Abstract
Brain-derived neurotrophic factor (BDNF) is a critical effector of depression-like behavior and antidepressant responses. Here, we show that VGF (non-acronymic), which is robustly regulated by BDNF/TrkB signaling, is downregulated in dorsal hippocampus (dHc) (male/female) and upregulated in nucleus accumbens (NAc) (male) in depressed human subjects and in mice subjected to chronic social defeat stress (CSDS). Adeno-associated virus (AAV)-Cre-mediated Vgf ablation in floxed VGF mice, in dHc or NAc, led to pro-depressant or antidepressant behaviors, respectively, while dHc or NAc AAV-VGF overexpression induced opposite outcomes. Mice with reduced VGF levels in the germline (Vgf+/−) or in dHc (AAV-Cre-injected floxed mice) showed increased susceptibility to CSDS and impaired responses to ketamine treatment in the forced swim test. Floxed mice with conditional pan-neuronal (Synapsin-Cre) but not those with forebrain (αCaMKII-Cre) Vgf ablation displayed increased susceptibility to subthreshold social defeat stress, suggesting that neuronal VGF, expressed in part in inhibitory interneurons, regulates depression-like behavior. Acute antibody-mediated sequestration of VGF-derived C-terminal peptides AQEE-30 and TLQP-62 in dHc induced pro-depressant effects. Conversely, dHc TLQP-62 infusion had rapid antidepressant efficacy, which was reduced in BDNF floxed mice injected in dHc with AAV-Cre, and in NBQX- and rapamycin-pretreated wildtype mice, these compounds blocking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and mammalian target of rapamycin (mTOR) signaling, respectively. VGF is therefore a critical modulator of depression-like behaviors in dHc and NAc. In hippocampus, the antidepressant response to ketamine is associated with rapid VGF translation, is impaired by reduced VGF expression, and as previously reported, requires coincident, rapid BDNF translation and release.
INTRODUCTION
Major depressive disorder (MDD) is a debilitating mental illness characterized by its high prevalence and resistance to treatment. Conventional antidepressant drugs have long-onset latency and limited response rates 1, and in ~70–80 % of MDD cases, fail to induce remission 2, 3. Antidepressant treatment is associated with increased brain-derived neurotrophic factor (BDNF) expression in the hippocampus 4. Interestingly, the actions of BDNF in the central nervous system (CNS) are highly region- and circuit-specific 5, 6, with BDNF having antidepressant efficacy in hippocampus and pro-depressant efficacy in ventral tegmental area (VTA)/nucleus accumbens (NAc) circuits.
VGF is a secreted protein and neuropeptide precursor that is robustly regulated by BDNF and neuronal activity in CNS neurons 7, 8. Hippocampal VGF expression is decreased in animal models of depression and is increased by exercise and antidepressant treatment 9–11. Heterozygous VGF knockout mice are characterized by depression-like phenotypes in the forced swim and tail suspension tests (FST and TST, respectively) 9. Moreover, intrahippocampal or intracerebroventricular (icv) infusion of C-terminal VGF-derived peptides AQEE-30 or TLQP-62 (named by their four N-terminal amino acids and length) attenuates depression-like behaviors 9, 10, 12. Acute and chronic TLQP-62 treatment modulates BDNF receptor TrkB phosphorylation 12–14, hippocampal neuronal progenitor proliferation, and synaptic plasticity 15, 16.
Ketamine, a noncompetitive glutamatergic N-methyl-D-aspartate receptor (NMDA) receptor antagonist, has recently emerged as a promising novel antidepressant. In clinical studies, a single intravenous subanesthetic infusion of ketamine significantly improves depression symptoms in MDD patients within 2 hours and can last weeks 17–19. Preclinical studies demonstrate that ketamine exerts its antidepressant effects by regulating synaptic plasticity, at least in part via activation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor 20, 21 and mammalian target of rapamycin (mTOR) signaling 20, increasing synthesis of synaptic proteins 20. Here we determined region-specific roles of VGF in regulating depression-like behaviors and ketamine response. We investigated the molecular mechanisms underlying the antidepressant efficacy of VGF-derived peptide TLQP-62 in dorsal hippocampus (dHc), which like ketamine, was dependent on BDNF, AMPA receptor and mTOR pathway activation.
METHODS
Animals
Animal protocols were approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai. Mice and procedures are described in the Supplemental Information.
Behavioral Studies
Chronic and subthreshold social defeat stress, social interaction testing, subchronic variable stress, and sucrose preference, forced swim, and open field tests, were performed as described 9, 22, 23 and detailed in the Supplemental Information.
Human postmortem tissues
Demographic characteristics associated with the human tissue samples, provided by the Dallas Brain Collection and Quebec Suicide Brain Bank, are listed in Supplemental Table 1.
Protein and RNA sample preparation, qPCR analysis, and western blotting
Mouse tissues, obtained by dissection (dHc) or brain punches (NAc), were either extracted by RNeasy Mini Kit (Qiagen) and RNA was reverse transcribed and subjected to qPCR, or were analyzed by SDS-PAGE and western blotting 13, as detailed in Supplemental Information.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7 and SPSS 25 software. Details of statistical analyses including test used, exact sample sizes and P values for each figure are included in the Figure Legends, Supplemental Information and/or in Supplemental Table 2.
RESULTS
Inverse regulation of VGF expression in hippocampus and nucleus accumbens in depressed patients and mice following chronic social defeat stress
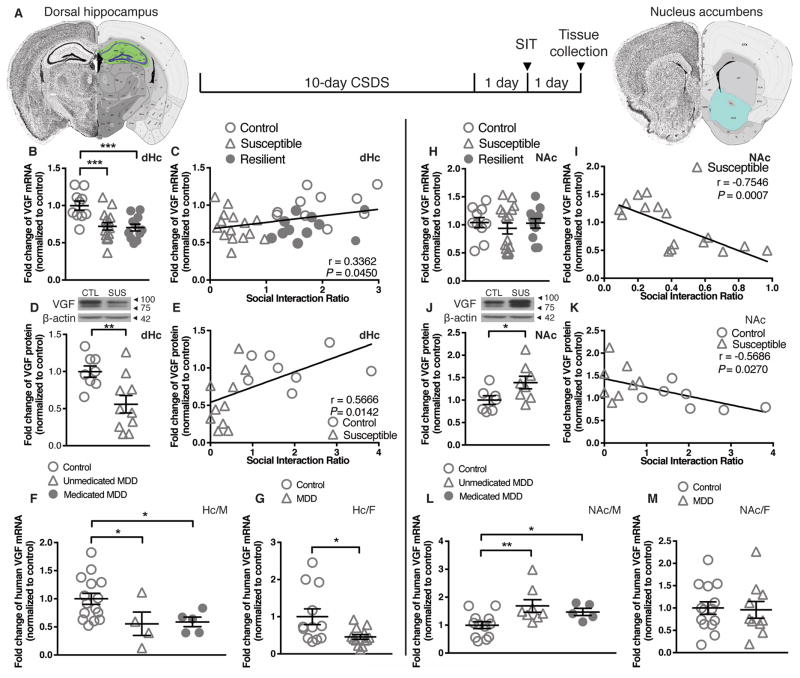
To investigate whether VGF expression is regulated in dHc and NAc by chronic social defeat stress (CSDS), we quantified VGF mRNA and protein levels in male mice 48h after the last defeat (Figure 1A) 24. Compared to unstressed control mice, VGF mRNA levels were significantly reduced in dHc (Figure 1B) of both susceptible and resilient wildtype mice and correlated with social avoidance behavior (Figure 1C, Supplemental Figure 1C – F). This pattern is similar to the BDNF exon IV mRNA expression pattern observed in dHc following CSDS (Supplemental Figure 1A, B). VGF protein levels were also reduced in dHc of susceptible mice (Figure 1D). In NAc, no significant changes in VGF mRNA levels among control, susceptible and resilient mice were detected (Figure 1H), however, a negative correlation between VGF mRNA levels and social interaction ratio within the susceptible group was found (Figure 1I). VGF protein levels were increased in NAc of susceptible mice (Figure 1J). VGF protein levels and social avoidance behavior were inversely correlated in dHc and NAc (Figure 1E, K, Supplemental Figure 1G – J). In female mice, subchronic variable stress (SCVS) induced depression-like behaviors (Supplemental Figure S2A – C), and reduced VGF mRNA levels in both dHc and NAc (Supplemental Figure S2D, E).
Figure 1.
VGF expression is regulated by depression in a region-specific manner. (A) Timeline of 10-day chronic social defeat (CSDS) experiment and coronal schematics of mouse brain highlighting dorsal hippocampus (dHc) and nucleus accumbens (NAc) collected. Brain atlas adapted from Allen Brain Institute 24. Social interaction test and tissue collection were performed 24h and 48h after the last social defeat session, respectively. (B) VGF mRNA levels were reduced in dHc of both susceptible and resilient mice at 48h after the last defeat session (n = 10 ~ 15/group). (C) VGF mRNA expression in mouse dHc was strongly correlated with social avoidance behavior. (D) VGF protein levels were reduced in dHc of susceptible mice at 48h after the last defeat session (n = 8 ~ 10/group). (E) VGF protein levels in mouse dHc were strongly correlated with social avoidance behavior. (F) Human VGF mRNA levels were reduced in postmortum hippocampus of both unmedicated and medicated male MDD subjects compared to controls (n = 4 ~ 15/group). (G) Human VGF mRNA levels were reduced in postmortum hippocampus of female MDD subjects compared to controls (n = 12 ~ 13/group). (H) VGF mRNA levels were unchanged in mouse NAc 48h after the last defeat session (n = 10 ~ 16/group). (I) VGF mRNA expression in mouse NAc was strongly correlated with social avoidance behavior within susceptible group. (J) VGF protein levels were increased in NAc of susceptible mice at 48h after the last defeat session (n = 7 ~ 8/group). (K) VGF protein levels in mouse NAc were strongly correlated with social avoidance behavior. (L) Human VGF mRNA levels were increased in NAc of both unmedicated and medicated male MDD subjects, compared to controls (n = 5 ~ 12/group). (M) Human VGF mRNA levels in postmortum NAc of female MDD subjects were unchanged compared to controls (n = 10 ~ 14/group). SIT: social interaction test; M: male; F: female. All data are presented as mean ± s.e.m. One-way ANOVA followed by Fisher’s LSD test for B, F; Kruskal-Wallis test followed by uncorrected Dunn’s test for H, L; Pearson’s r for C, E, I, K; Student’s t test for D, J, M; Mann-Whitney test for G (* P<0.05, ** P<0.01, *** P < 0.001).
To investigate whether VGF is similarly regulated in human subjects, VGF mRNA levels were determined in human postmortem hippocampus and NAc from patients with MDD and control subjects. Compared with respective controls, VGF mRNA levels were significantly decreased in hippocampus of both unmedicated and medicated male MDD subjects and female MDD subjects (Figure 1F, G), were increased in NAc of both unmedicated and medicated male MDD subjects (Figure 1L), while no differences were observed in NAc from female subjects (Figure 1M).
Germline Vgf gene ablation induces depression-like behaviors in male and female heterozygous knockout mice
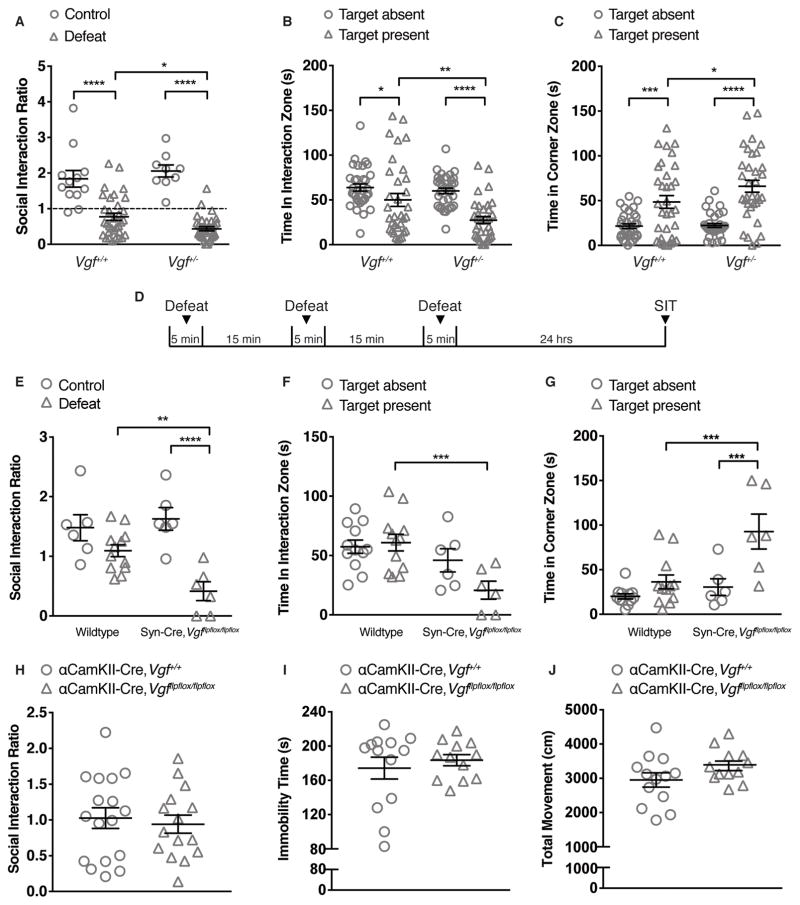
In germline Vgf+/− heterozygous knockout mice, partial deletion of VGF results in pro-depressant phenotypes in the FST 9, so we investigated whether male Vgf+/− mice showed increased susceptibility to CSDS. Compared to Vgf+/+ mice, Vgf+/− mice displayed a lower social interaction ratio, decreased time spent in the interaction zone and increased time spent in corner zone when the social target was present (Figure 2A – C; Supplemental Figure S3A – C). Importantly, a smaller percentage of Vgf+/− mice were resilient to CSDS (defined as social interaction ratio > 1; 6% Vgf+/− vs. 27% Vgf+/+) (Figure 2A). In addition, female Vgf+/− mice had increased immobility in the FST and reduced sucrose preference – anhedonia - at baseline (Supplemental Figure S3K, L). Social avoidance, despair and anhedonia in mice recapitulate the core symptoms of MDD in human patients 25.
Figure 2.
Germline and pan-neuronal VGF deficiency increase the susceptibility to social defeat stress-induced depression-like behaviors. (A) Social interaction ratio of Vgf+/+ and Vgf+/− mice 24 hr after the last session of CSDS. Defeated Vgf+/− mice showed increased susceptibility to social avoidance compared to defeated Vgf+/+ mice following CSDS. Nine of 33 defeated Vgf+/+ mice reached the threshold of resilience (social interaction ratio > 1) while only 2 of 34 defeated Vgf+/− mice were resilient after CSDS (n = 9 ~ 12/group for controls, n = 33 ~ 34/group for defeated mice). (B) Defeated Vgf+/− mice spent significantly less time in the interaction zone when the target was present compared to defeated Vgf+/+ mice (n = 33 ~ 34/group). (C) Defeated Vgf+/− mice spent significantly more time in the corner zone when the target was present compared to defeated Vgf+/+ mice (n = 33 ~ 34/group). (D) Schematic timeline of microdefeat paradigm. (E) Microdefeated Syn-Cre/+,Vgfflpflox/flpflox mice showed significantly lower social interaction ratios compared to unstressed control Syn-Cre/+,Vgfflpflox/flpflox mice and identifically microdefeated wildtype mice 24 hours after exposure to microdefeat [n = 6 for control, n = 6 for microdefeated (Syn-Cre/+,Vgfflpflox/flpflox), n = 12 for microdefeated wildtype (Syn-Cre/+,Vgf+/+; Syn-Cre/−,Vgf+/+; Syn-Cre/−,Vgfflpflox/flpflox)]. (F) Microdefeated Syn-Cre/+,Vgfflpflox/flpflox mice spent significantly less time in the interaction zone when the target was present compared to microdefeated wildtype mice (n = 6 for Syn-Cre/+,Vgfflpflox/flpflox, n = 12 for wildtype). (G) Microdefeated Syn-Cre/+,Vgfflpflox/flpflox mice spent significantly more time in the corner zone when the target was present compared to microdefeated wildtype mice (n = 6 for Syn-Cre/+,Vgfflpflox/flpflox, n = 12 for wildtype). (H) No difference in social interaction ratio was observed between wildtype (αCaMKII-Cre/+,Vgf+/+) and αCaMKII-Cre/+,Vgfflpflox/flpflox in the social interaction test after CSDS (n = 15 ~ 17/group). (I) No difference in immobility time was observed between wildtype (αCaMKII-Cre/+,Vgf+/+) and αCaMKII-Cre/+,Vgfflpflox/flpflox in the FST (n = 12 ~ 13/group) (I) No difference in locomotor activity was observed between wildtype (αCaMKII-Cre/+,Vgf+/+) and αCaMKII-Cre/+,Vgfflpflox/flpflox in the OFT (n = 12 ~ 13/group). SIT: social interaction test. All data are presented as mean ± s.e.m. Two-way ANOVA followed by Fisher’s LSD test for A – C, E – G; Student’s t test for H, J; Mann-Whitney test for I (* P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001).
Pan-neuronal embryonic Vgf gene ablation increases susceptibility to subthreshold social defeat stress, while conditional adult forebrain neuronal VGF ablation does not affect depression-like behaviors
To better localize VGF actions within the brain, and at the cellular level, Synapsin-Cre/+,Vgfflpflox/flpflox conditional knockout mice, with pan-neuronal embryonic ablation of Vgf were generated and subjected to subthreshold social defeat stress – microdefeat (Figure 2D), an abbreviated one-day social defeat paradigm used to reveal vulnerability to social stress 22. Twenty-four hours after microdefeat, Synapsin-Cre/+,Vgfflpflox/flpflox showed increased social avoidance compared to wildtype mice (Figure 2E – G, Supplemental Figure S3D – F), suggesting that neuronal VGF expression makes a critical contribution to depression-like behaviors, in the developing and/or adult nervous system.
To determine whether VGF expression in adult forebrain excitatory neurons regulates depression-like behaviors, we generated αCamKII-Cre/+,Vgfflpflox/flpflox mice, in which reduced VGF expression has been observed in hippocampal excitatory neurons at 3–4 months of age 13, and found that αCamKII-Cre/+,Vgfflpflox/flpflox conditional knockout and αCamKII-Cre/+,Vgf+/+ mice were indistinguishable in the social interaction and sucrose preference tests after CSDS, and in forced swim and open field tests at baseline (Figure 2H–J, Supplemental Figure S3G – J). These findings suggest that reduced VGF expression in adult forebrain excitatory neurons does not significantly contribute to depression-like behaviors, although contextual fear memory is impaired 13.
Opposing effects of VGF expression in adult hippocampus and nucleus accumbens on depression-like behaviors
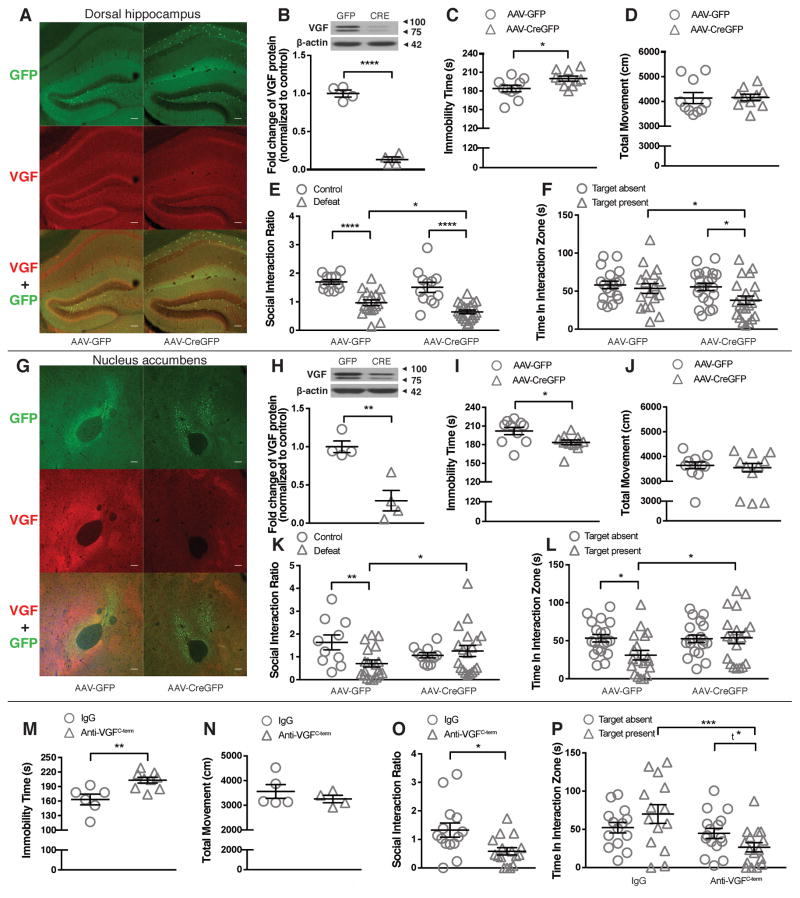
The regional and temporal specificity of VGF actions in regulating depression-like behaviors in adult mice was investigated utilizing targeted administration of AAV-CreGFP to homozygous floxed VGF mice (designated Vgfflpflox/flpflox) or AAV-VGF to wildtype mice, allowing local ablation or overexpression of VGF, respectively. AAV-CreGFP-mediated Vgf gene ablation in dHc (Figure 3A, B) increased immobility time in the FST (Figure 3C) without affecting locomotor activity (Figure 3D), and increased social avoidance (Figure 3E, F, Supplemental Figure S4A – C) following CSDS. Conversely, AAV-VGF-mediated VGF overexpresssion in dHc (Supplemental Figure S5A, B) significantly reduced immobility time in the FST (Supplemental Figure S5C, D).
Figure 3.
AAV-Cre-mediated VGF knockout in dorsal hippocampus (dHc) and nucleus accumbens (NAc) induce pro-depressant and antidepressant phenotypes, respectively. (A) Immunohistochemical staining showed decreased VGF expression in CA1 region and hilus of dHc of AAV-CreGFP-injected Vgf flpflox/flpflox mice [red: rabbit polyclonal anti-VGFC-term (C-terminal) antibody; green: GFP; scale bar 100 μm]. (B) Western blot analysis showed significantly decreased VGF protein levels in dHc of AAV-CreGFP-injected Vgf flpflox/flpflox mice (n = 4/group). (C) AAV-CreGFP injected Vgf flpflox/flpflox showed increased immobility time compared to AAV-GFP injected Vgf flpflox/flpflox mice in the FST (n = 10/group). (D) No significant difference in locomotor activity was observed between dHc-AAV-CreGFP- and dHc-AAV-GFP-injected Vgf flpflox/flpflox mice in the OFT (n = 10/group). (E) AAV-CreGFP-injected Vgf flpflox/flpflox mice showed reduced social interaction ratio compared to AAV-GFP-injected Vgf flpflox/flpflox mice following CSDS (n = 11 ~ 12/group for control, n = 18 ~ 21/group for defeated mice). (F) After CSDS, AAV-CreGFP-injected Vgf flpflox/flpflox mice spent significantly less time in the interaction zone when the target was present compared to AAV-GFP-injected Vgf flpflox/flpflox mice (n = 18 ~ 21/group). (G) Immunohistochemical staining showed decreased VGF expression in the core region of the NAc of AAV-CreGFP-injected Vgf flpflox/flpflox mice (red: anti-VGFC-term; green: GFP; scale bar 100 μm). (H) Western blot analysis showed significantly decreased VGF protein levels in NAc of AAV-CreGFP-injected Vgf flpflox/flpflox mice (n = 4/group). (I) Vgf flpflox/flpflox mice injected in NAc with AAV-CreGFP showed decreased immobility time compared to AAV-GFP-injected Vgf flpflox/flpflox mice in the FST (n = 10 ~ 11/group). (J) No significant difference in locomotor activity was observed between NAc-AAV-CreGFP- and NAc-AAV-GFP-injected Vgf flpflox/flpflox mice in the OFT (n = 10 ~ 11/group). (K) Vgf flpflox/flpflox mice injected in NAc with AAV-CreGFP showed increased social interaction ratio compared to AAV-GFP-injected Vgf flpflox/flpflox mice following CSDS (n = 10/group for control, n = 19/group for defeated mice). (L) After CSDS, Vgf flpflox/flpflox mice injected in NAc with AAV-CreGFP spent significantly more time in the interaction zone when the target was present compared to AAV-GFP-injected Vgf flpflox/flpflox mice (n = 19/group). (M) Acute intrahippocampal infusion of anti-VGFC-term antibody (0.5 μg/side), which functionally neutralizes TLQP-62 and AQEE-30, significantly decreased the immobility time in the FST 30 min after treatment (n = 6 ~ 9/group). (N) Acute intrahippocampal infusion of anti-VGFC-term antibody (0.5 μg/side) did not affect locomotor activity (4 ~ 5/group). (O) Acute intrahippocampal infusion of anti-VGFC-term antibody (0.5 μg/side), 30 min before microdefeat, significantly induced susceptibility to stress-induced social avoidance (n = 14 ~ 16/group). (P) Acute intrahippocampal infusion of anti-VGFC-term antibody (0.5 μg/side), 30 min before microdefeat, significantly decreased the time spent in the interaction zone when target was present (n = 14 ~ 16/group). All data are presented as mean ± s.e.m. Student’s t test for B, C, H – J, M, N; Mann-Whitney test for D; Welch’s t test for O; two-way ANOVA followed by Fisher’s LSD test or Student’s t test for E, F, K, L, P (t* P < 0.05, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001).
In NAc, AAV-CreGFP-mediated Vgf gene ablation (Figure 3G, H) decreased immobility time in the FST (Figure 3I) without affecting locomotor activity (Figure 3J) and reduced social avoidance following CSDS (Figure 3K, L, Supplemental Figure S4D – F). Conversely, AAV-VGF-mediated VGF overexpression in NAc (Supplemental Figure S6A, B) increased immobility time in the FST (Supplemental Figure S6C, D).
Role for endogenous hippocampal VGF C-terminal peptides in the control of depression-like behaviors: targeted dHc antibody-mediated sequestration has a pro-depressant effect
To investigate the role of endogenous VGF C-terminal peptides in dHc in regulating depression-like behaviors, we intrahippocampally infused mice 30 min before testing with anti-VGFC-term antiserum (0.5 μg/side), which functionally neutralizes C-terminal peptides AQEE-30 and TLQP-62 13, 26, and found increased immobility time in the FST, without affecting locomotor activity (Figure 3M, N). Moreover, mice that received bilateral intrahippocampal anti-VGFC-term infusions 30 min before the first session of microdefeat showed significantly increased susceptibility to subthreshold social defeat stress (Figure 3O – P, Supplemental Figure S4G – I) compared to mice receiving control IgG.
Antidepressant efficacy of the rapid–acting antidepressant ketamine is reduced by germline and dorsal hippocampal VGF ablation
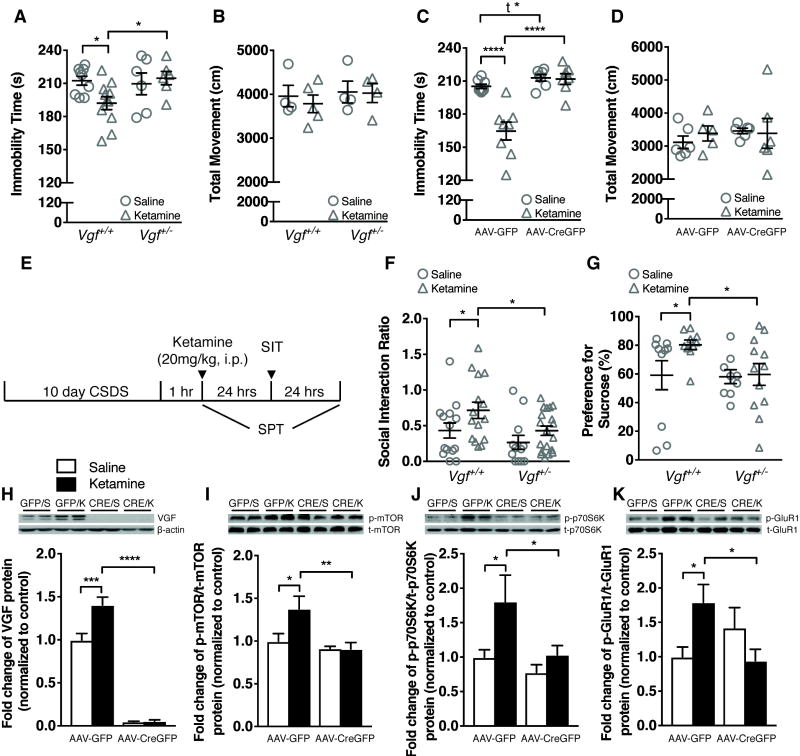
To determine whether VGF regulates the response to ketamine, we investigated the impact of VGF deficiency on the efficacy of acute ketamine treatment. Thirty min after ketamine treatment (20 mg/kg, i.p.), Vgf+/+ and AAV-GFP-injected Vgfflpflox/flpflox mice showed significant reduction in immobility time in the FST compared to saline-treated controls, while this effect was completely absent in heterozygous Vgf+/− knockout and intrahippocampal AAV-CreGFP-injected Vgfflpflox/flpflox mice (Figure 4A, C). Ketamine treatment did not affect locomotor activity (Figure 4B, D). Similarly, heterozygous Vgf+/− knockout and intrahippocampal AAV-CreGFP-injected Vgfflpflox/flpflox mice showed impaired responses to conventional antidepressant imipramine (Supplemental Figure S7A – E).
Figure 4.
VGF deficiencies in the germline and in dorsal hippocampus attenuate the efficacy of the rapid-acting antidepressant ketamine. (A) Acute ketamine treatment (20 mg/kg, i.p.) significantly reduced the immobility time in the FST in Vgf+/+ but not Vgf+/− mice, compared to saline treatment (n = 6 ~ 11/group). (B) Acute ketamine treatment (20 mg/kg, i.p.) did not affect the locomotor activity in both Vgf+/+ and Vgf+/− mice (n = 4 ~ 5/group). (C) Acute ketamine treatment (20 mg/kg, i.p.) significantly reduced the immobility time in the FST in dorsal hippocampal AAV-GFP but not AAV-CreGFP-injected Vgf flpflox/flpflox mice, compared to saline treatment (n = 7 ~ 8/group). (D) Acute ketamine treatment (20 mg/kg, i.p.) did not affect the locomotor activity in both dorsal hippocampal AAV-GFP- and AAV-CreGFP-injected Vgfflpflox/flpflox mice (n = 5 ~ 6/group). (E) Timeline of ketamine treatment paradigm and behavioral tests following CSDS. (F) Acute ketamine treatment (20 mg/kg, i.p.) 1 hr after the last defeat session of CSDS, increased social interaction ratio in Vgf+/+ but not Vgf+/− mice (n = 11 ~ 19/group). (G) Acute ketamine treatment (20 mg/kg, i.p.) 1 hr after the last defeat session of CSDS, increased sucrose preference in Vgf+/+ but not Vgf+/− mice (n = 9 ~ 12/group). (H) At 30 min time point, VGF expression was significantly increased in dHc total homogenates by ketamine treatment in AAV-GFP- but not AAV-CreGFP-injected Vgfflpflox/flpflox mice (n= 4 ~ 5/group). (I) At 30 min time point, the ratio of phospho-mTOR/total-mTOR was significantly increased in dHc total homogenates by ketamine treatment in AAV-GFP- but not AAV-CreGFP-injected Vgfflpflox/flpflox mice (n= 4 ~ 5/group). (J) At 30 min time point, the ratio of phospho-p70S6K/total-p70S6K was significantly increased in dHc total homogenates by ketamine treatment in AAV-GFP- but not AAV-CreGFP-injected Vgfflpflox/flpflox mice (n= 5/group). (K) At 30 min time point, the ratio of phospho-GluR1/total-GluR1was significantly increased in dHc total homogenates by ketamine treatment in AAV-GFP- but not AAV-CreGFP-injected Vgfflpflox/flpflox mice (n= 5/group). Densitometry images for panels (H–K) from left to right: duplicates of AAV-GFP/Saline, AAV-GFP/Ketamine, AAV-CreGFP/Saline and AAV-CreGFP/Ketamine. SIT: social interaction test; i.p.: intraperitoneal. All data are presented as mean ± s.e.m. Two-way ANOVA followed by Fisher’s LSD test or Student’s t test for A – D, F – K (t* P<0.05, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001).
Single dose ketamine treatment reverses social avoidance induced by CSDS 27. We found that a single dose of ketamine (20 mg/kg, i.p.) administered 1 hr after the last session of CSDS (Figure 4E) increased social interaction and sucrose preference in Vgf+/+ mice, compared to saline treatment (Figure 4F, G, Supplemental Figure S8A – C), but this effect was not observed in Vgf+/− mice. Ketamine efficacy is therefore significantly reduced by VGF ablation in the germline and in adult dorsal hippocampus.
Dorsal hippocampal VGF ablation blocks activation of the mTOR pathway and elevation of GluR1 phosphorylation at Ser845
To determine the molecular mechanisms underlying impaired ketamine response in intrahippocampal AAV-CreGFP-injected Vgfflpflox/flpflox mice, we examined mTOR signaling and phosphorylation of AMPA receptor subunit GluR1, as both have been implicated in ketamine’s antidepressant actions 20, 28. Western blot analysis demonstrated robustly increased VGF protein expression, and increased phosphorylation of mTOR (Ser2448) and its substrate p70S6K (Thr389) as well as GluR1 (Ser845), in dHc dissected from AAV-GFP-injected Vgfflpflox/flpflox mice 30 min after administration of ketamine (20 mg/kg, i.p.), compared to saline-treated controls (Figure 4H – K). No significant changes were observed in identically treated AAV-CreGFP-injected Vgfflpflox/flpflox mice.
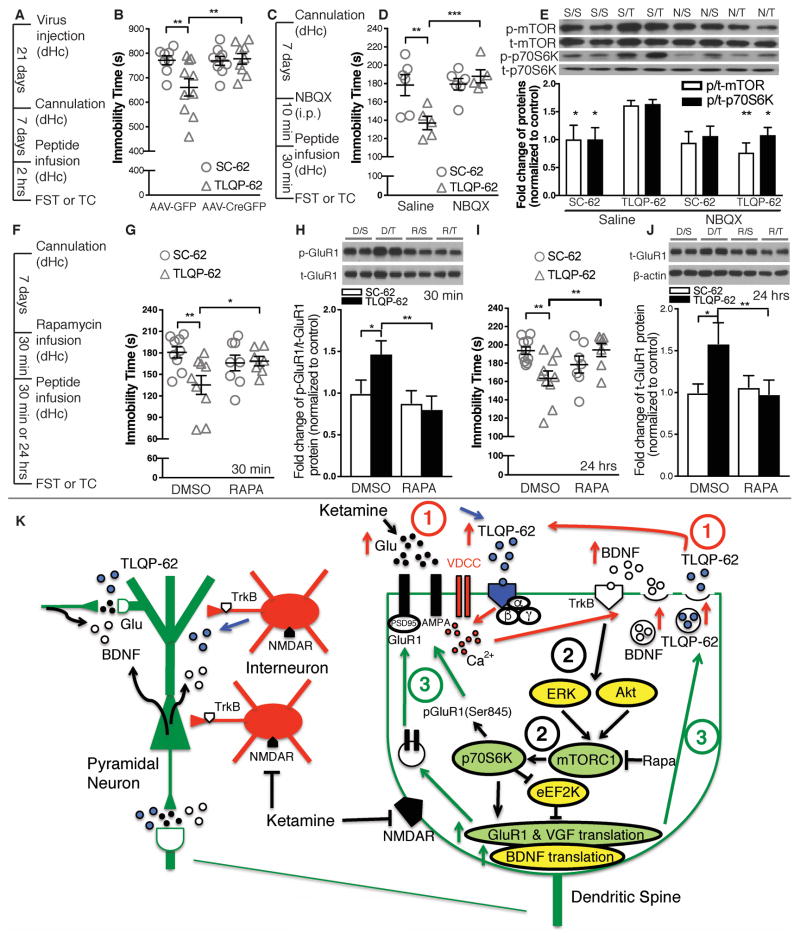
Similar to ketamine treatment, intrahippocampally administered TLQP-62 has acute antidepressant efficacy in the FST that requires BDNF, mTOR signaling, and AMPA receptor activation
Next, we tested whether the rapid antidepressant efficacy of TLQP-62 10, 12, 14 requires activation of AMPA receptor, mTOR signaling and BDNF, like ketamine 20, 21. Thirty min after intrahippocampal peptide administration, reduced immobility time was observed in the FST in TLQP-62 (0.5 μg/side)- but not scrambled peptide (SC-62)-infused mice, which was completely blocked by pretreatment with the AMPA receptor antagonist NBQX (10 min, 10 mg/kg, i.p.) (Figure 5C, D) and by intrahippocampal infusion of mTOR inhibitor rapamycin (30 min, 0.9 ng/side) (Figure 5F, G). Two hrs after intrahippocampal TLQP-62 infusion, an antidepressant effect was not detected in dorsal hippocampal AAV-CreGFP-injected BDNF floxed mice in the FST (Figure 5A, B), suggesting that dorsal hippocampal BDNF is required for TLQP-62’s behavioral effect. Conversely, intrahippocampal BDNF infusion and intraperitoneal injection of TrkB agonist 7,8-Dihydroxyflavone (7,8-DHF) were sufficient to induce antidepressant effects in both wildtype and Vgf +/− germline knockout mice (Supplemental Figure S9A, B). The antidepressant effect of dHc infused TLQP-62 lasted up to 24 hrs, which was completely blocked by local infusion of mTOR inhibitor rapamycin (Figure 5F, I).
Figure 5.
The antidepressant efficacy of acute intrahippocampal TLQP-62 infusion requires hippocampal BDNF, activation of AMPA receptor, and mTOR signaling. (A) Timeline of behavioral paradigm for AAV-CreGFP injected BDNFflox/flox mice receiving TLQP-62 infusion. (B) Dorsal hippocampal AAV-GFP injected, but not AAV-CreGFP injected BDNFflox/flox mice displayed reduced immobility time in the FST 2 hr after TLQP-62 (0.5 μg/side) infusion (n = 8 ~ 10/group) (15-min FST protocol). (C) Timeline of behavioral paradigm for wildtype mice receiving TLQP-62 infusion following pretreatment with NBQX. (D) Saline-pretreated but not NBQX-pretreated wildtype mice showed significantly decreased immobility time in the FST 30 min after TLQP-62 (0.5 μg/side) infusion (n = 5 ~ 6/group). (E) The ratio of phospho-mTOR/total-mTOR, and the ratio of phospho-p70S6K/total-p70S6K, were significantly increased in dHc total homogenates of saline-pretreated but not NBQX-pretreated wildtype mice, 30 min after TLQP-62 (0.5 μg/side) infusion (n = 4 ~ 5/group). Asterisks indicate significant changes (* P<0.05, ** P<0.01) as compared to Saline/TLQP-62 group. (F) Timeline of behavioral paradigm for wildtype mice receiving TLQP-62 infusion following pretreatment with rapamycin. (G) DMSO-pretreated but not rapamycin-pretreated wildtype mice showed significantly decreased immobility time in the FST, 30 min after TLQP-62 (0.5 μg/side) infusion (n = 7 ~ 9/group). (H) The ratio of phospho-GluR1/total-GluR1 was significantly increased in dHc total homogenates of DMSO-pretreated but not rapamycin-pretreated wildtype mice, 30 min after TLQP-62 (0.5 μg/side) infusion (n = 5 ~ 6/group). (I) DMSO-pretreated but not rapamycin-pretreated wildtype mice showed significantly decreased immobility time in the FST, 24 hours after TLQP-62 (0.5 μg/side) infusion (n = 7 ~ 10/group). (J) The total abundance of GluR1 was significantly increased in dHc synaptosome preparations from DMSO-pretreated but not rapamycin-pretreated wildtype mice, 24 hours after TLQP-62 (0.5 μg/side) infusion (n = 5 ~ 6/group). (K) Schematic model of VGF/TLQP-62 and BDNF actions in response to ketamine. Data supporting activation of pathways shaded in green is reported here, while modulation of those shaded in yellow has been inferred from published reports. We propose that ketamine and TLQP-62 trigger BDNF release (phase 1, red arrows, identified by the red number 1), stimulating BDNF/TrkB signaling and the mTOR pathway (phase 2, black arrows, identified by the black number 2), then rapid phosphorylation, local synthesis and insertion of GluR1, and rapid local synthesis and secretion of VGF (TLQP-62) and BDNF (phase 3, green arrows, identified by the green number 3), establishing a positive autoregulatory feedback loop in hippocampus, with antidepressant efficacy. Densitometry images for (E) from left to right: duplicates of Saline/SC-62, Saline/TLQP-62, NBQX/SC-62 and NBQX/TLQP-62, and for (H, J), duplicates of DMSO/SC-62, DMSO/TLQP-62, Rapamycin/SC-62 and Rapamycin/TLQP-62. dHc: dorsal hippocampus; FST: forced swim test; TC: tissue collection; i.p.: intraperitoneal; RAPA: rapamycin. All data are presented as mean ± s.e.m. Two-way ANOVA followed by Fisher’s LSD test for B, D, E, G - J (* P<0.05, ** P<0.01, *** P<0.001).
To determine the underlying molecular mechanism(s), the expression and phosphorylation levels of proteins in the mTOR pathway and GluR1 as well as VGF were examined by western blot analysis. Pretreatment with NBQX (10 min) blocked the elevation in mTOR and p70S6K phosphorylation and VGF level in dHc total homogenate 30 min after intrahippocampal TLQP-62 infusion (Figure 5E, Supplemental Figure S10A), suggesting that the antidepressant efficacy of TLQP-62 is associated with AMPA receptor-mediated mTOR pathway activation. At the same time point, TLQP-62 also increased Ser845 phosphorylation of GluR1 in dHc total homogenate, which together with increased VGF level, was abolished by local infusion of rapamycin prior to intrahippocampal TLQP-62 infusion (Figure 5H, Supplemental Figure S10B). Increased phosphorylation of mTOR and GluR1 lasted up to 2 hrs after TLQP-62 infusion, and was blocked by Cre-mediated BDNF knockout in dHc (Supplemental Figure S11A – C). As the behavioral effect of TLQP-62 infusion lasted 24 hrs, we measured GluR1 levels at this time point, and found elevation of GluR1 levels in synaptosomes 24 hrs after TLQP-62 infusion that was completely blocked by prior local infusion of rapamycin (Figure 5J), suggesting that TLQP-62 increases synaptic GluR1 expression in a rapamycin-sensitive manner.
DISCUSSION
Parallel region-specific regulation of VGF and BDNF expression is associated with depression-like behaviors in the human and mouse CNS
In mice, increased BDNF expression in and administration to hippocampus resulted in antidepressant outcomes 5, while in NAc, BDNF is pro-depressant 6. Here we demonstrate that VGF expression is decreased in hippocampus and increased in NAc in mice subjected to CSDS, consistent with VGF being regulated by the BDNF/TrkB signaling pathway that controls depression-like behaviors. Reduced VGF mRNA levels have been reported in leukocytes of depressed, unmedicated patients 11 and in the hippocampus and PFC (Brodmann area 9) of patients with bipolar disorder but not those with MDD 29. In our samples, VGF mRNA levels were downregulated in MDD patients in hippocampus (male and female), upregulated in male NAc and unchanged in female NAc, when compared to control healthy patients. Our finding of sexually dimorphic regulation of VGF expression in depressed patients is reminiscent of a previous meta-analysis of the BDNF Val66Met polymorphism in MDD 30–32, which found that this polymorphism is of greater importance in the development of MDD in men than in women 33. Male MDD patients have a greater symptomatic response to physical exercise than females, which is increased in older patients carrying the BDNF Met allele 34, while middle aged male carriers of the BDNF Met allele have greater depression symptoms than BDNF Val allele carriers 35. Interestingly, hippocampal VGF expression is robustly induced by prolonged voluntary exercise in male mice 9. VGF expression is therefore regulated in depressed human patients in a region-specific manner, like BDNF, that is sexually dimorphic in humans and is for the most part conserved in mouse models of depression, particularly in hippocampus. However in NAc, VGF mRNA levels are unchanged in susceptible male mice (VGF protein levels are elevated), while VGF mRNA levels are reduced in female mice exposed to SCVS. CSDS in male mice and SCVS in females are very different paradigms, so sex differences in VGF expression in mouse NAc could reflect sexual dimorphism or alternatively could result from the different types of stress applied in these models.
Like BDNF, VGF regulates depression-like behaviors in the CNS in a region-specific manner
We found that Vgf gene ablation in adult dHc induced susceptibility to social avoidance, while ablation in adult NAc increased resilience to CSDS-induced depression-like phenotypes. Importantly, VGF ablation in dHc and NAc in control unstressed mice did not change their social behaviors, suggesting that VGF-mediated effects on susceptibility require CSDS exposure. Consistent with these findings, phasic optogenetic activation of the VTA-NAc reward circuit only stimulates release of BDNF, synthesized in VTA and transported to NAc 6, 36, 37, in socially stressed but not stress-naïve mice, inducing social avoidance in the former but not the latter 37. In the FST, VGF overexpression achieved by local infusion of AAV-VGF, in adult dHc and NAc, had antidepressant and pro-depressant effects, respectively. Thus region-specific VGF function(s) in the adult limbic system parallel those of BDNF to regulate depression-like behaviors 5, 6. In hippocampus, down-regulation of BDNF or VGF results in a pro-depressant phenotype. Ablation of BDNF from the VTA, which projects to NAc, in which neuronal Bdnf gene expression is undetectable 38, has an antidepressant effect in CSDS 6 similar to AAV-Cre-mediated VGF ablation in NAc shown here. In contrast to targeted AAV-VGF infusion in adult NAc or dHc, more widespread VGF overexpression driven by the CMV-enhancer/chicken beta-actin (CAG) promoter in a single transgenic line resulted in working memory deficits, increased depression-like behavior, reduced striatal volume and brain weight, increased lateral ventricle volume, reduced anxiety, and hyperactivity, although random insertion of this Vgf transgene into Lingo2, variants of which are associated with Parkinson’s disease 39–41, resulting in reduced Lingo2 mRNA levels, could also potentially contribute to the phenotype of this line 42.
Previous studies have identified VGF mRNA and protein in NAc 43, 44, possibly in inhibitory interneurons, in which VGF is abundantly expressed in other brain regions including hippocampus 13. To gain insight into which VGF-expressing cell type(s) regulate depression-like behaviors, we determined that ablation of VGF in CNS neurons increased susceptibility to stress-induced social avoidance, while ablation in forebrain excitatory neurons did not, although it previously impaired fear memory 13. Together with the pro-depressant effect of AAV-Cre-mediated VGF ablation in dHc, our data suggest an important role for VGF, potentially synthesized in and secreted from inhibitory interneurons, on the excitatory circuits that express BDNF and control depression-like behaviors. GABAergic circuit dysfunction, reduced GABA release, and/or loss of parvalbumin-reactive GABA interneurons, in hippocampus, have been identified in other rodent models of depression 45, 46. Moreover, although representing only ~5% of total striatal neurons, local inhibitory interneurons are increasingly being investigated as critical modulators of the VTA-NAc reward circuit that regulates depression-like behaviors 47.
Expression of VGF in dorsal hippocampus is required for ketamine’s antidepressant efficacy and induction of mTOR signaling
Rapid-acting antidepressants such as ketamine, an NMDA receptor (NMDAR) antagonist, and GLYX-13, an NMDAR (glycine-site) partial agonist, with utility for treatment-resistant MDD 17, 48, stimulate expression of activity-regulated genes including Vgf [(Figure 4H) and 49], Bdnf, Arc, Dusp1 (dual specificity phosphatase 1), and Adnp (activity-dependent neuroprotective protein) 50–53, reversing susceptibility– and inducing resilience–associated molecular adaptations 51. Ketamine modulates mTOR signaling in prefrontal cortex 20 and eEF2 phosphorylation in hippocampus 54, increasing local translation of proteins that regulate synaptic plasticity, including BDNF 54. Antidepressant efficacy of ketamine was attenuated in BDNF conditional knockout and BDNFMet/Met mice, as well as in mice receiving anti-BDNF antibody infusion 54–56, suggesting that BDNF expression and release are required for drug efficacy. Our data indicate that germline and dorsal hippocampal VGF expression is required for ketamine’s antidepressant efficacy in the FST, that dHc VGF ablation prevents the activation of mTOR signaling in dHc, and thus suggests that ketamine may stimulate rapid, local synthesis of VGF in the absence of alterations in VGF mRNA levels. VGF mRNA has previously been detected in hippocampal synaptic neuropil, suggesting an association with axons and/or dendrites 57. Locally synthesized VGF could be a necessary component of the regulated secretory pathway 58 through which locally translated BDNF is released in response to ketamine treatment 54, 56, 59. Fast-acting glutamatergic antidepressants including ketamine and GLYX-13 activate mTORC1 and AMPA receptors, and rapidly stimulate extracellular signal-regulated kinase (ERK) signaling and BDNF release in primary cortical neuronal cultures 56, 59. A previously described key role for rapidly translated hippocampal BDNF in memory consolidation 60, together with a similar requirement for VGF and pro-cognitive efficacy of intrahippocampal TLQP-62 infusion 13, suggest the possibility that a related positive autoregulatory VGF/BDNF/TrkB feedback loop may be critical for antidepressant efficacy (Figure 5K). Thus rapid, local synthesis and regulated secretion of BDNF and VGF (and/or TLQP-62) at the synapse could be required for efficacy of ketamine and potentially other fast-acting glutamatergic antidepressants.
Intrahippocampal TLQP-62 produces antidepressant effects via mechanisms similar to ketamine
Previous studies have demonstrated an antidepressant response to administration of VGF-derived peptide AQEE30 9, and to chronic intrahippocampal treatment with TLQP-62, the latter associated with increased TrkB phosphorylation 12. Anti-VGFC-term antibody neutralizes hippocampal TLQP-62 and AQEE-30 (Figure 3M–P), binding epitopes within the C-terminal 19 amino acids of either peptide 26, which importantly suggests that endogenous, secreted VGF C-terminal TLQP-62 and/or AQEE-30 also have antidepressant efficacy, although this antibody does not allow the precise peptide to be discerned. Li et al. 14 recently reported that pretreatment with icv TLQP-62 prevents lipopolysaccharide (LPS)-induced depression- and anxiety-like behaviors in mice via a BDNF/TrkB-dependent pathway. Our data show that acute intrahippocampal infusion of TLQP-62 produces a rapid antidepressant effect in the FST within 30 min, which parallels the time course of ketamine treatment in animal studies 20, 54. BDNF/TrkB signaling in hippocampus 54 is required for the rapid antidepressant efficacy of ketamine, and acute ketamine treatment induces the translation of BDNF 54 and TrkB phosphorylation 61. Here we demonstrate a dependence of TLQP-62 antidepressant actions on BDNF and its signaling pathways in vivo, as noted previously in hippocampal slices, in vitro, where TLQP-62 treatment stimulated electrical excitability that was blocked by the BDNF scavenger TrkB-Fc 16.
Like ketamine 20, 21, antidepressant efficacy of TLQP-62 depended on AMPA receptor activation (blocked by NBQX) and mTOR signaling (blocked by rapamycin), and TLQP-62 infusion activated mTOR signaling (increased mTOR and p70S6K phosphorylation) via an AMPA receptor-mediated mechanism (phosphorylation blocked by NBQX). Acute intrahippocampal TLQP-62 infusion also altered markers of synaptic plasticity, increasing the Ser845 phosphorylation of GluR1 as early as 30 min. Phosphorylation of Ser845 regulates receptor conductance and synaptic delivery, and incorporation of GluR1-containing AMPA receptors 62. Similar to BDNF 63, TLQP-62 acts through mTOR signaling to regulate GluR1 phosphorylation, and induces the sustained up-regulation of GluR1 in dHc synaptosomes, in a rapamycin-sensitive manner, as does ketamine 20. Increased synaptic expression of GluR1 likely results in synaptic potentiation 64, which is consistent with TLQP-62 actions on hippocampal neurons and slices 8, 16. We propose that a positive autoregulatory feedback loop of rapidly translated BDNF and VGF is critically involved in ketamine’s mechanism of action (Figure 5K).
Supplementary Material
Acknowledgments
Supported in part by: NIH grants MH086499 (SRS), MH083496 (SRS), pilot grant on P50AT008661 (SRS; PI G.M. Pasinetti); MH090264 (SJR); Hope for Depression Research Foundation (SRS); BrightFocus Foundation (SRS); Brain and Behavior Research Foundation (SRS and CM); CM is a P&S Fund Investigator (Young Investigator Grant).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Contributions:
C.J., W.J.L, S.J.R., and S.R.S. designed research; C.J., W.J.L. and M.S. performed research; C.J., and W.J.L. analyzed data; C.A.T., G.T., C.M., M.L.P., S.J.R., B.L. and E.J.N. provided reagents/samples; C.J. and S.R.S. wrote the paper.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Keller MB, Gelenberg AJ, Hirschfeld RM, Rush AJ, Thase ME, Kocsis JH, et al. The treatment of chronic depression, part 2: a double-blind, randomized trial of sertraline and imipramine. J Clin Psychiatry. 1998;59(11):598–607. doi: 10.4088/jcp.v59n1107. [DOI] [PubMed] [Google Scholar]
- 3.Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 4.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 7.Bonni A, Ginty DD, Dudek H, Greenberg ME. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6(2):168–183. doi: 10.1006/mcne.1995.1015. [DOI] [PubMed] [Google Scholar]
- 8.Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23(34):10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13(12):1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 10.Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27(45):12156–12167. doi: 10.1523/JNEUROSCI.1898-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo A, Sesta A, Calabrese F, Nielsen G, Riva MA, Gennarelli M. The expression of VGF is reduced in leukocytes of depressed patients and it is restored by effective antidepressant treatment. Neuropsychopharmacology. 2010;35(7):1423–1428. doi: 10.1038/npp.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin P, Wang C, Xu B, Gao S, Guo J, Zhao X, et al. The VGF-derived peptide TLQP62 produces antidepressant-like effects in mice via the BDNF/TrkB/CREB signaling pathway. Pharmacol Biochem Behav. 2014;120:140–148. doi: 10.1016/j.pbb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Lin WJ, Jiang C, Sadahiro M, Bozdagi O, Vulchanova L, Alberini CM, et al. VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB-Dependent Mechanism. J Neurosci. 2015;35(28):10343–10356. doi: 10.1523/JNEUROSCI.0584-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Li M, Yu H, Shen X, Wang J, Sun X, et al. Neuropeptide VGF C-Terminal Peptide TLQP-62 Alleviates Lipopolysaccharide-Induced Memory Deficits and Anxiety-like and Depression-like Behaviors in Mice: The Role of BDNF/TrkB Signaling. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.7b00154. [DOI] [PubMed] [Google Scholar]
- 15.Thakker-Varia S, Behnke J, Doobin D, Dalal V, Thakkar K, Khadim F, et al. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res. 2014;12(3):762–777. doi: 10.1016/j.scr.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28(39):9857–9869. doi: 10.1523/JNEUROSCI.3145-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 18.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 19.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35(50):16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty TR, Tkalych O, Nanno D, Garcia AL, Devi LA, Salton SR. Quantification of VGF- and pro-SAAS-derived peptides in endocrine tissues and the brain, and their regulation by diet and cold stress. Brain Res. 2006;1089(1):21–32. doi: 10.1016/j.brainres.2006.02.124. [DOI] [PubMed] [Google Scholar]
- 27.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76(7):550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016;9(458):ra123. doi: 10.1126/scisignal.aai7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakker-Varia S, Jean YY, Parikh P, Sizer CF, Jernstedt Ayer J, Parikh A, et al. The neuropeptide VGF is reduced in human bipolar postmortem brain and contributes to some of the behavioral and molecular effects of lithium. J Neurosci. 2010;30(28):9368–9380. doi: 10.1523/JNEUROSCI.5987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25(26):6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 34.Dotson VM, Hsu FC, Langaee TY, McDonough CW, King AC, Cohen RA, et al. Genetic Moderators of the Impact of Physical Activity on Depressive Symptoms. J Frailty Aging. 2016;5(1):6–14. doi: 10.14283/jfa.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gujral S, Manuck SB, Ferrell RE, Flory JD, Erickson KI. The BDNF Val66Met polymorphism does not moderate the effect of self-reported physical activity on depressive symptoms in midlife. Psychiatry Res. 2014;218(1–2):93–97. doi: 10.1016/j.psychres.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2012 doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17(1):27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17(7):2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilarino-Guell C, Wider C, Ross OA, Jasinska-Myga B, Kachergus J, Cobb SA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010;11(4):401–408. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu YW, Prakash KM, Rong TY, Li HH, Xiao Q, Tan LC, et al. Lingo2 variants associated with essential tremor and Parkinson’s disease. Hum Genet. 2011;129(6):611–615. doi: 10.1007/s00439-011-0955-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Cao B, Yang J, Wei Q, Ou RW, Zhao B, et al. Analysis and meta-analysis of five polymorphisms of the LINGO1 and LINGO2 genes in Parkinson’s disease and multiple system atrophy in a Chinese population. J Neurol. 2015;262(11):2478–2483. doi: 10.1007/s00415-015-7870-9. [DOI] [PubMed] [Google Scholar]
- 42.Mizoguchi T, Minakuchi H, Ishisaka M, Tsuruma K, Shimazawa M, Hara H. Behavioral abnormalities with disruption of brain structure in mice overexpressing VGF. Sci Rep. 2017;7(1):4691. doi: 10.1038/s41598-017-04132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol. 1998;394(1):91–105. [PubMed] [Google Scholar]
- 44.van den Pol AN, Bina K, Decavel C, Ghosh P. VGF expression in the brain. J Comp Neurol. 1994;347(3):455–469. doi: 10.1002/cne.903470311. [DOI] [PubMed] [Google Scholar]
- 45.Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN, Wiborg O, et al. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2011;21(4):422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- 46.Peruga I, Hartwig S, Merkler D, Thone J, Hovemann B, Juckel G, et al. Endogenous ciliary neurotrophic factor modulates anxiety and depressive-like behavior. Behav Brain Res. 2012;229(2):325–332. doi: 10.1016/j.bbr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21(2):140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 49.Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, et al. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol. 2014;18(5) doi: 10.1093/ijnp/pyu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown BP, Kang SC, Gawelek K, Zacharias RA, Anderson SR, Turner CP, et al. In vivo and in vitro ketamine exposure exhibits a dose-dependent induction of activity-dependent neuroprotective protein in rat neurons. Neuroscience. 2015;290:31–40. doi: 10.1016/j.neuroscience.2014.12.076. [DOI] [PubMed] [Google Scholar]
- 51.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol Psychiatry. 2017;81(4):285–295. doi: 10.1016/j.biopsych.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue W, Wang W, Gong T, Zhang H, Tao W, Xue L, et al. PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of Yueju but not ketamine. Sci Rep. 2016;6:26331. doi: 10.1038/srep26331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS, et al. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A. 2015;112(51):15755–15760. doi: 10.1073/pnas.1513913112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74(3):453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin WJ, et al. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J. 2014;28(5):2120–2133. doi: 10.1096/fj.13-239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM. A positive autoregulatory BDNF feedback loop via C/EBPbeta mediates hippocampal memory consolidation. J Neurosci. 2014;34(37):12547–12559. doi: 10.1523/JNEUROSCI.0324-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reus GZ, Abaleira HM, Titus SE, Arent CO, Michels M, da Luz JR, et al. Effects of ketamine administration on the phosphorylation levels of CREB and TrKB and on oxidative damage after infusion of MEK inhibitor. Pharmacol Rep. 2016;68(1):177–184. doi: 10.1016/j.pharep.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6(2):136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 63.Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4(6):e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281(2):752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.