Abstract
Basal cell carcinoma (BCC) of the skin is driven by aberrant hedgehog signaling. Thus blocking this signaling pathway by small molecules such as vismodegib inhibits tumor growth. Primary cilium in the epidermal cells plays an integral role in the processing of hedgehog signaling-related proteins. Recent genomic studies point to the involvement of additional genetic mutations that might be associated with the development of BCCs, suggesting significance of other signaling pathways, such as WNT, NOTCH, mTOR, and Hippo, aside from hedgehog in the pathogenesis of this human neoplasm. Some of these pathways could be regulated by noncoding microRNA. Altered microRNA expression profile is recognized with the progression of these lesions. Stopping treatment with Smoothened (SMO) inhibitors often leads to tumor reoccurrence in the patients with basal cell nevus syndrome, who develop 10–100 of BCCs. In addition, the initial effectiveness of these SMO inhibitors is impaired due to the onset of mutations in the drug-binding domain of SMO. These data point to a need to develop strategies to overcome tumor recurrence and resistance and to enhance efficacy by developing novel single agent-based or multiple agents-based combinatorial approaches. Immunotherapy and photodynamic therapy could be additional successful approaches particularly if developed in combination with chemotherapy for inoperable and metastatic BCCs.
Keywords: BCC, hedgehog, photodynamic immunotherapy, therapeutics
1 | INTRODUCTION
Skin cancer is the most common form of malignancy in the world. A total of 3.5 million cases of nonmelanoma skin cancer (NMSC) are diagnosed each year, of which 80% represent basal cell carcinoma (BCC). The average cost of treatment for NMSC amounted to 4.8 billion dollars between 2007 and 2011.1 Additionally, there has been a 3–8% yearly increase in the incidence of NMSC worldwide since 1960.2 The incidence of BCC alone continues to increase by 10% per year.2 Forty to fifty percent of patients with a primary malignancy are likely to develop one or more BCC within 5 years.2 A recent retrospective cohort study found that locally advanced BCC could be identified in 0.8% of BCC cases and metastatic BCC occurred in 0.4% of the BCC cohort.3 BCC prevalence tends to be much higher in fair-skinned individuals with high cumulative ultraviolet (UV) susceptibility factors, such as light eyes, hair color, and the inability to tan.4 In fact, the lifetime risk for developing BCC ranges from 33 to 39% in Caucasian men and 23 to 28% in Caucasian women.5 The incidence of NMSCs directly increases depending on exposure to UV radiation.6,7 Intense solar UVB light exposure is the most important environmental risk factor for the development of NMSCs.7 UVB-induced DNA damage promotes the development of pyrimidine photoproducts that ultimately lead to mutations in key genes promoting the alterations of signal transduction pathways, cell cycle deregulation and immunosuppression.7 Our and other earlier reviews described in detail the molecular pathogenesis of NMSCs including BCCs.8,9 This review focuses on certain aspects of the molecular pathogenesis of BCCs, which have not been previously been discussed in greater detail while providing a cursory discussion of other aspects that have been reviewed earlier.
2 | CANONICAL HEDGEHOG SIGNALING
Mutations that lead to the up-regulation of hedgehog (HH) signaling are associated with the development of BCC. For instance, mutations in the tumor suppressor gene Patched (PTCH) are implicated in the growth of sporadic BCCs and those that develop due to Gorlin syndrome. Gorlin or Nevoid basal cell carcinoma syndrome (NBCC) is a rare autosomal dominant disorder characterized by the development of multiple BCCs from an early age. Individuals are typically affected by numerous clinically advanced BCC lesions (ranging from dozens to thousands), dyskeratotic palmar and plantar pitting, rib and spine abnormalities, premature calcification of the falx ceribri, frontal bossing, hypertelorism, macrocephaly, and cleft lip or palate. Patients have an increased disposition for developing other malignancies, such as medulloblastomas, rhabdomyosarcomas, benign ovarian cysts, cardiac fibromas, and mesenteric cyst.10
PTCH is a 12-pass transmembrane receptor protein that suppresses the HH signaling cascade. HH ligands, including Sonic hedgehog (SHH), Indian hedgehog (IHH), and Desert hedgehog (DHH), repress the functions of tumor suppressor, PTCH, upon binding. This binding interaction allows the release of the seven-pass transmembrane protein Smoothened (SMO), which migrates to the primary cilium and regulates the transcription of GLI transcription factors (GLI1, GLI2, and GLI3). In the absence of the HH ligand, PTCH blocks this migration of SMO into the primary cilium. The majority of sporadic BCCs have loss-of-function mutations in at least one allele of PTCH1, preventing repression of the HH cascade, and others have gain-of-function mutations in SMO, leading to over-activation of the pathway. A recently published study found that intrafollicular epidermal stem cells, rather than committed progenitor cells, are able to develop into BCC upon HH signaling due to their enhanced self-renewing ability. This leads to rapid clonal expansion and resistance to p53-mediated apoptosis.11 HH signaling also has some role in the development of squamous cell carcinoma since cells carrying mutant PTCH within the interfollicular epidermis, hair follicular bulge, and hair germ have the potential for developing into either of the two NMSCs.8 However, HH signaling is not considered to be a driver pathway for this neoplasm.
In the absence of ligand, GLI’s are phosphorylated, ubiquitinated and partly cleaved to generate repressor forms that prevent downstream HH signaling.12 Upon translocation of SMO into the primary cilium, such proteolytic processing is prevented and the lengthy active form of GLI allows for the transcription of target genes.12 The translocation of GLI 1/2 also involves the disassociation of the complex from its inhibitor suppressor of fused (SUFU) (Fig. 1). A loss-of-function mutation in SUFU, which has been found in sporadic BCCs, leads to increased GLI transcription.12 For an elaborate description of these events, please refer to our and other recent reviews.8,9
FIGURE 1.
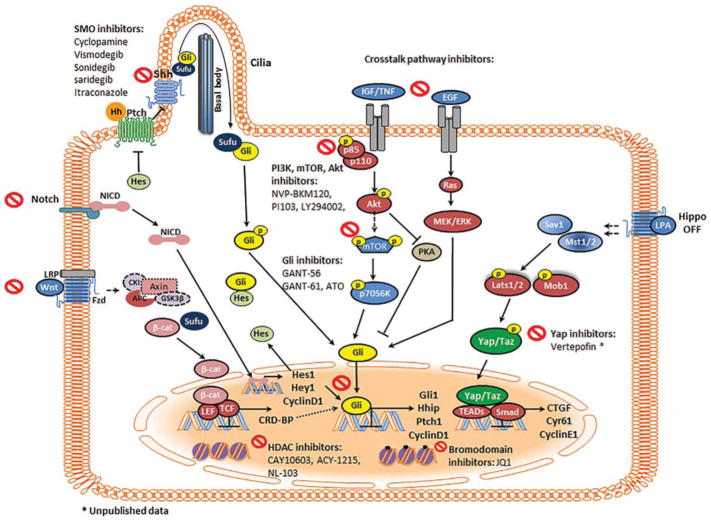
Molecular targets for therapy in the HH pathway web of collaboration. The HH pathway interacts with various other signaling networks that synergistically contribute to tumor development. PTCH is a 12-pass transmembrane receptor that generally represses SMO. HH binding to PTCH or inactivating PTCH mutations suppress this repressive response and allow for the translocation of SMO to the primary cilium to induce GLI transcription. The mTOR pathway helps release GLI from SUFU through S6K1 mediated GLI phosphorylation. The IGF/ PI3K/AKT and EGFR/MEK/ERK pathways modulate PKA-dependent phosphorylation of GLI. In the hippo pathway, MST1/2 kinases and SAV1 form a complex to phosphorylate and activate LATS1/2 and MOB1. In turn, LATS1/2 dephosphorylates YAP/TAZ, allowing the complex to translocate the nucleus and to interact with TEAD1-4 to induce the expression of genes that promote tumor progression. The aggrandizing effect of these various pathways results in BCC development, progression, and tumor resistance. Inhibiting various players within this crosstalk may lead to effective and resistance-proof treatment modalities. SMO antagonists, crosstalk pathway inhibitors, GLI antagonists, HDAC inhibitors, bromodomain inhibitors, and verteporfin target these various pathways to curb tumor development
3 | NONCANONICAL HEDGEHOG SIGNALING
GLI transcription also occurs via alternate pathways that comprise noncanonical HH signaling, as shown in Fig. 1. In this way, the characteristic ligand binding of PTCH1 and activation of SMO is bypassed to induce GLI expression.8 Epidermal growth factor receptor (EGFR) signaling through RAS/RAF/MEK/ERK synergistically modulates expression of downstream GLI target by activating JUN/AP-1, which cooperates with GLI to induce target gene expression.13 In addition, EGFR-induced activation of ERK1/2 also prevents proteasome mediated GLI2 degradation in keratinocytes.14 Transforming growth factor (TGFβ) signaling up-regulates GLI2 transcription by promoting SMAD and β-Catenin interaction at the GLI promoter site.15 Employing mouse BCC cell lines, activation of aPKC, an atypical protein kinase C, was shown to function downstream of SMO to phosphorylate and activate GLI1.16 WNT/β-Catenin regulates HH transcription by targeting Coding Region Determinant Binding Protein (CRD-BP), which binds with GLI1 mRNA and induces BCC development.17 SUFU binds to β-catenin and blocks its nuclear translocation.18 Kinases such as Cdc211, unc-51-like-kinase 3 (Ulk3), mitogen-activated protein kinase 10 (MAPK 10) and dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 2 (DYRK2) are considered important in the regulation of HH signaling.8,19,20 Stimulation of PI3K/AKT by IGF-1 induces GLI even in the presence of low SHH.21 AKT also regulates SHH signaling through PKA-mediated GLI inactivation.21 SHH signaling regulates metastasis through the activation of PI3K/AKT, which promotes epithelial-mesenchymal transition (EMT) and matrix metalloproteinase 9 (MMP-9).22 Snail, a direct transcriptional repressor of E-cadherin, drives skin cancer progression and metastasis.23 We also showed that there is an enhancement of Snail in BCCs.24 NF-κB is a transcription factor that is associated with cutaneous inflammation and carcinogenesis triggered by chemical or UVB.25 NF-κB allows for SMO independent GLI activation by binding to the GLI promoter in EMT and claudin-low cell lines.26 This complex network of mechanisms may underlie the pathogenesis of both slow growing as well as locally invasive BCCs into a rare metastatic disease.
Genomic analysis has identified novel mutations both downstream of GLI and independent of the HH pathway that may be considered important in the development and/or progression of BCC. Yes-associated protein (YAP) is a co-transcriptional activator with oncogenic potential that also has a role in maintaining basal epidermal progenitors, regulating hair follicles, and promoting proliferation in normal skin.27 In vitro studies demonstrated that YAP activation is associated with accelerated proliferation, diminishing apoptosis, and the suppression of differentiation of primary mouse keratinocytes.27 Upon Hippo signaling activation, YAP is phosphorylated and transported to the cytoplasm where it is sequestered by 14-3-3σ and can no longer induce target gene transcription.28,29 Bonilla et al30 analyzed 293 BCCs lesions and found that YAP target genes are significantly upregulated suggesting their role in tumorigenesis. PTPN14 and LATS1 loss-of-function mutations promote BCC through the nuclear localization and consequent transcriptional activation of YAP1.30 Two novel genes related to BCCs, serine/threonine protein phosphatase genes PPP6C and serine/threonine protein kinase gene STK19 have also been identified.30 PPP6C manifests an inhibitory effect on cyclin D1 and is involved in promoting phosphorylation-dependent activation of LATS1.31
Missense mutations in N-MYC, an oncogene associated with neuroblastoma and medulloblastoma, were found in 30% of BCCs analyzed.30 MYC is a key regulatory factor in embryonic development and plays a dominant role in oncogenesis, which occurs more frequently in highly recurrent BCCs.30,32 MYC genes are involved in regulating multiple cellular mechanisms such as DNA repair, proliferation, metabolism, cellular differentiation, regulation of noncoding RNA, and protein synthesis.33 The majority of mutations located in the MYC box 1 region (MB1) compromised the interaction of N-MYC with FBXW7, the substrate-binding component of an ubiquitin ligase complex that leads to proteasome-mediated degradation of MYC.30 Mutations in FBXW7 further augment the oncogenic impact of enhanced N-MYC stability in BCCs.30
Mutations in the conserved binding sites for the transcription factor GATA3 induce BCC genesis by disrupting epidermal differentiation.34 GATA3 knockout mice show increased proliferation in basal epidermal cells, decreased apoptosis, aberrant hair follicle, and epidermal differentiation along with an upregulation of NOTCH 1 and WNT signaling.35 Mutations in Kinastin (kinetochore-localized ASTRIN/SPAG5 binding protein (KNSTRN)), which encodes a kineto-chore-associated protein in the mitotic spindle that is vital to chromosomal segregation, are also implicated in BCC.30,36 Mutant KNSTRN in a murine BCC cell line disrupted sister chromatid cohesion during mitosis further emphasizing its role in promoting chromosomal instability.36 KNSTRN mutations seem to be acquired in the later stages in BCC development implicating their potential as biomarkers of aggressive disease, although this remains to be established.36
Oncogenic mutations noted in the genes of the MAPK pathway included ErbB2, Ras, PIK3CA, and RAC1. NOTCH1 and NOTCH2 mutations were observed in 26% and 29% of BCCs, respectively.30 Furthermore, single nucleotide polymorphisms associated with caspase 8 splice variants potentially inhibiting apoptosis are associated with BCCs.30,34 Inactivation of caspase-8 in basal epidermal keratinocytes triggers chronic skin inflammation and hyperproliferation in mouse skin.37
4 | PRIMARY CILIA AND HEDGEHOG SIGNALING PATHWAY
HH signaling is mediated in the primary cilium, a microtubule-based, membrane-enclosed structure.8 Primary cilia are nonmotile and sensory structures involved in trafficking receptors molecules to cilial membrane.38,39 Motile cilia participate in both generation and detection of mechanical signal during development.38 Both motile and nonmotile types of cilia have some common structural proteins such as core components of tubulin and intraflagellar transport (IFT) proteins. Some proteins such as inner and outer dynein arms (IDAs) and nexin-dynein regulatory complex (NDRC) and radial spokes are only present in motile cilia to regulate waveform and beat frequency.40,41 Ciliary and basal body proteins act as signaling hubs for various developmental as pathophysiological pathways.42 Morphogenesis and homeostasis of epidermis and hair follicles require involvement of multiple SHH, WNT, NOTCH, Hippo signaling pathways which have been closely linked with nonmotile ciliary proteins.42 Besides these pathways, ciliary proteins also participate in vesicle transport, cytoskeleton, signaling, ubiquitination.42 Lehman et al43 have demonstrated that ciliary function disruption of dermal cells of the skin results in loss of SHH or GLI2 function, and arrest of follicle development. Primary cilia’s temporally and spatially distinct functions participate in crosslink of signaling, proliferation and differentiation in epidermis, hair follicle, and in the bulge stem cells whose normal morphogenesis is relies on SHH and NOTCH signaling.44,45 Loss of epidermal cilia leads to hyperplasia, expanded clone size and modulate keratinocyte differentiation.44 In a recent affinity proteomic analysis, 217 tagged ciliary proteins were analyzed to identify the new disease-relevance.46 Lewis et al47 showed that growth arrest specific 8 (Gas8) is needed for cilial motile functions and its two missense variants (A391V and E199K) were detected in human primary ciliary dyskinesia (PCD) patients. The involvement of primary cilia and interactions of various ciliary proteins through which epidermis-mesenchymal cells receive signals remain elusive but it remains very interesting to understand the pathophysiology of PCD.
GORAB, a golgin that localizes at the golgi apparatus, induces formation of the primary cilium.48 For this reason, GORAB deficient dermal mesenchymal cells that exhibit defects in primary cilia development are unable to fully respond to HH signaling in vitro.48 SMO initially originates from the cell surface and translocates to the ciliary membrane.49 Various proteins participate in the extensive mechanisms involved in the process of ciliary translocation.50 The IFT machinery mediates the movement of SMO from the ciliary base to the tip.51 For instance, mutant IFT27 and IFT25 mice experienced impaired hair follicular morphogenesis in association with disruptions in the trafficking of SMO and impaired transcription of GLI.52 Following the translocation of SMO to the primary cilia, protein kinase A (PKA) induced repression of GLI transcription is suppressed and GLI proteins are released from their inhibitor SUFU.53
The ciliary accumulation of SMO following HH signaling activation forms the Evc-SMO complex at a distinct ciliary compartment known as the EvC zone. This critical association is required for the SMO mediated suppression of PKA and SUFU, the subsequent GLI3 repressor inhibition and GLI2/3 activator formation.54 EF-hand calcium binding domain 7 (EFCAB7) and IQ domain-containing protein E (IQCE) are two ciliary proteins that positively regulate HH signaling by anchoring the EVC-EVC2 complex in a signaling microdomain at the base of the cilia.55 Moreover, a heteromeric transient receptor potential channel, polycystic kidney disease like 1 (PKD1L1)-(PKD2L1) controls ciliary calcium concentration and regulates SMO mediated GLI activation.55 These data suggest the involvement of complex interactions of ciliary proteins and SHH signaling proteins. The physiological importance of many of these interactions is not yet clear.
The distribution of phosphatidylinositol 4-phosphate(PI(4)P) in the ciliary membrane and phosphatidylinositol 4,5 phosphate 2 (PI(4,5)P2) at the ciliary base is created by a ciliary phosphoinositide 5-phosphatase (INPP5E).56 This distribution is known to promote normal HH signaling by limiting the ciliary accumulation of G-protein coupled receptor 161 (GPR161), an inhibitor of HH signaling.56 Upon inactivation of INPP5E A, PI(4,5)P2 accumulates at the cilia tip and leads to the recruitment of PI(4,5)P2 interacting protein and Gpr161, which then represses GLI transcription.57 Thus, in the absence of signaling, GPR161 localizes to the cilium and may lead to the activation of PKA and subsequent processing of GLI3 to its repressor form.51 In the presence of signaling, GPR161 binds to β-arrestin and subsequently, clathrin-mediated endocytosis promotes its removal.58
Additionally, Jiang et al59 demonstrated that a phospholipid, (PI(4) P), shuttles between PTCH and SMO to mediate HH signaling. The binding of PI(4)P to the arginine motif in the SMO C-terminal tail promotes phosphorylation dependent activation of SMO and its ciliary localization. Studies also suggest that Pitchfork (PIFO) and the G protein-coupled receptor associated sorting protein 2 (GPRASP2) are integral components of the ciliary targeting complex that facilitates SMO translocation into the primary cilium.60 Kuzhandaivel et al61 identified that Costal (COS 2) and Fused (Fu) are required for SMO ciliary transport involved in Drosophila olfactory sensory neurons. These core components are conserved from Drospophila to vertebrates.
Other signaling pathways, such as WNT, NOTCH, mTOR, and Hippo that have been implicated in BCC promotion are also associated with the primary cilium. WNT signaling is required to modulate the cilia cytoskeleton suggesting that the cytoskeleton is vital to the onset of WNT signaling.62 The development of ciliopathies such as the Bardet-Biedl (associated with the knockdown of BBS1, BB4, and MKKS) have been linked to an overactive WNT response in cell cultures.63 Moreover, the presence of mutations in mice primary cilia has resulted in an increased canonical WNT signaling response.64 Canonical WNT signaling promotes stabilization of β-catenin in the cytoplasm, which then triggers the transcription of WNT target genes in the nucleus.62 Noncanonical WNT signaling, on the other hand, results in modification in cell shape and actin assembly.62 Cilia also function to regulate NOTCH signaling and, in doing so, preserve the balance between epidermal proliferation and differentiation.45 NOTCH receptors and NOTCH processing enzymes are localized within the cilia in epidermal cells and ciliary mutations lead to defects in NOTCH signaling.45 NOTCH regulates HH signaling by controlling the interaction of ciliary transport proteins with PTCH1 and SMO.65 miR-449 mediated inhibition of NOTCH is vital to the differentiation of ciliated cell progenitors.66 Moreover, cilia mediate cell size through downregulation of the mTOR pathway.66 The suppression of mTOR correlates with the activation of autophagy, which results in the inhibition of proteasome-mediated degradation of ciliary proteins.67 NPHP4, a cilia-associated protein, serves as a negative regulator of Hippo signaling by directly interacting with LATS1 and inhibiting LATS 1-mediated phosphorylation of YAP.68 NPHP4 acts upstream of NPHP9, another protein that localizes to the primary cilia, in a common pathway that ultimately activates YAP and promotes proliferation.69
5 | NONCODING RNA REGULATION
MicroRNAs (miRNA) are small regulatory RNA that have been implicated in the development of various forms of cancer.70–73 Mature miRNAs serve as post-transcriptional regulators of gene expression.74 DROSHA, an RNase III endonuclease involved with the initial stages of RNA processing in the nucleus, cleaves primary miRNA to precursor miRNA.75 DiGeorge syndrome critical region gene 8 (DGCR8), another essential component of the miRNA maturing microprocessor complex, stabilizes DROSHA and identifies the primary RNA substrate.75 Once the precursor miRNA is transported to the cytoplasm, DICER, an RNase III enzyme, further cleaves pre-miRNA to form mature strands that are incorporated into the RNA-induced silencing complexes (RISC).75 Incorporated miRNA guides RISC to its complementary mRNA so that translation can be interrupted.76 RISC is a multi-protein complex composed of several proteins; these include: the RISC core components argonaute-1 (AGO1) and argonaute-2 (AGO2), the RISC loading complex subunit TARBP, and the double-stranded RNA binding protein PACT.75 Altered expression of miRNA machinery components, such as the maturing microprocessor complex and RISC, may play a role in BCC development. mRNA expression levels of DROSHA, DGCR8, AGO1, AGO2, TARBP, and PACT were significantly upregulated in BCC tumor tissue as compared to the healthy skin controls.75,77 Conversely, DICER was found to be downregulated in BCC as compared to healthy skin.77 Phosphorylation of TRBP by MAPK leads to the stabilization of the miRNA complex, an upregulation of tumor promoting miRNAs and the downregulation of tumor suppressor miRNAs.78 Hence, modifications in this complex miRNA maturation process may lead to altered protein expression and resulting tumorigenesis.
Altered miRNA profiles in association with some of the key players in BCC pathogenesis provide a compelling story regarding the significance of noncoding RNA regulation in this context. Sand et al79 found 16 upregulated and 10 down regulated miRNA in BCC skin with connections to HH and MAPK signaling. miR-203 has been identified as a tumor suppressor that is suppressed by EGFR/MEK/ ERK/c-JUN signaling80 Heffelfinger et al81 found that distinctive miRNA expression correlates with nodular and infiltrative tumor BCC subtypes. Not only that but the expression level of miR-183, a miRNA that has been shown to inhibit metastasis in various other cancers, was consistently lower in the infiltrative than nodular BCCs.81 The upregulation of MiR-141, 200a, and 200c in nodular BCC may be linked to C-MYC and the WNT-β-catenin pathway.81,82
Oncogene cluster, oncomiR-1 cluster (miR-17-92) has been implicated in SHH pathway regulated medulloblastoma in a PTCH1 mouse model.83 miRNA belonging to this particular cluster were overexpressed in BCCs suggesting their role in BCC pathogenesis.79 Recently Sand et al84 identified differentially expressed cirRNAs in BCCs that regulate miRNA activity by sequestering target sequences. This study further describes the interaction of cirRNA with the OncomiR-1 cluster.84 Differentially expressed cirRNAs with microRNA response elements (MREs) for members of OncomiR-1, including hsa-miR-19b-1 and hsa-miR-92, were among the top 10 cirRNAs identified.84 Differentially expressed long noncoding RNA (lncRNAs) in BCCs including oncogenic and epidermis specific ones such as CASC15 or ANRIL have also been identified.85
6 | INTERVENTIONS
6.1 | SMO antagonists
Cyclopamine was the classic SHH inhibitor found to antagonize SMO and to prevent BCC development in a murine model of UVB carcinogenesis in our laboratory.86 Its toxic effects, poor oral bioavailability along with solubility, and stability issues8,87 led to the development of vismodegib (GDC-0449), a second generation cyclopamine derivative, which also directly binds SMO at the same location where cyclopamine binds.8 Similar to cyclopamine, vismodegib exposure during organogenesis is associated with embryo fetal death and congenital birth defects.88 It is currently approved to treat adults with metastatic or recurrent BCC who are not eligible for surgery or radiation therapy.6 According to a recent meta-analysis examining the clinical response to vismodegib, more than a quarter of the patients discontinued therapy due to adverse events such as muscle spasms, alopecia, dysgeusia, and amenorrhea.89 SMO inhibitors such as sonidegib (LDE225) and itraconazole are in phase II clinical trials to treat advanced or metastatic BCC.90,91 Other experimental agents in Phase I clinical trials are saridegib,92 TAK-441 XL-139 (NCT00670189), and LEQ506 (NCT01106508)92–97 (Table 1) (Fig. 1).
TABLE 1.
Chronology of hedgehog inhibitors development
| Agent | Trial | Patient population | Number of patients | Significance |
|---|---|---|---|---|
| Vismodegib | Phase 1102 | Patients with solid tumors refractory to current therapy including basal cell cancer | 68 | Established an acceptable safety profile at a recommended daily dose of 150 mg/d and an effective tumor response in patients with BCC |
| Phase II94 | Patients with Basal Cell Nevus Syndrome | 41 | At 1 month, vismodegib use had reduced the hedgehog target gene expression in BCCs by 90% and no residual BCC was histologically detectable in 83% of biopsy samples from clinically regressed basal cell carcinoma sites | |
| Phase II (ERIVSNCE: 12 month analysis)95 | Patients with metastatic BCC and locally advanced BCC | 104 | Objective response rate in patients with metastatic and locally advanced BCC was 33.3% and 46.7% respectively | |
| Phase II (ERIVSNCE 30 month analysis)96 | Patients with metastatic BCC and locally advanced BCC | 104 | Objective response rate by investigator review: 48.5% for metastatic BCC and 60.3% for locally advanced BCC | |
| Phase II STEVIE97 | Patients with metastatic BCC and locally advanced BCC | 499 | Overall response in 66.7% of patients with advanced BCC and 37.9 % of patients with metastatic BCC | |
| Meta-analysis89 | Patients with metastatic BCC and locally advanced BCC | 744 | Objective response for locally advanced and metastatic BCC was 64.7% and 31.1%, respectively. Complete response was as 31.1% for locally advanced BCC and only 3.9% for metastatic BCC | |
| Sonidegib (LDE225) | Basal cell carcinoma outcomes with LDE 225 (BOLT) trial90 | Patients with metastatic BCC and locally advanced BCC | 94 | Objective response rate of 57.6% for locally advanced BCC and 7.7% for metastatic BCC |
| Topical Sonidegib (LDE225) | Phase II101 | Patients with Basal Cell Nevus Syndrome | 8 | Out of 13 LDE225-treated BCCs: 3 showed a complete, 9 a partial, and only 1 no clinical response |
| Itraconazole | Phase II91 | Patients with one or more BCC tumor >4 mm in diameter | 29 | 4 patients experienced partial response and four had stable disease |
| Saradegib- IPI-926 | Phase I92 | Patients with solid tumors including BCC | 94 | 8 of 28 BCC patients naïve to previous SMO inhibitor therapy showed a response to IPI-926 at doses ≥130 mg |
| TAK-441 | Phase I93 | Patients with solid tumors including BCC | 34 | Best response was partial (1 patient with BCC) and stable disease (7 patients with various solid tumors) |
BCC, basal cell carcinoma; SMO, smoothened.
Itraconazole, a systemic antifungal, is a HH signaling antagonist that inhibits SMO in a manner that is distinct from cyclopamine and vismodegib and prevents the ciliary accumulation of SMO.98 Posaconazole, a second generation triazole anti-fungal agent that inhibits HH signaling by a mechanism similar to itraconazole, exhibits activity against drug resistant SMO mutants.99 It has been proposed to be a better chemopreventative drug for suppressing BCC growth as it does not significantly affect the expression/activity of drug metabolizing cytochrome P450s as compared to itraconazole, which manifests drug-drug interactions99.
A preclinical study examining the topical efficacy of SMO antagonists using a depilated mouse model concluded that even a single application of LDE-225 was sufficient to inhibit GLI and PTCH1 expression, making it the most desirable agent for topical therapy.100 A phase II trial showed that topical LDE-225 in NBCCs patients was well-tolerated and caused BCC regression101 (Table 1).
6.2 | Resistance to SMO-targeted therapy
Despite the high efficacy of vismodegib, certain tumors exhibit increased potential for drug resistance and are more capable of evading SMO inhibitor therapy.16 In a retrospective medical chart review, out of 28 patients with metastatic and locally advanced BCCs treated with vismodegib, 21% regrew at least one tumor during treatment within a mean time of 56 weeks.16 Primary resistance in these trials was defined by BCC tumors that do not respond to therapy, possibly due to variant genes downstream of SMO.103 In other cases, patients with advanced BCCs that initially respond to SMO inhibitor treatment, develop secondary resistance to therapy, which is attributed to SMO mutations in the drug-binding domain.104
Atwood et al105 and Sharpe et al106 by comparing DNA sequences from sporadic BCCs, Gorlin syndrome patients, vismodegib sensitive and resistant tumors demonstrated that the majority of vismodegib resistance in BCC cells is caused by mutations in SMO. In these studies, SMO variants are found in 15–33% of untreated BCCs and in 69–77% of resistant tumors following therapy.105,106 The SMO mutations that occurred in or proximal to the drug binding pocket were only present in resistant BCCs implying that such mutations are specifically selected for during therapy.105,106 Some of the drug-binding site mutations include of D473, H231, W281, Q477, V321, I408, and C469.105,106 In contrast, SMO mutations distal to the drug binding domain were found in both untreated and SMO inhibitor resistant tumors suggesting their inherent role as oncodrivers.105,106 Mutations outside of the drug-binding site (such as T241M, A459V, L412F, S533N, and W535L) destabilize SMO, reduce affinity for the antagonist, and increase basal activity of SMO.105,106 Mutation W535 interacts with V321, L412F, and F460L to constitutively activate SMO.105,106 SMO mutant cells resistant to vismodegib continued to proliferate when treated with other chemically distinct SMO antagonists.106 This underlying cross-resistance between different inhibitors indicated that combination therapy utilizing multiple SMO antagonists may not be highly effective against emerging resistance.106 Interestingly in some cases, resistant tumors without SMO mutations are still associated with increased HH induced GLI expression.106 Such mutations are likely due to mutations occurring downstream of SMO at the level of GLI2 or SUFU.106 For this reason, multiple hereditary infundibulocystic basal cell carcinoma (MHIBCC), which is associated with a germline SUFU mutation, does not respond to SMO inhibitors that target upstream of the mutated SUFU gene in this case.107 Sharpe et al found a loss-of-function mutation in a PI3Kpathway regulator, phosphatase and tensin homologue (PTEN), which has been previously implicated in lowering response to vismodegib treatment in MB models.106,108 Highly vismodegib-resistant tumors, with or without SMO mutations, may be better treated through combination therapies with agents that directly target GLI downstream of SMO.109
6.3 | SMO antagonists and the development of squamous cell carcinoma
Findings in a case control study showed that patients exposed to vismodegib have an increased risk for the development of a non-BCC malignancy, especially SCC with a hazard ratio of 8.12 (95% CI, 3.89–16.97; P < 0.001).110 Additionally, in medulloblastoma models, the RAS/MAPK pathway offers an alternative to promote tumor growth by bypassing SHH signaling.111 Mutations in RAS/MAPK that likely develop following treatment with SMO inhibitors circumvent canonical HH inhibition, promote resistance and alter the subsequent tumor characteristics.111 Typically, canonical HH signaling inhibits ERK activation in the RAS/ MAPK pathway, leading to basaloid expression and SCC suppression.112 Noncanonical GLI activation promotes proliferation and the squamous cell phenotype in RAS mutated BCC by stimulating the EGFR-MEK-ERK axis.112 Hence, the inhibition of canonical HH signaling through SMO antagonists and the subsequent compensatory upregulation of the noncanonical pathway promotes the development of SCCs in BCC patients with concurrent mutations in the RAS/MAPK pathway.112 Similarly, RAS mutations in melanoma patients treated with BRAF inhibitors have been associated with the development of SCC, which is also associated with the concurrent upregulation of MAPK signaling.113
6.4 | GLI antagonists
In the model systems, tumor development may be efficaciously targeted by directly inhibiting GLI downstream of SMO. Agents that selectively target the transcription activities of GLI1/2 may make the most effective inhibitors.114 GLI antagonists such as GANT56 and GANT61 have been shown to mediate GLI-mediated gene activation. However, they have not been tested in models of BCCs. In other models where GLI expression is found to be augmented, GANT 61, a GLI 1/2 inhibitor, inhibits proliferation of GLI over-expressing cancer cells such as those derived from rhabdomyosarcoma, osteosarcoma, osteosarcoma, neuroblastoma, and ovarian cancer.115 GANT61 more effectively suppressed HH signaling in neuroblastoma cells as compared to SMO blockers.116 Similarly, GANT61 was able to inhibit breast cancer survival, mRNA expression of GLI1, nuclear translocation of GLI1, ERK1/2, MAPK signaling, and EGFR expression more so than SMO antagonist.117 GANT61 also inhibited rhabdomyosarcoma tumor proliferation by 50% in mouse models and significantly diminished AKT/mTOR signaling.118 Additionally, GANT61 increased apoptosis in pancreatic cancer stem cells by activating caspase-3 as well as suppressed EMT by increasing E-cadherin expression and inhibiting EMT regulating transcription factors snail, slug, and zeb1119 (Fig. 1).
Inhibition of DYRKIB (dual-specificity-phosphorylation-regulated kinase 1B), a positive regulator of HH/GLI signaling downstream of SMO, reduces GLI signaling in SMO resistant BCC cells.120 More recently, arsenic trioxide, which impedes HH signaling by blocking ciliary accumulation of GLI, reduces BCC development.121 The anti-tumor activity of arsenic trioxide also occurs through the inactivation of NOTCH1 targets, BCL2 and NF-κB.122
6.5 | Bromodomain inhibition
Bromo and extra C-terminal (BET) domain family are composed of multiple members (BRD2, BRD3, BRD4, BRDT, and others) that bind to acetylated histones to induce transcription.123 These proteins induce transcription by binding to N-ε-acetylysines on histone tails and forming critical complexes.123 The BET protein, BRDR4, can directly bind to GLI1 and GLI2 promoter sites to induce transcription.123 Despite SMO inhibitor resistance, the BET inhibitor, JQ1, decreases tumor cell growth by reversing the occupancy of BRD4 at the GLI promotor site in medulloblastoma, atypical rhabdoid tumor, and BCC cells.123 BET inhibition through JQ1 was also efficacious against SMO resistance mechanisms including mutations of SMO, SUFU, or amplification of GLI2 or MYC (Fig. 1).
Studies using multiple myeloma models have established that bromodomain proteins facilitate c-MYC dependent transcription.124 Targeting c-MYC driven transcription through JQ1 provides a therapeutic opportunity to inhibit c-MYC driven medulloblastoma and neuroblastoma.125,126 A recent report, describing that JQ1 inhibits neuroblastoma tumor growth and induce apoptosis by altering MYCN driven transcription, suggests that BET inhibitors may have the potential to target multiple MYC family members.126 As described previously in this review, MYCN has also been identified as a potential driver in BCC development.29,33 Hence, BET inhibitors may lead to decreased BCC tumor viability by down regulating MYCN transcription.
A recent study demonstrated that BRD4 inhibitors exert their cytotoxic effect by triggering BAX/BAK dependent intrinsic apopto-sis.127 For this reason, in the absence of pro-apoptotic proteins, BAX and BAK, malignant hemopoietic cells remained resistant to the effects of JQ1, both in vitro and in vivo.127 BIM, a BH3-only protein, is an inducer of mitochondrial apoptosis that inhibits antiapoptotic BCL2 proteins and activates pro-apoptotic proteins BAX and BAK.128 JQ1 treatment partly increased BIM levels by suppressing miR-17-92 cluster, a negative regulator of BIM.127 BET inhibition is able to suppress miR-17-92, a transcriptional target of c-myc, even in the absence of c-MYC suggesting that JQ1 may counter tumorigenesis by directly altering mRNA expression associated with BCC.127
A recent study showed that BRD4 is expressed throughout the brain and promotes the transcription of critical genes and synaptic proteins regulating learning and memory.129 Thus, JQ1 inhibition of BRD4 resulted in deficits in memory consolidation and also decreased the seizure threshold in mice.129 On the other hand, a more recent study showed the potential of JQ1 to actually reduce neuroinflammation in mice models of Alzheimer’s disease.130 Additional research to elucidate any potential neurological impairments will need to be considered while evaluating the therapeutic benefits and possible toxicity of JQ1 therapy in the treatment of BCC. Further investigation of GLI inhibition by targeting BRD4 in BCC would be a worthwhile approach at least in a combinatorial setting.
6.6 | Other epigenetic inhibitors
Various epigenetic regulators mediating HH signaling are found mutated in the progression of neoplastic growth.131 It is known that promoter hypermethylation of key players that promote the SHH and WNT pathways drive BCC growth.132 The acetylation of GLI1 and GLI2 inhibits their recruitment to target promoter site. Upon activation, upregulated histone deacetylase 1 and 2 (HDAC1 and 2) deacetylates GLI 1 and 2 and enhances their functions.133 HDAC inhibitors have shown efficacy in targeting various malignancies in clinical and preclinical trials; such as T-cell lymphoma, multiple myeloma, pancreatic, breast, cervical, ovarian thyroid, and nonsmall cell lung cancer.134,135 HDAC6, which is overexpressed in murine medulloblastoma cells, induces maximal HH activation by stabilizing GLI3 and enhancing GLI2 expression.136 HDAC6 blockade by antagonists tabacin, CAY10603, and ACY-1215 reduced tumor growth in vitro and in vivo in allograft models.136 Compound NL-103, which contains both the structural elements of vismodegib and the general HDAC inhibitor vorinostat, was able to overcome SMO resistance by concurrently inhibiting HDAC function and HH signaling.137 EZH2, a histone methyltransferase of the polycomb repressive complex 2, methylates lysine 27 on histone H3 and is associated with tumor suppressor silencing in medulloblastoma.138 EZH2 expression is also elevated in aggressive BCC subtypes and correlates with the proliferation marker Ki67.139 EZH2 may be a potential target to inhibit BCC progression. Moreover, promoter hypermethylation of important regulators in the SHH and WNT pathways contributes to BCC tumor promotion and inhibition of such key players may lead to novel treatment options132 (Fig. 1).
Sirtunins (SIRT), NADH-dependent deacetylase, are involved with DNA repair and offer protection against DNA damage and oxidative stress.140 Reduced expression of mRNA levels of two SIRT family members (SIRT 2 and 3) has been found in BCCs as compared to nontumoral skin.141 SIRT3, which has been shown to affect NOTCH levels in gastric tumors, may likely affect NOTCH expression in BCCs too and also affect HH signaling in the primary cilia.141,142
6.7 | Immunotherapy
Immunotherapy is currently considered to be an effective modality to manage neoplastic growth. Recently, regressing tumors were shown to have increased infiltration of CD3+CD4+ T-cells and an altered cytokine profile as compared to nonregressing BCCs, suggesting that immunotherapy may be a valuable therapeutic modality for this neoplasm.143 Initial efforts to apply immunotherapy to this context included of sensitization with dinitrobenzene and microbial allergens in combination with cytokines.144,145 A recent study attempted to assess the effectiveness of intralesional candida antigen in treating BCCs by stimulating an immune response.146 While such treatments have shown some potential, they have not been further explored due to lower efficacy as compared to the gold standard.147 Treatment of BCC with IFN alpha has been shown to stimulate IL2 and inhibit IL10, leading to tumor regression.148 This treatment modality requires multiple intralesional injections and can cause flu-like symptoms.149
Topical immune response modifier imiquimod 5% cream, which stimulates a cytokine response and activates Toll like receptors (TLR7/ 8), is a promising option.150 The resulting innate immune response enhances IFN, TNF, IL1, IL12 and further stimulates an acquired immune response through the activation of TH1 cells.150 IFN alpha mediates apoptosis by inhibiting the RAS-ERK pathway induced by HH signaling.151 Additionally, IFN causes BCC cells to express CD95 receptor so that CD95 receptor CD95 ligand interaction can induce apoptosis.152 Imiquimod has also been shown to inhibit GLI transcription through adenosine receptors (ADORAs) that are known to regulate proliferation, cell death, and signaling throughout the body.152 ADORA promotes PKA activity leading to the phosphorylation of GLI and the selection of the repressor form of GLI.152 Imiquimod has been shown to stimulate p53-induced apoptosis through increased ROS production and stimulation of the ATM/ATR.153 However, this mechanism may not operate in BCCs carrying mutant p53. Tumor cells exhibit decreased BCL2 expression following treatment with Imiquimod, making them vulnerable to apoptosis.150 Treatment with imiquimod results in a massive increase in macrophages surrounding and infiltrating tumor islets.150 Since BCC tumor cells do not express MHC class I molecules and subsequently lack infiltration by CD8 cells, local imiquimod treatment has been shown to upregulate MHC I on tumor cells along with a surge in CD8 cells.154 Imiquimod also leads to keratinocytes differentiation by activating the NOTCH pathway through the upregulation of JAGGED1.155 Multiple phase III clinical trials have shown the utility of imiquimod in the initial and long-term clearance of superficial BCCs along with favorable cosmetic outcomes.156–159 Adverse reactions associated with imiquimod include cutaneous psoriasiform eruptions, and oral ulcerations.160–162
Peripheral tissues and tumor cells express PDL-1 which binds with the PD-1 receptor on T cells to deliver an inhibitory signal that suppresses T cell proliferation and TCR mediated activation of IL2.163 Immune checkpoint blockade with antibodies targeting PD-1 and its ligand PD-L1 enhances the anti-tumor immune response mediated by T cells and counters the immune-suppressive microenvironment in tumors.164 Blockade of the PD-1 pathways with humanized anti-PD-1 monoclonal antibody, pembrolizumab, has shown a modest tumor response rate in patients with advanced melanoma.165 A recently published case report shared a case of patient with metastatic BCC who was treated with pembrolizumab.166 Following the cessation of therapy, metastatic lung lesions stabilized.166 A phase I clinical trial is currently studying if pembrolizumab can be used with or without vismodegib to treat metastatic or unresectable BCCs (NCT02690948). Macular popular rash, pruritus, lichenoid dermatitis or psoriasis are immune related adverse events observed following anti-PD-1/PDL1 therapy.167
Sustained activation of CTLA4, a negative regulator of T cell activation, induces immune tolerance to the oncogenic antigens.168 CTLA-4 blocking antibodies such as, ipilimumab, promote T-cell recognition of tumors such as melanoma and nonsmall cell lung cancer.169,170 Treatment for recurrent nodular melanoma with ipilimumab incidentally also led to the regression of concurrent advanced BCC.171 This surprising finding suggests that impilimumab may be an effective agent to counter CTLA-4 mediated immunotherapy in BCCs.171
The inflammatory response produced by COX-2 induced prostaglandins appears important in the development of BCC. COX-2 overexpression, induced by ROS following UV exposure could be linked with BCC tumor proliferation and promotion at least in part.172 Enhanced COX-2 expression increases anti-apoptosis, angiogenesis and tumorigenesis in BCC,173 while NSAIDs and COX-2 selective inhibitors suppress tumor progression by inhibiting acute inflammation, proliferation of keratinocytes, and activation of epithelial neutrophil infiltration.172 Celecoxib therapy has been shown to partially reduce BCC tumor burden in experimental animals and decrease the development of new BCCs in patients with Gorlin syndrome.174 Clinical trials have shown that celecoxib may be effective for the prevention of NMSC, including BCCs, in high-risk skin cancer patients.175 Result of a recent phase II clinical trial demonstrated that topical diclofenac therapy promotes tumor regression of superficial BCCs and alleviates anti-apoptotic and proliferative markers.176
6.8 | Photodynamic therapy
Photodynamic therapy (PDT) involves the application of a photosensitizing drug, such as aminolevulinic acid (ALA) or methylaminolevulinate, to the skin.149 The accumulated photosensitizing compounds are converted to protoporphyrin IX once absorbed into the epithelium.177 Treatment efficacy using photodynamic therapy is limited by the penetration of the topical photosensitizers.149 Following its accumulation within intracellular membranes of organelles, protoporphyrin IX is activated by visible light to produce cytotoxic ROS that results in tumor cell damage and killing.177 PDT therapy exerts its effects by directly damaging tumor cells, indirectly disrupting tumor vasculature, and activating the immune responses.178 PDT invokes an inflammatory response characterized by localized edema at the target site through the generation of damage-associated molecular patterns (DAMPs), cell death-associated molecular patterns (CDAMPS) and the stimulation of dendritic cells.179 It is typically used to treat superficial BCCs and results in favorable cosmetic outcomes.173 Various clinical studies have demonstrated the efficacy of PDT in the treatment of nodular and superficial BCCs.180–183
Verteporfin is a second generation photosensitizer that has been approved by the FDA for the treatment of age related macular degeneration.184 Verteporfin in addition to its light sensitivity shows significant pharmacological activity. Verteporfin alone has been shown to inhibit the proliferation of hepatocellular carcinoma and retinoblastoma cells by inhibiting the YAP pathway.185,186 Verteporfin therapy leads to a decrease in YAP nuclear localization resulting and a concurrent increase in cytosolic YAP via its trapping by 14-3-3σ which may occur in a p53-dependent manner29 (Fig. 1). It is interesting to note that limiting YAP signaling in the skin increases epidermal differentiation and dampens proliferation by accelerating apoptosis. Our group is currently studying the effects of verteprofin administered as monotherapy and in combination with SMO inhibitors on BCC tumor regression (unpublished data).
6.9 | Combination therapies
Combination therapies targeting canonical HH signaling along with key crosstalk pathways offer a synergistic strategy to curb tumor development. Treatment with gefitinib, an EGFR inhibitor, in combination with either cyclopamine or GANT61 reduced cell growth of BCC cell line more effectively than any of the agents individually.13 Cetuximab, a monoclonal antibody inhibits EGFR and has shown potential in targeting NMSC.187 It may also be evaluated for the treatment of advanced BCCs along with HH pathway inhibitors.187
Crosstalk involving PI3K and SHH has been linked with acquired resistance to SMO inhibition.188 Thus, combination therapy involving PI3K/mTOR inhibition along with HH inhibition may be an effective therapeutic strategy. Adding a PI3K class I inhibitor to a potent SMO antagonist delayed the development of resistance in a medulloblastoma mouse model.189 Dijkgraaf et al190 demonstrated this therapeutic synergy when they found that SMO resistant medulloblastoma was still sensitive to PI3K inhibition. Similarly, the combined inhibition of SHH and mTOR signaling pathways together with standard care chemotherapy is capable of eliminating pancreatic cancer stem cells both in vitro and in vivo.191 Co-treatment with GANT61 and PI3K/ mTOR inhibitor, PI103, at subtoxic concentrations synergistically inhibited tumor growth in SHH-driven rhabdomyosarcoma model.192 A pilot trial to examine the efficacy of erismodegib (SMO inhibitor) and buparlisib (PI3K inhibitor) in metastatic or advanced BCCs is already underway (NCT02303041).
Chaudhary et al24 showed that the combined inhibition of SHH signaling and the inflammatory response using a COX-2 inhibitor, sulindac, and SMO antagonist, itraconazole, respectively, in a Gorlin syndrome murine model inhibited the growth of UVB-induced BCCs by more than 90%. These authors elucidated that the p50 subunit of NF-κB engages BCL3, an atypical member of the IκB family, to mediate transcription program, which may be important in the pathogenesis of BCCs.24 Moreover, they also confirmed the crosstalk between SHH signaling and BCL3 pathways by demonstrating the efficacy of targeting this crosstalk in effectively eliminating BCCs.24
Kim et al193 showed that the combination of itraconazole and arsenic trioxide inhibited HH signaling driven growth of drug resistant BCC and medulloblastoma in vivo and in vitro. A recent clinical study with five patients undergoing itraconazole and arsenic trioxide treatment led to inhibition of the HH pathway by 75% compared to baseline; however, while some patients did experience tumor arrest, none noticed tumor shrinkage.194 The lack of clinical efficacy in this trial may be due to suboptimal dosing, the short treatment length (approximately 3 months) and the small sample size.194
PDT therapy may also induce tumor cell resistance due to the activation of NF-κB, MAPK, protein kinase B/AKT, PI3K, and COX-2 following therapy.178 As a result, combination therapies along with PDT may increase efficacy and counter resistance. Studies have shown that neoadjuvant PDT therapy prior to surgical excision may significantly lower tumor burden, and result in a surgical excision with histologically clear margins.195,196 Substantially improved clinical outcomes were observed when complementing PDT with imiquimod.197–199 Future studies examining the synergy between PDT and other small molecules that target various pathways associated with BCCs are needed to further explore the utility of these therapeutic modalities.200,201
7 | SUMMARY AND FUTURE PROSPECTS
Hedgehog signaling has been extensively studied as a key player in the pathogenesis of both sporadic BCCs and those linked with NBCCS. Crosstalk of the HH pathway with EGFR, TGFβ, aPKC, mTOR, PI3K, and NF-κB amongst others maximizes the GLI response that leads to BCC tumor growth and perhaps metastasis. Recent studies have identified additional mutations that take part in BCC development. For instance, aberrant activation of the oncogenic YAP promotes BCC through nuclear localization and transcriptional activation of YAP1. The ciliary accumulation of SMO, which is mediated by critical proteins and phosphoinositides, is central to the activation of GLI proteins. Primary cilia also regulate other signaling pathways that are associated with BCC, such as WNT, NOTCH, mTOR, and Hippo. Altered miRNAs expression levels are associated with BCC, suggesting the role of noncoding RNA regulation in tumor promotion. Various clinical trials have illustrated the limited efficacy of SMO inhibitors on tumor suppression (Table 1). Despite some success, resistance tends to develop following therapy with SMO antagonists paving the need for additional agents. SMO resistance can be partially overcome by targeting HH pathway downstream of SMO through agents like GANT61, DYRKIB, arsenic trioxide, and bromodomain inhibitors; although novel more effective and less toxic agents are required to achieve the desired therapeutic resistance. Exploration of epigenetic regulators that mediate HH signaling in the context of BCC genesis may provide additional options to curb tumor development. Immunotherapy through imiquimod, PD-1/ PD-L1 inhibitors, ipilimumab and COX-2 inhibitors together and can be evaluated as valuable treatment options. Photodynamic therapy, especially through novel agents such as verteporfin, should be explored in the context of BCCs. Many of these therapeutic strategies succeed in outplaying BCCs by promoting epidermal cell differentiation and inducing p53-mediated apoptosis. Therapeutic cocktails that combine the various treatment modalities and target multiple pathways associated with BCCs may offer the unique opportunity for treating metastatic and advanced BCCs. Additionally, the finding that differentially expressed miRNAs was found in vismodegib treated BCC tissue warrants additional investigations to confirm the role noncoding RNA in promoting tumor resistance.202 Future studies can explore strategies to modulate miRNAs that are associated with BCC pathogenesis and resistance. Finally, the discovery of mutations beyond HH could be important to develop therapies that target the novel additional driver signaling pathways that could be central to BCC growth progression despite targeting HH.
Acknowledgments
Funding information
NIH/NIEHS, Grant number: 1RO1ESO26219-01A1(MA); NIH/NCI, Grant number: 1RO1CA193885-01A1(CAE)
Abbreviations
- ADORAs
adenosine receptors
- AGO1
argonaute-1
- AGO2
Argonaute-2
- ALA
aminolevulinic acid
- aPKC
atypical protein kinase C
- BCC
basal cell carcinoma
- BET
bromo and extra C-terminal
- CDAMPS
cell death-associated molecular patterns
- COX-2
cyclooxygenase-2
- CTLA-4
cytotoxic T-lymphocyte protein 4
- DAMPs
damage-associated molecular patterns
- DGCR8
DiGeorge syndrome critical region gene 8
- DYRKIB
dual-specificity-phosphorylation-regulated kinase 1B
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal regulated kinases
- EVC
Ellis-van Creveld syndrome
- Gpr161
G-protein coupled receptor 161
- Gprasp2
G protein-coupled receptor associated sorting protein 2
- HDAC
histone deacetylase
- HH
hedgehog
- IFN
interferon
- IFT
intraflagellar transport
- IGF
insulin like growth factor
- IL
interleukin
- lncRNAs
long noncoding RNA
- Inpp5e
phosphoinositide 5-phosphatase
- IQCE
IQ domain-containing protein E
- KNSTRN
kinetochore-localized astrin/SPAG5 binding protein
- MAPK
mitogen-activated protein kinase
- MHIBCC
multiple hereditary infundibulocystic basal cell carcinoma
- MMP-9
matrix metalloproteinase-9
- MREs
microRNA response elements
- mTOR
mammalian target of rapamycin
- NBCCS
Nevoid basal cell carcinoma syndrome
- NMSC
nonmelanoma skin cancer
- NSAID
nonsteroidal anti-inflammatory drug
- PDL-1
programmed death-ligand
- PDT
photodynamic therapy
- PI(4)P
phosphatidylinositol 4-phosphate
- PI(4,5)P2
phosphatidylinositol 4,5 phosphate 2
- PKA
protein kinase A
- PTCH
patched
- RISC
RNA-induced silencing complexes
- ROS
reactive oxygen species
- SCC
squamous cell cancer
- SHH
sonic hedgehog
- SIRT
sirtuins
- SMO
smoothened
- SUFU
suppressor of fused
- TGF-β
transforming growth factor beta
- TLR
toll like receptors
- TNF
tumor necrosis factor
- UV
ultraviolet
- YAP
yes-associated protein.
Footnotes
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
References
- 1.Guy GP, Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green A. Changing patterns in incidence of non-melanoma skin cancer. Epithelial Cell Biol. 1992;1:47–51. [PubMed] [Google Scholar]
- 3.Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957–966. e952. doi: 10.1016/j.jaad.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 6.Axelson M, Liu K, Jiang X, et al. U.S. Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289–2293. doi: 10.1158/1078-0432.CCR-12-1956. [DOI] [PubMed] [Google Scholar]
- 7.Nishisgori C. Current concept of photocarcinogenesis. Photochem Photobiol Sci. 2015;14:1713–1721. doi: 10.1039/c5pp00185d. [DOI] [PubMed] [Google Scholar]
- 8.Athar M, Li C, Kim AL, Spiegelman VS, Bickers DR. Sonic hedgehog signaling in Basal cell nevus syndrome. Cancer Res. 2014;74:4967–4975. doi: 10.1158/0008-5472.CAN-14-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signaling in skin development and cancer. Exp Dermatol. 2006;15:667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Bresler SCA-Ohoo, Padwa BL, Granter SR. Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome) Head Neck Pathol. 2016;10:119–124. doi: 10.1007/s12105-016-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Danés A, Hannezo E, Larsimont JC, et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature. 2016;536:298–303. doi: 10.1038/nature19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Magno L, Coni S, Di Marcotullio L, Canettieri G. Digging a hole under Hedgehog: downstream inhibition as an emerging anticancer strategy. Biochim Biophys Acta. 2015;1856:62–72. doi: 10.1016/j.bbcan.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Schnidar H, Eberl M, Klingler S, et al. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasper M, Schnidar H, Neill GW, et al. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283–6298. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennler S, Andre J, Verrecchia F, Mauviel A. Cloning of the human GLI2 Promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J Biol Chem. 2009;284:31523–31531. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atwood SX, Chang AL, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199:193–197. doi: 10.1083/jcb.201207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noubissi FK, Kim T, Kawahara TN, et al. Role of CRD-BP in the growth of human basal cell carcinoma cells. J Invest Dermatol. 2014;134:1718–1724. doi: 10.1038/jid.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X, Poon R, Zhang X, et al. Suppressor of fused negatively regulates beta-catenin signaling. J Biol Chem. 2001;276:40113–40119. doi: 10.1074/jbc.M105317200. [DOI] [PubMed] [Google Scholar]
- 19.Maloverjan A, Piirsoo M, Michelson P, Kogerman P, Osterlund T. Identification of a novel serine/threonine kinase ULK3 as a positive regulator of Hedgehog pathway. Exp Cell Res. 2010;316:627–637. doi: 10.1016/j.yexcr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Varjosalo M, Bjorklund M, Cheng F, et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell. 2008;133:537–548. doi: 10.1016/j.cell.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and akt are essential for sonic hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo YA, Kang MH, Lee HJ, et al. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 23.De Craene B, Denecker G, Vermassen P, et al. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21:310–320. doi: 10.1038/cdd.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhary SC, Tang X, Arumugam A, et al. Shh and p50/Bcl3 signaling crosstalk drives pathogenesis of BCCs in gorlin syndrome. Oncotarget. 2015;6:36789–36814. doi: 10.18632/oncotarget.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiDonato JA, Mercurio M, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 26.Colavito SA, Zou MR, Yan Q, Nguyen DX, Stern DF. Significance of glioma-associated oncogene homolog 1 (GLI1) expression in claudin-low breast cancer and crosstalk with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkappaB) pathway. Breast Cancer Res. 2014;16:444. doi: 10.1186/s13058-014-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 31.Couzens AL, Knight JD, Kean MJ, et al. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci Signal. 2013;6:rs15. doi: 10.1126/scisignal.2004712. [DOI] [PubMed] [Google Scholar]
- 32.Swartling FJ. Myc proteins in brain tumor development and maintenance. Ups J Med Sci. 2012;117:122–131. doi: 10.3109/03009734.2012.658975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 34.Stacey SN, Helgason H, Gudjonsson SA, et al. New basal cell carcinoma susceptibility loci. Nat Commun. 2015;6:6825. doi: 10.1038/ncomms7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurek D, Garinis GA, van Doorninck JH, van der Wees J, Grosveld FG. Transcriptome and phenotypic analysis reveals Gata3-dependent signaling pathways in murine hair follicles. Development. 2007;134:261–272. doi: 10.1242/dev.02721. [DOI] [PubMed] [Google Scholar]
- 36.Jaju PD, Nguyen CB, Mah AM, et al. Mutations in the Kinetochore Gene KNSTRN in Basal Cell Carcinoma. J Invest Dermatol. 2015;135:3197–3200. doi: 10.1038/jid.2015.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovalenko A, Kim JC, Kang TB, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drummond IA. Cilia functions in development. Curr Opin Cell Biol. 2012;24:24–30. doi: 10.1016/j.ceb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol. 2014;24:R973–R982. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125:1109–1117. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehman JM, Laag E, Michaud EJ, Yoder BK. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol. 2009;129:438–448. doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croyle MJ, Lehman JM, O’Connor AK, et al. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development. 2011;138:1675–1685. doi: 10.1242/dev.060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141s. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boldt K, van Reeuwijk J, Lu Q, et al. An organelle-specific protein landscape identifies novel diseases and molecular mechanisms. Nat Commun. 2016;7:11491. doi: 10.1038/ncomms11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis WR, Malarkey EB, Tritschler D, et al. Mutation of growth arrest specific 8 reveals a role in motile cilia function and human disease. PLoS Genet. 2016;12:e1006220. doi: 10.1371/journal.pgen.1006220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Snedecor ER, Choi YJ, et al. Gorab is required for dermal condensate cells to respond to hedgehog signals during hair follicle morphogenesis. J Invest Dermatol. 2016;136:378–386. doi: 10.1016/j.jid.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malicki J, Avidor-Reiss T. From the cytoplasm into the cilium: bon voyage. Organogenesis. 2014;10:138–157. doi: 10.4161/org.29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilgendorf KI, Johnson CT, Jackson PK. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol. 2016;39:84–92. doi: 10.1016/j.ceb.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang N, Li L, Eguether T, Sundberg JP, Pazour GJ, Chen J. Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis. Development. 2015;142:2194–2202. doi: 10.1242/dev.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell. 2012;23:823–835. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pusapati GV, Hughes CE, Dorn KV, et al. EFCAB7 and IQCE regulate hedgehog signaling by tethering the EVC-EVC2 complex to the base of primary cilia. Dev Cell. 2014;28:483–496. doi: 10.1016/j.devcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Gonzalo FR, Phua SC, Roberson EC, et al. Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev Cell. 2015;34:400–409. doi: 10.1016/j.devcel.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann SN. Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell. 2015;34:338–350. doi: 10.1016/j.devcel.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Pal K, Hwang SH, Somatilaka B, et al. Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212:861–875. doi: 10.1083/jcb.201506132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang K, Liu Y, Fan J, et al. PI(4)P promotes phosphorylation and conformational change of smoothened through interaction with its C-terminal tail. PLoS Biol. 2016;14:e1002375. doi: 10.1371/journal.pbio.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung B, Padula D, Burtscher I, et al. Pitchfork and Gprasp2 target smoothened to the primary cilium for hedgehog pathway activation. PLoS ONE. 2016;11:e0149477. doi: 10.1371/journal.pone.0149477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuzhandaivel A, Schultz SW, Alkhori L, Alenius M. Cilia-mediated hedgehog signaling in Drosophila. Cell Rep. 2014;7:672–680. doi: 10.1016/j.celrep.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 62.May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia. 2012;1:7. doi: 10.1186/2046-2530-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerdes JM, Liu Y, Zaghloul NA, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 64.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong JH, Yang L, Dessaud E, et al. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev Cell. 2015;33:373–387. doi: 10.1016/j.devcel.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcet B, Chevalier B, Coraux C, Kodjabachian L, Barbry P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle. 2011;10:2858–2864. doi: 10.4161/cc.10.17.17011. [DOI] [PubMed] [Google Scholar]
- 67.Wang S, Livingston MJ, Su Y, Dong Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy. 2015;11:607–616. doi: 10.1080/15548627.2015.1023983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habbig S, Bartram MP, Muller RU, et al. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol. 2011;193:633–642. doi: 10.1083/jcb.201009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Habbig S, Bartram MP, Sagmuller JG, et al. The ciliopathy disease protein NPHP9 promotes nuclear delivery and activation of the oncogenic transcriptional regulator TAZ. Hum Mol Genet. 2012;21:5528–5538. doi: 10.1093/hmg/dds408. [DOI] [PubMed] [Google Scholar]
- 70.Itesako T, Seki N, Yoshino H, et al. The microRNA expression signature of bladder cancer by deep sequencing: the functional significance of the miR-195/497 cluster. PloS ONE. 2014;9:e84311. doi: 10.1371/journal.pone.0084311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertoli G, Cava C, Castiglioni I. MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer. Int J Mol Sci. 2016;17:421. doi: 10.3390/ijms17030421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inamura K, Ishikawa Y. MicroRNA in lung cancer: novel biomarkers and potential tools for treatment. J Clin Med. 2016:5. doi: 10.3390/jcm5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L, Nadeem L, Connor K, Xu G. Mechanisms and Therapeutic Targets of microRNA-associated Chemoresistance in Epithelial Ovarian Cancer. Curr Cancer Drug Targets. 2016;16:429–441. doi: 10.2174/1568009616666160404121105. [DOI] [PubMed] [Google Scholar]
- 74.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 75.Sand M, Skrygan M, Georgas D, et al. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2012;51:916–922. doi: 10.1002/mc.20861. [DOI] [PubMed] [Google Scholar]
- 76.Dalmay T. MicroRNAs and cancer. J Intern Med. 2008;263:366–375. doi: 10.1111/j.1365-2796.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 77.Sand M, Gambichler T, Skrygan M, et al. Expression levels of the microRNA processing enzymes Drosha and dicer in epithelial skin cancer. Cancer Invest. 2010;28:649–653. doi: 10.3109/07357901003630918. [DOI] [PubMed] [Google Scholar]
- 78.Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sand M, Skrygan M, Sand D, et al. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847–855. doi: 10.1111/j.1365-2133.2012.11022.x. [DOI] [PubMed] [Google Scholar]
- 80.Sonkoly E, Loven J, Xu N, et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis. 2012;1:e3. doi: 10.1038/oncsis.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heffelfinger C, Ouyang Z, Engberg A, et al. Correlation of global microRNA expression with basal cell carcinoma subtype. G3 (Bethesda) 2012;2:279–286. doi: 10.1534/g3.111.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saydam O, Shen Y, Wurdinger T, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zindy F, Kawauchi D, Lee Y, et al. Role of the miR-17 approximately 92 cluster family in cerebellar and medulloblastoma development. Biol Open. 2014;3:597–605. doi: 10.1242/bio.20146734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sand M, Bechara FG, Sand D, et al. Circular RNA expression in basal cell carcinoma. Epigenomics. 2016;8:619–632. doi: 10.2217/epi-2015-0019. [DOI] [PubMed] [Google Scholar]
- 85.Sand M, Bechara FG, Sand D, et al. Long-noncoding RNAs in basal cell carcinoma. Tumour Biol. 2016;37:10595–10608. doi: 10.1007/s13277-016-4927-z. [DOI] [PubMed] [Google Scholar]
- 86.Athar M, Li C, Tang X, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 87.Lipinski RJ, Hutson PR, Hannam PW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Sci. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morinello E, Pignatello M, Villabruna L, Goelzer P, Burgin H. Embryofetal development study of vismodegib, a hedgehog pathway inhibitor, in rats. Birth Defects Res B Dev Reprod Toxicol. 2014;101:135–143. doi: 10.1002/bdrb.21093. [DOI] [PubMed] [Google Scholar]
- 89.Jacobsen AA, Aldahan AS, Hughes OB, Shah VV, Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies. JAMA Dermatol. 2016;152:816–824. doi: 10.1001/jamadermatol.2016.0780. [DOI] [PubMed] [Google Scholar]
- 90.Dummer R, Guminski A, Gutzmer R, et al. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016;75:113–125. e115. doi: 10.1016/j.jaad.2016.02.1226. [DOI] [PubMed] [Google Scholar]
- 91.Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 92.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19:2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldman J, Eckhardt SG, Borad MJ, et al. Phase I dose-escalation trial of the oral investigational Hedgehog signaling pathway inhibitor TAK-441 in patients with advanced solid tumors. Clin Cancer Res. 2015;21:1002–1009. doi: 10.1158/1078-0432.CCR-14-1234. [DOI] [PubMed] [Google Scholar]
- 94.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021–1026. e1028. doi: 10.1016/j.jaad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 96.Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332. doi: 10.1186/s12885-017-3286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729–736. doi: 10.1016/S1470-2045(15)70198-1. [DOI] [PubMed] [Google Scholar]
- 98.Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866–876. doi: 10.1158/1535-7163.MCT-15-0729-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lauressergues E, Heusler P, Lestienne F, et al. Pharmacological evaluation of a series of smoothened antagonists in signaling pathways and after topical application in a depilated mouse model. Pharmacol Res Perspect. 2016;4:e00214. doi: 10.1002/prp2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of Basal cell carcinomas in nevoid Basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 102.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of Hedgehog pathway inhibitors in Basal cell carcinoma. Mol Cancer Ther. 2015;14:633–641. doi: 10.1158/1535-7163.MCT-14-0703. [DOI] [PubMed] [Google Scholar]
- 104.Pricl S, Cortelazzi B, Dal Col V, et al. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2015;9:389–397. doi: 10.1016/j.molonc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342–353. doi: 10.1016/j.ccell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharpe HJ, Pau G, Dijkgraaf GJ, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015;27:327–341. doi: 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schulman JM, Oh DH, Sanborn JZ, Pincus L, McCalmont TH, Cho RJ. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323–327. doi: 10.1001/jamadermatol.2015.4233. [DOI] [PubMed] [Google Scholar]
- 108.Metcalfe C, Alicke B, Crow A, et al. PTEN loss mitigates the response of medulloblastoma to Hedgehog pathway inhibition. Cancer Res. 2013;73:7034–7042. doi: 10.1158/0008-5472.CAN-13-1222. [DOI] [PubMed] [Google Scholar]
- 109.Ridky TW, Cotsarelis G. Vismodegib resistance in basal cell carcinoma: not a smooth fit. Cancer Cell. 2015;27:315–316. doi: 10.1016/j.ccell.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 110.Mohan SV, Chang J, Li S, Henry AS, Wood DJ, Chang AL. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527–532. doi: 10.1001/jamadermatol.2015.4330. [DOI] [PubMed] [Google Scholar]
- 111.Zhao X, Ponomaryov T, Ornell KJ, et al. RAS/MAPK activation drives Resistance to smo inhibition, metastasis, and tumor evolution in shh pathway-dependent tumors. Cancer Res. 2015;75:3623–3635. doi: 10.1158/0008-5472.CAN-14-2999-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rubben A, Hilgers RD, Leverkus M. Hedgehog blockade for basal cell carcinoma: coming at a (secondary neoplastic) price. JAMA Dermatol. 2016;152:521–523. doi: 10.1001/jamadermatol.2015.5239. [DOI] [PubMed] [Google Scholar]
- 113.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palle K, Mani C, Tripathi K, Athar M. Aberrant GLI1 activation in DNA damage response, carcinogenesis and chemoresistance. Cancers (Basel) 2015;7:2330–2351. doi: 10.3390/cancers7040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel) 2016;8:22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wickstrom M, Dyberg C, Shimokawa T, et al. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int J Cancer. 2013;132:1516–1524. doi: 10.1002/ijc.27820. [DOI] [PubMed] [Google Scholar]
- 117.Benvenuto M, Masuelli L, De Smaele E, et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/ GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 2016;7:9250–9270. doi: 10.18632/oncotarget.7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Srivastava RK, Kaylani SZ, Edrees N, et al. GLI inhibitor GANT-61 diminishes embryonal and alveolar rhabdomyosarcoma growth by inhibiting Shh/AKT-mTOR axis. Oncotarget. 2014;5:12151–12165. doi: 10.18632/oncotarget.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu J, Rodova M, Roy SK, et al. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22–32. doi: 10.1016/j.canlet.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gruber W, Hutzinger M, Elmer DP, et al. DYRK1B as therapeutic target in Hedgehog/GLI-dependent cancer cells with Smoothened inhibitor resistance. Oncotarget. 2016;7:7134–7148. doi: 10.18632/oncotarget.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J, Lee JJ, Kim J, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci USA. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xia J, Li Y, Yang Q, et al. Arsenic trioxide inhibits cell growth and induces apoptosis through inactivation of notch signaling pathway in breast cancer. Int J Mol Sci. 2012;13:9627–9641. doi: 10.3390/ijms13089627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tang Y, Gholamin S, Schubert S, et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodo-main inhibition. Nat Med. 2014;20:732–740. doi: 10.1038/nm.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Venkataraman S, Alimova I, Balakrishnan I, et al. Inhibition of BRD4 attenuates tumor cell self-renewal and suppresses stem cell signaling in MYC driven medulloblastoma. Oncotarget. 2014;5:2355–2371. doi: 10.18632/oncotarget.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puissant A, Frumm SM, Alexe G, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3:308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu Z, Sharp PP, Yao Y, et al. BET inhibition represses miR17-92 to drive BIM-initiated apoptosis of normal and transformed hematopoietic cells. Leukemia. 2016;30:1531–1541. doi: 10.1038/leu.2016.52. [DOI] [PubMed] [Google Scholar]
- 128.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27:S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Korb E, Herre M, Zucker-Scharff I, Darnell RB, Allis CD. BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat Neurosci. 2015;18:1464–1473. doi: 10.1038/nn.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Magistri M, Velmeshev D, Makhmutova M, et al. The BET-bromodomain inhibitor JQ1 reduces inflammation and tau phosphorylation at Ser396 in the brain of the 3xTg model of Alzheimer’s disease. Curr Alzheimer Res. 2016;13:985–995. doi: 10.2174/1567205013666160427101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu D, Xie J. Non-canonical Hh signaling in cancer-current understanding and future directions. Cancers (Basel) 2015;7:1684–1698. doi: 10.3390/cancers7030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brinkhuizen T, van den Hurk K, Winnepenninckx VJ, et al. Epigenetic changes in Basal Cell Carcinoma affect SHH and WNT signaling components. PloS ONE. 2012;7:e51710. doi: 10.1371/journal.pone.0051710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coni S, Antonucci L, D’Amico D, et al. Gli2 acetylation at lysine 757 regulates hedgehog-dependent transcriptional output by preventing its promoter occupancy. PloS ONE. 2013;8:e65718. doi: 10.1371/journal.pone.0065718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoon S, Eom GH. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J. 2016;52:1–11. doi: 10.4068/cmj.2016.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 136.Dhanyamraju PK, Holz PS, Finkernagel F, Fendrich V, Lauth M. Histone deacetylase 6 represents a novel drug target in the oncogenic Hedgehog signaling pathway. Mol Cancer Ther. 2015;14:727–739. doi: 10.1158/1535-7163.MCT-14-0481. [DOI] [PubMed] [Google Scholar]
- 137.Zhao J, Quan H, Xie C, Lou L. NL-103, a novel dual-targeted inhibitor of histone deacetylases and hedgehog pathway, effectively overcomes vismodegib resistance conferred by Smo mutations. Pharmacol Res Perspect. 2014;2:e00043. doi: 10.1002/prp2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smits M, van Rijn S, Hulleman E, et al. EZH2-regulated DAB2IP is a medulloblastoma tumor suppressor and a positive marker for survival. Clin Cancer Res. 2012;18:4048–4058. doi: 10.1158/1078-0432.CCR-12-0399. [DOI] [PubMed] [Google Scholar]
- 139.Rao RC, Chan MP, Andrews CA, Kahana A. EZH2, proliferation rate, and aggressive tumor subtypes in cutaneous basal cell carcinoma. JAMA Oncol. 2016;2:962–963. doi: 10.1001/jamaoncol.2016.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852:2442–2455. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Temel M, Koc MN, Ulutas S, Gogebakan B. The expression levels of the sirtuins in patients with BCC. Tumour Biol. 2016;37:6429–6435. doi: 10.1007/s13277-015-4522-8. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, Wang WY, Cao LP. SIRT3 inhibits cell proliferation in human gastric cancer through down-regulation of Notch-1. Int J Clin Exp Med. 2015;8:5263–5271. [PMC free article] [PubMed] [Google Scholar]
- 143.Hunt MJ, Halliday GM, Weedon D, Cooke BE, Barnetson RS. Regression in basal cell carcinoma: an immunohistochemical analysis. Br J Dermatol. 1994;130:1–8. doi: 10.1111/j.1365-2133.1994.tb06873.x. [DOI] [PubMed] [Google Scholar]
- 144.Klein E. Immunotherapeutic approaches to skin cancer. Hosp Pract. 1976;11:107–116. doi: 10.1080/21548331.1976.11707033. [DOI] [PubMed] [Google Scholar]
- 145.Holtermann OA, Papermaster B, Rosner D, Milgrom H, Klein E. Regression of cutaneous neoplasms following delayed-type hypersensitivity challenge reactions to microbial antigens or lymphokines. J Med. 1975;6:157–168. [PubMed] [Google Scholar]
- 146.Aftergut K, Curry M, Cohen J. Candida antigen in the treatment of basal cell carcinoma. Dermatologic Surg. 2005;31:16–18. doi: 10.1111/j.1524-4725.2005.31002. [DOI] [PubMed] [Google Scholar]
- 147.Ghafouri-Fard S, Ghafouri-Fard S. Immunotherapy in nonmelanoma skin cancer. Immunotherapy. 2012;4:499–510. doi: 10.2217/imt.12.29. [DOI] [PubMed] [Google Scholar]
- 148.Kim J, Modlin RL, Moy RL, et al. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 149.Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. 2007;4:462–469. doi: 10.1038/ncponc0883. [DOI] [PubMed] [Google Scholar]
- 150.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Arch Dermatol. 2003;139:1325–1332. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 151.Li C, Chi S, He N, et al. IFNalpha induces Fas expression and apoptosis in hedgehog pathway activated BCC cells through inhibiting Ras-Erk signaling. Oncogene. 2004;23:1608–1617. doi: 10.1038/sj.onc.1207273. [DOI] [PubMed] [Google Scholar]
- 152.Wolff F, Loipetzberger A, Gruber W, Esterbauer H, Aberger F, Frischauf AM. Imiquimod directly inhibits Hedgehog signalling by stimulating adenosine receptor/protein kinase A-mediated GLI phosphorylation. Oncogene. 2013;32:5574–5581. doi: 10.1038/onc.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang SW, Chang SH, Mu SW, et al. Imiquimod activates p53-dependent apoptosis in a human basal cell carcinoma cell line. J Dermatol Sci. 2016;81:182–191. doi: 10.1016/j.jdermsci.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 154.Walter A, Barysch MJ, Behnke S, et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16:3562–3570. doi: 10.1158/1078-0432.CCR-09-3136. [DOI] [PubMed] [Google Scholar]
- 155.Wuest M, Dummer R, Urosevic M. Induction of the members of Notch pathway in superficial basal cell carcinomas treated with imiquimod. Arch Dermatol Res. 2007;299:493–498. doi: 10.1007/s00403-007-0785-2. [DOI] [PubMed] [Google Scholar]
- 156.Schulze HJ, Cribier B, Requena L, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol. 2005;152:939–947. doi: 10.1111/j.1365-2133.2005.06486.x. [DOI] [PubMed] [Google Scholar]
- 157.Ezughah FI, Dawe RS, Ibbotson SH, Fleming CJ. A randomized parallel study to assess the safety and efficacy of two different dosing regimens of 5% imiquimod in the treatment of superficial basal cell carcinoma. J Dermatolog Treat. 2008;19:111–117. doi: 10.1080/09546630701557965. [DOI] [PubMed] [Google Scholar]
- 158.Quirk C, Gebauer K, De’Ambrosis B, Slade HB, Meng TC. Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: Results of a prospective 5-year study. Cutis. 2010;85:318–324. [PubMed] [Google Scholar]
- 159.Gollnick H, Barona CG, Frank RG, et al. Recurrence rate of superficial basal cell carcinoma following treatment with imiquimod 5% cream: Conclusion of a 5-year long-term follow-up study in Europe. Eur J Dermatol. 2008;18:677–682. doi: 10.1684/ejd.2008.0519. [DOI] [PubMed] [Google Scholar]
- 160.Brown RS, Farquharson AA. A topical imiquimod-induced oral mucosal lichenoid reaction: a case report. J Am Dent Assoc. 2014;145:1141–1145. doi: 10.14219/jada.2014.88. [DOI] [PubMed] [Google Scholar]
- 161.Patel U, Mark NM, Machler BC, Levine VJ. Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br J Dermatol. 2011;164:670–672. doi: 10.1111/j.1365-2133.2010.10124.x. [DOI] [PubMed] [Google Scholar]
- 162.Smith WA, Siegel D, Lyon VB, Holland KE. Psoriasiform eruption and oral ulcerations as adverse effects of topical 5% imiquimod treatment in children: a report of four cases. Pediatr Dermatol. 2013;30:e157–e160. doi: 10.1111/j.1525-1470.2012.01780.x. [DOI] [PubMed] [Google Scholar]
- 163.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 164.Zheng P, Zhou Z. Human Cancer Immunotherapy with PD-1/PD-L1 Blockade. Biomark Cancer. 2015;7:15–18. doi: 10.4137/BIC.S29325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 166.Winkler JK, Schneiderbauer R, Bender C, et al. Anti-programmed cell death-1 therapy in nonmelanoma skin cancer. Br J Dermatol. 2017;176:498–502. doi: 10.1111/bjd.14664. [DOI] [PubMed] [Google Scholar]
- 167.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 168.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 169.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 171.Mohan SV, Kuo KY, Chang AL. Incidental regression of an advanced basal cell carcinoma after ipilimumab exposure for metastatic melanoma. JAAD Case Rep. 2016;2:13–15. doi: 10.1016/j.jdcr.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Muller-Decker K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011;30:343–361. doi: 10.1007/s10555-011-9306-z. [DOI] [PubMed] [Google Scholar]
- 173.Tjiu JW, Liao YH, Lin SJ, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–1151. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]
- 174.Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/− humans and mice. Cancer Prev Res (Phila) 2010;3:25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brinkhuizen T, Frencken KJ, Nelemans PJ, et al. The effect of topical diclofenac 3% and calcitriol 3 mμg/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J Am Acad Dermatol. 2016;75:126–134. doi: 10.1016/j.jaad.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 177.Szpringer E, Lutnicki K, Marciniak A. Photodynamic therapy—mechanism and employment. Ann Univ Mariae Curie Sklodowska Med. 2004;59:498–502. [PubMed] [Google Scholar]
- 178.Lucena SR, Salazar N, Gracia-Cazana T, et al. Combined treatments with photodynamic therapy for non-melanoma skin cancer. Int J Mol Sci. 2015;16:25912–25933. doi: 10.3390/ijms161025912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tehranchinia Z, Rahimi H, Ahadi MS, Ahadi MS. Aminolevulinic Acid-photodynamic therapy of Basal cell carcinoma and factors affecting the response to treatment: a clinical trial. Indian J Dermatol. 2013;58:327. doi: 10.4103/0019-5154.113968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Surrenti T, De Angelis L, Di Cesare A, Fargnoli MC, Peris K. Efficacy of photodynamic therapy with methyl aminolevulinate in the treatment of superficial and nodular basal cell carcinoma: an open-label trial. Eur J Dermatol. 2007;17:412–415. doi: 10.1684/ejd.2007.0239. [DOI] [PubMed] [Google Scholar]
- 182.Szeimies RM, Ibbotson S, Murrell DF, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol. 2008;22:1302–1311. doi: 10.1111/j.1468-3083.2008.02803.x. [DOI] [PubMed] [Google Scholar]
- 183.Rhodes LE, de Rie M, Enstrom Y, et al. Photodynamic therapy using topical methyl aminolevulinate vs surgery for nodular basal cell carcinoma: results of a multicenter randomized prospective trial. Arch Dermatol. 2004;140:17–23. doi: 10.1001/archderm.140.1.17. [DOI] [PubMed] [Google Scholar]
- 184.Henney JE. From the food and drug administration. JAMA. 1999;282:1995. [PubMed] [Google Scholar]
- 185.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brodowska K, Al-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Della Vittoria Scarpati G, Perri F, Pisconti S, et al. Concomitant cetuximab and radiation therapy: a possible promising strategy for locally advanced inoperable non-melanoma skin carcinomas. Mol Clin Oncol. 2016:4467–471. doi: 10.3892/mco.2016.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget. 2015;6:13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 191.Mueller MT, Hermann PC, Witthauer J, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 192.Graab U, Hahn H, Fulda S. Identification of a novel synthetic lethality of combined inhibition of hedgehog and PI3K signaling in rhabdomyosarcoma. Oncotarget. 2015;6:8722–8735. doi: 10.18632/oncotarget.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452–456. doi: 10.1001/jamadermatol.2015.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jeremic G, Brandt MG, Jordan K, Doyle PC, Yu E, Moore CC. Using photodynamic therapy as a neoadjuvant treatment in the surgical excision of nonmelanotic skin cancers: prospective study. J Otolaryngol Head Neck Surg. 2011;40:S82–S89. [PubMed] [Google Scholar]
- 196.Torres T, Fernandes I, Costa V, Selores M. Photodynamic therapy as adjunctive therapy for morpheaform basal cell carcinoma. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:23–25. [PubMed] [Google Scholar]
- 197.Devirgiliis V, Panasiti V, Curzio M, et al. Complete remission of nodular basal cell carcinoma after combined treatment with photodynamic therapy and imiquimod 5% cream. Dermatol Online J. 2008;14:25. [PubMed] [Google Scholar]
- 198.Osiecka B, Jurczyszyn K, Ziolkowski P. The application of Levulan-based photodynamic therapy with imiquimod in the treatment of recurrent basal cell carcinoma. Med Sci Monit. 2012;18:PI5–9. doi: 10.12659/MSM.882449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Requena C, Messeguer F, Llombart B, Serra-Guillen C, Guillen C. Facial extensive recurrent basal cell carcinoma: successful treatment with photodynamic therapy and imiquimod 5% cream. Int J Dermatol. 2012;51:451–454. doi: 10.1111/j.1365-4632.2011.05293.x. [DOI] [PubMed] [Google Scholar]
- 200.Weyergang A, Selbo PK, Berg K. Sustained ERK inhibition by EGFR targeting therapies is a predictive factor for synergistic cytotoxicity with PDT as neoadjuvant therapy. Biochim Biophys Acta. 2013;1830:2659–2670. doi: 10.1016/j.bbagen.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 201.Akita Y, Kozaki K, Nakagawa A, et al. Cyclooxygenase-2 is a possible target of treatment approach in conjunction with photodynamic therapy for various disorders in skin and oral cavity. Br J Dermatol. 2004;151:472–480. doi: 10.1111/j.1365-2133.2004.06053.x. [DOI] [PubMed] [Google Scholar]
- 202.Sand M, Bechara FG, Gambichler T, et al. Next-generation sequencing of the basal cell carcinoma miRNome and a description of novel microRNA candidates under neoadjuvant vismodegib therapy: an integrative molecular and surgical case study. Ann Oncol. 2016;27:332–338. doi: 10.1093/annonc/mdv551. [DOI] [PubMed] [Google Scholar]