Summary
Stereotaxic surgery is an invaluable tool to deliver a variety of gene therapy constructs to the non-human primate caudate and putamen in pre-clinical studies for the genetic, neurodegenerative disorder, Huntington’s disease (HD). Here we describe in detail how to perform this technique beginning with a pre-surgical magnetic resonance imaging scan to determine surgical coordinates followed by the stereotaxic surgical injection technique. In addition, we include methodology of a full necropsy including brain and peripheral tissue removal and a standard immunohistochemical technique to visualize the injected gene therapy agent.
Keywords: Huntington’s disease, Neurodegeneration, Stereotaxic surgery, Magnetic Resonance Imaging, Non-human Primate, Necropsy, Immunohistochemistry
1. Introduction
Huntington’s disease (HD) is a genetic, neurological disorder that is characterized by a devastating array of symptoms including a hyperkinetic movement disorder, changes in cognitive capabilities, psychiatric manifestations and a robust metabolic syndrome (1). Many of these symptoms are caused by neuronal loss in the caudate and putamen that results from a mutated HTT gene containing an abnormally long stretch of the DNA bases cytosine, adenine and guanine (CAG) (1, 2). Because of the dramatic cell loss observed in these brain regions, the caudate and putamen have historically been the go-to target structures in both pre-clinical studies as well as clinical trials investigating potential therapeutic strategies to treat HD.
Stereotaxic surgery is a powerful tool that has been used for decades to accurately inject therapeutic agents into different brain regions in a variety of different animal species. Prior to the identification of the mutant, disease-causing HTT gene in 1993 (1), stereotaxic surgery was commonly used to deliver excitotoxins into the non-human primate (NHP) caudate and putamen to create a large animal model of HD that replicated some of the key symptoms of the disease (3–6). More recently, lentiviral vectors (LVs) (7) and adeno-associated viral vectors (AAVs) (ongoing studies in our laboratory) have been used to deliver mutant HTT into the caudate and putamen, replicating many of the neuropathological and behavioral changes seen in humans with HD. Pre-clinical studies have employed stereotaxic surgical targeting of the caudate and putamen to evaluate potential therapeutic strategies in some of these NHP models including fetal tissue transplants and trophic factor administration (8–11). More recently, stereotaxic surgery has been used to target the naïve NHP putamen to assess the safety of partially reducing HTT expression via 1) AAVs expressing microRNA constructs (12, 13) or siRNAs delivered through a cannula placed into the putamen (14). With the recent creation of a transgenic HD monkey bearing a fragment of human mutant HTT (15), it is anticipated that stereotaxic surgery will be used to evaluate a variety of therapeutics in this NHP model in the near future.
Here, we describe in detail the methodology that our laboratory uses to surgically target the NHP caudate and putamen to deliver both mutant HTT (disease modeling) as well as gene therapeutics (AAV-RNAi) including 1) a pre-surgical magnetic resonance imaging (MRI) scan to determine surgical coordinates, 2) the stereotaxic surgical injection procedure including anesthesia monitoring and pain medication delivery, 3) post-surgical necropsy including brain, blood and peripheral tissue collection and 4) immunohistochemical processing of brain sections to evaluate the expression and spread of the injected agent.
2. Materials
2.1 Pre-surgical magnetic resonance imaging (MRI)
Personal protective equipment including scrubs, a surgical face mask, protective eye wear, nitrile gloves, a hair net, shoe covers and a water-resistant gown
MRI machine (1.5T or 3T) and associated imaging software
Ketamine HCl
Glycopyrrolate
Isoflurane anesthesia machine on a mobile, wheeled cart or transport board
Shaver
Endotracheal tube (4.0 to 5.5 mm i.d.) and umbilical tape
Ophthalmic ointment
22G cephalic vein catheter
Pulse oximeter
Heart rate monitor
Animal heating mechanisms (towels, water blanket, etc)
MRI-compatible non-human primate head frame containing ear bars, eye bars and a palate bar
Mineral oil to fill the ear bars
Allen wrenches of various sizes
Fiducial marker (Vitamin E capsule)
Surgical coordinate sheets
MRI surface/head coil
2.2 Stereotaxic injection into the caudate and putamen
PPE including scrubs, hair bonnets, water resistant gown, surgical masks, protective eyewear and gloves (surgical staff) as well as sterile gowns and sterile surgical gloves (surgeons)
Anesthesia cart and physiologic monitoring system
Ethylene oxide sterilizing system
Infusate (viral vector, cells, etc)
Bucket of ice or cooling block if infusate must remain cold prior to injection
Primate head frame
Sterile micromanipulator
Sterile Allen wrenches
Sterile T-square (PolySquare™)
Sterile Hamilton infusion syringes (10–100 ul or appropriate volume for infusion)
Sterile Hamilton surgical needles (22–25G, 25–38mm in length, blunt tip style)
Sterile stereotaxic infusion pump and controller box
Sterile AP Zeroing bar
Sterile towels and a sterile craniotomy drape
ChloraPrep preoperative skin preparation
Hydromorphone (2 mg/ml)
Cafazolin (250 mg/ml)
Buprenorphine (0.3 mg/ml)
Lidocaine (1%) and epinephrine
Bupivicaine (0.5%)
1 and 3 cc syringes with 25G needles
Lactated Ringer’s solution
Warm forced air system (Bair hugger) and warm water bags
Sterile Parafilm (several 2”x2” squares)
Sterile surgical drill and drill bit (2mm round, carbide burr)
Sterile gauze
Sterile surgical cotton swabs
Sterile pipettors and appropriately sized sterile pipette tips
Sterile surgical instruments (Adson forceps, scalpels, scalpel holders, hemostats, scissors, micro-dissection scissors, rongeurs, needle holders, elevators, Gelpi retractors
Sterile 22G needle
Sterile Sharpie marker
Sterile saline
Sterile 30ml syringe
Sterile suction tip and suction machine
Sterile Gelfoam
Sterile 4-0 Monocryl and 3-0 Vicryl suture
Biohazard waste container
Calculator, pencils and surgical coordinate sheets
2.3 Necropsy and tissue collection
PPE: Scrubs, water resistant gown, hair bonnet, nitrile gloves, facemask, protective eye wear
Peristaltic perfusion pump
Polyethylene tubing for perfusion pump (inside diameter: ¼ inch) attached to a 13G cannula
Board & masking tape for securing animal
Scalpel and scalpel blades, Metzenbaum scissors, forceps, assorted sizes of hemostats, heavy duty scissors, bone lever
Bottles of 70% EtOH and saline
Cutting boards
Weigh boats for organs
60cc syringe with 18 gauge needle for drawing terminal serum
Supply of scalpel blades, #22, blunt & sharp
Supply of 4×4 gauze
Pruning shears
T-bar
Bone cutting forceps
Pens (sharpie)/pencils for labeling tubes and foil packs
2 4ml serum blood tubes
Assorted syringes (1cc, 3cc, 5cc, 20cc, 30cc, 60cc) 7 needles (18–25G, 1” to 1.5”)
Tourniquet
Sharps containers
Waste disposal bags
Hand saw
Sodium pentobarbital
Ketamine hydrochloride
0.9% sterile saline
Paraformaldehyde
Sodium Phosphate Monobasic Monohydrate NaH2PO4 (FW137.99)
Sodium Phosphate Dibasic Anhydrous Na2HPO4 (FW141.96)
4% paraformaldehyde (40g Paraformaldehyde, 3.2g monobasic, 10.9g dibasic, 1L dH2O)
Ice bucket with wet ice
Dewar with liquid nitrogen
Aluminum foil packs labeled with ID, date & tissue for tissue bank
Microcentrifuge tubes (2.0ml with screw cap & o-ring/sterilized)
Brain jar on ice filled with sterile saline
Small jars on ice filled with sterile saline
Tissue biopsy cores (2mm)
Rhesus brain matrix
Tissue slicer blades
Sterile petri dishes
2.4 Cutting and immunohistochemical processing of brain
PPE: White lab coat, nitrile gloves, protective eye wear
Sliding microtome and microtome blade
Paint brushes for tissue cutting
Dry ice
Trizma® pre-set crystals (pH7.4, avg Mw: 151.6)
NaCl (FW58.44)
Triton®X-100
Sodium Phosphate Dibasic Anhydrous Na2HPO4 (FW141.96)
Sodium Phosphate Monobasic Monohydrate NaH2PO4 (FW137.99)
Serum (goat or donkey)
Sodium meta-Periodate INaO4 (FW213.89)
DAB (FW360.11)
Nickel(II) sulfate hexahydrate (FW262.85)
Vectastain® Elite® ABC Kit
Dilution media (7.46g Trizma, 8.77g NaCl, 0.5 ml TritonX-100, 1L dH2O)
TBS (7.46g Trizma, 8.77g NaCl, 1L dH2O)
PBS (5.47g Dibasic, 1.60 Monobasic, 9.26g NaCl, 1L dH2O)
Cryoprotectant solution (300g Sucrose, 300 mls ethylene glycol, 0.2g Sozium Azide, 500 mls PBS)
Netted staining dishes (with glass dishes to contain fluid)
Orbital shaker
Appropriate primary and secondary antibodies
6 well tissue culture plates
Large 24 well (4×6) compartmented tissue collection box
3. Methods
3.1 Pre-surgical magnetic resonance imaging (MRI)
Sedate the animal while in its home cage with 10–20 mg/kg Ketamine combined with 0.01–0.02 mg/kg Glycopyrrolate (intra-muscular-IM) and bring to the pre-operative procedure room (food withheld for 12 hours prior to MRI and surgery). Shave animal’s head from the brow ridge caudally to the foramen magnum and laterally down to the lateral canthus of each eye, place ophthalmic ointment in each eye, shave the left forearm and establish a 22G cephalic vein catheter for agent administration during surgery.
Place an endotracheal tube (for adult rhesus macaques, 4.0 to 5.5 mm i.d. endotracheal tubes are typically used), secure tube with umbilical tape and start the animal on 1.5% isoflurane. While waiting to go into MRI, monitor the animal’s pulse rate, blood oxygen saturation, and ETC02 levels.
Bring the animal to the MRI facility on a wheeled cart under isoflurane anesthesia.
In the MRI procedure room, place the animal’s head into the MRI-compatible stereotaxic head frame (Figure 1A) while still connected to the breathing tube and under anesthesia (Note 1). First place the animal in the earbars (filled with contrast dye or mineral oil) such that there is free movement of the animals head up and down (nodding motion), but no movement laterally. Center animal in the head frame both medial-laterally and dorso-ventrally. Next, place the palate bar into the animal’s mouth, resting against the hard palate, and secure into position such that the animal’s head is straight and its eyes are facing forward. Finally, secure the eye bars such that they fit tightly in the ventral grooves of the orbital socket. Ensure that ear bars, palate bar and eye bars are all secured in place.
Disconnect the animal’s breathing tube from the anesthesia machine and quickly transfer the animal placed in the head frame to the MRI table (still prone and head first) and reconnect to the anesthesia machine located in the MRI unit (Note 2).
Place a circulating warm water pad and towels under the animal on the MRI table and place extra towels over the animal order to maintain body temperature.
Attach peripheral physiological (pulse rate/oxygen saturation, ETCO2, respiration, and/or non-invasive blood pressure) monitors to the animal (digit cuffs) and observe for accuracy and reliability of readings. From the control room, an MRI technician should continue to monitor the animal’s respiratory rate, pulse rate, end tidal CO2 and oxygen saturation (Note 3).
Tape a fiducial marker to right side of the animal’s head to use as a reference on the MR image (Note 4).
Place the surface/head coil (Figure 1B) directly above and parallel to the animal’s head such that it is centered over the scanning area of interest (caudate and/or putamen, approximately 12–25 mm in front of the ear bars) for HD gene therapy studies. The coil should be approximately 0.5–1.0 cm above the surface of the head but not touching the skin. (Note 5). Center the animal’s head on MRI table using the laser crosshair feature on the MRI scanner and then send the animal into the center of the magnet.
First collect a quick, single-slice, three-axis localizer scan that gives a view of the animal’s head in the three scanner-frame axes (voxel size1.0 × 1.0 × 1.0). This ensures that the coil is functioning properly and allows for further refinement around the scanning area of interest (1–2 minute scan).
Next collect a T1-weighted structural scan (MPRAGE) scan to establish the surgical coordinates (voxel size: 0.5 × 0.5 × .05, 12 minute scan, Note 6). Coordinates are determined from coronal scans using the MRI software Distance Tool as follows: 1) Anterior/Posterior (AP) determined by establishing the MR image containing your injection region of interest (ie caudate or putamen) and calculating the distance (in millimeters (mm)) in front of or behind the MR image containing the earbars (earbar contrast dye is evident on scan), 2) Medial/Lateral (ML) established by determining the distance (mm) from the center of the two hemispheres to the proposed injection site (draw a vertical line through the center of the sagittal sinus vein as a reference) and 3) Dorsal/Ventral (DV) established by determining the distance from the pial surface to the proposed injection site (Figure 2, red asterisks indicates the sagittal sinus vein and the blue dot indicates the injection site in the rhesus putamen, Note 7). Record surgical coordinates on a piece of paper that will go with you into the operating room.
When the scan is finished, the animal can be removed from the magnet, disconnected from physiological monitors and anesthesia tubing, placed back onto the mobile anesthesia cart, reconnected to anesthesia on cart and wheeled into the operating room.
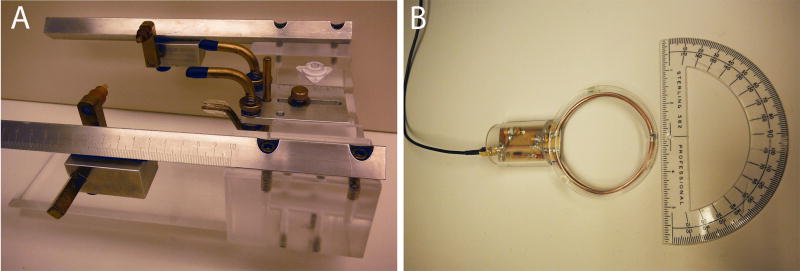
Figure 1.
Pre-surgical MRI to determine surgical coordinates. (A) Animals are anesthetized and fitted into an MRI-compatible stereotaxic head frame by first securing the animal into the earbars, followed by the palate and eye bars. (B) The scan is obtained using a surface coil that sits directly above the animal’s head.
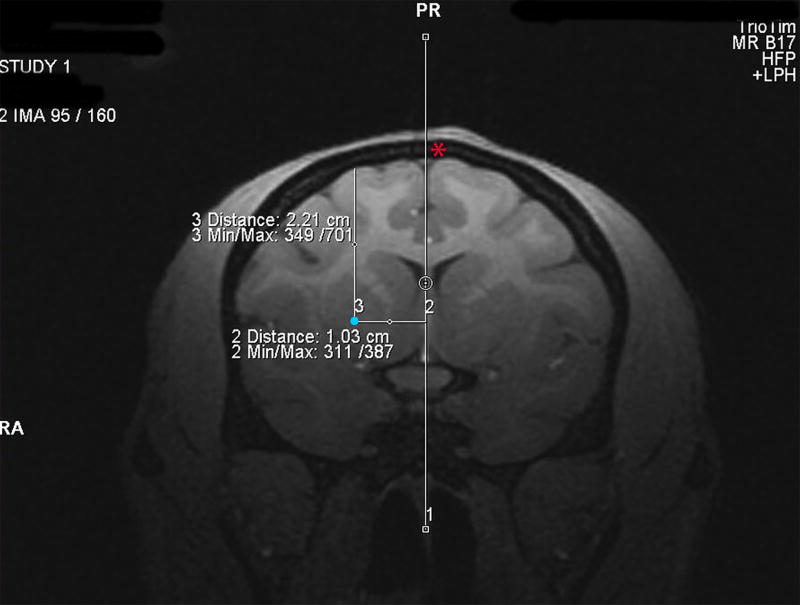
Figure 2.
Determining surgical coordinates from the MRI scan. Surgical coordinates an for injection site into the putamen (shown by a blue dot) are created by using the MRI software to establish 1) the distance in mm in front of the earbars (AP coordinate), 2) the distance in mm lateral to the center of the sagittal sinus vein (ML coordinate, shown by a red asterisks) and 3) the distance in mm ventral to the pial surface of the brain (DV coordinate).
3.2 Stereotaxic injection into the caudate and putamen
Disconnect animal from anesthesia and remove from mobile anesthesia cart. Place animal onto the surgical table and connect endotracheal tube to surgical anesthesia unit. Maintain animal on 1–2% Isoflurane combined with 100% oxygen administered at a rate of 1–1.5 L/min (Note 8). Secure the base of stereotaxic frame to the table with surgical tape (animal’s torso and legs must be delicately lifted up to achieve this, while head remains locked into frame) and place physiologic monitoring equipment on the animal.
Administer pre-operative doses of Hydromorphone HCl (2 mg/ml) (range of doses: <3kgs bodyweight, 0.5mgs, 0.25ml; 3–10kgs bodyweight, 1mg, 0.5ml; >10kgs bodyweight, 2mg, 1ml) intravenously and 25 mg/kg Cefazolin intravenously as well as every 2 hours intra-operatively (for both drugs).
Establish intravenous fluids (Lactated Ringers solution) @ 10–20 ml/kg/hr. Fluids may be increased based on hydration status of patient or due to blood loss during surgery.
Apply a ChlorPrep (a chlorhexadine/alcohol instant solution) to the entire surgical site and allow it to dry. Drape surgical areas with sterile towels.
Place a warm forced air system (Bair hugger) around the animal, as well as warm water bags, for the duration of the surgical procedure. Place a full table drape over the animal, towels and Bair hugger to avoid any potential breaks in aseptic technique due to the necessary manipulation of the stereotaxic manipulator.
Surgical staff should wear hair bonnets, surgical masks, protective eyewear and gloves prior to entering the operating room. Surgeons should perform a full pre-surgical sterile scrub with a chlorhexadine or betadine solution. Surgeons should wear sterile gowns and sterile surgical gloves prior to draping the patient and initiating the surgical procedure.
Open sterile (ethylene oxide sterilized) surgical packs containing instruments, drill, micromanipulator, AP zeroing bar, surgical pump, needles/syringes and supplies on a flat surface near the operating table.
Assemble needles and syringes and wet the syringe barrels by withdrawing and expelling injection vehicle (saline, PBS, buffer, etc) several times (Note 9).
Slide the micromanipulator onto the AP zeroing bar and tighten down anywhere.
Screw the AP slide attachment onto the micromanipulator and tighten down (use Allen wrench if necessary).
Screw the infusion pump onto the AP slide attachment and set/lock AP slide attachment to a set number (example- 50 or 0; all AP coordinate measurements from the MRI will be added or subtracted from this number, depending on whether the micromanipulator is placed on the left or right AP bar on the animal’s head frame).
Place the syringe/needle into the infusion pump and lock into place. Use a T-square (ex PolySquare) to make certain that the micromanipulator, pump, syringe and needle are straight and make adjustments if not (Figure 3).
Ensure that all components of the micromanipulator are tightened appropriately (use Allen wrenches).
Carefully loosen the micromanipulator from the AP zeroing bar and slide laterally to line up the tip of the needle with the point structure on the AP zeroing plate. Record the number on the AP zeroing bar (not the AP slide attachment). When the micromanipulator is placed at the same number on the animal’s head frame AP bar, the needle will be correctly positioned at the ear bars (ear bar zero).
Administer 25 mg/kg of Cefazolin intravenously prior to making the initial scalp incision for antibiotic coverage. Inject an intradermal, local block at the proposed incision site consisting of 0.4 ml (up to 0.8 ml depending on size of incision) Bupivicaine (0.5%) combined with 0.1 ml (up to 0.2 ml depending on size of incision) and Lidocaine (1%) with epinephrine.
Make a linear sagittal incision of approximately 6 cm length across the top of the animal’s scalp. Elevate the subcutis and periosteum and retract laterally to expose the skull. Sharply dissect muscle attachments and retract laterally.
Drill a 1 cm long line close to the planned injection site (caudate and/or putamen) perpendicular to the sagittal sinus. Extend the drill line down to the level of the dura to expose the dark sagittal sinus vein. The exposed sagittal sinus vein will be used to establish the ML zero point.
Bring the micromanipulator (connected to the pump, syringe and needle) from the AP zero bar to the animal’s head frame and lock into place on either the left or right AP bar at the number recorded earlier (Note 10). Use the AP slide attachement and the ML dials on the micromanipulator to bring the needle to the center of the saggital sinus vein and record the ML number as the zero point.
Calculate the AP and ML coordinates for each injection site (Note 11) and use the micromanipulator to bring the needle to those sites and mark the sites on the skull with a pencil or permanent marker (Sharpie).
Create oval craniotomies over the proposed injection sites using a high speed surgical drill with a 2 mm carbide burr (Figure 4A). The length and diameter of the craniotomies will vary depending on the number of proposed injection sites in each hemisphere. Use saline and suction to cool the drill bit and clear bone debris from the craniotomy area. Smooth the edges of the craniotomies with ronguers and incise the dura with a 22 gauge needle followed by micro-dissection scissors (Figure 4B).
Load the syringe with infusate without removing the syringe from the infusion pump (Note 12) and prime the needle by using the infusion pump to infuse until a small amount of liquid is visible at the tip of the needle. Swab the infusate away with gauze or a surgical swab.
At each injection site, carefully lower the needle down to the pial surface and record the DV value on the micromanipulator, which will serve as the DV zero value. Calculate the DV coordinate by subtracting the DV value obtained from the MRI from the DV zero value.
Slowly lower the needle to the DV coordinate and infuse at the desired rate (Figure 4C, Note 13).
After the infusion is complete, allow the needle to rest in place for an additional 3–10 minutes to allow the infusate to disperse from the needle tip. Slowly raise the needle out of the brain and proceed to the next injection site until all injections have been made.
Place Gelfoam sponge into the craniotomy sites (Figure 4D) and use Cruciate 3-0 Vicryl sutures to bring the muscle back to its original lateral position along the skull. Close the subcutis and appose the skin edges with simple interrupted and intradermal 4-0 Monocryl sutures. Take the animal out of the stereotaxic head frame, discontinue the isoflurane gas anesthesia while continuing oxygen support and then release the animal from the stereotaxic head frame. Allow the animal to recover on the OR table until extubation.
Administer the following post-surgical medications: Hydromorphone on the day of surgery every 4 hours IM until 2000 hours, Buprenorphine for 2 days IM SID at 2000 hours and Cefazolin for 3 days IM BID (25mg/kg, 250mg/ml) (Table 1).
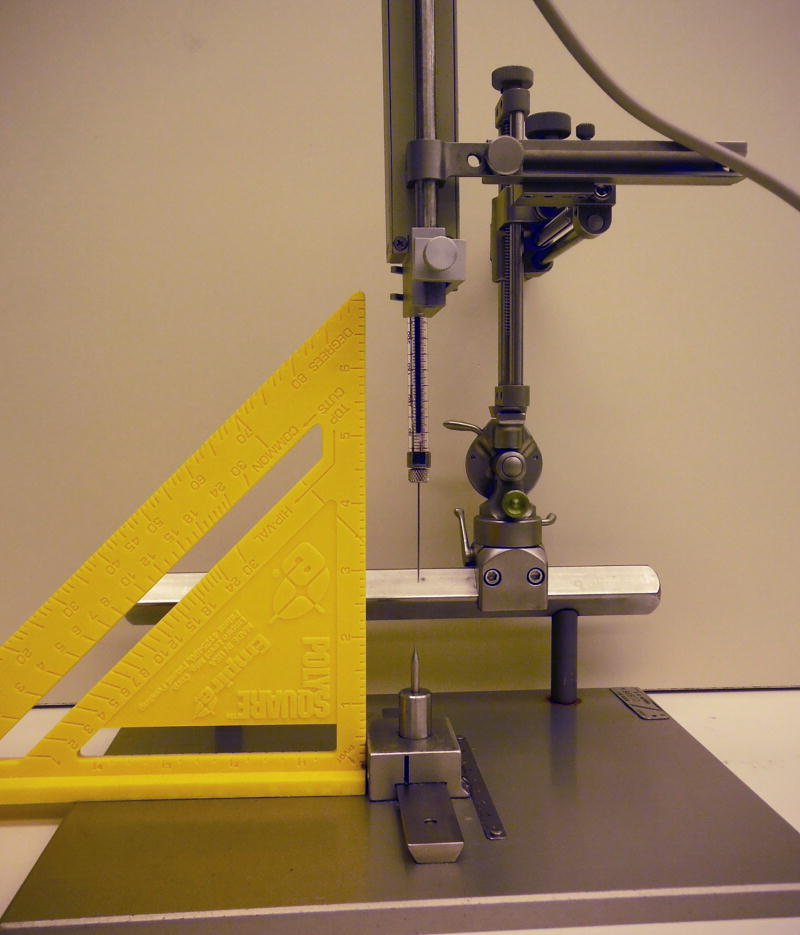
Figure 3.
Zeroing the AP slide bar. Prior to securing the micromanipulator on the animal’s head frame in the surgery room, the AP slide bar must be zeroed out by setting the AP slide bar to a pre-determined number (example, 50) and lining up the tip of the needle/cannula with the point structure on the AP zeroing plate. When the micromanipulator is placed into the exact position on the AP bar of the head frame, the needle tip will be located at the earbars. Using a T-square when zeroing the AP slide bar helps to ensure that the needle is straight.
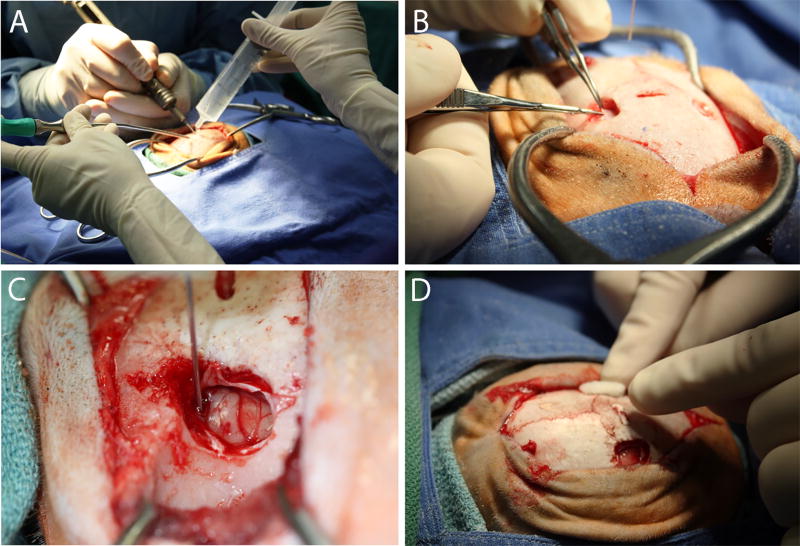
Figure 4.
Stereotaxic injection into the caudate and putamen. (A) A craniotomy that encompasses the proposed injection site(s) is established using a high speed drill and a 2 mm carbide burr. The edges are rounded using a rongeur. (B) The dura is excised using a micro-scissor and folded back to expose the pial surface of the brain. (C) The needle is slowly lowered to the calculated injection site coordinate and the infusate delivered using an infusion pump set to either a constant or ramping injection rate. (D) Post-injection, the needle is slowly raised and the craniotomy is filled with Gelfoam prior to suturing.
Table 1.
Pain medications administered post-surgery. Different doses of Hydromorphone and Buprenorphine are listed according to rhesus macaque weight. Hydromorphone is administered post-surgery every 4 hours IM until 2000 hours and Buprenorphine (IM) for 2 days post-surgery at 2000 hours (once per day).
| Animal Weight |
Hydromorphone (2mg/ml) |
Buprenorphine (0.3mg/ml) |
|---|---|---|
| <3kg | 0.5mg (0.25ml) | 0.15mg (0.5ml) |
| 3–10kg | 1mg (0.5ml) | 0.3mg (1ml) |
| >10kg | 2mg (1ml) | 0.3mg (1ml) |
3.3 Necropsy and tissue collection
Necropsy
-
1
Sedate the animal while in its home cage with 10–20 mg/kg ketamine (IM) and bring to the necropsy room. Secure animal to the board on the necropsy table with masking tape and anesthetize animal with sodium pentobarbital administered IV at 25mg/kg. Assess depth of anesthesia by loss of palpebral, corneal, pain and gag reflexes
-
2
Incise abdomen with scalpel after adequate anesthesia has been established and collect terminal blood samples from abdominal aorta or caudal vena cava if needed. Place blood in red top serum tubes. Sever aorta to effect exsanguination and euthanasia.
-
3
If necessary, collect cerebrospinal fluid (3cc syringe with 1.5” 22 gauge sterile needle) and place into sterile Microcentrifuge collection tubes.
-
4
Open the thoracic cavity and the perfuse brain and spinal cord with 1–2 liters of ice-cold, 0.9% sterile saline via the right carotid artery (Note 14).
-
5
Carefully saw around the entire skull in the axial plane using a hand saw and remove the skull cap using a prying motion with a bone lever. Gently remove the brain from the calvarium and place it in a brain jar (in saline) on ice. It will be necessary to dissect through the cranial nerves and spinal cord prior to removing the brain.
-
6
Collect relevant samples of tissues/organs (liver, spleen, gastroc, kidney, lung, lymph nodes, pancreas, intestine, adrenal, gonads) in microcentifuge tubes (smaller samples) and foil packs (larger samples for banking). Immediately place the samples in microfuge tubes on dry ice and the samples in foil packs in the Dewar filled with liquid nitrogen (Note 15).
-
7
If of interest, remove spinal cord, divide into 3 sections (Cervical, Thoracic & Lumbar), remove meninges, cut a 2 cm long segment from the middle of each section and place each in small jars with sterile saline on ice.
-
8
Transport the brain & spinal cord on ice back to the laboratory in an enclosed biohazard container. Transport the Dewar full of frozen tissue samples back to laboratory. Transport blood samples in red-top tubes back to the laboratory at room temperature in a biohazard container.
-
9
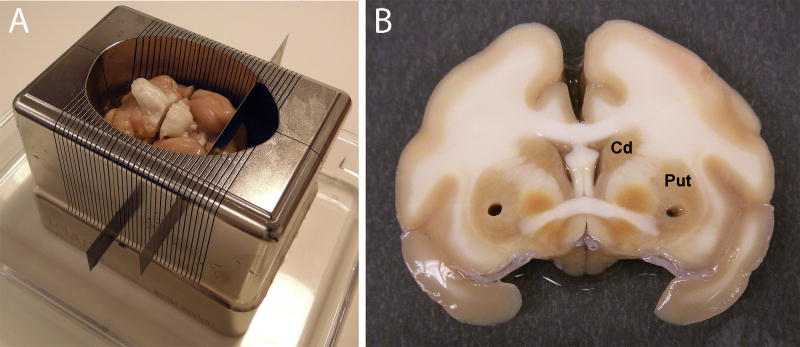
Place the brain into a non-human primate brain matrix and carefully cut into slabs (2–8mm thick slabs recommended) using tissue blades (Figure 5A, showing a rhesus macaque brain matrix). Place each brain slab into a separate, sterile saline-filled petri dish on ice.
-
10
Use tissue biopsy cores (2mm) to take samples from regions of interest for future molecular and/or biochemical analyses (Figure 5B, Note 16). Place samples in microcentrifuge tubes and place immediately on dry ice. Store samples at −80 degrees.
-
11
After collecting brain samples, place slabs of brain tissue in 4% paraformaldehyde for 48 hours for post-fixing. After post-fixing in 4% paraformaldehyde, place slabs of brain tissue in 30% sucrose until they have completely sunken to the bottom of the jar.
-
24
Cut brain slabs using a frozen microtome at a thickness of 40 um and collect tissues in large 24 well (4×6) compartmented tissue collection box filled with cryoprotectant solution.
Figure 5.
Brain processing post-necropsy. (A) Following removal at necropsy, the brain is placed in to a matrix and sectioned into slabs of varying thickness using tissue slicer blades. (B) The slabs are placed into petri dishes containing sterile saline and biopsy punches are taken from key areas to be used for molecular and biochemical studies. Brain slabs are then post-fixed in 4% paraformaldehyde for 48 hours and cryoprotected in 30% sucrose prior to immunohistochemical staining.
Blood processing
Allow blood to coagulate for 2 hours at room temperature.
Centrifuge red top serum tubes at 2500 rpm for 20 minutes at room temperature (25°C) using a tabletop centrifuge.
Pipet off serum and transfer to microcentrifuge tubes. Store serum samples at −80 degrees.
3.4 Immunohistochemical processing of brain to verify expression of gene therapy agent
Day 1
Wash tissue in dilution media in netted staining dishes (5 × 8 minutes).
Block endogenous peroxidase (2.13 g of sodium meta-periodate per 100 mls TBS) for 20 minutes in netted staining dishes.
Wash tissue in dilution media in netted staining dishes (5 × 8 minutes).
Block tissue in 5% of the appropriate serum (goat, donkey) in netted staining dishes (5 mls serum per 100 mls dilution media).
Incubate tissue in primary antibody solution (primary antibody solution: 3 mls serum and 400 ul triton-X per 100 mls PBS in 6 well tissue culture plates (either shaking at room temp overnight or at 4 degrees for 48 hours). Each primary antibody should be used at its own concentration.
Day 2
-
1
Wash tissue in dilution media in netted staining dishes (5 × 8 minutes).
-
2
Incubate tissue in secondary antibody solution in 6 well tissue culture plates (shaking at room temperature for 1 hour). Secondary antibody solution: 3 mls normal serum per 100 mls dilution media. Each secondary ab should be used at its own required concentration.
-
3
Wash tissue in dilution media in netted staining dishes (5 × 8 minutes).
-
4
Incubate tissue in ABC solution in netted staining dishes for 1 hour. (First add 3 mls serum per 100 mls of dilution media. Next add 4 drops of bottle A and 4 drops of bottle B to 10 mls of serum/dilution media solution and mix. After waiting 30 minutes, mix those 10 mls back with the other 90 mls and place tissue into the solution).
-
5
Wash tissue in TBS in netted staining dishes (3 × 8 minutes).
-
6
DAB reaction to develop color on the tissue: First add 50 mg DAB to 100 mls of TBS. Next add 2.5 g Nickel II Sulfate if nickel intensification needed (makes stain purple versus brown). Immediately prior to tissue incubation, add 20 ul of the 30% hydrogen peroxide solution. Incubate tissue in DAB solution until the stain develops (depends on the antibody, ranges from ∼ 1–10 minutes) (Note 17, Figure 6).
-
6
Wash tissue in TBS in netted staining dishes (3 × 8 minutes).
-
7
Store tissue at 4 degrees in PBS in netted staining dishes until able to mount onto slides.
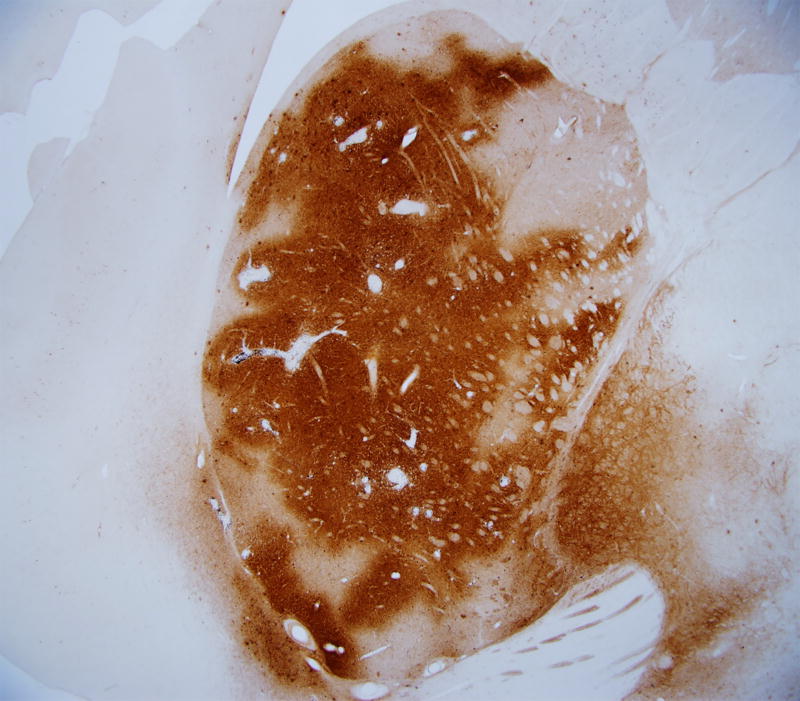
Figure 6.
Immunohistochemcal staining of brain sections to identify the injected agent. Anti-eGFP staining demonstrates accurate expression of the transgene following a 50 ul injection of AAV2/1-eGFP (5e12 vg/ml) into the putamen of an adult rhesus macaque.
Acknowledgments
This work was supported by NIH Grant NS069798 (JLM), and Oregon National Primate Research Center (ONPRC) Core Grants RR000163 and RR000163.
Footnotes
The animal is positioned in the frame in the prone/sphinx position, with the stomach facing downward, the head facing forward, the hind legs positioned behind the animal and the forelegs placed in front of the animal. While placing the animal in the frame, it is important to not disturb or move the endotracheal tube. It is best to carefully place the tube laterally and out of the way of the palate bar.
Due to the magnetic field in and around the MRI Scanner, a precision vaporizer is located outside of the MRI Scanner room, in the control room. A long (3 meter) non-rebreathing tube is utilized which extends from the anesthesia machine, through the wall of the control room, into the MRI room, where it connects to the subject. This set-up also allows for adjustments to the oxygen flow rate and anesthetic gas delivery to the subject from the control room. Because of the length of the non-rebreathing tube, high oxygen flow rates are necessary to avoid rebreathing of the exhaled gases in the tube. Typically the oxygen flow rate is set at 1–2 liters/minute.
Approximate normal values: Respiratory rate: 10–20 breaths/min, Heart rate: 110–170 beats/min, O2Sat (while breathing 100% O2): 95–100%; EtCO2: 35–45 mmHg
This can be anything that contains an MRI contrast such as a Vitamin E capsule or a small tube filled with contrast dye.
Surface/head coils must be affixed to a base that is placed near/next to the animal. In our laboratory, the head coil is attached to a Gorillapod (flexible tripod) that is affixed to a heavy utility bottle filled with water. The tripod affords flexibility when placing the coil directly above the head.
Scan details on our Siemen’s 3T MRI unit: TR- 2500, TI-1100, TE −3.9, BW-128, MF- 2.04
Carefully choose pathways to desired injection sites that do not pass through sulci (location of major blood vessels that may rupture) or pass through ventricular walls (may alter needle trajectory to target). The number of injection sites into the caudate and putamen will depend on numerous factors including the diffusion capacity of the infusate (ex, viruses versus cells), different viral vectors (ex, lentivirus versus adeno-associated virus), different viral vector serotypes (ex, AAV2 versus AAV5), different viral vector titers (ex, high versus low) different cell types (ex, stem cells versus progenitor cells).
The criteria used used to assess adequacy of anesthesia and animal intraoperative well-being during the procedure include the following: body temperature via esophageal temperature probe, heart rate and pulse character (fast or slow) via pulse oximetry and electrocardiography, blood pressure via indirect blood pressure cuff or direct percutaneous arterial line, oxygen saturation via pulse oximetry, respiratory rate and pattern, end tidal Carbon Dioxide, capillary refill time, absence of palpebral response to touching the medial canthus, jaw tone and the color of mucous membranes at the gums or conjunctiva.
When injecting viral vectors, we prime the needle at this point with a separate tube of the virus to coat the barrel of the syringe with viral particles (prevents the vector prep that injected into the brain from sticking to the barrel). This is done by flushing up and down several times and expelling the used vector into a biohazard container.
Ensure that the pump, syringe and needle and raised as high as possible before removing from the AP zero bar to ensure maximal clearance of the animal’s head when being placed onto the head frame. After the manipulator is locked onto the head frame, the manipulator, infusion pump, syringe and needle will remain sterile, while the end of the pump cord will become unsterile as it is attached to pump controller box located on a Mayo stand close to the surgical table. At this point, a non-scrubbed member of the surgical staff can program the infusion pump with the volume and rate of the infusion. The non-scrubbed staff can continue to operate the pump controller box or a sterile clear plastic drape can be placed over the box so that it can be controlled by the surgeon. We find the latter to be the easiest option.
AP coordinates: The set point on the AP slide attachment when the needle was zeroed on the zeroing bar (ex, 50 or 0) added or subtracted from the distance from the earbar to the injection site established from the MRI. Example 1: with the AP slide attachment set to 50, the micromanipulator placed on the left AP bar of the head frame and the distance of an injection site in the putamen 20 mm in front of the ear bars on the coronal MR image, the AP for this injection site is 50–20=30. Example 2: with the AP slide attachment set to 0, the micromanipulator placed on the right AP bar of the head frame and the distance of an injection site in the putamen 20 mm in front of the ear bars on the coronal MR image (the AP for this injection site is 0+20=20.
ML coordinates: ML value on the micromanipulator when the needle was zeroed on the animal’s sagittal sinus added or subtracted from the distance from the sagittal sinus to the injection site established from the MRI. Example 1: with the ML zero value at the sinus at 60, the micromanipulator placed on the left AP bar of the head frame and the distance of an injection site in the left putamen 15 mm lateral to the center of the sagittal sinus on the coronal MR image, the ML for this injection site is 60+15=75. For an injection site in the right putamen the calculation would be 60-15-55.
Loading the syringe with infusate can be achieved by using a sterile pipetteman and sterile pipette tips to pipette the desired amount of infusate onto a sterile piece of Parafilm, bringing the infusate on the Parafilm over to the needle and using the infusion pump to withdraw the infusate into the syringe. Any extra virus should be placed into a biohazard container.
Infusion rates will differ depending on type and expected spread of the infusate. A standard rate of 1–2 ul/minute works well for injections into both the caudate and putamen. Spread of infusate can be enhanced with a ramping infusion rate that begins at 0.5 ul/min and increases by 0.5–1.0 ul every 5 minutes.
The pumping rate should be a moderate pulsatile flow versus a steady stream so that blood vessels in the brain are not ruptured. Ruptured blood vessels can lead to background in future immunohistochemical staining processes.
Wash instruments (forceps & Metzenbaums) with Tergazyme, 70% ethanol and rinse in PBS between each tissue to prevent cross-contamination. It is advised to collect peripheral tissues to assess for potential distribution of the infusate out of the brain and into the periphery.
Spray biopsy cores and forceps with 70% ethanol and wiped clean with a Kimwipe between punches of different brain regions.
DAB is a carcinogen and should be handled with caution. It is essential that the hydrogen peroxide is not added until immediately prior to placing the tissue in the DAB solution because it will quickly break down into oxygen and water. Figure 6 depicts an example of a coronal section from a rhesus macaque that was injected with 50 ul of AAV2/1-eGFP (titered at 5e12 vg/ml) into the putamen. The MRI, surgery, necropsy and immunohistochemistry techniques used were those mentioned above and the tissue was stained using an anti-eGFP antibody (Invitrogen, 1:1000).
References
- 1.Huntington Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol.Exp.Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Isacson O, Riche D, Hantraye P, Sofroniew MV, Maziere M. A primate model of Huntington’s disease: cross-species implantation of striatal precursor cells to the excitotoxically lesioned baboon caudate-putamen. Exp.Brain Res. 1989;75:213–220. doi: 10.1007/BF00248544. [DOI] [PubMed] [Google Scholar]
- 4.Burns LH, Pakzaban P, Deacon TW, Brownell AL, Tatter SB, Jenkins BG, Isacson O. Selective putaminal excitotoxic lesions in non-human primates model the movement disorder of Huntington disease. Neuroscience. 1995;64:1007–1017. doi: 10.1016/0306-4522(94)00431-4. [DOI] [PubMed] [Google Scholar]
- 5.Hantraye P, Riche D, Maziere M, Isacson O. A primate model of Huntington’s disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp.Neurol. 1990;108:91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- 6.Roitberg BZ, Emborg ME, Sramek JG, Palfi S, Kordower JH. Behavioral and morphological comparison of two nonhuman primate models of Huntington’s disease. Neurosurgery. 2002;50:137–145. doi: 10.1097/00006123-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Palfi S, Brouillet E, Jarraya B, Bloch J, Jan C, Shin M, Conde F, Li XJ, Aebischer P, Hantraye P, Deglon N. Expression of mutated huntingtin fragment in the putamen is sufficient to produce abnormal movement in non-human primates. Mol.Ther. 2007;15:1444–1451. doi: 10.1038/sj.mt.6300185. [DOI] [PubMed] [Google Scholar]
- 8.Isacson O, Hantraye P, Maziere M, Sofroniew MV, Riche D. Apomorphine-induced dyskinesias after excitotoxic caudate-putamen lesions and the effects of neural transplantation in non-human primates. Prog.Brain Res. 1990;82:523–533. doi: 10.1016/s0079-6123(08)62643-6. [DOI] [PubMed] [Google Scholar]
- 9.Hantraye P, Riche D, Maziere M, Isacson O. Intrastriatal transplantation of cross-species fetal striatal cells reduces abnormal movements in a primate model of Huntington disease. Proc.Natl.Acad.Sci.U.S.A. 1992;89:4187–4191. doi: 10.1073/pnas.89.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerich D, Winn S, Hantraye P, Peschanski M, Chen E, Chu Y, McDermott P, Baetge E, Kordower J. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997;386:395–399. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 11.Emerich D, Thanos C, Goddard M, Skinner S, Geany M, Bell W, Bintz B, Schneider P, Chu Y, Babu R, Borlongan C, Boekelheide K, Hall S, Bryand B, Kordower J. Extensive neuroprotection by choroid plexus transplants in excitotoxin lesioned monkeys. Neurobiol Dis. 2006;23(2):471–80. doi: 10.1016/j.nbd.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 12.McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, Davidson BL. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol.Ther. 2011;19:2152–2162. doi: 10.1038/mt.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, Weatherspoon MR, Blum JL, Burright EN, Zhang Z, Kaemmerer WF. Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum. Brain. 2012;135:1197–1209. doi: 10.1093/brain/awr333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiles DK, Zhang Z, Ge P, Nelson B, Grondin R, Ai Y, Hardy P, Nelson PT, Guzaev AP, Butt MT, Charisse K, Kosovrasti V, Tchangov L, Meys M, Maier M, Nechev L, Manoharan M, Kaemmerer WF, Gwost D, Stewart GR, Gash DM, Sah DW. Widespread suppression of huntingtin with convection-enhanced delivery of siRNA. Exp.Neurol. 2012;233:463–471. doi: 10.1016/j.expneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Chan AW, Xu Y, Jiang J, Rahim T, Zhao D, Kocerha J, Chi T, Moran S, Engelhardt H, Larkin K, Neumann A, Cheng H, Li C, Nelson K, Banta H, Zola SM, Villinger F, Yang J, Testa CM, Mao H, Zhang X, Bachevalier J. A two years longitudinal study of a transgenic Huntington disease monkey. BMC.Neurosci. 2014;15:36. doi: 10.1186/1471-2202-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]