Abstract
Significance: Nicotinamide adenine dinucleotide (NAD+) participates in redox reactions and NAD+-dependent signaling processes, which involve the cleavage of NAD+ coupled to posttranslational modifications of proteins or the production of second messengers. Either as a primary cause or as a secondary component of the pathogenic process, mitochondrial dysfunction and oxidative stress are prominent features of several neurodegenerative diseases. Activation of NAD+-dependent signaling pathways has a major effect in the capacity of the cell to modulate mitochondrial function and counteract the deleterious effects of increased oxidative stress.
Recent Advances: Progress in the understanding of the biological functions and compartmentalization of NAD+-synthesizing and NAD+-consuming enzymes have led to the emergence of NAD+ metabolism as a major therapeutic target for age-related diseases.
Critical Issues: Three distinct families of enzymes consume NAD+ as substrate: poly(ADP-ribose) polymerases (PARPs), ADP-ribosyl cyclases (CD38/CD157) and sirtuins. Two main strategies to increase NAD+ availability have arisen. These strategies are based on the utilization of NAD+ intermediates/precursors or the inhibition of the NAD+-consuming enzymes, PARPs and CD38. An increase in endogenous sirtuin activity seems to mediate the protective effect that enhancing NAD+ availability confers in several models of neurodegeneration and age-related diseases.
Future Directions: A growing body of evidence suggests the beneficial role of enhancing NAD+ availability in models of neurodegeneration. The challenge ahead is to establish the value and safety of the long-term use of these strategies for the treatment of neurodegenerative diseases. Antioxid. Redox Signal. 28, 1652–1668.
Keywords: : NAD, mitochondria, oxidative stress, neurodegeneration, CD38, PARP
Introduction
Nicotinamide adenine dinucleotide (NAD+) has long been recognized as an essential redox molecule in metabolism. However, its role as cosubstrate for a series of enzymes that regulate fundamental biological processes has recently renewed the interest on this dinucleotide. In redox reactions, a hydride equivalent is reversibly transferred at the nicotinamide moiety causing a switch between oxidized (NAD+) and reduced (NADH) forms of the nucleotide. While this property of NAD+ is critical for redox reactions, it is not accompanied by net consumption of the dinucleotide. In contrast, NAD+-dependent signaling involves the cleavage of NAD+ (degradation) coupled to posttranslational modifications of proteins or the production of second messengers (5, 14, 78).
Activation of NAD+-dependent signaling pathways has a major effect in the capacity of the cell to modulate mitochondrial function and counteract the deleterious effects of increased oxidative stress. Either as a primary cause or as a secondary component of the pathogenic process, mitochondrial dysfunction and oxidative stress are prominent features of Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) (25, 45, 46, 121, 146, 155). As we learn more about the biological role of NAD+-synthesizing and NAD+-consuming enzymes and their compartmentalization, NAD+ metabolism has emerged as a key therapeutic target for neurodegenerative diseases.
NAD+-Dependent Signaling
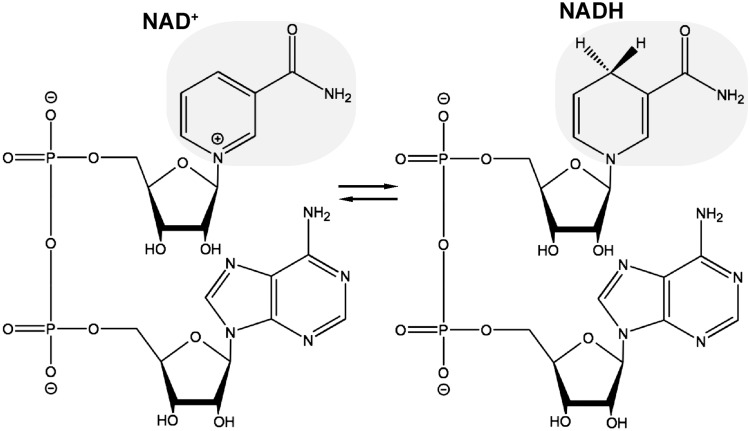
NAD+ is a dinucleotide generated by the covalent link between nicotinamide mononucleotide (NMN) and adenosine monophosphate. In redox reactions, the addition of two electrons and a proton to the nicotinamide moiety of NAD+ generates NADH (Fig. 1). This electron carrier function is critical for catabolic reactions and energy production in the cell but does not cause any net loss of NAD+ because it is reversible. On the contrary, NAD+-dependent signaling processes lead to its degradation. Three distinct families of enzymes consume NAD+ as substrate: poly(ADP-ribose) polymerases (PARPs), ADP-ribosyl cyclases, and sirtuins (Fig. 2).
FIG. 1.
NAD+ as an electron carrier. In redox reactions, a hydride equivalent is reversibly transferred at the nicotinamide moiety causing a switch between oxidized (NAD+) and reduced (NADH) forms of the dinucleotide.
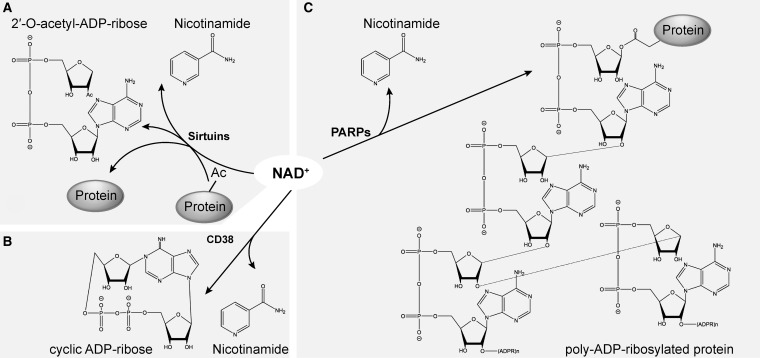
FIG. 2.
NAD+-consuming reactions. (A) NAD+ hydrolysis is coupled to deacetylation reactions by sirtuins. (B) Cyclic ADP-ribose production by ADP-ribosyl cyclases, including CD38 and CD157. (C) PARPs catalyze the addition of ADP-ribose to an acceptor protein following with extension and branching of the chain to form poly(ADP-ribose) polymers. Member of this family may also have mono-ADP-ribosylation activity, catalyzing the transfer of a single ADP-ribose moiety to the acceptor protein. All three reactions produce nicotinamide as a by-product. PARP, poly(ADP-ribose) polymerase.
Poly(ADP-ribose) polymerases
In humans, the PARP family of proteins consists of 17 proteins widely distributed in all tissues. PARPs hydrolyze NAD+ and transfer the ADP-ribose moiety to a receptor amino acid. PARPs transfer multiple ADP-ribose moieties leading to the formation of long, linear, or branched poly(ADP-ribose) polymers (Fig. 2). This family also includes members with mono-ADP-ribosylation activity, catalyzing the transfer of a single ADP-ribose moiety. PARPs regulate DNA damage repair, tumorigenesis, cell differentiation, and metabolism (14, 15, 36). PARP1 and PARP2 account for the majority of the basal and stimulated PARP activity in the cell (36). PARP1 activation is an integral part of the cellular response to oxidative stress-induced DNA damage.
Excessive activation of PARPs rapidly impairs mitochondrial function and is linked to cell death in a variety of situations. Multiple signaling pathways have been described to mediate the deleterious effect of PARP1 overactivation. In principle, PARP1 overactivation could lead to a catastrophic decrease in cytosolic NAD+, leading to direct glycolytic inhibition and cell death (6, 156). Energy depletion following PARP1 overactivation could also be mediated by poly(ADP-ribose)-dependent inhibition of the enzyme hexokinase (9). In addition, cell death induced by PARP1 overactivation can be mediated by poly(ADP-ribose)-dependent release of apoptosis inducing factor from the mitochondria, part of a specific caspase-independent cell death program known as parthanatos (177, 189, 190).
In addition, it has been shown that the expression of a mitochondria-targeted PARP1 decreases total cellular NAD+ and mitochondrial function, even in the presence of a compensatory increase in glycolytic activity (116). This result suggests that increased mitochondrial PARP activity is sufficient to directly impair mitochondrial function. Although PARP1 is predominantly localized to the nucleus, and despite the fact that PARP activity may or may not exist in the mitochondrial matrix, PARP activation remains a major component in oxidant-induced mitochondrial dysfunction (14, 29).
ADP-ribosyl cyclases
These unique enzymes catalyze the cyclization of NAD+ to cyclic ADP ribose (cADPR) (Fig. 2). The two members of this family, CD38 and CD157, have receptor functions in immune and myeloid cells (133), but are also multifunctional enzymes that catalyze the production of several second messengers (such as cADPR, adenosine dinucleotide phosphoribose, and nicotinic acid adenine dinucleotide phosphate) (100). These products then act as potent intracellular calcium-mobilizing agents to control chemotaxis of dendritic cells and activation of microglia (108, 124).
Despite this original role described in cells of the immune system, CD38 is ubiquitously expressed in mammalian tissues and its major enzymatic activity is the hydrolysis of NAD+. In fact, it has been estimated that CD38 will generate one molecule of cADPR for every 100 molecules of NAD+ hydrolyzed (52). CD38 is highly expressed in neurons and astrocytes (125, 184).
Originally described as an ectoenzyme, CD38 is also present in the endoplasmic reticulum and in the nuclear and mitochondrial membranes (5), suggesting that CD38 could be an important regulator of intracellular NAD+ pools, and therefore metabolic pathways, in both cell types. In the mouse central nervous system (CNS), the neuronal distribution of CD38 coincides with that of ryanodine receptors, supporting the involvement of CD38 in a cADPR-mediated calcium-mobilizing system in neurons (184). Highlighting the array of biological processes in which this enzyme seems to be involved, CD38 signaling has also been shown to mediate mitochondrial transfer from astrocytes to neurons (73).
CD157 also produces cADPR, however, compared to CD38, the catalytic efficiency of CD157 seems to be significantly lower (100). While CD157 has a role in immune development, it has also been recently associated with the development of late-onset PD in specific populations (110, 140, 145). In addition, during development, CD157 expression is observed in nestin-positive cells of the ventricular and subventricular zones, suggesting a yet unrecognized role during neuronal development (38, 75).
Sirtuins
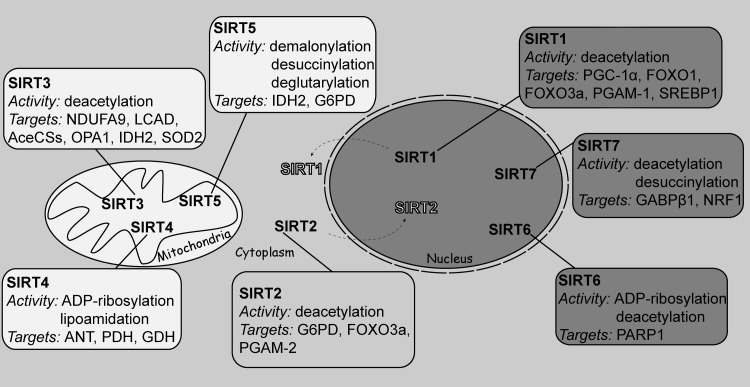
Sirtuins (Sir2-like enzymes) are NAD+-dependent deacylases that play a key role in transcription, DNA repair, metabolism, and oxidative stress resistance (78). There are seven mammalian sirtuins (SIRT1–7) with diverse subcellular localization, enzymatic activity, and protein substrates (Fig. 3). SIRT1, SIRT6, and SIRT7 localize to the nucleus, SIRT2 is cytoplasmic, and SIRT3, SIRT4, and SIRT5 are present in the mitochondria. Under specific circumstances, SIRT1 and SIRT2 may shuttle between their respective compartments (74).
FIG. 3.
Subcellular localization, enzymatic activity, and selected substrates of the seven mammalian sirtuins. SIRT1, SIRT6, and SIRT7 localize to the nucleus, SIRT2 is cytoplasmic, and SIRT3, SIRT4, and SIRT5 are mitochondrial. SIRT1 and SIRT2 may shuttle between their respective compartments. See the text for ANT, adenine nucleotide translocator; G6PD, glucose-6-phosphate dehydrogenase; GABPβ1, GA repeat binding protein beta 1; GDH, glutamate dehydrogenase; IDH2, isocitrate dehydrogenase 2; LCAD, long chain acyl-CoA dehydrogenase; NDUFA9, NADH:ubiquinone oxidoreductase subunit A9; NRF, nuclear respiratory factor; OPA1, optic atrophy 1; PDH, pyruvate dehydrogenase; PGAM-1, phosphoglycerate mutase-1; PGC-1?, peroxisome proliferator-activated receptor coactivator-1?; SIRT, sirtuin; SOD, superoxide dismutase; SREBP1, sterol regulatory element binding protein 1.
SIRT4 harbors ADP-ribosyltransferase and lipoamidase activity (70, 105). SIRT5 is an NAD+-dependent protein lysine demalonylase, deglutarylase, and desuccinylase (53). SIRT6 and SIRT7 display weak deacetylase activity in vitro and instead, SIRT6 can catalyze ADP-ribosylation and lysine defatty acylation (56, 80, 93), while SIRT7 function as an NAD+-dependent histone desuccinylase (92). SIRT1, SIRT2, and SIRT3 are the best-characterized members of the family. They display robust deacetylation activity. In this reaction, the hydrolysis of NAD+ is coupled to the removal of the acetyl group from the ɛ-amino group of acetylated lysine residues present in substrate proteins, generating nicotinamide and 2′-O-acetyl-ADP ribose as by-products (57).
It has become evident that lysine acetylation is a prevalent regulatory mechanism of protein function, and thousands of acetylated proteins from different subcellular compartments, including the nucleus, cytoplasm, mitochondria, and endoplasmic reticulum, have been identified by mass spectrometry (43, 99, 129). An estimate of 63% of mitochondrial proteins is subject to reversible lysine acetylation, and NAD+-dependent regulation of their acetylation status is key for optimal mitochondrial function (13). Although some metabolic enzymes display increased enzymatic activity following acetylation, lysine acetylation negatively affects the activity of most metabolic enzymes (182). Hence, in general, sirtuin-mediated deacetylation is associated with activation of enzymatic processes.
Sirtuins Link NAD+ Levels to Mitochondrial Function and Antioxidant Defenses
Sirtuins have a significant and ever growing number of protein targets and a comprehensive description of these targets is outside the scope of this review. However, some of these targets have a direct influence on mitochondrial function and antioxidant defenses and exemplify the potential therapeutic role of sirtuins in neurodegenerative diseases.
SIRT1 deacetylates histones at specific gene loci and thereby silences expression of the corresponding genes. In addition to its role as a true histone deacetylase, SIRT1 modulates the activity of several transcription factors and coactivators. In response to increased NAD+ levels, SIRT1 activates peroxisome proliferator-activated receptor coactivator 1α (PGC-1α) and forkhead box O1 (FOXO1) to increase mitochondrial biogenesis and function (16, 67, 114, 179). SIRT1 can also regulate mitophagy (79, 119) and the mitochondrial unfolded protein response (112), a transcriptional response that promotes cell survival and mitochondrial repair during mitochondrial dysfunction or accumulation of unfolded proteins within the mitochondria (82). Thus, SIRT1 activity affects the overall quantity and quality of mitochondria. Moreover, SIRT1 controls energy production by modulating glucose and lipid homeostasis through direct regulation of phosphoglycerate mutase-1 (PGAM-1) and sterol regulatory element binding protein 1 (SREBP1), respectively (40). SIRT1 may also have a direct role in regulating antioxidant defenses, by deacetylating the transcription factor Nrf2 (nuclear factor, erythroid 2 like 2) (84, 183), a master regulator of antioxidant and phase II enzymes (167). However, the exact biological significance of this observation remains to be elucidated.
All major mitochondrial processes, such as tricarboxylic acid cycle (TCA), fatty acid metabolism, oxidative phosphorylation, mitochondrial acetyl-CoA production, and antioxidant response, are regulated by acetylation. While the mechanisms leading to mitochondrial protein acetylation remain uncertain (120, 149, 172), SIRT3 has clearly emerged as the main deacetylase activity in the mitochondria (95). To date, over 1000 SIRT3 targets have been identified (13), including the 39-kDa NDUFA9 subunit of complex I (2, 34), long chain acyl-CoA dehydrogenase (LCAD), and acetyl-CoA synthases (AceCSs) (71, 76, 148). SIRT3 promotes mitochondrial function not only by regulating the activity of metabolic enzymes but also by regulating mitophagy (180), the mitochondrial unfolded protein response (61), and optic atrophy 1 (OPA1)-dependent mitochondrial dynamics (142).
SIRT3 activity also has a major role in the regulation of mitochondrial antioxidant defenses, by activating mitochondrial superoxide 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2). The activation of SOD2 has a direct effect on superoxide production by the mitochondria. Interestingly, overexpression of SOD2 alone does not reduce the levels of reactive oxygen species to the same extent as its coexpression with SIRT3 does (41, 132, 157). SIRT3-mediated IDH2 activation increases mitochondrial availability of NADPH, which is necessary for the regeneration of reduced glutathione. Reduced glutathione in turn is essential for mitochondrial antioxidant defenses (151, 191). While changes in protein acetylation affect the redox status of the cell, changes in the redox status of the cell can also change the acetylation status of several metabolic and antioxidant pathways, suggesting that these two phenomena are functionally linked (127).
Other members of the sirtuin family also have established and emerging roles regulating mitochondrial function and antioxidant defenses. For example, SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction, and hence increases the expression of SOD2 (173). In addition, SIRT2-mediated deacetylation and activation of glucose-6-phosphate dehydrogenase (G6PD) enhance the activity of the pentose phosphate pathway to supply cytosolic NADPH to counteract oxidative damage (178). Moreover, it has been recently shown that SIRT2 associates with the inner mitochondrial membrane and that loss of SIRT2 leads to increased oxidative stress, decreased ATP levels, and altered mitochondrial morphology in cells from the CNS (94).
Meanwhile, SIRT4-mediated lipoamidation inhibits the activity of pyruvate dehydrogenase (PDH), the enzyme linking glycolysis to the TCA cycle (105). SIRT4 also inhibits glutamate dehydrogenase (GDH) activity, leading to downregulation of insulin secretion in response to amino acids (70). Furthermore, by interacting with the adenine nucleotide translocator (ANT), SIRT4 may regulate the ATP:ADP ratio and membrane potential (3, 77).
SIRT5 activity also has a significant impact in the overall antioxidant defenses. In addition to its role in regulating various metabolic pathways, SIRT5 activates IDH2 through desuccinylation and G6PD through deglutarylation (194). SIRT6 mono-ADP-ribosylates PARP1 and stimulates PARP1-dependent repair of DNA double-strand breaks under oxidative stress (102, 164). Moreover, in a model of cellular senescence, SIRT6 appears to be a new coactivator of Nrf2, being required for the transactivation of Nrf2-driven antioxidant genes (122).
Finally, SIRT7 controls epigenetic and cellular homeostasis through deacetylation of histones and other nuclear proteins. SIRT7 also promotes, through histone desuccinylation, chromatin condensation and DNA double-strand breaks repair in a PARP1-dependent manner (92). Moreover, a significant role for SIRT7 on mitochondrial homeostasis has emerged over the recent years. SIRT7 deacetylates GA repeat binding protein beta 1 (GABPβ1), thus modulating the activity of the GABPα/GABPβ heterotetramer (also known as nuclear respiratory factor or NRF2), a master regulator of nuclear-encoded mitochondrial genes. Consequently, SIRT7 knockout mice display multisystemic mitochondrial dysfunction (139). In addition, SIRT7 can repress NRF1 activity to reduce the expression of the mitochondrial translation machinery, therefore promoting nutritional stress resistance (111).
Interaction Between NAD+-Consuming Enzymes
The Km of sirtuins for NAD+ seem to be in range with the estimated concentration of NAD+ in their respective compartments (33, 35). Regardless of whether sirtuins act as bona fide NAD+ sensors, in general the reported Km for PARP1 and CD38 are smaller than those of the sirtuins. Thus, PARP1 and CD38 activity limits endogenous sirtuin activation by reducing NAD+ availability, and inhibition of these main NAD+ consumers increases NAD+ levels. It has been shown that decreased PARP1 and CD38 activity effectively increases total NAD+ content, resulting in SIRT1 activation and protection against the development of metabolic disorders in mice. Protection is conferred through NAD+-dependent activation of the SIRT1/PGC-1α axis and increased mitochondrial metabolism (4, 16, 17).
Consistently with its rate-limiting role in the NAD+ salvage pathway, nicotinamide phosphoribosyltransferase (NAMPT) overexpression or its knockdown is associated with increased or reduced SIRT1 activity, respectively (60, 162, 163). Moreover, administration of NAD+ precursors or a CD38 inhibitor to mice increases NAD+ content and improves oxidative metabolism thorough an SIRT1/SIRT3-dependent mechanism (34, 54, 187). These early observations lead to the notion that modulating NAD+ availability, through inhibition of NAD+-consuming enzymes (PARP1 or CD38) or induction of NAD+ synthesis, can be a potential therapeutic approach for age-related diseases.
NAD+ Synthesis
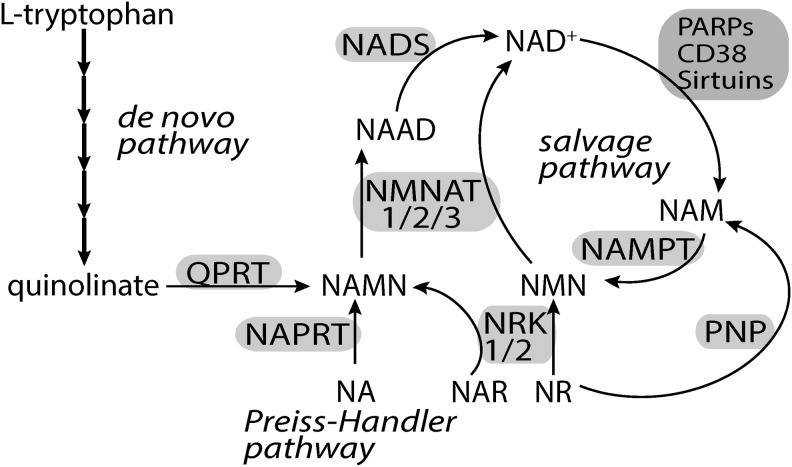
To maintain viability, cells have to continuously synthesize NAD+. NAD+ neosynthesis can occur from L-tryptophan (kynurenine pathway), nicotinic acid (Preiss-Handler pathway), or nicotinamide riboside (NR) (Fig. 4) (20, 23, 138). However, since all the major NAD+-consuming enzymes generate nicotinamide (NAM) as a by-product, eukaryotic cells have evolved a rescue pathway capable of resynthesizing NAD+ from NAM. The enzyme NAMPT catalyzes the conversion of NAM and 5′-phosphoribosyl-1-pyrophosphate to NMN; subsequently, nicotinamide mononucleotide adenylyl transferases (NMNATs) transfer adenine from ATP to NMN to generate NAD+ (48, 62). The relative contribution of the salvage pathway to the overall NAD+ synthesis rate is exemplified by the observation that homozygous deletion of the Nampt gene causes embryonic lethality (137), while deletion of the quinolinate phosphoribosyltransferase (Qprt) gene does not (159). All the biosynthetic pathways converge at the level of dinucleotide formation catalyzed by the NMNATs. However, NAMPT is the rate-limiting enzyme in the salvage pathway and overexpression of NAMPT, but not NMNATs, increases cellular NAD+ levels (117, 136, 186).
FIG. 4.
NAD+ biosynthetic pathways in mammals. Four building blocks may be used for NAD+ synthesis: L-tryptophan, NA, NR, and NAM. The de novo pathway uses L-tryptophan to generate, through several intermediate steps, quinolinate. Quinolinate is sequentially converted to NAMN, NAAD, and NAD+ by the action of QPRT, NMNATs, and NADS, respectively. In the Preiss-Handler pathway, NAPRT converts NA to NAMN, which is then used by NMNATs. NR can enter the salvage pathway either by the action of NRK, which generates NMN, or by the reaction catalyzed by PNP, which generates NAM. NAM is subsequently converted to NMN by NAMPT, the rate-limiting step in the salvage pathway. NRKs can also convert NAR to NAMN. All the biosynthetic pathways converge at the level of dinucleotide formation catalyzed by the NMNATs. NMNATs use NAMN and NMN with similar efficiency. NAD+-consuming reactions catalyzed by PARPs, CD38, and sirtuins release NAM that may be use to resynthesize NAD+ in the salvage pathway. NA, nicotinic acid; NAD+, nicotinamide adenine dinucleotide; NAAD, nicotinic acid adenine dinucleotide; NADS, NAD+ synthetase; NAM, nicotinamide; NAR, nicotinic acid riboside; NR, nicotinamide riboside; NRK, NR kinase; NMN, nicotinamide mononucleotide; NAMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinic acid phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyl transferase; PNP, purine nucleoside phosphorylase; QPRT, quinolinate phosphoribosyltransferase.
Extracellular NMN and NR can serve as NAD+ precursors and effectively increase total and mitochondrial NAD+ content in different cell types (152). However, an NMN transporter has not been identified in eukaryotic cells and it appears that the nucleotide is degraded to the nucleoside (NR) to enter the cell (117). The ectoenzyme CD73, a 5′-nucleotidase, has been shown to dephosphorylate NMN to produce NR (69). NR is then imported into the cell by the equilibrative nucleoside transporters (ENTs), and inhibition of ENTs prevents the increase in cellular NAD+ induced by NMN and NR treatments (117, 135). Once in the cytosol, NR can be phosphorylated by NR kinases (NRKs) to produce NMN (135). Cells are also capable of using NR in an NRK-independent way, in which the purine nucleoside phosphorylase (PNP) generates NAM that enters the salvage pathway (21).
In addition to NR, NRKs can also phosphorylate nicotinic acid riboside (NAR) to produce nicotinic acid mononucleotide (NAMN). NR and NAR can be produced and released by cells, suggesting that one cell may support NAD+ synthesis in a neighboring cell by providing ribosides as NAD+ precursors (89). This is an interesting possibility that could have important implications for neuroprotection, similar to the observation that release of glutathione by astrocytes boosts glutathione levels in cocultured neurons (167).
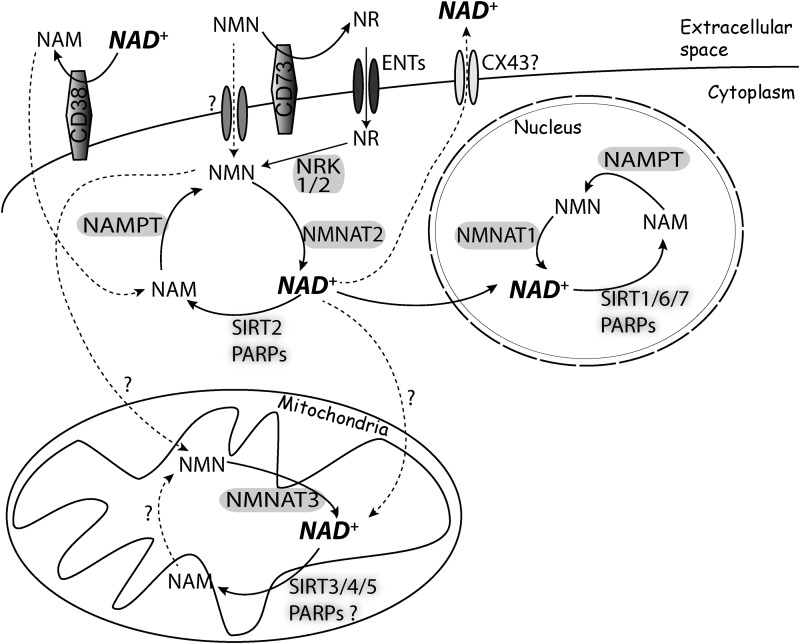
As the relative NAD+ concentration in different subcellular compartments has a decisive effect on redox and nonredox reactions, the compartmentalization of NAD+-synthesizing and NAD+-consuming enzymes has important functional implications (Fig. 5). In general, NAMPT localizes in the cytoplasm and the nucleus and is absent from the mitochondrial fraction (130, 152, 186). In addition, a form of extracellular NAMPT has been described. It is produced by some cell types and seems to have cytokine-like activity (63).
FIG. 5.
Compartmentalization of NAD+ synthesis and utilization. NMN and NR effectively increase total and mitochondrial NAD+. No NMN or NAD+ transporters have been identified in mammalian cells. CD73 dephosphorylate NMN to produce NR, which is then imported into the cell, probably by ENTs. Mitochondria can synthesize NAD+ from NMN, but transport of cytoplasmic NAD+ into the mitochondria also appears to contribute to the mitochondrial NAD+ pool. NAD+ is consumed intracellularly by PARPs and sirtuins, although CD38 has also been found in the endoplasmic reticulum and in the nuclear and mitochondrial membranes (not shown). NAD+ can also be transported outside of the cell, likely through connexin 43 hemichannels (30). CD38 and CD157 consume extracellular NAD+. Cells can also synthesize NAD+ from nicotinic acid and tryptophan (Fig. 4). Dashed lines and question marks indicate steps for which the exact mechanism has not been fully characterized. ENT, equilibrative nucleoside transporter.
The three different isoforms of NMNAT found in mammalian cells have distinctive subcellular localization. NMNAT1 is found in the nucleus, NMNAT2 is localized in the cytoplasm, and NMNAT3 is found in the mitochondria (22), although this has been recently challenged (58, 185). The relative expression levels of NMNAT3 may influence the role of this enzyme in the modulation of the mitochondrial NAD+ pool. Thus, in cells that express relatively high levels of NMNAT3, as in HEK293T cells, deletion significantly reduces mitochondrial NAD+ levels. In contrast, in cells that express NMNAT3 at low levels, as in HeLa cells, its deletion has no effect on the levels of NAD+ in the organelle (33, 165).
Mitochondrial NAD+ pool ranges from 40% to 70% of the total NAD+ content (33, 152). Since NAD+ is unable to cross the mitochondrial internal membrane, and a mammalian mitochondrial NAD+ transporter has not yet been identified, the origin of the mitochondrial NAD+ pool remains to be fully characterized. However, isolated mitochondria can synthesize NAD+ from NMN (18), and we have recently demonstrated that expression of a mitochondria-targeted NAMPT effectively increases total and mitochondrial NAD+ levels in astrocytes (72). This observation has several important implications. First, mitochondrial NAD+ degradation seems to account for a significant portion of cellular NAD+ turnover, and it is possible to modulate NAD+ levels by enhancing NAD+ salvage in a compartment-specific manner. Second, overexpression of NAMPT or the mitochondria-targeted NAMPT increases mitochondrial NAD+ to similar levels, suggesting that in basal conditions, cytoplasmic NAMPT activity determines mitochondrial NAD+ levels in astrocytes. Finally, the data provide additional evidence for the existence of a mitochondrial enzymatic activity capable of using NMN to synthetize NAD+.
NAD+ Levels in Aging
Aging is the most important risk factor for the development of many neurodegenerative diseases. It is defined by the reduced ability of the organism to maintain cellular homeostasis over time, leading to accumulated damage to DNA, proteins, membranes, and cellular organelles, including mitochondria. NAD+ concentration decreases during aging in animal models and in humans (26, 32, 112, 195). In vivo imaging data showed for the first time that both NAD+ levels and mitochondrial function decline in the brain of old healthy humans compared to younger subjects (195). Several mechanisms have been proposed to explain the decline of NAD+ concentration with aging. NAMPT expression itself may decline with aging, and ablation or overexpression of NAMPT, respectively, aggravates or prevents age-related changes (59, 153, 162). In addition, the decrease of NAD+ with aging could also be explained by the fact that NAMPT expression displays circadian oscillation; and since circadian oscillation dampens with aging, the overall total levels of NAMPT expression could be affected (39).
On the contrary, increased NAD+ degradation could also account for the decline in NAD+ levels observed in aging. As DNA damage accumulates with aging, PARP1 activation may lead to decreased NAD+ levels and metabolic impairment. However, since PARP1 activation contributes to maintain genomic integrity, the role of PARP1 activation during aging seems to be multifaceted (14). A key role for CD38 in age-related NAD+ decline has been recently proposed (32). In mice, aging is associated with an increase in CD38 activity, which negatively correlates with NAD+ levels and mitochondrial activity; an effect attributed at least, in part, to a decrease in SIRT3 activity. In contrast, CD38 knockout mice seem to be protected from this age-related decline in NAD+ and mitochondrial activity. (32). Since the activity of sirtuins declines with aging (78), these observations link the reduction of NAD+ levels observed during aging as a leading cause for decreased sirtuin activity and possibly the development of age-related diseases. Accordingly, increasing NAD+ availability counteracts the effects of aging in many different models (169). Indeed, NAD+ supplementation has been shown to increase mitochondrial and stem cell function leading to a significant increase in the life span of mice (193).
Circadian Rhythm
The circadian rhythm coordinates metabolism and behavior to recurring daily environmental changes such as light/dark cycles and food availability (147). The ability to anticipate these changes increases the fitness of the organism, while impairment of normal circadian rhythmicity leads to a range of metabolic defects (12, 19, 107, 154). The levels of NAD+ display circadian rhythmicity due to direct clock transcriptional control of NAMPT expression (113, 134). In turn, NAD+ completes a feedback loop by driving the activity of SIRT1, which regulates the activity of the circadian core clock transcription factor heterodimer CLOCK:BMAL1 (113, 134). SIRT6 also participates in circadian regulation by governing CLOCK:BMAL1 recruitment to circadian gene promoters. In addition, SIRT6 controls circadian lipid profiles in the liver by modulating SREBP1 promoter recruitment (104).
Moreover, as mentioned earlier, the activity of key enzymes involved in all major mitochondrial processes is regulated by lysine acetylation, which displays circadian oscillation (103, 115). Since NAD+ availability regulates the activity of the mitochondrial deacetylase SIRT3, circadian control of NAD+ availability directly modulates mitochondrial oxidative metabolism and antioxidant defenses (126). Accordingly, PARP1 and CD38 knockout mice display altered circadian metabolic rhythm (11, 141). A growing body of data obtained from both human and animal studies has documented disruption of circadian timekeeping at the physiological, molecular, and behavioral level in AD, PD, and HD (106, 170). A key question that remains unanswered is whether circadian disruption associated with neurodegeneration contributes to altered metabolism and redox homeostasis.
NAD+ Metabolism and Neurodegeneration
The role of sirtuins in the context of neurodegeneration has been widely studied (51, 74, 78). The following section reviews evidence showing that manipulation of NAD+ metabolism can be a potential therapeutic target for neurodegeneration. It is worth stressing that alterations in NAD+ metabolism can potentially affect a number of biological processes in a sirtuin-independent manner. For example, although SIRT3 activity can regulate mitochondria antioxidant defenses, the ratio and concentration of NADH and NAD+ directly determine the rate of superoxide production by mitochondrial complex I (90, 131). Thus, an increase in NAD+ availability can directly influence reactive oxygen species production by the mitochondria independently of a sirtuin-mediated increase in antioxidant defenses. As new data emerge on the effect of modulating NAD+ availability in models of neurodegeneration, it will be important to characterize with genetic and pharmacological approaches whether the beneficial effects are indeed linked to activation of sirtuin family members.
Perhaps the most recognized link between NAD+ metabolism and neuronal degeneration is the effect of the dominant mutation found in the “Wallerian degeneration slow” (Wlds)-mouse on axonal degeneration. In this mouse model, distal axonal degeneration following sciatic nerve transection is significantly delayed (44, 65). Overexpression of the Wlds gene seems to be protective in some PD and peripheral neuropathy models but fails to confer protection in ALS and spinal muscular atrophy models (44). The Wlds gene is a triplication of a chimeric gene that encodes full-length NMNAT1 fused to the first 70 amino acids from the ubiquitin conjugation factor E4 B (UBE4B).
While the exact mechanism is still debated, it is now clear that the protection conferred by the Wlds gene depends on the presence of NMNAT1 (50, 143). However, maintenance of high levels of NAD+ may not be critical. For example, the absence of PARP1 and CD38 does not confer protection against axonal degeneration despite the observed elevated NAD+ levels in these models (144). Instead, since axonal injury leads to rapid NMNAT2 depletion, the concomitant accumulation of NMN may be responsible for promoting axonal degeneration (50, 97, 144). Accordingly, reducing NMN levels delays Wallerian degeneration after axonal injury (49, 50, 143, 144). On the contrary, NAD+ degradation has also been directly linked to axonal degeneration. SARM1 (sterile alpha and TIR motif containing 1) is a central player in the degenerative program activated following axonal injury. SARM1 initiates a local destruction program through a process that involves the catastrophic depletion of axonal NAD+ (55, 64, 118, 143). Thus, the therapeutic potential of modulating NAD+ availability in the context of Wallerian degeneration remains to be settled.
In addition, it has been shown that NMNAT2 constitutes an essential survival factor for maintenance of healthy axons, and reduction of NMNAT2 expression below a threshold level triggers degeneration even in uninjured axons (66). Moreover, NMNAT overexpression provides neuroprotection in several models of neurodegeneration (8, 44). In addition to help maintain NAD+ levels, NMNATs can act as chaperones, a function that is believed to be responsible for the protection observed in some models of proteotoxicity (7, 192). Consistently with its rate-limiting role in NAD+ synthesis, NAMPT overexpression is also protective in several models of neurodegeneration (72, 81, 176). Interestingly, aminopropyl carbazole derivatives, previously shown to be neuroprotective in ALS and PD models, have been recently identified as NAMPT activators (47, 160, 174).
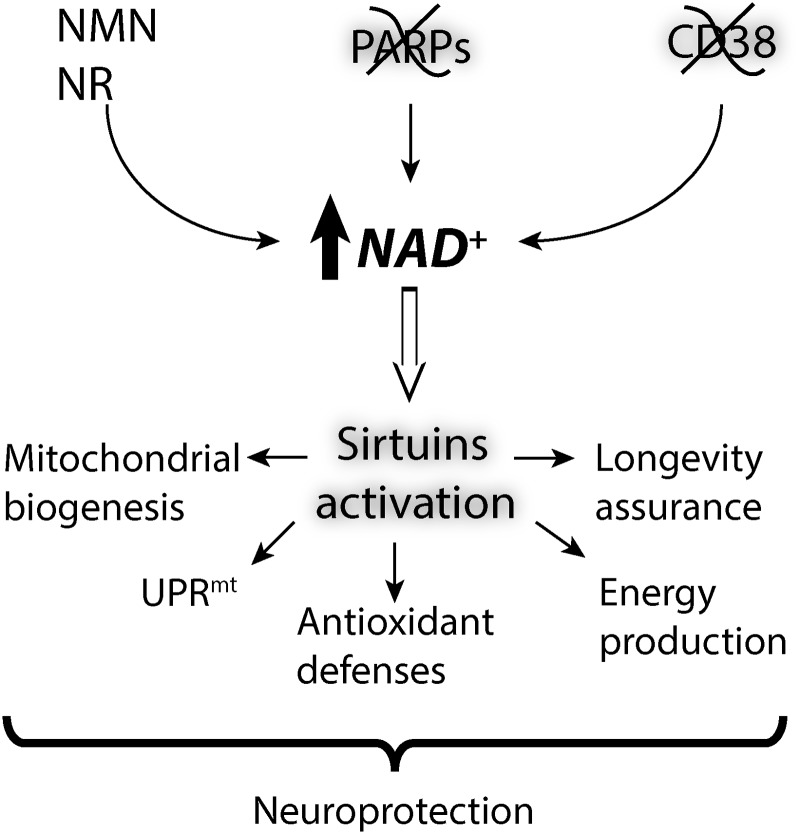
Two main strategies have arisen to increase NAD+ availability: (i) inhibition of NAD+-consuming enzymes and (ii) utilization of bioavailable NAD+ intermediates (Fig. 6).
FIG. 6.
Possible protective mechanisms responsible for the neuroprotection conferred by increasing NAD+ availability. Supplementation with NAD+ precursors/intermediates (NMN and NR) or inhibition of NAD+-consuming enzymes (PARP1 and CD38) leads to increased NAD+ availability and sirtuin activity. PARP1 inhibition could have additional effects not depicted in the figure (see NAD+-dependent signaling section for details).
PARP inhibition
Excessive PARP activation depletes NAD+ and impairs mitochondrial function leading to cell death (14). PARP inhibition improves axonal regeneration in Caenorhabditis elegans neurons and adult dorsal root ganglia neuronal cultures (27, 31). Interestingly, despite elevated neuronal NAD+ levels in PARP1(−/−) and CD38(−/−) mice, these deletions confer no protection against sciatic nerve transection (144). PARP1 activity appears to be upregulated in the CNS of AD, HD, and ALS patients, and PARP1 activation leads to progressive loss of dopaminergic neurons in PD models (86, 91, 98, 101, 171).
PARP1(−/−) mice are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamie toxicity (87, 101), suggesting that PARP1 inhibitors may be beneficial in the treatment of PD. Moreover, PARP inhibitors prevent mitochondrial membrane depolarization and neuronal death in astrocyte/neuronal cocultures exposed to amyloid β (1). Likewise, PARP1 knockout is protective against amyloid β-induced microglial activation and neurotoxicity (83). PARP1 inhibition is also protective in a mouse model of HD (R6/2), but PARP1 knockout aggravates onset and progression in a model of experimental autoimmune encephalomyelitis (37, 150). Comparably, one of the earliest developed PARP inhibitors failed to confer protection in an ALS animal model (10).
Since in neurodegenerative processes dying cells are exposed to increased oxidative stress, and PARPs play a central role in DNA damage repair, further work is needed to establish the value of long-term PARP1 inhibition as a therapeutic target in neurodegenerative diseases. It is worth stressing that in addition to prevent NAD+ depletion due to PARP1 overactivation, PARP1 inhibition will also prevent poly(ADP-ribose)-dependent deleterious effects (see NAD+-dependent signaling section). Thus, the relative contribution of these two components to the pathogenic process may differentially affect the outcome of PARP inhibition in different diseases.
CD38 inhibition
Ablation of CD38 results in a significant increase in the steady-state levels of NAD+ in the brain; with reported changes ranging from 2- to 10-fold increases (5, 188). All studies to date indicate that CD38 activity is constitutive, but can be upregulated following CNS injury. In focal cerebral ischemia, CD38 is upregulated, and CD38(−/−) mice display reduced cerebral injury and immune cell infiltration after ischemia (42, 88). Consistently with its role in the immune system (100), CD38 is likely to have a significant part in the activation of astrocytes and microglia. However, relative scarce data exist about the potential role of CD38 during chronic neurodegeneration. CD38 regulates macrophages and microglia migration to inflammatory mediators such as amyloid β (123). Knocking out CD38 significantly reduces amyloid burden and learning deficits in an AD mouse model (APP/PS) (24). Since CD38 plays a role in many complex biological processes such as stem cell differentiation, transfer of mitochondria between cells, and animal behavior (73, 85, 181), its inhibition can potentially affect neurodegenerative processes in hard to predict ways.
Precursor supplementation
Use of NAD+ biosynthetic intermediates/precursors (mainly NMN and NR) has proven to be an effective way to increase NAD+ availability and sirtuin activity in vitro and in vivo (35, 78, 152). Much of the available literature on precursor supplementation focuses on the capacity to enhance oxidative metabolism and protect against metabolic disease, but some examples have started to emerge in models of neurodegeneration. NMN administration restores mitochondrial function and cognition in AD animal models (96, 175). In addition, long-term NMN administration (12 months) mitigates age-associated physiological decline in wild-type mice, without any obvious toxicity or deleterious effects (109), highlighting the therapeutic potential of this strategy.
As mentioned earlier, NR is a naturally occurring NAD+ precursor that can be imported into the cell, where it is phosphorylated by NRKs to produce NMN (117, 135). NR has recently been shown to be orally available and to safely boost NAD+ levels in mice and humans (161). Dietary supplementation with NR promotes PGC-1α expression and reduction on amyloid β production in an AD mouse model (Tg2576) (68). Moreover, NR administration prevents noise-induced spiral ganglia neurite degeneration in an SIRT3-dependent manner (28).
Using a coculture model, we have shown that boosting NAD+ specifically in astrocytes could be a valid therapeutic approach to confer neuroprotection. Mouse astrocytes expressing ALS-linked SOD1 mutations and human astrocytes isolated from familial or sporadic ALS cases are toxic to cocultured motor neurons (158). Although there is no consensus on the nature of the toxic mediator, increasing antioxidant defenses in ALS astrocytes reverts this neurotoxic phenotype (128, 166, 168). Treatment of ALS astrocytes with NMN or NR decreases the production of reactive oxygen species by the mitochondria and rescues the toxicity toward motor neuron (72). Thus, increasing NAD+ availability may be a viable therapeutic approach to prevent astrocyte-mediated motor neuron death in ALS.
Conclusions
It has become clear that NAD+-dependent signaling has a major impact in many biological processes. Particularly, its manipulation can provide protection against pathologies linked to mitochondrial dysfunction and oxidative stress. Even though the detailed mechanism responsible for the observed protection is not yet established, increasing NAD+ availability is neuroprotective in several in vitro and in vivo models. The increase in NAD+ availability is likely to increase endogenous sirtuin activity and offset NAD+ depletion caused by PARP or CD38 overactivation. The emerging protective mechanism will most likely be relevant in the context of several, acute or chronic, pathological conditions of the CNS. Thus, therapies targeting NAD+ availability could contribute to the development of integral approaches for the treatment of ischemic injury, trauma, and chronic neurodegenerative diseases.
Abbreviations Used
- AceCSs
acetyl-CoA synthases
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- ANT
adenine nucleotide translocator
- BMAL1
brain and muscle ARNT-Like 1
- cADPR
cyclic ADP ribose
- CD38
CD38 antigen
- CLOCK
circadian locomotor output cycles kaput
- CNS
central nervous system
- ENTs
equilibrative nucleoside transporters
- FOXO1
forkhead box O1
- FOXO3a
forkhead box O3a
- G6PD
glucose-6-phosphate dehydrogenase
- GABPα
GA repeat binding protein, alpha
- GABPβ1
GA repeat binding protein, beta 1
- GDH
glutamate dehydrogenase
- HD
Huntington's disease
- IDH2
isocitrate dehydrogenase 2
- LCAD
long chain acyl-CoA dehydrogenase
- NAAD
nicotinic acid adenine dinucleotide
- NAD+
nicotinamide adenine dinucleotide
- NADH
nicotinamide adenine dinucleotide (reduced)
- NADS
NAD+ synthetase
- NAM
nicotinamide
- NAMN
nicotinic acid mononucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NAPRT
nicotinic acid phosphoribosyltransferase
- NAR
nicotinic acid riboside
- NDUFA9
NADH:ubiquinone oxidoreductase subunit A9
- NMN
nicotinamide mononucleotide
- NMNAT
nicotinamide mononucleotide adenylyl transferase
- NR
nicotinamide riboside
- NRF
nuclear respiratory factor
- Nrf2
nuclear factor, erythroid 2 like 2
- NRK
NR kinase
- OPA1
optic atrophy 1
- PARP
poly(ADP-ribose) polymerase
- PD
Parkinson's disease
- PDH
pyruvate dehydrogenase
- PGAM-1
phosphoglycerate mutase-1
- PGC-1α
peroxisome proliferator-activated receptor coactivator-1α
- PNP
purine nucleoside phosphorylase
- QPRT
quinolinate phosphoribosyltransferase
- SARM1
sterile alpha and TIR motif containing 1
- SIRT
sirtuin
- SOD
superoxide dismutase
- SREBP1
sterol regulatory element binding protein 1
- TCA
tricarboxylic acid cycle
- UBE4B
ubiquitin conjugation factor E4 B
- Wlds
Wallerian degeneration slow
Acknowledgments
We thank the reviewers for their comments and suggestions. This work was supported by the NIH grant NS089640.
References
- 1.Abeti R, Abramov AY, and Duchen MR. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134: 1658–1672, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, and Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, and Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem 282: 33583–33592, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, and Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun 349: 353–359, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Aksoy P, White TA, Thompson M, and Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 345: 1386–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, and Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci 30: 2967–2978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali YO, Allen HM, Yu L, Li-Kroeger D, Bakhshizadehmahmoudi D, Hatcher A, McCabe C, Xu J, Bjorklund N, Taglialatela G, Bennett DA, De Jager PL, Shulman JM, Bellen HJ, and Lu HC. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLoS Biol 14: e1002472, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali YO, Li-Kroeger D, Bellen HJ, Zhai RG, and Lu HC. NMNATs, evolutionarily conserved neuronal maintenance factors. Trends Neurosci 36: 632–640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagne JP, Poirier GG, Dawson VL, and Dawson TM. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A 111: 10209–10214, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreassen OA, Dedeoglu A, Friedlich A, Ferrante KL, Hughes D, Szabo C, and Beal MF. Effects of an inhibitor of poly(ADP-ribose) polymerase, desmethylselegiline, trientine, and lipoic acid in transgenic ALS mice. Exp Neurol 168: 419–424, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, and Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142: 943–953, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161: 84–92, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Baeza J, Smallegan MJ, and Denu JM. Mechanisms and Dynamics of Protein Acetylation in Mitochondria. Trends Biochem Sci 41: 231–244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai P. Biology of Poly(ADP-Ribose) Polymerases: the Factotums of Cell Maintenance. Mol Cell 58: 947–958, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Bai P. and Canto C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab 16: 290–295, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, and Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, and Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 21: 3629–3639, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Barile M, Passarella S, Danese G, and Quagliariello E. Rat liver mitochondria can synthesize nicotinamide adenine dinucleotide from nicotinamide mononucleotide and ATP via a putative matrix nicotinamide mononucleotide adenylyltransferase. Biochem Mol Biol Int 38: 297–306, 1996 [PubMed] [Google Scholar]
- 19.Bass J. Circadian topology of metabolism. Nature 491: 348–356, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Belenky P, Bogan KL, and Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci 32: 12–19, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Belenky P, Christensen KC, Gazzaniga F, Pletnev AA, and Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J Biol Chem 284: 158–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger F, Lau C, Dahlmann M, and Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 280: 36334–36341, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Bieganowski P. and Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117: 495–502, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MO, Hartmann T, Lund FE, Stein R, and Levy A. Alzheimer's disease pathology is attenuated in a CD38-deficient mouse model. Ann Neurol 78: 88–103, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose A. and Beal MF. Mitochondrial dysfunction in Parkinson's disease. J Neurochem 139 Suppl 1: 216–231, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, and Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 6: e19194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Brochier C, Jones JI, Willis DE, and Langley B. Poly(ADP-ribose) polymerase 1 is a novel target to promote axonal regeneration. Proc Natl Acad Sci U S A 112: 15220–15225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, and Jaffrey SR. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 20: 1059–1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunyanszki A, Szczesny B, Virag L, and Szabo C. Mitochondrial poly(ADP-ribose) polymerase: the Wizard of Oz at work. Free Radic Biol Med 100: 257–270, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzzone S, Guida L, Zocchi E, Franco L, and De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 15: 10–12, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Byrne AB, McWhirter RD, Sekine Y, Strittmatter SM, Miller DM, and Hammarlund M. Inhibiting poly(ADP-ribosylation) improves axon regeneration. Elife 5: pii: , 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camacho-Pereira J, Tarrago MG, Chini CC, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, and Chini EN. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab 23: 1127–1139, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, and Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352: 1474–1477, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, and Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canto C, Menzies KJ, and Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: a Balancing Act between Mitochondria and the Nucleus. Cell Metab 22: 31–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canto C, Sauve AA, and Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med 34: 1168–1201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardinale A, Paldino E, Giampa C, Bernardi G, and Fusco FR. PARP-1 Inhibition Is Neuroprotective in the R6/2 Mouse Model of Huntington's Disease. PLoS One 10: e0134482, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceni C, Muller-Steffner H, Lund F, Pochon N, Schweitzer A, De Waard M, Schuber F, Villaz M, and Moutin MJ. Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38-deficient mice. J Biol Chem 278: 40670–40678, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Chang HC. and Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153: 1448–1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang HC. and Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25: 138–145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, and Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 12: 534–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choe CU, Lardong K, Gelderblom M, Ludewig P, Leypoldt F, Koch-Nolte F, Gerloff C, and Magnus T. CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia. PLoS One 6: e19046, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 15: 536–550, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Conforti L, Gilley J, and Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 15: 394–409, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, and Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta 1820: 553–564, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozzolino M, Pesaresi MG, Gerbino V, Grosskreutz J, and Carri MT. Amyotrophic lateral sclerosis: new insights into underlying molecular mechanisms and opportunities for therapeutic intervention. Antioxid Redox Signal 17: 1277–1330, 2012 [DOI] [PubMed] [Google Scholar]
- 47.De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, Melito LM, Wang G, Williams NS, Ready JM, McKnight SL, and Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A 109: 17010–17015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Stefano M. and Conforti L. Diversification of NAD biological role: the importance of location. FEBS J 280: 4711–4728, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Di Stefano M, Loreto A, Orsomando G, Mori V, Zamporlini F, Hulse RP, Webster J, Donaldson LF, Gering M, Raffaelli N, Coleman MP, Gilley J, and Conforti L. NMN Deamidase Delays Wallerian Degeneration and Rescues Axonal Defects Caused by NMNAT2 Deficiency In Vivo. Curr Biol 27: 784–794, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, Tickle J, Patrick J, Webster JR, Marangoni M, Carpi FM, Pucciarelli S, Rossi F, Meng W, Sagasti A, Ribchester RR, Magni G, Coleman MP, and Conforti L. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ 22: 731–742, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Dousa TP, Chini EN, and Beers KW. Adenine nucleotide diphosphates: emerging second messengers acting via intracellular Ca2+ release. Am J Physiol 271: C1007–C1024, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, and Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334: 806–809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O'Neil L, White TA, Sinclair DA, and Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62: 1084–1093, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, and Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 93: 1334–1343 e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldman JL, Baeza J, and Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem 288: 31350–31356, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman JL, Dittenhafer-Reed KE, and Denu JM. Sirtuin catalysis and regulation. J Biol Chem 287: 42419–42427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felici R, Lapucci A, Ramazzotti M, and Chiarugi A. Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS One 8: e76938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frederick DW, Loro E, Liu L, Davila A, Jr., Chellappa K, Silverman IM, Quinn WJ, 3rd, Gosai SJ, Tichy ED, Davis JG, Mourkioti F, Gregory BD, Dellinger RW, Redpath P, Migaud ME, Nakamaru-Ogiso E, Rabinowitz JD, Khurana TS, and Baur JA. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab 24: 269–282, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, and Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14: 661–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, Kim B, Park YK, Piersigilli A, Pham TX, Yang Y, Ku CS, Koo SI, Fomitchova A, Canto C, Schoonjans K, Sauve AA, Lee JY, and Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 63: 1190–1204, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garten A, Petzold S, Korner A, Imai S, and Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab 20: 130–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, and Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11: 535–546, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, and Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348: 453–457, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerdts J, Summers DW, Milbrandt J, and DiAntonio A. Axon Self-Destruction: new Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 89: 449–460, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilley J. and Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol 8: e1000300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, and Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, and Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging 34: 1581–1588, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, and Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem 288: 25938–25949, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, and Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Hallows WC, Lee S, and Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A 103: 10230–10235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harlan BA, Pehar M, Sharma DR, Beeson G, Beeson CC, and Vargas MR. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1). J Biol Chem 291: 10836–10846, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, and Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535: 551–555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herskovits AZ. and Guarente L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23: 746–758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higashida H, Liang M, Yoshihara T, Akther S, Fakhrul A, Stanislav C, Nam TS, Kim UH, Kasai S, Nishimura T, Al Mahmuda N, Yokoyama S, Ishihara K, Gerasimenko M, Salmina A, Zhong J, Tsuji T, Tsuji C, and Lopatina O. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci 18: 35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr., Alt FW, Kahn CR, and Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, Saha AK, Nakamura K, Gut P, Verdin E, and Kolthur-Seetharam U. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY) 5: 835–849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imai S. and Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 24: 464–471, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jang SY, Kang HT, and Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem 287: 19304–19314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, and Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496: 110–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jing Z, Xing J, Chen X, Stetler RA, Weng Z, Gan Y, Zhang F, Gao Y, Chen J, Leak RK, and Cao G. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J Cereb Blood Flow Metab 34: 1613–1621, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jovaisaite V. and Auwerx J. The mitochondrial unfolded protein response-synchronizing genomes. Curr Opin Cell Biol 33: 74–81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kauppinen TM, Suh SW, Higashi Y, Berman AE, Escartin C, Won SJ, Wang C, Cho SH, Gan L, and Swanson RA. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid beta. J Neuroinflammation 8: 152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawai Y, Garduno L, Theodore M, Yang J, and Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 286: 7629–7640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim S, Kim T, Lee HR, Jang EH, Ryu HH, Kang M, Rah SY, Yoo J, Lee B, Kim JI, Lim CS, Kim SJ, Kim UH, Lee YS, and Kaang BK. Impaired learning and memory in CD38 null mutant mice. Mol Brain 9: 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SH, Engelhardt JI, Henkel JS, Siklos L, Soos J, Goodman C, and Appel SH. Widespread increased expression of the DNA repair enzyme PARP in brain in ALS. Neurology 62: 319–322, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Kim TW, Cho HM, Choi SY, Suguira Y, Hayasaka T, Setou M, Koh HC, Hwang EM, Park JY, Kang SJ, Kim HS, Kim H, and Sun W. (ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson's disease. Cell Death Dis 4: e919, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kristian T, Balan I, Schuh R, and Onken M. Mitochondrial dysfunction and nicotinamide dinucleotide catabolism as mechanisms of cell death and promising targets for neuroprotection. J Neurosci Res 89: 1946–1955, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Kulikova V, Shabalin K, Nerinovski K, Dolle C, Niere M, Yakimov A, Redpath P, Khodorkovskiy M, Migaud ME, Ziegler M, and Nikiforov A. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J Biol Chem 290: 27124–27137, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kussmaul L. and Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A 103: 7607–7612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC, Kim D, Tessarollo L, Dawson VL, and Dawson TM. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci 16: 1392–1400, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J, Cheng Z, Shi L, Shang Y, and Yu W. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun 7: 12235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liszt G, Ford E, Kurtev M, and Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 280: 21313–21320, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Liu G, Park SH, Imbesi M, Nathan WJ, Zou X, Zhu Y, Jiang H, Parisiadou L, and Gius D. Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy. Antioxid Redox Signal 26: 849–863, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, and Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, and Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol 15: 19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loreto A, Di Stefano M, Gering M, and Conforti L. Wallerian Degeneration Is Executed by an NMN-SARM1-Dependent Late Ca(2+) Influx but Only Modestly Influenced by Mitochondria. Cell Rep 13: 2539–2552, 2015 [DOI] [PubMed] [Google Scholar]
- 98.Love S, Barber R, and Wilcock GK. Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer's disease. Brain 122 (Pt 2): 247–253, 1999 [DOI] [PubMed] [Google Scholar]
- 99.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, and Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep 2: 419–431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, and Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88: 841–886, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, and Dawson TM. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A 96: 5774–5779, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, and Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332: 1443–1446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Masri S, Patel VR, Eckel-Mahan KL, Peleg S, Forne I, Ladurner AG, Baldi P, Imhof A, and Sassone-Corsi P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci U S A 110: 3339–3344, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, and Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158: 659–672, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T, and Cristea IM. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159: 1615–1625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattis J. and Sehgal A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab 27: 192–203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maury E, Hong HK, and Bass J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab 40: 338–346, 2014 [DOI] [PubMed] [Google Scholar]
- 108.Mayo L, Jacob-Hirsch J, Amariglio N, Rechavi G, Moutin MJ, Lund FE, and Stein R. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol 181: 92–103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, and Imai SI. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab 24: 795–806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyake Y, Tanaka K, Fukushima W, Kiyohara C, Sasaki S, Tsuboi Y, Yamada T, Oeda T, Shimada H, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M, and Fukuoka Kinki Parkinson's Disease Study G. Lack of association between BST1 polymorphisms and sporadic Parkinson's disease in a Japanese population. J Neurol Sci 323: 162–166, 2012 [DOI] [PubMed] [Google Scholar]
- 111.Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, and Chen D. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347: 1374–1377, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, and Auwerx J. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 154: 430–441, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakahata Y, Sahar S, Astarita G, Kaluzova M, and Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nemoto S, Fergusson MM, and Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, and Asher G. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S A 113: E1673–E1682, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Niere M, Kernstock S, Koch-Nolte F, and Ziegler M. Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol Cell Biol 28: 814–824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nikiforov A, Dolle C, Niere M, and Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem 286: 21767–21778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr., Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, and Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337: 481–484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ou X, Lee MR, Huang X, Messina-Graham S, and Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 32: 1183–1194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paik WK, Pearson D, Lee HW, and Kim S. Nonenzymatic acetylation of histones with acetyl-CoA. Biochim Biophys Acta 213: 513–522, 1970 [DOI] [PubMed] [Google Scholar]
- 121.Palomo GM. and Manfredi G. Exploring new pathways of neurodegeneration in ALS: the role of mitochondria quality control. Brain Res 1607: 36–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, and Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res 26: 190–205, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, Wang JM, and Lund FE. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol 172: 1896–1906, 2004 [DOI] [PubMed] [Google Scholar]
- 124.Partida-Sanchez S, Rivero-Nava L, Shi G, and Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol 590: 171–183, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Pawlikowska L, Cottrell SE, Harms MB, Li Y, and Rosenberg PA. Extracellular synthesis of cADP-ribose from nicotinamide-adenine dinucleotide by rat cortical astrocytes in culture. J Neurosci 16: 5372–5381, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pehar M, Ball LE, Sharma DR, Harlan BA, Comte-Walters S, Neely BA, and Vargas MR. Changes in protein expression and lysine acetylation induced by decreased glutathione levels in astrocytes. Mol Cell Proteomics 15: 493–505, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pehar M, Beeson G, Beeson CC, Johnson JA, and Vargas MR. Mitochondria-Targeted Catalase Reverts the Neurotoxicity of hSOD1G93A Astrocytes without Extending the Survival of ALS-Linked Mutant hSOD1 Mice. PLoS One 9: e103438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pehar M. and Puglielli L. Lysine acetylation in the lumen of the ER: a novel and essential function under the control of the UPR. Biochim Biophys Acta 1833: 686–697, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, and Chiarugi A. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem 285: 34106–34114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pryde KR. and Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem 286: 18056–18065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiu X, Brown K, Hirschey MD, Verdin E, and Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 133.Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, and Malavasi F. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 84: 207–217, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, and Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324: 651–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, and Canto C. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun 7: 13103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Revollo JR, Grimm AA, and Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, and Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6: 363–375, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, and Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8: 1–27, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Ryu D, Jo YS, Lo Sasso G, Stein S, Zhang H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, Schoonjans K, and Auwerx J. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab 20: 856–869, 2014 [DOI] [PubMed] [Google Scholar]
- 140.Saad M, Lesage S, Saint-Pierre A, Corvol JC, Zelenika D, Lambert JC, Vidailhet M, Mellick GD, Lohmann E, Durif F, Pollak P, Damier P, Tison F, Silburn PA, Tzourio C, Forlani S, Loriot MA, Giroud M, Helmer C, Portet F, Amouyel P, Lathrop M, Elbaz A, Durr A, Martinez M, Brice A, and French Parkinson's Disease Genetics Study G. Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum Mol Genet 20: 615–627, 2011 [DOI] [PubMed] [Google Scholar]
- 141.Sahar S, Nin V, Barbosa MT, Chini EN, and Sassone-Corsi P. Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging (Albany NY) 3: 794–802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, and Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34: 807–819, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sasaki Y, Nakagawa T, Mao X, DiAntonio A, and Milbrandt J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. Elife 5: pii: , 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sasaki Y, Vohra BP, Lund FE, and Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci 29: 5525–5535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, and Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet 41: 1303–1307, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Schapira AH, Olanow CW, Greenamyre JT, and Bezard E. Slowing of neurodegeneration in Parkinson's disease and Huntington's disease: future therapeutic perspectives. Lancet 384: 545–555, 2014 [DOI] [PubMed] [Google Scholar]
- 147.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, Demarque M, and Franken P. Clock-Talk: interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb Symp Quant Biol 80: 223–232, 2015 [DOI] [PubMed] [Google Scholar]
- 148.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, and Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A 103: 10224–10229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scott I, Webster BR, Li JH, and Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5 L1. Biochem J 443: 655–661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Selvaraj V, Soundarapandian MM, Chechneva O, Williams AJ, Sidorov MK, Soulika AM, Pleasure DE, and Deng W. PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis. J Biol Chem 284: 26070–26084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, and Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stein LR. and Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 23: 420–428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stein LR. and Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J 33: 1321–1340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stubblefield JJ, Terrien J, and Green CB. Nocturnin: at the crossroads of clocks and metabolism. Trends Endocrinol Metab 23: 326–333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a “complex” problem. Antioxid Redox Signal 9: 1591–1603, 2007 [DOI] [PubMed] [Google Scholar]
- 156.Tang KS, Suh SW, Alano CC, Shao Z, Hunt WT, Swanson RA, and Anderson CM. Astrocytic poly(ADP-ribose) polymerase-1 activation leads to bioenergetic depletion and inhibition of glutamate uptake capacity. Glia 58: 446–457, 2010 [DOI] [PubMed] [Google Scholar]
- 157.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, and Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor JP, Brown RH, Jr., and Cleveland DW. Decoding ALS: from genes to mechanism. Nature 539: 197–206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Terakata M, Fukuwatari T, Sano M, Nakao N, Sasaki R, Fukuoka S, and Shibata K. Establishment of true niacin deficiency in quinolinic acid phosphoribosyltransferase knockout mice. J Nutr 142: 2148–2153, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, McDaniel L, Knobbe W, Burket A, Tran S, Starwalt R, Morlock L, Naidoo J, Williams NS, Ready JM, McKnight SL, and Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 109: 17016–17021, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, and Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7: 12948, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, and Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem 282: 10841–10845, 2007 [DOI] [PubMed] [Google Scholar]
- 163.van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, and Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res 97: 25–34, 2005 [DOI] [PubMed] [Google Scholar]
- 164.Van Meter M, Simon M, Tombline G, May A, Morello TD, Hubbard BP, Bredbenner K, Park R, Sinclair DA, Bohr VA, Gorbunova V, and Seluanov A. JNK Phosphorylates SIRT6 to Stimulate DNA Double-Strand Break Repair in Response to Oxidative Stress by Recruiting PARP1 to DNA Breaks. Cell Rep 16: 2641–2650, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.VanLinden MR, Dolle C, Pettersen IK, Kulikova VA, Niere M, Agrimi G, Dyrstad SE, Palmieri F, Nikiforov AA, Tronstad KJ, and Ziegler M. Subcellular Distribution of NAD+ between Cytosol and Mitochondria Determines the Metabolic Profile of Human Cells. J Biol Chem 290: 27644–27659, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vargas MR, Johnson DA, Sirkis DW, Messing A, and Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 28: 13574–13581, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vargas MR. and Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med 11: e17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vargas MR, Pehar M, Cassina P, Beckman JS, and Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem 97: 687–696, 2006 [DOI] [PubMed] [Google Scholar]
- 169.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science 350: 1208–1213, 2015 [DOI] [PubMed] [Google Scholar]
- 170.Videnovic A, Lazar AS, Barker RA, and Overeem S. ‘The clocks that time us’—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 10: 683–693, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vis JC, Schipper E, de Boer-van Huizen RT, Verbeek MM, de Waal RM, Wesseling P, ten Donkelaar HJ, and Kremer B. Expression pattern of apoptosis-related markers in Huntington's disease. Acta Neuropathol 109: 321–328, 2005 [DOI] [PubMed] [Google Scholar]
- 172.Wagner GR. and Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem 288: 29036–29045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang F, Nguyen M, Qin FX, and Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6: 505–514, 2007 [DOI] [PubMed] [Google Scholar]
- 174.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, and McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158: 1324–1334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X, Hu X, Yang Y, Takata T, and Sakurai T. Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res 1643: 1–9, 2016 [DOI] [PubMed] [Google Scholar]
- 176.Wang X, Li H, and Ding S. Pre-B-cell colony-enhancing factor protects against apoptotic neuronal death and mitochondrial damage in ischemia. Sci Rep 6: 32416, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, Chen R, Wang JE, Kam TI, Jeong JS, Xie Z, Neifert S, Qian J, Andrabi SA, Blackshaw S, Zhu H, Song H, Ming GL, Dawson VL, and Dawson TM. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 354: pii: , 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, Ling ZQ, Hu FJ, Sun YP, Zhang JY, Yang C, Yang Y, Xiong Y, Guan KL, and Ye D. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J 33: 1304–1320, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, and Kaasik A. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284: 21379–21385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Webster BR, Scott I, Han K, Li JH, Lu Z, Stevens MV, Malide D, Chen Y, Samsel L, Connelly PS, Daniels MP, McCoy JP, Jr., Combs CA, Gucek M, and Sack MN. Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J Cell Sci 126: 4843–4849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wei W, Lu Y, Hao B, Zhang K, Wang Q, Miller AL, Zhang LR, Zhang LH, and Yue J. CD38 is required for neural differentiation of mouse embryonic stem cells by modulating reactive oxygen species. Stem Cells 33: 2664–2673, 2015 [DOI] [PubMed] [Google Scholar]
- 182.Xiong Y. and Guan KL. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol 198: 155–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, and Yan WJ. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res 309: 1–8, 2016 [DOI] [PubMed] [Google Scholar]
- 184.Yamada M, Mizuguchi M, Otsuka N, Ikeda K, and Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res 756: 52–60, 1997 [DOI] [PubMed] [Google Scholar]
- 185.Yamamoto M, Hikosaka K, Mahmood A, Tobe K, Shojaku H, Inohara H, and Nakagawa T. Nmnat3 Is Dispensable in Mitochondrial NAD Level Maintenance In Vivo. PLoS One 11: e0147037, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, and Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130: 1095–1107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yoshino J, Mills KF, Yoon MJ, and Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Young GS, Choleris E, Lund FE, and Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38-/- mouse. Biochem Biophys Res Commun 346: 188–192, 2006 [DOI] [PubMed] [Google Scholar]
- 189.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, and Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 103: 18314–18319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, and Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263, 2002 [DOI] [PubMed] [Google Scholar]
- 191.Yu W, Dittenhafer-Reed KE, and Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287: 14078–14086, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, and Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature 452: 887–891, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, and Auwerx J. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352: 1436–1443, 2016 [DOI] [PubMed] [Google Scholar]
- 194.Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu Q, Wang Y, Wang S, Xiong Y, Guan KL, Yang P, Yu H, and Ye D. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep 17: 811–822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu XH, Lu M, Lee BY, Ugurbil K, and Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A 112: 2876–2881, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]