Abstract
Hyperglycemia and other adverse exposures early in life that reprogram stem cells may lead to long-lasting phenotypic influences over the lifetime of an individual. Hyperglycemia and oxidative stress cause DNA damage when they exceed the protective capabilities of the cell, in turn affecting cellular function. DNA damage in response to hyperglycemia and oxidative stress was studied in human umbilical cord mesenchymal stem cells (hUC-MSCs) from large-for-gestational-age (LGA) infants of mothers with gestational diabetes mellitus (LGA-GDM) and control subjects. We tested the response of these cells to hyperglycemia and oxidative stress, measuring reactive oxygen species (ROS) levels and antioxidant enzyme activities. We find that hUC-MSCs from LGA-GDM infants have increased DNA damage when exposed to oxidative stress. With the addition of hyperglycemic conditions, these cells have an increase in ROS and a decrease in antioxidant glutathione peroxidase (GPx) activity, indicating a mechanism for the increased ROS and DNA damage. This study demonstrates that a memory of in utero hyperglycemia, mediated through downregulation of GPx activity, leads to an increased susceptibility to oxidative stress. The alteration of GPx function in self-renewing stem cells, can mediate the effect of intrauterine hyperglycemia to be propagated into adulthood and contribute to disease susceptibility.
Keywords: : gestational diabetes, mesenchymal stem cells, oxidative stress, DNA damage, glutathione peroxidase
Introduction
Type 2 diabetes mellitus is a chronic metabolic disease involving the dysfunction of many organs, such as pancreas, liver, muscle, and adipose tissue and resulting in hepatic and peripheral insulin resistance and ultimately hyperglycemia [1]. The incidence of type 2 diabetes continues to increase worldwide and nearly quadrupled in adults from 1980 to 2014 when nearly 422 million adults over the age of 18 were estimated to have diabetes [2]. Similarly, the number of reproductive-aged women with pregestational and gestational diabetes mellitus (GDM) has increased at an alarming rate with an estimated 9.2%–35.5% prevalence in the United States [3,4]. Diabetes during pregnancy is characterized by carbohydrate intolerance, hyperinsulinemia, and hyperglycemia if untreated.
The goals of glycemic control are generally tighter in pregnancy than outside of pregnancy due to the adverse fetal effects of excessive glucose. The fetus has a limited capacity for gluconeogenesis and relies heavily on maternal sources of glucose. A carrier-mediated transport system in the placenta fulfills the high fetal demand for the transfer of glucose from the maternal compartment [5]. Glucose transfer across the placenta is highly efficient and determined by maternal plasma glucose concentration and uterine/placental blood flow [6]. The fetal pancreas compensates for excesses in plasma glucose levels with increases in insulin synthesis and secretion. The hyperglycemia and trophic effects of insulin stimulate fat and protein production, which can lead to fetal macrosomia [7]. In the setting of maternal diabetes, large for gestational age (LGA) is often used as a marker of degree of maternal glycemic control. LGA infants of diabetic women have an enhanced risk for adverse perinatal outcomes as well as the development of type 2 diabetes, obesity, and metabolic syndrome later in life, when compared with appropriately grown infants or those born LGA from nondiabetic women [8,9]. How exposure to hyperglycemia in utero increases the risk for an array of chronic adult diseases in the offspring remains unclear.
High levels of glucose can increase cellular oxidative stress [10–12]. Within the cell, oxidative stress induces several response pathways, including enzymatic antioxidant clearance. The three primary enzymes that help to resolve reactive oxygen species (ROS) are superoxide dismutase (SOD), Catalase, and glutathione peroxidase (GPx). When intracellular levels of ROS exceed the capacity for cellular clearance provided by these enzymes, these damaging oxidative agents can affect the integrity of DNA, proteins and lipids, and potentially lead to cell death [13]. Oxidative stress associated with hyperglycemia results in DNA damage and is accompanied by decreased erythrocyte SOD activity in both rodents and humans [11,14]. We hypothesized that an inadequate cellular response to oxidative stress in LGA infants from diabetic mothers provides the link between in utero exposure to hyperglycemia and increased risk for development of chronic diseases in adulthood.
Oxidative stress modulates cellular functional phenotypes that can lead to disease [15]. Hydrogen peroxide treatment of adipocytes and muscle cells in vitro can induce or worsen insulin resistance [16,17]. Furthermore, increased ROS were found to precede the onset of pancreatic beta cell dysfunction [18]. Alterations in antioxidant enzymes have also been found in type 2 diabetes and hypertensive patients, continuing to decline with age, potentially contributing to increased susceptibility to tissue damage and diabetic complications over time [19]. The current study focuses on understanding the cellular stress response phenotype of multipotent stem cells previously exposed to excess glucose levels during intrauterine development. High glucose conditions in vitro is used to recapitulate the effect of in utero exposure and to assess the consequences of future exposure to glucose to determine if in utero exposure to high glucose influences the ability to respond to oxidative stress.
Materials and Methods
Human umbilical cord mesenchymal stem cell isolation and cell culture
This study was approved by the Montefiore Medical Center Institutional Review Board and Albert Einstein College of Medicine's Committee on Clinical Investigation and is in accordance with Health Insurance Portability and Accountability Act regulations. Written informed consent was obtained from all subjects before participation. Umbilical cord samples after delivery from neonates of appropriate for gestational age (AGA) or LGA growth were collected immediately and stored in Dulbecco's modified Eagle's medium (DMEM; 1 g/L glucose) with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2.5 μg/mL amphotericin B. Control (AGA) samples were collected from healthy mothers with normal glucose challenge testing, whereas LGA samples were collected from GDM mothers, identified by diagnosis code and verified in written notations. Within 16 h of collection, processing for mesenchymal stem cell (MSC) isolation and culture was initiated, as described previously [20]. Umbilical cord sections were washed with phosphate-buffered saline (PBS). The tissues surrounding the umbilical vein and arteries were dissected and cut into 1–2 mm pieces, then placed matrix side down, filling the surface of a 150-cm2 tissue culture plate. Complete medium (DMEM with 1 g/L glucose [5 mM] + GlutaMax [10567014; Gibco], 15% fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin, and 2.5 μg/mL amphotericin B) was added to cover the tissue pieces and plates were incubated at 37°C and 5% CO2. Explant culture proceeded for 10–12 days. Passages 1–3 were obtained by trypsinization of adherent cells and reseeded at 2,000 cells/cm2. Passage 3 was frozen into aliquots for further studies. All experimental assays were performed at passage 5. High glucose studies were conducted for 5 days with high glucose medium (DMEM with 4.5 g/L glucose [25 mM] + GlutaMax [10569010; Gibco], 15% fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin, and 2.5 μg/mL amphotericin B).
Flow cytometry for cell homogeneity
Cells were trypsinized and prepared in fluorescence-activated cell sorting (FACS; PBS +2% FBS) buffer and treated with FcR Blocking Reagent for 10 min on ice, except for an aliquot reserved for the unstained negative control. MSCs are characterized by the presence of CD105, CD90, CD44, and CD73 and absence of CD14, CD34, and CD45 [21]. Cells were stained with CD45-PerCP-Cy5.5, CD31-FITC, and CD90-APC for 30 min on ice to test for an MSC expression pattern. Single staining controls were made for fluorescence spectral overlap compensation. After staining, cells were washed with PBS and resuspended in 500 μL FACS buffer with 1 mg/mL DAPI. Fluorescence was recorded on BD LSR II Cell Analyzer (Becton Dickinson). Analysis was performed using FlowJo v10 software.
Cell viability and hydrogen peroxide survival
Human umbilical cord mesenchymal stem cells (hUC-MSCs) were seeded cells in a 96-well plate at 5 × 103 cells/well in 100 μL of culture medium with or without 25 mM glucose. Cells were cultured in a CO2 incubator at 37°C for 5 days; samples in each condition were then treated with 100 μM hydrogen peroxide for 30 min. To each well, 10 μL of WST-1 Mixture (10008883; Cayman Chemicals) was added. After mixing on orbital shaker, the plate was incubated in a CO2 incubator at 37°C for 2 h. Absorbance was measured using Multiskan FC microplate photometer (Thermo Scientific) at 450 nm. For the cell viability experiment, six Control and six LGA-GDM samples were used. For the H2O2 titration experiment, two samples from Control and LGA-GDM groups were used.
Comet assay
Five hUC-MSCs samples from Control and LGA-GDM groups were plated at 2.5 × 104 cells per well into six-well plates in complete culture medium with low or high glucose. Five days later, the cells (60%–80% confluence) were washed with PBS, then treated with 100 μM H2O2 for 30 min. Cells were washed with PBS, trypsinized, and counted by Trypan Blue exclusion. The comet assay was performed according to the Trevigen's (Gaithersburg, MD) Protocol and Kit (4250-050-K). For each sample area, 8,000 cells were embedded in 50 μL low-melting agarose. Immobilized cells were lysed and incubated in alkaline unwinding solution each for 1 h at 4°C in the dark. Slides were electrophoresed in alkaline conditions at 1 V/cm distance between electrodes for 30 min. Cells were stained with SYBR Green and at least 100 cells per sample were imaged. Analysis was performed using the ImageJ software plugin, OpenComet [22]. Scores for DNA damage were percent DNA in comet tail and tail moment. Scores were normalized by internal control sample for each day's assay that was performed.
Reactive oxygen species
For the detection of intracellular ROS, CM-H2DCFDA dye was utilized. CM-H2DCFDA reacts with a broad range of oxidizing species, including hydroxyl radicals, peroxynitrite and hypochlorous acid [23]. hUC-MSCs from six Control and seven LGA-GDM samples were plated at 2.5 × 104 cells per well into six-well plates in complete culture medium in low or high glucose, without phenol red. Five days later, the cells (60%–80% confluence) were washed with PBS and loaded with dye, 1 M of CM-H2DCFDA (C6827; Life Technologies) diluted in Hank's Buffered Salt Solution (HBSS) for 20 min, and incubated at 37°C in the dark. Cells were washed with HBSS and treated with 100 μM of H2O2 for 30 min, and incubated at 37°C in the dark. Cells were washed with PBS, trypsinized, and resuspended with 300–400 μL PBS and DAPI (final concentration 1 μg/mL) and placed on ice, in the dark. Flow cytometry analysis followed immediately; living cells (DAPI negative) were selected by FACS gating. Within the living cell population, fluorescence in the FITC channel (525 nm) was recorded on BD LSR II Cell Analyzer (Becton Dickinson). Two measures of ROS were analyzed using FlowJo v10 software; (1) the proportion of cells positive for fluorescence was determined from unstained negative control MSCs, and (2) the mean fluorescence intensity (MFI) of positive cells. Measurements for each group were normalized relative to the Control Low-Glucose group.
Antioxidant enzyme activities
The hUC-MSCs were seeded in T-75 flasks at 2,000–2,500 cells/cm2 at low glucose or high glucose for 5 days. Before cell collection, a flask for each sample in low glucose and high glucose were treated with 100 μM H2O2 for 30 min. Cells were collected by cell scraping in PBS, centrifuged at 2,000 rpm for 5 min at 4°C, and lysed with cell lysis buffer: 10 mM Tris HCl ph7.5, 10 mM NaCl, 2 mM EDTA, and 0.1% Triton X-100. Cell lysates were centrifuged at 14,000 rpm for 15 min at 4°C, supernatants were aliquoted (with 1 mM DTT for GPx), and stored at −80°C. Spectrophotometric analysis measured SOD (706002; Cayman Chemicals), Catalase (707002; Cayman Chemicals), and GPx (703102; Cayman Chemicals) at 450, 540, and 340 nm, respectively. One unit of SOD activity is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. One unit of Catalase activity is defined as the amount of enzyme that causes formation of 1 nmol of formaldehyde per minute. One unit of GPx is defined as the amount of enzyme that will cause the oxidative of 1 nmol of NADPH to NADP+ per minute. Enzyme activity was standardized by protein amount added to each well using the BCA Protein Assay Kit (Pierce; Thermo Scientific). Activity of SOD was analyzed in five Control and six LGA-GDM samples. Activity of Catalase was analyzed in five Control and seven LGA-GDM samples. Activity of GPx was analyzed in four Control and five LGA-GDM samples.
Statistical analysis
Two-way analysis of variance (ANOVA) of nonrepeated measures with Sidak's correction for multiple testing was used for statistical testing in all experiments, except for clinical comparisons, for which student's t-test was used. ANOVA was performed in two ways to match our hypothesis; first for differences between Controls and LGA-GDMs, then for effects of high glucose and H2O2 treatment within each group. For simplicity, all comparisons and significance are aggregated in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd), except the cell viability results from H2O2 titration due to the different comparisons.
Results
Clinical cohort and sample characteristics
The characteristics of the clinical cohort are described in Table 1. The hUC-MSCs were collected from six appropriately grown for gestational age (AGA) neonates from mothers with negative glucose tolerance screening (Controls) and seven LGA neonates from mothers with GDM, referred to as LGA-GDM. LGA-GDM neonates had significantly larger birth weights than controls by 836 ± 403 g (P < 0.05). Maternal weight and body mass index are significantly higher and glucose challenge test results are twice as high in GDM mothers (199.25 ± 87.2 mg/dL) compared with controls (99.4 ± 29.8 mg/dL; P < 0.05). The diagnosis of GDM was determined by the woman's physician. All GDM samples were identified by diagnosis code and verified in written notations in the medical record. We use increased birth weight and LGA status as markers of poorly controlled GDM and fetal exposure to hyperglycemia. Homogeneity of the hUC-MSCs was confirmed using flow cytometry analysis of CD90, an MSC marker. The hUC-MSCs were consistently above 95% positive for CD90 with an average of 98.18% ± 0.44% and negative for contaminating endothelial and blood cells measured by the absence of CD31 and CD45, respectively (Supplementary Fig. S1). The expression pattern of CD90 positivity and deficiency of CD31 and CD45 are concordant with an expected MSC expression pattern [21]. Similar results at passages three and five indicate that the cells were maintained as a relatively homogenous population up to passage 5 when experimental assays were performed.
Table 1.
Cohort Clinical Data
| Maternal | Infant | GDM diagnostic criteria | Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study group | Age (years) | BMI (kg/m2) | Sex | Race/ethnicity | Birth weight (g) | Gestational age (weeks) | GCT | GTT | None/diet/oral/insulin |
| Control | |||||||||
| Control 1 | 33 | 22.7 | F | African American | 3,375 | 39 | Negative | n/a | None |
| Control 2 | 31 | 24.3 | F | Hispanic | 3,820 | 39 | Negative | n/a | None |
| Control 3 | 37 | 30.0 | F | Hispanic | 3,560 | 40.2 | n/a | Normal | None |
| Control 4 | 24 | 27.7 | M | Hispanic | 3,395 | 38.5 | Positive | Normal | None |
| Control 5 | 20 | 23.4 | M | African American | 3,005 | 38 | n/a | n/a | None |
| Control 6 | 27 | 29.4 | F | Hispanic | 3,180 | 40.5 | Negative | n/a | None |
| Mean | 28.7 ± 6.2 | 26.3 ± 3.2 | 3,389 ± 285 | 39.0 ± 1.0 | |||||
| LGA-GDM | |||||||||
| LGA-GDM 1 | 36 | 37.5 | M | Asian | 4,265 | 38.6 | Positive | n/a | Insulin |
| LGA-GDM 2 | 28 | 40.2 | M | Hispanic | 4,495 | 39.5 | Positive | Abnormal | Diet |
| LGA-GDM 3 | 32 | 44.8 | M | Hispanic | 3,965 | 39.4 | n/a | Abnormal | Diet |
| LGA-GDM 4 | 21 | 35.7 | M | Asian | 4,320 | 40.3 | Positive | Abnormal | Diet |
| LGA-GDM 5 | 41 | 38.5 | F | Hispanic | 3,800 | 39.2 | n/a | Abnormal | Oral |
| LGA-GDM 6 | 36 | 34.0 | F | Hispanic | 4,505 | 40.6 | Negative | Abnormal | Diet |
| Mean | 32.3 ± 7.1 | 38.4 ± 3.8a | 4,225 ± 286a | 39.6 ± 0.7 | |||||
Clinical data of Control and LGA-GDM cohorts. Mean age, weight, BMI, birth weight, and gestational age, represented as mean ± standard deviation.
P value <0.05 measured by student's t-test.
BMI, body mass index; GCT, glucose challenge test; GDM, gestational diabetes mellitus; GTT, glucose tolerance test.
Cell viability and oxidative stress survival
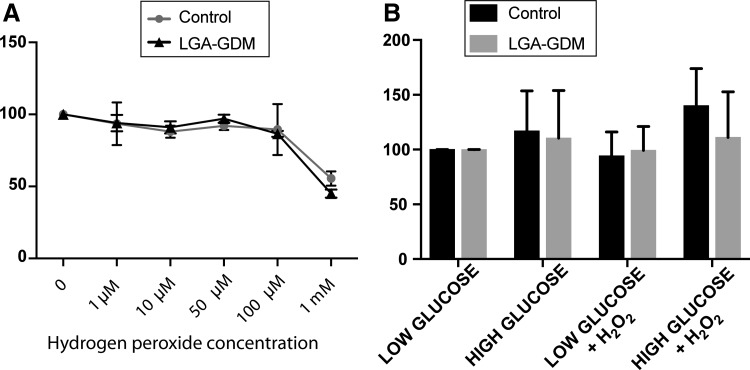
As oxidative stress is toxic for cells [24], oxidative stress treatment was tested to identify the dose that would induce cellular dysfunction without immediately producing cell death. The optimal hydrogen peroxide concentration was 100 μM, as determined by titration of concentrations and measuring the resulting cellular viability. At this concentration, ∼90% of cells survived in both Control and LGA-GDM hUC-MSCs, whereas the concentration of 1 mM decreased cell survival to ∼50% (Fig. 1A).
FIG. 1.
Cell viability related to H2O2 and glucose exposure. We found no difference in cell viability of MSCs between Controls (circles) and LGA-GDMs (triangles) exposed to various hydrogen peroxide (H2O2) concentrations; significant decrease was found in both Controls and LGA-GDMs with 1 mM H2O2 exposure that was not found with another concentration (A). Cell viability in low or HG with and without 100 μM H2O2 treatment for 30 min was comparable in Control (black bars) and LGA-GDM (gray bars) (B). Significance was determined by two-way ANOVA. ANOVA, analysis of variance; HG, GDM, gestational diabetes mellitus; high glucose; MSCs, mesenchymal stem cells.
A concentration of 25 mM glucose is commonly used in in vitro culture conditions [14,25]. This would be an extremely high glucose concentration in vivo, so we tested whether the hUC-MSCs were viable when exposed to 25 mM glucose with or without 100 μM hydrogen peroxide (Fig. 1B). Relative to the low glucose condition, the average viability proportions for Control and LGA-GDM with high glucose exposure were 116% ± 56% and 110% ± 43%, respectively. Combining hydrogen peroxide treatment with high glucose exposure, cell viability proportions for Control and LGA-GDM were 140% ± 61% and 111% ± 42%, respectively, with cell viability above 100% indicating increased proliferation. We confirm that the high glucose and hydrogen peroxide conditions selected allowed robust cell viability, with no difference found for the viability between hUC-MSCs from Controls and LGA-GDMs.
DNA damage
To assess the impact of intrauterine or in vitro high glucose exposure on oxidative stress response, DNA damage was measured using the comet assay under alkaline conditions, which measures both double- and single- strand DNA breaks [26]. The two most accepted and commonly used comet assay scores of DNA damage are percent DNA in the tail and tail moment, which is the product of tail length and amount of DNA in the tail. Under low or high glucose conditions, no difference in DNA damage between Controls and LGA-GDMs was found, regardless of the scoring method. As expected, DNA damage was induced with hydrogen peroxide treatment evidenced by the increase in the percent DNA in tail and tail moment scores in both Controls and LGA-GDMs under both glucose concentration conditions. Interestingly, following the hydrogen peroxide treatment, LGA-GDMs in low and high glucose exhibited a significantly greater extent of DNA damage compared with Controls, with more than a 30% increase in percent DNA in tail scores (Fig. 2A) and >50%–70% increase in tail moment scores (Fig. 2B).
FIG. 2.
Comet assay analysis. DNA damage are represented as Tail DNA percent (A) and DNA Tail Moment (B) in Controls (black open box) and LGA-GDMs (gray open box). Oxidative stress treatment with H2O2 resulted in increased DNA damage in both Controls and LGA-GDM, with more DNA damage in LGA-GDM samples. Significant differences (P < 0.05) were determined by two-way ANOVA comparing: Control-LG (a), Control-HG (b), Control-LG+H2O2 (c), Control-HG+H2O2 (d), LGA-GDM-LG (e), and LGA-GDM-HG (f). *Indicates comparisons highlighted in the text.
Reactive oxygen species
ROS levels in the cell were measured due to the known effects between intracellular ROS and DNA damage [27,28]. The proportion of cells with fluorescence due to ROS production was comparable between Control and LGA-GDM with or without hydrogen peroxide treatment (Fig. 3A). To quantify the differences in ROS in cells undergoing oxidative damage, the amount of fluorescence per positive cell was averaged to give the MFI for each group and treatment condition (Fig. 3B). Without hydrogen peroxide treatment, no difference in ROS between Controls and LGA-GDM was found in low glucose or high glucose exposures. As expected, treatment of hUC-MSCs with hydrogen peroxide increased intracellular ROS levels, but also revealed differences between samples. The largest increase in ROS was detected in the condition of combined high glucose and hydrogen peroxide treatments. Furthermore, LGA-GDMs showed a significant increase in ROS (>40%) in the combined treatment condition compared with Controls. Thus, the combined treatment reveals a diminished ability to combat this oxidative stress in hUC-MSCs with prior intrauterine glucose exposure.
FIG. 3.
Intracellular ROS levels were measured on the population of cells with positive fluorescence (A) and by MFI (B) in Controls (black bars) and LGA-GDM (gray bars). Proportion of cells fluorescently positive increased with H2O2 treatment in both Controls and LGA-GDMs. Combined treatment of HG and H2O2 revealed increased ROS in LGA-GDM samples compared with Controls. Significant differences (P < 0.05) were determined by two-way ANOVA comparing: Control-LG (a), Control-HG (b), Control-LG+H2O2 (c), Control-HG+H2O2 (d), LGA-GDM-LG (e), LGA-GDM-HG (f), and LGA-GDM-LG+H2O2 (g). *Indicates comparisons highlighted in the text. MFI, mean fluorescence intensity; ROS, reactive oxygen species.
Antioxidant enzyme activities
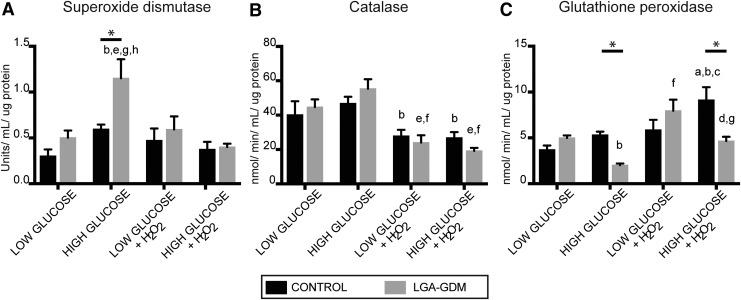
Antioxidant enzyme activities were examined in each of the cellular conditions to understand the differential response between Control and LGA-GDM. Control and LGA-GDM had similar levels of SOD, Catalase, and GPx activity in low glucose conditions, with and without hydrogen peroxide treatment. Under high glucose conditions, LGA-GDM hUC-MSCs exhibit increased SOD activity compared with Controls (Fig. 4A). However, this increase is attenuated with additional oxidative stress. With hydrogen peroxide treatment, Catalase activity in both Controls and LGA-GDMs is decreased globally (Fig. 4B). GPx activity is positively correlated to increased oxidative stress in Controls. In low glucose conditions, Control and LGA-GDM MSCs behave similarly. LGA-GDM with high glucose treatment shows a significant decrease of GPx activity, which was maintained when exposed to additional oxidative stress (Fig. 4C). Therefore, under high glucose conditions LGA-GDM MSCs show a blunted GPx response. This paradoxical decrease in GPx activity in LGA-GDM hUC-MSCs reveals an attenuated stress response in the setting of high glucose and additional oxidative stress.
FIG. 4.
Antioxidant enzymatic activities were measured in hUC-MSCs from Control (black bars) and LGA-GDM (gray bars) groups. Activity of SOD increased in LGA-GDM hUC-MSCs under HG that was lost with H2O2 treatment (A). Catalase activity decreased in response to H2O2 treatment and was comparable between both Controls and LGA-GDMs in all conditions (B). GPx activity was significantly decreased in the LGA-GDM group under HG conditions alone and in combination with H2O2 treatment when compared with Controls (C). Activity values were normalized in each well by microgram of protein tested. Significant differences (P < 0.05) was determined by two-way ANOVA comparing: Control-LG (a), Control-HG (b), Control-LG+H2O2 (c), Control-HG+H2O2 (d), LGA-GDM-LG (e), LGA-GDM-HG (f), LGA-GDM-LG+H2O2 (g), and LGA-GDM-HG+H2O2 (h). *Indicates comparisons highlighted in the text. GPx, glutathione peroxidase; hUC-MSCs, human umbilical cord mesenchymal stem cells; SOD, superoxide dismutase.
Discussion
Our study uniquely combines high glucose and H2O2 exposure in a long-lived stem cell population. We show that glucose and hydrogen peroxide have a combined effect on oxidative stress sensitivity in LGA-GDM hUC-MSCs; this evidences toward a glucose-sensitive cellular memory involved in the delayed consequences of early life exposures found in infants of diabetic mothers. The complexity of disease states, where multiple insults occur simultaneously, can be replicated by the combined treatment of high glucose and oxidative stress. For example, high glucose sensitizes hepatocytes to H2O2-induced cell death that was not induced by high glucose or H2O2 alone [29]. The impact of high glucose exposure in influencing a differential response to glucose re-exposure or oxidative stress-inducing conditions has recently been documented. Skin fibroblast cells from diabetic patients exhibited differential gene expression during in vitro high glucose exposure compared with nondiabetic control patients [25].
Our findings provide a mechanistic link for differential responses to later oxidative stress-inducing insults that occur with aging and age-related disease. With increasing age, declining tolerance to oxidative stress leads to accumulation of damage and ultimately cellular dysfunction [30]. When we mimic these conditions through hydrogen peroxide treatment and high glucose exposure, we observe differences between the stress responses of Control and LGA-GDM cells. The model suggested is one of LGA babies born to GDM mothers having an impaired ability to combat oxidative stress, a susceptibility propagated into adulthood by having imprinted their long-lived stem/progenitor cells. We demonstrated a similar model in rodents with maternal under- and overnutrition [31]. We showed that expression patterns of key metabolic genes in a 6-month exposed offspring were dysregulated at levels similar to advanced age animals compared with age-matched controls [31]. These observations cumulatively suggest that early exposure to hyperglycemia reprograms MSCs, affecting their oxidative stress-related mechanisms, and reducing their long-term capacity to handle further exposures. This can then trigger oxidative stress-related mechanisms of disease development, creating a vicious cycle of ROS-inducing cellular dysfunction.
After hydrogen peroxide treatment, we found major alterations in the oxidative stress response. As expected, DNA damage and ROS levels were increased in both groups, but to a greater degree in the LGA-GDMs. Our measurement of the antioxidant enzyme activities helps us to characterize the differential response in LGA-GDMs to high glucose and oxidative stress-inducing peroxide treatment. We observed a decrease in Catalase and SOD activities after peroxide treatment. These findings may seem counterintuitive, but previous studies have also demonstrated decreased activities of both of these enzymes under increased hydrogen peroxide treatment conditions [32–34]. Increased hydrogen peroxide appears to interact with the active site Cu2+ or histidine in SOD [32], whereas intermediates during the decomposition of hydrogen peroxide can induce reversible inactivation of Catalase [32]. Furthermore, the decreases in Catalase and SOD activities were demonstrated in both Controls and LGA-GDMs and therefore do not explain the differential response in LGA-GDMs. However, the reduction of GPx activity observed with high glucose in the LGA-GDMs is conserved after peroxide treatment, suggesting that without the increased activity of SOD and Catalase, the decreased GPx activity is sufficient to explain the increase in DNA damage detected in LGA-GDM cells. GPx activity has previously been associated with DNA damage. For example, GPx-1 knockout mice had five-fold more DNA damage compared with Controls [35]. In humans, decreased GPx activity is associated with increased DNA damage in older men [36]. Conversely, increased GPx activity enhanced protection from hydrogen peroxide-induced DNA damage [37]. However, this only explains our observations for the combined high glucose and oxidative stress conditions. We also see a differential response in DNA damage under low glucose conditions with hydrogen peroxide. Consequently, different mechanisms elicited from glucose-related and hydrogen peroxide-related stress may account for the differential response in DNA damage. Ultimately, our findings emphasize that the cellular stress response is subject to cellular memory, which imparts a dysfunctional stress response leading to damaging effects.
The Developmental Origins of Health and Disease (DOHaD) hypothesis [38] proposes that perturbations resulting from early adverse exposures are maintained throughout the entire life of the individual and contribute to an increased susceptibility to a wide range of diseases [39]. With this study, we provide a possible mechanism for this long-term risk, by demonstrating that after in utero exposure to gestational diabetes, hUC-MSCs have a compromised response to oxidative stress, and that this is exacerbated by concurrent high glucose exposure.
The memory of past cellular states is described as an epigenetic property. Studies from our group [31,40] and others [41,42] have demonstrated alterations in genomic regulatory modifications associated with extremes in fetal growth. Not only are these regulatory changes present at birth [40–42], they persist into adulthood [31,41,42]. Potential future steps of this study are the investigation of the regulatory mechanisms of epigenetic memory involved in the establishment of memory of hyperglycemia in these stem cells. Alterations of these regulatory mechanisms could influence their differential response to oxidative stress, which could also be carried into adulthood. Two epigenetic modifications of particular interest are cytosine methylation and histone O-linked Beta-N-acetyl glucosamine (O-GlcNAc). Cytosine methylation is environmentally responsive, but also stably passed to daughter cells. The O-GlcNAc modification on histones relays metabolic status within the cell to the regulation of chromatin structure, gene expression, and possibly DNA methylation or hydroxyl-methylation through its interactions with the methylcytosine dioxygenase (TET2) [43]. Therefore, studies can be designed to investigate the epigenetic effects of high glucose exposure in utero and how they may participate in the response to re-exposure to high glucose and oxidative stress.
In summary, our study is unique in its approach to find a differential stress response of LGA-GDM MSCs, not only to high glucose and hydrogen peroxide, but also with testing their combinatorial effect. We find that exposure to high glucose during fetal development impairs oxidative stress response in hUC-MSCs. The decreased activity of GPx in the setting of high glucose and additional oxidative stress leads to increased ROS and DNA damage. It is important to understand how these stem cells respond to stress, as they may pass this altered response to their downstream differentiated cell types, maintaining a cellular epigenetic memory over the lifetime of the individual.
Supplementary Material
Acknowledgments
This work was supported by Einstein's Medical Scientist Training Program NIH NIGMS T32 GM007288 and Einstein's Flow Cytometry Core Facility P30CA013330.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Scheen AJ. (2003). Pathophysiology of type 2 diabetes. Acta Clin Belg 58:335–341 [DOI] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). (2016). Global Report on Diabetes. WHO, Geneva [Google Scholar]
- 3.DeSisto CL, Kim SY. and Sharma AJ. (2014). Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duran A, Saenz S, Torrejon MJ, Bordiu E, del Valle L, Galindo M, Perez N, Herraiz MA, Izquierdo N, et al. (2014). Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care 37:2445–2450 [DOI] [PubMed] [Google Scholar]
- 5.Teasdale F. and Jean-Jacques G. (1985). Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from mid-gestation to term. Placenta 6:375–381 [DOI] [PubMed] [Google Scholar]
- 6.Illsley N, Lin H. and Verkman A. (1987). Lipid domain structure correlated with membrane protein function in placental microvillous vesicles. Biochemistry 26:446–454 [DOI] [PubMed] [Google Scholar]
- 7.Kc K, Shakya S. and Zhang H. (2015). Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66 (Suppl. 2):14–20 [DOI] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R. and Vohr BR. (2005). Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 9.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr., Liese AD, Vehik KS, Narayan KM, Zeitler P. and Hamman RF. (2008). Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TC, Hsu MF. and Wu KK. (2015). High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One 10:e0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F, Jia W, Yao Y, Hu Y, Lei L, Lin J, Sun X. and Liu L. (2007). Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed type 2 diabetes. Clin Sci (Lond) 112:599–606 [DOI] [PubMed] [Google Scholar]
- 12.Susztak K, Raff AC, Schiffer M. and Bottinger EP. (2006). Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55:225–233 [PubMed] [Google Scholar]
- 13.Fulda S, Gorman AM, Hori O. and Samali A. (2010). Cellular stress responses: cell survival and cell death. Int J Cell Biol 2010:214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simone S, Gorin Y, Velagapudi C, Abboud HE. and Habib SL. (2008). Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes 57:2626–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, et al. (2003). Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 88:4673–4676 [DOI] [PubMed] [Google Scholar]
- 16.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H. and Bashan N. (1998). Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 47:1562–1569 [DOI] [PubMed] [Google Scholar]
- 17.Kaneto H, Katakami N, Matsuhisa M. and Matsuoka TA. (2010). Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm 2010:453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiedge M, Lortz S, Drinkgern J. and Lenzen S. (1997). Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 19.Okoduwa SI, Umar IA, Ibrahim S, Bello F. and Habila N. (2015). Age-dependent alteration of antioxidant defense system in hypertensive and type-2 diabetes patients. J Diabetes Metab Disord 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinisch A. and Strunk D. (2009). Isolation and animal serum free expansion of human umbilical cord derived mesenchymal stromal cells (MSCs) and endothelial colony forming progenitor cells (ECFCs). J Vis Exp 32:1525–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hass R, Kasper C, Bohm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D. and Clement MV. (2014). OpenComet: an automated tool for comet assay image analysis. Redox Biol 2:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd and Ischiropoulos H. (2012). Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GY, Chen KJ, Lin-Shiau SY. and Lin JK. (1999). Peroxyacetyl nitrate-induced apoptosis through generation of reactive oxygen species in HL-60 cells. Mol Carcinog 25:196–206 [DOI] [PubMed] [Google Scholar]
- 25.Caramori ML, Kim Y, Natarajan R, Moore JH, Rich SS, Mychaleckyj JC, Kuriyama R, Kirkpatrick D. and Mauer M. (2015). Differential response to high glucose in skin fibroblasts of monozygotic twins discordant for type 1 diabetes. J Clin Endocrinol Metab 100:E883–E889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC. and Stetina R. (2008). The comet assay: topical issues. Mutagenesis 23:143–151 [DOI] [PubMed] [Google Scholar]
- 27.Alhama J, Ruiz-Laguna J, Rodriguez-Ariza A, Toribio F, Lopez-Barea J. and Pueyo C. (1998). Formation of 8-oxoguanine in cellular DNA of Escherichia coli strains defective in different antioxidant defences. Mutagenesis 13:589–594 [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian B, Pogozelski WK. and Tullius TD. (1998). DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci U S A 95:9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata H, Ichikawa T, Nakao K, Miyaaki H, Takeshita S, Akiyama M, Fujimoto M, Miuma S, Kanda S, Yamasaki H. and Eguchi K. (2008). A high glucose condition sensitizes human hepatocytes to hydrogen peroxide-induced cell death. Mol Med Rep 1:379–385 [PubMed] [Google Scholar]
- 30.Brandl A, Meyer M, Bechmann V, Nerlich M. and Angele P. (2011). Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res 317:1541–1547 [DOI] [PubMed] [Google Scholar]
- 31.Heo HJ, Tozour JN, Delahaye F, Zhao Y, Cui L, Barzilai N. and Hughes Einstein F. (2016). Advanced aging phenotype is revealed by epigenetic modifications in rat liver after in utero malnutrition. Aging Cell 15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray RC, Cockle SA, Fielden EM, Roberts PB, Rotilio G. and Calabrese L. (1974). Reduction and inactivation of superoxide dismutase by hyodrogen peroxide. Biochem J 139:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lardinois OM, Mestdagh MM. and Rouxhet PG. (1996). Reversible inhibition and irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim Biophys Acta 1295:222–238 [DOI] [PubMed] [Google Scholar]
- 34.Wijartne SSK, Cuppett SL. and Schlegel V. (2005). Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colone cells. J Agric Food Chem 53:8768–8774 [DOI] [PubMed] [Google Scholar]
- 35.Reddy VN, Lin LR, Ho YS, Magnenat JL, Ibaraki N, Giblin FJ. and Dang L. (1997). Peroxide-induced damage in lenses of transgenic mice with deficient and elevated levels of glutathione peroxidase. Ophthalmologica 211:192–200 [DOI] [PubMed] [Google Scholar]
- 36.Mendoza-Nunez VM, Beristain-Perez A, Perez-Vera SP. and Altamirano-Lozano MA. (2010). Age-related sex differences in glutathione peroxidase and oxidative DNA damage in a healthy Mexican population. J Womens Health 19:919–926 [DOI] [PubMed] [Google Scholar]
- 37.Sandstrom BE. and Marklund SL. (1990). Effects of variation in glutathione peroxidase activity on DNA damage and cell survival in human cells exposed to hydrogen peroxide and t-butyl hydroperoxide. Biochem J 271:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, Godfrey K, Gluckman P, Hanson M, et al. (2007). Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr Res 61:625–629 [DOI] [PubMed] [Google Scholar]
- 39.Bateson P, Barker DJ, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, et al. (2004). Developmental plasticity and human health. Nature 430:419–421 [DOI] [PubMed] [Google Scholar]
- 40.Delahaye F, Wijetunga NA, Heo HJ, Tozour JN, Zhao YM, Greally JM. and Einstein FH. (2014). Sexual dimorphism in epigenomic responses of stem cells to extreme fetal growth. Nat Commun 5:5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai M, Jellyman JK, Han G, Lane RH. and Ross MG. (2015). Programmed regulation of rat offspring adipogenic transcription factor (PPARgamma) by maternal nutrition. J Dev Orig Health Dis 6:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Stoffers DA, Nicholls RD. and Simmons RA. (2008). Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118:2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Chen Y, Bian C, Fujiki R. and Yu X. (2013). TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 493:561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.