Abstract
Background
Human saphenous veins used for arterial bypass undergo stretch injury at the time of harvest and pre-implant preparation. Vascular injury promotes intimal hyperplasia, the leading cause of graft failure, but the molecular events leading to this response are largely unknown. This study investigated adenosine triphosphate (ATP) as a potential molecular mediator in the vascular response to stretch injury, and the downstream effects of the purinergic receptor, P2X7R, and p38 MAPK activation.
Materials and Methods
A subfailure stretch rat aorta model was employed to determine the effect of stretch injury on release of ATP and vasomotor responses. Stretch-injured tissues were treated with apyrase, the P2X7R antagonist, A438079, or the p38 MAPK inhibitor, SB203580, and subsequent contractile forces were measured using a muscle bath. An exogenous ATP (eATP) injury model was developed and the experiment repeated. Change in p38 MAPK phosphorylation after stretch and eATP tissue injury was determined using Western blotting. Non-injured tissue was incubated in the p38 MAPK activator, anisomycin, and subsequent contractile function and p38 MAPK phosphorylation were analyzed.
Results
Stretch injury was associated with release of ATP. Contractile function was decreased in tissue subjected to subfailure stretch, eATP, and anisomycin. Contractile function was restored by apyrase, P2X7R antagonism, and p38-MAPK inhibition. Stretch, eATP, and anisomycin-injured tissue demonstrated increased phosphorylation of p38 MAPK.
Conclusions
Taken together these data suggest that the vascular response to stretch injury is associated with release of ATP and activation of the P2X7R/P38 MAPK pathway, resulting in contractile dysfunction. Modulation of this pathway in vein grafts after harvest and before implantation may reduce the vascular response to injury.
Keywords: ATP, vascular injury, P2X7R, subfailure stretch, p38 MAPK
INTRODUCTION
Human saphenous vein (HSV) is commonly used for coronary and peripheral artery bypass, yet vein graft failure rates remain high (45% and 39%, respectively, at 12–18 months per the PREVENT trials) (1, 2). After harvest, the vein is "prepared" on the back table and stored in solution prior to implantation. This laboratory has previously demonstrated that these preparation techniques are injurious to the vein and promote intimal hyperplasia (IH), the leading cause of graft failure (3–6). For example, the off-label surgical skin marker used to orient the HSV contains isopropyl alcohol and gentian violet, both of which are highly cytotoxic (7). During the work to identify a safe alternative dye, it was determined that erioglaucine (FCF), a food-dye derived from coal tars and P2X7R antagonist, was not only non-toxic to tissue, but improved the contractile function of HSV that had been harvested endoscopically (8, 9). Endoscopic vein harvest has been widely adopted to reduce the incidence of leg wound complications, however, analysis of the PREVENT IV data demonstrated that endoscopic vein harvest is associated with increased vein graft failure as compared to open harvest (10). These data suggested that HSV sustains injury during harvest which leads to functional impairment reversible by P2X7R antagonism.
P2X7R, commonly found on hematopoietic cells, are activated by ATP (EC50 of 300–800uM) and are unusual in that ATP stimulation of P2X7R leads to ATP release from P2X7R (11, 12). The feedback potentiation of P2X7R by ATP causes cellular activation of downstream pathways including p38 MAPK, which leads to apoptosis and inflammation (13–15). A growing list of P2X7R antagonists have been used in animal studies to ameliorate detrimental cascades initiated by injury in various tissues, but targeting P2X7R to treat vascular injury represents a new field (16–18).
ATP is a multifunctional nucleotide that serves as an energy source, a component of RNA, and substrate for intracellular signaling. ATP is normally retained within the cytoplasm and a steep ATP concentration gradient exists between the cytoplasm (10−3 to 10−2 M) and the extracellular space (10−9 to 10−8 M) (19). Thus, tissue damage resulting in breaks in the cell membrane would lead to rapid release of high local concentrations of ATP. Extracellular release of ATP and activation of P2X7R is associated with injury to epithelial, bronchiole, and neuronal cells but has not been described in vascular tissue (16–18).
The hypothesis of this investigation is that mechanical injury (surgical stretch) during endoscopic harvest of HSV leads to cellular damage, release of a molecular mediator (ATP), activation of a purinergic receptor (P2X7R), and downstream activation of p38 MAPK. To test this hypothesis, a model of subfailure stretch injury in rat aorta was used (20). This model recapitulates stretch injury that occurs with surgical manipulation of vessels (e.g. endoscopic vein harvest), but is above physiologic levels of stretch and well below stretch injury that leads to tissue failure. If the proposed hypothesis is correct, stretch injury would lead to measureable release of ATP. If contractile function associated with stretch injury is due to release of ATP, then P2X7R activation by exogenous ATP (eATP), and p38 MAPK activation by anisomycin would also lead to contractile dysfunction in RA and that treatment with pharmacologic antagonists would reverse this dysfunction. This would enable targeted inhibition of ATP (apyrase), P2X7R (A438079), or p38 MAPK (SB203580) as therapeutic options for the treatment of vein grafts after injury and prior to implantation to reduce the pathologic vascular response to harvest injury.
MATERIALS AND METHODS
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Procurement of Rat Aorta
Abdominal aorta was collected from female, 250–300g, Sprague-Dawley rats. Only female rats were used to decrease potential vascular physiologic variability between the sexes. Animal procedures followed study protocol approved by the Vanderbilt Institutional Animal Care and Use Committee (IACUC) and adhered to National Institute of Health Guidelines for the Care of Laboratory Animals. Immediately after euthanasia, the abdominal aorta was isolated via a midline incision using sharp dissection with minimal vessel manipulation. The tissue was placed at room temperature in heparinized PlasmaLyte (10units/mL) (HP) and taken to the laboratory for immediate treatment and testing.
Stretch Injury Model
The previously described subfailure overstretch injury protocol was followed (20). Subfailure overstretch represents the length that vein can be stretched until the haptic endpoint is reached. This level of stretch injury is described as subfailure overstretch injury to indicate that it is a pathologic stretch injury, but does not lead to disruption of the vessel. Briefly, the tissue was manually stretched to twice the ex vivo length and held for 10 seconds prior to release. This was repeated for a total of two stretch cycles. Tissues were then incubated in 200μL of HP at room temperature for one hour to mimic ‘back table’ graft storage. In some experiments, stretch-injured tissue was treated with either apyrase (4units/mL), A438079 (100μM), or SB203580 (20μM) for the 1 hour duration. Tissues were next suspended in the muscle bath to determine contractile function.
ATP Release after Stretch Injury
Non-stretched (control) and stretch injured RA were cut into segments (3–5mm) and placed in HP (60μL) supplemented with the ectonucleotidase inhibitor ARL67156 (50μM) and EDTA (2mM). ATP concentration in the perfusates was determined using the ATP Bioluminescence Assay Kit (FL-AAM; Sigma). The perfusates were collected for ATP measurements at 10 minutes and compared with non-stretched RA perfusates. ATP released was normalized to tissue weight.
ATP Injury Model
Non-stretched RA tissue was cut into 1–2mm rings, and incubated in HP in the presence of 0, 10, 20, or 25mM ATP for 1 hour at room temperature to determine dose dependency of the injury. In some experiments, tissues were co-treated with eATP plus apyrase (4u/mL), A438079 (100μM), or SB203580 (20μM) in HP for 1 hour at room temperature. The tissues were next suspended in the muscle bath to determine contractile function.
Viability assay
The methyl tetrazolium (MTT) assay was used to determine viability as previously described (3). Briefly, RA tissue was placed in MTT solution for 1 hour at 37° C to allow for tetrazole uptake and reduction to formazan in viable cells. Tissue was then incubated overnight in CelloSolve solution to dissolve the formazan. Optical density (OD) was measured using spectrophotography and the viability measured as OD/mg/mL. Non-injured (control) tissue was given a viability rating of 100% with treatment group viability calibrated relative to the control group.
Activation of p38 MAPK in RA
Similar to the eATP treatment described earlier, RA tissue rings were incubated in 200μM anisomycin for 1 hour prior to contractility studies and Western blot analysis.
Measurement of Contractile Function
RA rings were suspended in a muscle bath, washed, and equilibrated as previously described (21). The tissue rings were then contracted with phenylephrine (PE, 5×10−7 M). Force measurements were collected using the Radnoti force transducer (model 159901A) interfaced with a PowerLab data acquisition system. The diameter and weight of each tissue ring was measured. Force generated and tissue mass were used to calculate stress.
Western Blotting Analysis
After treatment incubations, RA tissues were immediately frozen in liquid nitrogen. Proteins were extracted using lysis buffer containing 50mM Tris. Cl pH 7.4, 140mM NaCl, 1% NP40, 1mM EDTA, 1mM EGTA, 0.5% deoxycholic acid with protease and phosphatase inhibitor cocktail. The proteins were separated using SDS-PAGE and transferred onto nitrocellulose membrane, followed by immunoblotting with antibodies against phospho-p38 MAPK Thr180/Tyr182 (Cell Signaling), p38 MAPK (Cell Signaling). The protein-antibody complexes were visualized and quantified using the Odyssey Infrared Imaging System. Phosphorylation was calculated as a ratio of the phosphorylated protein to total protein and was then normalized to the unstimulated control with the control value set as 1.0.
Statistical Analysis
Data were reported as mean response ± standard deviation. The statistical significant (p value) and achieved power of each experiment was determined using GraphPad Prizm version 5.0 and G*Power version 3.1.9.2, respectively. One-way ANOVA tests were used to analyze differences between three groups (control, injury, and injury plus treatment) from the same animal while paired t-tests were used to analyze differences between two-group, dependent studies. P values ≤0.05 were considered significant.
RESULTS
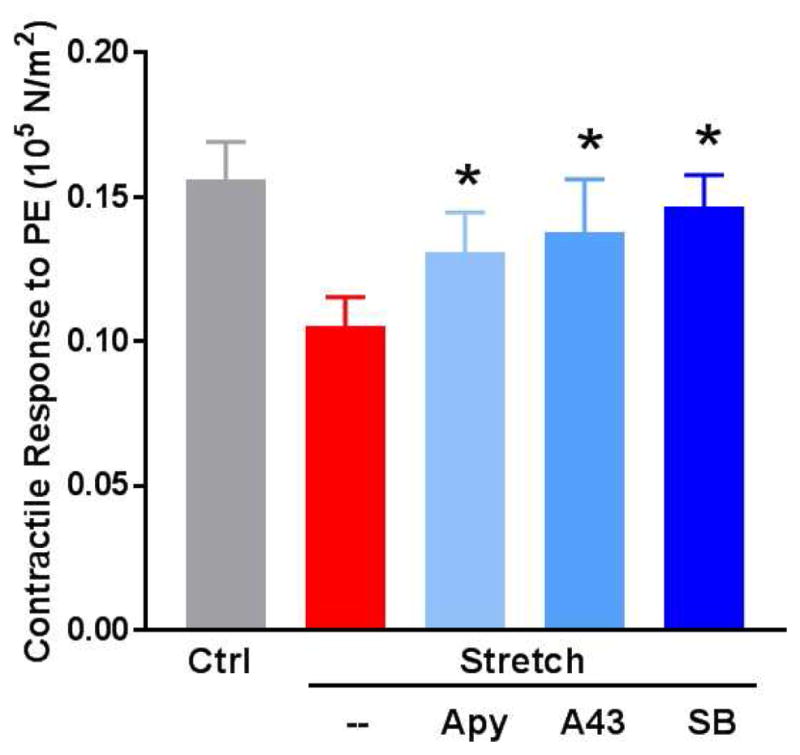
ATP/P2X7R pathway antagonism restores contractility in a rat aorta (RA) subfailure overstretch injury model
Previous data have shown that contractile function is decreased after subfailure stretch injury in RA (20). In this study, contractile function was restored in stretch-injured tissue by hydrolysis of ATP with apyrase (F [2, 71] = 3.73, p = 0.03) and competitive antagonism of P2X7R with A438079 (F [2, 76] = 3.55, p = 0.03) (Fig. 1).
Figure 1. ATP/P2X7R/p38 MAPK pathway antagonism restores contractility in a rat aorta (RA) subfailure overstretch injury model.
RA were untreated (Ctrl) or stretched to twice the ex vivo resting length (Stretch). The stretched segments were cut into 2mm rings and incubated for 1 hour in heparinized PlasmaLyte in the absence or presence of apyrase (Apy, 4 units/mL), the P2X7R inhibitor A438078 (A43, 100μM), or the p38 MAPK inhibitor SB203580 (SB, 20μM). Tissues were suspended on a muscle bath, contracted with phenylephrine (5×10−7M) and contractile responses were calculated (n=9–13, *p<0.05 compared to stretch injured tissue).
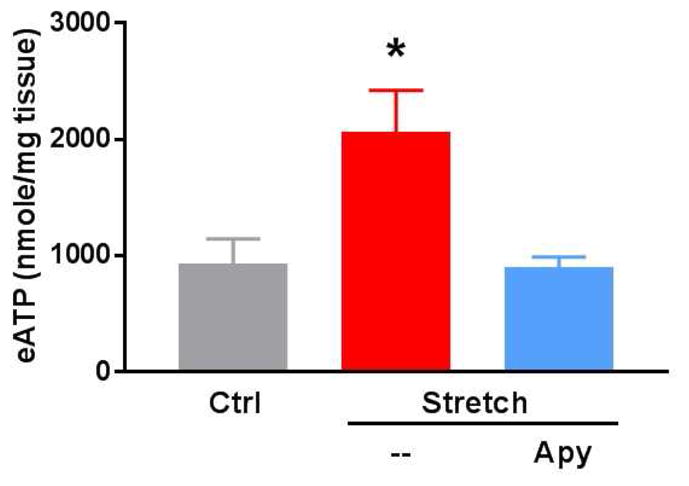
Subfailure stretch of rat aorta (RA) leads to release of extracellular ATP
To demonstrate that subfailure stretch of RA leads to release of eATP, RA perfusates were analyzed. Stretch-injured tissue treated with apyrase (4units/mL) for 10 minutes served as a negative control. Extracellular ATP release after stretch injury was higher than in non-stretched (2063 nmol/mg ± 801.2 versus 931.3 nmol/mg ± 475.2; p = 0.03) or apyrase-treated controls (903.2 nmol/mg ± 190.1; p = 0.02) (Fig. 2).
Figure 2. Subfailure overstretch mediates extracellular ATP release in the rat aorta (RA).

RA were untreated (Ctrl) or stretched to twice the ex vivo resting length (Stretch). The stretched segments were cut into 3–5mm segments. ATP released was measured in the perfusates in the absence or presence of apyrase (Apy) for 10 min (n=5, *p<0.05 compared to stretch injured tissue).
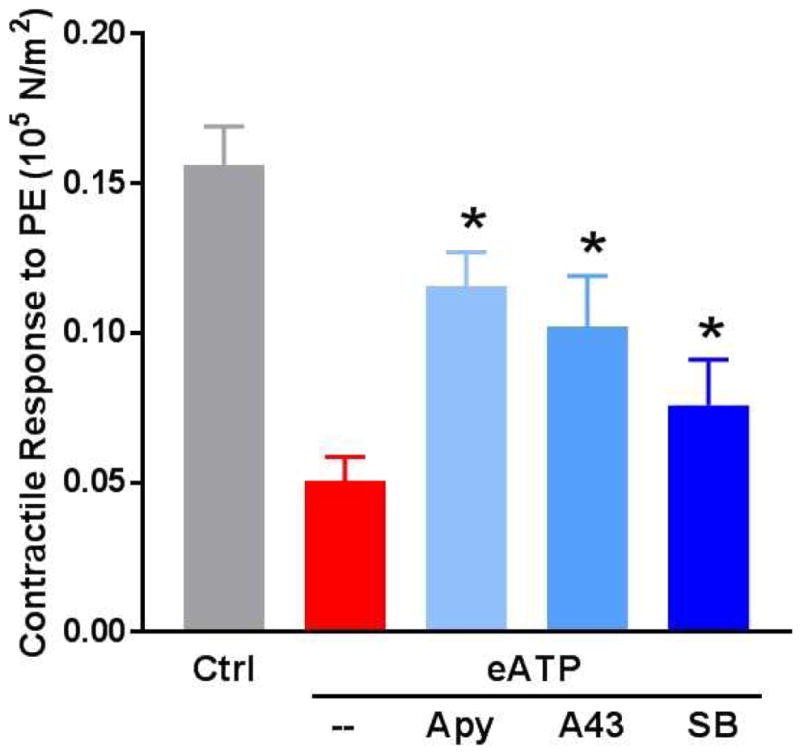
Exogenous ATP leads to reversible contractile dysfunction in RA without cytotoxicity
Since stretch injury leads to contractile dysfunction and release of ATP, we next determined if treatment with exogenous ATP would lead to contractile dysfunction. Treatment with low-doses of ATP (10mM) did not lead to decreases in contractile function in comparison to control tissue (0.13± 0.13 versus 0.17 ± 0.11; p = 0.15). Higher doses of ATP treatment (20 and 25mM) led to decreased contractility (0.05 ± 0.03, p < 0.01; 0.02 ± 0.02, p < 0.01, respectively when compared to the control) (Fig. 3).
Figure 3. Rat aorta (RA) contractile function is decreased with increasing concentrations of exogenous ATP.
RA were either left untreated (control, Ctrl) or incubated with exogenous ATP (eATP) in PlasmaLyte for 1 hour. Tissues were suspended on a muscle bath, contracted with phenylephrine (5×10−7) and contractile responses were calculated (n=7–13, *p<0.05 compared to control tissues).
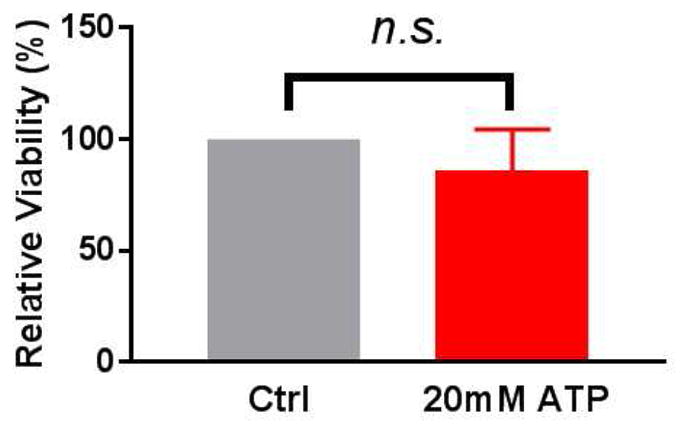
To ensure that ATP-induced contractile dysfunction was not due to cell death, a MTT assay was performed. Viability was not compromised with 20mM eATP (p = 0.49) (Fig. 4). This dose of ATP which led to physiologic dysfunction was chosen for subsequent mechanistic studies.
Figure 4. Rat aorta (RA) tissue viability is maintained after treatment with exogenous ATP.
RA segments were incubated in either heparinized PlasmaLyte alone (control) or with 20mM exogenous ATP for 1 hour at room temperature. The tissue was then subjected to a methyl tetrazolium (MTT) assay and the optical density was measured by spectrophotography. Percent viability was calculated with the control tissue set at 100% (n=5, p=0.49, n.s. = not significant).
Impaired contractile function in non-stretched RA injured with eATP (20mM) was restored with co-treatment of apyrase (F [2, 67] = 15.54, p < 0.01) and competitive antagonism of P2X7R with A438079 (F [2, 66] = 15.38, p < 0.01) similar to the restoration of function observed in the stretch injury model (Fig. 5).
Figure 5. Treatment of rat aorta (RA) with exogenous ATP leads to contractile dysfunction which is restored by ATP/P2X7R/p38 MAPK pathway antagonism.
Fresh RA segments were incubated in heparinized PlasmaLyte for 1 hour (control, Ctrl). Additional segments were cut into 2mm segments and treated with eATP (20mM) in the absence or presence of apyrase (Apy, 4units/mL), the P2X7R inhibitor A438078 (A43, 100μM), or the p38 MAPK inhibitor SB203580 (SB, 20μM) in heparinized PlasmaLyte for 1 hour. Tissue rings were suspended on a muscle bath, contracted with phenylephrine (5×10−7M) and contractile responses were calculated (n=4–6, *p<0.05 compared to ATP treated tissues).
Stretch and eATP injury of RA leads to increased p38 MAPK activation
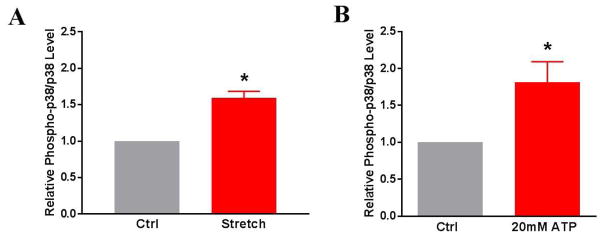
Because P2X7R activation has been linked to downstream activation of p38 MAPK, we next determined if stretch and eATP injury resulted in increased p38 MAPK phosphorylation. Each treatment group had a paired control with results presented as a relative ratio of phospho-p38 MAPK to p38 MAPK and the control values set at 1. Non-stretched control RA had less relative p38 MAPK activation than RA tissue after stretch injury (1.59 ± 0.24 fold, p < 0.01) or eATP treatment (1.812 ± .063 fold, p = 0.04) (Fig. 6).
Figure 6. p38 MAPK phosphorylation increases after stretch or eATP-injury of rat aorta (RA).
A) RA underwent subfailure overstretch or B) were treated with 20mM ATP in Plasmalyte for 1 hour at room temperature. Western Blotting technique was used to determine phospho-p38/p38 levels of tissue lysates from untreated (Ctrl) and treated tissues. (Stretch model: n=7, p<0.05; ATP-injury model: n=5, p<0.05 compared to control tissues).
p38 MAPK activation is associated with impaired contractility after RA stretch and ATP injury
Since vascular stretch injury and eATP treatment both resulted in increased activation of p38 MAPK, a relationship between phospho-p38 MAPK and contractile dysfunction was determined. Treatment with the selective p38 MAPK inhibitor, SB203580, in stretch-injured (F [2, 70] = 3.74, p = 0.03) (Fig. 1) and eATP-injured RA led to restoration of contractile function (F [2, 65] = 15.87, p < 0.01) (Fig. 5).
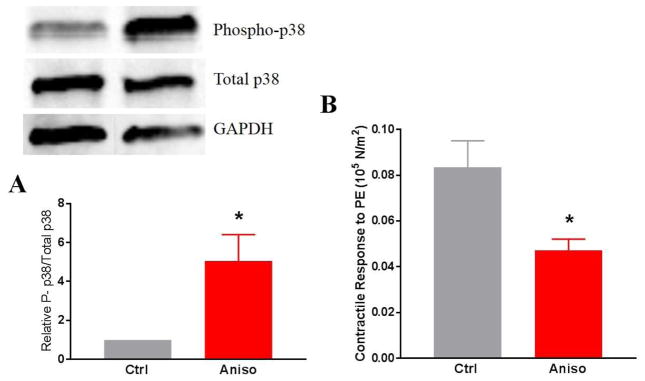
To confirm the role of p38 MAPK in contractile dysfunction, non-stretched RA were treated with a known p38 MAPK activator, anisomycin (22). Anisomycin treatment led to increases in the phosphorylation of p38 MAPK in RA (1 vs 5.05 ± 2.7, p < .05) and contractile function was decreased in anisomycin treated RA relative to untreated RA (0.05 ± 0.01 versus 0.8 ± 0.02, p = 0.02) (Fig. 7).
Figure 7. Anisomycin induces phosphorylation of p38 MAPK and decreases contractile function in rat aorta (RA).
RA rings were incubated with 200 μM of anisomycin for 15 minutes and either quick frozen in liquid nitrogen or suspended on a muscle bath. A) The proteins were extracted and phosphorylation of p38 MAPK was determined by SDS-PAGE and western blot analysis using antibodies against phospho and total p38 and compared to the loading control GAPDH (n=4, *p<0.05 compared to untreated control). B) Tissue rings were contracted with phenylephrine (PE, 5×10−7M) and contractile responses were calculated (n=4, *p<0.05 compared to control).
DISCUSSION
This study tested the hypothesis that mechanical stretch of vascular tissue, consistent with stretch induced during surgery, leads to cellular disruption, release of ATP into the extracellular space, and contractile dysfunction. Treatment of vascular tissue with P2X7R antagonists mitigates contractile dysfunction after stretch injury (20). This suggests that ATP released during surgical trauma activates P2X7R which leads to a cascade of events that leads to contractile dysfunction.
An increased concentration of extracellular ATP was measured in media after RA subfailure stretch injury. The causative relationship between this eATP release and vasomotor dysfunction was confirmed through physiologic muscle bath studies. Specifically, hydrolysis of extracellular ATP with apyrase resulted in restoration of tissue contractility after stretch injury. eATP, therefore, is not simply an indicator of mechanical stretch, but a molecular mediator of the subsequent physiologic response to injury.
These data led to the development of an ATP injury model to more specifically examine the effects of increased extracellular ATP on downstream signaling events in vascular tissue. An ideal model for eATP injury recapitulates the results after subfailure stretch injury: reversible vasomotor dysfunction without compromise to tissue viability. Initially, the lowest eATP concentration that resulted in contractile dysfunction in otherwise fresh, healthy RA tissue was determined (20mM) and viability confirmed with MTT assay. As a proof of injury extenuation, apyrase co-treatment was shown to restore vasomotor function. Finally, the selective P2X7R antagonist, A438079, demonstrated restoration of tissue contractility after eATP injury. Thus, direct stimulation of vascular tissue with eATP recapitulated stretch injury responses.
In neural and immune cell pathways, injury-induced activation of P2X7R is associated with activation of the stress activated protein kinase, P38 MAPK (15, 23). To confirm the presence and effect of downstream p38 MAPK activation within the ATP/P2X7R pathway of vascular injury, we used the subfailure stretch and eATP injury models. First, increased phospho-p38 MAPK levels after injury were observed in both models. When stretched or eATP-injured tissue was treated with a p38 MAPK inhibitor, SB203580, contractile function was restored. P38 MAPK activation with anisomycin led to increase in p38 MAPK phosphorylation which was associated with decreased contractile function analogous to that seen in the stretch and eATP injury models. Therefore, p38 MAPK activation may contribute to the molecular cascade linking injury to vasomotor dysfunction.
Limitations of this study include the use of rat arterial rather than venous tissue. However, the rat aortic stretch model is reproducible and well characterized (20). This study used vasomotor dysfunction as a functional endpoint and it is not clear if this early functional endpoint of injury correlates with later intimal hyperplasia. However, impaired vasomotor function is a reversible and relevant physiologic endpoint (24, 25). Finally, P2X7R inhibitors did not completely restore function after injury suggesting that there may be other pathways involved.
In conclusion, these studies suggest that ATP is a molecular mediator of vasomotor dysfunction after subfailure stretch injury. This injury is similar to that which occurs under typical surgical conditions. P2X7R/p38 pathway represents a potential therapeutic target for early intervention in the vascular response to injury that occurs in situations such as vein graft harvesting.
Footnotes
Author Contributions: Study conception and design: CG, JC, CB, PK
Acquisition of data: CG, WL, OJ, KC, PK
Analysis and interpretation of data: CG, WL, PK
Drafting of manuscript: CG, CB
Critical revision: CG, PK, JC, CB
Overall responsibility: CG
DISCLOSURES
This work was supported by the National Institutes of Health (R01HL70715-09) and the Veterans’ Affairs Merit Award (I01BX002036) to CB and R01HL105731-01 to JC. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 3.Osgood MJ, Hocking KM, Voskresensky IV, Li FD, Komalavilas P, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. J Vasc Surg. 2014;60:202–211. doi: 10.1016/j.jvs.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li FD, Eagle S, Brophy C, Hocking KM, Osgood M, et al. Pressure control during preparation of saphenous veins. JAMA Surg. 2014;149:655–662. doi: 10.1001/jamasurg.2013.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wise ES, Hocking KM, Eagle S, Absi T, Komalavilas P, et al. Preservation solution impacts physiologic function and cellular viability of human saphenous vein graft. Surgery. 2015;158:537–546. doi: 10.1016/j.surg.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise ES, Hocking KM, Luo W, Feldman DL, Song J, et al. Traditional graft preparation decreases physiologic responses, diminishes viscoelasticity, and reduces cellular viability of the conduit: A porcine saphenous vein model. Vasc Med. 2016;21:413–421. doi: 10.1177/1358863X16649040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocking KM, Luo W, Li FD, Komalavilas P, Brophy C, et al. Brilliant blue FCF is a nontoxic dye for saphenous vein graft marking that abrogates response to injury. J Vasc Surg. 2016;64:210–218. doi: 10.1016/j.jvs.2014.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osgood MJ, Sexton K, Voskresensky I, Hocking K, Song J, et al. Use of Brilliant Blue FCF during vein graft preparation inhibits intimal hyperplasia. J Vasc Surg. 2016;64:471–478. doi: 10.1016/j.jvs.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise ES, Hocking KM, Feldman D, Komalavilas P, Cheung-Flynn J, et al. An Optimized Preparation Technique for Saphenous Vein Graft. Am Surg. 2015;81:E274–276. [PMC free article] [PubMed] [Google Scholar]
- 10.Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, et al. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2014;130:1445–1451. doi: 10.1161/CIRCULATIONAHA.113.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North RA. Surprenant A Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 12.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. Faseb j. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 14.Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- 16.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Bai J, Zhou X, Tang J, Jiang C, et al. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol. 2015;308:C463–472. doi: 10.1152/ajpcell.00245.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Ishida Y, Ugawa S, Ueda T, Oka Y, et al. Upregulation of the P2X7 receptor after cryogenic injury to rat brain. Neuroreport. 2009;20:393–397. doi: 10.1097/WNR.0b013e328324ede7. [DOI] [PubMed] [Google Scholar]
- 19.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc. 2007;118:199–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Luo W, Guth CM, Jolayemi O, Duvall CL, Brophy CM, et al. Subfailure Overstretch Injury Leads to Reversible Functional Impairment and Purinergic P2X7 Receptor Activation in Intact Vascular Tissue. Front Bioeng Biotechnol. 2016;4:75. doi: 10.3389/fbioe.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voskresensky IV, Wise ES, Hocking KM, Li FD, Osgood MJ, et al. Brilliant blue FCF as an alternative dye for saphenous vein graft marking: effect on conduit function. JAMA Surg. 2014;149:1176–1181. doi: 10.1001/jamasurg.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junghae M, Raynes JG. Activation of p38 mitogen-activated protein kinase attenuates Leishmania donovani infection in macrophages. Infect Immun. 2002;70:5026–5035. doi: 10.1128/IAI.70.9.5026-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Li G, Huang LY. p38 MAPK mediates glial P2X7R-neuronal P2Y1R inhibitory control of P2X3R expression in dorsal root ganglion neurons. Mol Pain. 2015;11:68. doi: 10.1186/s12990-015-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li FD, Sexton KW, Hocking KM, Osgood MJ, Eagle S, et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res. 2013;180:e55–62. doi: 10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eagle S, Brophy CM, Komalavilas P, Hocking K, Putumbaka G, et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77:922–928. [PMC free article] [PubMed] [Google Scholar]