Abstract
Promising results in adult neurologic and psychiatric disorders are driving active research into transcranial brain stimulation techniques, particularly transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), in childhood and adolescent syndromes. TMS has realistic utility as an experimental tool tested in a range of pediatric neuropathologies such as perinatal stroke, depression, Tourette syndrome, and autism spectrum disorder (ASD). tDCS has also been tested as a treatment for a number of pediatric neurologic conditions, including ASD, attention-deficit/hyperactivity disorder, epilepsy, and cerebral palsy. Here, we complement recent reviews with an update of published TMS and tDCS results in children, and discuss developmental neuroscience considerations that should inform pediatric transcranial stimulation.
Keywords: Transcranial stimulation in children, TMS, tDCS, GABA, Cortical inhibition, Cortical excitability
Introduction
Transcranial brain stimulation, at present primarily with several forms of noninvasive electrical cortical stimulation, is under active investigation in child neurology and psychiatry, particularly in disorders where focal cortical over- or under-activation is presumed to be part of the pathophysiology [1, 2, 3•, 4•] While a number of transcranial neurostimulation techniques have been developed, two are undergoing the most active investigation: transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). In TMS, intracranial electrical currents are induced in the cortex by a fluctuating extracranial magnetic field, whereas in tDCS constant electrical currents are conducted to the brain via scalp electrodes. Both techniques share a capacity to modulate regional cortical excitability, and both are well-tolerated by children and adults [3•, 4•].
TMS in particular stands out among noninvasive brain stimulation techniques in that it has experimental, diagnostic, and therapeutic potential. With TMS, an operator may either measure or modulate cortical excitability. This is underscored by clearances from the US Food and Drug Administration (FDA) for TMS devices for preneurosurgical functional motor and language mapping, and for the treatment of major depression and migraine [5–7]. A rapidly growing body of research attests to the utility of TMS as a valuable tool for the study of normal neurophysiology, and to the safety and efficacy of TMS in clinical conditions where repetitive TMS (rTMS) is applied to either enhance or depress regional cortical excitability and distributed activity in specific brain networks [1, 3•, 8–11].
tDCS is also undergoing investigation as a plausible therapy for a range of neuropsychiatric disorders [4•]. However, to date, the FDA has not approved any devices for tDCS in the setting of any disorder. Interest in brain stimulation by direct current (DC) subsided after an initial spike following experiments in the early 1960s, which showed the polarity dependent DC-mediated modulation of cortical neuronal activity in animal experiments [12], but was rekindled at the beginning of the 21st century with a fairly rapid expansion of tDCS in humans [4•, 13]. Now, active tDCS research is driven in part by a very favorable tDCS safety profile [14••], the low cost of tDCS stimulators, and by fairly reproducible effects on the cortex, where (coarse) exposure to cathodal current leads to cortical suppression and exposure to anodal current leads to cortical activation [15].
Promising results in adult neurologic and psychiatric disorders have elicited interest in TMS and tDCS in childhood and adolescent syndromes, and a number of thorough recent reviews summarize the applications of these techniques in pediatric patients [1, 3•, 4•, 14••, 16, 17]. Yet, most clinical TMS and tDCS studies focus on adult populations, and extensive research into the clinical utility of TMS and tDCS in pediatrics remains an unmet needed. As relevant to the present review, the child’s brain is a unique physiological entity, and not merely a small adult brain. We therefore take this opportunity to complement recent reviews with an update of published TMS and tDCS results in children, and to discuss developmental neuroscience considerations that should inform pediatric transcranial stimulation.
TMS Investigational and Diagnostic Utility
TMS is unique among the neurostimulation methods in its roles as a therapeutic intervention as well as an experimental and diagnostic tool. Three TMS protocols have been used extensively to study, measure, and modulate cortical excitability as follows:
Single Pulse TMS
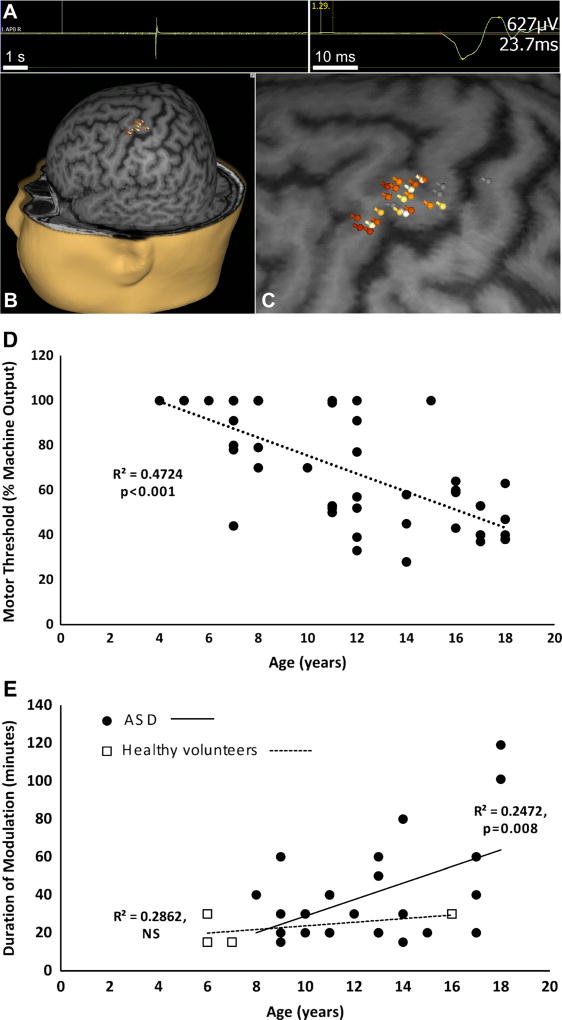
In single-pulse TMS (spTMS), the cortex is stimulated once to elicit an evoked response. In its most common embodiment, spTMS is delivered to the motor cortex to elicit a motor evoked potential (MEP) in a contralateral limb muscle, recorded via surface EMG. spTMS is thus gaining acceptance in preneurosurgical motor cortex mapping, and the Nexstim eXimia device (Nexstim Inc., Chicago IL) has been cleared by the FDA for this indication. During such functional mapping, the TMS operator, guided by a patient’s brain MRI and frameless stereotaxy, can test whether stimulation of a specified brain region evokes an MEP from a specific muscle group. These data are then registered to the patient’s MRI to generate a precise motor map (Fig. 1A–C) where the spatial resolution approximates that which can be obtained by intraoperative monitoring of MEPs [9, 18].
Fig. 1. Investigational and diagnostic utility of transcranial magnetic stimulation.
A–C, representative hand motor map in a child. A, abductor pollicis brevis (APB) MEP obtained by motor cortex single-pulse TMS. B, multiple MEPs are color-coded by amplitude, with white corresponding to maximum and gray corresponding to minimum, and projected onto the patient’s brain MRI. C, enlarged motor map shows APB localizing to the “hand knob” region of the precentral gyrus (unpublished data). D, correlation of age to resting motor threshold. Pearson correlation test shows significant negative correlation between rMT (in hemisphere contralateral to seizure focus) and age in children (N = 48) with epilepsy [148]. E, modulatory effect of continuous theta burst stimulation in ASD. Corticospinal excitability was assessed every 5–10 minutes to determine the duration of cTBS induced modulation in children with ASD (N = 27) and healthy controls (N = 4). Pearson correlation test in the ASD group shows significant positive correlation between age and duration of modulation. Only 4 healthy control participants have been assessed thus far, but there is currently no age-related correlation in this group (unpublished data)
Motor spTMS has also been utilized to study developmental corticospinal physiology, and also neurophysiological abnormalities in children with neuropsychiatric disease. Such physiologic insights can be obtained from several metrics derived from the MEP, such as its duration, amplitude, latency, and threshold to activation [1]. In early childhood corticospinal tract development, for instance, the stimulus intensity required to generate the MEP (motor threshold; MT) increases for the first 90 days after birth, stabilizes until about age 12 months, and then decreases throughout childhood to adult levels by age 16–18 years [1, 3•, 19, 20]. Data from our laboratory corroborate these earlier published studies, showing a significant negative correlation of age to resting MT in children with epilepsy (Fig. 1D).
Maturation of corticospinal tract lateralization has been extensively studied using spTMS. Stimulation often triggers bilateral MEPs in neonates, with shorter ipsilateral than contralateral latency, implying direct ipsilateral projections. Ipsilateral MEPs progressively decrease in amplitude with age, with increasing MT and MEP latency compared with contralateral, indicating progressive suppression or loss of direct uncrossed corticospinal projections [1, 3•, 19, 20]. In contrast, contralateral latency progressively decreases during childhood and early adolescence (this can be detected if the protocol is adjusted for height) [19, 21–26]. Even though ipsilateral projections persist in some adults, short latency ipsilateral responses do not occur in normal subjects past infancy [19, 20].
In contrast to the above-described normal corticospinal maturation patterns, early life brain injury can result in preservation of ipsilateral cortico-motor projections [19, 20, 27]. Thus, spTMS studies also provide insights into neural reorganization after unilateral brain injury and identify distinct recovery patterns, depending on age at the time of injury. Ipsilateral and contralateral tracts arising from the undamaged hemisphere persist following unilateral motor cortex or white matter injury early in development, eventually contributing to bilateral corticospinal connectivity in the contra-lesional hemisphere [1, 28]. Yet, such scenarios occur largely with perinatal injury. In 3 older patients (>2 years) with acquired hemiplegia caused by injury after the second year of life, spTMS applied to either hemisphere failed to elicit MEPs in the affected limb, suggesting absence of any healthy motor tracts in the lesioned hemisphere and also absence of compensatory preservation of ipsilateral motor connections in the undamaged hemisphere [29].
spTMS has also identified impaired facilitation of motor cortex excitability in adults with autism spectrum disorder (ASD) [30], has been used to detect corticospinal tract abnormalities not identified by imaging studies in patients with adrenoleukodystrophy and transverse myelitis [31, 32], and has shown promise as a diagnostic and prognostic tool in adolescents and adults with multiple sclerosis [33–35].
In separate applications, spTMS has allowed the study of interhemispheric inhibition in patients with attention-deficit/ hyperactivity disorder (ADHD) by measuring ipsilateral silent period (iSP, an arrest in activated EMG signal in a muscle ipsilateral to the stimulated hemisphere resultant from activation of transcallosal inhibitory circuits by motor cortex spTMS) metrics. iSP duration is shorter in children with ADHD, suggesting compromised inhibitory signaling, while iSP latency is prolonged [36], which may be due to compromised maturation of anterior callosal fibers [37–39], and correlates with more severe behavioral symptoms, particularly hyperactivity, and with worse motor scores [40]. Notably, in a similar cohort of children with ADHD treated with methylphenidate, increased iSP duration indicating improved cerebral inhibition correlated with clinical improvement of ADHD symptoms [41].
Paired-Pulse TMS
Paired-pulse TMS (ppTMS) paradigms provide measures of intracortical inhibition and facilitation, thought to be mediated by gamma-aminobutyric acid (GABA)-ergic and glutamatergic activity, respectively. In ppTMS, a “conditioning” TMS pulse is delivered before a succeeding “test” pulse, and limb muscle MEP responses for each stimulus are recorded. Condition and test pulses vary in intensity depending on the ppTMS paradigm. Short, 1–5 ms interstimulus intervals (ISI) with a subthreshold conditioning stimulus lead to a reduction of the MEP induced by the suprathreshold test pulse, and reflect intrahemispheric GABA-mediated short-interval intracortical inhibition (SICI). Longer ISIs (6–20 ms) with a subthreshold conditioning stimulus lead to facilitation of the MEP induced by the suprathreshold test stimulus, reflecting glutamatergic intracortical facilitation (ICF). ISIs of 50–300 ms with both stimuli of suprathreshold intensity measure long-interval intracortical inhibition (LICI), which is also mediated by GAB Aergic inhibition, although it may have a greater contribution from GABAB receptors than SICI [42, 43].
ppTMS measures may have a particularly valuable role in pediatric epilepsy. A decrease in LICI and SICI is seen in the early morning in some patients with generalized but not focal epilepsy [44], whereas SICI is reduced and ICF is increased in patients with generalized epilepsy caused by a GABAA receptor mutation [45]. A facilitative shift in the excitation-inhibition ratio (E:I) occurs in the pre-ictal period in epilepsy, and thus TMS may allow the identification of epochs of seizure vulnerability and prediction of timing and likelihood of seizures [44, 46]. ppTMS may also be used to track whether the brain’s E:I balance has shifted in a favorable direction with treatment (predicting seizure control) [47], addressing an important unmet need for a biomarker to guide dosing or for the identification of a therapeutic effect in advance of a clinical change, as in after vagus nerve stimulator (VNS) activation [48] or after initiation of the ketogenic diet [49].
In children with ADHD, SICI (ISI: 10 ms) in the dominant primary motor cortex may correlate with disease severity. SICI was reduced by 40% in 49 children with ADHD, correlating with symptom severity [50], and with prolonged iSP latency [40]. Both these metrics imply that children with ADHD have deficient inhibitory control.
Combining SICI and iSP measures with functional and behavioral studies may help in characterizing long-term prognostic profiles, and in identifying and developing more effective treatments. ppTMS studies have also demonstrated differences between ASD subgroups, such as deficits in SICI in those with early language delay compared with ASD patients without delay [51], and reduced cortical inhibition in high functioning autism compared with Asperger disorder [52].
ppTMS metrics thus provide neurophysiological biomarkers with plausible utility in managing diseases that lack definitive diagnostic and prognostic tools such as ADHD, ASD, and epilepsy, where clinicians currently must rely on subjective and complex clinical outcome measures.
Repetitive TMS
Whereas the physiologic effects of single and paired TMS pulses last on the scale of milliseconds, repetitive TMS (rTMS) modulates cortical excitability on the scale of minutes to hours, and has clinical effects that can last weeks to months. The magnitude and direction of change in cortical function following rTMS depends on the stimulation protocol, and the number and frequency of rTMS sessions. rTMS thus has potential roles as both a therapeutic intervention and an aide to measure cortical plasticity, which are discussed below.
Continuous and intermittent theta burst stimulation (cTBS, iTBS), specialized rTMS protocols, are often used to investigate and measure rTMS-mediated cortical plasticity [53, 54]. TBS involves the application of very high frequency rTMS (50 Hz) triplets, spaced 200 ms apart, either continuously for a total of 40 seconds (cTBS) or once every 8 seconds (iTBS) for about 3 minutes. The rationale for use of TBS protocols is in part the convenience of deploying a short stimulation train (in contrast to high-frequency and low-frequency rTMS trains that may take as long as 30 minutes) without any appreciable differences in the rate and severity of adverse events compared with single-pulse and paired-pulse TMS [55•], and in part their resemblance to in vitro stimulation protocols from which much insight into cortical plasticity mechanisms is derived [56].
In most normal subjects, iTBS and cTBS cause lasting facilitation or depression of motor cortical excitability, respectively [17]. When applied over the motor cortex of adults with ASD, both iTBS and cTBS provide a measure of synaptic plasticity that distinguishes ASD and control groups, with the ASD group experiencing more durable modulation of the motor response, possibly reflecting hyperplasticity in these individuals [57]. In 11- to 18-year-old children with ASD, while iTBS delivered to the dominant motor cortex produced an overall increase in excitability lasting for 30 minutes in both the ASD and control groups, MEP amplitude in patients with ASD was significantly lower 20 minutes after iTBS compared with controls, indicative, as in adults, of altered (although not necessarily hyperplastic) cortical synaptic plasticity in children with ASD [58••]. A complementary study from our laboratory indicates that the duration of cTBS-induced modulation increased with age in children (ages 9–18 years) with ASD, though whether such maturation occurs in neurotypical subjects is not known (Fig. 1E) [59••].
A related TMS protocol, paired associated stimulation (PAS), has also been used to study synaptic plasticity, and has been demonstrated to be safe and reproducible in pediatric subjects [17, 60]. In PAS, pairs of stimuli are delivered to the median nerve while simultaneously applying repeated single-pulse TMS to the primary motor cortex, such that the afferent signal from the median nerve arrives at the motor cortex at the same time as the TMS pulse is applied. The protocol produces a facilitation in cortical excitability in neurotypical subjects for up to an hour after stimulation [17] but does not increase MEP amplitudes in patients with high functioning autism and Asperger disorder [61••].
In normal brains, cTBS, iTBS, and PAS are believed to induce long-term potentiation (LTP)-like or long-term depression (LTD)-like plasticity [56, 62]. which the above-mentioned reports suggest is impaired in ASD. However, substantial variability in TMS-derived measures of plasticity has been reported even in healthy controls, and may be further confounded by the heterogeneity underlying ASD pathophysiology [58••]. Nevertheless, with additional research and adjustments, TMS protocols as a whole may help differentiate between neurotypical and ASD cohorts as well as distinguish between ASD subtypes [30, 51, 52, 57, 58••, 63].
TMS Therapeutic Potential
Cortical excitability may be suppressed or facilitated long-term by the application of trains of TMS pulses, as rTMS. The size and direction of the resultant effect can be controlled by altering the frequency, intensity, and location of stimulation, and the number and frequency of sessions [17]. Depending on the protocol, rTMS may alter cortical excitability, likely by both modulating regional GABAergic activity and by the induction of LTP-like or LTD-like changes in excitatory synaptic strength [17, 64–67]. In general, high frequency repetitive TMS (HF-rTMS, 5–20 Hz) induces to cortical facilitation, whereas low frequency rTMS (LF-rTMS, ≤1 Hz) reduces cortical excitability by mechanisms that are only partially understood but likely mirror those of use-dependent changes in synaptic strength that follow electrical repetitive cortical stimulation [68, 69].
In a preliminary trial investigating the use of low frequency rTMS (LF-rTMS) in chronic hemiparesis after subcortical pediatric ischemic stroke, 1 Hz rTMS at 100% resting motor threshold (rMT) for 20 minutes over contralesional motor cortex was demonstrated to be safe and well tolerated in 10 children. LF-rTMS treatment also resulted in improvements in grip strength in treated patients, which persisted 1 week beyond the intervention in some participants [28]. A larger clinical trial of 45 children with perinatal stroke showed that LF-rTMS (1 Hz, 90% rMT for 20 minutes) over contralesional motor cortex constraint induced movement therapy (CIMT), doubled the chances of clinically significant motor improvement in this cohort. Functional motor assessment 6 months after treatment indicated that the therapeutic effects were additive and greatest when LF-rTMS was coupled with CIMT [70••].
In adult major depression, high frequency rTMS (HF-rTMS) delivered to the region of the dominant dorsolateral prefrontal cortex (DLPFC) is presently an established therapeutic intervention, although precise mechanisms of action are not well understood [3•]. Trials in children are limited, despite evidence that younger adults with depression respond better to rTMS [71, 72]. One early study with 9 patients identified several adverse events after 10 Hz HF-rTMS at 80% rMT for 20 minutes over the left DLPFC over a period of 2 weeks, with 1 patient stopping treatment early due to anxiety and mood lability, another developing hypomanic symptoms, and a third attempting suicide after treatment [73]. A later study of 30 sessions of 10 Hz HF-rTMS at 120% rMT applied to the DLPFC in subjects taking selective serotonin reuptake inhibitors reported significant improvement from baseline, which persisted at follow-up 6 months later, with no cognitive decline in function compared with baseline [74]. Another recent report on neurocognitive outcomes in adolescents treated with 30 sessions of open-label DLPFC 10 Hz HF-rTMS at 120% rMT found a significant decrease in the severity of depression and improved memory and verbal recall, and no clinically meaningful changes in cognitive function were reported by patients [75•]. rTMS to the left DLPFC in children (10 Hz, 120% rMT, 15 sessions) has also been shown to increase regional glutamate (Glu) levels by 11% from baseline on MRS in 4 out of 6 patients with depression, corresponding to improved symptom severity [76].
TMS may also provide a novel therapy for other common developmental neuropsychiatric disorders. For instance, investigators applying 1 Hz LF-rTMS (110% rMT, 20 sessions) to the supplemental motor area in 25 patients under 16 years of age with Tourette syndrome observed significant reductions in symptoms 4 weeks after treatment, which correlated with a bilateral increase in resting motor for at least 6 months after treatment [77•].
Numerous studies have also targeted a range of brain regions and symptoms with both high and low frequency rTMS protocols in ASD. Sub-threshold LF-rTMS (0.5–1 Hz, 90% rMT, 6 sessions), suppresses cortical excitability in ASD when applied to the left (or sequential bilateral) DLPFC, and corresponds to improvements in Repetitive Behavior Scale – Revised (RBS-R), Aberrant Behavior Checklist (ABC) scores and autonomic measures [17]. One open-label trial with waitlist controls (control group assignment was not random and was in part dependent on patient preferences and availability) reported that LF-rTMS over the DLPFC bilaterally (1.0 Hz, 90% rMT, 6 sessions each side) integrated with neurofeedback (NFB) enhanced performance on the 3-stimuli oddball task with kanizsa figures and significantly improved EEG power in the gamma frequency band (psychophysiological biomarker of attention and executive function in humans). Treatment significantly reduced repetitive and compulsive behavior as measured by the RBS-R, and scores on the lethargy/social withdrawal and hyperactivity subscales of the ABC. rTMS-NFB also improved event-related potential (ERP) indices in patients with ASD [78]. However, HF-rTMS has also been used to increase excitability in inhibited cortical regions in children with ASD with intellectual disability. Investigators have reported significant improvement in eye-hand coordination, especially when behavioral training was paired with HF-rTMS (8 Hz, 90% rMT, 30 trains of 3.6 seconds, 3–10 sessions) applied to premotor cortex [79]. While these trials support plausible efficacy of rTMS in the relief of some ASD symptoms in children and adolescents, they are susceptible to placebo effects owing to their open-label nature. Large, randomized controlled clinical trials are required to translate these early findings to clinical practice.
tDCS Therapeutic Potential
tDCS has been tested as a treatment for a number of pediatric neurologic conditions [4•]. In a randomized crossover trial in 20 children with ASD, 1 mA anodal tDCS for 20 minutes over the left DLPFC (F3 on a 10–20 montage) increased peak alpha frequency (an EEG measure correlated to cognitive performance) and significantly improved scores on the Autism Treatment Evaluation Checklist (ATEC), with the results showing a positive correlation between the two [80]. In another crossover trial with 20 children showed significant improvements in both the Childhood Autism Rating Scale (CARS) and the ATEC with 20 minutes of left frontal 1 mA anodal tDCS [81]; 2 mA anodal stimulation for 30 minutes over the left DLPFC corresponded to improved syntax acquisition using the Bilingual Aphasia Test in 10 minimally verbal autistic children [82]. Moreover, 28 sessions of 1 mA tDCS for 20 minutes with the anode over F3 and cathode over F4 resulted in a month-long improvement in symptoms in a patient with ASD and drug-refractory catatonia [83].
As rTMS, tDCS has been investigated in the management of ADHD. One randomized crossover trial in 20 adolescents with ADHD who received 1.5 mA anodal, cathodal, and sham tDCS for 8 minutes over the left DLPFC in 3 separate sessions 72 hours apart found that anodal stimulation increased the accuracy of Go responses in a Go-No-Go task. Interestingly, cathodal stimulation improved No-Go accuracy compared with anodal and sham tDCS, indicating improved inhibitory control [84]. Another open-label study of 9 children with ADHD showed an improvement in selective attention and a decrease in errors in the inhibitory control task with 2 mA anodal tDCS over the left DLPFC [85•]. A third study in 14 children with ADHD using slow oscillating tDCS applied for 5 sessions of 5 minutes each separated by 1 minute, with anodal electrodes at F3 and F4 (current strength, 0–250 µA; oscillating frequency, 0.75 Hz) showed a shorter reaction time on a Go-No-Go task the morning after stimulation at non-REM sleep stage 2, whereas there was no difference in alertness between groups [86].
tDCS has also been used as an experimental treatment in childhood epilepsy, where the rationale for use is supported by preclinical animal studies [87, 88]. In one early case series, 18 young children, 13 with cerebral palsy and 5 with organic brain lesions, who received 0.3–0.7 mA tDCS with the anode over the posterior temporal cortex and the cathode over parietal cortex, for 20–40 minute sessions with a maximum of 15 sessions, showed reductions in seizure frequency after application [89]. One mA cathodal tDCS over the seizure focus for 20 minutes also resulted in a significant reduction in epileptiform discharges up to 2 days after application in 36 children with refractory focal epilepsy [90•], and 2 mA cathodal tDCS over the epileptic focus, 5 days a week for 2 weeks in a child with epilepsy was associated with a reduction in epileptiform discharges after stimulation [91]. Another case report of 2 children with refractory epilepsy showed a reduction of interictal epileptiform discharges after three 30-minute sessions of 1 mA cathodal tDCS over C5–C6 during slow-wave sleep [92].
Several randomized, controlled studies also report a beneficial effect of tDCS in patients with cerebral palsy. Single or multiple 20–50 minute sessions of 0.2–1 mA anodal tDCS over the primary motor cortex over the more affected hemisphere, either in combination with standard training or as a mono-therapeutic approach, seems to provoke an improvement in proprioception, mobility, body sway and balance, gait distance and velocity, and spasticity ranging from weeks to months [93–98].
Data on the efficacy and safety of tDCS in children, as with most data on pediatric therapeutic interventions, are sparse and employ heterogeneous stimulation protocols. Furthermore, there is a paucity of strictly conducted randomized, sham controlled clinical trials and a preponderance of open label use and case reports with relatively short follow-up periods, which makes it difficult to use these results to inform clinical practice.
Preclinical Insights into the E:I Ratio in Development
As summarized above and in recent reviews, clinical and investigational TMS and tDCS roles in humans are encouraging but underscore incomplete efficacy in many disease states [1, 3•, 4•]. Notably, the synaptic and molecular mechanisms of neuronal excitability and plasticity upon which the therapeutic TMS and tDCS effects rely are impractical to study in humans and, thus, are derived from preclinical animal model research [99–105]. For instance, given that seizures are a realistic, if improbable, potential side effect of transcranial stimulation (particularly rTMS) [106], and the immature brain’s vulnerability to seizures as indicated by experiments in rodent epilepsy models [102], insights into the maturation of the E:I balance in the developing brain may inform investigators whether and how to adjust adult neuromodulation protocols to trials in children.
Neuronal activity is critical for normal central nervous system development in utero and, thus, neuronal circuits are hyper-excitable in early life [103]. Yet, the hyperexcitable state is not compatible with mature brain function, and the E:I balance shifts toward progressively lower excitability with age. These shifts are reflected in well-studied maturation of glutamate and GABA biology as discussed below.
Glutamate released from presynaptic terminal acts on both ionotropic—N-methyl-D-aspartate receptors (NMDARs) and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs)—and metabotropic receptors (mGLuRs) on post-synaptic terminals to trigger intracellular signaling cascades.
Ionotropic receptors consist of distinct subunits, and the relative abundance of individual subunits within the receptor, which varies with age, has important functional consequences [101]. NMDARs are heterotetramers, usually consisting of an NR1 subunit and combinations of NR2 and NR3 subunit isoforms, and are a major source of calcium influx when activated by the glutamate ligand. NMDAR-mediated intracellular signaling cascades are critical for normal synaptic remodeling and likely for LTP-like and LTD-like rTMS and tDCS effects [107–109]. However, excessive NMDAR signaling can also contribute to glutamate excitotoxicity and to pathological processes, such as epileptogenesis, which result from excess glutamate-mediated neuronal activation [102].
NMDARs in the immature rodent brain contain predominantly NR2B subunits, compared with NR2A-predominant NMDARs in mature brains. Activated NR2B–NMDARs have prolonged synaptic current decay times [104, 110] leading to greater calcium influx per action potential. Other NR subunit isoforms highly expressed in the first 2 weeks after birth in rodents (human age: birth to 2 years [111])—NR2C, NR2D, and NR3A—reduce the voltage-dependent magnesium block of NMDA response normally seen in the adult rodent brain, and also contribute to a relatively increased excitability [112].
AMPARs are also heterotetrameric complexes of combinations of GluR1–4 subunits. The presence of GluR2 makes an AMPAR impermeable to calcium, and the subunit is present in most AMPARs in adult rodent brains. However, GluR2 is not well-expressed in the developing rodent brain up to the third postnatal week (human age: 2–3 years) leading to increased calcium influx via activated AMPARs [104].
Thus, the subunit composition of ionotropic receptors together with increased synaptic glutamate concentrations due to immature removal mechanisms in the developing rat brain renders it hyper-excitable. While this may be vital for normal developmental plasticity and synaptogenesis, it also allows extra-synaptic receptor activation and synaptic crosstalk, and results in lower thresholds for seizures and injury [101, 102, 104, 113, 114] and may, therefore, be a consideration during brain stimulation.
The maturational trajectory of the G-protein-coupled metabotropic glutamate receptors that modulate excitability and synaptic transmission via intracellular second messenger systems is less well studied. The abundance of presynaptic mGluR1α receptors in developing rat brains up to the ninth day after birth (human age: term infant), particularly on hippocampal interneurons [115], may facilitate presynaptic release of GABA, and may explain the resistance of the developing brain to seizure-induced damage in spite of increased susceptibility to seizures. mGluR2, mGluR3, and mGluR5 mature by 15 days after birth in rodents (human age: 0–24 months) [115]. Recent evidence indicates that cathodal tDCS-induced LTD is primarily mediated by mGluR5 receptors [116] and, thus, direct current stimulation protocols may not be less, if at all, effective during the first 2 years after birth in humans.
In addition to increased glutamate sensitivity, glutamate uptake from the synaptic cleft into astrocytes via glutamate transporter 1 (GLT-1; called excitatory amino acid transporter 2, EAAT-2 in humans) is also impaired in developing brains. GLT-1 is highly expressed and provides 95% of the total glutamate clearance capacity in the adult mammalian brain, providing the major glutamate removal mechanism [117, 118]. Astrocytic GLT-1 expression and glutamate uptake, however, do not reach adult levels in the rat neocortex and hippocampus till the 30th postnatal day (human age: 4–11 years) [113, 119]. Thus, glutamate may accumulate in the synaptic cleft in the immature brain and contribute to either compromised signaling or to neuronal injury.
The effectiveness of transcranial stimulation in adults also depends on the modulation of GABAergic inhibitory signaling [56, 120]. The powerful inhibitory mechanisms normally found in the adult brain are compromised at younger ages. Glutamic acid decarboxylase (GAD, the enzyme responsible for GABA synthesis) and GABA receptor (GABAR) expression reach adult levels only by the end of the first month after birth in rats (human age: 4–11 years) [102, 104, 121]. While the GABAA receptor (GABAAR), a transmembrane protein consisting of five subunits which determine its functional properties, is embryonically expressed, GABA signaling undergoes major developmental changes. In adults, GABAR activation causes chloride ions to move down their concentration gradient into the neuron, resulting in hyperpolarization and suppression of excitability. This gradient is established and maintained by KCC2, a neuron-specific transporter that utilizes the potassium ion gradient generated by the Na+/ K+ ATP pump to export chloride ions, keeping intracellular concentrations low. In the first week after birth in rodents (human age: 36–40 week gestation), however, increased expression of the chloride importer NKCC1 and decreased expression of KCC2 relative to the adult rat brain reverses chloride gradients [122–124], and thus GABAAR activation causes a net efflux of chloride from the neuron through the receptor paradoxically facilitating excitation [102–104, 125]. Increasing expression of neuronal KCC2 over the first 21 postnatal days in rodents (human age: 0–4 years) gradually establishes adult chloride concentration gradients and correlates with the gradual increase in GABAergic inhibitory tone. GABAARs in the developing brain also have lower quantities of the α1 subunit compared with adults, and exhibit diminished benzodiazepine (BZD) sensitivity and slower kinetics [103, 104].
It is difficult to predict which neuromodulation protocols will be most impacted by the maturational trajectory of GABAergic inhibitory mechanisms, and those that rely on altered cortical GABAergic inhibition (such as TBS) may not work as predicted in the very immature brain [66, 126]. As such, GABA biology is an important consideration when stimulating the developing nervous system.
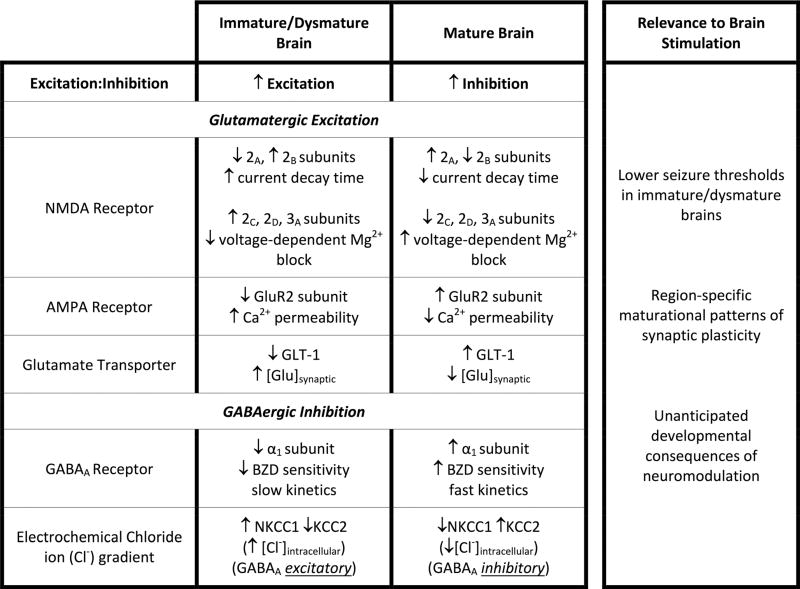
Given increased glutamate sensitivity, reduced glutamate clearance, and incomplete GABA-mediated inhibition in the developing brain, it follows that immature neuronal circuits are more prone to seizures and injury (Table 1), which is consistent with clinical observations in children. Yet, not all basic research indicates that brain stimulation poses heightened risks to children. Young rodents are also less vulnerable to neuronal damage after seizures compared with adults. Kainate exposure in adult rodents, for example, causes hippocampal and limbic neuronal death and results in new recurrent excitatory circuits between surviving neurons [127, 128], while developing brains do not show cell death after kainate-induced seizures [129, 130] regardless of kainate dose. The absence of well-developed hippocampal mossy fiber terminals in immature brains that amplify excitatory signaling and help sustain epileptic seizures may be one plausible explanation for this protection [131], which is seen in multiple animal models. Recurrent seizures in the developing brain may still, however, disrupt vital neurodevelopmental milestones and result in deranged networks, leading to long-term cognitive and behavioral changes, and lower seizure thresholds in adulthood [131].
Table 1.
Distinctions in excitatory and inhibitory mechanisms between immature and mature brains
BZD benzodiazepine; [Glu]synaptic synaptic glutamate concentration; [Cl−]intracellular intracellular chloride concentration
Adapted with permission from Hameed M.Q., Sanchez M.J., Gersner R., Rotenberg A. Insights into pediatric brain stimulation protocols from preclinical research. In: Kirton A., Gilbert D.L., Eds. Pediatric Brain Stimulation. Academic Press: 2016:117–130
In summary, implications of E:I maturation and the immature brain’s response to stimulation should be borne in mind while designing pediatric transcranial stimulation protocols, particularly in pediatric rTMS as seizures-a plausible, if unlikely, side effect [106]-may have distinct effects on the immature brain. In addition, given developmental changes in brain function, test-retest reliability and assessment of physiologic effects to properly interpret behavioral or therapeutic effects is important.
Another important consideration is that immature neurobiology in humans may be either chronological or pathological due to failure of appropriate maturation, with the brain being dysmature into adulthood as seen in a range of prevalent neurologic disorders. For example, mutations resulting in GABAARs deficient in α1 subunits (as in the immature brain) are seen in some patients with Dravet syndrome lacking the typical sodium channel mutation [132, 133]. GABAAR α1 subunit expression is also decreased in cortical tubers removed from human tuberous sclerosis complex (TSC) patients, which may account for the benzodiazepine insensitivity reported in these patients. Tubers also exhibit lower levels of KCC2 and higher NKCC1 expression, resulting in immature excitatory GABAAR responses [134]. Similarly, a dysmature NKCC1:KCC2 ratio is seen in early life in a mouse Fragile X Syndrome (FXS) model [135, 136]. Cortical and cerebellar α1 expression is also reduced in ASD, schizophrenia, and major depressive disorder [137].
Dysmature GABAergic signaling is thus found in a range of neuropsychiatric pathology. As stimulation protocols are developed across ranges of disease states, including those that affect primarily young patients, such immature physiology should also be considered in the design of therapeutic neuromodulation protocols for adults with these conditions.
Synaptic Plasticity in the Developing Brain
An assumption made while designing transcranial stimulation protocols is that the neurobiologic mechanisms underlying the desired effects are similar to those responsible for the innate capacity to register and store experience as memories or changes in specific neuronal functions. Such neuroplasticity is believed to depend on LTP and LTD of synaptic strength. Thus, investigators and clinicians in the neuromodulation field should consider insights into the neurodevelopmental regulation of synaptic plasticity gained from animals during the design of pediatric protocols.
Use-dependent enhancement of excitatory synaptic strength (LTP), as well as the mechanistically inverse LTD, can be reliably reproduced in isolated rodent brain slices [138–140]. While LTP-like potentiation and LTD-like depression of human corticospinal responses can now be reliably produced by transcranial stimulation protocols [10, 141], the developmental timeline of these phenomena has not been extensively studied across the range of pediatric ages in humans, and translational research can offer valuable insights here as well, although extrapolating clinical relevance from animal LTP/LTD studies is challenging.
Maturation of synaptic plasticity follows multiple, distinct, region-specific trajectories in the rodent brain. LTP is present at birth in rat barrel cortex, reaches maximal levels between postnatal days 3 and 5 (human age: 32–36 weeks’ gestation), and is undetectable by 2 weeks of age (human age: 0–24 months) [99]. On the other hand, isolated rat hippocampal slices first demonstrate LTP in response to TBS 12 days after birth (0–24 months in humans), and the response improves with age up to the 35th postnatal day (human age: 11 years) [100]. The visual cortex’s capacity for LTP is greater in adult mice than at 4–5 weeks of life (human age: 4–11 years) [142]. In contrast, LTD magnitude is enhanced in developing rats compared with adults. Maximal in rat brains less than 14 days after birth (human age: <2 years), hippocampal LTD progressively declines and reaches adult levels by the postnatal day 35 (human age: 4–11 years) [143, 144]. In addition, younger rodents have a lower threshold for LTD induction [143].
Experiments designed to investigate differences between immature and mature brain physiology in vivo are sparse. However, one study shows that iTBS causes functional modification of cortical GABAergic parvalbumin positive (PV+) interneurons in rats only after 32 days of life (human age: 4–11 years), reaching a maximal effect by postnatal day 40 (human age: 12–18 years). This may be because immature PV+ cells have not developed enough synaptic connections to receive the level of excitatory input required to be affected by iTBS [105].
Whether such region-specific maturational trajectories of plasticity are present in human brains is unknown, and could potentially be tested with TMS and tDCS. For instance, altered plasticity is reported in human FXS patients in a recently published study from our laboratory, where a single application of cTBS failed to modulate MEP amplitude in FXS but a second cTBS application, 24 hours after the first, caused paradoxical facilitation of excitability [145]. These data, along with classic reports of critical periods of cortical plasticity [146, 147], demonstrate that modulation of cortical excitability in immature and dysmature brains may require stimulation protocols that are distinct from those used for healthy adult subjects.
Conclusions
Noninvasive transcranial stimulation is an intensive area of ongoing basic science and translational research in pediatric neurology aimed at identifying new therapeutic options for a variety of suboptimally treated neurologic diseases in children. The authors hope that the data discussed above provide insights that merit consideration in pediatric brain stimulation. Further research is required to investigate the effects of age-related differences in basic neurologic mechanisms on the safety and efficacy of brain stimulation in the pediatric brain. However, although the characteristics of the child’s brain pose challenges to the design and execution of transcranial stimulation protocols, the unique opportunity these modalities offer for studying and modulating pediatric neuropathology and neuroplasticity is unmatched.
Acknowledgments
This work was supported by NIMH R01100186 (Alexander Rotenberg, Alvaro Pascual-Leone, Lindsay M. Oberman) and grants from the Boston Children’s Hospital Translational Research Program (Alexander Rotenberg). Alexander Rotenberg receives support form NIH NINDS R01NS088583, The Assimon Family Foundation, Sage Pharmaceuticals, Eisai Pharmaceuticals, Massachusetts Life Sciences, Neuroelectrics, and Brainsway. Alvaro Pascual-Leone was supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R21 NS082870, R01HD069776, R01NS073601, R21 MH099196, R21 NS085491, R21 HD07616), Harvard Catalyst and the Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758).
Alvaro Pascual-Leone has consulted for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Magstim, Neosync and Axilum Robotics, and is a co-inventor of a patent for real-time integration of TMS, EEG and MRI.
Alexander Rotenberg is a co-founder and consults for Neuro’motion Inc., consults for NeuroRex Inc., and a co-inventor of a patent for realtime integration TMS and EEG. He also receives or has received research funding from Sage Pharmaceuticals, Eisai Pharmaceuticals, Neuropace, and Brainsway.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Mustafa Q. Hameed, Sameer C. Dhamne, Roman Gersner, Harper L. Kaye, and Lindsay M. Oberman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent In figure 1, unpublished preliminary human data is used to illustrate important points in the text. Data collection is being performed under a study protocol approved by the IRB at Boston Children’s Hospital (IRB-P00020115). Informed consent was obtained from all subjects. We cite multiple other published papers by the authors, but do not report any new animal or human findings here.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Frye RE, Rotenberg A, Ousley M, Pascual-Leone A. Transcranial magnetic stimulation in child neurology: current and future directions. J Child Neurol. 2008;23(1):79–96. doi: 10.1177/0883073807307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziemann U, Paulus W, Nitsche MA, et al. Consensus: motor cortex plasticity protocols. Brain Stimul. 2008;1(3):164–82. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3•.Rajapakse T, Kirton A. Noninvasive brain Stimulation in children: applications and future directions. Transl Neurosci. 2013;4(2):217–233. doi: 10.2478/s13380-013-0116-3. Rajapakse and Kirton reviewed translational approaches in adults and children using TMS, including developmental neurophysiology, stroke, cerebral palsy, epilepsy, and neuropsychiatric diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Palm U, Segmiller FM, Epple AN, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm (Vienna) 2016;123(10):1219–34. doi: 10.1007/s00702-016-1572-z. Palm et al reviewed several computational modeling studies addressing tDCS dosing in children and adolescents, as well as several clinical trials on the use of tDCS for a variety of neurologic and neuropsychiatric disorders. Overall, tDCS seems to be safe in children, and more studies are needed to confirm preliminary results. [DOI] [PubMed] [Google Scholar]
- 5.Schwedt TJ, Vargas B. Neurostimulation for treatment of migraine and cluster headache. Pain Med. 2015;16(9):1827–34. doi: 10.1111/pme.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein MM, Treister R, Raij T, et al. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain. 2015;156(9):1601–14. doi: 10.1097/j.pain.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eldaief MC, Press DZ, Pascual-Leone A. Transcranial magnetic stimulation in neurology: a review of established and prospective applications. Neurol Clin Pract. 2013;3(6):519–26. doi: 10.1212/01.CPJ.0000436213.11132.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller PA, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with pathologic positive sensory phenomena: a review of literature. Brain Stimul. 2012;5(3):320–9. doi: 10.1016/j.brs.2011.05.003. e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115(8):1730–9. doi: 10.1016/j.clinph.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–82. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9(5):641–61. doi: 10.1016/j.brs.2016.06.004. Evidence-based review of dosing metrics and dose-response for tDCS. The authors report that, to date, the use of tDCS protocols in thousand of patients have not resulted in any reports of a serious adverse effect or irreversible injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehmann R, Sczesny-Kaiser M, Lenz M, et al. Polarity-specific cortical effects of transcranial direct current stimulation in primary somatosensory cortex of healthy humans. Front Hum Neurosci. 2016;10:208. doi: 10.3389/fnhum.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvey MA, Gilbert DL. Transcranial magnetic stimulation in children. Eur J Paediatr Neurol. 2004;8(1):7–19. doi: 10.1016/j.ejpn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Oberman LM, Enticott PG, Casanova MF, et al. Transcranial magnetic stimulation in autism spectrum disorder: challenges, promise, androadmap for future research. Autism Res. 2016;9(2):184–203. doi: 10.1002/aur.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picht T, Schmidt S, Brandt S, et al. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011;69(3):581–8. doi: 10.1227/NEU.0b013e3182181b89. discussion 588. [DOI] [PubMed] [Google Scholar]
- 19.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57(9):1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 20.Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10(1–2):93–106. doi: 10.1155/NP.2003.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fietzek UM, Heinen F, Berweck S, et al. Development of the corticospinal system and hand motor function: central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42(4):220–7. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- 22.Heinen F, Fietzek UM, Berweck S, Hufschmidt A, Deuschl G, Korinthenberg R. Fast corticospinal system and motor performance in children: conduction proceeds skill. Pediatr Neurol. 1998;19(3):217–21. doi: 10.1016/s0887-8994(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 23.Muller K, Homberg V. Development of speed of repetitive movements in children is determined by structural changes in corticospinal efferents. Neurosci Lett. 1992;144(1–2):57–60. doi: 10.1016/0304-3940(92)90715-j. [DOI] [PubMed] [Google Scholar]
- 24.Muller K, Homberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of corticomotoneuronal projections. Electroencephalogr Clin Neurophysiol. 1991;81(1):63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- 25.Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42(5):705–11. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- 26.Nezu A, Kimura S, Takeshita S. Topographical differences in the developmental profile of central motor conduction time. Clin Neurophysiol. 1999;110(9):1646–9. doi: 10.1016/s1388-2457(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 27.Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31(8):1136–49. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7(6):507–13. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- 29.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(Pt 5):1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 30.Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol. 2005;15(3):R84–5. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Nezu A, Kimura S, Kobayashi T, et al. Transcranial magnetic stimulation in an adrenoleukodystrophy patient. Brain Dev. 1996;18(4):327–9. doi: 10.1016/0387-7604(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi Y, Okubo O, Fuchigami T, Fujita Y, Harada K. Motor-evoked potentials in a child recovering from transverse myelitis. Pediatr Neurol. 2000;23(5):436–8. doi: 10.1016/s0887-8994(00)00211-3. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Martinez A, Gonzalez-Orodea JI, Lopez Pajares R, Arpa J. Disability in multiple sclerosis. The role of transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2000;40(7):441–7. [PubMed] [Google Scholar]
- 34.Schmierer K, Irlbacher K, Grosse P, Roricht S, Meyer BU. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology. 2002;59(8):1218–24. doi: 10.1212/wnl.59.8.1218. [DOI] [PubMed] [Google Scholar]
- 35.Dan B, Christiaens F, Christophe C, Dachy B. Transcranial magnetic stimulation and other evoked potentials in pediatric multiple sclerosis. Pediatr Neurol. 2000;22(2):136–8. doi: 10.1016/s0887-8994(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 36.Buchmann J, Wolters A, Haessler F, Bohne S, Nordbeck R, Kunesch E. Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) Clin Neurophysiol. 2003;114(11):2036–42. doi: 10.1016/s1388-2457(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 37.Giedd JN, Castellanos FX, Casey BJ, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151(5):665–9. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 38.Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–40. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- 39.Steere JC, Arnsten AF. Corpus callosum morphology in ADHD. Am J Psychiatry. 1995;152(7):1105–6. doi: 10.1176/ajp.152.7.1105b. [DOI] [PubMed] [Google Scholar]
- 40.Wu SW, Gilbert DL, Shahana N, Huddleston DA, Mostofsky SH. Transcranial magnetic stimulation measures in attention-deficit/ hyperactivity disorder. Pediatr Neurol. 2012;47(3):177–85. doi: 10.1016/j.pediatrneurol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchmann J, Gierow W, Weber S, et al. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH) Neurosci Lett. 2006;405(1–2):14–8. doi: 10.1016/j.neulet.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Rotenberg A. Prospects for clinical applications of transcranial magnetic stimulation and real-time EEG in epilepsy. Brain Topogr. 2010;22(4):257–66. doi: 10.1007/s10548-009-0116-3. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh TH, Dhamne SC, Chen JJ, Pascual-Leone A, Jensen FE, Rotenberg A. A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J Neurophysiol. 2012;107(3):966–72. doi: 10.1152/jn.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology. 2009;73(3):218–22. doi: 10.1212/WNL.0b013e3181ae7ca6. [DOI] [PubMed] [Google Scholar]
- 45.Fedi M, Berkovic SF, Macdonell RA, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18(3):664–9. doi: 10.1093/cercor/bhm100. [DOI] [PubMed] [Google Scholar]
- 46.Badawy R, Macdonell R, Jackson G, Berkovic S. The peri-ictal state: cortical excitability changes within 24 h of a seizure. Brain. 2009;132(Pt 4):1013–21. doi: 10.1093/brain/awp017. [DOI] [PubMed] [Google Scholar]
- 47.Badawy RA, Macdonell RA, Berkovic SF, Newton MR, Jackson GD. Predicting seizure control: cortical excitability and antiepileptic medication. Ann Neurol. 2010;67(1):64–73. doi: 10.1002/ana.21806. [DOI] [PubMed] [Google Scholar]
- 48.Di Lazzaro V, Oliviero A, Pilato F, et al. Effects of vagus nerve stimulation on cortical excitability in epileptic patients. Neurology. 2004;62(12):2310–2. doi: 10.1212/01.wnl.0000131743.45131.ae. [DOI] [PubMed] [Google Scholar]
- 49.Cantello R, Varrasi C, Tarletti R, et al. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia. 2007;48(9):1756–63. doi: 10.1111/j.1528-1167.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76(7):615–21. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. GABAergic activity in autism spectrum disorders: an investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology. 2013;68:202–9. doi: 10.1016/j.neuropharm.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol. 2010;52(8):e179–83. doi: 10.1111/j.1469-8749.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- 53.Benali A, Trippe J, Weiler E, et al. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31(4):1193–203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stagg CJ, Wylezinska M, Matthews PM, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101(6):2872–7. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Hong YH, Wu SW, Pedapati EV, et al. Safety and tolerability of theta burst stimulation vs. single and paired pulse transcranial magnetic stimulation: a comparative study of 165 pediatric subjects. Front Hum Neurosci. 2015;9:29. doi: 10.3389/fnhum.2015.00029. A comparative analysis of data from 165 participants aged 6–18 years, from 2009–2014, indicated that TBS appears to be as safe as single and paired pulse TMS in terms of rate and severity of adverse events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 57.Oberman L, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A. Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. Eur J Neurosci. 2012;36(6):2782–8. doi: 10.1111/j.1460-9568.2012.08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Pedapati EV, Gilbert DL, Erickson CA, et al. Abnormal cortical plasticity in youth with autism spectrum disorder: a transcranial magnetic stimulation case-control pilot study. J Child Adolesc Psychopharmacol. 2016;26(7):625–31. doi: 10.1089/cap.2015.0183. Pedapati et al. reported that subthreshold iTBS may be a potential neurophysiological biomarker of cortical plasticity in children and adolescents with ASD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627. doi: 10.3389/fnhum.2014.00627. Oberman et al. reported a positive linear relationship between age and duration of modulation of corticospinal excitability after cTBS in patients with ASD, and a paradoxical facilitatory response in a subset of the study population, suggesting aberrant plasticity and GABAergic dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damji O, Keess J, Kirton A. Evaluating developmental motor plasticity with paired afferent stimulation. Dev Med Child Neurol. 2015;57:548–55. doi: 10.1111/dmcn.12704. [DOI] [PubMed] [Google Scholar]
- 61••.Jung NH, Janzarik WG, Delvendahl I, et al. Impaired induction of long-term potentiation-like plasticity in patients with high-functioning autism and Asperger syndrome. Dev Med Child Neurol. 2013;55(1):83–9. doi: 10.1111/dmcn.12012. Jung et al., reported significantly impaired LTP-like plasticity via PAS in patients with ASD, suggesting abberant synaptic connectivity and sensory-motor integration. [DOI] [PubMed] [Google Scholar]
- 62.Player MJ, Taylor JL, Alonzo A, Loo CK. Paired associative stimulation increases motor cortex excitability more effectively than theta-burst stimulation. Clin Neurophysiol. 2012;123(11):2220–6. doi: 10.1016/j.clinph.2012.03.081. [DOI] [PubMed] [Google Scholar]
- 63.Oberman LM, Enticott PG, Casanova MF, Rotenberg A, Pascual-Leone A, McCracken JT. Transcranial magnetic stimulation (TMS) therapy for autism: an international consensus conference held in conjunction with the international meeting for autism research on May 13th and 14th, 2014. Front Hum Neurosci. 2014;8:1034. doi: 10.3389/fnhum.2014.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed Z, Wieraszko A. Modulation of learning and hippocampal, neuronal plasticity by repetitive transcranial magnetic stimulation (rTMS) Bioelectromagnetics. 2006;27(4):288–94. doi: 10.1002/bem.20211. [DOI] [PubMed] [Google Scholar]
- 65.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180(4):583–93. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 66.Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199(3–4):411–21. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- 67.Funke K, Benali A. Cortical cellular actions of transcranial magnetic stimulation. Restor Neurol Neurosci. 2010;28(4):399–417. doi: 10.3233/RNN-2010-0566. [DOI] [PubMed] [Google Scholar]
- 68.Theodore WH. Transcranial magnetic stimulation in epilepsy. Epilepsy Curr. 2003;3(6):191–7. doi: 10.1046/j.1535-7597.2003.03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 70••.Kirton A, Andersen J, Herrero M, et al. Brain stimulation and constraint for perinatal stroke hemiparesis: the PLASTIC CHAMPS Trial. Neurology. 2016;86(18):1659–67. doi: 10.1212/WNL.0000000000002646. Kirton et al provide class II evidence that combined repetitive TMS and constraint-induced movement therapy increases the functional motor gains seen after intensive rehabilitation therapy in children with hemiparetic cerebral palsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figiel GS, Epstein C, McDonald WM, et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci. 1998;10(1):20–5. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 72.Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9(6):641–54. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 73.Bloch Y, Grisaru N, Harel EV, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. 2008;24(2):156–9. doi: 10.1097/YCT.0b013e318156aa49. [DOI] [PubMed] [Google Scholar]
- 74.Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263–9. doi: 10.4088/JCP.11m07003. [DOI] [PubMed] [Google Scholar]
- 75•.Wall CA, Croarkin PE, McClintock SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in adolescents with major depressive disorder. Front Psych. 2013;4:165. doi: 10.3389/fpsyt.2013.00165. This open label study reports that rTMS treatment decreased symptom severity and significantly improved memory and delayed verbal recall in 18 adolescent patients with major depressive disorder, with no reported changes in neurocognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang XR, Kirton A, Wilkes TC, et al. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT. 2014;30(3):242–7. doi: 10.1097/YCT.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 77•.Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257–62. doi: 10.1016/j.jocn.2012.01.049. Le et al report that LF-rTMS improved clinical symptoms in 25 children with Tourette Syndrome for six months after treatment. [DOI] [PubMed] [Google Scholar]
- 78.Sokhadze EM, El-Baz AS, Tasman A, et al. Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: an exploratory study. Appl Psychophysiol Biofeedback. 2014;39(3–4):237–57. doi: 10.1007/s10484-014-9264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panerai S, Tasca D, Lanuzza B, et al. Effects of repetitive transcranial magnetic stimulation in performing eye-hand integration tasks: four preliminary studies with children showing low-functioning autism. Autism. 2014;18(6):638–50. doi: 10.1177/1362361313495717. [DOI] [PubMed] [Google Scholar]
- 80.Amatachaya A, Jensen MP, Patjanasoontorn N, et al. The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: a randomized crossover controlled trial. Behav Neurol. 2015;2015:928631. doi: 10.1155/2015/928631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amatachaya A, Auvichayapat N, Patjanasoontorn N, et al. Effect of anodal transcranial direct current stimulation on autism: a randomized double-blind crossover trial. Behav Neurol. 2014;2014:173073. doi: 10.1155/2014/173073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneider HD, Hopp JP. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon. 2011;25(6–7):640–54. doi: 10.3109/02699206.2011.570852. [DOI] [PubMed] [Google Scholar]
- 83.Costanzo F, Menghini D, Casula L, et al. Transcranial direct current stimulation treatment in an adolescent with autism and drug-resistant catatonia. Brain Stimul. 2015;8(6):1233–5. doi: 10.1016/j.brs.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord. 2015 Dec 20; doi: 10.1177/1087054715618792. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85•.Bandeira ID, Guimaraes RS, Jagersbacher JG, et al. Transcranial direct current stimulation in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a pilot study. J Child Neurol. 2016;31(7):918–24. doi: 10.1177/0883073816630083. This open-label study showed that tDCS therapy increased processing speed and stimulus detection, and enhanced the ability to switch tasks in 9 children with ADHD. [DOI] [PubMed] [Google Scholar]
- 86.Munz MT, Prehn-Kristensen A, Thielking F, Molle M, Goder R, Baving L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci. 2015;9:307. doi: 10.3389/fncel.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liebetanz D, Klinker F, Hering D, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47(7):1216–24. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 88.Dhamne SC, Ekstein D, Zhuo Z, et al. Acute seizure suppression by transcranial direct current stimulation in rats. Ann Clin Transl Neurol. 2015;2(8):843–56. doi: 10.1002/acn3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shelyakin AM, Preobrazhenskaya IG, Kassil MV, Bogdanov OV. The effects of transcranial micropolarization on the severity of convulsive fits in children. Neurosci Behav Physiol. 2001;31(5):555–60. doi: 10.1023/a:1010487201282. [DOI] [PubMed] [Google Scholar]
- 90•.Auvichayapat N, Rotenberg A, Gersner R, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013;6(4):696–700. doi: 10.1016/j.brs.2013.01.009. A study of 36 children with focal epilepsy showed that cathodal tDCS is well-tolerated, and improves EEG. [DOI] [PubMed] [Google Scholar]
- 91.Yook SW, Park SH, Seo JH, Kim SJ, Ko MH. Suppression of seizure by cathodal transcranial direct current stimulation in an epileptic patient: a case report. Ann Rehabil Med. 2011;35(4):579–82. doi: 10.5535/arm.2011.35.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faria P, Fregni F, Sebastiao F, Dias AI, Leal A. Feasibility of focal transcranial DC polarization with simultaneous EEG recording: preliminary assessment in healthy subjects and human epilepsy. Epilepsy Behav. 2012;25(3):417–25. doi: 10.1016/j.yebeh.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 93.Aree-uea B, Auvichayapat N, Janyacharoen T, et al. Reduction of spasticity in cerebral palsy by anodal transcranial direct current stimulation. J Med Assoc Thai. 2014;97(9):954–62. [PubMed] [Google Scholar]
- 94.Grecco LA, Duarte NA, Zanon N, Galli M, Fregni F, Oliveira CS. Effect of a single session of transcranial direct-current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: a randomized sham-controlled study. Braz J Phys Ther. 2014;18(5):419–27. doi: 10.1590/bjpt-rbf.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grecco LA, de Almeida Carvalho Duarte N, Mendonca ME, et al. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: a randomized controlled double-blind clinical trial. Res Dev Disabil. 2014;35(11):2840–8. doi: 10.1016/j.ridd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 96.Duarte Nde A, Grecco LA, Galli M, Fregni F, Oliveira CS. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PLoS One. 2014;9(8):e105777. doi: 10.1371/journal.pone.0105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lazzari RD, Politti F, Santos CA, et al. Effect of a single session of transcranial direct-current stimulation combined with virtual reality training on the balance of children with cerebral palsy: a randomized, controlled, double-blind trial. J Phys Ther Sci. 2015;27(3):763–8. doi: 10.1589/jpts.27.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collange Grecco LA, de Almeida Carvalho Duarte N, Mendonca ME, Galli M, Fregni F, Oliveira CS. Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: a pilot, randomized, controlled, double-blind, clinical trial. Clin Rehabil. 2015;29(12):1212–23. doi: 10.1177/0269215514566997. [DOI] [PubMed] [Google Scholar]
- 99.An S, Yang JW, Sun H, Kilb W, Luhmann HJ. Long-term potentiation in the neonatal rat barrel cortex in vivo. J Neurosci. 2012;32(28):9511–6. doi: 10.1523/JNEUROSCI.1212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao G, Harris KM. Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal areaCA1 of the rat. J Neurophysiol. 2012;107(3):902–12. doi: 10.1152/jn.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep. 2015;15(5):27. doi: 10.1007/s11910-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5(7):380–91. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanchez RM, Jensen FE. Maturational aspects of epilepsy mechanisms and consequences for the immature brain. Epilepsia. 2001;42(5):577–85. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- 104.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62(2):112–20. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 105.Mix A, Hoppenrath K, Funke K. Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol. 2015;75(1):1–11. doi: 10.1002/dneu.22205. [DOI] [PubMed] [Google Scholar]
- 106.Rosa MA, Picarelli H, Teixeira MJ, Rosa MO, Marcolin MA. Accidental seizure with repetitive transcranial magnetic stimulation. J ECT. 2006;22(4):265–6. doi: 10.1097/01.yct.0000244236.72049.9e. [DOI] [PubMed] [Google Scholar]
- 107.Sui L, Huang S, Peng B, Ren J, Tian F, Wang Y. Deep brain stimulation of the amygdala alleviates fear conditioning-induced alterations in synaptic plasticity in the cortical-amygdala pathway and fear memory. J Neural Transm. 2014;121(7):773–82. doi: 10.1007/s00702-014-1183-5. [DOI] [PubMed] [Google Scholar]
- 108.Tawfik VL, Chang SY, Hitti FL, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. 2010;67(2):367–75. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaieb L, Antal A, Paulus W. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front Neurosci. 2015;9:125. doi: 10.3389/fnins.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 111.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blanke ML, VanDongen AMJ. Activation Mechanisms of the NMDA Receptor. In: Van Dongen AM, editor. Biology of the NMDA Receptor, Chapter 13. Boca Raton (FL): CRC Press/ Taylor & Francis; 2009. [PubMed] [Google Scholar]
- 113.Hanson E, Armbruster M, Cantu D, et al. Astrocytic glutamate uptake is slow and does not limit neuronal NMDA receptor activation in the neonatal neocortex. Glia. 2015;63(10):1784–96. doi: 10.1002/glia.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez RM, Jensen FE. Modeling hypoxia-induced seizures and hypoxic encephalopathy in the neonatal period. In: Pitkanen A, Moshe SL, Schwartzkroin PA, editors. Models of seizures and epilepsy. San Diego: Elsevier; 2006. [Google Scholar]
- 115.Avallone J, Gashi E, Magrys B, Friedman LK. Distinct regulation of metabotropic glutamate receptor (mGluR1 alpha) in the developing limbic system following multiple early-life seizures. Exp Neurol. 2006;202(1):100–11. doi: 10.1016/j.expneurol.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 116.Sun Y, Lipton JO, Boyle LM, et al. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol. 2016;80(2):233–46. doi: 10.1002/ana.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Danbolt NC, Storm-Mathisen J, Kanner BI. An [Na+ + K+ ]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51(2):295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- 118.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18(21):8751–7. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain gluta-mate transporter proteins GLAST and GLT. Eur J Neurosci. 1997;9(8):1646–55. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 120.Amadi U, Allman C, Johansen-Berg H, Stagg CJ. The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABAA activity. Brain Stimul. 2015;8(5):898–905. doi: 10.1016/j.brs.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swann JW, Brady RJ, Martin DL. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience. 1989;28(3):551–61. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 122.Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33(6):781–95. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 123.Rivera C, Voipio J, Payne JA, et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 124.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 125.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15(6):1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 126.Labedi A, Benali A, Mix A, Neubacher U, Funke K. Modulation of inhibitory activity markers by intermittent theta-burst stimulation in rat cortex is NMDA-receptor dependent. Brain Stimul. 2014;7(3):394–400. doi: 10.1016/j.brs.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 127.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 128.Epsztein J, Represa A, Jorquera I, Ben-Ari Y, Crepel V. Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci. 2005;25(36):8229–39. doi: 10.1523/JNEUROSCI.1469-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984;13(4):1073–94. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- 130.Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience. 1984;13(4):1051–72. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- 131.Ben-Ari Y. The developing cortex. Handb Clin Neurol. 2013;111:417–26. doi: 10.1016/B978-0-444-52891-9.00045-2. [DOI] [PubMed] [Google Scholar]
- 132.Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015;86(5):1119–30. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 133.Carvill GL, Weckhuysen S, McMahon JM, et al. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology. 2014;82(14):1245–53. doi: 10.1212/WNL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talos DM, Sun H, Kosaras B, et al. Altered inhibition in tuberous sclerosis and type IIb cortical dysplasia. Ann Neurol. 2012;71(4):539–51. doi: 10.1002/ana.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile X mice. J Neurosci. 2014;34(2):446–50. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tyzio R, Nardou R, Ferrari DC, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343(6171):675–9. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 137.Fatemi SH, Folsom TD. GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr Res. 2015;167(1–3):42–56. doi: 10.1016/j.schres.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lomo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):617–20. doi: 10.1098/rstb.2002.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4(3):389–99. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 140.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 141.Nitsche MA, Paulus W. Transcranial direct current stimulation– update 2011. Restor Neurol Neurosci. 2011;29(6):463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 142.Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci U S A. 1997;94(7):3380–3. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13(7):2910–8. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lante F, Cavalier M, Cohen-Solal C, Guiramand J, Vignes M. Developmental switch from LTD to LTP in low frequency-induced plasticity. Hippocampus. 2006;16(11):981–9. doi: 10.1002/hipo.20228. [DOI] [PubMed] [Google Scholar]
- 145.Oberman LM, Ifert-Miller F, Najib U, et al. Abnormal mechanisms of plasticity and metaplasticity in autism spectrum disorders and fragile X syndrome. J Child Adolesc Psychopharmacol. 2016;26(7):617–24. doi: 10.1089/cap.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Froemke RC. Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci. 2015;38:195–219. doi: 10.1146/annurev-neuro-071714-034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 148.Gersner R, Kaye HL, Oberman L, et al. Safety and tolerability of transcranial magnetic stimulation for motor and language mapping in children with epilepsy; 6th International Conference on Transcranial Brain Stimulation; 2016; Göttingen, Germany. [Google Scholar]