduction
Foxp3+ regulatory T cells (Tregs) are critical for maintaining immune homeostasis and preventing the development of tissue-specific autoimmunity. Their functional relevance in type 1 diabetes (T1D) can be observed in IPEX patients, who have mutations in FOXP3, and develop T1D at a high frequency (1). Likewise, deletion of Foxp3+ T cells in TCR transgenic or retrogenic (Rg) mice specific for beta-cell antigens leads to accelerated diabetes (2, 3). Consequently, Treg-centric immunotherapies have been vigorously pursued for prevention or treatment of T1D. However, it remains unclear whether boosting overall Treg numbers will be sufficient, or whether therapeutic approaches will need to focus on a subpopulation of functional Tregs. It is largely accepted that Tregs develop in response to stronger TCR signals, and are presumed to exhibit an overall higher degree of self-reactivity compared to conventional T cells (4–6). Moreover, recent work has shown that continuous TCR signaling is necessary for optimal Treg function (7, 8). Although it is tempting to assume that high affinity T cells are generally more functional, emerging literature suggests an equal and important role for low affinity effector T cells (Teffs) in responses against pathogens, in autoimmunity, and in tumor surveillance (9–11). However, studies addressing the role of low affinity Tregs in immune homeostasis have not been performed; thus, it remains unclear whether TCR affinity is correlated with Treg recruitment, accumulation, and function in autoimmunity.
In our previous analysis of mice expressing eight TCRs with variable affinity for the immunodominant insulin epitope B:9–23, deletion of Tregs in mice expressing higher affinity TCRs resulted in accelerated autoimmune diabetes, whereas in mice harboring lower affinity TCRs the rate of disease was unaffected by Treg-depletion (3). We therefore hypothesized that low affinity Tregs might not be functional in autoimmune diabetes. However, since in single TCR Rg mice both Teffs and Tregs possessed the same TCR, it remains unclear whether higher affinity Tregs were more functional or whether low affinity Teffs were resistant to suppression. In order to directly compare high and low affinity Tregs in vivo, here we utilized a mixed TCR Rg bone marrow chimera model. In this competitive setting, we were able to assess the relative accumulation and capacity of high and low affinity Tregs to control the same population of effector T cells.
Materials and Methods
Mice
NOD/ShiLtJ (NOD), NOD.B6-Ptprcb (NOD.CD45.2), NOD.CB17-Prkdcscid/J (NOD.scid), NOD.129P2(C)-Tcrαtm1Mjo/DoiJ (NOD.TCRα−/−) and NOD.Cg-Foxp3sf/DoiJ (NOD.scurfy) mice were obtained directly from the Jackson Laboratories and maintained at our facility. NOD.scurfy mice were crossed with NOD.scid at our facility. All mice were housed in specific-pathogen-free conditions. The studies were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Generation of two-TCR Rg mice
Two-TCR Rg mice were generated as previously described (12). Briefly, bone marrow (BM) was harvested from NOD.scid and NOD.scid.scurfy mice, transduced with retroviral TCR vectors expressing either a GFP or Ametrine fluorescent reporter, and transferred into recipient NOD.Tcrα−/− mice (Supplemental Fig. 1G, 1I). Mice were either monitored for diabetes development or analyzed 5–6.5 weeks post-bone marrow transfer, at which point the T cell reconstitution was assessed (Supplemental Fig. 1H, 1J). For some experiments, NOD.CD45.2 bone marrow was added at 10% of the total cell number prior to injection.
Assessment of Diabetes
Diabetes incidence was monitored weekly with Diastix (Bayer, Elkhart, IN), and confirmed with Breeze2 glucometer (Bayer, Elkhart, IN). Mice were considered diabetic if their blood glucose was >400 mg/dl.
Isolation of Pancreatic Islets
Pancreata were digested with collagenase IV (Worthington, Lakewood, NJ), and single islets were isolated for further analysis as previously described (3).
Flow Cytometry and Antibodies
Flow cytometry analyses were performed on LSRFortessa II (BD Biosciences), and data were analyzed with FlowJo software (Tree Star Inc.). Monoclonal antibodies against the following molecules were used: Foxp3 (FJK-16s), Vβ12 (MR11-1), and TIGIT (GIGD7) from eBioscience; CD5 (53-7.3), Ki67 (B56), and Vβ11 (RR3-15) from BD Biosciences; CD3 (145-2C11), CD4 (GK1.5), CD25 (PC61), CTLA-4 (UC10-4B9), CD8 (53-6.7), GITR (YGITR 765), Vβ2 (B20.6), and IL-10 (JES5-16E3) from Biolegend.
RNAseq
Tregs were sorted from pancreatic islets and spleens of wt/wt two-TCR Rg mice based on Ametrine or GFP TCR fluorescent reporter and CD4+CD3+GITR+CD25+ gating strategy (Supplemental Fig. 2G). Samples were sorted with an average purity of 92.6% Foxp3+ for 4–8, and 92.5% Foxp3+ for 12-4.4m1. cDNA was synthesized using the SMARTer Ultra Low Input RNA Kit (Clonetech). Library preparation was performed with the Illumina Nextera XT kit before paired-end RNA-sequencing using the Illumina NextSeq500 platform for 150 cycles (NextSeq500 Mid Output Kit). Sequencing reads were aligned to the mm10 genome using TopHat Alignment Trapnell, et al. (13) and gene expression was quantified by FPKM. Cufflinks Assembly & DE (14) were used to compute differential expression (q<0.05) between groups, with Benjamini-Hochberg correction for multiple testing. Heatmaps and principle component analysis (PCA) were generated in R (version 3.2.3) using pheatmap from gplots package (version 2.17.0) with viridis (version 0.4.0), and ggbiplots (15). Data Resources: The accession number for the raw data reported in this paper is GSE106467 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE106467).
Statistical Analysis
Diabetes incidence was subjected to Log-rank Mantel-Cox test. Group comparisons were performed using two-tailed Mann-Whitney nonparametric test in Figures 2 and 3, and Wilcoxon matched-pairs test in Figure 4. The mean ± SEM is shown, ns = not significant, *p<0.05, **p<0.005, ***p<0.0005. Statistical analyses were performed using Prism (La Jolla, CA).
Figure 2.
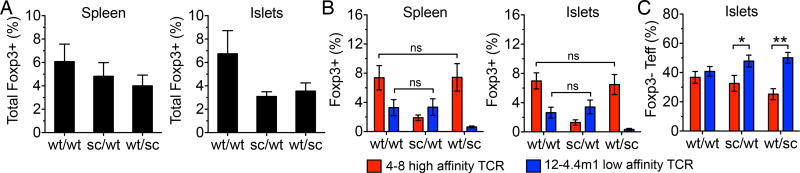
Treg frequencies are regulated by TCR intrinsic mechanisms and are not affected by changing the size of the regulatory compartment. (A) Total Foxp3+ Treg frequencies in the spleens and islets of 4–8/12-4.4m1 two-TCR Rg BM chimeras. Analysis is gated on CD4+CD3+ (n=13–20 mice per group). (B) Frequencies of 4–8 or 12-4.4m1 Foxp3+ T cells in the spleens and islets of two-TCR Rg BM chimeras. Analysis is gated on CD4+CD3+Vβ2+ or Vβ12+ (n=13–20 mice per group). (C) Relative frequencies of 4–8 or 12-4.4m1 Foxp3– Teff cells in the islets of two-TCR Rg BM chimeras. Analysis is gated on CD4+CD3+Foxp3–Vβ2+ or Vβ12+ (n=13–20 mice per group). Mice were analyzed 5–6.5 weeks post-bone marrow transfer. Data are pooled from six independent experiments.
Figure 3.
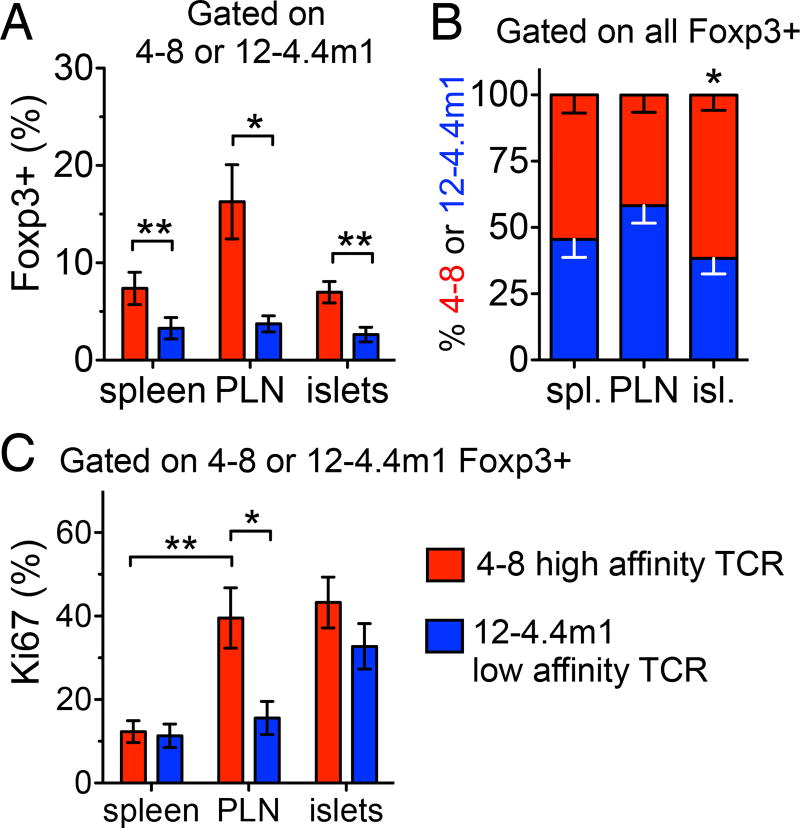
Increased activation in PLN and preferentially accumulation in the islets by high affinity Tregs. (A) Intra-TCR frequencies of 4–8 and 12-4.4m1 Foxp3+ Tregs in wt/wt two-TCR Rg BM chimeras. CD4+CD3+ T cells are initially separated based on Vβ2+ or Vβ12+ expression, followed by analysis of Foxp3+ frequencies within each TCR population (n=17–20 mice per group). (B) Relative frequencies of 4–8 and 12-4.4m1 cells within the whole Foxp3+ Treg population in wt/wt two-TCR Rg BM chimeras. Analysis is first gated on all CD4+CD3+Foxp3+ cells, followed by the analysis of relative frequency of Vβ2+ or Vβ12+ cells within all Foxp3+ T cells (n=17–20 mice per group). (C) Percent Ki67+ Tregs in wt/wt two-TCR Rg BM chimeras. (n=17–20 mice per group). Mice were analyzed 5–6.5 weeks post-bone marrow transfer. Data are pooled from six independent experiments.
Figure 4.
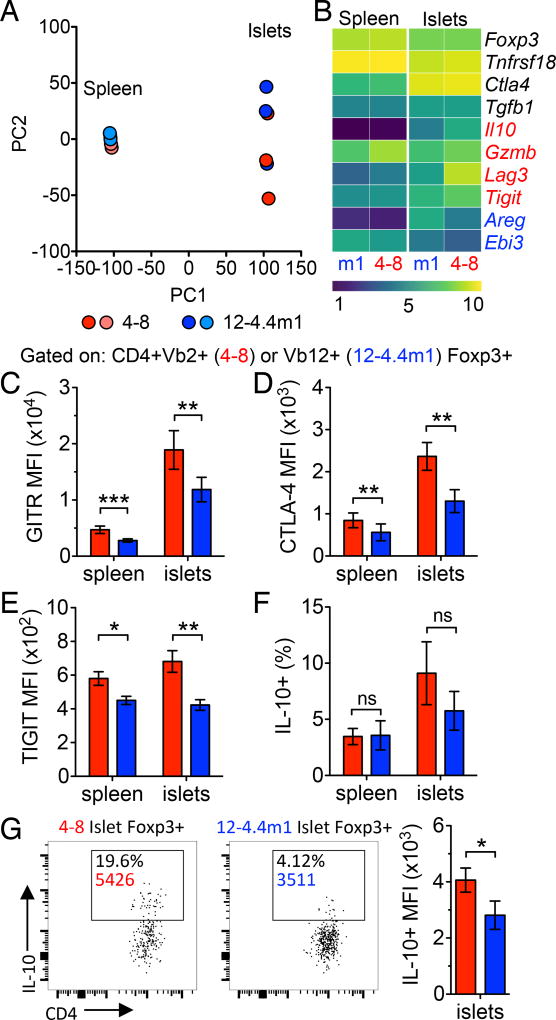
High and low affinity Tregs are transcriptionally distinct. (A) Principle component analysis of 4–8 and 12-4.4m1 Tregs isolated from the spleens and islets of wt/wt two-TCR Rg BM chimeras (n=3). (B) rlog transformed heatmap of Treg functional genes (n=3). Genes in black had no statistical difference between 4–8 and 12-4.4m1 Tregs in the islets. Red (increased in 4–8 Tregs) and blue (increased in 12-4.4m1 Tregs) genes had a significant q-value (q<0.05) between 4–8 and 12-4.4m1 Tregs in the islets. (C) GITR MFI (n=16), (D) CTLA-4 MFI (n=7), (E) TIGIT MFI (n=10) and (F) percent IL-10+ (n=7) of 4–8 and 12-4.4m1 Tregs in wt/wt two-TCR Rg BM chimeras. (G) Representative gating of IL-10+ 4–8 and 12-4.4m1 Tregs in wt/wt two-TCR Rg BM chimeras and quantification of IL-10+ MFI (n=7). Mice were analyzed 5–6.5 weeks post-bone marrow transfer. (C–D) Data are pooled from at least 3 independent experiments.
Results and Discussion
High and low affinity Tregs cooperate to control autoimmune diabetes
Although some studies suggest that Treg development can be supported by TCRs with a wide range of affinities for self-antigens (16), there appears to be a positive correlation between TCR affinity and Treg development (4, 6). In order to determine whether TCR affinity for self governs Treg function in autoimmunity, we generated two-TCR Rg mice with mixed bone marrow from NOD.scid (‘wt’) and NOD.scid.scurfy (‘scurfy’) mice. In this system, we transduced either wt or scurfy bone marrow with low or high affinity TCR, and mixed the two bone marrows at an equal ratio prior to injection into the TCRα−/− recipients (Fig. 1A). Scurfy mice carry a missense mutation in the Foxp3 gene, resulting in the complete absence of functional Tregs (17). Therefore, this mixed bone marrow chimera allowed us to limit Treg development to the TCR that was expressed on the NOD.scid background, while the Teff population was derived from both NOD.scid and NOD.scid.scurfy bone marrows (Fig. 1B and Supplemental Fig. 1A).
Figure 1.
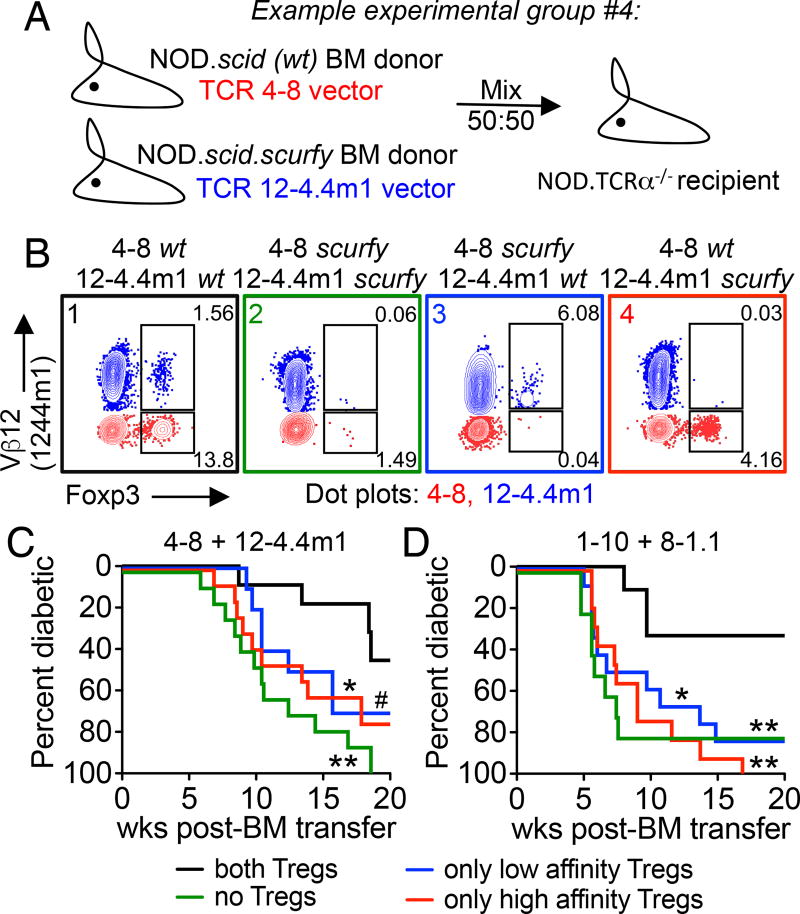
Both high and low affinity Tregs contribute to protection during autoimmunity. (A) An example of a two-TCR retrogenic chimera experimental group where Foxp3+ Treg development is limited to 4–8 TCR expressing T cells. (B) Representative flow plots of Foxp3+ Tregs from the spleen of 4–8/12-4.4m1 two-TCR Rg BM chimeras. Analysis is gated on CD4+CD3+ cells, Vβ12+ (blue) are 12-4.4m1, and Vβ12– (red) are 4–8 T cells. Mice were analyzed 5.3 weeks post-bone marrow transfer. (C and D) Diabetes incidence for two-TCR Rg chimeras expressing either 4–8 and 12-4.4m1 (C) or 1–10 and 8-1.1 TCRs (D). Mice were monitored for spontaneous diabetes development for 20 weeks (n=10–13 mice per group, #p=0.057). Data are pooled from six (C) and nine (D) independent experiments.
We chose to study two pairs of high and low affinity TCRs, which were selected based on their ability to support efficient Treg development. The TCRs were stratified into “high” and “low” affinity based on their biophysical 2D affinities, functional responses to the wild type insulin (InsB:9–23) and agonist InsB:9–23(R22E) peptides, as well as insulin tetramer staining (Supplemental Fig. 1B–E) (3).
We first generated two-TCR Rg mice expressing a combination of the high and the low InsB:9–23 reactive TCRs: 4–8 and 12-4.4m1 (Supplemental Fig.1G, H). The two TCRs were chosen because when expressed individually, they yield similar frequencies of islet-infiltrating Foxp3+ Tregs (12% and 9%, respectively), and have similar composition of helioshi or thymically derived Tregs (Supplemental Fig. 1F). Importantly, the two TCRs lead to significantly different disease patterns (3). In single-TCR Rg mice, the 4–8 TCR results in spontaneous diabetes development in about 60% of mice. By contrast, low affinity 12-4.4m1 TCR mice are free from diabetes, despite T cell infiltration of the pancreas (3). The lack of disease in 12-4.4m1 TCR mice could not be solely explained by the presence of Tregs, as Treg ablation in Foxp3DTR or in scurfy 12-4.4m1 TCR mice did not lead to diabetes development (data not shown). On the other hand, Treg depletion in 4–8 TCR mice resulted in significant acceleration of diabetes (3). Therefore, we hypothesized that unlike the high affinity 4–8 Tregs, the 12-4.4m1 low affinity Tregs lack sufficient levels of TCR signaling to regulate pathogenic T cells, such as 4–8 Teffs.
In the 4–8 and 12-4.4m1 NOD.scid mixed bone marrow chimeras, where both TCRs gave rise to Tregs, mice were partially protected from diabetes, with only 40% developing disease by 20 weeks post bone marrow transfer (Fig. 1C, black line). However, when both TCRs were expressed on the NOD.scid.scurfy background, we observed an accelerated disease course with 100% penetrance (Fig. 1C, green line). Surprisingly, the absence of either low or high affinity Tregs resulted in similar acceleration of disease, with about 70% of mice developing diabetes (Fig. 1C, blue and red lines), indicating that both Treg populations were critical for protection and acted in a cooperative manner.
In order to confirm our observations with a different set of InsB:9–23 specific TCRs, we used a combination of the high affinity TCR 1–10 and low affinity 8-1.1, both of which displayed similar frequencies of Tregs when expressed individually (9% and 8%, respectively), and were both pathogenic (Supplemetal Fig. 1I, J) (3). As with the first pair of TCRs, we observed similar levels of helios expression, suggesting equal distribution of thymically and peripherally derived cells within the two Treg populations (Supplemental Fig. 1F). Importantly, as observed with the first set of TCRs, disease onset was accelerated when both or either of the two Treg populations were absent (Fig. 1D). Taken together, these data indicate that both high and low affinity Tregs can contribute to regulation of autoimmunity.
Treg frequencies are regulated by TCR intrinsic mechanisms
Since elimination of either Treg population accelerated diabetes development comparable to the scurfy/scurfy group with no functional Tregs, we considered the possibility that the absence of one Treg population might have a negative effect on the survival and homeostasis of the remaining Tregs in the increasingly inflammatory environment. Therefore, we assessed the frequencies of Foxp3+ Tregs in the spleens and pancreatic islets of 4–8/12-4.4m1 two-TCR chimeras. Indeed, we observed an overall decrease in Treg frequencies in wt/scurfy chimeras; however, in general, the decrease in Treg frequencies and numbers did not exceed 50% loss (Fig. 2A, Supplemental Fig. 2A). Intra-TCR analysis confirmed that the absence of either Treg population had minimal effect on the frequencies or numbers of the other population (Fig. 2B, Supplemental Fig. 2B). This suggests that Treg frequencies are likely determined by the strength of TCR signaling during thymic development and tonic TCR signaling in the periphery, and not affected by the size or composition of the regulatory T cell compartment. Overall, these data indicate that a net Treg to Teff ratio, rather than Treg TCR affinity, is more important for controlling the pathogenic Teff population in autoimmunity.
Tregs have been known to take on specialized functional characteristics appropriate for suppression of specific T helper subsets (18, 19). We considered that low and high affinity Tregs might be specialized for controlling either high or low affinity effector populations. Therefore, we asked whether deletion of high or low affinity Tregs resulted in preferential expansion of either 4–8 or 12-4.4m1 effectors. Contrary to our expectations, we observed a relative increase in low affinity 12-4.4m1 Teffs when either Treg population was removed, indicating perhaps that low affinity Teffs were generally more susceptible to regulation by either Treg population (Fig. 2C).
Low affinity Tregs are competitive in a polyclonal environment
While both high and low affinity Tregs infiltrated the pancreas, high affinity TCRs supported an overall larger frequency of Tregs in periphery (Fig. 3A). Moreover, when we analyzed the relative contribution of high and low affinity Tregs to the whole Treg population by first gating on Foxp3+ T cells and then separating high and low affinity cells based on Vβ expression, the relative proportion of high affinity 4–8 Tregs was increased at the site of inflammation (Fig. 3B). In order to examine whether the increase of high affinity Tregs is reflected in their increased proliferation we compared expression of Ki67, a marker of cell cycle, between the two populations. Only the high affinity Tregs exhibited signs of activation and proliferation in the draining pancreatic lymph nodes (PLN), the site of initial antigen exposure, based on the increase of Ki67+ cells (Fig. 3C). Once in the islets, however, both Treg populations reached similar high levels of proliferation. This observation suggested that competition for antigen between the two Treg populations was limited to the site of initial antigen exposure – draining pancreatic LN.
Since we observed unequal Treg expansion in the draining lymph nodes, potentially driven by local competition for antigen, we needed to determine whether there was a similar competition within the single islet microenvironment that was obscured by pooling the islets for analysis. We considered the possibility that low affinity Tregs preferentially accumulated and expanded in the islets with lower numbers of high affinity cells - an environment with reduced competition for antigen and IL-2. To this end, we analyzed single pancreatic islets, and observed consistent presence of both Treg populations within the same microenvironment (Supplemental Fig. 2C). Of 27 islets analyzed from 7 different mice, both Treg populations were detected in 24 islets (88.9%). The observed co-existence of high and low affinity Tregs in individual islet microenvironments suggested that low affinity Tregs are competitive at the site of inflammation.
Next, we considered the possibility that in a limited two-TCR system low affinity Tregs had an artificial advantage, and would be less competitive in a polyclonal environment. Alternatively, a diverse repertoire could result in reduced competition for antigen, thus expanding the insulin reactive Treg developmental niche, potentially favoring low affinity T cells (5, 20). We therefore co-transferred two-TCR Rg bone marrow with congenically marked NOD.CD45.2 polyclonal bone marrow cells (Supplemental Fig. 2D). By 5 weeks post-transfer, the sub-population of insulin specific Rg T cells accumulated at the site of antigen in the draining LN and pancreatic islets (3.8% in spleen vs 9.2% in PLN and 10.4% in the islets) (Supplemental Fig. 2D). The expansion of the antigenic niche resulted in a preferential increase of low affinity Tregs. Compared to the lymphopenic environment, the presence of polyclonal T cells resulted in a 1.59-fold increase of low affinity Tregs in PLN (from 3.7% to 5.9%) and 1.74-fold increase in pancreatic islets (from 2.6% to 4.6%) (Fig. 3A and Supplemental Fig. 2E). Although, the relative proportion of high affinity 4–8 and low affinity 12-4.4m1 Tregs was largely unchanged by the expansion of the antigenic niche (Supplemental Fig. 2F). These data suggest that in a polyclonal setting Tregs with low TCR affinity can successfully compete for the developmental niche and accumulate in the inflammatory tissue.
High and low affinity insulin-specific Tregs are poised to utilize distinct suppressive functions
In order to elucidate the suppressive mechanisms utilized by high and low affinity Tregs in the tissue site, we assessed the transcriptional landscape of 4–8 (high affinity TCR) and 12-4.4m1 (low affinity TCR) Tregs isolated from spleens and pancreatic islets. Principle component analysis showed tight clustering of the two Treg populations in the spleens, while the islet infiltrating Treg populations were more variable in their genetic profile and significantly distinct from the spleen (Fig. 4A). Further analysis revealed similar expression of Treg functional genes including Foxp3, Tnfrsf18 (GITR), Ctla4, and Tgfb1 (Fig. 4B) (21). Interestingly, several genes associated with Treg suppressive function had distinct expression in either high or low affinity Tregs. High affinity Tregs preferentially expressed Il10, Gzmb, Lag3, and Tigit, all previously described to be important for Treg suppression of Th1 responses, including autoimmune diabetes (22–25). On the other hand, low affinity Tregs exhibited significantly higher levels of Areg and Ebi3 (a subunit of heterodimeric IL-35) transcripts, which are known to be important for tissue repair, Treg survival, and suppression of autoimmune responses (26–29). To confirm the RNAseq results, we assessed protein expression of GITR, CTLA-4, TIGIT, and IL-10. Interestingly, the slight difference observed in Gitr and Ctla4 transcript expression was significantly enhanced at the protein level (Fig. 4C, 4D and Supplemental Fig. 2H). Although both Treg populations upregulated GITR and CTLA-4 upon entry into the pancreas, high affinity Tregs displayed enhanced expression of these functional markers. Consistent with the transcriptional analysis, expression of TIGIT and IL-10 was significantly higher in 4–8 high affinity Tregs (Fig. 4E–G and Supplemental Fig. 2H). While there was some variability in the frequency of IL-10 expressing Tregs, high affinity Tregs generally expressed greater levels of IL-10 based on MFI (Fig. 4F, 4G). Taken together these data suggest that neither Treg population alone is sufficient to control autoimmune diabetes, and high and low affinity Tregs have the potential to utilize distinct non-redundant suppressive mechanisms for combined effective control of tissue-specific autoimmune responses.
Studies performed in polyclonal and single TCR systems expressing Treg-derived TCRs revealed that Tregs preferentially express TCRs with higher affinity for self-antigens, and unperturbed TCR signaling is critical for optimal Treg function (30–32). The intensity of TCR signaling during Treg activation dictates the expression levels of several key genes involved in Treg homeostasis and function, including CD25 and CTLA4 (7, 30). Thus, it has been widely accepted that functional potential of Tregs is directly correlated with their affinity for self. However, there is little direct evidence to show whether TCR affinity has a direct effect on Treg function in autoimmunity. Importantly, we found that the simultaneous presence of high and low affinity Tregs was necessary to delay the onset of diabetes (Fig. 1C, D). This unexpected observation suggests that while tissue specificity and affinity is necessary for optimal Treg infiltration (Fig. 3B) (33), TCR affinity for tissue antigen might be important in activating distinct regulatory programs. Upon entry into the site of autoimmune inflammation, both populations increased the expression of Treg functional mediators, suggesting at least partial, if not equal, contribution of low affinity Tregs to regulation of autoimmunity.
Interestingly, in the recipients of haplodeficient bone marrow, disease kinetics and incidence were similar to scurfy bone marrow recipient mice, which were completely devoid of functional Tregs. These results are in contrast to previous studies of polyclonal haplodeficient or insufficient systems where the remaining Tregs are able to expand and compensate for the deficiency (34). Treg frequencies are regulated by both TCR intrinsic factors and the availability of IL-2 (8, 20, 35). Although in a polyclonal system partial deletion of Tregs presumably relieves IL-2 sources that drive Treg expansion to fill the niche, in our two-TCR system Treg frequencies do not change within each TCR population in response to reductions in the overall Treg compartment (Fig. 2B). Therefore, within the context of fixed antigen availability intra-TCR Treg frequencies seem to be limited by TCR intrinsic parameters and remain stable.
Recently, we began to appreciate the heterogeneity of the Foxp3+ Treg population, which often mirrors effector T cells in their ability to utilize a wide range of tissue specific and context dependent responses (36, 37). Heterogeneity of effector T cell responses is primarily regulated at the level of TCR signaling, which dictates the level of activation, as well as instructs the type and relative proportion of helper lineage development (38, 39). It is likely that the range of Treg phenotype and function is similarly dependent on the level of TCR activation. Moreover, regulatory mechanisms employed by Tregs are differentially dependent on TCR signaling, and some of these are induced by inflammatory cytokines rather than TCR activation. Perhaps not surprisingly one of these genes, Areg or amphiregulin (26), was preferentially upregulated in low affinity insulin reactive Tregs (Fig. 4B), suggesting that in the absence of strong TCR signaling Tregs are more likely to utilize non-TCR dependent suppressive functions. While on the other hand, high affinity Tregs preferentially upregulated TCR-dependent regulatory molecules including CTLA-4, TIGIT, and IL-10 (Fig. 4D–G) (7, 30). Given the dramatic disease acceleration in mice devoid of either the high or the low affinity Tregs, it is tempting to postulate that the two Treg populations utilize distinct regulatory mechanisms and both are necessary for regulation of autoimmunity (37). An alternative explanation is that the regulation of autoimmunity is highly dependent on Teff to Treg ratio, and once that ratio is compromised the regulation fails completely. Collectively, our data suggest that functional Tregs span a range of TCR affinities, and high and low affinity populations cooperatively prevent autoimmune pathology. These results might have important implications for the development of Treg-based approaches for the monitoring and treatment of autoimmune diseases.
Supplementary Material
Acknowledgments
This study was funded by NIH R01 AI125301-01A1 and P30 DK079638-05, ADA 7-14-JF-07, JDRF 1-FAC-2014-243-A-N, T32 AI053831 Immunology Scientist Training Grant to M.L.S., AAI Career Development Fellowship to I.S., and The Robert and Janice McNair Foundation.
We would like to thank NIH Tetramer Core Facility for providing peptide and MHC monomers, the BCM Cytometry and Cell Sorting Core for assistance with cell sorting, and Richard (Aaron) Cox for helpful discussion.
References
- 1.Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol. 2008;28(Suppl 1):S11–19. doi: 10.1007/s10875-008-9176-5. [DOI] [PubMed] [Google Scholar]
- 2.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettini M, Blanchfield L, Castellaw A, Zhang Q, Nakayama M, Smeltzer MP, Zhang H, Hogquist KA, Evavold BD, Vignali DA. TCR affinity and tolerance mechanisms converge to shape T cell diabetogenic potential. J Immunol. 2014;193:571–579. doi: 10.4049/jimmunol.1400043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 5.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebeisen M, Rufer N, Oberle SG, Speiser DE, Zehn D. Signaling Mechanisms that Balance Anti-viral, Auto-reactive, and Antitumor Potential of Low Affinity T Cells. Journal of Clinical & Cellular Immunology. 2013;01 [Google Scholar]
- 11.Martinez RJ, Andargachew R, Martinez HA, Evavold BD. Low-affinity CD4+ T cells are major responders in the primary immune response. Nat Commun. 2016;7:13848. doi: 10.1038/ncomms13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T, Shevchenko I, Sprouse ML, Bettini M, Bettini ML. Retroviral transduction of bone marrow progenitor cells to generate T-cell receptor retrogenic mice. Journal of Visual Experiments. 2016;113 doi: 10.3791/54196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York: 2009. [Google Scholar]
- 16.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, Abbas AK, Sharpe AH, Sansom DM, Walker LSK. CTLA-4 Controls Regulatory T Cell Peripheral Homeostasis and Is Required for Suppression of Pancreatic Islet Autoimmunity. The Journal of Immunology. 2008;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 22.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gondek DC, DeVries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation Survival Is Maintained by Granzyme B+ Regulatory Cells and Adaptive Regulatory T Cells. The Journal of Immunology. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DA. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J Immunol. 2011;187:3493–3498. doi: 10.4049/jimmunol.1100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 29.Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;8213:8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. The Journal of experimental medicine. 2008:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DH, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toomer KH, Yuan X, Yang J, Dee MJ, Yu A, Malek TR. Developmental Progression and Interrelationship of Central and Effector Regulatory T Cell Subsets. J Immunol. 2016;196:3665–3676. doi: 10.4049/jimmunol.1500595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyss L, Stadinski BD, King CG, Schallenberg S, McCarthy NI, Lee JY, Kretschmer K, Terracciano LM, Anderson G, Surh CD, Huseby ES, Palmer E. Affinity for self antigen selects Treg cells with distinct functional properties. Nat Immunol. 2016 doi: 10.1038/ni.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.