Abstract
Emerging evidence demonstrates that megakaryocytes (MK) play key roles in regulating skeletal homeostasis and hematopoiesis. To test if the loss of MK negatively impacts osteoblastogenesis and hematopoiesis, we generated conditional knockout mice where Mpl, the receptor for the main MK growth factor, thrombopoietin, was deleted specifically in MK (Mplf/f;PF4cre). Unexpectedly, at 12 weeks of age, these mice exhibited a 10-fold increase in platelets, a significant expansion of hematopoietic/mesenchymal precursors, and a remarkable 20-fold increase in femoral midshaft bone volume. We then investigated whether MK support hematopoietic stem cell (HSC) function through the interaction of MK with osteoblasts (OB). LSK cells (Lin−Sca1+CD117+, enriched HSC population) were co-cultured with OB+MK for 1 week (1wk OB+MK+LSK) or OB alone (1wk OB+LSK). A significant increase in colony-forming units was observed with cells from 1wk OB+MK cultures. Competitive repopulation studies demonstrated significantly higher engraftment in mice transplanted with cells from 1wk OB+MK+LSK cultures compared to 1wk OB+LSK or LSK cultured alone for 1 week. Furthermore, single-cell expression analysis of OB cultured±MK revealed adiponectin as the most significantly upregulated MK-induced gene, which is required for optimal long-term hematopoietic reconstitution. Understanding the interactions between MK, OB, and HSC can inform the development of novel treatments to enhance both HSC recovery following myelosuppressive injuries, as well as bone loss diseases, such as osteoporosis.
Keywords: : megakaryocytes, osteoblasts, hematopoietic stem cells, bone regeneration, hematopoiesis
Introduction
Megakaryocytes (MK) are hematopoietic stem cell (HSC)-derived cells and are the largest and one of the rarest cells (0.05%–0.1%) found in the bone marrow (BM) [1]. They produce platelets, which stop bleeding and minimize blood vessel injury. Within the BM, they are primarily located centrally, near sinusoidal endothelial cells, where they release platelets into the circulation [2]. MK also play significant roles in the maintenance/quiescence of HSC and bone formation [3,4].
Recent whole-mount imaging and computational studies in mice show that HSC are frequently located adjacent to MK. This suggests a functional relationship, and MK have been postulated to regulate HSC quiescence and pool size [3,4]. Using genetic strategies to deplete MK, two groups observed that in the absence of MK, HSC proliferation increased, suggesting that MK maintain HSC quiescence in the niche [3,4]. MK expression of PF4 (platelet factor 4, also known as CXCL4) and transforming growth factor β was implicated in this process. Of importance, these studies genetically regulated MK in vivo; therefore, they could result from direct MK activities on HSC or through indirect interactions between MK and other components of the niche.
Work from Nilsson's laboratory describes an indirect effect of MK on HSC quiescence [5]. This group has shown that mature MK produce factors that generate thrombin, which in turn cleaves osteopontin (an extracellular matrix protein found at the endosteal surface), opening a cryptic binding site for α4β1 and α9β1 integrins. HSC binding to this site resulted in maintenance of HSC quiescence through inhibiting proliferation and differentiation [5]. These studies elucidate the role of MK in supporting HSC maintenance.
The endosteal niche is a complex structure lining the interiors of cortical bone and trabecular bone surfaces that contain several mesenchymal lineage cells. Endosteal osteoblasts (OB) are derived from mesenchymal precursors and are involved in the regulation of bone formation and HSC function [6,7]. Our group and others have demonstrated that less mature OB-lineage cells better support hematopoiesis than more mature cells [8–10]. Thus, it is likely that, increased osteoblastogenesis alters the BM microenvironment/HSC niche. Indeed, our group has previously demonstrated that MK regulate bone formation by increasing osteoblastogenesis through direct cell-to-cell contact [11]. A portion of this effect is mediated by β1 integrin and Pyk2 signaling pathways [12,13].
MK regulation of bone formation in vivo was demonstrated when mice, deficient in transcription factors required for normal MK development (GATA-1 or NF-E2), exhibited significant increases in immature MK numbers and trabecular bone [11]. In addition, mice overexpressing thrombopoietin (TPO), the major growth factor for MK, also have increased numbers of MK and present with an osteosclerotic bone phenotype [14]. Therefore, mouse models with increased numbers of MK in BM consistently induce a high bone mass phenotype. Paradoxically, MK inhibit OB differentiation in vitro. Specifically, OB-lineage cells in long-term co-cultures (14 days) showed that MK inhibited the expression of OB differentiation markers [15]. These data demonstrate that MK regulate OB proliferation and differentiation [11,15].
To further understand the impact that interactions between MK and OB-lineage/osteoprogenitor cells have on bone formation and hematopoiesis, we generated mice where the TPO receptor, the cellular homologue of the myeloproliferative leukemia virus oncogene (Mpl), was specifically deleted in MK (Mplf/f;PF4cre) to examine the effect of the loss of MK on OB-mediated hematopoiesis. Our results are consistent with work by Ng et al. [16] who recently characterized the hematopoietic phenotype of a similar mouse model. Although some of our data confirm what Ng et al. documented, we demonstrate a previously unknown interaction between MK and OB that leads to enhanced OB-mediated support of hematopoiesis. This effect is likely mediated through MK-induced expression of adiponectin by OB-lineage cells. Unexpectedly, we also confirm the ability of MK to regulate bone formation by generating a mouse model of megakaryocytosis, resulting in a high bone mass phenotype.
Materials and Methods
Animals
Commercially available, C57BL/6, B6.SJL-Pt_cqPep3b/BoyJ (BoyJ), C57BL/6 X BoyJ (F1), and C57BL/6-Tg (PF4-icre) Q3Rsko/J (The Jackson Laboratory, Bar Harbor, ME) mice were used in this study. Mice were bred and housed in the animal facility at Indiana University. For transplantation studies, recipient mice received 1,000 cGy ionizing radiation from a cesium source (650 and 350 cGy, split dose). All procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and followed National Institutes of Health guidelines.
Generation of Mpl conditional knockout mice
The conditional knockout strategy used in preparing these mice involved the introduction of loxP sites flanking coding exon 3 of the Mpl gene by homologous recombination (Ingenious Targeting Laboratory, Ronkonkoma, NY). Briefly, a 7.78 kb region used to construct the targeting vector was first subcloned from a positively identified C57BL/6 BAC clone (RP23: 118J14) into the backbone vector (pSP72; Promega, Madison, WI). The 5′ homology arm extends about 5.31 kb and contains the first two exons. The 5′ loxP site, containing an engineered EcoRV, Msc I, and Sca I site for Southern blot analysis, was inserted upstream of exon 3. The loxP-FRT-flanked pGK-gb2-Neo cassette was inserted downstream of exon 3 with the 3′arm extending 1.9 kb. The targeting construct was verified by restriction analysis and sequencing after each modification step.
Targeting of the Mpl locus was confirmed by Southern blot of EcoRV-digested genomic DNA hybridized with a 5′ probe from targeted iTL BA1(C57BL/6 × 129/SvEv) hybrid embryonic stem cells microinjected into C57BL/6 blastocysts. Resulting chimeras with a high percentage agouti coat color were mated to C57BL/6 FLP mice to remove the Neo cassette. Mice were genotyped by polymerase chain reaction (PCR) using the following primers: (1) 5′-ctgcctctctacctgctcaagctc-3′ and (2) 5′-gaggaaggttgcctgtcctg-3′. Mice homozygous for the Mpl floxed allele (Mplf/f) on a mixed C57BL/6-Sv129 genetic background were crossed with PF4Cre on a C57BL/6 background. Unless specified, male and female Mplf/f;PF4cre and their littermate counterparts (Mplf/f, Mplf/+;PF4cre) were used at 8, 12, and 17 weeks of age.
Quantitative real-time PCR
Total RNA was isolated using the RNAeasy kit (Qiagen, Valencia, CA) and was used to generate complementary DNA (cDNA) by reverse transcription according to the manufacturer's instructions (Transcriptor First Strand cDNA Synthesis kit; Roche, Indianapolis, IN). PCR were performed in an MX3000 detection system using SYBR green PCR reagents following the manufacturer's instructions (Life Technologies, Warrington, UK). For Mpl, and GAPDH expression, their specific pairs of primers were used, a calibration curve was performed, and all oligonucleotides were tested to ensure specificity and sensitivity. For each sample, arbitrary units obtained using the standard curve and the expression of GAPDH was used to normalize the amount of the investigated transcript.
Mpl 5′ tcaccttggtgactgctctg 3′ 5′ ggacttagggctgcagtgtc 3′
GAPDH 5′ accacagtccatgccatcac 3′ 5′ tccaccaccctgttgctgta 3′
TPO serum concentrations
Whole blood was collected, serum separated, and stored at −80°C until assayed. The Mouse Thrombopoietin Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) was used to determine the TPO serum concentration, following the manufacturer's instructions.
Complete blood counts
Whole blood was collected and complete blood counts were performed using a validated HEMAVET® 950FS Hematology System (Drew Scientific, Waterbury, CT).
Histology
Femurs were dissected and fixed in 10% neutral buffered formalin for 48 h before being transferred to 70% ethanol for storage. Static histomorphometric measurements were performed on von Kossa stained (AgNO3; Sigma-Aldrich, St. Louis, MO) and toluidine blue (Sigma-Aldrich) counterstained sections to determine OB and MK number [17]. Tartrate-resistant alkaline phosphatase (TRAP) staining was performed to visualize osteoclasts (OC).
Ex vivo microcomputed tomography
Microcomputed tomography (μCT, Skyscan 1172; SkyScan, Kontich, Belgium) was used to determine properties of the trabecular and cortical bone from both the distal and midshaft of the right femur. Parameters used were as follows: 60 kV, 0.5 mm aluminum filter, and 6 μm pixel size [18]. Trabecular bone values from the distal femur were obtained from a 0.5 mm thick region, 0.5 mm proximal from the distal growth plate, and included the following: bone volume/tissue volume (BV/TV; %), trabecular number (Tb.N; mm−1), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm). Cortical and midshaft trabecular bone values were obtained from a 1 mm thick region covering an area 0.5 mm proximal and distal from the midpoint of the femur. Cortical parameters included were as follows: bone area (B.Ar; mm2), cortical area (Ct.Ar; mm2), marrow area (M.Ar; mm2), BV/TV, mean polar moment of inertia (MMI polar; mm4), and cortical thickness (Ct.Th; mm). Midshaft trabecular parameters measured were the same as for the distal femur.
Preparation of OB and MK
Calvarial OB were prepared after a modification of published methods [19,20]. Briefly, calvariae from C57BL/6 mice <48 h old were dissected, pretreated with EDTA (4 mM; Sigma-Aldrich) in phosphate-buffered saline (PBS) for 30 min, and then subjected to sequential collagenase digestions (200 U/mL; Worthington Biomedical, Lakewood, NJ) at 37°C. Fractions 3–5 (collected between 45–60, 60–75, and 75–90 min through the digestion) were collected and used as OB. These cells are ∼95% OB or OB precursors and 5% osteal macrophages. Freshly prepared OB were used for all studies. To isolate MK, livers from E13 to E15 fetal mice were collected and single-cell suspensions were prepared and cultured in Dulbecco's modified Eagle's medium (Life Technologies) with 10% fetal calf serum (Hyclone, Logan, UT) and 1% conditioned medium from a murine TPO-secreting fibroblast cell line. MK were separated from other cells using a one-step albumin gradient (Probumin; Millipore, Kankakee, IL) [21].
Flow cytometry for HSC, mesenchymal stem cell, and OB phenotyping
Low-density BM cells were washed once with stain wash (PBS, 1% bovine calf serum, and 1% penicillin-streptomycin), followed by antibody staining for 15 min on ice with phycoerythrin (PE)-conjugated CD3, CD4, CD45R, Ter119, and Gr1; Alexa Fluor 700 (AF700)-conjugated c-Kit (CD117); (PE)-Cy7-conjugated Sca1; fluorescein isothiocyanate (FITC)-conjugated CD34; Allophycocyanin (APC)-conjugated Flk2 (CD135); PerCP-Cy5.5-conjugated IL7Ra (CD127); and APC-Cy7 conjugated anti-Fc-γR (CD16/32). All monoclonal antibodies were obtained from BD Biosciences (San Jose, CA), except CD127 from eBioscience (San Diego, CA) and anti-Fc-γR from BioLegend (San Diego, CA). Cells were washed once more with stain wash buffer, and data were acquired on BD LSRII (BD Biosciences). Progenitor cell content was gated and analyzed as described before [22], using FlowJo software (FlowJo, LLC, Ashland, OR).
For mesenchymal stem cell (MSC) phenotyping, BM cells were washed once with stain wash followed by antibody staining for 15 min on ice with PE-Cy7-conjugated CD45; FITC-conjugated CD31 and Ter119; PE-conjugated CD51 (BD Biosciences); and APC-conjugated PDGFRa (BioLegend). Lineage (CD45, CD31, and Ter119)-negative cells were analyzed for the presence of CD51 and PDGFRa [23]. For MK phenotyping, BM cells were treated as above, stained with PE-conjugated CD41 and FITC-conjugated CD61 (BD Biosciences), and analyzed for the presence of CD41 and CD61. Cell sorting was performed on a BD fluorescence-activated cell sorting (FACS) Aria and flow cytometric analysis was done on a BD LSRII.
For OB phenotyping, OB were generated from femurs and the epiphyses were removed from the diaphysis, forming two separate groups for analysis. The BM from the diaphysis was then flushed. After flushing, the bone pieces from both the groups were minced into segments (<1-mm), and then the bone segments were washed once with PBS. Bone segments were then subjected to five consecutive 15 min collagenase digestions. Cells were harvested after each digestion, pooled, and then stained with FITC-conjugated CD45, Ter119, and CD31 (BioLegend); APC: Cy7-conjugated Sca-1 (BioLegend); and PE-conjugated CD166 (eBioscience). CD45−Ter119−CD31−Sca-1− cells were gated as OB and then analyzed for the presence or absence of CD166 on a BD Fortessa (BD Biosciences). Data were analyzed and plots were made using FlowJo software.
Cell co-culture and colony-forming unit assay
Neonatal 2-day calvarial OB and fetal liver MK were prepared as detailed above and as previously described [19,20]. Calvarial OB were cultured alone (60,000 cells/well) or with MK (100,000 cells/well) for 7 days in α-minimum essential medium (α-MEM; Life Technologies) with 10% fetal bovine serum (FBS; Hyclone), supplemented with ascorbic acid (50 μg/mL; Sigma-Aldrich). MK were removed from the wells (four washes) as described before [19,20]. Freshly isolated OB, OB cultured for 1 week, OB co-cultured with MK for 1 week, and MK alone were seeded in 12-well plates for ∼24 h before co-culture with 1,000 freshly sorted murine LSK (Lin−Sca1+CD117+, enriched HSC population) cells from BoyJ (CD45.1) mice.
The medium consisted of 1:1 mix of Iscove's modified Dulbecco's medium (Life Technologies) and α-MEM supplemented with a cocktail of cytokines containing recombinant murine stem cell factor (SCF) and IL-3 (10 ng/mL; PeproTech, Rocky Hill, NJ), IGF-1 and TPO (20 ng/mL; PeproTech), IL-6 and fms-related tyrosine kinase 3 (Flt3; 25 ng/mL; PeproTech), and osteopontin (50 ng/mL; R&D Systems). Cultures were maintained for 7 days and hematopoietic cells were then harvested and plated in duplicate in 3 cm Petri dishes containing 1 mL of methylcellulose with included cytokines (recombinant human IL-6, erythropoietin, and insulin; recombinant mouse SCF, IL-3; MethoCult™ GF M3434; STEMCELL Technologies, Vancouver, Canada) for colony-forming unit (CFU) assay. Cultures were maintained at 37°C in a humidified incubator at 5% CO2, and colonies were counted on an inverted microscope after 7 days.
Bone marrow repopulating assay
Cultures of 1wkOB+LSK, 1wkOB+MK+LSK, and LSK were maintained in 12-well plates for 5 days and then co-transplanted with 100,000 C57BL/6 (CD45.2) competitor cells through intravenous injection into lethally irradiated CD45.2 × CD45.1 F1 recipients (4–5 recipients/group). Chimerism was assessed monthly for 4 months using peripheral blood (PB). After red blood cells were lysed with RBC lysis buffer (BioLegend), cells were stained with FITC-conjugated CD45.1 and PE-conjugated CD45.2, and chimerism was assessed as [CD45.2/(CD45.2 + CD45.1)]/100. This calculation was therefore independent of any residual host-derived HSC activity. The mice were euthanized at 4 months and marrow flushed from the femurs was stained as described above with CD45.1 and CD45.2 antibodies, and chimerism was determined.
Microfluidic chip-based single-cell analysis
Calvarial OB cultures were plated±MK and were collected at day 4. Co-cultures were washed four times with PBS to remove MK, trypsinized, and FACS sorted. CD45− OB-lineage single cells from both cultures were used for single-cell quantitative PCR (qPCR) on the Fluidigm platform (San Jose, CA). Briefly, on a C1 integrated fluidic circuit chip (IFC), 56–84 individual cells were captured, cDNA synthesized, and product preamplification was performed. Individual cell qPCR was done using a Biomark 96.96 dynamic array. Initial data analysis was completed using Fluidigm real-time PCR analysis software. Differential gene expression was determined and violin plots generated with the FluidigmSC R-package software.
Statistical analysis
Data are presented as mean ± standard deviation and where applicable, triplicate samples were measured. Two-tailed Student's t-tests were performed when two groups were compared. One-way and two-way analysis of variance (ANOVA) were used for multiple group comparisons, followed by Tukey's honest significant difference (HSD) or Bonferroni's post hoc t-tests. Significance was set at 0.05.
Results
Development of Mpl−/− MK conditional knockout mice
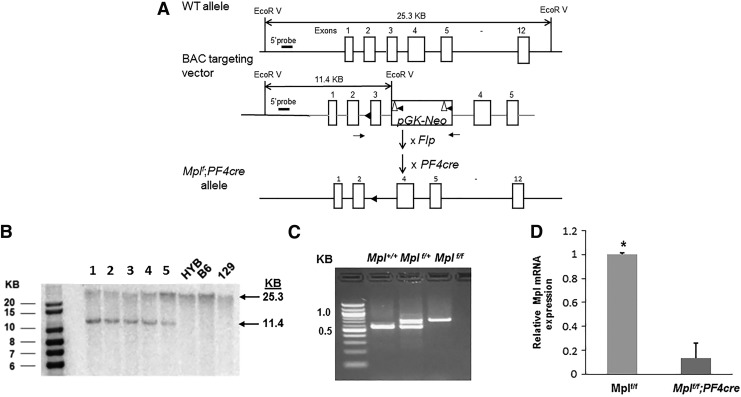
We generated conditional knockout mice where Mpl was deleted specifically in MK (Mplf/f;PF4cre). Our strategy involved the introduction of loxP sites flanking coding exon 3 of the Mpl gene by homologous recombination (Fig. 1A), similar to the strategy used to generate the global Mpl knockout mice (Mpl−/−) [24]. Southern blot analysis of embryonic stem cell clones (Fig. 1B) and PCR of mouse tail DNA (Fig. 1C) were used to confirm homologous recombination. We mated homozygous Mplf/f mice with PF4cre mice; PF4 is a platelet-specific chemokine that is expressed by MK and platelets [25]. qPCR was used to evaluate the expression of Mpl in MK isolated from Mplf/f and Mplf/f;PF4cre, which confirmed a decrease of 90%–95% in MK from Mplf/f;PF4cre mice (Fig. 1D).
FIG. 1.
Gene targeting of exon 3 in the Mpl gene. (A) Schematic of the targeting strategy. The 5′ loxP site was inserted upstream of exon 3, followed by the insertion of an Frt-pGK-loxP-Neo-Frt-loxP selection cassette. The expected EcorV-digested fragments for wild-type and targeted alleles are indicated by large arrows. The 5′ external probe is indicated by a short line and the 5′ and 3′ homology arms are shown in gray. LoxP and Frt sites are shown by filled triangles and empty triangles, respectively. Genotyping primers are shown as small arrows. Flp, Flp recombinase mice; PF4cre, Platelet factor 4 cre recombinase mice. (B) Southern blot analysis with the 5′ probe. 1–5, Mplf/+ mouse lines; HYB, C57BL/6/129 hybrid; B6, C57BL/6; 129, 129/SvEv mice. (C) PCR analysis of mouse tail DNA obtained from Mplf/+ intercrosses, indicating transmission of the targeted (KO) allele. (D) qPCR of cDNA from MK showing expression of Mpl, n = 3–5 mice per genotype, Student's t-test, *P < 0.001. cDNA, complementary DNA; KO, knockout; MK, megakaryocytes; qPCR, quantitative polymerase chain reaction.
Mplf/f;PF4cre mice exhibit thrombocytosis and megakaryocytosis
Complete blood cell counts revealed that Mplf/f;PF4cre mice did not exhibit thrombocytopenia as is seen in global Mpl−/− mice [24,26]. Instead, there was a significant 10-fold increase in the number of platelets compared to Mplf/f and Mplf/+;PF4cre mice (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). In Mplf/+;PF4cre, one wild-type Mpl allele appeared to rescue the increase in platelet levels. MK and platelets are thought to be the major consumers of TPO through Mpl and a decrease in the expression of Mpl on MK shifts the steady state of TPO levels higher in the circulation as shown in Mpl−/− mice [24]. Therefore, we analyzed the serum levels of TPO in Mplf/f;PF4cre mice. Unexpectedly, the concentration of TPO in the serum of Mplf/f;PF4cre was not significantly different than in Mplf/f or the Mplf/+;PF4cre mice (Supplementary Fig. S1) [16]. HSC express Mpl [27] and recently, we have shown that OB and OC precursors also express Mpl and are responsive to TPO [28]. As the TPO serum concentrations between the three genotypes were not different, we surmise that TPO serum levels are regulated, in part, by a process independent of MK/platelet consumption.
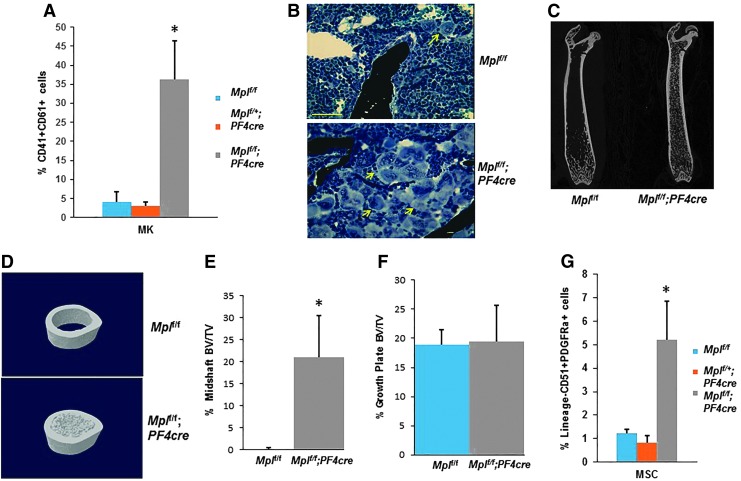
While Mplf/f;PF4cre mice are healthy and appear no different from controls, they displayed significant splenomegaly compared to Mplf/f and Mplf/+;PF4cre mice (Supplementary Table S2). FACS analysis of BM cells indicated a sevenfold increase in the percentage of MK (CD41+CD61+) in the Mplf/f;PF4cre mice compared to Mplf/f mice (Fig. 2A), and is consistent with the high numbers of platelets in the mutant mice (Supplementary Table S1). Histological staining also confirmed the expansion of the MK pool in the mutant BM (Fig. 2B).
FIG. 2.
Skeletal and mesenchymal stem cell phenotype of the Mpll/f;PF4cre mice. (A) The percentage of MK in the BM. (B) Histological sections of the BM stained with von Kossa and toluidine blue. Yellow arrows indicate MK. Scale bar = 100 μm. (C, D) Longitudinal and cross-sectional μCT images of Mplf/f and Mplf/f;PF4cre femurs. BV/TV in the femoral midshaft (E) and near the growth plate (F). n = 6–8 mice/genotype, Student's t-test, *P < 0.001 (Table 1). (G) FACS analysis of the mesenchymal stem cell populations in the BM. N = 3–5 mice/genotype. One-way ANOVA, *P < 0.002. μCT, microcomputed tomography; ANOVA, analysis of variance; BM, bone marrow; BV/TV, bone volume/tissue volume. FACS, fluorescence-activated cell sorting.
High bone mass phenotype in Mplf/f;PF4cre mice
To determine if the increased BM megakaryopoiesis impacted bone volume, we investigated the skeletal phenotype of Mplf/f;PF4cre mice. μCT of femurs from female Mplf/f;PF4cre mice revealed a medullary canal replete with trabecular bone at 12 weeks of age, compared to the Mplf/f mice (Fig. 2C, D). This striking phenotype represents a 20-fold increase in femoral BV/TV in Mplf/f;PF4cre animals at the diaphysis (Fig. 2E, F and Table 1). The skeletal phenotype of the Mplf/+;PF4cre was similar to the Mplf/f mice (data not shown). The Tb.Th, Tb.N, and Tb.Sp were also significantly different in the Mplf/f;PF4cre compared to the Mplf/f mice at the diaphysis (Table 1). At the distal epiphysis, there were no differences in the BV/TV, and Th.Sp; however, Tb.Th and Tb.N were significantly different between the genotypes (Table 1).
Table 1.
Skeletal Phenotype of 12-Week-Old Mplf/f;PF4cre Female Mice
| μCT | Static histomorphometry | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | BV/TV | Tb.Th | Tb.N | Tb.Sp | N.Ob/mm | N.Oc/mm | |
| Epiphysis | Mplf/f | 17 ± 2 | 0.03 ± 0.001 | 5.8 ± 0.7 | 0.17 ± 0.03 | 10.0 ± 2.5 | 2.9 ± 0.3 |
| Mplf/f;PF4cre | 20 ± 3 | 0.02 ± 0.003a | 8.4 ± 1.8a | 0.15 ± 0.02 | 5.8 ± 0.6a | 1.9 ± 0.6a | |
| Diaphysis | Mplf/f | 0.02 ± 0.04 | 0.02 ± 0.01 | 0.03 ± 0.0 | 0.72 ± 0.04 | N/D | N/D |
| Mplf/f;PF4cre | 25 ± 6a | 0.03 ± 0.01a | 4.6 ± 1.1a | 0.10 ± 0.02a | 12.0 ± 2.7 | 2.7 ± 0.8 | |
μCT n = 6–8, static histomorphometry n = 3–5 mice. Results are presented as mean ± SEM. Significant difference compared to Mplf/f.
p < 0.05.
μCT, microcomputed tomography; BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; N.Ob/mm, number of osteoblasts/mm; N.Oc/mm, osteoclast number/mm; SEM, standard error of the mean.
In addition, histomorphometric analysis indicated that there was a significant 42% and 34% reduction in the number of OB/bone surface (N.Ob/BS) and OC/bone surface (N.Oc/BS), respectively, in the Mplf/f;PF4cre mice compared to controls, suggesting low bone turnover (Table 1). That said, the total number of OB and OC in the distal femur (N.Ob/T.Ar and N.Oc/T.Ar) was higher in the mutant Mplf/f;PF4cre mice because of the increased bone surface in these animals (Table 1). We also analyzed the N.Ob/BS and N.Oc/BS at the epiphysis and diaphysis in only the Mplf/f;PF4cre mice, and found no differences in their numbers (Supplementary Table S3). It should be noted that N.Ob/T.Ar and N.Oc/T.Ar were not reported as the defined regions, were different due to differences in bone geometry.
Next, we examined the cortical bone phenotype at the midshaft using μCT. As shown in Fig. 2D and as reported in Table 2, at 12 weeks of age, female Mplf/f;PF4cre mice had a small, but significantly higher amount of cortical bone, as reflected by BV/TV and bone area at this site. Because there was no significant increase in cortical area (Ct.Ar), this indicates that the increase in bone mass was primarily driven by increased endosteal, not periosteal bone mass.
Table 2.
Cortical Bone Phenotype for 12-Week-Old Female Mplf/f;PF4cre Mice
| Genotype | N | BV/TV | Ct.Ar. | B.Ar. | M.Ar. | MMI (polar) | Ct.Th. |
|---|---|---|---|---|---|---|---|
| Mplf/f | 6 | 45.39 ± 0.99 | 1.75 ± 0.041 | 0.792 ± 0.021 | 0.954 ± 0.033 | 0.350 ± 0.0146 | 0.178 ± 0.00442 |
| Mplf/f;PF4cre | 7 | 49.80 ± 1.08a | 1.85 ± 0.071 | 0.919 ± 0.031a | 0.930 ± 0.049 | 0.418 ± 0.0300 | 0.191 ± 0.00544 |
Results are presented as mean ± SEM. Significant difference compared to Mplf/f.
p < 0.05.
B.Ar., bone area; Ct.Ar., cortical area; Ct.Th., cortical thickness; M.Ar., marrow area; MMI (polar), polar moment of inertia.
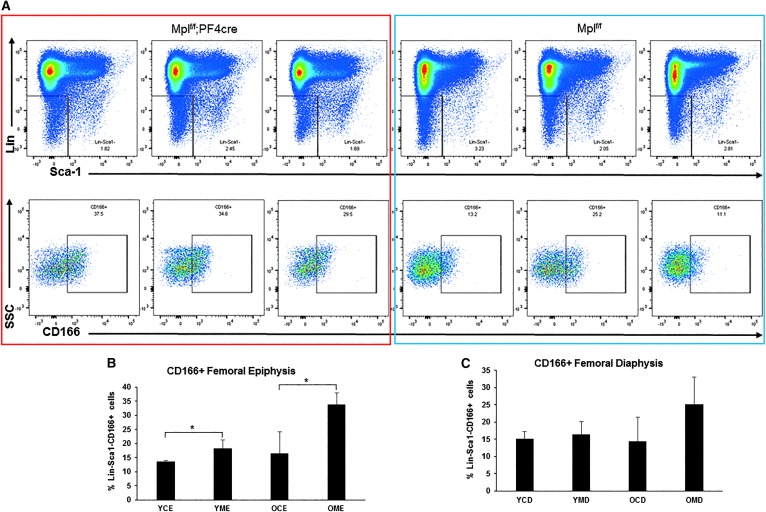
Due to the increased amount of bone present in the marrow space, we performed FACS analysis on the MSC fraction (CD51+ PDGFRa+) in the BM (which contains OB-lineage cells) and found a fourfold increase in the MSC population in Mplf/f;PF4cre mice (Fig. 2G). As we have shown that MK maintain OB in an immature state [11,15], this raised the question, why was there increased bone with increased numbers of MK in the marrow space? Therefore, we investigated variation in the maturity levels of OB at the epiphyses and the diaphyses in Mplf/f;PF4cre mice. We have previously demonstrated that Activated Leukocyte Cell Adhesion Molecule (ALCAM, CD166) is positively associated with immature OB [29]. FACS analysis was performed on OB from the femoral diaphyses and the epiphyses of 8- and 17-week-old Mplf/f and Mplf/f;PF4cre mice (Fig. 3A). The percentage of Lin−Sca1−CD166+ cells was significantly higher at the epiphysis of the Mplf/f;PF4cre mice compared to the Mplf/f mice at 8 and 17 weeks of age (Fig. 3B). This may be due to higher numbers of CD51+PDGFRa+ osteoprogenitors in the Mplf/f;PF4cre mice. However, there were no differences in Lin−Sca1−CD166+ cell number within the diaphysis between the genotypes (Fig. 3C). There were also no significant differences in Lin−Sca1−CD166+ cell number between the epiphysis and diaphysis within either genotype (Fig. 3B, C and Supplementary Fig. S3A–C).
FIG. 3.
FACS analysis of OB maturity in Mpll/f;PF4cre mice femurs. (A) Dotplots showing the Lin−Sca1−CD166+ cells within the epiphyseal region of femurs from 17-week-old Mpll/f;PF4cre and Mpll/f mice. (B) Percentage of CD166+ OB within the femoral epiphyses. YCE, young (8-week old) control (Mplf/f) epiphysis; YME, young (8-week old) mutant (Mplf/f;PF4cre) epiphysis; OCE, old (17-week old) control (Mplf/f) epiphysis; OME, old (17-week old) mutant (Mplf/f;PF4cre) epiphysis. Student's t-test *P < 0.05. (C) Percentage of CD166+ OB within the femoral diaphyseal region. YCD, young (8-week old) control (Mplf/f) diaphysis; YMD, young (8-week old) mutant (Mplf/f;PF4cre) diaphysis; OCD, old (17-week old) control (Mplf/f) diaphysis; OMD, old (17-week old) mutant (Mplf/f;PF4cre) diaphysis. n = 3–5 mice per genotype, One-way ANOVA, *P < 0.05. OB, osteoblasts.
In an effort to assess the divergence in the development of the high bone mass phenotype between females and males, we examined the percentage of males and females at 8 and 12 weeks of age, which presented with atypical bone (>1% BV/TV) within the marrow cavity at the midshaft (Table 3). At 8 weeks, 40% of females had high bone mass at the midshaft, while this was not observed in the males. At 12 weeks, 100% of the females assessed demonstrated high bone mass at the midshaft compared to 30% of the males. It appears that male mice develop this bone phenotype 4–5 weeks later than females.
Table 3.
Development of High Bone Mass Phenotype in Female and Male Mplf/f;PF4cre Mice
| Age (weeks) | Sex | N | High bone mass phenotype (%) |
|---|---|---|---|
| 8 | Female | 2/5 | 40 |
| Male | 0/5 | 0 | |
| 12 | Female | 6/6 | 100 |
| Male | 2/7 | 30 |
The altered HSC phenotype in Mplf/f;PF4cre mice
Erythroid cell number was normal in the PB of all genotypes; however, lymphocyte and monocyte numbers were significantly increased in Mplf/f;PF4cre mice (Supplementary Table S1). To investigate these increases, we analyzed the HSC phenotype using FACS analysis. Mplf/f;PF4cre mice exhibited a two-to-fivefold significant increase in LSK, long- and short-term HSC, multipotent progenitors (MPP), common myeloid progenitors (CMP), and granulocyte/macrophage progenitors (GMP), compared to Mplf/f and Mplf/+;PF4cre mice (Supplementary Fig. S2A). Common lymphoid progenitors (CLP) were not different (Supplementary Fig. S2B). There was a trend for increased MK/erythroid progenitor (MEP; Lin−Il7rα−Sca1−c-Kit+CD16/32−-CD34−) population, although this did not reach statistical significance. Of note, Ng et al. used alternate cell differentiation markers CD150+FcyR+ [30] to distinguish the MEP population and found this population to be significantly increased [16].
MK enhances OB-lineage cell support of hematopoiesis
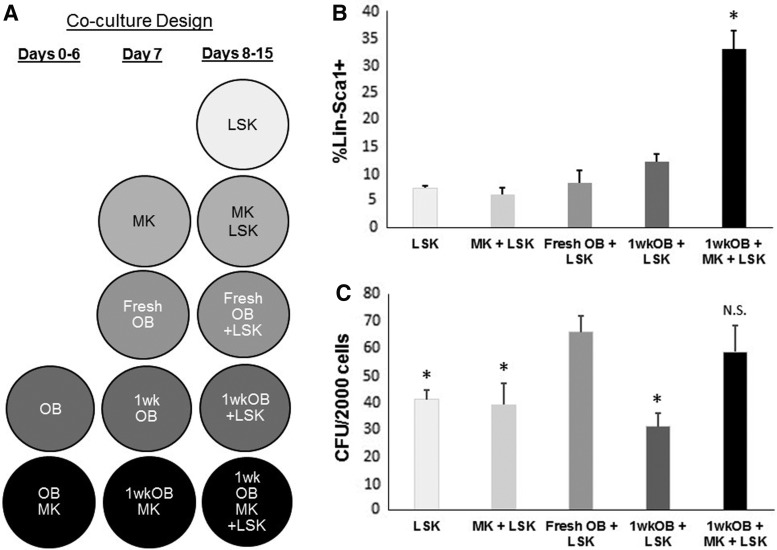
We have previously shown that MK augment OB-lineage proliferation and suppress maturation [11,14]. We have also shown that immature OB have higher hematopoiesis-enhancing activity (HEA) than mature OB [8,9]. We therefore sought to determine whether MK can promote OB-mediated HEA. To accomplish this, we cultured OB for 1 week either alone (1wkOB) or with MK (1wkOB+MK) (Fig. 4A). On day 7, 1wkOB and 1wkOB+MK were reseeded at the same concentrations into separate wells, and fresh MK were added to the latter group. At this time, fresh OB and MK were prepared and seeded into separate wells. A day later (day 8), all wells were seeded with freshly isolated LSK cells. After 1 week (day 15), LSK progeny were harvested and several hematopoietic parameters were analyzed.
FIG. 4.
Hematopoietic enhancing effect of MK and OB. (A) Experimental design of LSK, OB, and MK co-culture. Fresh OB, OB matured in vitro for 1 week (1wkOB), OB+MK co-cultured for 1 week (1wkOB+MK), and fresh MK were co-cultured with fresh LSK for 1 week, LSK were also cultured alone for 1 week, as controls. (B) Progeny were assayed for Lin−Sca1+ cells or (C) CFU, *P < 0.05 compared to fresh OB+LSK, N.S., not significantly different from fresh OB+LSK, n = 3. CFU, colony-forming unit.
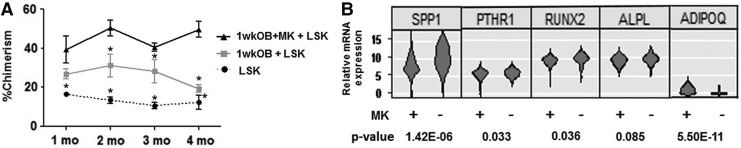
Figure 4B shows that the highest percentage of cells expressing Lin−Sca1+ phenotype was detected in the cultures with 1wkOB+MK+LSK, indicating that MK can preserve the HEA of OB matured in vitro for 1 week. It should be noted that c-Kit expression is lost when cells are exposed to SCF in vitro [31]. The CFU generated from 1wkOB+MK+LSK cultures were significantly elevated compared to 1wkOB+LSK or LSK alone, again indicating the ability of MK to sustain the HEA of OB matured in vitro for 1 week (Fig. 4C). Note that the number of CFU in co-cultures of LSK+MK were very similar to LSK only, suggesting that MK do not modulate LSK function directly, but through OB instead. These results suggest that LSK progeny from cultures with 1wkOB+MK+LSK may have higher BM repopulating potential. To investigate this, LSK progeny from 1wk OB+MK+LSK, 1wk OB+LSK, and LSK maintained alone in culture were harvested at day 5 of culture and assessed in a competitive repopulation assay in irradiated F1 recipient mice. Chimerism was measured monthly for 4 months. Figure 5A illustrates that 1wk OB+MK+LSK co-cultures sustained the highest level of chimerism over a period of 4 months, confirming that MK sustain the HEA of OB matured in vitro. In addition, MK+LSK results are not different from LSK alone (data not shown). These observations indicate that MK can, in part, regulate HSC function indirectly through their effects on OB proliferation and/or differentiation.
FIG. 5.
MK augment OB effect on engraftment and mRNA expression. (A) Percent of donor-derived chimerism from progeny of LSK, 1wkOB+LSK, and 1wkOB+MK+LSK cultures. *P < 0.05 versus corresponding values from 1wkOB+MK+LSK cells, mean ± standard error, n = 3, two-way ANOVA, followed by Bonferroni's post hoc t-test. (B) Single-cell gene expression profiles of osteopontin (SPPI), PTHR1, RUNX2, ALPL, and adiponectin (ADIPOQ) of OB cultured with or without MK, n = 84 and 56 cells per treatment were analyzed, respectively. ALPL, alkaline phosphatase; mRNA, messenger RNA; PTHR1, parathyroid hormone receptor 1; RUNX2, runt-related transcription factor 2.
In an effort to identify factors from the OB±MK co-cultures that enhance hematopoiesis, we used single-cell expression analysis to analyze 90 genes in fluorescence-activated cell sorting of calvarial OB cells cultured±MK for 4 days (Supplementary Table S4). Of these, adiponectin (ADIPOQ) expression was found to have the highest statistically significant percent increase in the OB+MK co-cultures, compared to OB cultures. (Fig. 5B). Consistent with MK-mediated inhibition of OB differentiation [14], the OB expression of differentiation markers osteopontin (SPP1), parathyroid hormone receptor 1 (PTHR1), runt-related transcription factor 2 (RUNX2), and alkaline phosphatase (ALPL) were all downregulated in the OB cultures containing MK compared to the cultures containing OB alone (Fig. 5B and Supplementary Table S3). The violin plots shown in Fig. 4B display relative expression on the y-axis; and the x-axis represents the probability that an individual cell will express a gene at the relative expression value indicated on the y-axis.
Discussion
The long-term goal of this research is to understand the interactions between MK and OB-lineage/osteoprogenitor cells, which result in the regulation of bone formation and hematopoiesis. To better dissect the interactions between these cells, which increase bone formation, we created mice with an MK-specific deletion of the TPO receptor, Mpl (Mplf/f;PF4cre). There are conflicting reports as to the specificity of PF4 expression in MK and platelets, with two studies addressing this issue [16,32]. Ng et al. used lineage tracing with Rosa26 EYFP;PF4cre transgenic mice and an IRES-GFP introduced to the Mpl locus and found there was no expression of the reporter in the myeloid or lymphoid lineages [16]. Calaminus et al., used lineage tracing with the Rosa26dtomato;PF4cre transgenic mouse and found that PF4 is expressed not only in MK and platelets but also in myeloid and lymphoid lineages, and primitive hematopoietic progenitor cells [32]. This may be due to issues with “leaky” expression of reporters or variability of reporter constructs. As we had generated a similar mouse model and confirmed Ng et al.'s findings, we believe that the PF4 expression is found primarily in the MK lineage cells in our model.
Our approach to the creation of the Mplf/f mouse was different compared with Ng et al. [16]. We deleted exon 3 of the Mpl gene, whereas they deleted exons 11 and 12 and created a mouse with a virtually identical phenotype. In this study, we not only confirmed their findings but extended them to include the skeletal phenotype.
TPO is considered to be the main MK growth factor, and thus thought to be necessary for normal MK and platelet development/function. In Mplf/f;PF4cre mice, we had expected to see a reduction in the number of MK, platelets, and HSC, and increased levels of TPO, as observed in mice lacking Mpl globally [24,26]. The reason for the discrepancy in platelet and MK numbers between the global knockout and the Mplf/f;PF4cre mice may be, as postulated by Ng et al., that in the Mplf/f;PF4cre mice, MK and platelets could not consume TPO, leaving it available for other cells. They speculate that TPO binds to HSC, which in turn increases MK progenitors, resulting in greater numbers of MK and platelets [16]. OB and OC precursors also express TPO receptors; thus, it may be that these cells also bind excess TPO.
As stated previously, several mouse models with high numbers of MK display a high bone mass phenotype. The positive role of estrogen in bone formation and maintenance has been well described [33], and we posit the effect of estrogen accounts for the earlier skeletal phenotype in female mice, possibly owing to the presence of the estrogen receptor in MK and the ability of estrogen to stimulate MK differentiation [34]. Our data revealed that 100% of female 3-month-old Mplf/f;PF4cre mice develop a high bone mass phenotype, while in male mice, the skeletal phenotype is delayed by approximately by 4–5 weeks. Thus, there are now five mouse models with increased numbers of MK in BM yielding mice with a strikingly high bone mass phenotype [11,14,18]. These data demonstrate that MK increase osteoblastogenesis, bone formation, and likely a modified BM microenvironment/HSC niche.
In contrast to the distal femur, we observed a striking increase in BV/TV at the femoral midshaft (Fig. 2C–E) in the conditional knockout mice compared to controls. In addition, we measured large increases in MK (Fig. 2A, B) and large increases in Lin−CD51+PDGFRa+ mesenchymal OB progenitors (Fig. 2G) residing in the marrow space of the mutant mice. This is consistent with other models in which MK stimulate bone formation. Of interest, we found that OB and OC numbers per bone surface were significantly decreased in the epiphysis of the mutants, which is consistent with a low bone turnover phenotype. This does not result in a decrease of BV/TV in this compartment because of the increase in the number of trabeculae and the overall increase in OB-lineage cells. FACS analysis indicated that the expression of CD166+ was higher in the OB within the epiphyseal bone in the Mplf/f;PF4cre mice compared to that observed in control mice. This indicates that the OB in the epiphyses were less mature in mutant mice than that observed in control mice. The greater number of immature (CD166+) OB in the epiphyseal region is consistent with the known role that MK play in delaying OB maturation. However, our results indicate that these cells maintain the ability to differentiate into bone forming mature OB cells. This skeletal phenotype confirms that MK play a significant role in bone mass regulation.
Importantly, patients with high numbers of MK in the BM and consequently thrombocytosis may be diagnosed with essential thrombocythemia (ET), a rare chronic myeloproliferative blood disorder, which may be due to mutations in Mpl and its downstream signaling of the JAK-STAT pathway. Patients with these conditions may progress to idiopathic myelofibrosis and develop an osteosclerotic phenotype [35,36]. Ng et al. investigated the role of TPO in Mplf/f;PF4cre mice and postulated that the role of Mpl on MK and platelets is to prevent myeloproliferation due to chronic exposure to high levels of TPO [16].
We also hypothesize that in addition to the MK direct effects on HSC (as evidenced by Bruns et al. and Zhao et al.) [3,4], MK signaling can also regulate HSC numbers and function indirectly through their regulation of OB proliferation/differentiation. This is based on the following findings: (1) our previous data demonstrating that MK stimulate OB proliferation in vitro and in vivo, as well as bone formation in vivo [12,37,38]; (2) our data showing that the Mplf/f;PF4cre mice also exhibit a high bone mass phenotype; (3) data from our group and others demonstrating that OB-lineage cells are known to support hematopoiesis [8–10]; (4) data from Horwitz's group demonstrating that following irradiation, surviving MK migrate to endosteal bone surfaces and stimulate a twofold increase in OB number, which augmented endosteal HSC niches, and this in turn was posited to enhance HSC recovery [39,40]; and (5) our data demonstrating that MK preserve OB HEA, as was shown here in our co-culture experiments.
We have also found that the expression of ADIPOQ in OB-lineage cells is significantly increased when cultured with MK. OB-specific expression of ADIPOQ is regulated by Fra-2/AP-1 and influences the maintenance of bone mass [41]. In addition, ADIPOQ has been observed to increase HSC proliferation, to keep HSC in an immature state, and is reported to be required for long-term hematopoietic reconstitution [42]. Future studies are required to investigate whether OB-derived ADIPOQ plays a functional role in the MK-induced increases in HEA observed in our transplantation studies.
Together, our data support the hypothesis that MK modulate bone formation in vivo and also regulate HSC function indirectly through OB. Furthermore, our findings suggest that the latter may be due to an increased expression of adiponectin. Targeting MK-mediated OB proliferation and hematopoietic supporting pathways may offer novel treatment approaches for bone loss diseases such as osteoporosis and recovery of hematopoiesis in the context of chemotherapy and BM transplantation.
Supplementary Material
Acknowledgments
This work was sponsored by Developing Diverse Researchers with InVestigative Expertise from IU-Purdue University Indianapolis, T32 DK007519, Ralph W. and Grace M. Showalter Research Trust Fund, and the NIH: R01 AR060332, R01 AR060863, U54 DK106846, and P30 DK090948. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Disclosure Statement
The authors declare no competing or conflicting financial interests.
References
- 1.Harker LA. and Finch CA. (1969). Thrombokinetics in man. J Clin Invest 48:963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp HG, Avecilla ST, Hooper AT. and Rafii S. (2005). The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology 20:349–356 [DOI] [PubMed] [Google Scholar]
- 3.Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A. and Frenette PS. (2014). Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J. and Li L. (2014). Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20:1321–1326 [DOI] [PubMed] [Google Scholar]
- 5.Storan MJ, Heazlewood SY, Heazlewood CK, Haylock DN, Alexander WS, Neaves RJ, Oteiza A. and Nilsson SK. (2015). Factors released by megakaryocytes thrombin cleave osteopontin to negatively regulate hematopoietic stem cells. Stem Cells 33:2351–2357 [DOI] [PubMed] [Google Scholar]
- 6.Wang LD. and Wagers AJ. (2011). Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol 12:643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SJ. and Scadden DT. (2014). The bone marrow niche for haematopoietic stem cells. Nature 505:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YH, Chitteti BR, Streicher DA, Morgan JA, Rodriguez-Rodriguez S, Carlesso N, Srour EF. and Kacena MA. (2011). Impact of maturational status on the ability of osteoblasts to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res 26:1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitteti BR, Cheng YH, Streicher DA, Rodriguez-Rodriguez S, Carlesso N, Srour EF. and Kacena MA. (2010). Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function. J Cell Biochem 111:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846 [DOI] [PubMed] [Google Scholar]
- 11.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA. and Horowitz MC. (2004). Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res 19:652–660 [DOI] [PubMed] [Google Scholar]
- 12.Kacena MA, Eleniste PP, Cheng YH, Huang S, Shivanna M, Meijome TE, Mayo LD. and Bruzzaniti A. (2012). Megakaryocytes regulate expression of Pyk2 isoforms and caspase-mediated cleavage of actin in osteoblasts. J Biol Chem 287:17257–17268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemieux JM, Horowitz MC. and Kacena MA. (2010). Involvement of integrins and alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem 109:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chagraoui H, Tulliez M, Smayra T, Komura E, Giraudier S, Yun T, Lassau N, Vainchenker W. and Wendling F. (2003). Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood 101:2983–2989 [DOI] [PubMed] [Google Scholar]
- 15.Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ. and Kacena MA. (2009). The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone 44:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng AP, Kauppi M, Metcalf D, Hyland CD, Josefsson EC, Lebois M, Zhang JG, Baldwin TM, Di Rago L, Hilton DJ. and Alexander WS. (2014). Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci U S A 111:5884–5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feher A, Koivunemi A, Koivunemi M, Fuchs RK, Burr DB, Phipps RJ, Reinwald S. and Allen MR. (2010). Bisphosphonates do not inhibit periosteal bone formation in estrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone 46:203–207 [DOI] [PubMed] [Google Scholar]
- 18.Olivos DJ, 3rd, Alvarez M, Cheng YH, Hooker RA, Ciovacco WA, Bethel M, McGough H, Yim C, Chitteti BR, et al. (2017). Lnk deficiency leads to TPO-mediated osteoclastogenesis and increased bone mass phenotype. J Cell Biochem 118:2231–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz MC, Fields A, DeMeo D, Qian HY, Bothwell AL. and Trepman E. (1994). Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology 135:1032–1043 [DOI] [PubMed] [Google Scholar]
- 20.Wong GL. and Cohn DV. (1975). Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A 72:3167–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drachman JG, Sabath DF, Fox NE. and Kaushansky K. (1997). Thrombopoietin signal transduction in purified murine megakaryocytes. Blood 89:483–492 [PubMed] [Google Scholar]
- 22.Chitteti BR, Kobayashi M, Cheng YH, Zhang H, Poteat BA, Broxmeyer HE, Pelus LM, Hanenberg H, Zollman A, et al. (2014). CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood 124:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y. and Frenette PS. (2013). PDGFRα and CD51 mark human nestin+sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 210:1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitteti BR, Cheng YH, Kacena MA. and Srour EF. (2013). Hierarchical organization of osteoblasts reveals the significant role of CD166 in hematopoietic stem cell maintenance and function. Bone 54:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurney AL, Carver-Moore K, de Sauvage FJ. and Moore MW. (1994). Thrombocytopenia in c-mpl-deficient mice. Science 265:1445–1447 [DOI] [PubMed] [Google Scholar]
- 26.Yu M. and Cantor AB. (2012). Megakaryopoiesis and thrombopoiesis: an update on cytokines and lineage surface markers. Methods Mol Biol 788:291–303 [DOI] [PubMed] [Google Scholar]
- 27.Alexander WS, Roberts AW, Maurer AB, Nicola NA, Dunn AR. and Metcalf D. (1996). Studies of the c-Mpl thrombopoietin receptor through gene disruption and activation. Stem Cells 14(Suppl 1):124–132 [DOI] [PubMed] [Google Scholar]
- 28.de Graaf CA. and Metcalf D. (2011). Thrombopoietin and hematopoietic stem cells. Cell Cycle 10:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijome TE, Baughman JT, Hooker RA, Cheng YH, Ciovacco WA, Balamohan SM, Srinivasan TL, Chitteti BR, Eleniste PP, et al. (2016). C-Mpl is expressed on osteoblasts and osteoclasts and is important in regulating skeletal homeostasis. J Cell Biochem 117:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng AP, Kauppi M, Metcalf D, Di Rago L, Hyland CD. and Alexander WS. (2014). Characterization of thrombopoietin (TPO)-responsive progenitor cells in adult mouse bone marrow with in vivo megakaryocyte and eythroid potential. Proc Natl Acad Sci U S A 109:2364–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee NS, Hsiau CW, Serve H, Vosseller K. and Besmer P. (1994). Mechanism of down-regulation of c-kit receptor. Roles of receptor tyrosine kinase, phosphatidylinositol 3′-kinase, and protein kinase C. J Biol Chem 269:31991–31998 [PubMed] [Google Scholar]
- 32.Calaminus SDJ, Guitart A, Sinclair A, Schachtner H, Watson SP, Holyoake TL, Kranc KR. and Machesky LM. (2012). Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PLoS One 7:e51361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khosla S, Oursler MJ. and Monroe DG. (2012). Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bord S, Vedi S, Beavan SR, Horner A. and Compston JE. (2000). Megakaryocyte population in human bone marrow increases with estrogen treatment: a role in bone remodeling? Bone 27:397–401 [DOI] [PubMed] [Google Scholar]
- 35.Sangle N, Cook J, Perkins S, Teman CJ, Bahler D, Hickman K, Wilson A, Prchal J. and Salama ME. (2014). Myelofibrotic transformations of polycythemia vera and essential thrombocythemia are morphologically, biologically, and prognostically indistinguishable from primary myelofibrosis. Appl Immunohistochem Mol Morphol 22:663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen QJ, Yang Q, Goldenson B, Malinge S, Lasho T, Schneider RK, Breyfogle LJ, Schultz R, Gilles L, et al. (2015). Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med 21:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciovacco WA, Cheng YH, Horowitz MC. and Kacena MA. (2010). Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem 109:774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng YH, Streicher DA, Waning DL, Chitteti BR, Gerard-O'Riley R, Horowitz MC, Bidwell JP, Pavalko FM, Srour EF, Mayo LD. and Kacena MA. (2015). Signaling pathways involved in megakaryocyte-mediated proliferation of osteoblast lineage cells. J Cell Physiol 230:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M. and Horwitz EM. (2013). Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121:5238–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, et al. (2009). Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114:2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozec A, Bakiri L, Jimenez M, Rosen ED, Catala-Lehnen P, Schinke T, Schett G, Amling M. and Wagner EF. (2013). Osteoblast-specific expression of Fra-2/AP-1 controls adiponectin and osteocalcin expression and affects metabolism. J Cell Sci 126:5432–5440 [DOI] [PubMed] [Google Scholar]
- 42.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U. and Reya T. (2007). Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol 178:3511–3520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.