Abstract
Background
CDC guidelines recommend caution in prescribing opioids for chronic pain. The characteristics of opioid prescription (OpRx) among kidney transplant (KTx) recipients has not been described in a national population.
Methods
We assessed OpRx prevalence among prevalent KTx recipients, and associated duration (chronic, defined as ≥90 days in a year) and dosing (in morphine milligram equivalents per day, MME, of <50, 50–89, and ≥90) with outcomes, death and graft loss, among incident KTx recipients using 2006–2010 US Renal Data System files, including Medicare Part D for medication ascertainment. Cox models controlled for recipient factors.
Results
Of 36,486 KTx recipients in the 2010 prevalent cohort, approximately 14.6% had chronic OpRx. The strongest association with chronic OpRx after KTx was chronic OpRx before KTx (64%; adjusted odds ratio, 95% confidence interval [CI]: 95.2, 74.2–122.1). Incident KTx recipients with chronic OpRx had increased risk of mortality and graft loss compared with those without OpRx or short-term OpRx after KTx. This risk was highest among recipients with chronic OpRx doses of ≥90 MME (adjusted hazard ratio, 95% CI: 1.61, 1.24–2.10 for death, and 1.33, 1.05–1.67 for graft loss, respectively).
Conclusions
In contrast to either no or short-term OpRx, chronic, and especially chronic high dose OpRx is associated with increased risk of death and graft loss in US KTx recipients. Causal relationships cannot be inferred, and OpRx may be an illness marker. Nevertheless, efforts to treat pain effectively in KTx recipients with less toxic interventions and decrease OpRx deserve consideration.
INTRODUCTION
A substantial literature exists on use of opioid prescriptions in the pretransplant period for both liver and kidney transplants and among kidney donors.1–4 More than a quarter of kidney transplant recipients filled a narcotic prescription in the year prior to transplant.3 The highest quartile (≥23.8 mg/kg) of narcotic use prior to transplantation was associated with increased morbid outcomes after transplantation. The prevalence, predictors, and prognosis of opioid prescriptions after transplantation has not been reported in a national population. Chronic opioid prescription (generally defined as ≥90 days in a calendar year5) is not considered appropriate, particularly for noncancer pain for which a comprehensive benefit to harm evaluation is recommended.6
We showed opioid medication prescriptions are more common among dialysis patients than the general Medicare population, and chronic opioid prescriptions are associated with increased risk of mortality, termination of dialysis, and hospitalizations in a dose related manner.7 A single-center report8 indicated 11.4% of 1,045 kidney transplant recipients from 2004–2008 continued to receive outpatient prescription of opioid analgesics during the year after kidney transplant, mostly for nonsurgery related pain. Continued prescription of opioids was associated with more frequent hospitalization, but not with acute rejection rates or overall mortality. The authors concluded continued opioid prescription in this setting was not associated with mortality or other adverse outcomes, based on small sample size and relatively brief followup.
We assessed the prevalence and duration of opioid medication prescriptions in US kidney transplant recipients, factors associated with prescription, and determined associations between dose of such prescription and mortality and graft loss, using the most recently available data from the United States Renal Data System (USRDS) and the Centers for Medicare & Medicaid Services (CMS), including Medicare Part D files for prescription information.
MATERIALS and METHODS
Study design, data sources, and sample selection
Using standard analysis files from the USRDS, a national end-stage renal disease (ESRD) registry, we performed a retrospective cohort study to 1) describe trends in the proportion of prevalent transplant recipients who received chronic opioid medication prescriptions, overall and for specific opioids, from 2006 to 2010, 2) examine factors associated with chronic opioid medication prescriptions in 2010 prevalent transplant recipients, and 3) examine associations of all-cause death and of graft loss with dose of filled chronic opioid prescription in recipients having a first transplant in 2007–2009.
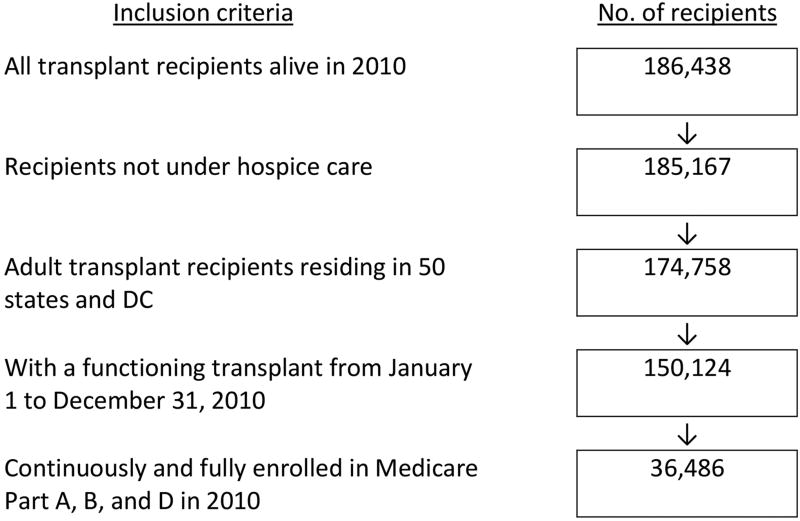
Two sets of cohorts (prevalent transplant cohorts for the first and second objectives, and an incident transplant cohort for the third) were identified. We identified annual cohorts of adult recipients ≥20 years old, who resided in one of the 50 states or Washington, DC, and were not under hospice care (identified through claims with the place of service listed as hospice) in 2006–2010. To ensure complete claims data for recipients, the cohorts were further limited to recipients with a full year functioning graft, who had Medicare as their primary payer and full Part A, B, and D coverage in each study year. The selection of transplant recipients in year 2010 is illustrated (Figure 1). Based on the same criteria, all living and eligible recipients with a functioning transplant from January 1 to December 31 in each calendar year were included in the remaining annual cohorts for 2006–2009.
Figure 1.
Selection of prevalent transplant recipients continuously enrolled in Medicare Part A, B and D in 2010
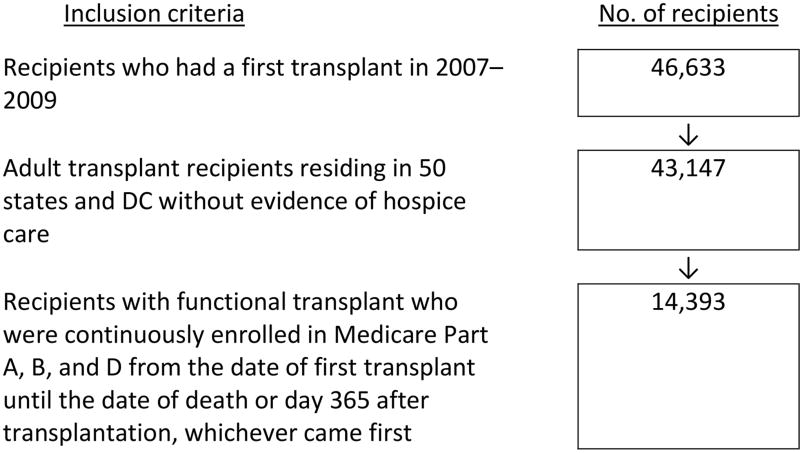
For the third objective, we identified adult recipients of a first kidney transplant in 2007–2009 who were not under hospice care and resided in one of the 50 states or Washington, DC. To ensure complete claims data to define recipients’ status of filled opioid prescription after transplantation, the cohort was further limited to recipients with full and continuous Part A, B, and D coverage for whom Medicare was the primary payer from the date of transplant until the date of death or day 365 after transplantation, whichever came first. The selection of the incident cohort is illustrated in Figure 2.
Figure 2.
Selection of incident transplant recipients who had a first transplant in 2007–2009
Study measures
For each recipient, Part D prescription claims data were used to determine whether a recipient had ever filled an opioid medication prescription (Table S1), and the days’ supply for each prescription obtained from an outpatient pharmacy during a given calendar year or in a specified observation period.
For the prevalent cohorts, the total days’ supply per year is the sum of the days’ supply for all the recipient’s opioid prescriptions during the year, whether or not the dates of the prescriptions overlapped or continued. We identified a recipient as having a chronic opioid prescription if the recipient’s total supply for a study year was ≥90 days, as having a short-term opioid prescription if the total supply for the year was 1 to 89 days, and as having no prescription if the total days’ supply for the year was 0, consistent with previous definitions of chronicity.5
For the incident cohort, we identified recipients with no, short-term, or chronic opioid prescriptions before transplant among recipients with continuous Part D coverage for at least 1 year prior to transplant. For recipients’ opioid prescriptions after transplant, we identified the date of the 90th cumulative day with a filled opioid prescription as the date of chronic opioid prescription, and calculated the morphine milligram equivalent (MME) dosage for each opioid prescription, using established conversion tables.9,10 We considered overlapping opioid prescriptions per day and calculated a daily MME from the total MME for all opioid prescriptions filled from the first through the 90th cumulative day within 1 year after transplant dividing by 90. We did not have information regarding the indication for which an opioid was prescribed.
Medicare inpatient billing data were used to 1) identify recipients’ diagnosis of cancer, 2) whether they had at least 1 pain-related or 1 mental health–related hospitalization, and 3) the number of hospitalizations in each calendar year. Indicator variables were set for recipients with at least 1 hospitalization for cancer, at least 1 hospitalization with a pain diagnosis, and at least 1 hospitalization with a mental health diagnosis (Tables S2 and S3). In additional to inpatient claims, recipients’ cancer status was also identified using the CMS Medical Evidence Form (CMS-2728). Recipients’ date of cancer diagnosis was assigned as the first hospitalization with a cancer diagnosis or, for recipients identified as having cancer by CMS-2728, the date of ESRD initiation, whichever came first. Recipients’ total number of hospitalizations in each study year were counted (1–2, 3–4, or 5 or more hospital admissions).
Medicare physician/supplier billing data were used to indicate whether a recipient resided in a nursing home, identified by at least one claim with the place of service listed as nursing home.
We assigned recipients dual status if they were eligible for insurance coverage by both Medicare and Medicaid in Medicare Part A, B, or D for at least 1 month in each study year.7,11
Recipient characteristics were taken from the CMS-2728 and from information collected by the Organ Procurement and Transplantation Network, including self-reported gender, race (White or non-White), education (high school or less, some college or higher), employment and smoking status, as well as comorbid conditions.7,12 Recipient age was calculated as of January 1 of each relevant year, or at the date of transplantation (20–44, 45–64, or 65 and over). Based on the year of the first transplant, recipients’ kidney transplant vintage for each year was assigned.13 For the incident cohort, based on the date of ESRD initiation, recipients’ ESRD vintage on the date of the first transplant was also assigned. We dichotomized type of residential area into rural area and other, using the National Center for Health Statistics Urban–Rural Classification Scheme for counties.14 Each recipient was assigned a median household income (<$45,000, $45,000–$74,999, or ≥$75,000) based on residential ZIP code, using 2007–2011 U.S. Census American Community Survey 5-year estimates.15
Outcomes
Outcomes of interest for the incident transplant cohort were all-cause graft loss and death. Recipients were followed from the date of transplant for all-cause graft loss and censored on December 31, 2013. All-cause graft loss included re-transplant, return to regular dialysis, and death. Recipients were also followed for all-cause death and censored on December 31, 2013. The death outcome was not censored at graft loss, and included deaths that occurred after re-transplant or return to dialysis.
Statistical analysis
Baseline characteristics of the cohorts were summarized using descriptive statistics and reported as percentages or mean ± standard deviation. We calculated annual percentage distributions for age group, gender, race, education, employment status, income, dual status, residential area, transplant vintage, nursing home residence, cancer status, pain-related hospitalization, mental health–related hospitalization, and number of hospitalizations in the 2006–2010 prevalent transplant cohorts. The annual proportion of transplant recipients with chronic opioid prescriptions, overall and for specific opioids, during 2006–2010 was also determined.
Logistic regression models were used to identify factors associated with chronic prescription of any opioid medication in the 2010 prevalent transplant cohort and factors associated with chronic opioid prescription after transplantation in the incident cohort. Cox regression models were specified to assess associations of time to all-cause death and time to graft loss, separately, with dose of filled chronic opioid prescriptions, accounting for changes in the status with dosage of chronic opioid prescriptions during follow-up. The mean MME for the first through 90th cumulative filled days of opioid prescriptions after transplant was used to indicate the dose of the prescription from the date of the chronic opioid prescription through the end of follow-up. All recipient-level demographic characteristics, ZIP code-level median income, medical conditions, and if a new cancer occurred after transplantation (as a time-dependent covariate) were included in multivariate-adjusted Cox models. Sensitivity analyses that limited follow-up to December 31 of 2010, 2011, and 2012 were performed. Statistical significance was defined as p<0.05 using two-tailed tests. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 shows the annual cohorts of adult transplant recipients with continuous functioning grafts, and who were continuously enrolled in Medicare Part A, B, and D in a specific year, by selected characteristics. There were approximately 21,000–36,500 transplant recipients each year during 2006–2010.
Table 1.
Distribution by selected characteristics of adult transplant recipients with continuous enrollment in Medicare Part A, B, and D, 2006–2010*
| Characteristics | 2006 (n=21,030) |
2007 (n=30,299) |
2008 (n=32,586) |
2009 (n=34,153) |
2010 (n=36,486) |
|---|---|---|---|---|---|
| Age group | |||||
| 20–44 | 35.1% | 30.3% | 28.5% | 27.0% | 25.3% |
| 45–64 | 44.9% | 45.8% | 45.6% | 45.3% | 45.7% |
| 65+ | 20.0% | 23.9% | 25.9% | 27.7% | 29.0% |
| Gender | |||||
| Male | 55.9% | 57.9% | 58.0% | 58.1% | 58.3% |
| Female | 44.1% | 42.1% | 42.0% | 41.9% | 41.7% |
| Race | |||||
| White | 68.8% | 70.1% | 70.3% | 70.2% | 69.9% |
| Non-White | 31.2% | 29.9% | 29.7% | 29.8% | 30.1% |
| Education | |||||
| High school or less | 44.6% | 44.7% | 45.6% | 46.6% | 47.0% |
| Some college or higher | 21.3% | 23.7% | 25.2% | 26.3% | 27.4% |
| Unknown/Missing | 34.1% | 31.6% | 29.2% | 27.1% | 25.6% |
| Employment status | |||||
| Working | 22.0% | 22.4% | 21.7% | 21.2% | 21.1% |
| Not working | 60.9% | 60.7% | 61.7% | 63.0% | 63.8% |
| Unknown/Missing | 17.1% | 16.9% | 16.5% | 15.9% | 15.1% |
| ZIP code median income | |||||
| < $45,000 | 48.4% | 47.1% | 46.4% | 45.9% | 45.5% |
| $45,000 – $74,999 | 41.8% | 42.4% | 42.7% | 43.0% | 43.1% |
| ≥ $75,000 | 9.8% | 10.6% | 11.0% | 11.2% | 11.5% |
| Dual status† | 80.0% | 64.0% | 61.5% | 59.9% | 58.4% |
| Residential area: rural | 20.6% | 21.0% | 20.8% | 20.5% | 20.4% |
| Nursing home residence‡ | 2.4% | 2.1% | 2.3% | 2.3% | 2.6% |
| Transplant vintage | |||||
| 1–5 years | 46.9% | 45.0% | 44.8% | 43.5% | 42.1% |
| 6–10 years | 25.7% | 26.7% | 26.6% | 27.5% | 28.0% |
| 11+ years | 27.4% | 28.3% | 28.6% | 29.0% | 29.9% |
| Cancer§ | 2.4% | 2.7% | 3.0% | 3.0% | 3.1% |
| Pain-related hospitalization | 3.1% | 3.0% | 3.4% | 3.4% | 3.6% |
| Mental health-related hospitalization | 9.4% | 8.4% | 9.4% | 8.8% | 9.9% |
| Number of hospitalizations | |||||
| 0 | 62.1% | 64.0% | 63.7% | 64.8% | 65.0% |
| 1–2 | 27.6% | 26.9% | 26.7% | 26.2% | 26.2% |
| 3–4 | 6.8% | 6.2% | 6.3% | 6.0% | 5.9% |
| 5 or more | 3.5% | 3.0% | 3.3% | 3.0% | 2.9% |
The same patient might have been included in multiple years.
Dual status in Medicare and Medicaid
On the basis of one or more claims in physician/carrier files
On the basis of one or more inpatient stays with a cancer diagnosis and Form 2728
The prevalent cohort (n=36,486 in 2010; Figure 1) was different from the parent ESRD transplant population (n=186,438) in that it had proportionally more women, Black recipients, recipients with a greater number of hospitalizations in 2010, and older recipients (data not shown). The study population was predominantly male, and White, consistent with the Medicare ESRD transplant population. About 10% lived in neighborhoods with ZIP code median household incomes ≥$75,000, over 45% in neighborhoods with incomes <$45,000, and fewer than 45% in neighborhoods with incomes $45,000–$74,999. About 60% were dual eligible for Medicare and Medicaid, reflecting the fact that all dual coverage beneficiaries receive Part D coverage, while it is optional for other recipients. Over half had transplant vintage greater than 5 years. About 35% had one or more hospital admissions, 2% were nursing home residents, and 20% lived in rural areas. Information on education level was unavailable for about 30% of the study population. Information on employment status was unavailable for more than 15%. However, among those for whom information was available, about 35% had some college or higher and 25% were employed (Table 1).
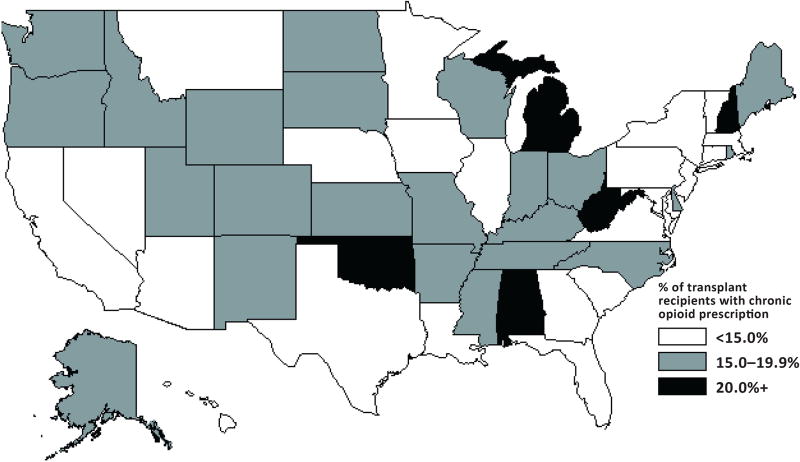
During 2006–2010, about 48% of transplant recipients had a prescription filled for an opioid medication (48.3% in 2006 and 47.9% in 2010; data not shown). During the study period, about 14% of transplant recipients (13.7% in 2006 and 14.6% in 2010) had ≥90 days of filled chronic opioid prescriptions (Table 2). Chronic opioid prescription ranged from 4.2% of recipients in Hawaii to 23.1% of recipients in West Virginia in 2010 (with 22.5% in Oklahoma, 21.6% in Alabama, 20.9% in Michigan, and 20.7% in New Hampshire) (Figure 3). Highest opioid prescription rates were among women, recipients 45–64 years old, recipients living in poorer neighborhoods or rural areas, and nursing home residents. Dual eligible status and unemployment were also associated with higher rates of chronic opioid prescription. Both pain-related and mental health–related hospitalizations were associated with higher chronic opioid prescription, as was a greater number of hospitalizations.
Table 2.
Percent of adult transplant recipients continuously enrolled in Medicare Part A, B and D who had prescriptions for ≥ 90 days of an opioid, by year
| Characteristics | 2006 (n=21,030) |
2007 (n=30,299) |
2008 (n=32,586) |
2009 (n=34,153) |
2010 (n=36,486) |
|---|---|---|---|---|---|
| Total | 13.7% | 13.6% | 14.3% | 14.4% | 14.6% |
| Age group | |||||
| 20–44 | 11.0% | 10.9% | 12.0% | 12.2% | 12.5% |
| 45–64 | 16.0% | 15.5% | 16.6% | 16.8% | 17.1% |
| 65+ | 13.1% | 13.1% | 12.6% | 12.6% | 12.5% |
| Gender | |||||
| Male | 11.7% | 11.7% | 12.5% | 12.4% | 12.8% |
| Female | 16.2% | 16.1% | 16.7% | 17.1% | 17.1% |
| Race | |||||
| White | 14.8% | 14.8% | 15.4% | 15.4% | 15.5% |
| Non-White | 11.3% | 10.8% | 11.6% | 11.9% | 12.6% |
| Education | |||||
| High school or less | 14.0% | 13.5%* | 14.1%* | 14.1%* | 14.9% |
| Some college or higher | 12.4% | 12.9%* | 13.5%* | 13.6%* | 13.1% |
| Employment status | |||||
| Working | 12.7% | 12.2% | 12.8% | 13.2% | 13.1% |
| Not working | 14.1% | 14.0% | 14.8% | 14.8% | 15.1% |
| ZIP code median income | |||||
| < $45,000 | 14.6% | 14.4% | 15.2% | 15.6% | 15.9% |
| $45,000 – $74,999 | 13.2% | 13.3% | 13.8% | 13.8% | 14.1% |
| ≥ $75,000 | 10.7% | 11.0% | 11.2% | 11.5% | 11.3% |
| Dual status† | |||||
| No | 10.8% | 10.7% | 11.2% | 11.6% | 11.3% |
| Yes | 14.4% | 15.2% | 16.2% | 16.2% | 16.9% |
| Residential area | |||||
| Rural | 16.2% | 15.4% | 16.4% | 16.7% | 16.6% |
| Non-rural | 13.1% | 13.1% | 13.7% | 13.8% | 14.1% |
| Nursing home residence‡ | |||||
| No | 13.5% | 13.4% | 14.0% | 14.2% | 14.3% |
| Yes | 23.6% | 23.0% | 23.9% | 22.4% | 25.4% |
| Transplant vintage | |||||
| 1–5 years | 11.6% | 11.6% | 12.2% | 12.2% | 12.5% |
| 6–10 years | 14.4% | 14.1% | 14.8% | 14.8% | 14.9% |
| 11+ years | 16.6% | 16.1% | 16.9% | 17.2% | 17.3% |
| Cancer§ | |||||
| No | 13.5% | 13.4% | 14.1% | 14.3% | 14.4% |
| Yes | 20.5% | 20.5% | 20.4% | 17.2% | 19.5% |
| Pain-related hospitalization | |||||
| No | 13.1% | 12.8% | 13.3% | 13.3% | 13.6% |
| Yes | 32.2% | 38.1% | 40.6% | 43.6% | 41.3% |
| Mental health-related hospitalization | |||||
| No | 12.4% | 12.3% | 12.8% | 13.0% | 13.1% |
| Yes | 25.8% | 26.7% | 28.4% | 28.7% | 27.8% |
| Number of hospitalizations | |||||
| 0 | 10.5% | 10.4% | 10.9% | 11.0% | 11.3% |
| 1–2 | 16.4% | 16.6% | 17.3% | 17.7% | 18.4% |
| 3–4 | 21.5% | 24.7% | 25.5% | 24.8% | 25.3% |
| 5 or more | 33.1% | 31.5% | 33.4% | 35.7% | 33.0% |
The percentages of recipients who had chronic prescriptions were significantly different across groups in each characteristic (p<0.05 for all comparisons), unless otherwise indicated.
The difference in percentages of recipients who had chronic prescriptions between recipients with different education levels was not significantly different.
Dual status in Medicare and Medicaid
On the basis of one or more claims in physician/carrier files
On the basis of one or more inpatient stays with a cancer diagnosis and Form 2728
Figure 3.
Geographic variation in the percentage of transplant recipients with chronic opioid prescription in 2010, by state
The opioids most commonly prescribed for ≥90 days during 2006–2010 were hydrocodone (increasing from 5.7% in 2006 to 6.4% in 2010) and oxycodone (increasing from 3.2% to 3.8%). The third most commonly prescribed opioid for ≥ 90 days was propoxyphene in 2006–2007 (approximately 1.5%) and tramadol in 2008–2010 (1.6% – 1.8%) (Table 3).
Table 3.
Percent of transplant recipients who had a prescription for ≥ 90 day of a specific opioid, by year
| Opioid | 2006 (n=21,030) |
2007 (n=30,299) |
2008(n=32,586) | 2009 (n=34,153) |
2010 (n=36,486) |
|---|---|---|---|---|---|
| Hydrocodone | 5.7% | 5.6% | 6.1% | 6.3% | 6.4% |
| Oxycodone | 3.2% | 3.3% | 3.5% | 3.6% | 3.8% |
| Propoxyphene | 1.6% | 1.5% | 1.4% | 1.2% | 1.0% |
| Tramadol | 1.3% | 1.4% | 1.6% | 1.7% | 1.8% |
| Codeine | 0.6% | 0.5% | 0.5% | 0.4% | 0.4% |
| Morphine | 0.6% | 0.6% | 0.6% | 0.7% | 0.7% |
| Hydromorphone | 0.3% | 0.3% | 0.3% | 0.4% | 0.4% |
| Fentanyl | 0.8% | 0.8% | 0.7% | 0.7% | 0.8% |
Chronic prescription is not mutually exclusive; a recipient could have ≥ 90 days prescription for more than 1 opioid medication
Table 4 shows factors associated with chronic prescription of any opioid in 2010 transplant recipients, adjusting for all listed characteristics. Chronic opioid prescription was independently associated with female gender (odds ratio [OR] and 95% confidence interval [CI]: 1.33, 1.25–1.41), White race (compared with other race; 1.35, 1.26–1.45), unemployment (1.16, 1.07–1.26), dual status (1.47, 1.37–1.58), mental health–related hospitalizations in 2010 (1.39, 1.26–1.53), and pain-related hospitalizations in 2010 (2.62, 2.30–2.97). Compared with recipients ≥ age 65, the ORs (95% CI) in age groups 20–44 and 45–64 were 0.87 (0.79–0.95) and 1.31 (1.22–1.42), respectively. Compared with recipients with 1–5 years transplant vintage, the odds of receiving a chronic opioid prescription were 43% (95% CI, 1.33–1.55) greater for recipients with 11 or more years’ transplant vintage. Recipients living in rural areas (OR [95% CI], 1.12 [1.04–1.21]) and poorer neighborhoods (1.22 [1.09–1.36] for median household incomes $45,000–$74,999 and 1.36 [1.22–1.53] for those <$45,000) had greater likelihood of chronic opioid prescription than those living in nonrural areas and in richer neighborhoods (median household incomes ≥$75,000). The adjusted model showed greater likelihood of opioid prescription associated with greater numbers of hospitalizations in 2010 (OR [95% CI], increasing from 1.45 [1.34–1.56] for 1–2 admissions to 2.18 [1.86–2.56] for more than 4 admissions).
Table 4.
Factors associated with opioid medication prescription for ≥ 90 days in adult transplant recipients continuously enrolled in Medicare Part A, B and D, 2010 (n = 35,682)
| Characteristics | OR | (95% CI) | P value | |
|---|---|---|---|---|
| Age group | ||||
| 20–44 | 0.87 | (0.79, 0.95) | 0.003 | |
| 45–64 | 1.31 | (1.22, 1.42) | <0.001 | |
| 65+ | 1.00 | |||
| Gender | ||||
| Male | 1.00 | |||
| Female | 1.33 | (1.25, 1.41) | <0.001 | |
| Race | ||||
| White | 1.35 | (1.26, 1.45) | <0.001 | |
| Non-White | 1.00 | |||
| Education | ||||
| High school or less | 1.00 | |||
| Some college or higher | 0.95 | (0.88, 1.03) | 0.18 | |
| Unknown/Missing | 0.98 | (0.90, 1.06) | 0.58 | |
| Employment status | ||||
| Working | 1.00 | |||
| Not working | 1.16 | (1.07, 1.26) | <0.001 | |
| Unknown/Missing | 1.09 | (0.98, 1.21) | 0.13 | |
| ZIP code median income | ||||
| < $45,000 | 1.36 | (1.22, 1.53) | <0.001 | |
| $45,000 – $74,999 | 1.22 | (1.09, 1.36) | <0.001 | |
| ≥$75,000 | 1.00 | |||
| Dual status† | ||||
| No | 1.00 | |||
| Yes | 1.47 | (1.37, 1.58) | <0.001 | |
| Residential area | ||||
| Rural | 1.12 | (1.04, 1.21) | 0.004 | |
| Non-rural | 1.00 | |||
| Nursing home residence‡ | ||||
| No | 1.00 | |||
| Yes | 1.15 | (0.98, 1.35) | 0.10 | |
| Transplant vintage | ||||
| 1–5 years | 1.00 | |||
| 6–10 years | 1.22 | (1.13, 1.31) | <0.001 | |
| 11+ years | 1.43 | (1.33, 1.55) | <0.001 | |
| Cancer§ | ||||
| No | 1.00 | |||
| Yes | 1.16 | (0.99, 1.37) | 0.06 | |
| Pain-related hospitalization in 2010 | ||||
| No | 1.00 | |||
| Yes | 2.62 | (2.30, 2.97) | <0.001 | |
| Mental health-related hospitalization in 2010 | ||||
| No | 1.00 | |||
| Yes | 1.39 | (1.26, 1.53) | <0.001 | |
| Number of hospitalizations in 2010 | ||||
| 0 | 1.00 | |||
| 1–2 | 1.45 | (1.34, 1.56) | <0.001 | |
| 3–4 | 1.84 | (1.63, 2.08) | <0.001 | |
| 5 or more | 2.18 | (1.86, 2.56) | <0.001 |
OR, odds ratio; CI, confidence interval.
All variables presented in the table were adjusted in the logistic regression model.
Dual status in Medicare and Medicaid
On the basis of one or more claims in physician/carrier files
On the basis of one or more inpatient stays with a cancer diagnosis and Form 2728
In the incident cohort (Figure 2), the mean age of the 14,393 transplant recipients was 50.8 ± 14.0 years and 60.5% were men (Table 5). In the 365 days before transplantation, 4,618 recipients (32.1%) had no opioid prescriptions, 4,720 (32.8%) had opioid prescriptions for <90 cumulative days and 1,641 (11.4%) had ≥90 days of filled opioid prescriptions. Data on filled prescriptions for the 1 year before transplantation were not available in 23.7% of recipients.
Table 5.
Selected characteristics of adult transplant recipients who had a first transplant in 2007–2009 (n=14,393), and associations with chronic opioid prescriptions after transplantation
| Characteristics | Percent | Multivariate logistic regression | ||
|---|---|---|---|---|
|
| ||||
| OR | (95% CI) | P value | ||
| Demographic characteristics | ||||
| Age at transplant | ||||
| 20–44 | 33.9% | 1.41 | (1.13, 1.76) | 0.002 |
| 45–64 | 45.8% | 1.56 | (1.28, 1.90) | <0.001 |
| 65+ | 20.3% | 1.00 | ||
| Male gender | 60.5% | 0.89 | (0.78, 1.01) | 0.08 |
| White race | 59.9% | 1.06 | (0.92, 1.23) | 0.42 |
| Education | ||||
| High school or less | 55.9% | 1.00 | ||
| Some college or higher | 30.8% | 0.99 | (0.85, 1.15) | 0.87 |
| Unknown/Missing | 13.3% | 0.93 | (0.76, 1.14) | 0.47 |
| Employment status | ||||
| Working | 12.5% | 1.00 | ||
| Not working | 75.7% | 1.32 | (1.05, 1.66) | 0.02 |
| Unknown/Missing | 11.8% | 1.33 | (1.00, 1.78) | 0.05 |
| ZIP code median income | ||||
| < $45,000 | 48.3% | 1.19 | (0.93, 1.53) | 0.17 |
| $45,000 – $74,999 | 41.0% | 1.29 | (1.01, 1.65) | 0.04 |
| ≥$75,000 | 10.7% | 1.00 | ||
| Dual status† | 73.4% | 1.12 | (0.94, 1.34) | 0.21 |
| Residential area: rural | 17.8% | 1.23 | (1.04, 1.46) | 0.01 |
| Nursing home residence‡ | 3.0% | 1.22 | (0.86, 1.73) | 0.26 |
| Clinical characteristics | ||||
| ESRD vintage | ||||
| 0 years | 8.4% | 1.79 | (1.34, 2.39) | <0.001 |
| 1–3 years | 45.1% | 1.07 | (0.93, 1.24) | 0.35 |
| 4+ years | 46.5% | 1.00 | ||
| Opioid prescription, before transplant | ||||
| None | 32.1% | 1.00 | ||
| Short-term | 32.8% | 3.81 | (2.95, 4.91) | <0.001 |
| Chronic | 11.4% | 95.2 | (74.2, 122.1) | <0.001 |
| No data | 23.7% | 3.13 | (2.33, 4.20) | <0.001 |
| Comorbidities | ||||
| Cancer§ | 4.1% | 1.11 | (0.81, 1.53) | 0.51 |
| Congestive heart failure | 12.4% | 0.98 | (0.80, 1.20) | 0.86 |
| Peripheral vascular disease | 4.6% | 1.36 | (1.01, 1.82) | 0.04 |
| Cerebrovascular disease | 3.5% | 1.06 | (0.75, 1.50) | 0.75 |
| Chronic obstructive pulmonary disease | 1.7% | 1.28 | (0.81, 2.01) | 0.29 |
| Atherosclerotic heart disease | 8.4% | 1.16 | (0.91, 1.48) | 0.23 |
| Diabetes | 40.0% | 1.05 | (0.92, 1.21) | 0.47 |
| AIDS | 0.3% | 1.64 | (0.54, 4.97) | 0.38 |
OR, odds ratio; CI, confidence interval.
All variables presented in the table were adjusted in the logistic regression model.
None opioid prescription: 0 days filled opioid prescription; short-term opioid prescription: 1–89 days filled opioid prescription; chronic opioid prescription: 90 days or more filled opioid prescription
Dual status in Medicare and Medicaid before transplant
On the basis of one or more claims before transplant in physician/carrier files
On the basis of one or more inpatient stays with a cancer diagnosis before transplant and Form 2728
Up to the date of death or day 365 posttransplant, whichever came first, 2,432 recipients (16.9%) did not have an opioid prescription and 1,644 (11.4%) had ≥90 cumulative days of filled opioid prescriptions. Among the remaining 10,317 recipients (71.7%) having <90 cumulative days, 64.0% had 1–14, 18.8% had 15–30, 12.1% had 31–60, and 5.2% had 61–89 cumulative days of filled opioid prescriptions. Among 1,644 recipients having chronic opioid prescriptions, 62.2% had <50 MME/day, 21.8% had 50–89 MME/day, and 16.0% had ≥90 MME/day. Percentages did not substantially differ when patients with preexisting cancer or cancer after transplantation were excluded (data not shown).
Among 1,641 recipients having chronic opioid prescriptions before transplantation, 64.1% had chronic opioid prescriptions after transplantation. In contrast, only 1.7% of the 4,618 recipients without opioid prescription and 6.4% of the 4,720 recipients with short-term opioid prescriptions before transplantation had chronic opioid prescriptions after transplantation (p<0.001; data not shown). Table 5 also shows factors associated with chronic opioid prescriptions after transplantation. Chronic opioid prescription before transplantation was the overwhelmingly dominant factor (adjusted OR of 95.2) for a chronic opioid prescription after transplantation.
During a median 1,894 days follow-up for all-cause death (interquartile range, 1,609 to 2,217) after transplantation, 2,495 deaths occurred. In the Cox regression model analyzing dosage of chronic opioid prescriptions as a time-varying covariate with no and short-term opioid prescriptions as the referent, the adjusted hazard ratios of death were 1.24 (95% CI, 1.05–1.46) for recipients having <50 MME/day, 1.45 (1.15–1.84) for those having 50–89 MME/day, and 1.61 (1.24–2.10) for those having ≥90 MME/day. Similarly, during a median 1,800 days follow-up for graft loss (interquartile range, 1,490 to 2,149) after transplantation, 4,150 graft losses occurred. The adjusted hazard ratios of graft loss were 1.06 (CI, 0.92–1.22) for recipients having <50 MME/day, 1.27 (1.03–1.56) for 50–89 MME/day, and 1.33 (1.05–1.67) for ≥90 MME/day (Table 6). Findings from sensitivity analyses that limited follow-up duration were similar to those from the primary analysis (data not shown).
Table 6.
Adjusted hazard ratios of death and graft loss associated with ≥ 90 days opioid prescription after transplant in transplant recipients who had their first transplant in 2007–2009 (n=13,925)
| Characteristics | Outcome = death | Outcome = graft loss | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | (95% CI) | P value | HR | (95% CI) | P value | |
| Opioid prescription, after transplant * | ||||||
| None and short-term | 1.00 | 1.00 | ||||
| Chronic, <50 MME/day | 1.24 | (1.05, 1.46) | 0.01 | 1.06 | (0.92, 1.22) | 0.44 |
| Chronic, 50–89 MME/day | 1.45 | (1.15, 1.84) | 0.002 | 1.27 | (1.03, 1.56) | 0.02 |
| Chronic, 90+ MME/day | 1.61 | (1.24, 2.10) | <0.001 | 1.33 | (1.05, 1.67) | 0.02 |
| Age at transplant | ||||||
| 20–44 | 1.00 | 1.00 | ||||
| 45–64 | 1.69 | (1.51, 1.89) | <0.001 | 0.95 | (0.88, 1.02) | 0.19 |
| 65+ | 2.85 | (2.51, 3.25) | <0.001 | 1.25 | (1.14, 1.38) | <0.001 |
| Gender | ||||||
| Male | 1.12 | (1.03, 1.21) | 0.01 | 1.05 | (0.98, 1.12) | 0.15 |
| Female | 1.00 | 1.00 | ||||
| Race | ||||||
| White | 1.00 | 1.00 | ||||
| Non-White | 1.05 | (0.96, 1.14) | 0.26 | 1.26 | (1.18, 1.35) | <0.001 |
| Education | ||||||
| High school or less | 1.00 | 1.00 | ||||
| Some college or higher | 1.03 | (0.94, 1.13) | 0.56 | 1.04 | (0.97, 1.11) | 0.33 |
| Unknown/Missing | 1.05 | (0.93, 1.18) | 0.45 | 0.97 | (0.89, 1.07) | 0.58 |
| Employment status | ||||||
| Working | 1.00 | 1.00 | ||||
| Not working | 1.39 | (1.20, 1.61) | <0.001 | 1.21 | (1.09, 1.34) | <0.001 |
| Unknown/Missing | 1.31 | (1.09, 1.58) | 0.004 | 1.14 | (1.00, 1.30) | 0.05 |
| ZIP code median income | ||||||
| < $45,000 | 1.08 | (0.94, 1.24) | 0.26 | 1.15 | (1.03, 1.28) | 0.02 |
| $45,000 – $74,999 | 1.03 | (0.90, 1.18) | 0.68 | 1.08 | (0.96, 1.20) | 0.19 |
| ≥$75,000 | 1.00 | 1.00 | ||||
| Dual status† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.96 | (0.87, 1.06) | 0.46 | 1.06 | (0.98, 1.14) | 0.18 |
| Residential area | ||||||
| Rural | 1.08 | (0.97, 1.20) | 0.17 | 1.02 | (0.94, 1.11) | 0.66 |
| Non-rural | 1.00 | 1.00 | ||||
| Nursing home residence‡ | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.77 | (1.50, 2.08) | <0.001 | 1.53 | (1.32, 1.78) | <0.001 |
| ESRD vintage | ||||||
| 0 years | 1.00 | 1.00 | ||||
| 1–3 years | 1.13 | (0.94, 1.35) | 0.19 | 1.27 | (1.10, 1.48) | 0.001 |
| 4+ years | 1.42 | (1.17, 1.71) | <0.001 | 1.48 | (1.27, 1.74) | <0.001 |
| Opioid prescription, before transplant | ||||||
| None and short-term | 1.00 | 1.00 | ||||
| Chronic | 1.35 | (1.18, 1.55) | <0.001 | 1.22 | (1.10, 1.36) | <0.001 |
| No data | 0.93 | (0.82, 1.05) | 0.22 | 0.95 | (0.87, 1.05) | 0.32 |
| Cancer, after transplant*§ | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 6.23 | (5.46, 7.10) | <0.001 | 4.75 | (4.19, 5.39) | <0.001 |
| Cancer, before transplant¶ | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.48 | (1.25, 1.74) | <0.001 | 1.29 | (1.12, 1.49) | <0.001 |
| Congestive heart failure | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.28 | (1.15, 1.42) | <0.001 | 1.13 | (1.04, 1.24) | 0.006 |
| Peripheral vascular disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.14 | (0.98, 1.33) | 0.10 | 1.09 | (0.95, 1.25) | 0.22 |
| Cerebrovascular disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.30 | (1.09, 1.55) | 0.004 | 1.18 | (1.01, 1.37) | 0.03 |
| Chronic obstructive pulmonary disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.30 | (1.02, 1.66) | 0.03 | 1.37 | (1.11, 1.69) | 0.003 |
| Atherosclerotic heart disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.35 | (1.20, 1.52) | <0.001 | 1.24 | (1.11, 1.38) | <0.001 |
| Diabetes | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.51 | (1.39, 1.64) | <0.001 | 1.22 | (1.14, 1.30) | <0.001 |
| AIDS | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.31 | (0.62, 2.75) | 0.48 | 1.40 | (0.89, 2.21) | 0.14 |
HR, hazard ratio; CI, confidence interval; MME, morphine milligram equivalent.
All variables presented in the table were adjusted in the Cox regression model.
None opioid prescription: 0 days filled opioid prescription; short-term opioid prescription: 1–89 days filled opioid prescription; chronic opioid prescription: 90 days or more filled opioid prescription
As a time-dependent variable in the Cox regression model
Dual status in Medicare and Medicaid before transplant
On the basis of one or more claims before transplant in physician/carrier files
On the basis of one or more inpatient stays with a cancer diagnosis after transplant; the date of the first inpatient stay with a cancer diagnosis defined as the date of cancer occurrence after transplant
On the basis of one or more inpatient stays with a cancer diagnosis before transplant and Form 2728
DISCUSSION
Approximately 14% of prevalent US kidney transplant recipients had opioid prescriptions for ≥ 90 days each year. 11% of incident recipients had chronic opioid prescriptions after transplantation. Of those, almost 22% were prescribed a daily dose of 50–89 MME, which the CDC indicates is associated with increased risk of overdose. 16% had a dose ≥90 MME, which the CDC recommends should be avoided.16 The overwhelming risk factor for chronic opioid prescriptions after transplantation was receipt of chronic opioid prescriptions before transplantation (64%, and an OR of 95) compared to recipients who were not prescribed opioid medications (1.7%) or had short-term opioid prescriptions before transplantation (6.4%), consistent with reports of an enduring impact of initial opioid prescriptions.17,18
After transplantation, compared to no or short-term prescription, chronic prescription was associated with an increased risk of death and graft loss. The magnitude of all associations increased sequentially with higher (MME 90 or higher) compared to lower doses of chronic opioid prescriptions. Using the same definition, chronic prescription of opioids increased in the general Medicare population, from 4.6% in 2007 to 7.4% in 2012.5 We also found that only 5 states accounted for >20% prescription of chronic opioids among transplant recipients, consistent with geographic concentration reported by the CDC and others.19
While the largest single-center study of opioid use after transplantation found most opioids were prescribed for nonsurgical musculoskeletal pain,8 in a cross-sectional comparative study in 164 hemodialysis patients and 114 stable deceased donor kidney transplant recipients using the modified McGill Pain Questionnaire,20 over 60% of both hemodialysis patients and kidney transplant recipients reported pain.
Transplant recipients have specific if not unique etiologies of pain including calcineurin inhibitor associated pain21,22 and avascular necrosis of bone.23,24 While neither are reportedly common, chronic opioid therapy does not represent optimal or even appropriate therapy in either case.
The CDC recently released clinical guidelines for opiate prescription,25 including checklists for provider prescription. Transplant recipients were not specifically addressed, and were not explicitly considered as a group at greater risk of harm, although recipients with renal insufficiency were included in this category. The lowest dose and duration of opioid treatment is recommended, and nonopioid approaches are considered essential. This last point is critical, because many previous assessments of pain control, including in the ESRD population, primarily considered analgesic and opioid drug prescriptions as appropriate treatment for pain, and gave less emphasis to nonpharmacological therapies, such as cognitive behavioral therapy (CBT), psychotherapy and biofeedback techniques.26–28 This is a crucial shortcoming given our finding that chronic opioid prescription before transplantation is a predictor of chronic opioid prescription after transplantation, which is associated with adverse outcomes. Current guidelines for transplant screening do not make management recommendations for candidates with chronic opioid use. At a minimum, given the conflicting findings of causes of transplant related pain, the etiology and management of such pain among transplant candidates should be an urgent topic of investigation.
In contrast to the general population, where CBT has been assessed and found effective for pain management in clinical trials,29,30 no reports of its efficacy have been published among transplant recipients.31 In addition, transplant candidates and recipients with pain should be assessed for the presence of coexisting diagnoses, such as depression, anxiety and sleep disorders, since pain is often linked to these conditions.32–34 Perception of pain can be intensified by depression and anxiety, both of which occur frequently in the ESRD population.26,31–33 These may be responsive to both pharmacologic and nonpharmacologic therapies, such as antidepressants or CBT, which may not be considered typical interventions for pain. Treatment addressing these conditions may ameliorate perceived pain. In addition to research using buprenorphine and naloxone,35,36 newer therapies for patients using opioid medications are being developed.37
Retrospective analysis does not allow distinction between whether these medications are prescribed in candidates or recipients at high risk of death, with terminal conditions, or whether these medications may causally contribute to their increased risk of death. Pain itself, rather than, or in addition to the prescription of medication, may be in the causal pathway of mortality.38 However, the strong link between opioid prescription before and after transplantation argues against opioid prescription in terminal or severely morbid conditions, since such patients would presumably be much less likely to undergo transplantation.
Our study was limited to those recipients with full Medicare Part A, B and D coverage. To the extent that prescription rates and outcomes are different in the remaining transplant population, our results may not be fully applicable to the universe of transplant recipients. Only filled prescriptions, and not actual prescription or consumption of opioids were assessed in this study. The observational and retrospective nature of this analysis establishes associations only, not causation. Findings of increased risk of death and graft loss among recipients prescribed opioids could be related to their prescription in recipients with preexisting higher risk of mortality or those who already had progressive allograft failure, although these associations persisted despite adjustment for comorbid conditions and other factors known to be associated with mortality. We do not know the cause of death or graft loss in the recipients in this study.
In summary, we report a high rate of chronic opioid prescription in the prevalent kidney transplant population. Chronic opioid prescriptions are associated with increased risk of mortality and graft loss, although a causal role cannot be established.
A “transplant specific” interpretation of CDC guidelines for opioid use could include an initial discussion with the candidate or recipient with similar level of detail given to immunosuppressive medications. The findings that providers usually continue pretransplant prescriptions of long-term, high-dose opioids after transplant should prompt a reemphasis on evaluating all medication use, including opioids, at the candidate’s transplant evaluation. To the extent that opioid drugs may cause death in transplant recipients, appropriate clinical interventions to reduce drug prescription, and dose level, and offer nonpharmacological options, while keeping in mind recipient satisfaction and comfort, are warranted. The impact of chronic opioid prescription on other aspects of transplant recipient care, such as quality of life, immunosuppressive medication adherence, or adherence generally, is unknown and is a worthy subject of further investigation. Nonpharmacologic therapies for pain control, and approaches which minimize drug doses and duration of therapy deserve further study in this population, as they may result in lower recipient mortality and morbidity.
Supplementary Material
Acknowledgments
Funding: Dr. Fwu is supported by a contract from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN276201200161U).
ABBREVIATIONS
- CBT
cognitive behavioral therapy
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- ESRD
end-stage renal disease
- MME
morphine milligram equivalents
- OR
odds ratio
- USRDS
United States Renal Data System
Footnotes
Authorship:
All authors were involved in the design of the study, interpretation of the data, and the writing of the manuscript. In addition,
Drs. Chyng-Wen Fwu, Kevin C. Abbott, and Paul W. Eggers had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Drs. Chyng-Wen Fwu, Kevin C. Abbott, Anne W. Eggers, and Paul W. Eggers contributed to the statistical analysis.
Drs. Kevin C. Abbott, Chyng-Wen Fwu, and Paul L. Kimmel contributed to the drafting of the manuscript.
Drs. Paul L. Kimmel, Paul W. Eggers, Prudence P. Kline, and Anne W. Eggers contributed to the critical revision of the manuscript for important intellectual content.
Disclosure: The authors declare no conflicts of interest
References
- 1.Lentine KL, Lam NN, Schnitzler MA, et al. Predonation Prescription Opioid Use: A Novel Risk Factor for Readmission After Living Kidney Donation. Am J Transplant. 2017;17(3):744–753. doi: 10.1111/ajt.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randall HB, Alhamad T, Schnitzler MA, et al. Survival implications of opioid use before and after liver transplantation. Liver Transpl. 2017;23(3):305–314. doi: 10.1002/lt.24714. [DOI] [PubMed] [Google Scholar]
- 3.Lentine KL, Lam NN, Xiao H, et al. Associations of pre-transplant prescription narcotic use with clinical complications after kidney transplantation. Am J Nephrol. 2015;41(2):165–176. doi: 10.1159/000377685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015;99(1):187–196. doi: 10.1097/TP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 5.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in Opioid Prescriptions Among Part D Medicare Recipients From 2007 to 2012. Am J Med. 2016;129(2):221.e221–230. doi: 10.1016/j.amjmed.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW. Opioid Prescription, Morbidity, and Mortality in United States Dialysis Patients. J Am Soc Nephrol. 2017;28(12):3658–3670. doi: 10.1681/ASN.2017010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulshrestha S, Barrantes F, Samaniego M, Luan FL. Chronic opioid analgesic usage post-kidney transplantation and clinical outcomes. Clin Transplant. 2014;28(9):1041–1046. doi: 10.1111/ctr.12414. [DOI] [PubMed] [Google Scholar]
- 9.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CMS. Opioid Morphine Equivalent Conversion Factors. [Accessed December 13 2016]; www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-March-2015.pdf.
- 11.Kimmel PL, Fwu CW, Abbott KC, Ratner J, Eggers PW. Racial Disparities in Poverty Account for Mortality Differences in US Medicare Beneficiaries. SSM Popul Health. 2016;2:123–129. doi: 10.1016/j.ssmph.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol. 2013;24(2):293–301. doi: 10.1681/ASN.2012070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg JP, Chasan-Taber S, Blair A, et al. Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial. Arthritis Rheum. 2005;52(1):290–295. doi: 10.1002/art.20781. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DD, Franco SJ. NCHS urban-rural classification scheme for counties. Vital Health Stat 2. 2012;(154):1–65. [PubMed] [Google Scholar]
- 15.United States Census Bureau. Median Household Income in the past 12 months: 2007–2011 American Community Survey 5-year Estimates. [Accessed September 20 2017]; < https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_11_5YR_B19013&prodType=table>.
- 16.CDC Guideline for Prescribing Opioids for Chronic Pain. [Accessed September 28 2017]; www.cdc.gov/drugoverdose/pdf/guidelines_at-a-glance-a.pdf.
- 17.Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21–27. doi: 10.1007/s11606-016-3810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett ML, Olenski AR, Jena AB. Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med. 2017;376(7):663–673. doi: 10.1056/NEJMsa1610524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. Opioid Prescribing: Where You Live Matters. [Accessed September 28 2017]; www.cdc.gov/vitalsigns/pdf/2017-07-vitalsigns.pdf.
- 20.Masajtis-Zagajewska A, Pietrasik P, Krawczyk J, et al. Similar prevalence but different characteristics of pain in kidney transplant recipients and chronic hemodialysis patients. Clin Transplant. 2011;25(2):E144–151. doi: 10.1111/j.1399-0012.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- 21.Breitenstein A, Stumpe KD, Gnannt R, Fehr T, Etter C. Calcineurin inhibitor-induced pain syndrome after kidney transplantation-a rare but disabling condition. NDT plus. 2011;4(1):63–66. doi: 10.1093/ndtplus/sfq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prommer E. Calcineurin-inhibitor pain syndrome. Clin J Pain. 2012;28(6):556–559. doi: 10.1097/AJP.0b013e31823a67f1. [DOI] [PubMed] [Google Scholar]
- 23.Abbott KC, Oglesby RJ, Agodoa LY. Hospitalized avascular necrosis after renal transplantation in the United States. Kidney Int. 2002;62(6):2250–2256. doi: 10.1046/j.1523-1755.2002.00667.x. [DOI] [PubMed] [Google Scholar]
- 24.Abbott KC, Koff J, Bohen EM, et al. Maintenance immunosuppression use and the associated risk of avascular necrosis after kidney transplantation in the United States. Transplantation. 2005;79(3):330–336. doi: 10.1097/01.tp.0000149894.95435.7f. [DOI] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. JAMA. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 26.Cheatle MD. Biopsychosocial Approach to Assessing and Managing Patients with Chronic Pain. Med Clin North Am. 2016;100(1):43–53. doi: 10.1016/j.mcna.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Garland EL. Treating chronic pain: the need for non-opioid options. Expert Rev Clin Pharmacol. 2014;7(5):545–550. doi: 10.1586/17512433.2014.928587. [DOI] [PubMed] [Google Scholar]
- 28.Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30(2):155–166. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Castro MM, Daltro C, Kraychete DC, Lopes J. The cognitive behavioral therapy causes an improvement in quality of life in patients with chronic musculoskeletal pain. Arq Neuropsiquiatr. 2012;70(11):864–868. doi: 10.1590/s0004-282x2012001100008. [DOI] [PubMed] [Google Scholar]
- 30.Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behav Res Ther. 2012;50(11):685–689. doi: 10.1016/j.brat.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cukor D, Ver Halen N, Asher DR, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol. 2014;25(1):196–206. doi: 10.1681/ASN.2012111134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimmel PL. The weather and quality of life in ESRD patients: everybody talks about it, but does anybody do anything about it? Semin Dial. 2013;26(3):260–262. doi: 10.1111/sdi.12063. [DOI] [PubMed] [Google Scholar]
- 33.Davison SN, Jhangri GS. The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J Pain Symptom Manage. 2005;30(5):465–473. doi: 10.1016/j.jpainsymman.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Davison SN. Chronic kidney disease: psychosocial impact of chronic pain. Geriatrics. 2007;62(2):17–23. [PubMed] [Google Scholar]
- 35.Weiss RD, Potter JS, Griffin ML, et al. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Depend. 2015;150:112–119. doi: 10.1016/j.drugalcdep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 38.Harris TJ, Nazir R, Khetpal P, et al. Pain, sleep disturbance and survival in hemodialysis patients. Nephrol Dial Transplant. 2012;27(2):758–765. doi: 10.1093/ndt/gfr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.