INTRODUCTION
Progressive fibrosis is the most important predictor for liver-related outcomes among patients with nonalcoholic fatty liver disease (NAFLD). Signaling by extracellular nucleotides, such as extracellular ATP (eATP) and adenosine, are ubiquitous pathways involved in immune regulation and tissue regeneration.1 Ectonucleoside triphosphate diphosphohydrolases (ENTPD) rapidly phosphohydrolyze eATP and eADP to AMP, which is then converted to adenosine by 5′-nucleotidase (ecto-5′-NT). Adenosine deaminase (ADA) converts adenosine or deoxyadenosine to inosine and derivatives. Two ADA enzymes exist in humans. While ADA1 is ubiquitous both as an intracellular enzyme and an ecto-enzyme on the surface of selected lymphocytes and endothelial cells through CD26, ADA2 is a largely secreted protein produced by activated monocytes and dendritic cells.2 Recent studies showed that loss-of-function mutations in ADA2 render a proinflammatory phenotype in macrophages, manifesting clinically in vasculitis and early stroke.3, 4
METHODS
We measured the rate of three tandem enzyme activities in eADP metabolism in the serum of 100 biopsy-proven NAFLD patients and studied their cross-sectional association to liver fibrosis (Figure 1A). These patients were selected from a previously described prospective NAFLD registry to approximate an equal distribution of fibrosis stages.5 Blood samples were collected on the index visit. Liver biopsies performed within three months of enrollment were reviewed by a hepatobiliary pathologist to ensure the consistency of pathological interpretation. ADPase and 5′-nucleotidase activities were measured using 14C-ADP and 14C-AMP as substrate as described previously.6 Total ADA and ADA2 activities were measured using Adenosine Deaminase Assay kits (Diazyme). Immunohistochemistry was performed on formalin-fixed, paraffin-embedded biopsies using a rabbit anti-ADA2 polyclonoal antibody (Sigma-Aldrich). Lab researchers were blinded to the histopathological diagnosis. All statistical analyses were performed using Stata13.1.
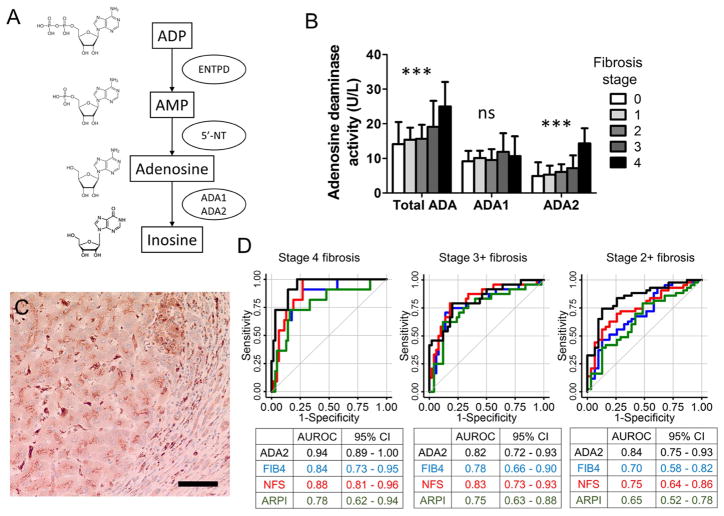
Figure 1.
A. A schematic diagram of extracellular ADP metabolism shows the three enzymatic steps measured in this study. B. The serum activity of ADA2 increases with the stage of liver fibrosis in NAFLD (***, p < 0.001). C. An immunohistochemistry of ADA2 in a liver biopsy with stage 4 fibrosis. Scale bar represents 50 μm. D. ROC analysis comparing the predictive value of serum ADA2 activity (black) for liver fibrosis with established fibrosis indices: FIB4 (blue), NFS (red) and APRI (Green).
RESULTS
Among 100 patients in the study, 41 were female, 89 were diagnosed with NASH, 57 had stage 2–4 fibrosis, and 12 had cirrhosis. Among the three enzymatic steps, the rate of conversion from adenosine to inosine via ADA was significantly higher among those with stage 2–4 fibrosis compared to those with stage 0–1 fibrosis (18.7 ± 6.9 vs. 14.8 ± 5.0 U/L), whereas the rates of conversion from ADP to AMP and AMP to adenosine were not significantly different. Between the two ADA isoforms, ADA2 accounted for this difference in the deaminase activity (Figure 1B). In a sensitivity analysis excluding stage 4 fibrosis, ADA2 activity remains strongly associated with the stage of fibrosis (p = 0.001). There was no ADA2 activity difference between those with and without NASH, although the number of patients with simple steatosis was small (n=11). Immunohistochemistry of the liver tissue demonstrated intracellular ADA2 staining in mononuclear cells in the portal area, fibrotic bands and sinusoidal cells, consistent with the distribution of macrophages and Kupffer cells (Figure 1C).
To evaluate the diagnostic value of serum ADA2 activity, receiver operating curve (ROC) analysis was performed. Compared to conventional indices of liver fibrosis, the performance of serum ADA2 activity was similar or superior than FIB4, NFS and APRI in predicting cirrhosis, stage 3+ and stage 2+ liver fibrosis with an area under the ROC (AUROC) of 0.94, 0.82 and 0.84 respectively (Figure 1D).
DISCUSSION
This study showed a positive cross-sectional association between the circulating activity of ADA2, a macrophage-derived deaminase, and liver fibrosis in NAFLD. The emerging association between ADA2 deficiency and vasculitis suggests that a potential mechanistic link underscoring this association, in which ADA2 modulates macrophage phenotype and impacting fibrogenesis.
ADA2 expression was observed in both infiltrative and resident macrophages in the liver. Defects in ADA2 have been linked to impaired macrophage M2 polarization in vitro, a phenotype that has been thought to be largely anti-inflammatory, but can also lead to fibrosis during repair and regeneration.4, 7 A positive association between serum ADA2 activity and liver fibrosis is potentially driven by a shift of macrophage polarization toward an anti-inflammatory, but profibrotic M2 phenotype. The molecular mechanism underlying ADA2-driven M2 polarization and its impact on liver fibrosis remains to be delineated.
Our study demonstrated that serum ADA2 activity is of diagnostic value in predicting liver fibrosis. Its performance is non-inferior to validated noninvasive fibrosis indices such as FIB4, NFS and APRI in our cohort. It will be interesting to know whether ADA2 activity correlates with soluble CD163 (sCD163), a biomarker for liver fibrosis in various etiologies that is also related to macrophage activity.8 Further study is needed to clarify the molecular mechanism of ADA2 in regulating fibrosis and to validate its diagnostic value in NAFLD as well as other forms of chronic liver disease, and in larger cohorts that can reflect the disease spectrum in the community.
Acknowledgments
Grant support
This work is in part supported by a Clinical Research Award from the American College of Gastroenterology (ACG) and the Alan Hoffman Clinical and Translational Research Award from the American Association for the Study of Liver Diseases (AASLD) to ZGJ, National Institute of Health (NIH) grants to SCR (P01HL107152, R21CA164970) and ML (K23DK083439).
Footnotes
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Author contributions
ZGJ, EUY, EC, BS, LF, SM, JH contributed to the experimental design and data collection; ML is involved in the management of patient registry; ZGJ, EUY, BS, LF, NHA, SCR, and ML contributed to manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367(24):2322–33. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zavialov AV, Engstrom A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391(Pt 1):51–7. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–31. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–20. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang ZG, Tapper EB, Connelly MA, et al. Steatohepatitis and liver fibrosis are predicted by the characteristics of very low density lipoprotein in nonalcoholic fatty liver disease. Liver Int. 2016 doi: 10.1111/liv.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang ZG, Wu Y, Csizmadia E, et al. Characterization of circulating microparticle-associated CD39 family ecto-nucleotidases in human plasma. Purinergic Signal. 2014;10(4):611–8. doi: 10.1007/s11302-014-9423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braga TT, Agudelo JS, Camara NO. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazankov K, Barrera F, Moller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60(2):521–30. doi: 10.1002/hep.27129. [DOI] [PubMed] [Google Scholar]