Abstract
OBJECTIVES
Depression is prevalent in inflammatory bowel disease (IBD) patients. The impact of depression on IBD is not well-studied. It is unknown how providers should assess depression.
METHODS
We used data from the Sinai–Helmsley Alliance for Research Excellence cohort, to assess methods of diagnosing depression and effects of baseline depression on disease activity at follow-up. A patient health questionnaire (PHQ-8) score ≥5 was consistent with mild depression. Relapse was defined as a modified Harvey–Bradshaw Index ≥5 or Simple Clinical Colitis Activity Index >2. We performed binomial regression to calculate adjusted risk ratios (RRs).
RESULTS
We included 2,798 Crohn’s disease (CD) patients with 22-month mean follow-up and 1,516 ulcerative colitis (UC) patients with 24-month mean follow-up. A total of 64% of CD patients and 45% of UC patients were in remission at baseline. By self-report, 20% of CD and 14% of UC patients were depressed. By PHQ-8, 38% of CD and 32% of UC patients were depressed (P <0.01). Adjusted for sex, remission, and disease activity, CD patients with baseline depression were at an increased risk for relapse (RR: 2.3; 95% confidence interval (CI): 1.9–2.8), surgery, or hospitalization (RR: 1.3 95% CI: 1.1–1.6) at follow-up. UC patients with baseline depression were also at increased risk for relapse (RR: 1.3; 95% CI: 0.9–1.7), surgery, or hospitalization (RR: 1.3; 95% CI: 1.1–1.5) at follow-up.
CONCLUSIONS
Baseline depression is associated with a higher risk for aggressive IBD at follow-up. A single question is not a sensitive method of assessing depression. Providers should consider administering the PHQ-8 to capture those at greater risk for aggressive disease.
INTRODUCTION
Depression is prevalent in patients with inflammatory bowel diseases (IBDs) (1–3). The rate of depression in IBD patients is not well determined; one prior study showed that IBD patients experience rates of depression that are three times that of the general population (4). In IBD patients, depression is associated with a lower quality of life, sexual dysfunction, worsened disease activity, and increased frequency of flares (5–9). However, even IBD patients in clinical remission can have depression (10). Depression may have serious consequences, including an increased rate of suicide among IBD patients (11). Depression may also prompt more medical testing in patients and has been shown to be a risk factor for radiation exposure in IBD patients (12). Therefore, assessing and addressing depression in IBD patients is an important component of clinical practice.
The majority of prior studies on depression in IBD patients have been cross-sectional in nature and focused on the prevalence of depression (13–19). Only two studies prospectively followed IBD patients with depression to assess for changes in disease activity at follow-up; (6,9) both demonstrated that depression at baseline can result in poorly controlled disease at follow-up. However, there are no studies to date on how to assess depression in an IBD practice.
We used data from a large, prospective, multi-center cohort, to determine the effects of depression at baseline on disease activity at follow-up. We also aimed to compare a single item question on depression to a standardized depression scale for assessing self-reported depression in the practice of IBD.
METHODS
Data source
The Sinai–Helmsley Alliance for Research Excellence cohort is a prospective observational cohort of patients with IBD. The cohort was established with the goal of creating a database of clinical information, as well as a repository of biological specimens for genetic, molecular, and microbiological research, to better understand IBD and develop new therapies. Patients were recruited from seven US academic centers: Mount Sinai, University of Chicago, Massachusetts General Hospital, Cedars-Sinai, Mayo Clinic, Rochester, University of North Carolina at Chapel Hill, and Washington University, Saint Louis. The institutional review boards of all institutions approved the study.
Patients who were under the age of 18 years, those unable to understand or provide informed consent, and those who did not have a confirmed diagnosis of IBD in their medical records were excluded. Consented patients provided demographic information, medical history, surgical history (including perianal procedures), family history, medication use, and extra-intestinal manifestations via a baseline interview with a study coordinator. Disease characteristics as defined by the Montreal classification, medication use, extra-intestinal manifestations, and laboratory results were obtained from the medical record. For the purposes of this study, we excluded anyone who did not have a follow-up visit. A modified Harvey–Bradshaw Index or Simple Clinical Colitis Activity Index were completed during the study visit. Remission was defined as a Harvey–Bradshaw Index <5 or a Simple Clinical Colitis Activity Index ≤2 (20,21).
Depression was assessed in two ways. Patients were asked whether they had depression as a single question, which we defined as self-reported depression in analyses. A very common method of screening for depression is with the patient health questionnaire (PHQ) scale. The PHQ in its full form is a nine-question scale, including a question assessing suicidality, which some propose is not as useful in a population with chronic disease (22). Furthermore, studies have shown a very strong correlation between the PHQ-8 and PHQ-9 scales (23,24). Therefore, we elected to use a PHQ-8 scale to assess depression in this IBD population. A PHQ-8 score of ≥5 was consistent with at least mild depression. A score of ≥10 was consistent with moderate to severe depression (25).
Patients were followed prospectively with follow-up questionnaires, similar to the baseline questionnaire, administered either on the phone, via the Internet, or during a subsequent clinic visit. In addition to updated information from the baseline study visit, patients were asked whether they had a hospitalization, for any reason, or surgery on their bowels since their last visit.
Statistical analysis
We determined demographics, disease behavior, medical history, and baseline PHQ-8 scores for enrolled subjects at baseline. We compared the prevalence of self-reported depression to depression defined by the PHQ-8 scale. We created a composite variable for more aggressive disease that consisted of hospitalization or IBD-related surgery at follow-up. We conducted binomial regression models to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for five distinct outcomes at follow up: relapse by disease activity index, new IBD-related surgery, new hospitalization, and new prescriptions for biologics, steroids, and narcotics. All models were adjusted for three potential confounders: sex, disease behavior by Montreal classification, and remission status at baseline. These confounders were selected based on clinical judgement. All models were stratified by IBD subtype (Crohn’s disease (CD) or ulcerative colitis (UC)) and were restricted to patients depressed at baseline defined as a PHQ-8 scale score ≥5. For sensitivity analysis, we estimated the same adjusted RRs only for those with moderate to severe depression, PHQ-8 scale score ≥10. We conducted a survival analysis of the composite endpoint of IBD-related surgery and hospitalization stratified by depression status at baseline, using log-rank tests to compare Kaplan–Meier curves. All analyses were done in Stata 14.0 (College Station, TX).
RESULTS
Among the 2,798 CD patients in this cohort, 56% were women. The mean age was 41 years and the mean disease duration was 13 years. The average duration of follow-up was 22 months (Table 1). According to the Montreal classification, 48% of CD patients had inflammatory disease at baseline, 28% had stricturing disease, 24% had penetrating disease, and 20% had perianal disease. The majority of CD patients (56%) had ileo-colonic disease. The median Harvey–Bradshaw Index was three with 64% of patients in remission by Harvey–Bradshaw Index.
Table 1.
Characteristics of the study population
| Crohn’s disease (n =2798) | Ulcerative colitis (n =1516) | |
|---|---|---|
| Female | 56 | 49 |
| Mean age in years (s.d.) | 41 (±15) | 42 (±15) |
| Mean disease duration in years (s.d.) | 13 (±11) | 10 (±10) |
| Mean follow up duration in months (s.d.) | 22 (±10) | 24 (±10) |
| White | 94 | 96 |
| Current smokers | 27 | 11 |
| History of GI surgery | 52 | 16 |
| Disease behavior | ||
| Inflammatory (B1) | 48 | |
| Stricturing (B2) | 28 | |
| Penetrating (B3) | 24 | |
| Perianal phenotype | 20 | |
| Disease location | ||
| Ileal (E1) | 23 | |
| Colonic (E2) | 21 | |
| Ileo-colonic (E3) | 56 | |
| Upper GI | 6 | |
| Median HBI (IQR) | 3 (1–6) | |
| Remission by HBI | 64 | |
| Disease extent | ||
| Proctitis (E1) | 11 | |
| Left-sided colitis (E2) | 30 | |
| Extensive colitis (E3) | 59 | |
| Median SCCAI (IQR) | 3 (1–5) | |
| Remission by SCCAI | 45 | |
| Medications at baseline | ||
| Mesalamine | 17 | 50 |
| Immunomodulator | 41 | 33 |
| Anti-TNF | 54 | 27 |
| Steroids | 11 | 17 |
| Reported depression | 20 | 14 |
| Depressed by PHQ-8 | 38 | 32 |
GI, gastrointestinal; HBI, Harvey–Bradshaw Index; IQR, interquartile range; PHQ, patient health questionnaire; SCCAI, Simple Clinical Colitis Activity Index; TNF, tumor necrosis factor.
All numbers are percents unless otherwise specified.
Of the 1,516 UC patients in this cohort, 49% were women. The mean age was 42 years and mean disease duration was 10 years (Table 1). The average duration of follow-up was 24 months. At baseline, 11% of UC patients had proctitis, 30% had left-sided colitis, and 59% had extensive colitis. The median Simple Clinical Colitis Activity Index score was 3, with 45% of patients in remission by Simple Clinical Colitis Activity Index.
In this cohort of IBD patients, 18% reported being depressed at baseline and 36% scored positive for depression by the PHQ-8 scale (P <0.01). In this cohort of patients, using the PHQ-8 scale as a gold standard, self-reported depression had a sensitivity of 31% and a specificity of 90%.
Patients with CD, who were depressed at baseline according to the PHQ-8 score, had a significantly higher risk for worse disease at follow-up, adjusting for sex, remission status at baseline, and disease activity by Montreal classification (Table 2). They had a higher risk for relapse defined by their disease activity index (RR: 2.3, 95% CI: 1.9–2.8). New biologic or steroid prescriptions at follow-up were more common in those patients who were depressed. Depressed CD patients also had a higher risk of hospitalization (RR: 1.3, 95% CI: 1.2–1.5) and IBD-related surgery at follow-up (RR: 1.3; 95% CI: 1.1–1.6). Similar trends were seen when analyzing the risk for aggressive disease in CD patients with moderate-to-severe depression (PHQ ≥10). Patients who had active disease at baseline were significantly more likely to be depressed compared with patients who were in remission (59 vs. 26%, P <0.01).
Table 2.
Risk of developing more aggressive IBD at follow-up
| Depressed with Crohn’s disease RR (95% CI) |
Depressed with ulcerative colitis RR (95% CI) |
|
|---|---|---|
| Relapse by disease activity index a | 2.3 (1.9–2.8) | 1.3 (0.9–1.8) |
| New biologic prescription | 1.8 (1.4–2.3) | 1.6 (1.1–2.3) |
| New steroid prescription | 1.8 (1.1–3.2) | 1.8 (0.9–3.8) |
| Hospitalization | 1.3 (1.2–1.5) | 1.3 (1.1–1.5) |
| IBD-related surgery | 1.3 (1.1–1.6) | 1.8 (1.2–2.6) |
Risk of developing more aggressive IBD at follow-up, adjusting for sex, remission status, and disease activity, for those who were depressed based on Patient Health Questionnaire (PHQ)-8 score at baseline.
CI, confidence interval; IBD, inflammatory bowel disease; RR, risk ratio.
Depression is defined as a PHQ-8 score of ≥5.
Modified Harvey-Bradshaw Index ≥5 for Crohn’s disease and Simple Clinical Colitis Activity Index >2 for ulcerative colitis, for those with lower scores at baseline.
UC patients who were depressed by PHQ-8 at baseline had a higher risk for more aggressive disease at follow-up, adjusting for sex, remission status at baseline, and disease activity by Montreal classification (Table 2). They were at a higher risk for having a new biologic prescription at follow-up (RR: 1.6; 95% CI: 1.1–2.3). Depressed UC patients also had a higher risk of being hospitalized (RR: 1.3, 95% CI: 1.1–1.5) or having IBD-related surgery (RR: 1.8; 95% CI: 1.2–2.6). Similar trends were seen when analyzing the risk for aggressive disease in UC patients with moderate-to-severe depression. Patients who had active disease at baseline were significantly more likely to be depressed compared with patients who were in remission (45 vs. 16%, P <0.01).
Patients who were depressed at baseline by PHQ-8 score were significantly more likely to be taking narcotics at follow-up, adjusting for sex, and remission by disease activity index. For CD patients the risk was 2.5 times higher (95% CI: 1.1–6.3) and for UC patients the risk was 3.6 times higher (95% CI: 1.1–11.4).
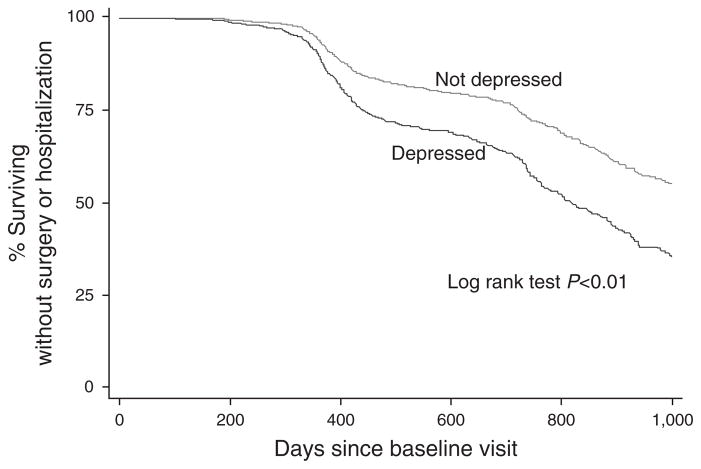
Survival analysis for CD patients revealed that those who were depressed at baseline relapsed (P <0.01), had a new biologic prescribed (P <0.01), a new steroid prescribed (P =0.02), were hospitalized (P <0.01), and had surgery (P <0.01) significantly sooner than those who were not depressed at baseline. CD patients who were depressed at baseline achieved a composite endpoint for surgery or hospitalization significantly sooner than CD patients who were not depressed at baseline (P <0.01, Figure 1).
Figure 1.
Kaplan–Meier curve for Crohn’s disease (CD) patients comparing the time to inflammatory bowel disease (IBD)-related surgery or hospitalization for those with and without baseline depression as defined by the patient health questionnaire (PHQ-8) depression scale.
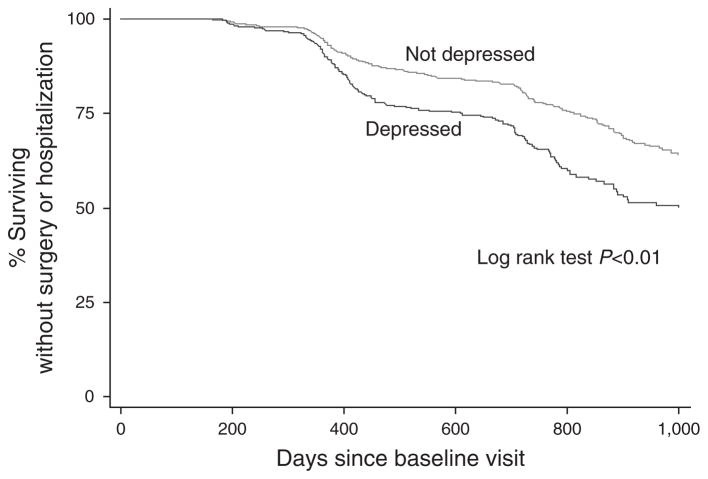
Survival analysis for UC patients revealed that those who were depressed at baseline relapsed (P <0.01), had a new biologic prescription (P <0.01), were hospitalized (P <0.01), and had surgery (P <0.01) significantly sooner than those who were not depressed at baseline. UC patients who were depressed at baseline achieve a composite endpoint for surgery or hospitalization significantly sooner than UC patients who are not depressed at baseline (P <0.01, Figure 2).
Figure 2.
Kaplan–Meier curve for ulcerative colitis (UC) patients comparing the time to inflammatory bowel disease (IBD)-related surgery or hospitalization at follow up for those with and without baseline depression as defined by the patient health questionnaire (PHQ-8) depression scale.
DISCUSSION
We present data from a large longitudinal study of IBD patients assessing the impact of depression on disease activity over time. We found that IBD patients who were depressed at baseline were at significantly greater risk for more aggressive disease, such as new biologic and steroid prescriptions, hospitalizations, and surgery, over time. We observed that those who were depressed developed more aggressive disease significantly sooner than those who were not depressed. In additions, we found that asking patients a single question whether they were depressed is not a sensitive method of assessing depression. Given the important correlation between depression and worsening disease activity over time, assessing depression accurately is critical to the care of IBD patients.
Our results confirm the findings from the Swiss IBD cohort that depression is associated with clinical recurrence of IBD (6). The Swiss study used the Hospital Anxiety and Depression Scale to assess depression. Although the PHQ-8 and Hospital Anxiety and Depression Scale have similar test characteristics for screening for depression, the PHQ-8 has been shown to be better at detecting moderate to severe depression (26,27). Furthermore, the PHQ scale is a multi-purpose instrument that can also be used to diagnose, monitor and measure the severity of depression (28). Our study demonstrates that higher PHQ-8 scores can predict worse outcomes.
Depression has been shown to be a risk factor for worsening disease in other inflammatory conditions (29–31). One of the confounders of assessing depression, especially in inflammatory states, is that disease-related somatic symptoms may be misclassified as depression. Nonetheless, the relationship between depression and inflammation may be an important one. Inducing depression in mice with quiescent colitis reactivated inflammation possibly due to impaired cholinergic inhibition of pro-inflammatory cytokines by macrophages (32). Therefore, depression in itself may be a risk factor for worsening inflammation. Depression has also been shown to be a risk factor for poor medication adherence, which is another pathway by which depression may increase a patient’s risk for worsening IBD (13,33).
We are among the first to compare separate methods of assessing depression via self-report in patients with IBD. We show that simply asking a patient whether they are depressed is not an adequate means of identifying those who are depressed and at increased risk for worsening IBD over time. Although this method is specific for depression, it lacks sensitivity and therefore is not an appropriate screening tool. The PHQ-8 is a scale that patients can complete themselves and is simple to calculate. Providers caring for IBD patients should consider administering the PHQ-8 as part of their routine clinical practice to screen patients for depression. If a patient screens positive for depression, a qualified professional should conduct a more detailed evaluation to confirm depression.
Identifying patients with depression should prompt treatment or referral for treatment of depression. One small study of IBD patients demonstrated that treating mental illness with a psychiatric medication was associated with significant improvements in depression and anxiety scales, hemoglobin, and Crohn’s Disease Activity Index, and a trend for lower modified Mayo scores in just 6 months; this study did not have a comparison arm (5). A study in pediatric IBD patients showed that psychotherapy for co-morbid depression was associated with decreased gastroenterology-related health care utilization (34). Despite such data, a Canadian study reported that only 40% of IBD patients with depression were being treated with an anti-depressant. The same large Canadian study also reported that 47% of depressed IBD patients contemplated suicide at some time (4). A Danish population-based study found that people with IBD had a higher rate of suicide compared with matched controls (11). Therefore, appropriately screening for depression in IBD patients may not only prevent worsening disease, but may help save patients’ lives. The IBD community could consider adding evaluating for depression as a quality metric in the care of IBD patients. Furthermore, depression is a chronic medical condition, like all others, that should be treated. Additional work is needed on the role of anti-depressant therapies, both psychotherapy and pharmacotherapy, in IBD and whether these will affect IBD-specific outcomes.
We have also shown that IBD patients who are depressed at baseline were at significantly higher risk for a new narcotic prescription at follow-up. Other studies found that narcotic use was higher in depressed IBD patients (35,36). In IBD patients, narcotics have been associated with increased disease complications and mortality (37). Therefore, appropriately identifying depressed IBD patients and referring them for treatment of depression may also result in decreased narcotic use.
There were numerous strengths to this large longitudinal study of depression in IBD patients. The data were validated from the medical record, with accurate depiction of disease phenotype. The sample drew from geographically diverse locations throughout the United States. The present study also has some limitations. All the patients were treated at tertiary care centers across the US, thus limiting generalizability. We previously reported variability in medication approaches in these centers, demonstrating diverse practice patterns which may be consistent with variability seen nationally (38). In addition, we did not have objective data such inflammatory markers or endoscopic scales of disease activity in our cohort. We also did not have data on all co-morbidities, such as chronic pain and anxiety, non-IBD medications, specifically anti-depressant medications, which may confound the measurement of depression.
Depression at baseline is associated with higher risks for worsening disease at follow-up in IBD patients. Asking patients whether they are depressed is not a sensitive way to assess depression in this population. Providers should consider administering a PHQ-8 scale as part of their care for IBD patients, in order to capture those who are at greater risk for worsening disease over time. Consideration should be given to treating depression, in order to improve quality of life and perhaps IBD-specific outcomes, in patients with IBD.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Depression is prevalent in patients with inflammatory bowel diseases (IBD).
Depression is associated with lower quality of life in IBD patients.
Depression is associated with more aggressive disease in cross-sectional studies.
WHAT IS NEW HERE
Asking IBD patients whether they are depressed is not a sensitive method of assessing depression in clinical practice.
IBD patients who were depressed at baseline had a significantly higher risk for more aggressive disease at follow-up.
Those who are depressed develop more aggressive disease significantly sooner than those who are not depressed.
Acknowledgments
Financial support: This research was supported by grants from the National Institutes of Health (P30DK034987, T32DK07634, T32DK007130, and UL1TR000448) and by the Helmsley Charitable Trust.
Footnotes
Potential competing interests: Bharati Kochar: none. Edward L. Barnes: none. Millie D. Long: Consultant Abbvie, Takeda, and Theravance. Kelly C. Cushing: none. Joseph Galanko: none. Christopher F. Martin: none. Laura E. Raffals: none. Robert S. Sandler: none.
Specific author contributions: Bharati Kochar: planning and conducting the study, interpreting data, drafting the manuscript, and critical revisions of the manuscript. Edward L. Barnes: interpreting data, drafting the manuscript, and critical revisions of the manuscript. Millie D. Long: planning and conducting the study, collecting the data, interpreting data, and critical revisions of the manuscript. Kelly C. Cushing: interpreting data, and critical revisions of the manuscript. Joseph Galanko: conducting the study, interpreting data, and critical revisions of the manuscript. Christopher F. Martin: collecting the data, conducting the study, interpreting data, and critical revisions of the manuscript. Laura E. Raffals: collecting the data, interpreting data, and critical revisions of the manuscript. Robert S. Sandler: planning and conducting the study, interpreting data, and critical revisions of the manuscript. All authors approved the final manuscript.
References
- 1.Patten SB, Beck CA, Kassam A, et al. Long-term medical conditions and major depression: strength of association for specific conditions in the general population. Can J Psychiatry. 2005;50:195–202. doi: 10.1177/070674370505000402. [DOI] [PubMed] [Google Scholar]
- 2.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–18. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 3.Hauser W, Janke KH, Klump B, et al. Anxiety and depression in patients with inflammatory bowel disease: comparisons with chronic liver disease patients and the general population. Inflamm Bowel Dis. 2011;17:621–32. doi: 10.1002/ibd.21346. [DOI] [PubMed] [Google Scholar]
- 4.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. 2006;12:697–707. doi: 10.1097/00054725-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Yanartas O, Kani HT, Bicakci E, et al. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr Dis Treat. 2016;12:673–83. doi: 10.2147/NDT.S106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikocka-Walus A, Pittet V, Rossel JB, et al. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:829–835e1. doi: 10.1016/j.cgh.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Bel LG, Vollebregt AM, Van der Meulen-de Jong AE, et al. Sexual dysfunctions in men and women with inflammatory bowel disease: the influence of IBD-related clinical factors and depression on sexual function. J Sex Med. 2015;12:1557–67. doi: 10.1111/jsm.12913. [DOI] [PubMed] [Google Scholar]
- 8.Panara AJ, Yarur AJ, Rieders B, et al. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment Pharmacol Ther. 2014;39:802–10. doi: 10.1111/apt.12669. [DOI] [PubMed] [Google Scholar]
- 9.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- 10.Kim MC, Jung YS, Song YS, et al. Factors associated with anxiety and depression in Korean patients with inactive inflammatory bowel disease. Gut Liver. 2016;10:399–405. doi: 10.5009/gnl15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradus JL, Qin P, Lincoln AK, et al. Inflammatory bowel disease and completed suicide in Danish adults. Inflamm Bowel Dis. 2010;16:2158–61. doi: 10.1002/ibd.21298. [DOI] [PubMed] [Google Scholar]
- 12.Englund H, Liden KK, Lind T, et al. Radiation exposure in patients with inflammatory bowel disease and irritable bowel syndrome in the years 2001–2011. Scand J Gastroenterol. 2016:1–6. doi: 10.1080/00365521.2016.1252945. [DOI] [PubMed] [Google Scholar]
- 13.Long MD, Kappelman MD, Martin CF, et al. Risk factors for depression in the elderly inflammatory bowel disease population. J Crohns Colitis. 2014;8:113–9. doi: 10.1016/j.crohns.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addolorato G, Capristo E, Stefanini GF, et al. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand J Gastroenterol. 1997;32:1013–21. doi: 10.3109/00365529709011218. [DOI] [PubMed] [Google Scholar]
- 15.Szigethy EM, Youk AO, Benhayon D, et al. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:574–81. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang CK, Hewett J, Hemming J, et al. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1732–9. doi: 10.1097/MIB.0b013e318281f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahon S, Lahmek P, Durance C, et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2086–91. doi: 10.1002/ibd.22888. [DOI] [PubMed] [Google Scholar]
- 18.Filipovic BR, Filipovic BF, Kerkez M, et al. Depression and anxiety levels in therapy-naive patients with inflammatory bowel disease and cancer of the colon. World J Gastroenterol. 2007;13:438–43. doi: 10.3748/wjg.v13.i3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurina LM, Goldacre MJ, Yeates D, et al. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716–20. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey RF, Bradshw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 21.Walmsley RS, Ayers RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razykov I, Ziegelstein RC, Whooley MA, et al. The PHQ-9 versus the PHQ-8--is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J Psychosom Res. 2012;73:163–8. doi: 10.1016/j.jpsychores.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Corson K, Gerrity MS, Dobscha SK. Screening for depression and suicidality in a VA primary care setting: 2 items are better than 1 item. Am J Manag Care. 2004;10:839–45. [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–15. [Google Scholar]
- 26.Cameron IM, Crawford JR, Lawton K, et al. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Pract. 2008;58:32–6. doi: 10.3399/bjgp08X263794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417–24. doi: 10.1016/j.genhosppsych.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Matcham F, Ali S, Irving K, et al. Are depression and anxiety associated with disease activity in rheumatoid arthritis? A prospective study. BMC Musculoskelet Disord. 2016;17:155. doi: 10.1186/s12891-016-1011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matcham F, Norton S, Scott DL, et al. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology (Oxford) 2016;55:268–78. doi: 10.1093/rheumatology/kev306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aberra TM, Joshi AA, Lerman JB, et al. Self-reported depression in psoriasis is associated with subclinical vascular diseases. Atherosclerosis. 2016;251:219–25. doi: 10.1016/j.atherosclerosis.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghia JE, Blennerhassett P, Deng Y, et al. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288. e1–4. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 33.Brandstetter S, Riedelbeck G, Steinmann M, et al. Depression moderates the associations between beliefs about medicines and medication adherence in patients with rheumatoid arthritis: cross-sectional study. J Health Psychol. 2016 doi: 10.1177/1359105316646440. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Keerthy D, Youk A, Srinath AI, et al. Effect of psychotherapy on healthcare utilization in children with inflammatory bowel disease and depression. J Pediatr Gastroenterol Nutr. 2016;63:658–64. doi: 10.1097/MPG.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long MD, Barnes EL, Herfarth HH, et al. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2012;18:869–76. doi: 10.1002/ibd.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–9. doi: 10.1111/j.1572-0241.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Ananthakrishnan AN, Kwon J, Raffals L, et al. Variation in treatment of patients with inflammatory bowel diseases at major referral centers in the United States. Clin Gastroenterol Hepatol. 2015;13:1197–200. doi: 10.1016/j.cgh.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]