Abstract
Inability to appropriately process afferent interoceptive stimuli may contribute to initiation and/or escalation of substance use. An aversive interoceptive stimulus probed neural processing in problem stimulant users (PSU; n=19), 18 desisted stimulant users (DSU; n=18), and healthy comparison subjects (CTL; n=21). Participants completed a continuous performance task while they anticipated and experienced 40 cm H20/L/sec inspiratory breathing loads during functional magnetic resonance imaging. PSU exhibited lower left dorsolateral prefrontal cortex and inferior frontal gyrus (IFG) activation than DSU and CTL across trials. Greater lifetime drug use due to stimulants was also linked to lower activation in these regions. In addition, PSU displayed lower right IFG and insula activation during breathing load than DSU and CTL. Findings suggest that transition to stimulant use disorders is marked by weakened attentional salience of aversive stimuli.
Introduction
Over 1 million people use cocaine and amphetamine recreationally, making stimulant use a major health problem (SAMHSA, 2012). Approximately 1 out of 5 individuals who use stimulants progress to dependence (Lopez-Quintero et al., 2011), raising a crucial but experimentally difficult question: what are behavioral and neurological markers leading to stimulant dependence? While research has identified several neural substrates in frontal, striatal, and interoceptive brain regions (Goldstein & Volkow, 2011; Koob, 2005), little is known about differences in these regions prior to the development of dependence. This knowledge is necessary to reduce the personal, financial, and societal burden of stimulant use (Nicosia, 2009; ONDCP, 2004).
Interoception, the processing and integration of afferent signals from inside the body to motivate approach and withdrawal behaviors, has recently been implicated in substance dependence more generally, wherein drug-related cues are thought to heighten interoceptive responses (e.g., craving, urges) at the expense of non-drug related-cues (Craig, 2002; Naqvi & Bechara, 2010; Paulus, Tapert, & Schulteis, 2009). During interoceptive processing, middle/posterior insula receives somatosensory activity from thalamocortical pathways, while anterior insula assists by integrating this information with emotionally salient activity from anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC), motivating action to eliminate homeostatic imbalances (Craig, 2002, 2009). Recent research in stimulant dependent individuals supports the assertion that brain regions involved in interoceptive processing may be hypoactive to non-drug related reward cues (e.g., reduced frontocingulate and thalamic responses to sexual images in cocaine dependence: Asensio et al., 2010; attenuated frontal, thalamic, and insular responses to soft touch brush strokes in methamphetamine dependence: May, Stewart, Migliorini, Tapert, & Paulus, 2013; attenuated thalamic processing to monetary reward as a function of abstinence in cocaine dependence: Jia et al., 2011). With respect to unpleasant stimuli, cocaine dependent individuals exhibit frontocingulate hypoactivation during non-drug related stress-induced imagery (Sinha et al., 2005) and insular/cingulate hypoactivation during monetary punishment (Hester, Bell, Foxe, & Garavan, 2013) but show thalamic, insular, and cingulate hyperactivation to stressful imagery paired with drug cues (Duncan et al., 2007). Taken together, the available literature suggests that stimulant dependence is linked to attenuated neural processing of non-drug related cues, regardless of appetitive or aversive context.
Frontocingulate regions including DLPFC are also structurally and functionally altered in stimulant dependence (Bechara, 2005; Ersche et al., 2012; Franklin et al., 2002; Goldstein & Volkow, 2002, 2011; Li & Sinha, 2008; Schwartz et al., 2010) and both ACC and DLPFC are active during interoceptive processing in healthy individuals, likely as a part of a monitoring and top-down inhibitory circuit regulating reactions to aversive stimuli (Critchley, Wiens, Rotschtein, Ohman, & Dolan, 2004; Paulus et al., 2012). It has been argued that lowered bodily responses to valenced non-drug stimuli in substance dependent individuals more generally may not adequately motivate action to adjust ongoing behavior (Verdejo-Garcia & Bechara, 2009), whereas heightened bodily responses to drug stimuli may reinforce drug seeking and taking behaviors (Paulus & Stewart, 2013). At this time, however, it is unclear whether this process is a function of chronic stimulant dependence or is also evident in individuals who have recently developed symptoms of stimulant abuse and/or dependence.
As noted by Verdejo-Garcia, Clark, and Dunn (2012), there is likely a complex relationship between interoceptive processing and the development, maintenance, and recurrence of substance use disorders such as stimulant dependence. It may be that in young adults predisposed to initiate stimulant use, bodily signals are registered too weakly in the brain, a situation which may motivate stimulant use to correct perceived imbalances or lack of feeling. In contrast, perhaps bodily signals lessen in potency over time as a consequence of increased use, due to narrowed focus on appetitive/aversive bodily signals linked specifically to stimulant consumption/withdrawal, and if this is the case: (1) only young adults who transition to problems with stimulant use show neural attenuations while processing non-drug related aversive bodily perturbations, whereas young adults who experiment with stimulants do not; or (2) blunted neural processing of non-drug related aversive bodily changes is only a result of years of chronic stimulant use, so that young adults who have recently developed problems do not show these deficits. Individuals who show attenuated processing of internal body states may be at higher risk for stimulant dependence because they are not able to utilize “gut feelings” to guide their decision-making (Paulus & Stewart, 2013; Stewart et al., 2013; Verdejo-Garcia & Bechara, 2009). In particular, aversive body state signals may reduce an individual’s propensity to engage in risky activities such as drug use. In comparison, engaging in risky activities such as drug taking may result from attenuated insular processing of potentially aversive states, which–in turn–results in inadequate cognitive control modulation implemented by DLPFC (Paulus et al., 2009; Verdejo-Garcia & Bechara, 2009; Verdejo-Garcia et al., 2012). As of yet, however, few studies have employed interoceptive manipulations to examine neural changes as a function of stages of stimulant use.
The present study focused on a cohort of recreational stimulant users, tracked for three years after an initial assessment. During this time, 19 individuals progressed to problematic use, defined as at least two symptoms of DSM-IV (American Psychiatric Association, 2000) stimulant abuse and/or dependence, and 18 subjects desisted using. These groups were compared with 21 healthy volunteers. During functional magnetic resonance imaging (fMRI), participants completed an inspiratory breathing load paradigm, a simple and powerful way to induce and image a negative interoceptive state (Lopata, La Fata, Evanich, & Lourenco, 1977). Given prior work on attenuated frontocingulate, insular, and/or thalamic processing in stimulant dependence (e.g., Asensio et al., 2010; Hester et al., 2013; May et al., 2013; Sinha et al., 2005), if blunted brain response to an interoceptive perturbation is a marker of transition to stimulant dependence as well as a marker of chronic stimulant dependence, it was predicted that neural activity subserving interoception and executive functioning would be attenuated in problem stimulant users when compared to desisted users and healthy individuals during the aversive interoceptive manipulation.
Method
Participants
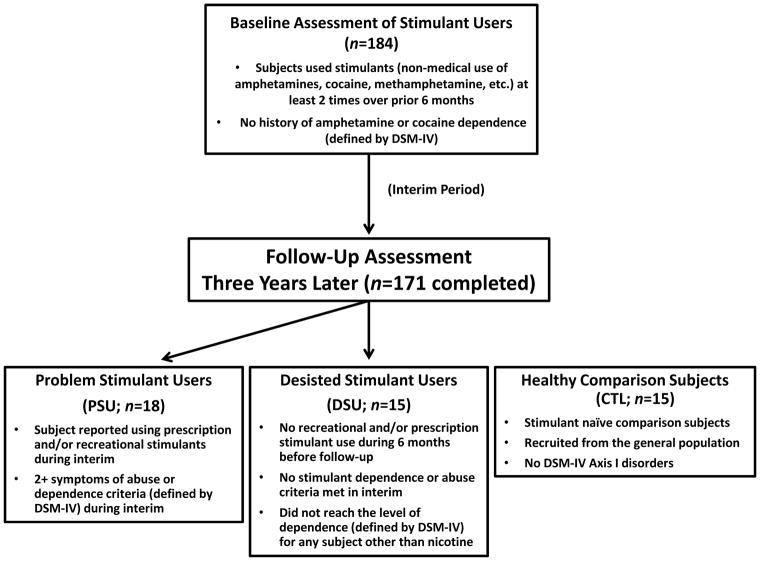
The study protocol was approved by the local Human Subjects Review Board (University of California, San Diego) and was carried out in accordance with the Declaration of Helsinki. Individuals were informed that this study was aimed to examine brain functioning of people who use stimulants, and all subjects gave written informed consent. Recreational, non-dependent male and female stimulant users were recruited and defined by methods described in previous studies (Reske, Delis, & Paulus, 2011; Stewart et al., 2013). Among this original cohort of 184 subjects, these individuals were contacted three years after their initial lab visit, with an overall follow-up rate of 93% (171 followed up; 10 unreachable; 3 refused to participate). Each individual underwent a standardized interview during the three year follow-up assessment to examine the extent of drug use, allowing us to identify subjects in this cohort who developed problems associated with stimulant use and others who had desisted using stimulants. Thus, two stimulant user groups were formed for the present study, termed problem stimulant users (PSU) and desisted stimulant users (DSU).
Specifically, PSU were a priori defined by: (1) continued use of prescription and/or recreational stimulants (e.g., dextroamphetamine, cocaine, methylphenidate) since the initial visit, and (2) endorsement of 2+ symptoms of DSM-IV amphetamine and/or cocaine abuse or dependence criteria (American Psychiatric Association, 2000) as defined by the Semi Structured Assessment for the Genetics of Alcoholism II (SSAGA II) (Bucholz et al., 1994) occurring together during at least 6 contiguous months since the initial visit (M=4.74 symptoms; SD=1.97; range: 2–8). In comparison, DSU were defined as having (1) no 6-month periods of time of 3+ uses of reported prescription and/or recreational stimulants, and (2) no endorsement of symptoms of stimulant abuse or dependence (other than nicotine) in the interim as defined by SSAGA II. Healthy comparison subjects (CTL) were recruited from the general population and endorsed no lifetime history of substance dependence as determined by SSAGA II (see Figure 1 for schematic overview). No subjects from any group were current regular nicotine smokers. The final cohort of the present study consisted of 19 PSU, 18 DSU, and 21 CTL subjects, all right handed as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects then completed two sessions: (1) a clinical interview and questionnaire session; and (2) an fMRI session wherein subjects completed a continuous performance task (CPT) with a breathing load manipulation (described below).
Figure 1.
Timeline of subject recruitment. Occasional stimulant users were followed up 3 years later to determine which individuals escalated stimulant use (Problem Stimulant Users; PSU) or desisted stimulant use (Desisted Stimulant Users; DSU). Age and education-matched stimulant-naïve healthy comparison subjects (CTL) were also recruited.
Clinical Interview Session
Subjects were assessed by experienced interviewers using the SSAGA II and diagnoses were based on consensus meetings (accredited clinician M.P.P. and trained study personnel). The following were exclusion criteria for all groups: (1) incorporated metal or any other factor that precludes use of fMRI; (2) head injuries or loss of consciousness for longer than 5 minutes; (3) prescription medication for attention deficit hyperactivity disorder (ADHD), depression, bipolar disorder, anxiety and other psychiatric disorders taken currently and/or within the past three years; (4) any diagnosed neurological disorder (including ADHD); (5) evidence for lifetime psychosis (e.g., schizophrenia, bipolar disorder) or antisocial personality disorder; (6) current and/or past six month episodes of DSM-IV anxiety disorders or unipolar depression; and (7) a positive urine toxicology screen for any substance other than marijuana at the time of the fMRI session (given that marijuana can be present in urine as long as six weeks after use).
At the time of the clinical interview, several personality and symptom assessment questionnaires known to correlate with substance use disorders were administered, including the Sensation Seeking Scale (SSS) (Zuckerman, 2007), the Barratt Impulsiveness Scale (BIS) (Barratt & Patton, 1983), the State-Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, Lushene, & Vagg, 1983) and the Beck Depression Inventory II (BDI-II) (Beck, Steer, & Brown, 1996). To assess trait interoceptive awareness, sensitivity, and responses to stress, subjects completed the Body Perception Questionnaire (BPQ) (Porges, 1993).
fMRI Session
Urine testing
All subjects were required to abstain from drugs for 72 hours prior to the fMRI session. Thirteen subjects tested positive for marijuana on the pre-fMRI urine toxicology screen (n=7 PSU; n=6 DSU: PSU and DSU did not differ in percentage of subjects testing positive for marijuana: χ2 (1)=.05, p=.82) but no subjects tested positive for any other substances.
Breathing load apparatus
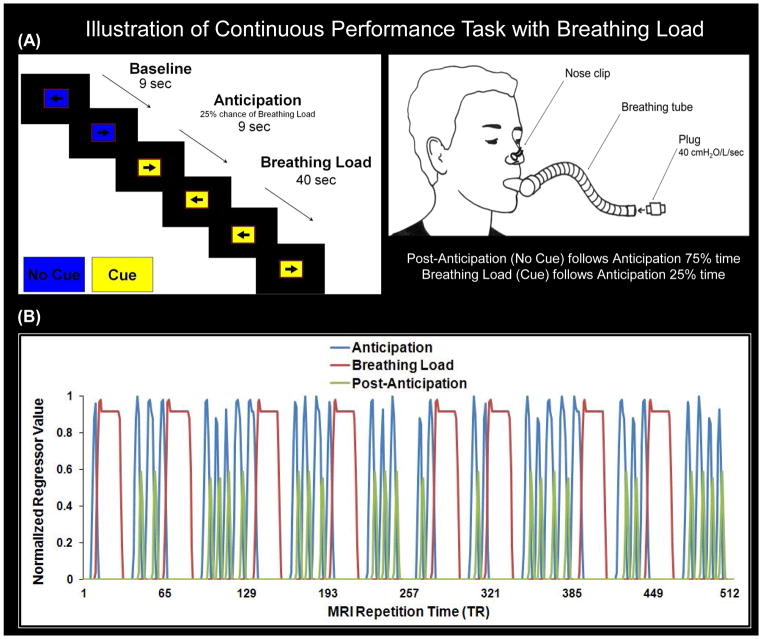
During the fMRI session, subjects wore a nose clip (see Figure 2A) and respired through a mouthpiece and non-rebreathing valve (2600 series, Hans Rudolph). The apparatus was attached to the fMRI scanner head coil to eliminate the need for the subject to contract mouth muscles while maintaining an airtight seal. The resistance load was a stainless steel screen mesh disk placed in a Plexiglas tube (loading manifold), closed with a stoppered port. Subjects were given a 40 cmH2O/L/sec inspiratory load applied to only the inspiratory port of the non-rebreathing valve for 40 seconds at a time. Prior to scanning, subjects were given instructions about the task and experienced four 1-minute segments of the breathing load. After the fMRI session, subjects completed Visual Analog Scale (VAS) questionnaires, on which they were asked to rate the breathing load experience on a 10 cm scale anchored from “not at all” (0) to “extremely” (10) on the following dimensions: pleasantness, unpleasantness, and intensity, corresponding to items used in prior studies (Chan & Davenport, 2008; Davenport & Vovk, 2009).
Figure 2.
Illustration of continuous performance task (CPT) with breathing load. A: Participants wore a nose clip and respired through a breathing tube while reclining in the scanner. Subjects were instructed to press a button corresponding to the direction pointed by an arrow on the screen left arrow = left button, right arrow = right button). Each trial lasted 3 s; the arrows appeared for 2.5 s and the subject was allowed to respond during the entire 3-s interval. The background color of the arrow served as a cue to the impending presentation of the breathing load; change of color indicated a 25% chance of load presence. Group analysis consisted of four conditions: (1) baseline: subject performs the CPT with a color background signifying no cue; (2) anticipation: a change in color background (cue) signals 25% chance of an impending resistive loaded breathing period; (3) breathing load: 25% of the periods following the anticipation condition, subject continues to view the colored cue and experiences 40-s period of resistive loaded breathing (plug at 40 cm H2O/L/sec); (4) post anticipation: 75% of the periods following the anticipation condition, subject performs the task with the original color background present (no cue). B: Depiction of condition regressors of interest (anticipation, breathing load, post anticipation) that were compared to the baseline condition (% signal change from baseline) in linear mixed model analysis. MRI repetition time (TR) was 2 s; thus, each trial consisted of 1.5 TRs. Subjects experienced 24 periods of anticipation, 8 periods of the breathing load, and 16 periods of post anticipation.
CPT
During fMRI, participants performed a simple attention task while undergoing periods of inspiratory loaded breathing (see Figure 2A). Prior to testing, subjects were trained on the task. Subjects were instructed to press a button corresponding to the direction pointed by an arrow on the screen (left arrow = left button, right arrow = right button). Each trial lasted 3 sec; each arrow appeared for 2.5 sec and the subject was allowed to respond during the entire 3 sec trial interval. Both accuracy and reaction time (RT) were recorded via button press. The background color of the stimulus served as a cue to the impending presentation of the breathing load; blue indicated that there would be no load, and yellow indicated a 25% chance of load presence. We introduced this probability to maximize the opportunity to measure the effect of anticipating an aversive interoceptive event. Throughout the task, five conditions were presented: (1) baseline: subject performs task with a blue background signifying no cue; (2) anticipation: a yellow background (cue) signals 25% chance of an impending resistive loaded breathing period; (3) breathing load: 25% of the periods following the anticipation condition, subject continues to view the yellow cue and experiences 40-second period of resistive loaded breathing (plug at 40 cm H2O/L/sec); (4) post-anticipation: 75% of the periods following the anticipation condition, subject performs the task with the blue background present (no cue); and (5) post-breathing load (not included in Figure 2A): immediately after the breathing load condition, subject performs the task with the blue background present (no cue).
Experimental design
This paradigm was presented in an event-related fMRI design consisting of 2 runs, each containing 170 trials (56 baseline, 46 anticipation, 52 breathing load, 12 post-anticipation, and 4 post-breathing load) and 256 repetition times (TR = 2 sec), yielding a total duration of 17 min and 4 sec. Each trial corresponded to 1.5 TR. Across runs, each subject was presented with 34 baseline conditions and 32 anticipation conditions of varying length (average: 3 trials each). Eight of the anticipation conditions were followed by the breathing load condition, consisting of 40 sec (13 trials) inspiratory breathing-load episodes (see Figure 2B). The remaining 24 anticipation conditions were followed by the post-anticipation condition (1 trial). All breathing load periods were followed by post-breathing condition (1 trial). Durations of baseline (range: 2–7 trials) and anticipation conditions (range: 2–4 trials) were jittered in time to permit optimal resolution of the hemodynamic response function. During the CPT, carbon dioxide (CO2) levels were also collected at a rate of 40 Hz for each subject via nasal cannula (InVivo Corporation, Orlando, FL). The main dependent measures of interest were reaction time (RT), accuracy, CO2 levels, and brain activation during the anticipation, breathing load, and post-anticipation conditions relative to the baseline condition.
Neuroimaging Acquisition and Analysis
Image acquisition
Imaging experiments were performed on a 3T GE CXK4 Magnet at the UCSD Imaging Center, which is equipped with 8 high-bandwidth receivers that allow for shorter readout times and reduced signal distortions and ventromedial signal dropout. Each one-hour session consisted of a three-plane scout scan and a standard anatomical protocol consisting of a sagittally acquired spoiled gradient recalled (SPGR) sequence (field of view, or FOV: 25.6 cm; matrix: 192x256; 172 sagittally acquired slices thickness: 1 mm; TR: 8ms; echo time, or TE: 3 ms; flip angle =12°). We used an 8-channel brain array coil to axially acquire T2*-weighted echo-planar images (EPI). The parameters for the EPI scans were: FOV: 24 cm; 64X64 matrix; 40 3.0 mm thick slices; 1.4 mm gap; TR=2 sec, TE=30 ms, flip angle = 90°. Rapid image acquisition was obtained via GE’s ASSET scanning, a form of sensitivity encoding (SENSE) which uses parallel imaging reconstruction to allow for sub k-space sampling.
Image analysis pathway
All subject-level structural and functional image processing was done with the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996). The multivariate regressor approach detailed below was used to relate changes in EPI intensity to differences in task characteristics (Haxby, Petit, Ungerleider, & Courtney, 2000). EPI images were co-registered using a 3D-coregistration algorithm (Eddy, Fitzgerald, & Noll, 1996) that was developed to minimize the amount of image translation and rotation relative to all other images. Six motion parameters (dx, dy, dz, roll, pitch, and yaw) were obtained across the time series for each subject. The latter three motion parameters were used as regressors in the deconvolution to adjust EPI intensity changes due to motion artifacts and increase power in detecting task-related activation (Skudlarski, Constable, & Gore, 1999). Groups did not differ in motion during the CPT. EPI slices were temporally aligned following registration to assure that different relationships with the regressors were not due to the acquisition of different slices at different times during each TR. EPI images then underwent automatic coregistration to the high-resolution anatomical images and each dataset was manually inspected to confirm successful alignment. New outliers were generated for the volume-registered dataset. If > 10% voxels were marked as outliers within a particular TR, that time point was then excluded from further analysis.
Multiple regressor analyses
Regressors of interest were generated to delineate conditions (anticipation, breathing load, post-anticipation, and post-breathing load). To that end, a 0–1 reference function of the particular time interval for each condition was convolved with a gamma variate function (Boynton, Engel, Glover, & Heeger, 1996) modeling a prototypical hemodynamic response (6–8 second delay; (Cohen, 1997; Friston, Frith, Turner, & Frackowiak, 1995). Three movement regressors (roll, pitch, yaw), a baseline and linear drift regressor, and normalized decision-making regressors (anticipation, breathing load, post-anticipation, post-breathing load), were included in the AFNI program 3dDeconvolve to estimate the goodness of fit between model estimates and BOLD responses for each subject. The baseline condition, wherein participants were performing the CPT but not experiencing anticipation, breathing load, post-anticipation, or post-breathing load conditions, served as the baseline for this analysis.
Following deconvolution, voxels were resampled into 4 x 4 x 4mm3 space and whole-brain voxel-wise normalized percent signal change, the main dependent measure, was determined by dividing the beta coefficient for each of the three decision predictors of interest (anticipation, breathing load, post-anticipation) by the beta coefficient for the baseline regressor and multiplying by 100. Next, a Gaussian spatial filter (4 mm full width at half maximum) was used to spatially blur percent signal change values to account for anatomical differences, and this output was then normalized to Talairach coordinates as defined by AFNI’s built-in atlases. Finally, individual subject percent (%) signal change scaled beta weight values for anticipation, breathing load, and post-anticipation conditions were extracted for their use as dependent measures in group analyses. Although the post-breathing load condition was included in the deconvolution to account for nuisance variance, it was not included in further analysis due to too few trials (8 across the entire task).
Group level analyses
For each voxel, a linear mixed effects (LME) model in R (Pinheiro et al., 2013) was calculated on % signal change values, with group (PSU, DSU, CTL) and condition (anticipation, breathing load, post-anticipation) modeled as fixed factors, and subject modeled as a random factor. Once these voxel-wise statistics were calculated, a threshold adjustment method based on Monte-Carlo simulations was employed to guard against identifying false positive areas of activation. Based on simulations implemented in the AFNI program AlphaSim, given a per voxel p < 0.0001 threshold, it was determined that the whole-brain volume threshold was 768 μL (12 contiguous voxels) for a clusterwise p <.05 corrected for multiple comparisons. The voxelwise threshold was based on the following LME degrees of freedom and F values thresholded at p<.05: (1) group main effect: F(2,55)=3.17; (2) condition main effect: F(2,110)=3.08; and (3) group by condition interaction: F(4,110)=2.45. Cohen’s d was calculated to determine effect sizes for significant differences between groups and conditions.
Questionnaire and Interview Analysis
Non-imaging statistical analyses were performed in SPSS (PASW Version 18 Statistics for Windows, Chicago, IL). For questionnaire data and VAS ratings, a univariate analysis of variance (ANOVA) was performed for each measure, with group as the between-subjects variable. Significance (p <.05) was determined by post-hoc independent sample t-tests between each set of groups (e.g., PSU vs. DSU, PSU vs. CTL, DSU vs. CTL). Lifetime drug use variables were compared pair-wise between groups with non-parametric Mann-Whitney U tests due to non-normal distributions.
RT, Accuracy, and CO2 Analysis
RT and accuracy were calculated for each condition per participant. CO2 data were visually inspected for artifacts and down sampled by 80 (40 Hz * 2 seconds per TR) to obtain one value per TR per fMRI run A total of 42/58 (72%) of subjects (12 PSU, 11 DSU, 19 CTL) had usable CO2 data as determined via visual inspection. For these subjects, CO2 values were averaged for each condition separately. Separate repeated measures ANOVAs were performed for RT, accuracy, and CO2, wherein % change from baseline was the dependent variable, condition (anticipation, breathing load, post-anticipation) was the within-subjects variable, and group was the between-subjects variable. Greenhouse-Geisser corrections were calculated and reported for cases of non-normality. Follow up independent t-tests were employed to test significant group differences, whereas dependent t-tests were used to clarify significant condition differences.
Results
Subject Characteristics
Although groups did not differ in age, education, gender, or race, PSU and DSU had lower verbal IQ scores than CTL (Table 1). PSU endorsed higher STAI trait anxiety and BDI-II depression scores than DSU, although neither group differed from CTL on these indices. As expected, PSU and DSU both reported higher lifetime amphetamine, cocaine, and marijuana use than CTL. PSU reported greater lifetime amphetamine use than DSU, but PSU and DSU endorsed commensurate cocaine and marijuana use. Similarly, more PSU and DSU met criteria for marijuana abuse than CTL, although groups did not differ in problem alcohol use. By definition, only PSU met criteria for current DSM-IV stimulant abuse and/or dependence.
Table 1.
Group Personality, Drug Use, and Emotion Characteristics.
| PSU (n=19) | DSU (n=18) | CTL(n=21) | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Gender, Race | % | % | % | χ2(2) | p | Description | |||
| Women | 47.62 | 38.89 | 47.37 | 0.37 | 0.83 | ns | |||
| Caucasian | 73.68 | 88.89 | 66.66 | 2.69 | 0.26 | ns | |||
|
| |||||||||
| Questionnaires | M | SD | M | SD | M | SD | F1 | p | Post-Hoc (p<.05)2 |
|
| |||||||||
| Demographics | |||||||||
| Age (years) | 24.58 | 1.50 | 24.39 | 1.46 | 24.10 | 1.97 | 0.42 | 0.66 | ns |
| Education (years) | 15.68 | 1.00 | 15.72 | 1.41 | 16.00 | 1.45 | 0.35 | 0.71 | ns |
| WTAR Verbal IQ | 111.28 | 8.094 | 112.61 | 8.197 | 117.78 | 6.53 | 3.63 | 0.03 | CTL>PSU CTL>DSU |
| Personality | |||||||||
| BIS | 66.76 | 10.85 | 60.94 | 7.41 | 59.33 | 9.06 | 3.12 | 0.05 | ns |
| SSS | 24.94 | 4.66 | 23.82 | 6.01 | 20.00 | 6.71 | 3.33 | 0.04 | ns |
| STAI Trait | 36.00 | 8.57 | 29.89 | 4.59 | 33.80 | 7.53 | 3.46 | 0.04 | PSU>DSU |
| STAI State | 30.21 | 6.59 | 26.72 | 5.36 | 28.20 | 5.14 | 1.74 | 0.19 | ns |
| BDI-II | 5.00 | 6.29 | 1.00 | 1.41 | 2.29 | 2.65 | 4.93 | 0.01 | PSU>DSU |
| Body Perception BPQ | |||||||||
| Body Awareness | 111.47 | 50.21 | 118.29 | 48.59 | 116.59 | 52.53 | 0.08 | 0.92 | ns |
| Stress Response | 24.71 | 10.24 | 26.35 | 11.04 | 26.24 | 9.96 | 0.13 | 0.88 | ns |
| ANS Reactivity | 35.18 | 7.42 | 39.18 | 11.94 | 34.38 | 7.37 | 1.31 | 0.28 | ns |
| VAS Ratings | |||||||||
| Pleasantness | 2.06 | 2.11 | 1.51 | 1.33 | 1.51 | 1.48 | 0.67 | 0.52 | ns |
| Unpleasantness | 5.83 | 2.92 | 7.58 | 1.71 | 6.50 | 2.42 | 2.39 | 0.10 | ns |
| Intensity | 3.77 | 2.73 | 4.79 | 2.96 | 3.57 | 3.06 | 0.91 | 0.41 | ns |
|
| |||||||||
| Drug Use at 1st Visit3 | M | SD | M | SD | M | SD | Mann-Whitney U Test (p<.05) | ||
|
| |||||||||
| Amphetamine | 35.79 | 48.11 | 6.83 | 6.75 | N/A | N/A | PSU>DSU | ||
| Cocaine | 35.26 | 83.67 | 27.33 | 49.67 | N/A | N/A | ns | ||
| Marijuana | 891.59 | 1370.67 | 922.72 | 1523.20 | N/A | N/A | ns | ||
|
| |||||||||
| Lifetime Drug Use3 | M | SD | M | SD | M | SD | Mann-Whitney U Test (p<.05) | ||
|
| |||||||||
| Amphetamine | 203.00 | 287.35 | 8.78 | 7.59 | 0.00 | 0.00 | PSU>CTL; DSU>CTL; PSU>DSU | ||
| Cocaine | 552.74 | 1246.35 | 32.22 | 51.47 | 0.00 | 0.00 | PSU>CTL; DSU>CTL | ||
| Marijuana | 1549.84 | 2044.02 | 1329.94 | 1811.30 | 18.43 | 38.47 | PSU>CTL; DSU>CTL | ||
|
| |||||||||
| Current Diagnosis | % | % | % | χ2(2) | p | Description | |||
|
| |||||||||
| Abuse | |||||||||
| Alcohol | 57.89 | 44.44 | 28.57 | 3.52 | 0.18 | ns | |||
| Marijuana | 52.63 | 61.11 | 4.76 | 15.67 | <001 | PSU/DSU ↑ | |||
| Amphetamine | 52.63 | 0 | 0 | 24.80 | <0.01 | PSU ↑ | |||
| Cocaine | 52.63 | 0 | 0 | 24.80 | <0.01 | PSU ↑ | |||
| Dependence | |||||||||
| Alcohol | 15.79 | 5.55 | 0 | 3.95 | 0.14 | ns | |||
| Marijuana | 15.79 | 0 | 0 | 6.49 | 0.04 | PSU ↑ | |||
| Amphetamine | 31.58 | 0 | 0 | 13.74 | <0.01 | PSU ↑ | |||
| Cocaine | 26.32 | 0 | 0 | 11.23 | <0.01 | PSU ↑ | |||
Note:
Degrees of freedom for F-tests were 2, 55 except for specific measures containing missing data (listed below).
Specific group differences were determined by post-hoc independent sample t-tests.
Measured as the number of distinct sessions each drug was used.
Due to non-normal distributions for lifetime use, non-parametric Mann-Whitney U tests were performed pair-wise to examine group differences. PSU = Problem Stimulant Users. DSU = Desisted Stimulant Users. CTL = Healthy Comparison Subjects. WTAR = Wechsler Test of Adult Reading. BIS = Barratt Impulsivity Scale. SSS = Sensation Seeking Scale. STAI = State Trait Anxiety Inventory. BDI-II = Beck Depression Inventory II. BPQ = Body Perception Questionnaire. ANS = Autonomic Nervous System. VAS = Visual Analog Scale. ns = non-significant. The following measures contained missing data: WTAR (3 CTL, 1 PSU); BIS (3 CTL; 1 DSU; 2 PSU); SSS (4 CTL, 1 DSU, 2 PSU), STAI (1 CTL); VAS scales (1 DSU); BPQ (4 CTL, 2 DSU 1 PSU) and 1 CTL was >3 SD outlier on ANS reactivity and therefore the score was removed from analysis). N/A = not applicable for CTL subjects.
Behavioral and Physiological Results
There were no significant effects for RT or accuracy. However, breathing load was associated with lower CO2 levels, which was due to increased expiratory tidal volume relative to anticipation and post-anticipation (see Table 2). No group differences were significant for behavioral or CO2 data.1
Table 2.
Behavioral Performance and Physiology
| PSU | DSU | CTL | |
|---|---|---|---|
|
| |||
| M (SD) | M (SD) | M (SD) | |
| RT (msec) | |||
| Baseline | 785.01 (168.26) | 805.23 (215.97) | 802.93 (190.34) |
| Anticipation | 775.55 (179.66) | 803.05 (215.25) | 815.62 (186.77) |
| Breathing Load | 773.10 (184.21) | 783.46 (207.53) | 793.24 (216.52) |
| Post-Anticipation | 770.78 (189.59) | 791.43 (211.79) | 790.66 (186.36) |
| Accuracy (%) | |||
| Baseline | 99 (1) | 98 (5) | 96 (11) |
| Anticipation | 99 (2) | 98 (7) | 94 (13) |
| Breathing Load | 100 (1) | 98 (6) | 95 (13) |
| Post-Anticipation | 99 (2) | 96 (8) | 95 (15) |
| CO2 | |||
| Baseline | 1.56 (0.50) | 1.37 (0.36) | 1.34 (0.37) |
| Anticipation | 1.56 (0.50) | 1.35 (0.35) | 1.32 (0.38) |
| Breathing Load | 1.28 (0.55) | 1.18 (0.36) | 1.16 (0.33) |
| Post-Anticipation | 1.53 (0.50) | 1.32 (0.31) | 1.32 (0.39) |
| RT ANOVA: % Change From Baseline | |||
| Group | 2, 52 | 0.21 | 0.81 |
| Condition | 2, 104 | 1.83 | 0.18 |
| Group x Condition | 4, 104 | 0.44 | 0.73 |
| Accuracy ANOVA: % Change From Baseline | |||
| Group | 2, 52 | 0.66 | 0.52 |
| Condition | 2, 104 | 1.55 | 0.22 |
| Group x Condition | 4, 104 | 0.39 | 0.74 |
| CO2 ANOVA: % Change From Baseline | |||
| Group | 2, 39 | 0.39 | 0.67 |
| Condition | 2, 78 | 38.55 | <0.001* |
| Group x Condition | 4, 78 | 1.00 | 0.39 |
Note: RT = reaction time. ANOVA = Repeated analysis of variance. CO2 = carbon dioxide. Significance values reflect Greenhouse-Geisser corrections. PSU = problem stimulant users. DSU = desisted stimulant users. CTL = healthy comparison subjects. Behavioral analyses include: 17 PSU, 18 DSU, and 20 CTL. CO2 analyses include 12 PSU, 11 DSU, and 19 CTL.
Breathing load was associated with significantly lower CO2 % signal change from baseline than anticipation and post-anticipation conditions across subjects.
fMRI LME Results
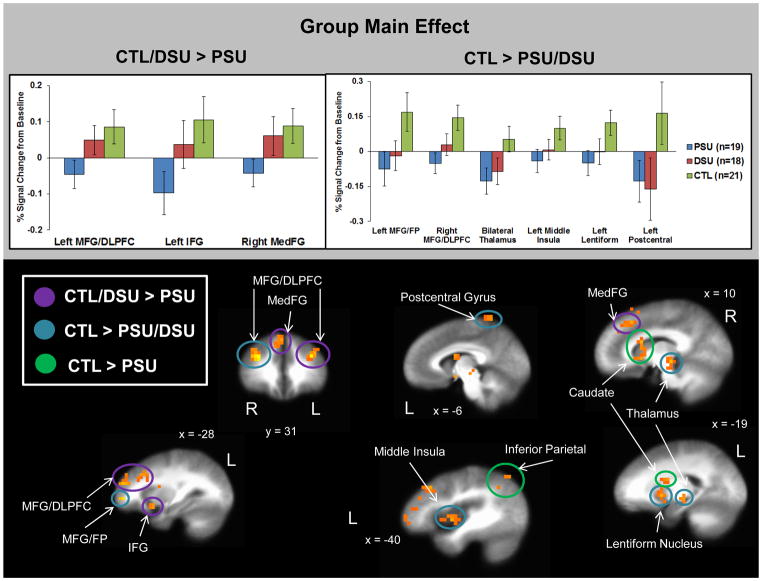
Main effect of group
First, Figure 3 demonstrates that PSU exhibited lower activation than the other two groups in three regions: (1) left middle frontal gyrus/dorsolateral prefrontal cortex (MFG/DLPFC), (2) left inferior frontal gyrus (IFG), and (3) right medial frontal gyrus (MedFG). Second, in regard to brain patterns linked to any lifetime use of stimulants, regardless of current patterns of use, Figure 3 demonstrates that both PSU and DSU displayed lower activation than CTL in six regions: (1) left MFG/frontopolar (FP) cortex, (2) right MFG/DLPFC, (3) bilateral thalamus, (4) left middle insula, (5) left lentiform nucleus, and (6) left postcentral gyrus.
Figure 3.
Results for the group main effect indicate that problem stimulant users (PSU) exhibited lower activation than desisted stimulant users (DSU) and healthy comparison subjects (CTL) in three regions: left middle frontal gyrus/dorsolateral prefrontal cortex (MFG/DLPFC), left inferior frontal gyrus (IFG), and right medial frontal gyrus (MedFG). Furthermore, PSU and DSU groups both displayed lower activation than CTL in six regions: left MFG/frontal pole (FP), right MFG/DLPFC, bilateral thalamus, left middle insula, left lentiform nucleus, and left postcentral gyrus. Error bars display ±1 standard error.
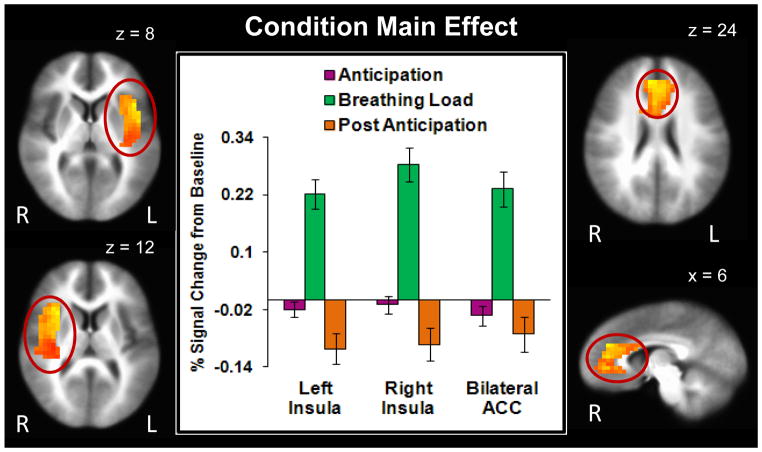
Main effect of condition
Since results for the p<.05 corrected threshold consisted of widespread activation in one large cluster (e.g., 16,664 voxels), clusters were re-extracted at a p<.001 corrected threshold (F(2,110)=7.32) for regions previously shown to be involved in interoceptive processing (bilateral insula and ACC). Figure 4 illustrates that across subjects, the breathing load condition elicited higher bilateral anterior/posterior insula and bilateral ACC activation than the anticipation condition (Table 3). Moreover, the anticipation condition was associated with greater bilateral insula activation than the post-anticipation condition.
Figure 4.
Findings for the condition main effect show that breathing load was associated with greater bilateral anterior and posterior insula (peak coordinates: left insula x = 42, y = 15, z = 8; right insula x = 30, y = 15, z = 12) and anterior cingulate cortex (ACC; peak coordinates: x = 6, y = 39, z = 24) activation than the anticipation condition. Bilateral insula activation was also greater for anticipation than post anticipation. Error bars display ±1 standard error.
Table 3.
fMRI Results for Group Main Effect, Condition Main Effect, and Group by Condition Interaction.
|
Group Main Effect
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vol(μL) | Voxels | x | y | z | Hem | Regions in Cluster | BA | CTL>PSU | CTL>DSU | DSU>PSU | Linked to Stimulant Use |
| 13312 | 208 | 37 | 27 | 30 | R | MFG/DLPFC | 9 | d=1.29 | d=0.75 | ns | No |
| 5184 | 81 | −35 | 34 | 28 | L | MFG/DLPFC | 9 | d=1.18 | ns | d=0.78† | Yes |
| 3264 | 51 | 3 | −21 | −5 | L/R | Thalamus | - | d=1.16 | d=0.88 | ns | No |
| 2048 | 32 | 6 | 29 | 44 | R | MedFG | 8 | d=1.21 | ns | d=0.70† | No |
| 1664 | 26 | −19 | 7 | 2 | L | Lentiform Nucleus | - | d=1.05 | d=0.74 | ns | No |
| 1664 | 26 | −16 | 1 | 18 | L | Caudate | - | d=0.98 | ns | ns | No |
| 1536 | 24 | −40 | −1 | 0 | L | Middle Insula | 13 | d=1.02 | d=0.71 | ns | Yes |
| 1472 | 23 | −6 | −42 | 67 | L | Postcentral Gyrus | 5 | d=0.97 | d=0.85 | ns | No |
| 1216 | 19 | −35 | 50 | 0 | L | MFG/FP | 10 | d=1.08 | d=0.80 | ns | No |
| 1152 | 18 | 11 | 11 | 12 | R | Caudate | - | d=1.15 | ns | ns | No |
| 1088 | 17 | −10 | −3 | −6 | L | Lentiform Nucleus | - | d=1.20 | d=0.75 | ns | No |
| 960 | 15 | −28 | 11 | −10 | L | IFG | 13 | d=1.06 | ns | d=0.76† | Yes |
| 896 | 14 | −42 | −60 | 45 | L | Inferior Parietal Lobule | 40 | d=0.99 | ns | ns | No |
| 768 | 12 | 14 | 17 | 58 | R | Superior Frontal Gyrus | 6 | d=1.08 | ns | ns | No |
| Condition Main Effect | |||||||||||
|
| |||||||||||
| Vol(μL) | Voxels | x | y | z | Hem | Regions in Cluster | BA | BL>A | BL>PA | A>PA | Linked to Stimulant Use |
|
| |||||||||||
| 22784 | 356 | −39 | −3 | 10 | L | Anterior/Posterior Insula | 13 | d=1.34 | d=1.36 | d=0.45 | N/A |
| 20352 | 318 | 39 | −3 | 11 | R | Anterior/Posterior Insula | 13 | d=1.42 | d=1.41 | d=0.42 | N/A |
| 17536 | 274 | −1 | 34 | 12 | L/R | Anterior Cingulate | 24 | d=1.21 | d=1.09 | ns | N/A |
| Group x Condition Interaction | |||||||||||
|
| |||||||||||
| Vol (μL) | Voxels | x | y | z | Hem | Regions in Cluster | BA | A | BL | PA | Linked to Stimulant Use |
|
| |||||||||||
| 2304 | 36 | 41 | 17 | −6 | R | IFG/Anterior Insula | 47,13 | ns | CTL>PSU d=1.07 DSU>PSU d=0.80 |
ns | No |
Note:
Reduced to marginal or non-significance (.06 < p < .12) when subjects with comorbid alcohol dependence (n=3 PSU; n=1 DSU), comorbid marijuana dependence (n=3 PSU), or non-dependence on stimulants (n=8 PSU) were excluded from analysis
This table lists regions that differed between groups in whole-brain linear mixed effects model analysis. Post-hoc independent sample t-tests were computed to determine which groups differed from each other (threshold p<.05). Vol = volume. Hem = hemisphere. BA = Brodmann Area. CTL = healthy comparison subjects. PSU = problem stimulant users. DSU = desisted stimulant users. MFG = middle frontal gyrus. DLPFC = dorsolateral prefrontal cortex. MedFG = medial frontal gyrus. FP = frontal pole. IFG = inferior frontal gyrus. A = Anticipation condition. BL = Breathing Load condition. PA = Post-Anticipation condition. d = Cohen’s d effect size reported for significant differences. ns = groups or conditions do not significantly differ. Coordinates (x, y, z) reflect center of mass. Linked to Stimulant Use = if Yes, then higher lifetime percentage of drug use attributable to stimulants (as opposed to marijuana) correlated with lower activation in these brain regions in PSU and DSU subjects (p<.05 uncorrected exploratory analysis); N/A = not applicable because correlations were not performed on brain regions not resulting from initial group differences.
Group by condition interaction
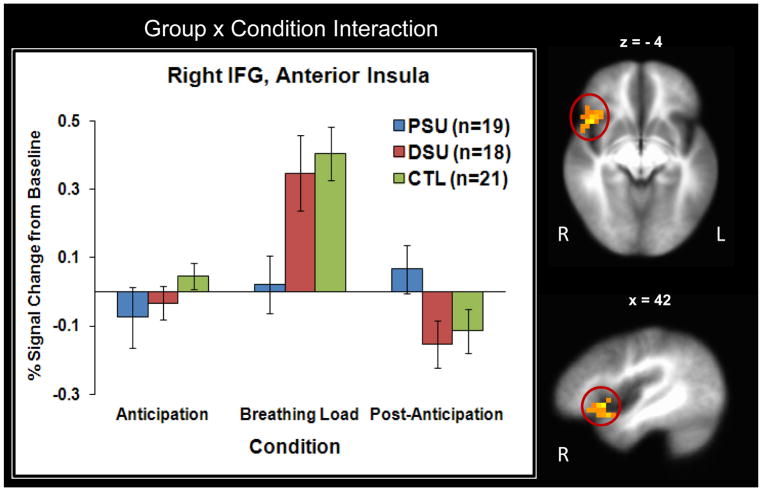
Figure 5 illustrates that during breathing load, PSU exhibited lower right IFG and right anterior insula activation than the other two groups.
Figure 5.
Results for the Group × Condition interaction demonstrate that problem stimulant users (PSU) displayed lower right inferior frontal gyrus (IFG) and right anterior insula activation than desisted stimulant users (DSU) and healthy comparison subjects (CTL) during breathing load (peak coordinates : x = 42, y = 15, z = 4). Groups did not differ in activation during anticipation or post anticipation. Error bars display ±1 standard error.
Follow-Up Analyses2
Percentage of drug use due to stimulants
Since the majority of PSU and DSU reported substantial marijuana use in addition to stimulant use, the percentage of lifetime drug use attributable to stimulants was calculated by the following formula: (lifetime cocaine use + lifetime amphetamine use) / (lifetime marijuana use + lifetime cocaine use + lifetime amphetamine use) x 100. This percentage was then correlated with brain activation from the LME analysis across PSU and DSU subjects (using Pearson correlations in SPSS).
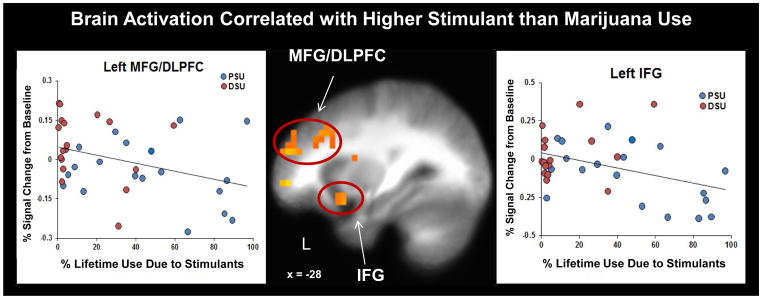
Results demonstrated that PSU had a significantly higher percentage of drug use attributable to stimulants (M=46.30%, SD=31.69%) than DSU (M=13.24%, SD=17.70%; independent samples Mann-Whitney U Test p<.05). Whereas 8 PSU had greater than 50% of their overall drug use due to stimulants, only 1 DSU met this threshold. Figure 6 illustrates that across PSU and DSU subjects, greater percentage of drug use due to stimulants than marijuana was associated with lower activation in left MFG/DLPFC (r= -.36, p=.03) and left IFG (r= −.40, p=.02) as well as left middle insula (r= −.34, p=.04). Within PSU alone, greater percentage of drug use due to stimulants was also linked to lower left IFG activation (r= −.52, p=.02).
Figure 6.
Greater percentage of lifetime drug use attributable to stimulants (amphetamine and cocaine as opposed to marijuana) was associated with lower left middle frontal gyrus/dorsolateral prefrontal cortex (MFG/DLPFC; r = .36, p = .03) and left inferior frontal gyrus (IFG; (r = .40, p = .02) activation across problem stimulant user (PSU) and desisted stimulant user (DSU) subjects, as well as lower left IFG activation within the PSU group alone (r = .52, p = .02).
Influence of comorbid substance dependence/positive marijuana urine screen
Univariate ANOVAs examining group differences in personality (depression and anxiety), IQ, and brain activation (e.g., significant group LME differences) were computed four times in SPSS, each time excluding a different set of subjects from analysis: (1) n=3 PSU and n=1 DSU with comorbid alcohol dependence to minimize the influence of severe alcohol use; (2) n=3 PSU with comorbid marijuana dependence to minimize the influence of severe marijuana use; (3) n=8 PSU who did not meet criteria for cocaine or amphetamine dependence (meaning that they met criteria for cocaine and/or amphetamine abuse but not dependence) to emphasize the influence of severe stimulant use; and (4) n=7 PSU and n=6 DSU who tested positive for marijuana at the time of the scan.
Results indicated that first, when alcohol dependent subjects were removed, differences between PSU and DSU became non-significant for right MedFG and left IFG (both p=.11). Second, when marijuana dependent subjects were removed, differences between PSU and DSU in the right MedFG was reduced to a trend (p=.06). Third, when non-stimulant dependent PSU were removed, differences between PSU and DSU were reduced to a trend in left MFG/DLPFC (p=.07) and right MedFG (p=.10). Finally, when marijuana-positive PSU and DSU were removed, differences between PSU and DSU were reduced to marginal significance for right anterior insula/IFG (p=.08) and right MedFG (p=.07). All other group differences remained significant.
Voxel-wise regression involving drug use
A robust whole-brain voxel-wise regression (Huber, 1981) was performed in R across all subjects who had used stimulants (PSU and DSU: n=37) to examine which brain regions were uniquely associated with amphetamine, cocaine and marijuana use specifically during subjects’ response to the aversive interoceptive stressor itself. This regression was subject to a bootstrapping procedure in order to obtain estimated bias coefficients and t statistics (random sampling with replacement; n=25 bootstraps, n=50 maximum iterations) for each of three lifetime substance use regressors, all natural log transformed + 1 due to non-normal distributions: (1) amphetamine; (2) cocaine; and (3) marijuana. The dependent variable was percent signal change during breathing load. Analogous AlphaSim values were applied to this analysis as the LME analysis (threshold = 12 contiguous voxels).
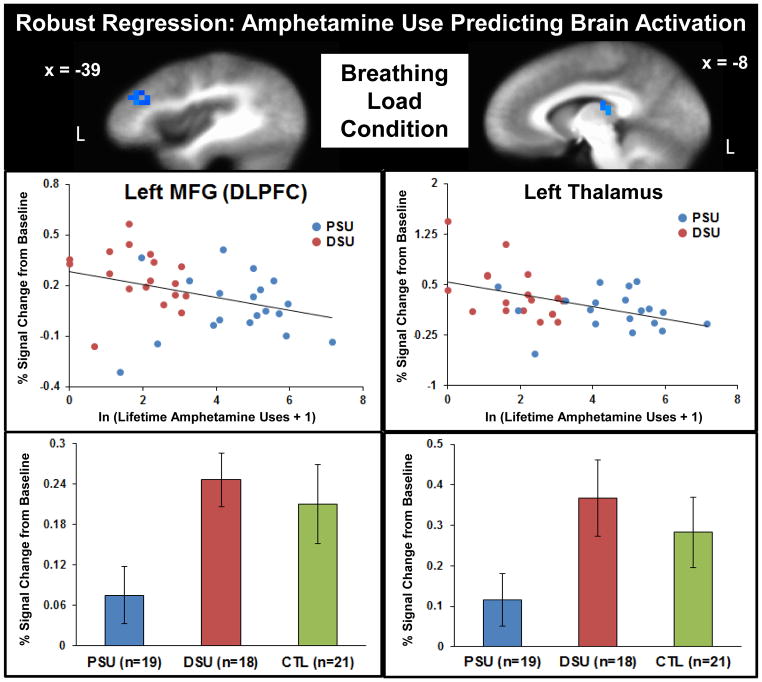
Figure 7 illustrates that higher lifetime amphetamine use was linked to lower left MFG/DLPFC and left thalamus activation during breathing load, and within these two particular regions, PSU also exhibited lower activation than the other two groups. In contrast, higher lifetime marijuana use was linked to lower right middle temporal gyrus, right postcentral gyrus, right thalamus, right caudate, and left cingulate gyrus activation, brain patterns that did not differ between PSU and DSU.
Figure 7.
Robust regression findings across problem stimulant user (PSU) and desisted stimulant user (DSU) subjects demonstrated that higher lifetime amphetamine use was uniquely associated with lower left middle frontal gyrus/dorsolateral prefrontal cortex (MFG/DLPFC) and left thalamus activation during the experience of breathing load. Bottom: Activation in these regions also differentiated groups, such that PSU exhibited lower neural responses during breathing load than DSU and healthy comparison subjects (CTL). Error bars display ±1 standard error.
Discussion
This investigation examined the question whether interoceptive processing differs in young adults who go on to develop problems with stimulants relative to those who do not. It was hypothesized that PSU would exhibit lower activation in brain regions involved in cognitive control and interoception than DSU and CTL while making decisions during an aversive interoceptive experience. Supporting this prediction, PSU exhibited lower right anterior insula and IFG activation than DSU and CTL specifically during the breathing load condition, an activation pattern that was not correlated with number of lifetime stimulant uses. These findings suggest that PSU are not registering the salience of threat signals appropriately, and that this appears to be independent of the quantity of amphetamine and cocaine use. Attenuated right anterior insula is suggestive of reduced awareness of bodily feeling states (Craig, 2011) as well as impaired coordination/evaluation of task demands (Eckert et al., 2009) and weakened attention salience (Menon & Uddin, 2010). Similarly, reduced right IFG may indicate less resources deployed to cope with emotional distractions (Wang et al., 2008) as well as reduced attentional salience of aversive stimuli (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010). Prior work also indicates that right anterior insula and IFG activations are linked to greater harm avoidance in healthy individuals during risky decision making (Bossaerts, 2010; Christopoulos, Tobler, Bossaerts, Dolan, & Schultz, 2009; Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003), suggesting that PSU may be susceptible to riskier decision-making, although more research is warranted to address this hypothesis.
In addition to attenuated right insula/IFG activation during the breathing load condition, reductions in three other brain regions differentiated PSU from the other two groups across anticipation, breathing load, and post-anticipation conditions: left MFG/DLPFC, left IFG, and right MedFG. PSU reductions in these three regions may be indicative of a more general deficit in cognitive control, given that they were not specific to the aversive interoceptive experience itself. Furthermore, across all subjects who had ever used stimulants: (1) higher lifetime stimulant use, particularly due to amphetamine, was associated with lower left DLPFC activation; and (2) greater lifetime drug use attributable to stimulants (as opposed to marijuana) was linked to lower activations in left IFG and left DLPFC. Lifetime amphetamine use and percentage of lifetime use due to stimulants were both higher in PSU than DSU, indicating that PSU were exhibiting the greatest reductions in resources implicated in cognitive control processes. Finally, PSU and DSU had similar patterns of lifetime marijuana use, so lower activation in left DLPFC, left IFG, and right MedFG cannot be attributed to greater marijuana consumption in PSU than DSU.
Research indicates that left IFG is recruited during spatial working memory tasks (McCarthy et al., 1996) and left DLPFC is associated with several aspects of cognitive control, including attention switching and the resolution of interference during competing task processes (Sylvester et al., 2003). Working memory, attention switching, and suppression of distractors are likely involved in monitoring the potential for aversive threat while performing our spatial CPT. Finally, the portion of the MedFG attenuated in PSU (BA 8) has been implicated in the processing of uncertainty (Volz, Schubotz, & von Cramon, 2005), which is also relevant to the present study, given that participants were unsure of when exactly they would experience the aversive interoceptive stimulus during the CPT. On the whole, these findings suggest that attenuated neural resources involving goal maintenance and attention are markers for recent transition to stimulant dependence.
In contrast to hypotheses, however, both PSU and DSU exhibited lower activation than CTL across task conditions in several brain regions, including bilateral thalamus, left middle insula and right MFG/DLPFC, and both PSU and DSU both endorsed substantially greater lifetime marijuana use than CTL. Although no significant correlations emerged between lifetime marijuana use and patterns of neural results across PSU and DSU participants, frequent marijuana use has previously been linked to reductions in left middle insula (Jacobus et al., 2012), right MFG (Cousijn et al., 2012; Li, Milivojevic, Constable, & Sinha, 2005), and right thalamus (Padula, Schweinsburg, & Tapert, 2007). Across PSU and DSU subjects: (1) greater lifetime amphetamine use was uniquely linked to lower left thalamus activation, whereas greater lifetime marijuana use was uniquely linked to lower right thalamus activation; and (2) greater lifetime use attributable to stimulants rather than marijuana was associated with lower left middle insula activation. Reduced thalamic resources linked to sensory processing (Craig, 2002; Padula et al., 2007), attenuated left middle insula resources involved in the interoceptive relay system (Craig, 2002, 2009), and hypoactive right MFG involved in affective stress regulation and attention (Cousijn et al., 2012; Li et al., 2005) may be markers of a predisposition in young adults open to both stimulant and marijuana experimentation, with impairments in these regions worsening as a function of increased drug use over time. Perhaps these young adults initially seek out drugs to heighten sensory and attentional experiences, although additional data are needed to examine this hypothesis.
This investigation, although novel in many respects, has several limitations. First, relatively small sample sizes may have limited power to detect differences between DSU and the other two groups. In addition, removal of PSU and DSU with comorbid alcohol and marijuana dependence reduced the statistical significance of left IFG and right MedFG differences between PSU and DSU groups, but these statistical reductions could also be related to the impact of diminished sample sizes in follow-up analyses. Second, groups did not differ in behavioral performance (RT, accuracy) within the context of this aversive interoceptive manipulation. It may be that interoceptive perturbations disrupt performance in more complex decision making tasks than the simple CPT used in the present study. Third, only 72% of participants had usable CO2 data collected during the CPT. There was a slight reduction in CO2 concentration during the breathing load, although CO2 change did not correlate with fMRI signal change across all subjects. Nevertheless, it cannot be completely ruled out that some of these findings are a consequence of attenuation of CO2 concentration. Fourth, although the study of the post-breathing load condition may have implications for addiction with respect to the spatial extent of neural recovery after interoceptive perturbations, the limited number of trials included in this CPT condition precluded group analysis. Future incarnations of this task will include a substantially higher number of trials in order to examine neural changes as a function of interoceptive recovery. Fifth, although participants were not instructed to regulate any feelings arising from the aversive interoceptive manipulation, it is possible that individual differences in emotion regulation strategies might have influenced imaging results, particularly for the breathing load condition. Inquiry of participant strategies to reduce negative emotion should be implemented in future work. Lastly, groups did not differ in self-reported interoceptive awareness/sensitivity or VAS breathing load experience, despite differences in brain activation within the context of the aversive stimulus. It may be the case that self-reported interoceptive experience changes only as a function of chronic stimulant use, although future studies could employ VAS measures administered in real time during the breathing load experience to determine whether state ratings distinguish groups better than post-scan retrospective ratings, or additional behavioral indicators of interoceptive sensitivity and awareness such as heartbeat detection (Garfinkel et al., 2013) could be included.
Despite these limitations, findings from the present study strongly suggest that the recent transition to stimulant dependence is marked by: (1) attenuated left DLPFC/IFG and right MedFG resources, implicated in cognitive control and uncertainty processing, that are not specific to the experience of aversive interoceptive stimuli; and (2) attenuated right inferior frontal/insular resources allocated to the experience of an aversive interoceptive state. These results suggest that individuals transitioning to stimulant dependence are not attending to signals within the context of uncertainty or threat, which may heighten risky behaviors such as increased drug consumption.
Table 4.
Robust Regression Results: Lifetime Drug Use Predicting Brain Activation during Breathing Load across PSU and DSU
| Substance | Vol (μL) | Voxels | x | y | z | Hem | Regions in Cluster | BA | Correlation | CTL/DSU>PSU? |
|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamine | 1152 | 18 | −26 | −16 | 24 | L | Caudate, Middle/Posterior Insula | 13 | Negative | No |
| 960 | 15 | −39 | 32 | 24 | L | MFG/DLPFC* | 46 | Negative | Yes | |
| 768 | 12 | −8 | −23 | 14 | L | Thalamus* | - | Negative | Yes | |
| Cocaine | 1088 | 17 | 34 | −5 | 25 | R | Precentral Gyrus, Posterior Insula | 13 | Positive | No |
| Marijuana | 1280 | 20 | 32 | −24 | 40 | R | Postcentral Gyrus | 3 | Negative | No |
| 1024 | 16 | −6 | −8 | 25 | L | Cingulate Gyrus | 24 | Negative | No | |
| 960 | 15 | 30 | −52 | 20 | R | Middle Temporal Gyrus | 22 | Negative | No | |
| 768 | 12 | 5 | −1 | 5 | R | Thalamus, Caudate | - | Negative | No |
Note:
Illustrated in Figure 7.
Vol = volume. Hem = hemisphere. BA = Brodmann Area. PSU = problem stimulant users. DSU = desisted stimulant users. MFG = middle frontal gyrus. DLPFC = dorsolateral prefrontal cortex. Coordinates (x, y, z) reflect center of mass. Lifetime drug use reflects total number of sessions.
Acknowledgments
Grant Support: This work was supported by grants from the National Institute on Drug Abuse (Grant Nos. R01-DA016663, P20-DA027834, R01-DA027797, and R01-DA018307 to Martin Paulus).
Footnotes
Across subjects, CO2 condition % signal change from baseline did not correlate with any regions that emerged for the group main effect, the condition main effect, or the group by condition interaction.
PSU endorsed higher anxiety and depression symptoms than DSU but both groups did not differ from CTL. However, both PSU and DSU exhibited lower IQ than CTL. PSU also reported greater lifetime uses of amphetamine than DSU and CTL, but both PSU and DSU groups both reported greater lifetime uses of cocaine and marijuana than CTL. It has been argued that it is inappropriate to attempt to “control” for these group differences using analysis of covariance, since removal of variance associated with these constructs might also remove important variance inherent to group membership itself (Miller & Chapman, 2001). Instead, we examined the covariation between these constructs and differences in neural processing within groups: Pearson correlations were performed between brain regions emerging as significant between groups in the LME (Table 3) and: (1) verbal IQ scores across PSU and DSU; (2) STAI trait anxiety scores within PSU; (3) BDI-II depression scores within PSU; (4) lifetime amphetamine, cocaine, and marijuana uses separately (log-transformed due to non-normality) within PSU; and (5) lifetime amphetamine, cocaine and marijuana uses separately (log-transformed) across PSU and DSU. Results indicated that no correlations were significant at p<.05 uncorrected.
References
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, … Romero FJ. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addiction Biology. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Patton JH. Impulsivity: Cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Bases of Sensation Seeking, Impulsivity, and Anxiety. Hillsdale, NJ: Erlbaum; 1983. pp. 77–116. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Structure and Function. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. Journal of Applied Physiology. 2008;105:1106–1113. doi: 10.1152/japplphysiol.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience. 2009;29:12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective FMRI study. PloS one. 2012;7:e42394. doi: 10.1371/journal.pone.0042394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computer Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Annuals of the New York Academy of Science. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respiratory Physiology and Neurobiology. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, … Kilts C. An fMRI Study of the Interaction of Stress and Cocaine Cues on Cocaine Craving in Cocaine-Dependent Men. The American Journal on Addictions. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Human Brain Mapping. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy WF, Fitzgerald M, Noll DC. Improved image registration by using Fourier interpolation. Magnetic Resonance Medicine. 1996;36:923–931. doi: 10.1002/mrm.1910360615. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, … Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Structure and Function. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology. 2013;50:505–512. doi: 10.1111/psyp.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:145–156. doi: 10.1006/nimg.1999.0527. [DOI] [PubMed] [Google Scholar]
- Hester R, Bell RP, Foxe JJ, Garavan H. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug and Alcohol Dependence. 2013;133:86–93. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber PJ. Robust statistics. New York: Wiley; 1981. [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. London: Academic Press; 2005. [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavior Reviews. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CSR, Milivojevic V, Constable RT, Sinha R. Recent cannabis abuse decreased stress-induced BOLD signals in the frontal and cingulate cortices of cocaine dependent individuals. Psychiatry Research: Neuroimaging. 2005;140:271–280. doi: 10.1016/j.pscychresns.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Lopata M, La Fata J, Evanich MJ, Lourenco RV. Effects of flow-resistive loading on mouth occlusion pressure during CO2 rebreathing. American Review of Respiratory Disease. 1977;115:73–81. doi: 10.1164/arrd.1977.115.1.73. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–669. doi: 10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Dependence. 2013;131:238–246. doi: 10.1016/j.drugalcdep.2013.05.029S0376-8716(13)00215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable T, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insular function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia M, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methampetamine Use in the United States, 2005. Santa Monica, CA: RAND Corporation; 2009. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- ONDCP. The economic costs of drug abuse in the United States, 1992–2002. Washington, DC: Executive Office of the President; 2004. [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21:478. doi: 10.1037/0893-164X.21.4.478. doi:10.1037%2F0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Flagan T, Simmons AN, Gillis K, Kotturi S, Thom N, … Swain JL. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS One. 2012;7:e29394. doi: 10.1371/journal.pone.0029394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacology Biochemistry and Behavior. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, Saikat D, Deepayan S the R Development Core Team. nlme: Linear and nonlinear mixed effects models. R package Version 3.1–111 2013 [Google Scholar]
- Porges SW. Body perception questionnaire. Laboratory of Developmental Assessment, University of Maryland; 1993. [Google Scholar]
- Reske M, Delis DC, Paulus MP. Evidence for subtle verbal fluency deficits in occasional stimulant users: quick to play loose with verbal rules. Journal of Psychiatric Research. 2011;45:361–368. doi: 10.1016/j.jpsychires.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2011 national survey on drug use and health: Summary of national findings. 2012. [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage. 1999;9:311–329. doi: 10.1006/nimg.1999.0402. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR. State-Trait Anxiety Inventory (STAI) BiB. 1983;2010:180. [Google Scholar]
- Stewart JL, Flagan TM, May AC, Reske M, Simmons AN, Paulus MP. Young adults at risk for stimulant dependence show reward dysfunction during reinforcement-based decision making. Biological Psychiatry. 2013;73:235–241. doi: 10.1016/j.biopsych.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CYC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/S0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience & Biobehavioral Reviews. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Variants of uncertainty in decision making and their neural correlates. Brain Research Bulletin. 2005;67:403–412. doi: 10.1016/j.brainresbull.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, … McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research: Neuroimaging. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The sensation seeking scale V (SSS-V): Still reliable and valid. Personality and Individual Differences. 2007;43:1303–1305. doi: 10.1016/j.paid.2007.03.021. [DOI] [Google Scholar]