Abstract
Significance: Essential metals such as copper, iron, manganese, and zinc play a role as cofactors in the activity of a wide range of processes involved in cellular homeostasis and survival, as well as during organ and tissue development. Throughout our life span, humans are also exposed to xenobiotic metals from natural and anthropogenic sources, including aluminum, arsenic, cadmium, lead, and mercury. It is well recognized that alterations in the homeostasis of essential metals and an increased environmental/occupational exposure to xenobiotic metals are linked to several neurological disorders, including neurodegeneration and neurodevelopmental alterations.
Recent Advances: The redox activity of essential metals is key for neuronal homeostasis and brain function. Alterations in redox homeostasis and signaling are central to the pathological consequences of dysfunctional metal ion homeostasis and increased exposure to xenobiotic metals. Both redox-active and redox-inactive metals trigger oxidative stress and damage in the central nervous system, and the exact mechanisms involved are starting to become delineated.
Critical Issues: In this review, we aim to appraise the role of essential metals in determining the redox balance in the brain and the mechanisms by which alterations in the homeostasis of essential metals and exposure to xenobiotic metals disturb the cellular redox balance and signaling. We focus on recent literature regarding their transport, metabolism, and mechanisms of toxicity in neural systems.
Future Directions: Delineating the specific mechanisms by which metals alter redox homeostasis is key to understand the pathological processes that convey chronic neuronal dysfunction in neurodegenerative and neurodevelopmental disorders. Antioxid. Redox Signal. 28, 1669–1703.
Keywords: : neurodegeneration, redox, essential metals, heavy metals, neurotoxicity
Introduction
Most elements in the periodic table are considered metals due to their propensity to lose electrons and react with molecular oxygen (O2) to form oxides. Metals in biological systems may be broadly divided into four groups: alkali and alkaline-earth metals, such as sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca); essential transition metals, such as copper (Cu), manganese (Mn), iron (Fe), and zinc (Zn); and xenobiotic heavy metals such as mercury (Hg), lead (Pb), and cadmium (Cd). In addition, metalloids such as arsenic (As) are present in the environment and have chemical and physical properties of both metal and nonmetal elements. Some authors include aluminum (Al) and selenium (Se) as metalloids. For simplicity, herein we will refer to metalloids as metals. Importantly, while essential metals participate in normal biological functions, alterations in their handling or their increased accumulation are well reported to exert neurotoxicity (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ars).
Prospective epidemiological studies have associated cognitive, motor, and behavioral alterations to environmental exposure to metals and metalloids (153, 158, 250, 371), effects that are exacerbated when environmental exposures occur chronically and during development (153, 158, 250). Long-term effects of either environmental metal exposure or alterations in metal homeostasis in the central nervous system (CNS) and peripheral nervous system (PNS) have been proposed to play a role in neurodegenerative disorders (379). Importantly, alterations in the cellular redox environment of the cell are central to the toxic effects of metals.
Previous reviews address the general role of metals in neurodegeneration, or the mechanisms by which metals produce oxidative stress or neurotoxicity (85, 86, 89, 163, 355). In this work, we present an integrated review on recent advances in (a) the metabolism of both essential and xenobiotic metals; (b) the mechanisms by which distinct metals determine or modify the cellular redox homeostasis; (c) the link between metal redox activity and function in neural systems; and (d) how alterations in metal homeostasis or intracellular/extracellular levels participate in neurotoxicity and neurodegeneration.
Overview of Oxidative Stress and Redox Homeostasis
Reactive species is a term used to describe compounds that can receive or provide a couple of electrons or one electron participating in nucleophilic, electrophilic, or redox metabolic reactions, respectively. Reactive oxygen species (ROS) are molecules derived from O2, an obligate component of aerobic organisms. The reduction of O2 is one of the primary reactions that sustain aerobic life, yet it is also the main source for ROS. ROS include free (•) and nonfree radical species such as hydroxyl radicals (•OH), superoxide anion radicals (O2•−), and hydrogen peroxide (H2O2). Reactive nitrogen species (RNS) contain both nitrogen (N) and O (oxygen atom), and thus can be categorized as ROS. RNS include nitric oxide (NO•), nitrogen dioxide radical (NO2), and peroxynitrite (OONO−) (120, 252).
A major source of intracellular ROS production are the mitochondrial electron transport complexes, primarily the one-electron reduction of O2 to O2•− by complex I (ubiquinone: NADH oxidoreductase), by the semiquinone of ubiquinone (coenzyme Q), and by complex III (cytochrome bc1 complex or CoQH2-cytochrome c reductase). O2•− undergoes rapid dismutation into H2O2 through the action of superoxide dismutases (SODs). Three types of SODs exist in mammalian cells that use an essential metal as cofactor. Cu/Zn-dependent SOD1 and 3 are localized in the cytosol (SOD1), the extracellular space (SOD3) and to a lesser extent, in the inner membrane space of the mitochondria (SOD1). MnSOD (SOD2) is solely localized in the mitochondrial matrix. The transition metal (Cu or Mn) in the active site of SODs is required for the breakdown of O2•− by catalyzing both the one-electron oxidation and one-electron reduction of separate O2•− to give the overall disproportionation reaction that produces O2 and H2O2. Binding of Zn to SOD1 or 3 is not essential for O2•− dismutation reaction but confers higher thermal stability to the proteins (64).
One to 2% of the total mitochondrial O2 consumed is leaked and contributes to the formation of ROS. Usually, this occurs at a slow rate and can be counteracted by mitochondrial antioxidant systems, but in damaged or aged mitochondria, increased ROS formation occurs. O2•− and H2O2 fuel •OH formation through Fenton/Haber–Weiss reactions, where H2O2 oxidizes a redox-active metal (Fe or Cu) leading to the formation of •OH. Then, the oxidized metal is reduced back by O2•− or other cellular reductants promoting metal-catalyzed free radical chain reactions (100, 120, 252).
Other sources of ROS are the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidases (NOX), enzymes whose principal function is to generate O2•− or H2O2. The formation of RNS begins with the synthesis of NO•, catalyzed by nitric oxide synthases (NOSs), which are Fe dependent. Zn is an important structural element of NOS enzymes and is also known to inhibit their activity. NO• reacts with O2•− to produce OONO−, which is a strong oxidizing agent. Microsomes and peroxisomes are important sources of ROS due to the presence of NOX and NOS (302). In addition, ROS production can be mediated by the activity of enzymes such as xanthine oxidase (that contains an Fe-sulfur [S] cluster), the heme proteins cyclooxygenases, cytochrome P450 enzymes, lipoxygenases, and myeloperoxidases, as well as the protein folding machinery in the endoplasmic reticulum (ER) (96, 120, 252).
ROS/RNS act as signaling molecules affecting the stability, expression, function, and activity of a multiplicity of proteins controlling almost all cellular functions, including proliferation, cell survival, metabolism, and signaling. An adequate balance between the formation and elimination of ROS/RNS facilitates the signaling role of these reactive species. However, an imbalance between an increase in the steady-state levels of ROS/RNS and the ability of the cell to metabolize/detoxify them leads to a nonhomeostatic state referred to as oxidative stress. Oxidative stress results in the irreversible oxidative modification of biomolecules with the concomitant loss of function of proteins, damage to cellular organelles, and eventual cell death (96, 100, 252, 282). Polyunsaturated fatty acids are one of the preferred oxidation targets for ROS; particularly, free radicals that are potent initiators of lipid peroxidative chain reactions. Lipid peroxidation products, including malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE), can react further with DNA bases and proteins (83). DNA bases are also susceptible to direct oxidation by free radicals that can cause mutations as well as deletions in both nuclear and mitochondrial DNA (328).
Protein oxidation can be a reversible or irreversible phenomenon depending on the type of modification, ROS involved, and the extent of oxidation. Tyrosine (Tyr) nitration, protein carbonylation, and protein crosslinkage generated by adduct formation between oxidized proteins, lipid peroxides, or glycative products are irreversible modifications that promote a loss of function, aggregation, and degradation of the targeted protein, and in some cases, the formation of toxic by-products (71). In contrast, reversible oxidative modifications in the sulfur-containing amino acids methionine (Met) and cysteine (Cys) act as sensors and transducers of ROS/RNS-mediated signaling. Thiol-based oxidoreductases thioredoxins (Trxs), glutaredoxins (Grxs), and Met sulfoxide reductases reduce such modifications acting as the OFF-switch for redox signaling processes (101, 160). On the contary, both Trxs and peroxiredoxins (Prxs) have been proposed to act as redox sensors, buffers, and relays for H2O2- and NO•-mediated signal transduction (46, 195, 268, 323). Cys are also targeted by electrophiles generating irreversible modifications, which are thought to be a primary mechanism of toxicity by xenobiotics (193).
Cells are equipped with enzymatic and nonenzymatic antioxidant systems to counteract the toxic effects of ROS/RNS and maintain redox homeostasis (96, 100, 120, 252). The reducing power of glutathione (GSH) is essential for the detoxification of peroxides by GSH peroxidases (GPX) with the resultant conversion of GSH to GSH disulfide (GSSG). GSSG is reduced back by GSH reductase (GR) in an NADPH-dependent manner (105). Catalases also detoxify peroxides but their localization is primarily restricted to peroxisomes. Other endogenous nonenzymatic antioxidants include uric acid, lipoic acid, and ubiquinol (or reduced coenzyme Q), and those obtained from the diet, such as vitamins and flavonoids (96, 100, 120, 252).
The CNS is particularly sensitive to oxidative damage, from which neurons and oligodendrocytes seem to be more susceptible than astrocytes and microglia. The basis for this increased sensitivity is linked to the high levels of O2 consumption (and electron leakage as a consequence), the low levels of antioxidant defenses when compared to other cells, and the abundance of lipids or fatty acids (262, 295).
Higher levels of endogenous antioxidants and antioxidant systems in astrocytes are explained by the activation of the nuclear factor erythroid-2-related factor 2 (Nrf2) transcription factor (314). Nrf2 recognizes antioxidant response elements to trigger the transcription of antioxidant systems. The ubiquitin ligase Kelch-like ECH-associated protein 1 (Keap1) negatively regulates Nrf2 signaling by inducing its ubiquitination and degradation. Upon modification of specific Cys residues within Keap1 by oxidants or electrophiles (including metals), Nrf2 is released from Keap1 and translocates to the nucleus to induce gene expression dependent on antioxidant response elements (ARE). Nrf2 signaling in neurons has been reported to be epigenetically silenced (27), and induction of the Nrf2 pathway does not seem to be able to promote antioxidant protection (148). Astrocytes also have higher levels of NADPH and glucose 6-phosphate dehydrogenase (G6PD) (109). In contrast, antioxidant genes in neurons seem to be transcriptionally regulated, independent from Nrf2 by synaptic activity, through the triggering of the activating transcription factor 4 (ATF4) and the activator protein 1 (AP-1) (23, 177). Furthermore, while both neurons and astrocytes can synthesize GSH, neurons depend on the supply of GSH precursors via GSH efflux (13, 26).
Neurotoxicity of Metals and Metalloids
Neurotoxicity is defined as a damaging effect on the nervous system caused by a biological or chemical agent. The neurotoxic effects of chemicals are the result of a series of events that include the following: the entry and/or changes in the distribution of a chemical into the brain, interactions with specific cellular targets (neurons and glia), and the initiation of biochemical changes, resulting in structural and functional changes of the nervous system (270). Environmental neurotoxicants include organic and inorganic chemical compounds, such as heavy metals, organic solvents, and cytotoxic substances that can also contain heavy metal mixtures (e.g., pesticides, cigarette smoke, diesel exhaust particles,). The neurotoxic effect of environmental agents is determined by their chemical composition, metabolic function, and pathologican consequence, differing widely according to the brain region targeted and the mechanism(s) of action (270).
Essential Metals
Micronutrients are defined by their essentiality and very limited quantity in humans, where their deficiency results in the impairment of biological functions (103, 205). Some metals are essential for the maintenance of cellular homeostasis. Essential metals display important roles as signaling agents or cofactors and, in particular, as activators or redox system components (Supplementary Table S1) (103, 205). Traditionally, cellular osmotic balance and signaling (including synaptic communication and excitability) are associated with nontransition metal ions, such as Na+, K+, and Ca2+, which form complexes with proteins using low-affinity binding sites, are found at high concentrations, and move quickly across cellular compartments. On the contrary, transition metal ions are known as catalytic cofactors or structural elements in enzymes. Transition metals are present in lower concentration (“trace elements”) and are usually coordinated to proteins at high-affinity binding sites. In the last decade, a role for Zn2+ ion as a second messenger has been recognized; however, the role of redox-active metals, such as Cu, Fe, and Mn, in cellular signaling is less explored.
Cu, Fe, and Mn are cofactors of many enzymes that catalyze redox reactions. Although the high reactivity of these metals is essential for life, they can also be involved in uncontrolled redox reactions associated with oxidative stress and cellular damage. Hence, a highly conserved network of proteins strictly regulates the homeostasis of redox-active metal ions, by controlling their uptake, intracellular distribution, storage, and export (205). In the following sections, we describe how brain homeostasis of redox reactive metal ions requires close communication between the blood-brain barrier (BBB), neurons, astrocytes, oligodendrocytes, and microglia. The cases where metal trafficking is tightly linked to the cellular redox environment are highlighted, while the potential role of metal redox cycling in redox signaling is also discussed. Finally, for each essential metal ion, we review how the disruption of its homeostasis may cause two major features associated with neurodegenerative diseases: dysfunction of metalloproteins and aberrant metal-protein interactions that can lead to protein aggregation and uncontrolled ROS production.
Copper
Cu is present in biological systems as Cu+ (cuprous ion) and Cu2+ (cupric ion) (Supplementary Table S1). Cu is a redox-active metal and a cofactor of many enzymes involved in cellular respiration, radical detoxification, as well as biosynthesis of neurotransmitters, neuropeptides, and hormones. For example, Cu is required as cofactor of several important enzymes in the brain, such as peptidylglycine monooxygenase (PHM), dopamine β-monooxygenase (DBM), tyrosinase (TYR), and cytochrome C oxidase (COX). Cu can activate O2 for reduction and although its high reactivity with O2 is essential for life, if uncontrolled it can promote oxidative stress and cellular damage. Cu+ can react with H2O2 to produce highly reactive •OH. Cu also induces microglial activation and mitochondrial ROS formation (137).
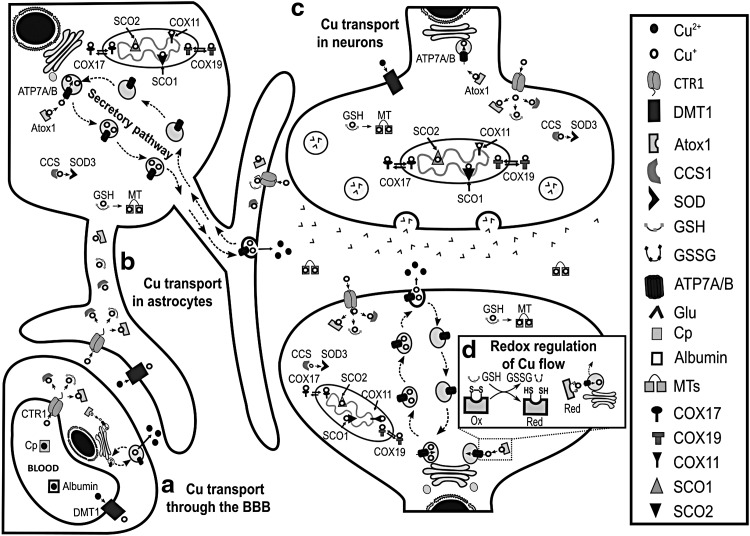
The control of Cu homeostasis in the brain requires a close interrelationship between the BBB, neurons, and astrocytes (300) (Fig. 1). Astrocytes regulate the properties of the BBB, which is the entry point for Cu into the brain from the blood stream, where Cu is bound to albumin or ceruloplasmin (Cp) (58) (Fig. 1a). At the same time, neurons require Cu as a cofactor and neuromodulator, while astrocytes are key players in synaptic transmission and Cu homeostasis (58). The Cu trafficking machineries of the BBB endothelial cells, neurons, and astrocytes resemble those of other extensively studied mammalian cells (Fig. 1a–c). Extracellular Cu is primarily transported into cells as Cu+ via the Cu transporter 1 (CTR1) (58). Cu2+ reduction to Cu+, and Cu uptake from Cp via CTR1, has been proposed to involve a reduction step but no Cu2+ reductase has been identified (281). CTR1-independent mechanisms have also been proposed. The divalent metal transporter 1 (DMT1) seems to play a compensatory role for Cu uptake under certain conditions such as in the absence of CTR1 or under low Fe conditions (147, 231). Interestingly, DMT1 loss promotes brain Cu accumulation and oxidative stress (122). Other potential candidates recently proposed to mediate Cu uptake are the Zrt (Zn-regulated transporter)- or Irt (Fe-regulated transporter)-like protein 4 (ZIP4) (11, 29).
FIG. 1.
Cu homeostasis in the brain and its redox control. Regulation of Cu homeostasis is a complex process requiring intercommunication between endothelial cells of the BBB, astrocytes, and neurons. These three types of cells have the same protein machinery as other mammalian cells: CTR1 and DMT1 for Cu uptake; Atox1, CCS1, and GSH for Cu delivery to ATP7(A/B), SOD3, and MT, respectively; COX17 and COX19 for Cu delivery to mitochondria, where SCO1, SCO2, and COX11 load Cu into COX; while ATP7 (A/B) participates in Cu export through the secretory pathway (a–c). (a) Cp- and albumin- bound Cu enters into the brain by crossing endothelial cells of BBB, which are in tight communication with astrocytes. (b) Astrocytes serve as an interface to deliver Cu to (c) neurons. (d) Recently, a redox control of Cu efflux was elucidated in neurons during differentiation: the redox state of Atox1 controls Cu delivery to the secretory pathway. ATP7A, ATPase copper transporting alpha; ATP7B, ATPase copper transporting beta; Atox1, antioxidant protein 1; BBB, blood-brain barrier; CCS1, copper chaperone for superoxide dismutase 1; COX, cytochrome C oxidase; Cp, ceruloplasmin; CTR1, copper transporter 1; Cu, copper; DMT1, divalent metal transporter 1; Glu, glutamate; GSSG, glutathione disulfide; GSH, glutathione; MT, metallothionein; SCO, cytochrome c oxidase assembly protein; SOD, superoxide dismutase.
Intracellular Cu distribution depends on the relative concentration and metal affinity of chaperones or chelators (18) (Fig. 1b, c). The antioxidant protein 1 (Atox1), the Cu chaperone for superoxide dismutase 1 (CCS1), and GSH have been proposed to take Cu+ from CTR1 (197). Chaperones not only bind Cu but they also deliver it to specific targets. CCS1 transfers Cu to SOD1, where its reactivity with O2 is required for SOD1 maturation via the formation of a disulfide bridge (17). Copper chaperones COX19 and COX17 deliver Cu to the COX assembly proteins (SCO1 and SCO2) and COX11. Finally, Atox1 transports Cu to the ATPase copper transporting alpha (ATP7A) and beta (ATP7B) in the secretory pathway, where cuproenzymes such as SOD3 are metalized (Fig. 1b, c). Upon an excess in cytosolic Cu levels, vesicles in the secretory pathway are loaded with Cu and trafficked to the plasma membrane, where Cu is released into the extracellular space (196, 197).
Strikingly, although GSH has a lower affinity for Cu, compared to Atox1 and CCS1, the rate of Cu entry into the cell via CTR1 is affected by GSH, but not by Atox1 or CCS1 depletion, likely due to the higher concentration of GSH (208). Thus, GSH is the most important cytosolic first acceptor of Cu from CTR1, providing a tight link between cellular Cu uptake and cellular redox homeostasis. GSH in turn is known to transfer Cu ions to metallothioneins (MTs), small Cys-rich proteins that play a major role as scavengers for metal ions (93) (Fig. 1b, c). Three distinct isoforms of MTs are expressed in the human brain. MT-I, MT-II, and MT-III are found in astrocytes, while MT-III is the main isoform expressed in neurons. MTs can be secreted, playing a crucial role in modulating Cu homeostasis and protecting the cell from oxidative damage (299).
Chaperones use Cys residues to coordinate Cu+ in their reduced state. Thus, Cys oxidation affects Cu dynamics. Atox1 coordinates Cu using a CysXXCys motif that can form a disulfide bond, which can be reduced directly by GSH or by Grx1 in a GSH-dependent manner (Fig. 1d) (41, 127). During neuronal differentiation, the GSH/GSSG ratio increases promoting a more reductive environment, which in turn reduces Cys residues at the Cu binding site of Atox1. These events enable Cu transport from Atox1 to ATP7A/B and enhance Cu availability to load the active sites of newly synthetized cuproenzymes (128) (Fig. 1d). After neuronal differentiation, both Cu and MT-III levels increase (249).
The recent development of fluorescent sensors has revealed new important roles of intracellular Cu in neuronal activity (78); for example, in the spine neck of hippocampal neurons, Cu is essential for the control of the dendritic actin cytoskeleton (269). Cu export by ATP7A has been reported to be triggered by the activation of glutamate (Glu)/N-methyl-d-aspartate receptors (NMDARs) (301) (Fig. 2a). At the synapse, Cu can modulate many neurotransmitter receptors (66). For example, Cu inhibits NMDAR activity by Cys nitros(yl)ation, a neuroprotective mechanism associated with neuronal plasticity that requires the participation of the cellular prion protein (PrPC) (110) (Fig. 2b). Accordingly, selective depletion of ATP7A in neurons and glia increases the susceptibility to NMDA seizures (132).
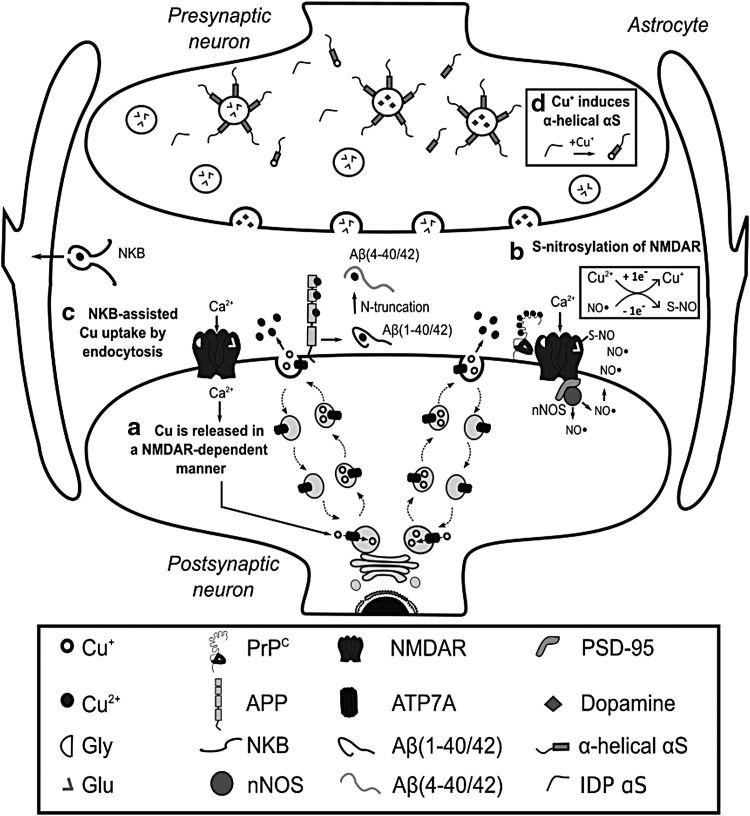
FIG. 2.
Cu at synapse and redox signaling. (a) After activation of NMDAR, Cu is released at the synapse through the secretory pathway. Several Cu binding proteins are present in the synaptic cleft, including PrPC, APP, Aβ peptides, and NKB. (b) The Cu-PrPC interaction is particularly important for the modulation of NMDAR activity via S-nitros(yl)ation. In turn, NO production is dependent on the activation of NMDAR and its interaction with nNOS through the PSD-95. (c) While NKB can assist Cu uptake into astrocytes, Cu distribution among Cu-binding proteins at the synapse likely depends on their relative concentration and affinities for Cu. (d) On the contrary, α-synuclein is an important presynaptic Cu-binding protein found in neurons; it is an IDP that can interact with membranes when it acquires α-helix structure at N-terminal region. Interestingly, Cu+ binding to the N-Terminal of α-synuclein enhances its α-helical conformation. Aβ, amyloid beta; APP, amyloid precursor protein; Cu+, cuprous ion; IDP, intrinsically disordered protein; NKB, neurokinin B; NMDAR, glutamate/N-methyl-d-aspartate receptor; nNOS, neural nitric oxide synthetase; PrPC, cellular prion protein; PSD-95, postsynaptic density protein 95.
Cu trafficking at the synapse is complex and involves several Cu binding proteins, such as the membrane-bound PrPC, the amyloid precursor protein (APP), and the amyloid beta (Aβ) peptide from neurons, or the neurokinin B (NKB) peptide from astrocytes (Fig. 2). PrPC has several Cu binding sites and it might be involved in Cu sensing and transport into neurons (Fig. 3a). During synaptic transmission, the extracellular Cu concentration may reach ∼100 μM. Cu binding to PrPC induces its endocytosis, possibly contributing to Cu delivering into the cytosol (263). APP has been reported to regulate Cu efflux, as the APP knockout mice display higher levels of Cu in the brain and in neurons, while APP overexpression leads to decreased intracellular Cu concentrations (28, 330, 375). In contrast, Aβ peptides produced from the proteolytic cleavage of APP have been proposed to act as Cu scavengers (264), particularly those cleaved to yield 4–40 peptides that have higher affinity for Cu and make up to 50% of the Aβ in plaques (374). NKB has been suggested to compete for Cu from PrPC and transport it into astrocytes by endocytosis (Fig. 2c) (308). PrPC and Aβ contain intrinsically disordered regions with Cu binding sites that have the capacity to adopt different Cu coordination modes, some of which have been proposed to activate O2 and produce ROS (Fig. 3a) (184, 350).
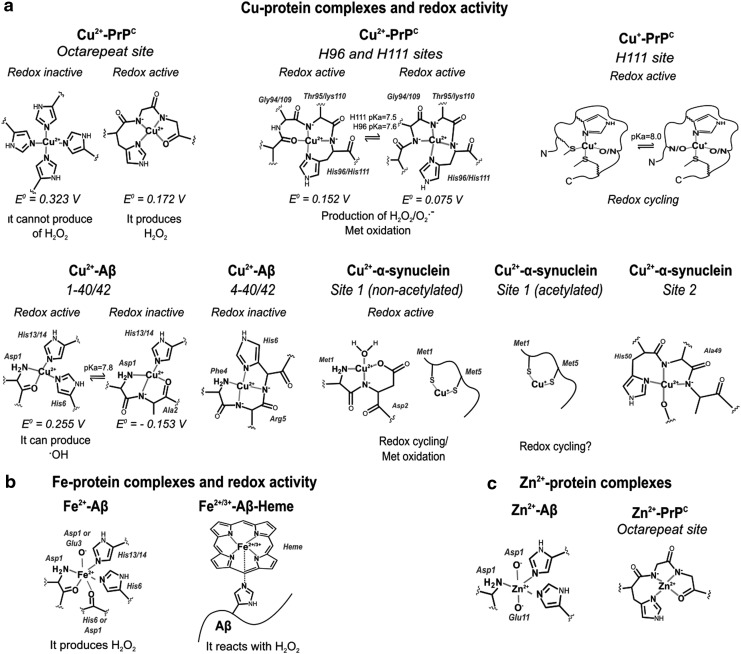
FIG. 3.
Structural features and redox properties of relevant metal-protein complexes in neurodegenerative diseases. (a) Cu can interact with PrPC, Aβ, and α-synuclein. PrPC has three coordination sites: histidine (His) His96, His111, and an octarepeat. Cu2+ coordination to His96 and His111 yields very similar complexes different to those generated upon Cu+ binding to His111. The octarepeat region coordinates up to four Cu2+ ions depending on the Cu2+/PrPC stoichiometric ratio. At low Cu:protein ratios, four His residues coordinate Cu2+, while at high ratios each His can bind one Cu2+ ion. The different coordination chemistry of these Cu-PrPC complexes is reflected in their redox properties. Similarly, Cu2+ coordination to Aβ (1–40/42) yields two different coordination modes that display different redox properties. Recently, coordination to Aβ (4–40/42) has also been described; this site displays higher affinity for Cu2+ than Aβ (1–40/42), and it yields a redox silent complex. On the contrary, nonacetylated α-synuclein has two high-affinity binding sites for Cu2+: site one involving residues at the N-terminal, and site two at His50. Acetylation of α-synuclein, as described to occur in vivo, abolishes Cu2+ binding at site 1, although both forms of α-synuclein can bind Cu+. (b) Fe2+ can interact with Aβ, but the redox properties of Fe-Aβ complexes remain unclear. Interestingly, recent studies propose that heme can bind to Aβ, yielding a heme (Fe)-Aβ complex with peroxidase activity. (c) Finally, Zn2+ can bind to the octarepeat region of PrPC and with Aβ. Cu2+, cupric ion; Fe, iron; Fe2+, ferrous; Zn, zinc.
A dysfunction in Cu homeostasis is reported to alter neuronal function and lead to disease progression, including neurodegeneration (Supplementary Table S1). Menkes disease and Wilson disease are caused by mutations or partial deletions in ATP7A and ATP7B, respectively. These Cu transporters have different patterns of expression in the CNS, explaining the distinct pathological features of each disease. ATP7B is found in the visual cortex, anterior cingulate cortex, caudate, putamen, substantia nigra (SN), and cerebellum. ATP7A is detected in astrocytes and neurons from the hippocampus and cerebellum, the BBB and choroid plexus, and during neural development (338). Wilson patients display parkinsonism, underscoring the importance of Cu homeostasis in the motor controlling systems. On the contrary, the ubiquitous expression of ATP7A has challenged mechanistic investigations in Menkes disease. However, a recent study shows that depleting ATP7A in neurons and glia does not lead to neurodegeneration, but to an increased susceptibility to NMDA seizures (132), underscoring its neuroprotective role as described above (Fig. 2a,b). Although neurodegeneration is clearly linked to alterations in Cu homeostasis in Menkes and Wilson diseases, the mechanisms are still unknown.
Neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS), Huntington's disease (HD), Parkinson's disease (PD), and Alzheimer's disease (AD) have been associated with alterations in Cu homeostasis, but a link to specific genetic alterations in Cu transport or handling is missing (Supplementary Table S1). These neurodegenerative diseases are associated with the formation of amyloid aggregates composed of proteins that are either a Cu-dependent antioxidant enzyme, such as SOD1 in ALS, or Cu-binding proteins, such as Aβ and α-synuclein in AD and PD, respectively.
ALS is characterized by the degeneration of motor neurons, and mouse models show increased intracellular Cu levels and the formation of protein aggregates composed of SOD1 and Cu transport proteins such as Ctr1, CCS, Atox1, and Cox17 (344, 346). SOD1 aggregation has been associated with an alteration in protein stability, which is impacted by metallation and Cu-dependent dimerization. Although SOD1 plays an important role in clearing O2•−, studies have demonstrated that the mechanism is likely other than the alteration in the antioxidant capacity of SOD1 (312). Increased intracellular Cu levels have been reported in ALS models (346). Consistently, Cu chelators or MT-I overexpression extend the life span and slow disease progression of the SOD1 (G93A) ALS mouse model (345).
HD is an autosomal dominant genetic disorder caused by polyglutamine (polyQ) repeat expansions near the N-terminus of the huntingtin (Htt) protein. HD is characterized by movement dysfunction, psychiatric and cognitive alterations linked to the degeneration of striatal spiny neurons. Cu accumulation in the striatum of HD transgenic mice has been reported (102). Mutant Htt forms toxic aggregates, but the mechanisms of toxicity are still unclear. A putative Cu binding site in Htt involving Met8 and His82 has been identified, and this interaction promotes polyQ aggregate formation (102, 381), which is reduced by MT-III overexpression (123).
PD is characterized by the degeneration of dopaminergic neurons in the SN, which are rich in Cu. PD patients show decreased Cu content in the SN (70) without changes in Cu levels in serum, plasma, and cerebrospinal fluid (CSF) (203). Accordingly, decreased levels of CTR1 (70) have been reported in the SN, while MT-I and MT-II levels in active astrocytes are increased, reflecting a glial response to the loss of Cu homeostasis in PD (226). In contrast, occupational exposure to Cu has been linked to an increased risk to develop PD (115).
The accumulation of intracellular protein inclusions (Lewy bodies), where α-synuclein is the main protein component, is another hallmark of PD (362). α-Synuclein is a small intrinsically disordered protein (IDP) enriched in presynaptic terminals and nucleus that can interact with cytoskeleton components and lipid membranes (170). IDPs are proteins that can adopt different conformations, and thus respond to changes in their biochemical environment. This property allows them to engage in interactions with multiple protein targets (23). Cu2+ and Cu+ ions are capable of binding to α-synuclein at three different sites (20, 33, 228, 361) (Fig. 3a). Interestingly, the H50Q SNCA/α-synuclein mutation linked to hereditary PD abolishes one Cu binding site altering Cu-induced α-synuclein aggregation (361). Moreover, Cu binding to the high-affinity Cu-binding site at the N-terminal region of α-synuclein accelerates its amyloid aggregation in vitro (20, 33); although this effect is abolished in acetylated α-synuclein (234), which is found in Lewy bodies (10).
While several studies have suggested that Cu-induced aggregation of α-synuclein is directly linked to its neurotoxicity, recent studies suggest a lack of correlation between protein aggregation and cytotoxicity (361); in fact, it has been demonstrated that α-synuclein potentiates the toxicity of Cu in dopaminergic cells in the absence of enhanced accumulation of protein aggregates (8). Clearly, further investigations are needed to completely understand the structural impact of Cu-α-synuclein interactions and their role in PD. On the contrary, acetylation and Cu+ binding to α-synuclein are two synergistic events that turn the intrinsically disordered N-terminal region into an α-helix conformation (228) (Fig. 2d), which displays higher affinity for membranes (75). These observations suggest a potential link between Cu+-α-synuclein interactions and the proposed function of α-synuclein in vesicle trafficking; a link that might be perturbed in PD.
Different redox modifications of α-synuclein are found in Lewy bodies, including Met oxidation, Tyr nitration (67), and formation of di-Tyr-linked α-synuclein dimers. Cu+-α-synuclein complexes have been implicated in these modifications, as they are capable of activating O2, leading to Met oxidation and di-Tyr bond formation (5, 227) (Fig. 3a). In contrast, a recent report suggests that Cu+ complexes with oligomeric or fibrillar α-synuclein reduce metal-catalyzed ROS formation (264). Cu has also been shown to potentiate oxidative damage induced by the dopamine (DA) analog 6-hydroxydopamine (63). Cu also interacts with the early-onset PD recessive genes (protein) PARK7 (DJ-1) and PARK2(Parkin). Cu binding to DJ-1 protects against metal toxicity, possibly acting as a chaperone for SOD1 (35, 114); while Parkin mutations have been reported to increase the cytotoxic effect of heavy metals, including Cu (1).
AD is a neurodegenerative disease associated with the degeneration of hippocampal and cortical neurons and eventual loss of memory and progressive dementia (326). Decreased levels of Cu are found in AD brains (42), while the total Cu content and labile nonprotein-bound Cu fraction are increased in the plasma of AD patients (325). Interestingly, polymorphisms in ATP7B have been linked to AD (326). AD is associated with the formation of extracellular amyloid plaques composed of Cu bound to Aβ peptides 1–40 and 1–42 fragments produced by cleavage of the APP, as well as N-truncated forms 4–40 and 4–42, and to a lesser extent 11–40/42 (210, 273, 364). The Cu binding features of Aβ 4–40/42 and 11–40/42 are different from those of 1–40/42, leading to the formation of Cu-Aβ complexes with distinct redox properties (Fig. 3a) (229). Different aggregation properties have also been described, as illustrated by the faster fiber assembly rate of Aβ 11–40/42 when compared with 1–40/42 (21). Substoichiometric Cu2+ concentrations trigger Aβ aggregation through a different pathway that involves the formation of oligomers more neurotoxic than those generated by the peptide alone (25, 204). On the contrary, the redox activity of Cu-Aβ complexes has also been proposed to lead to the generation of ROS (52, 214), but contradictory results exist as well (264). Interestingly, interaction of Cu with N-truncated 4–40 and 4–42 peptides yields redox-inactive Cu-Aβ complexes (229, 350). A dysfunction in Cu homeostasis in AD is also evidenced by decreased levels of MT-III (390). MTs are capable of exchanging Cu2+ with Aβ1–40/42, reducing Cu and stabilizing it. This Cu exchange by MTs has been proposed as a redox-silencing mechanism that prevents ROS formation by Cu-Aβ complexes (223).
The impact of dysfunctional Cu homeostasis in AD might go beyond the neurotoxicity of Cu-Aβ complexes. APP, whose mutations are associated with AD, is a type 1 transmembrane protein that displays three Cu binding sites in its extracellular domain (22, 140). One Cu-binding site is located in the growth factor-like domain, and has been implicated in Cu-induced dimerization of APP, a process that would be important in cell adhesion and signaling (22). Cu was found to induce APP phosphorylation at Thr668 promoting its localization to the axonal membrane, suggesting an important link between Cu and APP functions at the synapse that might be perturbed in AD. Aβ peptides can also interfere with Cu-PrPC interactions implicated in the regulation of NMDAR activity (Fig. 2b) (389). In addition, Cu has been reported to promote the degradation of the low-density lipoprotein receptor-related protein 1 (LRP1) via Tyr nitration and proteasomal degradation, which was linked to a decrease of Aβ clearance and its resultant accumulation in brain vasculature (319). Clearly, Cu plays important roles in neuromodulation and signaling processes, which would be perturbed in AD.
Iron
Fe is found in biological systems primarily as ferrous (2+) and ferric (3+) ions (Supplementary Table S1). Fe is a redox-active metal involved in several redox reactions that catalyze the formation of ROS. Fe is tightly bound to Fe storage and transport proteins, while <5% is present as labile redox-active Fe bound to low-affinity molecules. Fe is required as cofactor of several important enzymes for respiration and synthesis of neurotransmitters, including tryptophan hydroxylase (serotonin) and Tyr hydroxylase (norepinephrine and DA), cholesterol, and fatty acids; the latter particularly important for nerve myelination (161, 342). Electron transfer in many Fe enzymes, including the mitochondrial respiratory complexes, is facilitated by heme and Fe/S clusters (291). Fe is also important as cofactor for peroxidases and catalases, which are important for cellular redox homeostasis (6).
Fe is heterogeneously distributed in the brain; it is highly concentrated in the SN, hippocampus, striatum, interpeduncular nuclei (125), and myelin (329) (Supplementary Table S1). Fe homeostasis is regulated by communication between the BBB and astrocytes. In the blood stream, Fe3+ is found coordinated to transferrin (Tf) or ferritin (Ft), and as heme. Fe uptake into the BBB can occur by two pathways: (a) by direct transport of Fe2+ into the cytosol via DMT1; or (b) by endocytosis of Tf-bound Fe3+ via the Tf receptor (TfR), where the low pH of the endosome causes the release of Fe3+ from Tf. In both cases, Fe3+ is reduced to Fe2+ by the duodenal cytochrome b (Dcytb) or by the six transmembrane epithelial antigen of the prostate 2 (Steap2) ferrireductases and then transported by DMT1 (Fig. 4a) (215, 218).
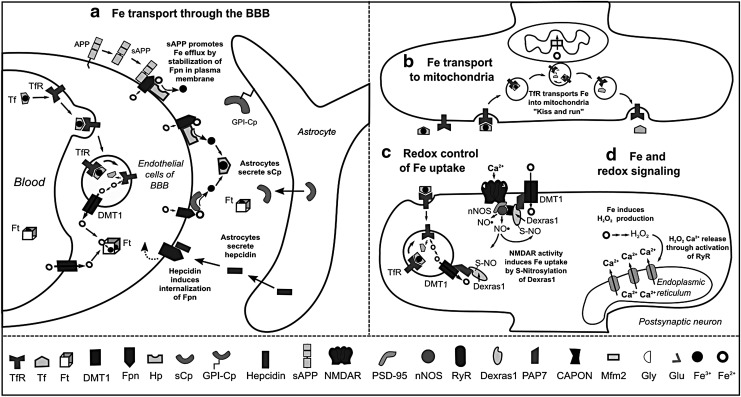
FIG. 4.
Fe transport and redox signaling. (a) Fe transport across the BBB occurs via DMT1 or by endocytosis of Tf- or Ft- bound Fe3+; followed by Fe release via the concerted action of Fpn with a ferroxidase, either Hp or sCp. Astrocytes modulate Fe transport through the production of sCp, sAPP, and hepcidin. sAPP stabilizes Fpn, while hepcidin induces its internalization. (b) Mitochondria are the organelles with highest demand of Fe. Although Fe transport into mitochondria is not fully understood, the recently demonstrated interaction between mitochondria and TfR-containing endosomes supports the “kiss and run” hypothesis. Inside the mitochondria, Fe crosses the inner membrane by Mfrn2. (c) In hippocampal neurons, NMDAR activation induces Ca2+ entry and NO• production by nNOS, causing S-nitros(yl)ation of Dexras1, which in turn induces Fe uptake through DMT1 and TfR. (d) Increased intracellular Fe also induces H2O2 production, inducing Ca2+ release from ER via RyR, which is important for neuronal plasticity. Dexras1, Ras-related dexamethasone induced 1; ER, endoplasmic reticulum; Fe3+, ferric; Fpn, ferroportin; Ft, ferritin; H2O2, hydrogen peroxide; Hp, hephaestin; Mfrn2, mitoferrin-2; NO•, nitric oxide; PAP7, peripheral benzodiazepine receptor-associated protein 7; RyR, ryanodine receptor; sAPP, soluble fragment of APP; sCp, soluble ceruloplasmin; Tf, transferrin; TfR: transferrin receptor.
Fe efflux from endothelial cells to the interstitial space occurs via the coordinated activity of the Fe2+ transporter ferroportin (Fpn) and the Cu-dependent ferroxidases hephaestin (Hp) and soluble ceruloplasmin (sCp), which oxidize Fe2+ to Fe3+. Astrocytes regulate the release of Fe from BBB by either secretion of sCp, which stimulates Fe release, or by production of hepcidin, a peptide that induces internalization and ubiquitination of Fpn and thus, decreased Fe efflux (216). Astrocytes also express a glycosylphosphatidylinositol (GPI)-anchored form of Cp, which interacts with Fpn and participates in Fe efflux (Fig. 4a) (144). In general, oligodendrocytes, astrocytes, microglia, and neurons have the same machinery for Fe efflux, involving the concerted action of Fpn with Cp or Hp (60).
The mechanisms of Fe uptake differ between brain cell types. Fe, Tf, and Ft are primarily found in oligodendrocytes (61). Most Tf in the brain is synthesized and secreted by oligodendrocytes as Fe-free Tf or apo-Tf and is required for Fe mobilization within the interstitial fluid brain (87). Although oligodendrocytes and astrocytes can accumulate high levels of Fe, they do not express TfR. Fe uptake into oligodendrocytes has been recently proposed to involve the internalization of H-Ft by a mucin-domain containing protein (Tim-2) (343). In astrocytes, ascorbate-dependent Fe2+ uptake is mediated by DMT1 (167) and transient-receptor potential channels (265).
In neurons, the mechanisms involved in Fe uptake are still unclear. DMT1 is found in human neurons and its expression levels are negatively regulated by Fe exposure via ubiquitination and degradation (134). However, DMT1 seems to colocalize in cytoplasmic vesicles with TfR and to primarily contribute to Tf-bound Fe uptake (232, 266). The ZIP8 and ZIP14 members of the ZIP family of metal transporters have also been demonstrated to mediate Fe2+ uptake (188) and to be expressed in the brain (113). A recent report demonstrates that ZIP8 at the plasma membrane is the primary transporter involved in non-Tf-bound Fe into neurons (145).
In brain cells, the ferrireductases Dcytb and stromal cell-derived receptor, SDR2, are expressed in astrocytes (192, 351), while SDR2 and Steap2 are found in neurons (145). In all cases, it is important to note that Fe uptake and efflux always require redox cycling between Fe2+ and Fe3+ oxidation states.
Cytosolic Fe is stored by Ft, which is a protein complex formed by 24 subunits of heavy-ferritin (H-Ft) and light-ferritin (L-Ft) chains. Ft genes are regulated by Nrf2 (272). While H-subunits have ferroxidase activity and participate in Fe uptake, L-subunits are involved in Fe mineralization and long-term storage. Ft can store ∼4500 Fe ions in its core. However, the mechanism by which Fe is released from Ft remains unclear (47). Although Fe is mainly stored in the cytosol, mitochondria are the organelles with the highest Fe demand, as they require Fe-S clusters and heme groups for electron transfer during respiration. Similarly, mitochondria are also considered the main sources of ROS under physiological conditions. Therefore, mitochondrial Fe homeostasis must be tightly regulated to prevent uncontrolled ROS production.
The mechanism(s) involved in mitochondrial Fe uptake are still unclear. Fe is delivered by a direct “kiss and run” interaction between the endosomes containing Tf-bound Fe and mitochondria (69). Subsequently, the metal crosses the inner membrane via the Fe importer mitoferrin-2 (Mfrn2) (Fig. 4b). In the mitochondrial matrix, Fe is either used for the biogenesis of prosthetic groups, Fe-S clusters, or heme, or it is stored by mitochondrial ferritin (FtMt), which is homologous to H-Ft, and it displays the same Fe uptake efficiency but lower ferroxidase activity. Fe distribution between mitochondria and cytosol depends on FtMt expression and the export of Fe prosthetic groups (106, 176).
Heme is a component of globins, a superfamily of heme-containing proteins involved in binding and/or transporting O2, and a cofactor for cytochromes, catalase, NOX, NOS, and myeloperoxidase, which is also found in microglia (59, 151, 198). Hemoglobin (Hb) is involved in O2, NO, and carbon dioxide (CO2) transport in cells of erythroid lineage, but Hbα and β transcripts have been found in dopaminergic neurons, oligodendrocytes, and cortical or hippocampal astrocytes as well (32). Brain Hb levels are altered during neurodegeneration (94), but their functional consequences are unclear. Neuroglobin is another globin expressed in the CNS and PNS and found in both neurons and astrocytes. Neuroglobin has a higher affinity for O2 than Hb and exerts a protective effect against oxidative and ischemic insults (7, 43, 81, 179, 331, 368). Oxidative stress has the potential to release heme from Hb, and labile heme can induce oxidative damage via Fenton reactions or NOX activation (165, 239). Heme oxygenases (HOs) catalyze heme degradation. HO-1 transcription is induced by oxidative stress, inflammation, hypoxia, and metal exposure, while HO-2 is constitutively expressed. HO activity has both antioxidant and pro-oxidant effects that relate to the ability of the cell to detoxify labile Fe released from heme (286, 369).
Cellular Fe trafficking is controlled by the iron regulatory proteins (IRP) IRP1 and IRP2 which regulate translation of proteins involved in Fe storage (H-Ft and L-Ft), Fe uptake (TfR), and Fe efflux (Fpn). Under conditions of Fe depletion, IRPs can bind to messenger RNAs (mRNAs), decreasing the levels of Ft and Fpn and promoting the translation of TfR via the stabilization of its mRNA. Conversely, Fe overload prevents mRNA binding to IRPs, promoting Ft and Fpn translation while reducing TfR levels. The ability of IRP1 and IRP2 to bind mRNAs exerts an important redox control via two distinct mechanisms. IRP1 has two conformations: (a) a closed one, triggered via an Fe-S cluster that prevents mRNA binding and has aconitase activity; and (b) an open conformation that is favored when NO•, O2, and H2O2 cause dissociation of the Fe-S cluster. In contrast, the ability of IRP2 to bind mRNAs is controlled by proteasomal degradation, involving the ubiquitin ligase F-box/LRR-repeat protein (FBXL5), which is also an Fe and O2 sensing protein. FBXL5 has a binuclear nonheme Fe site, and it is stabilized on Fe and O2 binding, promoting IRP2 degradation. Together, these two mechanisms illustrate the redox control of cellular Fe trafficking (164).
In neurons, Fe uptake is induced by a redox signaling cascade that starts with the activation of the NMDAR (Fig. 4c). Increased intracellular Ca2+ induces NO• production by the neuronal (n) NOS, leading to S-nitros(yl)ation of the small GTPase Dexras1 (Ras-related dexamethasone induced 1), which in turn induces Fe uptake through DMT1 and TfR (51). In hippocampal neurons, NMDAR activation increases intracellular Fe and H2O2 production, which activates the redox-sensitive ryanodine receptor (RyR) and promotes Ca2+ release from the ER (237) (Fig. 4d). Thus, Fe uptake is clearly part of a redox signaling mechanism that might be important for neuronal plasticity.
Cell death induced by an increase in the labile Fe pool within cells has been defined as a specific entity named ferroptosis. Ferroptotic cell death is a necrotic-like cell death characterized by Fe-dependent lipid peroxidation due to either the formation of •OH and H2O2 via Fenton-like reactions or the activation of lipoxygenases. As such, ferroptosis is counteracted by Fe-chelators and the GSH/GPX4 system. GPX4 knockout in neurons induces motor neuron degeneration, paralysis (53), and cognitive impairment (121). During ferroptosis, Tf-dependent Fe uptake and release of Fe from lysosomal compartments have been shown to act as important sources for Fe. Interestingly, lysosomal permeabilization is a common phenomenon observed in a number of neurodegenerative disorders, including PD (40, 73). However, during pathological conditions such as neurodegeneration and hemorrhagic stroke, cell death is likely to involve a combination of different pathways and a complex balance between them, including apoptosis, necrosis, autophagic cell death, and ferroptosis as well (77, 143, 357, 398).
During aging, accumulation of Fe in the frontal lobes and striatum is associated with motor dysfunction, loss of myelin sheaths, and memory decline (2, 327). Inflammation, a common hallmark of many brain disorders, increases the expression of DMT1 and hepcidin (354).
Abnormal accumulation of Fe is a common feature of many neurodegenerative diseases, including a group of twelve diseases known as neurodegeneration with brain iron accumulation (NBIA) (225). The most common clinical features of NBIA are movement disorders, such as ataxia, parkinsonism, and dystonia. Although in NBIA Fe is usually accumulated in the globus pallidus, and in some cases in the SN and cerebellum, only two types of NBIA involve the dysfunction of a protein that participates in Fe trafficking: aceruloplasminemia (lack of Cp) and neuroferritinopathy (loss of function mutations in L-Ft) (65, 261, 290, 360). Another neurodegenerative disease associated with disturbed Fe homeostasis is Friederich ataxia, which is linked to mutations in frataxin, an Fe-chaperone involved in Fe-S cluster biogenesis (55).
Altered Fe homeostasis and mitochondrial dysfunction are also hallmarks of PD. While no significant differences in Fe levels have been found in the blood, serum, and CSF (203), Fe is increased in the SN of PD patients (365). Neuromelanin is an Fe-rich pigment found in the dopaminergic neurons targeted in PD (A9) and it has been suggested that its presence makes this neuronal population vulnerable to oxidative damage (91). Decreased levels of serum Cp or its oxidation has also been proposed to exacerbate Fe accumulation in PD (149, 251). DMT1 is found increased in the SN of PD brains (294). Interestingly, Parkin regulates DMT1 expression levels via ubiquitination and proteasomal degradation (289).
Fe accumulation in SN might be associated with a dysfunctional delivery of Fe to mitochondria through the “kiss and run” interaction mentioned above (Fig. 4b) (69). Indeed, a Tf/TfR2-dependent mechanism for Fe transport into the mitochondria of dopaminergic neurons has been described (211), while a role for Tf and TfR2 genes in Fe accumulation and mitochondrial dysfunction in PD has been implicated as well (285).
Mitochondrial dysfunction in PD might lead to decreased synthesis of Fe-S clusters, which in turn would activate IRP1 binding to mRNAs, resulting in augmented Fe accumulation (142). Accordingly, knockdown of mitochondrial Grx2 impairs Fe-S cluster biogenesis in dopaminergic cells, decreases the activity of Complex I and aconitase, and increases the activation of IRP1 (171). Loss of PINK1/PARK6, another autosomal early-onset PD-related gene, has also been reported to inactivate Fe-S clusters via O2•− formation. As such, overexpression of FtMt exerts a protective effect on mitochondrial dysfunction and oxidative stress induced by loss of PINK1 (88).
HO-1 protects against neuronal cell death induced by PD-related mitochondrial toxins and α-synuclein, suggesting a role of heme in dopaminergic cell loss. However, HO-1 becomes toxic at high levels, but this effect is counteracted by FtMt (391). Interestingly, the pathogenic PINK1 mutation G309D impairs the induction of HO-1 on oxidative stress (56).
Fe2+ can interact with the negatively charged C-terminal region of α-synuclein, accelerating its amyloid aggregation (34). Conversely, α-synuclein overexpression also enables Fe accumulation (254). In PD, oxidative stress has been linked to intracellular Fe levels due to the redox activity of Fe-DA and Fe-neuromelanin complexes (400). Furthermore, overexpression of FtMt protects against neuronal cell death induced by 6-hydroxydopamine (313). Interestingly, a recent report demonstrates that depletion of Fpn has no consequence on the survival of dopaminergic neurons. In contrast, loss of TfR causes Fe deficiency and a PD-like neurodegeneration in mice (212). Previous findings have also suggested a link between Fe deficiency and predisposition to PD, while Fe overload seems to be protective (157, 190, 271). Thus, the exact role of Fe homeostasis in PD is still far from being understood.
A role of Fe in AD has also been proposed based on observations showing that Fe levels are decreased in the serum of AD patients (324), and increased in AD-related brain areas such as the hippocampus, neocortex, and basal ganglia (168). Mitochondrial dysfunction is also observed in AD, which might be related to a disruption in Fe homeostasis. Accordingly, overexpression of FtMt protects against the toxicity of Aβ (380). Fe is accumulated in amyloid plaques in the AD brain (38, 194), consistent with the observation that Aβ is able to bind Fe2+ in vitro (Fig. 3b) (39). Fe bound to Aβ plaques catalyzes H2O2 formation (321).
A novel mechanism for controlling Fe efflux from the BBB was recently discovered, involving the soluble fragment of APP (sAPP), which interacts with Fpn, stabilizing its membrane location (219) and counteracting the effects of hepcidin (216). APP expression is also negatively regulated by IRP1 (57), and production of sAPP and Aβ is increased in AD. Thus, the proposed role of sAPP in Fe movement from BBB into the brain is consistent with the accumulation of Fe in AD brains and the observation of higher levels of Aβ plaques surrounding the brain blood vessels. Indeed, BBB damage is a common feature in AD (217).
Heme metabolism also contributes to AD. APP binds and inhibits HO-1 activity and this effect is enhanced by pathogenic APP mutations (334). In addition, Aβ can form a complex with heme that displays peroxidase activity (16, 112). Conversely, neuroglobin has been proposed to protect against the toxicity of 1–42 Aβ peptides (180). Overall, disruption of Fe homeostasis in AD is likely linked to oxidative stress, and it may also impair NMDAR- and Fe- dependent redox signaling mechanisms (Fig. 4d) impacting neuronal plasticity and memory.
Manganese
Mn is an essential metal required for the activity of a plethora of enzymes, including hydrolases, isomerases, ligases, lyases, oxidoreductases, and transferases, involved in diverse metabolic functions such as amino acid (arginase and glutamine synthetase [GS]), lipid, protein, and carbohydrate metabolism (phosphoenolpyruvate decarboxylase), as well as protein glycosylation, energy production, and redox homeostasis (SOD2). The main source for Mn intake is food, but occupational/environmental exposures also occur associated with mining, smelting, welding, alloy, battery, pesticide, and electrical industries. Ingestion and inhalation are the primary routes of Mn exposure. Importantly, inhalation can transfer Mn directly to the brain. The brain is a major target for chronic Mn intoxication (manganism) where Mn is accumulated in nonheme Fe-rich regions. Manganism is defined as a parkinsonism that results in dystonia, hypokinesia, and rigidity as a consequence of impaired neurotransmitter function (133, 352).
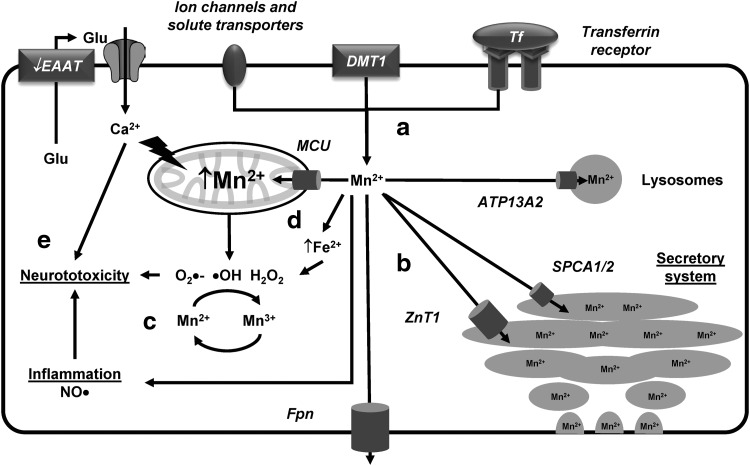
Mn can be transported via Tfr and DMT1 as it competes with Fe for its binding sites (333). Other proposed Mn transporters include the Zn carriers ZIP-8 and ZIP-14, voltage-regulated, store-operated, and ionotropic Glu receptor Ca2+ channels, and the Mn-citrate complex shuttle (Fig. 5a) (352). Mn is efficiently detoxified by Fpn at the plasma membrane. In addition, Mn is also detoxified by sequestration in the Golgi via the solute carrier family 30 member 10 or human Zn transporter 1 (SLC30A10/hZnT1) transporter whose mutations are directly associated with manganism (178). Alternatively, the Ca2+/Mn2+ ATPases SPCA1 and SPCA2 also detoxify Mn via the secretory pathway (356). Finally, the autosomal recessive early-onset PD-related gene ATP13A2/PARK9 mediates sequestration of Mn in lysosomes (Fig. 5b) (336). Recently, direct comparison of different detoxification proteins demonstrated that hZnT1 and SPCA1, but not ATP13A2, are involved in Mn detoxification and resistance (246).
FIG. 5.
Mn homeostasis and toxicity. (a) Mn can be transported via Tf and DMT1, as well as via Zn carriers, ion channels, and shuttle systems. (b) Mn is detoxified by Fpn at the plasma membrane or by its sequestration in the secretory system via the ZnT1 transporter or the Ca2+/Mn2+ ATPases SPCA1/2. ATP13A2 mediates sequestration of Mn in lysosomes. (c) Mn enhances ROS generation via Mn-catalyzed autoxidation of DA that involves the redox cycling of Mn2+ and Mn3+ and the generation of ROS and DA-o-quinones. (d) Mn accumulates in the mitochondria via the MCU and increases the accumulation of labile Fe. (e) Mn-induced neurotoxicity is linked to oxidative damage and mitochondrial dysfunction, and also to a decrease in Glu uptake by the EAATs in astrocytes (arrow in EAAT indicates reversal of Glu transport) leading to excitotoxicity in neurons, as well as the induction of inflammation. DA, dopamine; EAAT, excitatory amino acid transporter; Glu, glutamate; MCU, mitochondrial Ca2+ uniporter; Mn, manganese; ROS, reactive oxygen species; SPCA, secretory pathway Ca2+ ATPase; ZnT, zinc transporter.
Mn2+ is the predominant species found in cells that can be oxidized to the more reactive and toxic species Mn3+. Neither Mn2+ nor Mn3+ can generate free radicals via Fenton-type reactions. However, it has been proposed that Mn enhances ROS generation via the Mn-catalyzed autoxidation of DA that involves the redox cycling of Mn2+ and Mn3+ and the generation of ROS and DA-o-quinone (Fig. 5c) (79, 89). Mn accumulates in the mitochondria via the mitochondrial Ca2+ uniporter (MCU) (111) and increases the accumulation of labile Fe. Both mitochondrial dysfunction and Fe lead to ROS formation and oxidative damage (Fig. 5d) (54, 206). Mn specifically generates H2O2 but not O2•− in the mitochondria via complex II (92, 186, 322). Furthermore, Mn impairs oxidative phosphorylation and ATP production.
Astrocytes seem to have a high capacity to accumulate Mn (14). Mn-induced neurotoxicity has been linked to a decrease in Glu uptake by astrocytes (156) leading to excitotoxicity in neurons, as well as the induction of inflammation and increased activity of NOS (Fig. 5e) (95, 185).
Zinc
Zn is a redox-inactive transition metal ion with an oxidation state of +2. The majority of intracellular Zn is bound to proteins and is distributed in the cytoplasm (∼50%) and nucleus (∼40%) (304) (Supplementary Table S1). The function of Zn as enzyme cofactor is limited to structural roles (e.g., SOD1) (312), or as a Lewis acid that activates substrates for nucleophilic attack (e.g., carbonic anhydrase, where Zn2+ catalyzes the hydration of CO2 to form bicarbonate [HCO3−]) (138). Enzymes with Zn-dependent catalytic activity control many cellular processes, including DNA synthesis and brain development. Zn also plays an important role in cell signaling associated with development and learning. In the brain, Zn is highly concentrated in the hippocampus and cortex (304).
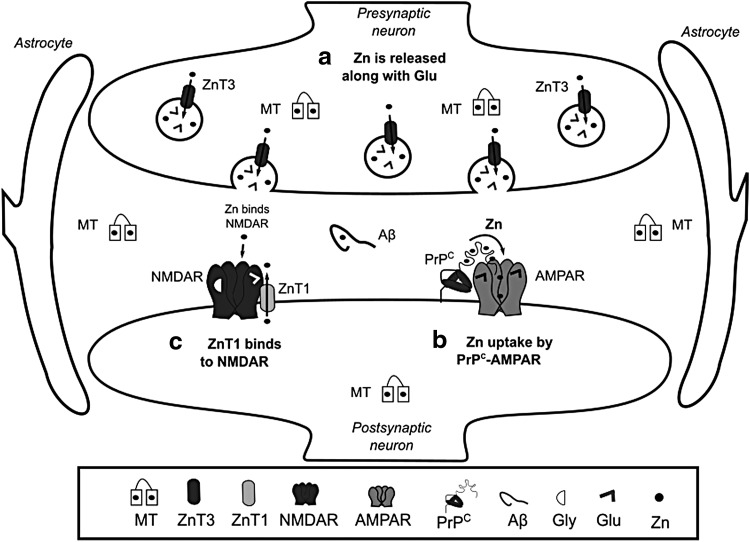
The key players in Zn homeostasis are the ZIP transporters that mediate Zn uptake into the cytosol, the zinc transporters (ZnT) that participate in Zn efflux, and MTs involved in Zn chelation. ZIP1 is expressed in astrocytes and microglia (303). In neurons Zn uptake is mediated by, voltage-gated Ca2+ channels, ZIP1 and 3 transporters, as well as Ca2+ and the Zn2+ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor (AMPAR) (146, 278, 335). Interestingly, Zn has been reported to be transported into postsynaptic neurons through a complex formed by PrPC, which is evolutionary linked to ZIP proteins, and the AMPAR (Fig. 6b) (373). ZnT1 is expressed in astrocytes, microglia, and oligodendrocytes, and its expression levels are directly modulated by Zn (247). Hypoxia decreases the levels of ZnT1 in astrocytes inducing the accumulation of cytosolic Zn (257). At the postsynaptic density, the ZnT1 transporter interacts with NMDAR and this complex is regulated during synaptic plasticity (Fig. 6c) (222, 373).
FIG. 6.
Zn trafficking at the synapse. (a) Zn ions are loaded into neurotransmitter vesicles by ZnT3, and are coreleased with Glu during synaptic transmission. In the synaptic cleft, Zn can bind to MT, Aβ, PrPC, and NMDAR. (b) Recently, two links between synaptic activity and Zn transport have been discovered: Zn uptake by the postsynaptic terminal occurs via a complex formed by PrPC and AMPAR, (c) while ZnT1 interacts with NMDAR and modulates spine morphology. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor.
Cytosolic Zn is distributed to different organelles, including synaptic vesicles, Golgi, ER, and mitochondria. Similarly, Zn uptake by these organelles is performed by ZnTs, while ZIPs participate in Zn efflux into the cytosol (155). Zn modulates cellular signaling pathways and acts as a neuromodulator. ZnT3 participates in the transport and accumulation of Zn within synaptic vesicles of glutamatergic terminals (Fig. 6a) (125). During synaptic transmission, Zn is released with Glu, where it inhibits the activity of NMDAR and AMPAR (9, 154), modulating neuronal excitability and long-term synaptic plasticity (long-term potentiation [LTP] and long-term depression). In addition, Zn regulates the activation of the tropomyosin kinase receptor B (TrkB) (304). A metabotropic Zn-sensing receptor (mZnR) has also been reported (31). At the synapse, Zn can also bind to MTs, Aβ, and PrPC (Figs. 3c and 6).
Although Zn2+ is a nonredox-active metal ion, it participates in redox signaling through several mechanisms. MTs bind about a fifth of the intracellular Zn with a stoichiometry of 1:7. MTs and proteins containing Zn-finger domains use Cys residues to bind to Zn2+ ions. Zn coordination stabilizes the reduced state of Cys thiol groups preventing their oxidation and subsequent formation of disulfide bonds. Transcription of MTs is induced by oxidative stress via Nrf2, and by heavy metal exposure via metal-response elements (MREs). MREs are recognized by the Zn-finger domain containing protein metal-responsive transcription factor-1 (MTF1, also known as MRE-binding transcription factor-1 or metal regulatory transcription factor-1). MTF1 senses Zn levels. Furthermore, via Zn displacement from MTs or Cys oxidation, MTF1 also senses heavy metal toxicity (Cd) and oxidative stress. In addition to MTs, MTF1 regulates the transcription of a number of genes involved in redox homeostasis and metal ion detoxification, including Zn transporters, Fr, Fpn, ATP7(A/B), Trx, selenoproteins, and γ-glutamate-cysteine ligase (GCL), a rate-limiting enzyme in GSH synthesis (119). The role of MTF1 in brain function and redox homeostasis is unclear. MTF1 has been shown to regulate the expression of β-synuclein (220), which is thought to act as a negative regulator of its homologue α-synuclein (126). Interestingly, deletion of MTF1 induces lethality in Parkin-deficient flies (Drosophila melanogaster) (293).
Altered Zn levels have been reported to promote neuronal injury. Upon Cys oxidation, Zn is released from MTs (255). Cellular acidification also releases intracellular Zn in neurons (159). In a recent report, AMPA-induced oligodendrocyte cell death was shown to be linked to Zn mobilization from mitochondria and protein-bound pools that were mediated by cytosolic acidification, independently from ROS (213). Exposure of mitochondria to Zn promotes increased ROS formation (305). While no specific mitochondrial Zn transporter(s) has been identified, potential candidates include the MCU, ZIP8, and Znt2 transporters (30, 199, 307). Zn also increases NOX-derived ROS formation and NOS activity (162). High extracellular Zn enhances microglia activation and ROS formation (130). Zn deficiency also induces oxidative stress via a reduction in the activity of SOD1 (378), and an impairment in the transcriptional regulation of GCL by Nrf2 (253).
Alterations in Zn homeostasis are associated to neurodegenerative diseases. Serum levels of Zn are decreased in AD patients (358), while Zn is enriched in Aβ plaques (364). Furthermore, a decrease in the levels of Znt3 and MT-III (390) is found in AD. Importantly, the predominant localization of Aβ plaques in Zn-containing glutamatergic synapses might explain why they are primarily found in the neocortex (304). Zn promotes a rapid, but reversible, aggregation of Aβ that is different to the aggregation of Aβ or Aβ-Cu complexes (25). Zn also reduces the toxicity of Cu-induced Aβ aggregates (214). ZnT3 knockout increases soluble Aβ in transgenic APP mice corroborating the role of extracellular Zn in plaque formation (172).
Elevated Zn levels have been found in the SN of PD brains (74), while reduced levels of Zn in serum and plasma have been linked to an increased risk for PD (80). Zn has been shown to potentiate the toxicity of DA as well (189). Furthermore, Zn chelation reduces the toxicity of mitochondrial PD-related toxins (310). Recently, ATP13A2 was identified as a Zn transporter localized to multivesicular bodies. Loss of function mutations of ATP13A2 induces alterations in Zn homeostasis and mitochondrial dysfunction (259).
Xenobiotic Metals
Measurable concentrations of xenobiotic metals with no physiological functions are present in humans (Supplementary Table S1) (103). In addition, environmental or occupational exposures to xenobiotic metals may take place by inhalation, ingestion, or skin penetration and are often linked to the development of toxicity and pathological conditions (Supplementary Table S1). Metals can reach the CNS from the vascular lumen affecting neuronal and glial function. Metals hijack transport systems of essential metals to pass through the BBB and enter neuronal tissues (molecular mimicry). Metal toxicity is largely attributable to their physicochemical properties, which mediate their interference with cellular biochemical systems, including redox-related processes (205, 355).
Environmental or occupational exposure to xenobiotic metals has been reported to contribute to neuronal dysfunction (cognitive, motor, and behavioral) and in some cases, neurodegeneration. However, the mechanisms involved are largely unclear. We next review the sources and routes of exposure to xenobiotic metals; the metabolic pathways involved in their transport and activation, and the mechanisms by which they alter cellular redox balance to promote neurotoxicity.
Arsenic
As is naturally present in air, water, and soil and is the 20th most abundant element in the earth's crust and 12th in the human body. This metal is named inorganic As (iAs) when found combined with other elements such as O2, chlorine (Cl), and sulfur (S). Combined with carbon (C) and hydrogen (H) is referred to as organic As. In the environment and within the human body, iAs predominantly exists in two oxidation states: arsenite +3 (or AsIII, found as arsenic trioxide [As2O3], sodium arsenite [NaAsO2], and arsenic trichloride [AsCl3]), and arsenate +5 (or AsV found as arsenic pentoxide [As2O5], arsenic acid [H3AsO4], and arsenates [PbHAsO4, Ca3(AsO4)2]).
iAs has been widely used as a therapeutic agent to treat leukemia. Currently, iAs compounds are predominantly used in pesticides, herbicides, cotton desiccants, wood preservatives, alloys for batteries, and in semiconductors and light-emitting diodes. Millions of individuals are currently exposed to iAs across the world due to natural groundwater contamination. Fish and crustaceans contain very high levels of organic arsenobetaine but no toxicity has been reported in vivo (382). The concentration of iAs in natural surface and groundwater is generally about 1 parts per billion (ppb) of water but it may exceed 1000 ppb in contaminated areas or where iAs soil levels are high (118, 200) (Supplementary Table S1).
While As is considered a carcinogen, in the brain, acute exposure to iAs can induce encephalopathy, with symptoms such as confusion, hallucinations, reduced memory, and emotional lability (exaggerated changes in mood or affect). Long-term exposure to lower levels of iAs can lead to the development of peripheral neuropathies.
There are reports of neurobehavioral alterations (cognitive function, verbal abilities, long-term memory, and motor skills) in children exposed to As concentrations ranging from 5 to 50 ppb in water, in Bangladesh (260), Mexico (45, 287), and in the United States (370). Although scientific understanding of the developmental neurotoxicity of As is still evolving, epidemiological and toxicological studies clearly show that As is a developmental neurotoxicant that affects intellectual function. Moreover, exposures even below current safety guidelines are associated with decrements in full-scale intelligence quotient (IQ) and memory (90, 347). Evidence in experimental models, including mice, rats, Caenorhabditis elegans (worm), and Danio rerio (zebrafish), has replicated many of the observations in humans supporting the notion that As can lead to cognitive, locomotor, and neurological impairment (85). Gestational exposure to NaAsO2 leads to a significant iAs accumulation in the mice offspring's brain (280). As neurotoxicity has been linked to changes in neurotransmitter metabolism and synaptic transmission (85, 276, 280). However, the mechanisms involved remain unclear.
In the environment, oxygenated water contains iAsV species, while in reducing environments iAsIII species are prevalent. iAsV enters cells through phosphate transporters to be subsequently reduced to iAsIII, while iAsIII is transported via aqua(glycerol)porins (AQP), organic anion transporters, and glucose transporters (GLUT) (Fig. 7a) (44, 187, 348). Once in the cytoplasm, iAsIII is methylated by different mechanisms. Oxidative methylation (Fig. 7b) is mediated by arsenite methyltransferase (AS3MT) that uses S-adenosylmethionine (AdoMet) as a cosubstrate. AS3MT methylates iAsIII to monomethylarsonic acid or arsonate (MMAV) that is reduced to monomethylarsonous acid (MMAIII) before being methylated again to dimethylarsinic acid (DMAV) by AS3MT (353, 372). Finally, DMAV is reduced generating dimethylarsinous acid (DMAIII). The reduction of pentavalent arsenicals (iAsV, MMAV, and DMAV) in this pathway is now well recognized to be mediated by the Trx/TR system, but GSH seems to increase the methylation rates by an unknown mechanism (76).
FIG. 7.
iAs biochemistry, redox signaling, and neurotoxicity. (a) iAsIII species prevail in anaerobic conditions, while aerobic environments contain iAsV species. iAsIII enters cells through AQP9/7 and GLUT, while iAsV uses phosphate transporters. Three mechanisms for iAs methylation have been proposed. (b) Oxidative methylation refers to the progressive reduction of AsV to AsIII metabolites by the Trx/TR system and the oxidative methylation of AsIII species by AS3MT. (c) The GSH conjugation pathway is based on the formation of As(GS)3 by the GSTs. As(GS)3 is subsequently methylated by AS3MT. (d) The protein thiol conjugation mechanism suggests that instead of As(GS)3, iAsIII binds to protein thiols and is methylated while still being conjugated to proteins. iAsIII methylation is likely to alter epigenetic signatures by alterations in AdoMet, a cosubstrate required for methyl group transfer. (e) Methylated metabolites and GSH/protein complexes are highly reactive and are exported through the MRPs. (f) DMA(GS) can form DMAH and react with O2 to form DMAH• and DMAOO•. DMAIII also reacts with O2 and forms DMAOOH. These reactive species lead to lipid peroxidation and protein carbonylation (oxidative stress). (g) AsV can replace phosphate in several metabolic pathways (arsenylation) where the end product is reduced AsIII. AsV binds ADP via ATP-synthase and uncouples oxidative phosphorylation and ATP formation in the mitochondria. AsV is also arsenylated by GAPDH to generate iAsV-3-P-glycerate from G3P. (h) iAsIII toxicity generates ER stress likely via thiol depletion and alterations in redox balance. (i) AsIII binds to thiol containing molecules (coenzyme A, DLA, GSH) and protein thiols inactivating enzymatic function. (j) iAsIII toxicity is counteracted by Nrf2 and MTF1 that mediate the transcriptional-dependent induction of antioxidant systems and MTs. AdoMet, S-adenosylmethionine; AQP, aqua(glycerol)porin; AS3MT, arsenite methyltransferase; AsIII, arsenite +3; As(SG)3, arsenic triglutathione; AsV, arsenate +5; DLA, dihydrolipoamide; DMA(GS), dimethylarsinic GSH; DMAH, dimethylarsine; DMAH•, DMAH radical; DMAIII, dimethylarsinous acid; DMAOO•, dimethylarsine peroxyl radical; DMAOOH, dimethylated arsenic peroxide; G3P, glyceraldehyde 3-phosphate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GLUT, glucose transporters; GSTs, glutathione-S transferases; iAs, inorganic As; iAsV-3-P-glycerate, 1-arsenato-3-phospho-D-glycerate; MRPs, multidrug resistance proteins; MTF1, metal-responsive transcription factor-1; Nrf2, nuclear factor erythroid-2-related factor 2; O2, molecular oxygen; Trx, thioredoxin.
Developmental exposure to As alters the methylation patterns of genes involved in neuroplasticity likely due to changes in AdoMet, but its long-term implications are unclear (207). Recent in vivo studies demonstrated that the alterations in synaptic plasticity (LTP), memory, and learning induced by gestational exposure to iAs were associated with an increase in extracellular Glu levels and downregulation of AMPAR subunits (244).
As methylation via the GSH conjugation mechanism is based on the formation of GSH complexes with iAsIII resulting in arsenic triglutathione [As(SG)3] (Fig. 7c). Conjugation of iAsIII with GSH has been proposed to occur nonenzymatically, but enzymatically as well by the activity of glutathione-S transferases (GST isoforms GSTO1, GSTM1, or GSTP1) (173, 372). As(GS)3 is subsequently methylated by AS3MT to form monomethylarsinic diglutathione [MMA(GS)2] and then again to generate dimethylarsinic GSH [DMA(GS)]. At low GSH levels, As(GS) conjugates are hydrolyzed and then oxidized to generate MMAV and DMAV (372). A third mechanism for iAsIII methylation has been recently proposed, where instead of As(GS) conjugate formation, iAsIII binds to protein-Cys (thiol) and is methylated while still being conjugated to proteins (Fig. 7d). This hypothesis is supported by the preferential binding of iAsIII to protein-Cys when compared to GSH (284).
Methylated (and maybe unmethylated) As metabolites are exported through the multidrug resistance proteins (MRP1, MRP2, or MRP4) (173, 317, 388) (Fig. 7e). AS3MT is ubiquitously expressed in all brain regions, and animal studies have shown that the iAs that crosses the BBB is methylated and accumulated across the brain, with the highest accumulation observed in the pituitary gland (297). Interestingly, knockout mouse for P-glycoprotein accumulates more As in the brain (183). Endothelial cells and astrocytes feet surrounding capillaries are the first barrier of detoxification of xenobiotics entering from the circulation. We (unpublished data) and others have observed that the resistance of astrocytes to iAsIII is mediated by MRPs (332).
iAs generates ROS and dimethylarsenic or peroxyl radicals that in turn lead to lipid peroxidation and the accumulation of oxidized by-products (MDA and HNE) (Fig. 7f). Importantly, MMAIII and DMAIII are proposed to be more potent toxicants than iAsIII due to their increased ability to generate radicals (392). Oxidative stress has been reported in brain regions of different animal models and in neurons and glial cell cultures exposed to As compounds (48, 107, 108, 243, 394).
Mitochondria have been proposed to be a primary source for ROS formation by iAs (Fig. 7g) (97, 150). Chronic iAs exposure generates mitochondrial oxidative stress in the rat brain by impairment of mitochondrial complexes I, II, and IV activities followed by increased ROS generation, lipid peroxidation, and protein carbonylation (275). Mitochondrial pyruvate dehydrogenase is also directly inhibited by iAs (136). In addition, iAs reduces the levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), downstream targets Nrf1 and Nrf2, and the mitochondrial transcription factor A (TFAM) decreasing mitochondrial biogenesis (274). ER stress has also been shown to contribute to iAs toxicity, but the mechanisms involved remain unclear (Fig. 7h) (182).
iAs toxicity has also been attributed to the ability of AsV to replace phosphate in several metabolic pathways (arsenylation) where the end product is the reduction of AsV to AsIII, because the arsenylated by-product is more readily reduced than AsV itself (Fig. 7g). AsV uncouples oxidative phosphorylation and ATP formation in the mitochondria by binding to ADP via ATP synthase. Replacement of phosphate in glycolysis also impairs carbon flux and ATP production. Reaction of AsV with glucose generates glucose 6-arsenate, an analog of glucose 6-phosphate that is suggested to act as an inhibitor of hexokinase. AsV is also arsenylated by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to produce the unstable product 1-arsenato-3-phospho-D-glycerate (iAsV-3-P-glycerate). Purine nucleoside phosphorylase, glycogen phosphorylase, and mitochondrial ornithine carbamoyl transferase (OCT) have also been shown to arsenylate AsV (139, 245, 340). Thus, energy failure, alterations in central carbon metabolism, and mitochondrial dysfunction are consequences of AsV toxicity.
AsIII binds to thiol containing molecules (coenzyme A, GSH, and dihydrolipoamide also known as dihydrolipoic acid [DLA]) and protein-Cys thiols inactivating enzyme function (Fig. 7d, i). AsIII has higher affinity for dithiols than monothiols as demonstrated by the transfer of AsIII from the GSH-adduct to 2,3-dimercaptosuccinic acid (DMSA) a sulfhydryl-containing metal chelator used to treat heavy metal toxicity. In addition, AsIII conjugated with GSH has the ability to bind protein thiols, which highlights the importance of detoxification of GSH-As adducts from the cell (230). Dithiol molecules such as the cofactor DLA and dithiol oxidoreductases Trxs, Trx reductase (TrxR), Prxs (except for monothiol Prx6), Grx, and GR, as well as proteins with adjacent Cys (MT), have been reported to avidly bind AsIII (Fig. 7d, j) (50, 279, 311, 393, 397). In addition to binding AsIII, Zn finger domains have been shown to be oxidized upon As binding (396). Binding of AsIII to DLA (Fig. 7i) is expected to interfere with the TCA cycle and energy production as DLA is a cofactor for the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes that catalyze the synthesis of acetyl-CoA and succinyl-CoA, respectively. DLA reverses protein oxidation and loss of protein-SHs in the brains of rats exposed to high levels of iAs (82, 296, 315, 316).
As activates the cystine/Glu exchanger system (xCT) in microglia to increase extracellular Glu levels (320), while in astrocytes it decreases the expression levels and activity of GS and Glu transporters (GLAST/excitatory amino acid transporter (EAAT) 1 and GLT-1/EAAT2) (48, 395). Importantly, these effects were linked to an increase in GSH levels and Nrf2 activity (Fig. 7i), but not oxidative stress (48). Accordingly, activation of Nrf2 by As seems to involve a noncanonical pathway where inhibition of autophagy leads to the accumulation of the ubiquitin-binding protein/adaptor p62 that sequesters Keap1 (169). iAsIII toxicity is also counteracted by the transcriptional regulation of MT via MTF1 (Fig. 7j) (129).
Lead
Inorganic Pb remains one of the most studied toxic elements due to several reasons. To begin with, human contact with Pb started very early in human civilization, and its toxic effects were also known since then. However, more importantly, Pb is neurotoxic leading to lower IQ even at lower doses than those recommended by the World Health Organization (10 ppb in drinking water). Human exposure to Pb not only occurs occupationally but also environmentally. The presence of Pb in the environment has multiple sources such as gasoline, industrial processes, paint, water pipes, and solder in canned food. It is present in air, household dust, soil, water, and food (Supplementary Table S1). Environmental Pb levels have fortunately decreased, especially in those countries where the Pb addition to gasoline and paints was banned. This prohibition was enforced after several studies associated the presence of high blood levels of Pb with impaired or diminished cognitive functions. Epidemiological studies have clearly shown that exposure to Pb in early stages of development is associated with significant deficits in neurobehavioral performance, including lower IQ, attention deficits, and aggressiveness later in life. Despite all the enforced restrictions, Pb contamination is still a major public health concern. For example, in November 2000 in Washington DC, there was a “lead drinking water crisis” triggered by a change in the disinfectant used to clean the water, this contamination affected hundreds of kids for 3 years. The health consequences of the recent crisis of Pb-contaminated water in Flint Michigan (United States, 2015) are still to be revealed in the future (68, 84, 124, 298).
Inhalation and ingestion of Pb and Pb-containing particles or products are the main routes of Pb entry into the body. Young children are especially vulnerable because they show higher gastrointestinal absorption than adults. Inhaled Pb particles are quickly absorbed in alveoli and distributed to other organs through the circulation. Thus, blood lead levels (BLL) are reliable biomarkers of exposure and risk. However, BLL do not reflect the total Pb body burden because Pb is absorbed in bones where it can be stored for several years (68). Currently, the acceptable BLL for children is lower than 10 μg/dl (0.48 μM) (49, 376), but due to the devastating effects that might occur later in life, there is a consensus to recommend efficient surveillance methods for children protection to reduce BLL to the lowest possible level (141). Pb binds with high affinity to erythrocytes' δ-aminolevulinic acid dehydratase (ALAD) that catalyzes the second step in the porphyrin and heme biosynthetic pathway, causing the accumulation of aminolevulinic acid (ALA) in both plasma and urine, which is used as a biomarker of exposure (248).
Pb can cross the BBB and cell membrane because of its ability to mimic Ca2+ and Fe2+ ions (Fig. 8a) (205, 298). In children, due to a more permeable BBB and a lower bone storage capacity for Pb, the amount of Pb passing into the nervous system is higher than in adults. The highest accumulation of Pb has been reported in the hippocampus, amygdala (116), and choroids plexus (201). The PNS may accumulate considerably more Pb than the CNS. Animals chronically exposed to Pb had impaired dendritic spines and synapse formation (306). Developmental exposure to Pb impacts the prefrontal cerebral cortex, hippocampus, and cerebellum regions, which can lead to neurological disorders, mental retardation, behavioral problems, and nerve damage (242). Early life exposure to Pb has also been linked to neurodegenerative diseases such as AD and PD (62, 209).
FIG. 8.
Pb neurotoxicity and synaptic signaling. (a) Pb enters cells due to its ability to mimic Ca2+ and Fe2+ ions. (b) Inside the cell, Pb can trigger oxidative stress by different mechanisms, including the accumulation of ALA; the formation of Pb2+–O2•− complexes; and the release of labile Fe2+ from Ft. (c) Pb can also form complexes with GSH [Pb(GSH2–3)] and bind to protein thiols. (d) Mitochondrial dysfunction and oxidative stress induced by Pb have also been linked to MCU. (e) Pb can displace Zn2+ and impair Zn2+ binding protein function. Furthermore, Pb interferes with the Se-dependent redox processes. (f) Finally, Pb can prevent CaM activation and thus reduce NOS activity. ALA, aminolevulinic acid; CaM, Ca2+/calmodulin; NOS, nitric oxide synthase; O2•−, superoxide anion radical; Pb, lead; Se, selenium.
The mechanisms of Pb toxicity include the ability of Pb to bind SH groups of proteins Cys and to mimic or compete with Ca2+, Fe2+, and Zn2+ (Fig. 8b, c, e, f) (99, 283). Zn deficiency increases the toxicity of Pb (4). The generation of oxidative damage by Pb in vitro and in vivo suggests that ROS also participate in Pb toxicity. For example, Pb acetate induces the opening of the mitochondrial permeability transition pore in human neuroblastoma SH-SY5Y cells via ROS (384). Pb can form a Pb2+–O2•− complex with higher oxidizing capacity than O2•− (3). In addition, accumulated δ-ALA by Pb-induced ALAD inhibition can be subsequently oxidized to generate O2•−, •OH, and H2O2. Pb per se has been reported to stimulate Fe2+-initiated lipid peroxidation (Fig. 8b) (337). Early postnatal exposure of rats to Pb leads to a higher accumulation of oxidative DNA damage in the cerebral cortical tissue when compared with aged controls or aged mice exposed acutely to Pb (36).
Perinatal exposure to Pb acetate inhibits the activity of brain acid and alkaline phosphatases, catalase, acetylcholinesterase, and ATPases (12). Similar observations have been made for the activities/levels of SOD1, GPX1, and GPX4 in the hippocampus, and for mitochondrial SOD2 and GSH, both in the cortex and hippocampus (19). Antioxidant nutrients such as vitamin E, vitamin C, vitamin B6, β-carotene, and DLA, as well as metal chelators such as DMSA, or replenishment of displaced metals has been shown to be beneficial against Pb-induced oxidative stress in the brain (98, 236, 256, 258, 277, 367). Diet supplementation with Zn and Se, which participates in the regulation of the GSH and Trx antioxidant systems, can effectively outcompete Pb binding to Zn- and Se-binding sites (Fig. 8e) (135).
Pb interferes with and disrupts Ca2+ signaling and homeostasis leading to excitotoxicity. In addition, Glu potentiates Pb-induced cell death in PC12 cells (267). Recently, oxidative stress induced by Pb has been shown to be linked to changes in the levels of MCU (Fig. 8d) (383). Other important intracellular targets of Pb in the brain are both neural NOS and endothelial NOS due to an impairment in their Ca2+/calmodulin (CaM)-dependent activation (Fig. 8f) (241). Importantly, Pb amplifies Glu-induced oxidative stress in a Ca2+-independent manner, but neither Ca2+ nor ROS seem to be essential for the enhanced cytotoxicity of combined exposure to Glu and Pb (191, 238).
Mercury
Hg is a transition metal that exists as elemental, inorganic, and organic Hg (Fig. 9a). Hg is ubiquitously found in the environment as sulfide compounds generated from volcanic activity and erosion, or released by anthropogenic sources such as fuel combustion, waste disposal, and industrial activities (Supplementary Table S1). Elemental or metallic Hg (Hg0) used in thermometers and amalgams is primarily absorbed via inhalation, while inorganic mercury (Hg1+ or 2+) used in medicine and everyday life products is partially absorbed through the gut. Organic Hg (ethylmercury [EtHg or C2H5Hg] and methylmercury [MeHg or CH3Hg]) is originated from atmospheric sources that are deposited in water body surfaces to be biomethylated and magnified in the food chain (Fig. 9a). Around 95% of MeHg is absorbed by the gastrointestinal tract making it the most toxic Hg species. Neurotoxic signs of Hg intoxication are vast and include ataxia, dizziness, insomnia, speech impairment, arthralgia, cognitive and behavioral changes, seizures, fatigue, and sensory disruption. While there has been an association between Hg exposure and neurodegeneration or autism, the neurological effects of chronic exposure to Hg are largely unclear. However, research has clearly demonstrated that Hg impairs neuronal development, communication, and myelination (89).
FIG. 9.
Hg redox-related toxicity mechanisms in brain cells. (a) Hg species are highly transformed (oxidation or methylation) before they are absorbed into organisms. MeHg and EtHg form a complex with Cys (CH3HgCys) and are transported across the membranes via LAT. (b) MeHg suffers dealkylation and thiol exchange to form Hg2+. (c) Thiol exchange reactions of CH3HgCys complexes with low-molecular-weight thiols (GSH) and protein thiols alter the cellular redox balance. MeHg also has a strong affinity for selenol groups. (d) GSH-MeHg adduct formation (CH3HgGS) is catalyzed by the GSTs and adducts are detoxified by MRPs. (e) MeHg also induces mitochondrial ROS and energy failure. Furthermore, MeHg-induced neurotoxicity has been ascribed to a reduction in Glu uptake by astrocytes via EAAT triggering neuronal excitotoxicity (arrow in EAAT indicates reversal of Glu transport). Cys, cysteine; Hg, mercury; EtHg, ethylmercury; Hg1+, inorganic mercurous ions; Hg2+, inorganic mercuric ions; LAT, L-type neutral amino acid transporter; MeHg, methylmercury.
Most of the studies regarding the mechanisms involved in Hg neurotoxicity have been done using MeHg. MeHg and EtHg are potent electrophiles that form a complex with Cys (CH3HgCys or C2H5HgCys), and then transported across the BBB and into neuronal cells via L-type neutral amino acid transporters (LAT1 and 2) (Fig. 9a) (318, 385, 399).
A high percentage of Hg in individuals intoxicated with MeHg is found as Hg2+, suggesting that dealkylation of MeHg is an important mechanism for the high persistence of Hg in the brain (Fig. 9b) (72). Thiol exchange from CH3HgCys to low-molecular-weight thiols (GSH) and protein thiols has been proposed to be central mechanisms by which MeHg induces GSH depletion, inhibition of thiol-dependent antioxidant systems, and alters the activity or function of proteins with redox-sensitive Cys (signaling proteins, metabolic enzymes, neurotransmitter receptors, and transporters) (Fig. 9c) (89). MeHg also has a stronger affinity for selenol groups (selenohydryl groups in selenocysteines) compared with thiol groups. As such, selenoproteins are important targets for direct electrophilic attack of MeHg or transfer from thiol adducts (CH3HgCys, CH3HgGS, or CH3HgPS [protein-Cys adduct]) (Fig. 9c) (104, 221). GSTs have been proposed to mediate the formation of CH3HgGS adducts, which are detoxified by MRP1-mediated transport (Fig. 9d). GSH synthesis, GST, and MRP1 levels are regulated transcriptionally by the Nrf2 antioxidant system (152, 292, 349).
MeHg induces mitochondrial ROS and energy failure (175, 233). Neurotoxicity induced by MeHg has also been ascribed to its inhibitory effect on Glu uptake by astrocytes, triggering neuronal excitotoxicity (15, 235) (Fig. 9e).
Hg0 absorbed through the respiratory tract is oxidized to inorganic mercurous (Hg1+) and mercuric ions (Hg2+) (Fig. 9a). While inorganic Hg ions have limited access to the CNS, they induce profound neurotoxic alterations that seem to be mediated as well by their binding to thiol groups (89). Accordingly, MTs exert protective effects against Hg0-induced neurotoxicity (387) (Fig. 9b).
Other xenobiotic metals
Aluminum
Al is one of the most abundant metals in the earth's crust (8.1%). Al has a plethora of uses in industry and manufacturing, as well as in food additives. As such, human exposure is primarily originated from food and drinking water. Importantly, pharmaceuticals have higher levels of Al compared to food. Occupational exposures to Al are related to mining, processing, and welding (359) (Supplementary Table S1). While Al is poorly absorbed in the gut, inhalation mediates direct transfer to the brain via the olfactory system (341). Importantly, ∼85% of Al in blood is bound to Tf, which is considered to mediate its transport across the BBB (Fig. 10a) (288), but Tf-independent Al transport also exists. Interestingly, monocarboxylate and xCT transporters have also been proposed to mediate the transport of Al-citrate complexes (240, 386). Acute Al toxicity occurs as a result of occupational exposure or chronic renal failure and is known to target the nervous system. Al is neurotoxic in animal models triggering the accumulation of neurofibrillary tangles and impairment of cognitive, behavioral, and motor functions. Al promotes Aβ aggregation, mitochondrial dysfunction, and triggers neuroinflammation (Fig. 10b, c) (24, 202, 309). However, conflicting results exist regarding the association of Al with any human disease, including AD (37, 359, 377).
FIG. 10.
Mechanisms of Al and Cd neurotoxicity. (a) Al is bound to Tf, which mediates its transport across the BBB via the Tfr, but a Tf-independent Al transport also exists. (b) Al promotes Aβ aggregation and (c) mitochondrial dysfunction trigging neuroinflammation and NO• formation. (d) Al3+ has been proposed to react with H2O2 to produce AlO2•− that can promote oxidative damage. (e) Al toxicity is also related to its ability to displace other metals (Ca or Fe). (f) Cd transport across membranes is through molecular mimicry via several transporters such as Cu/Zn transporters, DMT1, and Ca2+-channels. (g) High concentrations of Cd can block Ca2+ currents as well. (h) Cd toxicity is linked to its ability to bind thiol containing molecules such GSH, and protein-Cys (Trx and MT), as well as the displacement of redox-active metals such as Fe. Cd detoxification of cells is facilitated by the activity of GST and the detoxification of GSH-Cd adducts via MRPs. Al, aluminum; AlO2•−, aluminum superoxide radicals; Ca, calcium; Cd, cadmium.
Al exists primarily in a trivalent state (Al3+). While Al has no redox capacity, Al toxicity is linked to oxidative damage. Al3+ has been proposed to react with H2O2 to produce Al superoxide radicals (AlO2•−) that can deplete mitochondrial Fe and promote generation of ROS (Fig. 10d) (166). However, because of its high reactivity, Al is primarily found forming insoluble oxides whose toxicity seem to be related with the displacement of other biological cations (Ca2+, Fe2+, or Mg2+) (Fig. 10e) (377).
Cadmium
Cd is a transition metal whose use in industry has increased dramatically in the recent years. Cd is widely used in batteries, alloys, and pigments, and produced as a by-product from the extraction of other metals from ores. Food is the major source for Cd exposure as both animals and plants accumulate high levels of Cd. Inhalation is the prevalent route of Cd exposure due to industrial emissions and occupational activities (tobacco) (Supplementary Table S1). Cd neurotoxicity seems to occur only during development, before complete BBB formation, or in association with BBB dysfunction. Cd transport across membranes is thought to be mediated by molecular mimicry via several transporters and receptors for essential metals such as Cu/Zn transporters, DMT1, and Ca2+-channels (Fig. 10f) (117, 131, 174, 224, 339). Importantly, at high concentrations, Cd also has the ability to block Ca2+ currents (Fig. 10g).
Cd toxicity is linked to its ability to bind thiol containing molecules such as GSH, and protein-Cys (Trxs and MT) and as a consequence, displacement of redox-active metals and mitochondrial and metabolic dysfunction (Fig. 10h) (363). Cd detoxification of cells is facilitated by the activity of GST and the detoxification of GSH-Cd adducts via MRPs (Fig. 10h) (181, 339). Accordingly, resistance to Cd-toxicity is directly associated with the Nrf2-mediated antioxidant response (366).
Conclusions and Perspectives
Metals are important for brain function and human health. Thus, alterations in their content and/or distribution are expected to exert neurotoxicity. Both alterations in the homeostasis of essential metals and environmental exposure to xenobiotic metals can have silent chronic effects leading to neurodegeneration and neurological dysfunction (behavioral and cognitive alterations). The neurotoxic mechanisms by which metals impact neuronal or glial function are starting to become elucidated.
In this review, we have summarized how essential metals are trafficked in the brain. It is interesting to note that the mechanisms involved in metal transport and homeostasis are strongly linked to the chemical properties of each metal ion, while their coordination chemistry preferences also allow them to share some metal transport routes. For instance, Cu and Fe go through several redox cycles during their transport, while Mn and Zn remain in the same oxidation state. Metal trafficking in the cell is tightly regulated to control the high reactivity toward O2 of some metal ions, such as Fe2+ and Cu+, or to keep in solution otherwise insoluble species such as Cu+ and Fe3+. Moreover, while similar O-based ligand coordination preferences of Mn2+, Fe2+, and Fe3+ allow them to share some transport systems, the affinity of Zn2+ and Cu+ for Cys ligands makes MTs important players in their homeostasis. In fact, a close relationship between cellular redox environment and metal transport has been recently demonstrated for Cu and Fe (Figs. 1D and 4C).
Since metal trafficking machineries in neurons and astrocytes resemble those of other extensively studied mammalian cells, our understanding of intracellular metal homeostasis has advanced significantly. In contrast, metal trafficking at the synapse and the role in neuromodulation have just begun to be revealed, and point to a close inter-relationship among Zn, Cu, and Fe. For example, the synaptic release of Zn and Glu ultimately leads to the activation of NMDAR and the activation of signaling pathways that lead to postsynaptic Cu release and Fe uptake. Clearly, the close interplay between these metals at the synapse must play an important role in neuromodulation, while disturbed metal trafficking in neurodegenerative diseases would impact these processes.
We have also illustrated the differential alteration of metal homeostasis that occurs in neurodegenerative disorders as well as the key metal-protein interactions that might be involved in protein aggregation and metal-mediated oxidative damage. Understanding these interactions at the molecular level will shed light into the role of essential metals in neurodegenerative diseases.
Metals are transported across the BBB and into brain cells by selective transport systems for essential metals, while xenobiotic metals hijack those transporters via molecular mimicry (Supplementary Fig. S1a). The ability of xenobiotic metals to be transported and/or react with cellular targets is strongly determined by their metabolism via reduction/oxidation reactions, methylation, or adduct formation (Mt0→Mt1→Mt2). Phase II enzyme systems, GSH/GST, and MTs are essential for the detoxification of metals (Supplementary Fig. S1b). Metals induce or enhance ROS and RNS formation leading to oxidative stress (Supplementary Fig. S1c). While oxidative damage is one of the causative mechanisms involved in cellular damage induced by metals, it is now clear that other redox processes participate as well. The intrinsic reactivity of xenobiotic metals with thiol and selenol groups (Supplementary Fig. S1d) and their capacity to displace essential metals (Supplementary Fig. S1e) are also central to their capacity to promote energy failure (mitochondrial dysfunction), protein damage/aggregation, and metabolic alterations that challenge neuro/glial function and survival and trigger excitotoxic and inflammatory processes (Supplementary Fig. S1f). Cells have the capacity to respond by activating redox- or metal-dependent transcriptional regulation of antioxidant and anti-inflammatory responses (Supplementary Fig. S1g).
The aim of this review was to provide an integrated overview of the recent advances regarding how dysfunctional metal ion homeostasis of essential metals and exposure to xenobiotic metals alter cellular function to promote chronic neurodegeneration and neurotoxicity. Although the mechanisms involved in these processes are still being elucidated, the studies highlighted here are a starting point toward a better understanding of the pathological consequences of alterations in metal ion homeostasis, justifying the need of further studies regarding their metabolism and its impact on cellular homeostasis, function, and survival.
Supplementary Material
Abbreviations Used
- Aβ
amyloid beta
- AD
Alzheimer's disease
- AdoMet
S-adenosylmethionine
- Al
aluminum
- ALA
aminolevulinic acid
- ALAD
δ-aminolevulinic acid dehydratase
- AlO2•−
aluminum superoxide radicals
- ALS
amyotrophic lateral sclerosis
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor
- APP
amyloid precursor protein
- AQP
aqua(glycerol)porin
- As
arsenic
- AS3MT
arsenite methyltransferase
- AsIII
arsenite +3
- As(SG)3
arsenic triglutathione
- AsV
arsenate +5
- Atox1
antioxidant protein 1
- ATP7A
ATPase copper transporting alpha
- ATP7B
ATPase copper transporting beta
- BBB
blood-brain barrier
- BLL
blood lead levels
- Ca
calcium
- CaM
Ca2+/calmodulin
- CCS1
copper chaperone for superoxide dismutase 1
- Cd
cadmium
- CNS
central nervous system
- CO2
carbon dioxide
- COX
cytochrome C oxidase
- Cp
ceruloplasmin
- CSF
cerebrospinal fluid
- CTR1
copper transporter 1
- Cu
copper
- Cu+
cuprous ion
- Cu2+
cupric ion
- Cys
cysteine
- DA
dopamine
- Dcytb
duodenal cytochrome b
- Dexras1
Ras-related dexamethasone induced 1
- DLA
dihydrolipoamide or dihydrolipoic acid
- DMAIII
dimethylarsinous acid
- DMAV
dimethylarsinic acid
- DMSA
2,3-dimercaptosuccinic acid
- DMT1
divalent metal transporter 1
- EAAT
excitatory amino acid transporter
- ER
endoplasmic reticulum
- EtHg or C2H5Hg
ethylmercury
- FBXL5
F-box/LRR-repeat protein
- Fe
iron
- Fe2+
ferrous
- Fe3+
ferric
- Fpn
ferroportin
- Ft
ferritin
- FtMt
mitochondrial ferritin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCL
γ-glutamate-cysteine ligase
- Glu
glutamate
- GLUT
glucose transporters
- GPX
glutathione peroxidases
- GR
glutathione reductase
- Grxs
glutaredoxins
- GS
glutamine synthetase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione-S transferase
- H2O2
hydrogen peroxide
- Hb
hemoglobin
- HD
Huntington's disease
- H-Ft
heavy-ferritin
- Hg
mercury
- Hg0
elemental or metallic Hg
- Hg1+
inorganic mercurous ions
- Hg2+
inorganic mercuric ions
- HNE
4-hydroxy-2-nonenal
- HO
heme oxygenase
- Hp
hephaestin
- Htt
huntingtin
- iAs
inorganic As
- iAsV-3-P-glycerate
1-arsenato-3-phospho-Dglycerate
- IDP
intrinsically disordered protein
- IQ
intelligence quotient
- IRP
iron regulatory proteins
- K
potassium
- Keap1
kelch-like ECH-associated protein 1
- LAT
L-type neutral amino acid transporter
- L-Ft
light-ferritin
- LTP
long-term potentiation
- MCU
mitochondrial Ca2+ uniporter
- MDA
malondialdehyde
- MeHg or CH3Hg
methylmercury
- Met
methionine
- Mfrn2
mitoferrin-2
- Mg
magnesium
- MMAIII
monomethylarsonous acid
- MMAV
monomethylarsonic acid or arsonate
- Mn
manganese
- MRE
metal response element
- mRNA
messenger RNA
- MRPs
multidrug resistance proteins
- MT
metallothionein
- MTF1
metal-responsive transcription factor-1
- Na
sodium
- NaAsO2
sodium arsenite
- NADPH
nicotinamide adenine dinucleotide phosphate
- NBIA
neurodegeneration with brain iron accumulation
- NKB
neurokinin B
- NMDAR
glutamate/N-methyl-d-aspartate receptor
- NO•
nitric oxide
- NOS
nitric oxide synthase
- NOX
NADPH oxidases
- Nrf1/2
nuclear factor erythroid-2-related factor 1 or 2
- O2
molecular oxygen
- O2•−
superoxide anion radical
- •OH
hydroxyl radical
- OONO−
peroxynitrite
- Pb
lead
- PD
Parkinson's disease
- PNS
peripheral nervous system
- polyQ
polyglutamine
- ppb
parts per billion
- PrPC
cellular prion protein
- Prxs
peroxiredoxins
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- S
sulfur
- sAPP
soluble fragment of APP
- SCO
cytochrome c oxidase assembly protein
- sCp
soluble ceruloplasmin
- SDR2
stromal cell-derived receptor
- Se
selenium
- SLC30A10/hZnT1
solute carrier family 30 member 10 or human Zn transporter 1
- SN
substantia nigra
- SOD
superoxide dismutase
- SPCA
secretory pathway Ca2+ ATPase
- Steap2
six transmembrane epithelial antigen of the prostate 2
- Tf
transferrin
- TfR
transferrin receptor
- Trxs
thioredoxins
- Tyr
tyrosine
- xCT
cystine/glutamate exchanger system
- ZIP
Zrt-(Zn-regulated transporter)- or Irt (Fe-regulated transporter)-like proteins
- Zn
zinc
- ZnT
zinc transporters
Acknowledgments
This work was supported by the National Institutes of Health Grant P20RR17675, Centers of Biomedical Research Excellence (COBRE), the Interdisciplinary Grant from the Research Council, the Life Sciences Grant Program of the University of Nebraska-Lincoln, the Mexican Academy of Sciences (AMC) (R.F.), and the National Council for Science and Technology in Mexico (CONACYT) via grant 221134 (L.Q.). PhD fellowships to Y.P. (308512) and C.G.-L. (290116) were from CONACYT. This work was performed in partial fulfillment of the requirements for the PhD degree in the posgrado en Ciencias Biomédicas at the Universidad Nacional Autónoma de México.
References
- 1.Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, and Bowman AB. PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper. Neurobiol Dis 73: 204–212, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, and Nestor PJ. In vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci 36: 364–374, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adonaylo VN. and Oteiza PI. Pb2+ promotes lipid oxidation and alterations in membrane physical properties. Toxicology 132: 19–32, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Aimo L. and Oteiza PI. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci 91: 184–191, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Al-Hilaly YK, Biasetti L, Blakeman BJ, Pollack SJ, Zibaee S, Abdul-Sada A, Thorpe JR, Xue WF, and Serpell LC. The involvement of dityrosine crosslinking in alpha-synuclein assembly and deposition in Lewy bodies in Parkinson's disease. Sci Rep 6: 39171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfonso-Prieto M, Vidossich P, and Rovira C. The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Arch Biochem Biophys 525: 121–130, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Amri F, Ghouili I, Amri M, Carrier A, and Masmoudi-Kouki O. Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J Neurochem 140: 151–169, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Anandhan A, Rodriguez-Rocha H, Bohovych I, Griggs AM, Zavala-Flores L, Reyes-Reyes EM, Seravalli J, Stanciu LA, Lee J, Rochet JC, Khalimonchuk O, and Franco R. Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways. Neurobiol Dis 81: 76–92, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CT, Radford RJ, Zastrow ML, Zhang DY, Apfel UP, Lippard SJ, and Tzounopoulos T. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc Natl Acad Sci U S A 112: E2705–E2714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, and Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281: 29739–29752, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Antala S. and Dempski RE. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry 51: 963–973, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Antonio MT, Corredor L, and Leret ML. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol Lett 143: 331–340, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Aoyama K, Watabe M, and Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci 108: 227–238, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Aschner M, Gannon M, and Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem 58: 730–735, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Aschner M, Yao CP, Allen JW, and Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37: 199–206, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Atamna H. and Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease. Proc Natl Acad Sci U S A 103: 3381–3386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banci L, Bertini I, Cantini F, Kozyreva T, Massagni C, Palumaa P, Rubino JT, and Zovo K. Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc Natl Acad Sci U S A 109: 13555–13560, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, and Palumaa P. Affinity gradients drive copper to cellular destinations. Nature 465: 645–648, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, Marchetti C, Kurzawski M, Dziedziejko V, Kolasa A, Olszewska M, Rybicka M, Safranow K, Nowacki P, Wiszniewska B, and Chlubek D. Disrupted pro- and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res 1435: 56–71, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Barnes N, Tsivkovskii R, Tsivkovskaia N, and Lutsenko S. The copper-transporting ATPases, Menkes and Wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem 280: 9640–9645, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Barritt JD. and Viles JH. Truncated amyloid-beta(11–40/42) from Alzheimer disease binds Cu2+ with a femtomolar affinity and influences fiber assembly. J Biol Chem 290: 27791–27802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumkotter F, Schmidt N, Vargas C, Schilling S, Weber R, Wagner K, Fiedler S, Klug W, Radzimanowski J, Nickolaus S, Keller S, Eggert S, Wild K, and Kins S. Amyloid precursor protein dimerization and synaptogenic function depend on copper binding to the growth factor-like domain. J Neurosci 34: 11159–11172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter PS. and Hardingham GE. Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes. Free Radic Biol Med 100: 147–152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becaria A, Lahiri DK, Bondy SC, Chen D, Hamadeh A, Li H, Taylor R, and Campbell A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J Neuroimmunol 176: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Beck MW, Oh SB, Kerr RA, Lee HJ, Kim SH, Kim S, Jang M, Ruotolo BT, Lee J-Y, and Lim MH. A rationally designed small molecule for identifying an in vivo link between metal-amyloid-[small beta] complexes and the pathogenesis of Alzheimer's disease. Chem Sci 6: 1879–1886, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belanger M, Allaman I, and Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14: 724–738, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Markus NM, Baxter P, Deighton RF, Serio A, Bilican B, Chowdhry S, Meakin PJ, Ashford ML, Wyllie DJ, Scannevin RH, Chandran S, Hayes JD, and Hardingham GE. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun 6: 7066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellingham SA, Ciccotosto GD, Needham BE, Fodero LR, White AR, Masters CL, Cappai R, and Camakaris J. Gene knockout of amyloid precursor protein and amyloid precursor-like protein-2 increases cellular copper levels in primary mouse cortical neurons and embryonic fibroblasts. J Neurochem 91: 423–428, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Belloni-Olivi L, Marshall C, Laal B, Andrews GK, and Bressler J. Localization of zip1 and zip4 mRNA in the adult rat brain. J Neurosci Res 87: 3221–3230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, and Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, and Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci 29: 2890–2901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, Simone R, Vlachouli C, Plessy C, Bertin N, Beltrami A, Kobayashi K, Gallo V, Santoro C, Ferrer I, Rivella S, Beltrami CA, Carninci P, Raviola E, and Gustincich S. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A 106: 15454–15459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binolfi A, Quintanar L, Bertoncini CW, Griesinger C, and Fernández CO. Bioinorganic chemistry of copper coordination to alpha-synuclein: relevance to Parkinson's disease. Coord Chem Rev 256: 2188–2201, 2012 [Google Scholar]
- 34.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, and Fernandez CO. Interaction of alpha-synuclein with divalent metal ions reveals key differences: a link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc 128: 9893–9901, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Bjorkblom B, Adilbayeva A, Maple-Grodem J, Piston D, Okvist M, Xu XM, Brede C, Larsen JP, and Moller SG. Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity. J Biol Chem 288: 22809–22820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, and Cardozo-Pelaez F. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J 20: 788–790, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Bondy SC. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer's disease and age-related neurodegeneration. Neurotoxicology 52: 222–229, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Bourassa MW, Leskovjan AC, Tappero RV, Farquhar ER, Colton CA, Van Nostrand WE, and Miller LM. Elevated copper in the amyloid plaques and iron in the cortex are observed in mouse models of Alzheimer's disease that exhibit neurodegeneration. Biomed Spectrosc Imaging 2: 129–139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bousejra-ElGarah F, Bijani C, Coppel Y, Faller P, and Hureau C. Iron(II) binding to amyloid-beta, the Alzheimer's peptide. Inorg Chem 50: 9024–9030, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Bove J, Martinez-Vicente M, Dehay B, Perier C, Recasens A, Bombrun A, Antonsson B, and Vila M. BAX channel activity mediates lysosomal disruption linked to Parkinson disease. Autophagy 10: 889–900, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brose J, La Fontaine S, Wedd AG, and Xiao Z. Redox sulfur chemistry of the copper chaperone Atox1 is regulated by the enzyme glutaredoxin 1, the reduction potential of the glutathione couple GSSG/2GSH and the availability of Cu(I). Metallomics 6: 793–808, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Bucossi S, Ventriglia M, Panetta V, Salustri C, Pasqualetti P, Mariani S, Siotto M, Rossini PM, and Squitti R. Copper in Alzheimer's disease: a meta-analysis of serum,plasma, and cerebrospinal fluid studies. J Alzheimers Dis 24: 175–185, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Burmester T, Weich B, Reinhardt S, and Hankeln T. A vertebrate globin expressed in the brain. Nature 407: 520–523, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Calatayud M, Barrios JA, Velez D, and Devesa V. In vitro study of transporters involved in intestinal absorption of inorganic arsenic. Chem Res Toxicol 25: 446–453, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Calderon J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, and Diaz-Barriga F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res 85: 69–76, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Calvo IA, Boronat S, Domenech A, Garcia-Santamarina S, Ayte J, and Hidalgo E. Dissection of a redox relay: H2O2-dependent activation of the transcription factor Pap1 through the peroxidatic Tpx1-thioredoxin cycle. Cell Rep 5: 1413–1424, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Carmona F, Palacios Ò, Gálvez N, Cuesta R, Atrian S, Capdevila M, and Domínguez-Vera JM. Ferritin iron uptake and release in the presence of metals and metalloproteins: chemical implications in the brain. Coord Chem Rev 257: 2752–2764, 2013 [Google Scholar]
- 48.Castro-Coronel Y, Del Razo LM, Huerta M, Hernandez-Lopez A, Ortega A, and Lopez-Bayghen E. Arsenite exposure downregulates EAAT1/GLAST transporter expression in glial cells. Toxicol Sci 122: 539–550, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Chandran L. and Cataldo R. Lead poisoning: basics and new developments. Pediatr Rev 31: 399–405; quiz 406, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Chang YY, Kuo TC, Hsu CH, Hou DR, Kao YH, and Huang RN. Characterization of the role of protein-cysteine residues in the binding with sodium arsenite. Arch Toxicol 86: 911–922, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, and Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51: 431–440, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheignon C, Jones M, Atrian-Blasco E, Kieffer I, Faller P, Collin F, and Hureau C. Identification of key structural features of the elusive Cu-A[small beta] complex that generates ROS in Alzheimer's disease. Chem Sci 8: 5107–5118, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Hambright WS, Na R, and Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J Biol Chem 290: 28097–28106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, and Aschner M. Manganese homeostasis in the nervous system. J Neurochem 134: 601–610, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang S, Kovacevic Z, Sahni S, Lane DJ, Merlot AM, Kalinowski DS, Huang ML, and Richardson DR. Frataxin and the molecular mechanism of mitochondrial iron-loading in Friedreich's ataxia. Clin Sci (Lond) 130: 853–870, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Chien WL, Lee TR, Hung SY, Kang KH, Lee MJ, and Fu WM. Impairment of oxidative stress-induced heme oxygenase-1 expression by the defect of Parkinson-related gene of PINK1. J Neurochem 117: 643–653, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, and Rogers JT. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 285: 31217–31232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi BS. and Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res 1248: 14–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, and Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J Neurosci 25: 6594–6600, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Codazzi F, Pelizzoni I, Zacchetti D, and Grohovaz F. Iron entry in neurons and astrocytes: a link with synaptic activity. Front Mol Neurosci 8: 18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connor JR, Menzies SL, St Martin SM, and Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res 27: 595–611, 1990 [DOI] [PubMed] [Google Scholar]
- 62.Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, and Gorell J. Whole-body lifetime occupational lead exposure and risk of Parkinson's disease. Environ Health Perspect 114: 1872–1876, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cruces-Sande A, Mendez-Alvarez E, and Soto-Otero R. Copper increases the ability of 6-hydroxydopamine to generate oxidative stress and the ability of ascorbate and glutathione to potentiate this effect: potential implications in Parkinson's disease. J Neurochem 141: 738–749, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Culotta VC, Yang M, and O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta 1763: 747–758, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, and Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 28: 350–354, 2001 [DOI] [PubMed] [Google Scholar]
- 66.D'Ambrosi N. and Rossi L. Copper at synapse: release, binding and modulation of neurotransmission. Neurochem Int 90: 36–45, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Danielson SR. and Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med 44: 1787–1794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dapul H. and Laraque D. Lead poisoning in children. Adv Pediatr 61: 313–333, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Das A, Nag S, Mason AB, and Barroso MM. Endosome-mitochondria interactions are modulated by iron release from transferrin. J Cell Biol 214: 831–845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JP, Reyes S, Halliday GM, Mercer JF, and Double KL. Copper pathology in vulnerable brain regions in Parkinson's disease. Neurobiol Aging 35: 858–866, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Davies MJ. Protein oxidation and peroxidation. Biochem J 473: 805–825, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, and Clarkson TW. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol 35: 680–688, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, and Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 30: 12535–12544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, and Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114 (Pt 4): 1953–1975, 1991 [DOI] [PubMed] [Google Scholar]
- 75.Dikiy I. and Eliezer D. N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound alpha-synuclein and increases its affinity for physiological membranes. J Biol Chem 289: 3652–3665, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding L, Saunders RJ, Drobna Z, Walton FS, Xun P, Thomas DJ, and Styblo M. Methylation of arsenic by recombinant human wild-type arsenic (+3 oxidation state) methyltransferase and its methionine 287 threonine (M287T) polymorph: role of glutathione. Toxicol Appl Pharmacol 264: 121–130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, and Devedjian JC. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis 94: 169–178, 2016 [DOI] [PubMed] [Google Scholar]
- 78.Dodani SC, Firl A, Chan J, Nam CI, Aron AT, Onak CS, Ramos-Torres KM, Paek J, Webster CM, Feller MB, and Chang CJ. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc Natl Acad Sci U S A 111: 16280–16285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donaldson J, McGregor D, and LaBella F. Manganese neurotoxicity: a model for free radical mediated neurodegeneration? Can J Physiol Pharmacol 60: 1398–1405, 1982 [DOI] [PubMed] [Google Scholar]
- 80.Du K, Liu MY, Zhong X, and Wei MJ. Decreased circulating zinc levels in Parkinson's disease: a meta-analysis study. Sci Rep 7: 3902, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duong TT, Witting PK, Antao ST, Parry SN, Kennerson M, Lai B, Vogt S, Lay PA, and Harris HH. Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury. J Neurochem 108: 1143–1154, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Dwivedi N, Flora G, Kushwaha P, and Flora SJ. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ Toxicol Pharmacol 37: 7–23, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Eckl PM. and Bresgen N. Genotoxicity of lipid oxidation compounds. Free Radic Biol Med 111: 244–252, 2017 [DOI] [PubMed] [Google Scholar]
- 84.Edwards M, Triantafyllidou S, and Best D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001–2004. Environ Sci Technol 43: 1618–1623, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Escudero-Lourdes C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: role of oxidative stress and inflammatory responses. Neurotoxicology 53: 223–235, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Eskici G. and Axelsen PH. Copper and oxidative stress in the pathogenesis of Alzheimer's disease. Biochemistry 51: 6289–6311, 2012 [DOI] [PubMed] [Google Scholar]
- 87.Espinosa de los Monteros A, Chiapelli F, Fisher RS, and de Vellis J. Transferrin: an early marker of oligodendrocytes in culture. Int J Dev Neurosci 6: 167–175, 1988 [DOI] [PubMed] [Google Scholar]
- 88.Esposito G, Vos M, Vilain S, Swerts J, De Sousa Valadas J, Van Meensel S, Schaap O, and Verstreken P. Aconitase causes iron toxicity in Drosophila pink1 mutants. PLoS Genet 9: e1003478, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farina M, Avila DS, da Rocha JB, and Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 62: 575–594, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farzan SF, Karagas MR, and Chen Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272: 384–390, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, and Hirsch EC. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson's disease. J Neurochem 86: 1142–1148, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Fernandes J, Hao L, Bijli KM, Chandler JD, Orr M, Hu X, Jones DP, and Go YM. From the cover: manganese stimulates mitochondrial H2O2 production in SH-SY5Y human neuroblastoma cells over physiologic as well as toxicologic range. Toxicol Sci 155: 213–223, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferreira AM, Ciriolo MR, Marcocci L, and Rotilio G. Copper(I) transfer into metallothionein mediated by glutathione. Biochem J 292 (Pt 3): 673–676, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferrer I, Gomez A, Carmona M, Huesa G, Porta S, Riera-Codina M, Biagioli M, Gustincich S, and Aso E. Neuronal hemoglobin is reduced in Alzheimer's disease, argyrophilic grain disease, Parkinson's disease, and dementia with Lewy bodies. J Alzheimers Dis 23: 537–550, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Filipov NM, Seegal RF, and Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci 84: 139–148, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51: 257–281, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Flora SJ, Pande M, and Mehta A. Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 145: 267–280, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Flora SJ, Saxena G, and Mehta A. Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca(2+). J Pharmacol Exp Ther 322: 108–116, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Forman HJ. Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med 97: 398–407, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forman HJ, Davies MJ, Kramer AC, Miotto G, Zaccarin M, Zhang H, and Ursini F. Protein cysteine oxidation in redox signaling: caveats on sulfenic acid detection and quantification. Arch Biochem Biophys 617: 26–37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fox JH, Kama JA, Lieberman G, Chopra R, Dorsey K, Chopra V, Volitakis I, Cherny RA, Bush AI, and Hersch S. Mechanisms of copper ion mediated Huntington's disease progression. PLoS One 2: e334, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med 26: 235–244, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, and Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med 47: 449–457, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Franco R. and Cidlowski JA. Glutathione efflux and cell death. Antioxid Redox Signal 17: 1694–1713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao G. and Chang YZ. Mitochondrial ferritin in the regulation of brain iron homeostasis and neurodegenerative diseases. Front Pharmacol 5: 19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Chavez E, Jimenez I, Segura B, and Del Razo LM. Lipid oxidative damage and distribution of inorganic arsenic and its metabolites in the rat nervous system after arsenite exposure: influence of alpha tocopherol supplementation. Neurotoxicology 27: 1024–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Chavez E, Santamaria A, Diaz-Barriga F, Mandeville P, Juarez BI, and Jimenez-Capdeville ME. Arsenite-induced formation of hydroxyl radical in the striatum of awake rats. Brain Res 976: 82–89, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Nogales P, Almeida A, and Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem 278: 864–874, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Gasperini L, Meneghetti E, Pastore B, Benetti F, and Legname G. Prion protein and copper cooperatively protect neurons by modulating NMDA receptor through S-nitrosylation. Antioxid Redox Signal 22: 772–784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gavin CE, Gunter KK, and Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol 115: 1–5, 1992 [DOI] [PubMed] [Google Scholar]
- 112.Ghosh C, Seal M, Mukherjee S, and Ghosh Dey S. Alzheimer's disease: a heme-abeta perspective. Acc Chem Res 48: 2556–2564, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, and Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Girotto S, Cendron L, Bisaglia M, Tessari I, Mammi S, Zanotti G, and Bubacco L. DJ-1 is a copper chaperone acting on SOD1 activation. J Biol Chem 289: 10887–10899, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, and Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology 20: 239–247, 1999 [PubMed] [Google Scholar]
- 116.Grandjean P. Regional distribution of lead in human brains. Toxicol Lett 2: 65–69, 1978 [Google Scholar]
- 117.Gu C, Chen S, Xu X, Zheng L, Li Y, Wu K, Liu J, Qi Z, Han D, Chen G, and Huo X. Lead and cadmium synergistically enhance the expression of divalent metal transporter 1 protein in central nervous system of developing rats. Neurochem Res 34: 1150–1156, 2009 [DOI] [PubMed] [Google Scholar]
- 118.Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, Rohl C, Schupp T, Wollin KM, and Hengstler JG. High exposure to inorganic arsenic by food: the need for risk reduction. Arch Toxicol 89: 2219–2227, 2015 [DOI] [PubMed] [Google Scholar]
- 119.Gunther V, Lindert U, and Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta 1823: 1416–1425, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Halliwell B. and Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10): 5–12, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hambright WS, Fonseca RS, Chen L, Na R, and Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12: 8–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han M, Chang J, and Kim J. Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. J Neurochem 138: 918–928, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hands SL, Mason R, Sajjad MU, Giorgini F, and Wyttenbach A. Metallothioneins and copper metabolism are candidate therapeutic targets in Huntington's disease. Biochem Soc Trans 38: 552–558, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Hanna-Attisha M. and Kuehn BM. Pediatrician sees long road ahead for flint after lead poisoning crisis. JAMA 315: 967–969, 2016 [DOI] [PubMed] [Google Scholar]
- 125.Hare DJ, Lee JK, Beavis AD, van Gramberg A, George J, Adlard PA, Finkelstein DI, and Doble PA. Three-dimensional atlas of iron, copper, and zinc in the mouse cerebrum and brainstem. Anal Chem 84: 3990–3997, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto M, Rockenstein E, Mante M, Mallory M, and Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron 32: 213–223, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Hatori Y, Clasen S, Hasan NM, Barry AN, and Lutsenko S. Functional partnership of the copper export machinery and glutathione balance in human cells. J Biol Chem 287: 26678–26687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hatori Y, Yan Y, Schmidt K, Furukawa E, Hasan NM, Yang N, Liu CN, Sockanathan S, and Lutsenko S. Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat Commun 7: 10640, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He X. and Ma Q. Induction of metallothionein I by arsenic via metal-activated transcription factor 1: critical role of C-terminal cysteine residues in arsenic sensing. J Biol Chem 284: 12609–12621, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Higashi Y, Aratake T, Shimizu S, Shimizu T, Nakamura K, Tsuda M, Yawata T, Ueba T, and Saito M. Influence of extracellular zinc on M1 microglial activation. Sci Rep 7: 43778, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hinkle PM, Kinsella PA, and Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem 262: 16333–16337, 1987 [PubMed] [Google Scholar]
- 132.Hodgkinson VL, Zhu S, Wang Y, Ladomersky E, Nickelson K, Weisman GA, Lee J, Gitlin JD, and Petris MJ. Autonomous requirements of the Menkes disease protein in the nervous system. Am J Physiol Cell Physiol 309: C660–C668, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horning KJ, Caito SW, Tipps KG, Bowman AB, and Aschner M. Manganese is essential for neuronal health. Annu Rev Nutr 35: 71–108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Howitt J, Putz U, Lackovic J, Doan A, Dorstyn L, Cheng H, Yang B, Chan-Ling T, Silke J, Kumar S, and Tan SS. Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons. Proc Natl Acad Sci U S A 106: 15489–15494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu PC. and Guo YL. Antioxidant nutrients and lead toxicity. Toxicology 180: 33–44, 2002 [DOI] [PubMed] [Google Scholar]
- 136.Hu Y, Su L, and Snow ET. Arsenic toxicity is enzyme specific and its affects on ligation are not caused by the direct inhibition of DNA repair enzymes. Mutat Res 408: 203–218, 1998 [DOI] [PubMed] [Google Scholar]
- 137.Hu Z, Yu F, Gong P, Qiu Y, Zhou W, Cui Y, Li J, and Chen H. Subneurotoxic copper(II)-induced NF-kappaB-dependent microglial activation is associated with mitochondrial ROS. Toxicol Appl Pharmacol 276: 95–103, 2014 [DOI] [PubMed] [Google Scholar]
- 138.Huang CC, Lesburg CA, Kiefer LL, Fierke CA, and Christianson DW. Reversal of the hydrogen bond to zinc ligand histidine-119 dramatically diminishes catalysis and enhances metal equilibration kinetics in carbonic anhydrase II. Biochemistry 35: 3439–3446, 1996 [DOI] [PubMed] [Google Scholar]
- 139.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Hureau C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 1: an overview. Coord Chem Rev 256: 2164–2174, 2012 [Google Scholar]
- 141.International Lead Management Center. 1999. OECD declaration on lead risk reduction. The LEAD Group, Inc; 2016. www.lead.org.au/lanv7n1/L71–L79.html (November 28 2016) [Google Scholar]
- 142.Isaya G. Mitochondrial iron-sulfur cluster dysfunction in neurodegenerative disease. Front Pharmacol 5: 29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ito K, Eguchi Y, Imagawa Y, Akai S, Mochizuki H, and Tsujimoto Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov 3: 17013, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jeong SY. and David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 278: 27144–27148, 2003 [DOI] [PubMed] [Google Scholar]
- 145.Ji C. and Kosman DJ. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem 133: 668–683, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jia Y, Jeng JM, Sensi SL, and Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol 543: 35–48, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang L, Garrick MD, Garrick LM, Zhao L, and Collins JF. Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr 143: 1927–1933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jimenez-Blasco D, Santofimia-Castano P, Gonzalez A, Almeida A, and Bolanos JP. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ 22: 1877–1889, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin L, Wang J, Zhao L, Jin H, Fei G, Zhang Y, Zeng M, and Zhong C. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson's disease. Brain 134: 50–58, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, and Valko M. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31: 95–107, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Jucaite A, Svenningsson P, Rinne JO, Cselenyi Z, Varnas K, Johnstrom P, Amini N, Kirjavainen A, Helin S, Minkwitz M, Kugler AR, Posener JA, Budd S, Halldin C, Varrone A, and Farde L. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson's disease. Brain 138: 2687–2700, 2015 [DOI] [PubMed] [Google Scholar]
- 152.Julvez J. and Grandjean P. Genetic susceptibility to methylmercury developmental neurotoxicity matters. Front Genet 4: 278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, and Canfield RL. Blood lead concentrations <10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect 116: 243–248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kalappa BI, Anderson CT, Goldberg JM, Lippard SJ, and Tzounopoulos T. AMPA receptor inhibition by synaptically released zinc. Proc Natl Acad Sci U S A 112: 15749–15754, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kambe T, Tsuji T, Hashimoto A, and Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 95: 749–784, 2015 [DOI] [PubMed] [Google Scholar]
- 156.Karki P, Webb A, Smith K, Johnson J, Jr., Lee K, Son DS, Aschner M, and Lee E. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol 34: 1280–1289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kasten M, Tadic V, Klein C, Rocca WA, Savica R, Eric Ahlskog J, and Grossardt BR. Anemia or low hemoglobin levels preceding Parkinson disease: a case-control study. Neurology 74: 1655; author reply 1655–1656, 2010 [DOI] [PubMed] [Google Scholar]
- 158.Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, Graziano JH, and Factor-Litvak P. Manganese exposure from drinking water and children's academic achievement. Neurotoxicology 33: 91–97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kiedrowski L. Cytosolic acidification and intracellular zinc release in hippocampal neurons. J Neurochem 121: 438–450, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim G, Weiss SJ, and Levine RL. Methionine oxidation and reduction in proteins. Biochim Biophys Acta 1840: 901–905, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim J. and Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem 25: 1101–1107, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim YH. and Koh JY. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol 177: 407–418, 2002 [DOI] [PubMed] [Google Scholar]
- 163.Koskenkorva-Frank TS, Weiss G, Koppenol WH, and Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 65: 1174–1194, 2013 [DOI] [PubMed] [Google Scholar]
- 164.Kuhn LC. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 7: 232–243, 2015 [DOI] [PubMed] [Google Scholar]
- 165.Kumar S. and Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett 157: 175–188, 2005 [DOI] [PubMed] [Google Scholar]
- 166.Kumar V. and Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41: 154–166, 2014 [DOI] [PubMed] [Google Scholar]
- 167.Lane DJ, Robinson SR, Czerwinska H, Bishop GM, and Lawen A. Two routes of iron accumulation in astrocytes: ascorbate-dependent ferrous iron uptake via the divalent metal transporter (DMT1) plus an independent route for ferric iron. Biochem J 432: 123–132, 2010 [DOI] [PubMed] [Google Scholar]
- 168.Langkammer C, Ropele S, Pirpamer L, Fazekas F, and Schmidt R. MRI for iron mapping in Alzheimer's disease. Neurodegener Dis 13: 189–191, 2014 [DOI] [PubMed] [Google Scholar]
- 169.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, and Zhang DD. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol 33: 2436–2446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lautenschlager J, Kaminski CF, and Kaminski Schierle GS. alpha-Synuclein—regulator of exocytosis, endocytosis, or both? Trends Cell Biol 27: 468–479, 2017 [DOI] [PubMed] [Google Scholar]
- 171.Lee DW, Kaur D, Chinta SJ, Rajagopalan S, and Andersen JK. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson's disease. Antioxid Redox Signal 11: 2083–2094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee JY, Cole TB, Palmiter RD, Suh SW, and Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A 99: 7705–7710, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leslie EM, Haimeur A, and Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem 279: 32700–32708, 2004 [DOI] [PubMed] [Google Scholar]
- 174.Leslie EM, Liu J, Klaassen CD, and Waalkes MP. Acquired cadmium resistance in metallothionein-I/II(-/-) knockout cells: role of the T-type calcium channel Cacnalpha1G in cadmium uptake. Mol Pharmacol 69: 629–639, 2006 [DOI] [PubMed] [Google Scholar]
- 175.Levesque PC. and Atchison WD. Disruption of brain mitochondrial calcium sequestration by methylmercury. J Pharmacol Exp Ther 256: 236–242, 1991 [PubMed] [Google Scholar]
- 176.Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, Arosio P, and Drysdale J. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem 276: 24437–24440, 2001 [DOI] [PubMed] [Google Scholar]
- 177.Lewerenz J. and Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284: 1106–1115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, and Mukhopadhyay S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J Neurosci 34: 14079–14095, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li RC, Guo SZ, Lee SK, and Gozal D. Neuroglobin protects neurons against oxidative stress in global ischemia. J Cereb Blood Flow Metab 30: 1874–1882, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y, and Gozal D. Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiol Aging 29: 1815–1822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li ZS, Szczypka M, Lu YP, Thiele DJ, and Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 271: 6509–6517, 1996 [DOI] [PubMed] [Google Scholar]
- 182.Lin AM, Chao PL, Fang SF, Chi CW, and Yang CH. Endoplasmic reticulum stress is involved in arsenite-induced oxidative injury in rat brain. Toxicol Appl Pharmacol 224: 138–146, 2007 [DOI] [PubMed] [Google Scholar]
- 183.Liu J, Liu Y, Powell DA, Waalkes MP, and Klaassen CD. Multidrug-resistance mdr1a/1b double knockout mice are more sensitive than wild type mice to acute arsenic toxicity, with higher arsenic accumulation in tissues. Toxicology 170: 55–62, 2002 [DOI] [PubMed] [Google Scholar]
- 184.Liu L, Jiang D, McDonald A, Hao Y, Millhauser GL, and Zhou F. Copper redox cycling in the prion protein depends critically on binding mode. J Am Chem Soc 133: 12229–12237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu X, Sullivan KA, Madl JE, Legare M, and Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci 91: 521–531, 2006 [DOI] [PubMed] [Google Scholar]
- 186.Liu Y, Barber DS, Zhang P, and Liu B. Complex II of the mitochondrial respiratory chain is the key mediator of divalent manganese-induced hydrogen peroxide production in microglia. Toxicol Sci 132: 298–306, 2013 [DOI] [PubMed] [Google Scholar]
- 187.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, and Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A 99: 6053–6058, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liuzzi JP, Aydemir F, Nam H, Knutson MD, and Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A 103: 13612–13617, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lo HS, Chiang HC, Lin AM, Chiang HY, Chu YC, and Kao LS. Synergistic effects of dopamine and Zn2+ on the induction of PC12 cell death and dopamine depletion in the striatum: possible implication in the pathogenesis of Parkinson's disease. Neurobiol Dis 17: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 190.Logroscino G, Chen H, Wing A, and Ascherio A. Blood donations, iron stores, and risk of Parkinson's disease. Mov Disord 21: 835–838, 2006 [DOI] [PubMed] [Google Scholar]
- 191.Loikkanen JJ, Naarala J, and Savolainen KM. Modification of glutamate-induced oxidative stress by lead: the role of extracellular calcium. Free Radic Biol Med 24: 377–384, 1998 [DOI] [PubMed] [Google Scholar]
- 192.Loke SY, Siddiqi NJ, Alhomida AS, Kim HC, and Ong WY. Expression and localization of duodenal cytochrome b in the rat hippocampus after kainate-induced excitotoxicity. Neuroscience 245: 179–190, 2013 [DOI] [PubMed] [Google Scholar]
- 193.Lopachin RM. and Decaprio AP. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol Sci 86: 214–225, 2005 [DOI] [PubMed] [Google Scholar]
- 194.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, and Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci 158: 47–52, 1998 [DOI] [PubMed] [Google Scholar]
- 195.Lu J. and Holmgren A. The thioredoxin superfamily in oxidative protein folding. Antioxid Redox Signal 21: 457–470, 2014 [DOI] [PubMed] [Google Scholar]
- 196.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol 14: 211–217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lutsenko S. Copper trafficking to the secretory pathway. Metallomics 8: 840–852, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, and Reynolds WF. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J Biol Chem 284: 3158–3169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Malaiyandi LM, Vergun O, Dineley KE, and Reynolds IJ. Direct visualization of mitochondrial zinc accumulation reveals uniporter-dependent and -independent transport mechanisms. J Neurochem 93: 1242–1250, 2005 [DOI] [PubMed] [Google Scholar]
- 200.Mandal BK. and Suzuki KT. Arsenic round the world: a review. Talanta 58: 201–235, 2002 [PubMed] [Google Scholar]
- 201.Manton WI, Kirkpatrick JB, and Cook JD. Does the choroid plexus really protect the brain from lead? Lancet 2: 351, 1984 [DOI] [PubMed] [Google Scholar]
- 202.Mantyh PW, Ghilardi JR, Rogers S, DeMaster E, Allen CJ, Stimson ER, and Maggio JE. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J Neurochem 61: 1171–1174, 1993 [DOI] [PubMed] [Google Scholar]
- 203.Mariani S, Ventriglia M, Simonelli I, Donno S, Bucossi S, Vernieri F, Melgari JM, Pasqualetti P, Rossini PM, and Squitti R. Fe and Cu do not differ in Parkinson's disease: a replication study plus meta-analysis. Neurobiol Aging 34: 632–633, 2013 [DOI] [PubMed] [Google Scholar]
- 204.Marquez M, Blancas-Mejia LM, Campos A, Rojas L, Castaneda-Hernandez G, and Quintanar L. A bifunctional non-natural tetrapeptide modulates amyloid-beta peptide aggregation in the presence of Cu(ii). Metallomics 6: 2189–2192, 2014 [DOI] [PubMed] [Google Scholar]
- 205.Martinez-Finley EJ, Chakraborty S, Fretham SJ, and Aschner M. Cellular transport and homeostasis of essential and nonessential metals. Metallomics 4: 593–605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Martinez-Finley EJ, Gavin CE, Aschner M, and Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med 62: 65–75, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martinez L, Jimenez V, Garcia-Sepulveda C, Ceballos F, Delgado JM, Nino-Moreno P, Doniz L, Saavedra-Alanis V, Castillo CG, Santoyo ME, Gonzalez-Amaro R, and Jimenez-Capdeville ME. Impact of early developmental arsenic exposure on promotor CpG-island methylation of genes involved in neuronal plasticity. Neurochem Int 58: 574–581, 2011 [DOI] [PubMed] [Google Scholar]
- 208.Maryon EB, Molloy SA, and Kaplan JH. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am J Physiol Cell Physiol 304: C768–C779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Masoud AM, Bihaqi SW, Machan JT, Zawia NH, and Renehan WE. Early-life exposure to lead (Pb) alters the expression of microrna that target proteins associated with Alzheimer's disease. J Alzheimers Dis 51: 1257–1264, 2016 [DOI] [PubMed] [Google Scholar]
- 210.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, and Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82: 4245–4249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, and Greenamyre JT. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol Dis 34: 417–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Matak P, Matak A, Moustafa S, Aryal DK, Benner EJ, Wetsel W, and Andrews NC. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc Natl Acad Sci U S A 113: 3428–3435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mato S, Sanchez-Gomez MV, Bernal-Chico A, and Matute C. Cytosolic zinc accumulation contributes to excitotoxic oligodendroglial death. Glia 61: 750–764, 2013 [DOI] [PubMed] [Google Scholar]
- 214.Mayes J, Tinker-Mill C, Kolosov O, Zhang H, Tabner BJ, and Allsop D. beta-Amyloid fibrils in Alzheimer disease are not inert when bound to copper ions but can degrade hydrogen peroxide and generate reactive oxygen species. J Biol Chem 289: 12052–12062, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McCarthy RC. and Kosman DJ. Mechanistic analysis of iron accumulation by endothelial cells of the BBB. Biometals 25: 665–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McCarthy RC. and Kosman DJ. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One 9: e89003, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McCarthy RC. and Kosman DJ. Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci 72: 709–727, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McCarthy RC. and Kosman DJ. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front Mol Neurosci 8: 31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.McCarthy RC, Park YH, and Kosman DJ. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep 15: 809–815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McHugh PC, Wright JA, and Brown DR. Transcriptional regulation of the beta-synuclein 5′-promoter metal response element by metal transcription factor-1. PLoS One 6: e17354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Meinerz DF, Branco V, Aschner M, Carvalho C, and Rocha JB. Diphenyl diselenide protects against methylmercury-induced inhibition of thioredoxin reductase and glutathione peroxidase in human neuroblastoma cells: a comparison with ebselen. J Appl Toxicol 37: 1073–1081, 2017 [DOI] [PubMed] [Google Scholar]
- 222.Mellone M, Pelucchi S, Alberti L, Genazzani AA, Di Luca M, and Gardoni F. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J Neurochem 132: 159–168, 2015 [DOI] [PubMed] [Google Scholar]
- 223.Meloni G, Sonois V, Delaine T, Guilloreau L, Gillet A, Teissie J, Faller P, and Vasak M. Metal swap between Zn7-metallothionein-3 and amyloid-beta-Cu protects against amyloid-beta toxicity. Nat Chem Biol 4: 366–372, 2008 [DOI] [PubMed] [Google Scholar]
- 224.Menon AV, Chang J, and Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology 339: 58–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Meyer E, Kurian MA, and Hayflick SJ. Neurodegeneration with brain iron accumulation: genetic diversity and pathophysiological mechanisms. Annu Rev Genomics Hum Genet 16: 257–279, 2015 [DOI] [PubMed] [Google Scholar]
- 226.Michael GJ, Esmailzadeh S, Moran LB, Christian L, Pearce RK, and Graeber MB. Up-regulation of metallothionein gene expression in parkinsonian astrocytes. Neurogenetics 12: 295–305, 2011 [DOI] [PubMed] [Google Scholar]
- 227.Miotto MC, Rodriguez EE, Valiente-Gabioud AA, Torres-Monserrat V, Binolfi A, Quintanar L, Zweckstetter M, Griesinger C, and Fernandez CO. Site-specific copper-catalyzed oxidation of alpha-synuclein: tightening the link between metal binding and protein oxidative damage in Parkinson's disease. Inorg Chem 53: 4350–4358, 2014 [DOI] [PubMed] [Google Scholar]
- 228.Miotto MC, Valiente-Gabioud AA, Rossetti G, Zweckstetter M, Carloni P, Selenko P, Griesinger C, Binolfi A, and Fernandez CO. Copper binding to the N-terminally acetylated, naturally occurring form of alpha-synuclein induces local helical folding. J Am Chem Soc 137: 6444–6447, 2015 [DOI] [PubMed] [Google Scholar]
- 229.Mital M, Wezynfeld NE, Fraczyk T, Wiloch MZ, Wawrzyniak UE, Bonna A, Tumpach C, Barnham KJ, Haigh CL, Bal W, and Drew SC. A functional role for abeta in metal homeostasis? N-truncation and high-affinity copper binding. Angew Chem Int Ed Engl 54: 10460–10464, 2015 [DOI] [PubMed] [Google Scholar]
- 230.Mizumura A, Watanabe T, Kobayashi Y, and Hirano S. Identification of arsenite-and arsenic diglutathione-binding proteins in human hepatocarcinoma cells. Toxicol Appl Pharmacol 242: 119–125, 2010 [DOI] [PubMed] [Google Scholar]
- 231.Monnot AD, Zheng G, and Zheng W. Mechanism of copper transport at the blood-cerebrospinal fluid barrier: influence of iron deficiency in an in vitro model. Exp Biol Med (Maywood) 237: 327–333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol 375: 675–692, 1996 [DOI] [PubMed] [Google Scholar]
- 233.Mori N, Yasutake A, and Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol 81: 769–776, 2007 [DOI] [PubMed] [Google Scholar]
- 234.Moriarty GM, Minetti CA, Remeta DP, and Baum J. A revised picture of the Cu(II)-alpha-synuclein complex: the role of N-terminal acetylation. Biochemistry 53: 2815–2817, 2014 [DOI] [PubMed] [Google Scholar]
- 235.Morken TS, Sonnewald U, Aschner M, and Syversen T. Effects of methylmercury on primary brain cells in mono- and co-culture. Toxicol Sci 87: 169–175, 2005 [DOI] [PubMed] [Google Scholar]
- 236.Moshtaghie AA, Ani M, Aghadavod E, and Fazilati M. Protective effects of selenium and zinc on changes in catecholamine levels of brain regions in lead intoxified rat. Pak J Biol Sci 10: 2964–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 237.Munoz P, Humeres A, Elgueta C, Kirkwood A, Hidalgo C, and Nunez MT. Iron mediates N-methyl-d-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J Biol Chem 286: 13382–13392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Naarala JT, Loikkanen JJ, Ruotsalainen MH, and Savolainen KM. Lead amplifies glutamate-induced oxidative stress. Free Radic Biol Med 19: 689–693, 1995 [DOI] [PubMed] [Google Scholar]
- 239.Nagababu E. and Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal 6: 967–978, 2004 [DOI] [PubMed] [Google Scholar]
- 240.Nagasawa K, Ito S, Kakuda T, Nagai K, Tamai I, Tsuji A, and Fujimoto S. Transport mechanism for aluminum citrate at the blood-brain barrier: kinetic evidence implies involvement of system Xc- in immortalized rat brain endothelial cells. Toxicol Lett 155: 289–296, 2005 [DOI] [PubMed] [Google Scholar]
- 241.Nava-Ruiz C, Mendez-Armenta M, and Rios C. Lead neurotoxicity: effects on brain nitric oxide synthase. J Mol Histol 43: 553–563, 2012 [DOI] [PubMed] [Google Scholar]
- 242.Neal AP. and Guilarte TR. Molecular neurobiology of lead (Pb(2+)): effects on synaptic function. Mol Neurobiol 42: 151–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Negishi T, Takahashi M, Matsunaga Y, Hirano S, and Tashiro T. Diphenylarsinic acid increased the synthesis and release of neuroactive and vasoactive peptides in rat cerebellar astrocytes. J Neuropathol Exp Neurol 71: 468–479, 2012 [DOI] [PubMed] [Google Scholar]
- 244.Nelson-Mora J, Escobar ML, Rodríguez-Durán L, Massieu L, Montiel T, Rodríguez VM, Hernández-Mercado K, and Gonsebatt ME. Gestational exposure to inorganic arsenic (iAs3+) alters glutamate disposition in the mouse hippocampus and ionotropic glutamate receptor expression leading to memory impairment. Arch Toxicol 92: 1037–1048, 2018 [DOI] [PubMed] [Google Scholar]
- 245.Nemeti B, Regonesi ME, Tortora P, and Gregus Z. Polynucleotide phosphorylase and mitochondrial ATP synthase mediate reduction of arsenate to the more toxic arsenite by forming arsenylated analogues of ADP and ATP. Toxicol Sci 117: 270–281, 2010 [DOI] [PubMed] [Google Scholar]
- 246.Nishito Y, Tsuji N, Fujishiro H, Takeda TA, Yamazaki T, Teranishi F, Okazaki F, Matsunaga A, Tuschl K, Rao R, Kono S, Miyajima H, Narita H, Himeno S, and Kambe T. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J Biol Chem 291: 14773–14787, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nolte C, Gore A, Sekler I, Kresse W, Hershfinkel M, Hoffmann A, Kettenmann H, and Moran A. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48: 145–155, 2004 [DOI] [PubMed] [Google Scholar]
- 248.Nordberg G, Fowler BA, and Nordberg M. Handbook on the Toxicology of Metals. Amsterdam: Academic Press, 2014, p. 1 online resource [Google Scholar]
- 249.Ogra Y, Tejima A, Hatakeyama N, Shiraiwa M, Wu S, Ishikawa T, Yawata A, Anan Y, and Suzuki N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci Rep 6: 33007, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oken E. and Bellinger DC. Fish consumption, methylmercury and child neurodevelopment. Curr Opin Pediatr 20: 178–183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Olivieri S, Conti A, Iannaccone S, Cannistraci CV, Campanella A, Barbariga M, Codazzi F, Pelizzoni I, Magnani G, Pesca M, Franciotta D, Cappa SF, and Alessio M. Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J Neurosci 31: 18568–18577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Olsen LF, Issinger OG, and Guerra B. The Yin and Yang of redox regulation. Redox Rep 18: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Omata Y, Salvador GA, Supasai S, Keenan AH, and Oteiza PI. Decreased zinc availability affects glutathione metabolism in neuronal cells and in the developing brain. Toxicol Sci 133: 90–100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ortega R, Carmona A, Roudeau S, Perrin L, Ducic T, Carboni E, Bohic S, Cloetens P, and Lingor P. alpha-Synuclein over-expression induces increased iron accumulation and redistribution in iron-exposed neurons. Mol Neurobiol 53: 1925–1934, 2016 [DOI] [PubMed] [Google Scholar]
- 255.Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med 53: 1748–1759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pachauri V, Saxena G, Mehta A, Mishra D, and Flora SJ. Combinational chelation therapy abrogates lead-induced neurodegeneration in rats. Toxicol Appl Pharmacol 240: 255–264, 2009 [DOI] [PubMed] [Google Scholar]
- 257.Pan R. and Liu KJ. ZNT-1 expression reduction enhances free zinc accumulation in astrocytes after ischemic stroke. Acta Neurochir Suppl 121: 257–261, 2016 [DOI] [PubMed] [Google Scholar]
- 258.Pande M. and Flora SJ. Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 177: 187–196, 2002 [DOI] [PubMed] [Google Scholar]
- 259.Park JS, Koentjoro B, Veivers D, Mackay-Sim A, and Sue CM. Parkinson's disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet 23: 2802–2815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan K, Kline J, Ahsan H, and Graziano JH. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect 119: 1665–1670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, and David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci 22: 6578–6586, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37: 768–778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pauly PC. and Harris DA. Copper stimulates endocytosis of the prion protein. J Biol Chem 273: 33107–33110, 1998 [DOI] [PubMed] [Google Scholar]
- 264.Pedersen JT, Chen SW, Borg CB, Ness S, Bahl JM, Heegaard NH, Dobson CM, Hemmingsen L, Cremades N, and Teilum K. Amyloid-beta and alpha-synuclein decrease the level of metal-catalyzed reactive oxygen species by radical scavenging and redox silencing. J Am Chem Soc 138: 3966–3969, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pelizzoni I, Zacchetti D, Campanella A, Grohovaz F, and Codazzi F. Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochim Biophys Acta 1832: 1326–1333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pelizzoni I, Zacchetti D, Smith CP, Grohovaz F, and Codazzi F. Expression of divalent metal transporter 1 in primary hippocampal neurons: reconsidering its role in non-transferrin-bound iron influx. J Neurochem 120: 269–278, 2012 [DOI] [PubMed] [Google Scholar]
- 267.Penugonda S, Mare S, Lutz P, Banks WA, and Ercal N. Potentiation of lead-induced cell death in PC12 cells by glutamate: protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol Appl Pharmacol 216: 197–205, 2006 [DOI] [PubMed] [Google Scholar]
- 268.Perkins A, Nelson KJ, Parsonage D, Poole LB, and Karplus PA. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 40: 435–445, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Perrin L, Roudeau S, Carmona A, Domart F, Petersen JD, Bohic S, Yang Y, Cloetens P, and Ortega R. Zinc and copper effects on stability of tubulin and actin networks in dendrites and spines of hippocampal neurons. ACS Chem Neurosci 8: 1490–1499, 2017 [DOI] [PubMed] [Google Scholar]
- 270.Philbert MA, Billingsley ML, and Reuhl KR. Mechanisms of injury in the central nervous system. Toxicol Pathol 28: 43–53, 2000 [DOI] [PubMed] [Google Scholar]
- 271.Pichler I, Del Greco MF, Gogele M, Lill CM, Bertram L, Do CB, Eriksson N, Foroud T, Myers RH, Nalls M, Keller MF, Benyamin B, Whitfield JB, Pramstaller PP, Hicks AA, Thompson JR, and Minelli C. Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study. PLoS Med 10: e1001462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pietsch EC, Chan JY, Torti FM, and Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem 278: 2361–2369, 2003 [DOI] [PubMed] [Google Scholar]
- 273.Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, Zetterberg H, Winblad B, and Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer's disease. Acta Neuropathol 120: 185–193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Prakash C. and Kumar V. Arsenic-induced mitochondrial oxidative damage is mediated by decreased PGC-1alpha expression and its downstream targets in rat brain. Chem Biol Interact 256: 228–235, 2016 [DOI] [PubMed] [Google Scholar]
- 275.Prakash C, Soni M, and Kumar V. Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol Trace Elem Res 167: 121–129, 2015 [DOI] [PubMed] [Google Scholar]
- 276.Prakash C, Soni M, and Kumar V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol 36: 179–188, 2016 [DOI] [PubMed] [Google Scholar]
- 277.Prasanthi RP, Devi CB, Basha DC, Reddy NS, and Reddy GR. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci 28: 161–167, 2010 [DOI] [PubMed] [Google Scholar]
- 278.Qian J, Xu K, Yoo J, Chen TT, Andrews G, and Noebels JL. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J Neurosci 31: 97–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Qu W. and Waalkes MP. Metallothionein blocks oxidative DNA damage induced by acute inorganic arsenic exposure. Toxicol Appl Pharmacol 282: 267–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ramos-Chavez LA, Rendon-Lopez CR, Zepeda A, Silva-Adaya D, Del Razo LM, and Gonsebatt ME. Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci 9: 21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ramos D, Mar D, Ishida M, Vargas R, Gaite M, Montgomery A, and Linder MC. Mechanism of copper uptake from blood plasma ceruloplasmin by mammalian cells. PLoS One 11: e0149516, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Reczek CR. and Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol 33: 8–13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Reddy GR. and Zawia NH. Lead exposure alters Egr-1 DNA-binding in the neonatal rat brain. Int J Dev Neurosci 18: 791–795, 2000 [DOI] [PubMed] [Google Scholar]
- 284.Rehman K. and Naranmandura H. Arsenic metabolism and thioarsenicals. Metallomics 4: 881–892, 2012 [DOI] [PubMed] [Google Scholar]
- 285.Rhodes SL, Buchanan DD, Ahmed I, Taylor KD, Loriot MA, Sinsheimer JS, Bronstein JM, Elbaz A, Mellick GD, Rotter JI, and Ritz B. Pooled analysis of iron-related genes in Parkinson's disease: association with transferrin. Neurobiol Dis 62: 172–178, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rogers B, Yakopson V, Teng ZP, Guo Y, and Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med 35: 872–881, 2003 [DOI] [PubMed] [Google Scholar]
- 287.Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamano M, Cebrian ME, and Stoltzfus RJ. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect 115: 1371–1375, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Roskams AJ. and Connor JR. Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci U S A 87: 9024–9027, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Roth JA, Singleton S, Feng J, Garrick M, and Paradkar PN. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J Neurochem 113: 454–464, 2010 [DOI] [PubMed] [Google Scholar]
- 290.Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14: 551–564, 2013 [DOI] [PubMed] [Google Scholar]
- 291.Rouault TA. Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat Rev Mol Cell Biol 16: 45–55, 2015 [DOI] [PubMed] [Google Scholar]
- 292.Rush T, Liu X, Nowakowski AB, Petering DH, and Lobner D. Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1. Neurotoxicology 33: 476–481, 2012 [DOI] [PubMed] [Google Scholar]
- 293.Saini N, Georgiev O, and Schaffner W. The parkin mutant phenotype in the fly is largely rescued by metal-responsive transcription factor (MTF-1). Mol Cell Biol 31: 2151–2161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, and Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci U S A 105: 18578–18583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360: 201–205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Samuel S, Kathirvel R, Jayavelu T, and Chinnakkannu P. Protein oxidative damage in arsenic induced rat brain: influence of dl-alpha-lipoic acid. Toxicol Lett 155: 27–34, 2005 [DOI] [PubMed] [Google Scholar]
- 297.Sanchez-Pena LC, Petrosyan P, Morales M, Gonzalez NB, Gutierrez-Ospina G, Del Razo LM, and Gonsebatt ME. Arsenic species, AS3MT amount, and AS3MT gene expression in different brain regions of mouse exposed to arsenite. Environ Res 110: 428–434, 2010 [DOI] [PubMed] [Google Scholar]
- 298.Sanders T, Liu Y, Buchner V, and Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24: 15–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Santos CR, Martinho A, Quintela T, and Goncalves I. Neuroprotective and neuroregenerative properties of metallothioneins. IUBMB Life 64: 126–135, 2012 [DOI] [PubMed] [Google Scholar]
- 300.Scheiber IF. and Dringen R. Astrocyte functions in the copper homeostasis of the brain. Neurochem Int 62: 556–565, 2013 [DOI] [PubMed] [Google Scholar]
- 301.Schlief ML, Craig AM, and Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci 25: 239–246, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schrader M. and Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta 1763: 1755–1766, 2006 [DOI] [PubMed] [Google Scholar]
- 303.Segawa S, Tatsumi N, Ohishi A, Nishida K, and Nagasawa K. Characterization of zinc uptake by mouse primary cultured astrocytes and microglia. Metallomics 7: 1067–1077, 2015 [DOI] [PubMed] [Google Scholar]
- 304.Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, and Hershfinkel M. The neurophysiology and pathology of brain zinc. J Neurosci 31: 16076–16085, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, and Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A 100: 6157–6162, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Senut MC, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, and Ruden D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4: 665–674, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Seo YA, Lopez V, and Kelleher SL. A histidine-rich motif mediates mitochondrial localization of ZnT2 to modulate mitochondrial function. Am J Physiol Cell Physiol 300: C1479–C1489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shahzad R, Jones MR, Viles JH, and Jones CE. Endocytosis of the tachykinin neuropeptide, neurokinin B, in astrocytes and its role in cellular copper uptake. J Inorg Biochem 162: 319–325, 2016 [DOI] [PubMed] [Google Scholar]
- 309.Sharma DR, Sunkaria A, Wani WY, Sharma RK, Kandimalla RJ, Bal A, and Gill KD. Aluminium induced oxidative stress results in decreased mitochondrial biogenesis via modulation of PGC-1alpha expression. Toxicol Appl Pharmacol 273: 365–380, 2013 [DOI] [PubMed] [Google Scholar]
- 310.Sheline CT, Zhu J, Zhang W, Shi C, and Cai AL. Mitochondrial inhibitor models of Huntington's disease and Parkinson's disease induce zinc accumulation and are attenuated by inhibition of zinc neurotoxicity in vitro or in vivo. Neurodegener Dis 11: 49–58, 2013 [DOI] [PubMed] [Google Scholar]
- 311.Shen S, Li XF, Cullen WR, Weinfeld M, and Le XC. Arsenic binding to proteins. Chem Rev 113: 7769–7792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, and Valentine JS. Superoxide dismutases and superoxide reductases. Chem Rev 114: 3854–3918, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Shi ZH, Nie G, Duan XL, Rouault T, Wu WS, Ning B, Zhang N, Chang YZ, and Zhao BL. Neuroprotective mechanism of mitochondrial ferritin on 6-hydroxydopamine-induced dopaminergic cell damage: implication for neuroprotection in Parkinson's disease. Antioxid Redox Signal 13: 783–796, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, and Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23: 3394–3406, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Shila S, Kokilavani V, Subathra M, and Panneerselvam C. Brain regional responses in antioxidant system to alpha-lipoic acid in arsenic intoxicated rat. Toxicology 210: 25–36, 2005 [DOI] [PubMed] [Google Scholar]
- 316.Shila S, Subathra M, Devi MA, and Panneerselvam C. Arsenic intoxication-induced reduction of glutathione level and of the activity of related enzymes in rat brain regions: reversal by dl-alpha-lipoic acid. Arch Toxicol 79: 140–146, 2005 [DOI] [PubMed] [Google Scholar]
- 317.Shukalek CB, Swanlund DP, Rousseau RK, Weigl KE, Marensi V, Cole SP, and Leslie EM. Arsenic triglutathione [As(GS)3] transport by multidrug resistance protein 1 (MRP1/ABCC1) is selectively modified by phosphorylation of Tyr920/Ser921 and glycosylation of Asn19/Asn23. Mol Pharmacol 90: 127–139, 2016 [DOI] [PubMed] [Google Scholar]
- 318.Simmons-Willis TA, Koh AS, Clarkson TW, and Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-l-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J 367: 239–246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD, Deane RJ, Zhong E, Parisi M, Ciszewski J, Kasper RT, and Deane R. Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc Natl Acad Sci U S A 110: 14771–14776, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Singh V, Gera R, Kushwaha R, Sharma AK, Patnaik S, and Ghosh D. Hijacking microglial glutathione by inorganic arsenic impels bystander death of immature neurons through extracellular cystine/glutamate imbalance. Sci Rep 6: 30601, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Smith MA, Harris PL, Sayre LM, and Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 94: 9866–9868, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Smith MR, Fernandes J, Go YM, and Jones DP. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem Biophys Res Commun 482: 388–398, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sobotta MC, Liou W, Stocker S, Talwar D, Oehler M, Ruppert T, Scharf AN, and Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11: 64–70, 2015 [DOI] [PubMed] [Google Scholar]
- 324.Squitti R, Salustri C, Siotto M, Ventriglia M, Vernieri F, Lupoi D, Cassetta E, and Rossini PM. Ceruloplasmin/transferrin ratio changes in Alzheimer's disease. Int J Alzheimers Dis 2011: 231595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Squitti R, Simonelli I, Ventriglia M, Siotto M, Pasqualetti P, Rembach A, Doecke J, and Bush AI. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer's disease. J Alzheimers Dis 38: 809–822, 2014 [DOI] [PubMed] [Google Scholar]
- 326.Squitti R, Ventriglia M, Gennarelli M, Colabufo NA, El Idrissi IG, Bucossi S, Mariani S, Rongioletti M, Zanetti O, Congiu C, Rossini PM, and Bonvicini C. Non-ceruloplasmin copper distincts subtypes in Alzheimer's disease: a genetic study of ATP7B frequency. Mol Neurobiol 54: 671–681, 2017 [DOI] [PubMed] [Google Scholar]
- 327.Steiger TK, Weiskopf N, and Bunzeck N. Iron level and myelin content in the ventral striatum predict memory performance in the aging brain. J Neurosci 36: 3552–3558, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Storr SJ, Woolston CM, Zhang Y, and Martin SG. Redox environment, free radical, and oxidative DNA damage. Antioxid Redox Signal 18: 2399–2408, 2013 [DOI] [PubMed] [Google Scholar]
- 329.Stuber C, Morawski M, Schafer A, Labadie C, Wahnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, Spemann D, and Turner R. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage 93 (Pt 1): 95–106, 2014 [DOI] [PubMed] [Google Scholar]
- 330.Suazo M, Hodar C, Morgan C, Cerpa W, Cambiazo V, Inestrosa NC, and Gonzalez M. Overexpression of amyloid precursor protein increases copper content in HEK293 cells. Biochem Biophys Res Commun 382: 740–744, 2009 [DOI] [PubMed] [Google Scholar]
- 331.Sun Y, Jin K, Mao XO, Zhu Y, and Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A 98: 15306–15311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tadepalle N, Koehler Y, Brandmann M, Meyer N, and Dringen R. Arsenite stimulates glutathione export and glycolytic flux in viable primary rat brain astrocytes. Neurochem Int 76: 1–11, 2014 [DOI] [PubMed] [Google Scholar]
- 333.Tai YK, Chew KC, Tan BW, Lim KL, and Soong TW. Iron mitigates DMT1-mediated manganese cytotoxicity via the ASK1-JNK signaling axis: implications of iron supplementation for manganese toxicity. Sci Rep 6: 21113, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Takahashi M, Dore S, Ferris CD, Tomita T, Sawa A, Wolosker H, Borchelt DR, Iwatsubo T, Kim SH, Thinakaran G, Sisodia SS, and Snyder SH. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer's disease. Neuron 28: 461–473, 2000 [DOI] [PubMed] [Google Scholar]
- 335.Takeda A, Fuke S, Minami A, and Oku N. Role of zinc influx via AMPA/kainate receptor activation in metabotropic glutamate receptor-mediated calcium release. J Neurosci Res 85: 1310–1317, 2007 [DOI] [PubMed] [Google Scholar]
- 336.Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, and Zhang Z. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J Biol Chem 286: 29654–29662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tang L, Zhang Y, Qian Z, and Shen X. The mechanism of Fe(2+)-initiated lipid peroxidation in liposomes: the dual function of ferrous ions, the roles of the pre-existing lipid peroxides and the lipid peroxyl radical. Biochem J 352 (Pt 1): 27–36, 2000 [PMC free article] [PubMed] [Google Scholar]
- 338.Telianidis J, Hung YH, Materia S, and Fontaine SL. Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Front Aging Neurosci 5: 44, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Thevenod F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 23: 857–875, 2010 [DOI] [PubMed] [Google Scholar]
- 340.Thomas DJ. Arsenolysis and thiol-dependent arsenate reduction. Toxicol Sci 117: 249–252, 2010 [DOI] [PubMed] [Google Scholar]
- 341.Tjalve H. and Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology 20: 181–195, 1999 [PubMed] [Google Scholar]
- 342.Todorich B, Pasquini JM, Garcia CI, Paez PM, and Connor JR. Oligodendrocytes and myelination: the role of iron. Glia 57: 467–478, 2009 [DOI] [PubMed] [Google Scholar]
- 343.Todorich B, Zhang X, Slagle-Webb B, Seaman WE, and Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 107: 1495–1505, 2008 [DOI] [PubMed] [Google Scholar]
- 344.Tokuda E, Okawa E, and Ono S. Dysregulation of intracellular copper trafficking pathway in a mouse model of mutant copper/zinc superoxide dismutase-linked familial amyotrophic lateral sclerosis. J Neurochem 111: 181–191, 2009 [DOI] [PubMed] [Google Scholar]
- 345.Tokuda E, Okawa E, Watanabe S, and Ono S. Overexpression of metallothionein-I, a copper-regulating protein, attenuates intracellular copper dyshomeostasis and extends lifespan in a mouse model of amyotrophic lateral sclerosis caused by mutant superoxide dismutase-1. Hum Mol Genet 23: 1271–1285, 2014 [DOI] [PubMed] [Google Scholar]
- 346.Tokuda E, Okawa E, Watanabe S, Ono S, and Marklund SL. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol Dis 54: 308–319, 2013 [DOI] [PubMed] [Google Scholar]
- 347.Tolins M, Ruchirawat M, and Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 80: 303–314, 2014 [DOI] [PubMed] [Google Scholar]
- 348.Torres-Avila M, Leal-Galicia P, Sanchez-Pena LC, Del Razo LM, and Gonsebatt ME. Arsenite induces aquaglyceroporin 9 expression in murine livers. Environ Res 110: 443–447, 2010 [DOI] [PubMed] [Google Scholar]
- 349.Toyama T, Shinkai Y, Yasutake A, Uchida K, Yamamoto M, and Kumagai Y. Isothiocyanates reduce mercury accumulation via an Nrf2-dependent mechanism during exposure of mice to methylmercury. Environ Health Perspect 119: 1117–1122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Trujano-Ortiz LG, Gonzalez FJ, and Quintanar L. Redox cycling of copper-amyloid beta 1–16 peptide complexes is highly dependent on the coordination mode. Inorg Chem 54: 4–6, 2015 [DOI] [PubMed] [Google Scholar]
- 351.Tulpule K, Robinson SR, Bishop GM, and Dringen R. Uptake of ferrous iron by cultured rat astrocytes. J Neurosci Res 88: 563–571, 2010 [DOI] [PubMed] [Google Scholar]
- 352.Tuschl K, Mills PB, and Clayton PT. Manganese and the brain. Int Rev Neurobiol 110: 277–312, 2013 [DOI] [PubMed] [Google Scholar]
- 353.United States. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. Atlanta, GA: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 2007, p. 1 online resource (xx, 500 p.) [Google Scholar]
- 354.Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, and Nunez MT. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126: 541–549, 2013 [DOI] [PubMed] [Google Scholar]
- 355.Valko M, Jomova K, Rhodes CJ, Kuca K, and Musilek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90: 1–37, 2016 [DOI] [PubMed] [Google Scholar]
- 356.Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, and Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J Biol Chem 280: 22800–22808, 2005 [DOI] [PubMed] [Google Scholar]
- 357.Venderova K. and Park DS. Programmed cell death in Parkinson's disease. Cold Spring Harb Perspect Med 2: a009365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ventriglia M, Brewer GJ, Simonelli I, Mariani S, Siotto M, Bucossi S, and Squitti R. Zinc in Alzheimer's disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis 46: 75–87, 2015 [DOI] [PubMed] [Google Scholar]
- 359.Verstraeten SV, Aimo L, and Oteiza PI. Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82: 789–802, 2008 [DOI] [PubMed] [Google Scholar]
- 360.Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, and Ghetti B. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 28: 60–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Villar-Pique A, Lopes da Fonseca T, Sant'Anna R, Szego EM, Fonseca-Ornelas L, Pinho R, Carija A, Gerhardt E, Masaracchia C, Abad Gonzalez E, Rossetti G, Carloni P, Fernandez CO, Foguel D, Milosevic I, Zweckstetter M, Ventura S, and Outeiro TF. Environmental and genetic factors support the dissociation between alpha-synuclein aggregation and toxicity. Proc Natl Acad Sci U S A 113: E6506–E6515, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wakabayashi K, Tanji K, Mori F, and Takahashi H. The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27: 494–506, 2007 [DOI] [PubMed] [Google Scholar]
- 363.Wang B. and Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev 2013: 898034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wang H, Wang M, Wang B, Li M, Chen H, Yu X, Yang K, Chai Z, Zhao Y, and Feng W. Immunogold labeling and X-ray fluorescence microscopy reveal enrichment ratios of Cu and Zn, metabolism of APP and amyloid-beta plaque formation in a mouse model of Alzheimer's disease. Metallomics 4: 1113–1118, 2012 [DOI] [PubMed] [Google Scholar]
- 365.Wang JY, Zhuang QQ, Zhu LB, Zhu H, Li T, Li R, Chen SF, Huang CP, Zhang X, and Zhu JH. Meta-analysis of brain iron levels of Parkinson's disease patients determined by postmortem and MRI measurements. Sci Rep 6: 36669, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wang L. and Gallagher EP. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharmacol 266: 177–186, 2013 [DOI] [PubMed] [Google Scholar]
- 367.Wang Q, Luo W, Zhang W, Dai Z, Chen Y, and Chen J. Iron supplementation protects against lead-induced apoptosis through MAPK pathway in weanling rat cortex. Neurotoxicology 28: 850–859, 2007 [DOI] [PubMed] [Google Scholar]
- 368.Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, and Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke 39: 1869–1874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wang X, Mori T, Sumii T, and Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke 33: 1882–1888, 2002 [DOI] [PubMed] [Google Scholar]
- 370.Wasserman GA, Liu X, Loiacono NJ, Kline J, Factor-Litvak P, van Geen A, Mey JL, Levy D, Abramson R, Schwartz A, and Graziano JH. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Health 13: 23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Loiacono NJ, Levy D, Cheng Z, and Graziano JH. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect 115: 285–289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Watanabe T. and Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol 87: 969–979, 2013 [DOI] [PubMed] [Google Scholar]
- 373.Watt NT, Taylor DR, Kerrigan TL, Griffiths HH, Rushworth JV, Whitehouse IJ, and Hooper NM. Prion protein facilitates uptake of zinc into neuronal cells. Nat Commun 3: 1134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wezynfeld NE, Stefaniak E, Stachucy K, Drozd A, Plonka D, Drew SC, Krezel A, and Bal W. Resistance of Cu(Abeta4-16) to copper capture by metallothionein-3 supports a function for the abeta4-42 peptide as a synaptic Cu(II) scavenger. Angew Chem Int Ed Engl 55: 8235–8238, 2016 [DOI] [PubMed] [Google Scholar]
- 375.White AR, Reyes R, Mercer JF, Camakaris J, Zheng H, Bush AI, Multhaup G, Beyreuther K, Masters CL, and Cappai R. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res 842: 439–444, 1999 [DOI] [PubMed] [Google Scholar]
- 376.WHO. Childhood Lead Poisining. World Health Organization Press, 2010. http://www.who.int/ceh/publications/leadguidance.pdf [Google Scholar]
- 377.Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, and Krewski D. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol 44 (Suppl 4): 1–80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wu CY, Steffen J, and Eide DJ. Cytosolic superoxide dismutase (SOD1) is critical for tolerating the oxidative stress of zinc deficiency in yeast. PLoS One 4: e7061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, and Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28: 3–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Wu WS, Zhao YS, Shi ZH, Chang SY, Nie GJ, Duan XL, Zhao SM, Wu Q, Yang ZL, Zhao BL, and Chang YZ. Mitochondrial ferritin attenuates beta-amyloid-induced neurotoxicity: reduction in oxidative damage through the Erk/P38 mitogen-activated protein kinase pathways. Antioxid Redox Signal 18: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 381.Xiao G, Fan Q, Wang X, and Zhou B. Huntington disease arises from a combinatory toxicity of polyglutamine and copper binding. Proc Natl Acad Sci U S A 110: 14995–15000, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Yamauchi H, Kaise T, and Yamamura Y. Metabolism and excretion of orally administered arsenobetaine in the hamster. Bull Environ Contam Toxicol 36: 350–355, 1986 [DOI] [PubMed] [Google Scholar]
- 383.Yang X, Wang B, Zeng H, Cai C, Hu Q, Cai S, Xu L, Meng X, and Zou F. Role of the mitochondrial Ca(2)(+) uniporter in Pb(2)(+)-induced oxidative stress in human neuroblastoma cells. Brain Res 1575: 12–21, 2014 [DOI] [PubMed] [Google Scholar]
- 384.Ye F, Li X, Li F, Li J, Chang W, Yuan J, and Chen J. Cyclosporin A protects against Lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology 57: 203–213, 2016 [DOI] [PubMed] [Google Scholar]
- 385.Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, and Aschner M. The methylmercury-l-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem 107: 1083–1090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Yokel RA, Wilson M, Harris WR, and Halestrap AP. Aluminum citrate uptake by immortalized brain endothelial cells: implications for its blood-brain barrier transport. Brain Res 930: 101–110, 2002 [DOI] [PubMed] [Google Scholar]
- 387.Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, and Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett 155: 361–368, 2005 [DOI] [PubMed] [Google Scholar]
- 388.Yoshino Y, Yuan B, Kaise T, Takeichi M, Tanaka S, Hirano T, Kroetz DL, and Toyoda H. Contribution of aquaporin 9 and multidrug resistance-associated protein 2 to differential sensitivity to arsenite between primary cultured chorion and amnion cells prepared from human fetal membranes. Toxicol Appl Pharmacol 257: 198–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.You H, Tsutsui S, Hameed S, Kannanayakal TJ, Chen L, Xia P, Engbers JD, Lipton SA, Stys PK, and Zamponi GW. Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-d-aspartate receptors. Proc Natl Acad Sci U S A 109: 1737–1742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Yu WH, Lukiw WJ, Bergeron C, Niznik HB, and Fraser PE. Metallothionein III is reduced in Alzheimer's disease. Brain Res 894: 37–45, 2001 [DOI] [PubMed] [Google Scholar]
- 391.Yu X, Song N, Guo X, Jiang H, Zhang H, and Xie J. Differences in vulnerability of neurons and astrocytes to heme oxygenase-1 modulation: implications for mitochondrial ferritin. Sci Rep 6: 24200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Zamora PL, Rockenbauer A, and Villamena FA. Radical model of arsenic(III) toxicity: theoretical and EPR spin trapping studies. Chem Res Toxicol 27: 765–774, 2014 [DOI] [PubMed] [Google Scholar]
- 393.Zhang HN, Yang L, Ling JY, Czajkowsky DM, Wang JF, Zhang XW, Zhou YM, Ge F, Yang MK, Xiong Q, Guo SJ, Le HY, Wu SF, Yan W, Liu B, Zhu H, Chen Z, and Tao SC. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc Natl Acad Sci U S A 112: 15084–15089, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zhang Y, Duan X, Li J, Zhao S, Li W, Zhao L, Li W, Nie H, Sun G, and Li B. Inorganic arsenic induces NRF2-regulated antioxidant defenses in both cerebral cortex and hippocampus in vivo. Neurochem Res 41: 2119–2128, 2016 [DOI] [PubMed] [Google Scholar]
- 395.Zhao F, Liao Y, Jin Y, Li G, Lv X, and Sun G. Effects of arsenite on glutamate metabolism in primary cultured astrocytes. Toxicol In Vitro 26: 24–31, 2012 [DOI] [PubMed] [Google Scholar]
- 396.Zhou X, Cooper KL, Sun X, Liu KJ, and Hudson LG. Selective sensitization of zinc finger protein oxidation by reactive oxygen species through arsenic binding. J Biol Chem 290: 18361–18369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhou X, Sun X, Mobarak C, Gandolfi AJ, Burchiel SW, Hudson LG, and Liu KJ. Differential binding of monomethylarsonous acid compared to arsenite and arsenic trioxide with zinc finger peptides and proteins. Chem Res Toxicol 27: 690–698, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, and Ratan RR. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48: 1033–1043, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zimmermann LT, Santos DB, Naime AA, Leal RB, Dorea JG, Barbosa F, Jr., Aschner M, Rocha JB, and Farina M. Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake. Neurotoxicology 38: 1–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L, and Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol 155: 96–119, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.