Abstract
Background
Immunotherapy is emerging as the cornerstone for treatment of patients with advanced cancer, but significant toxicity (immune-related adverse events [irAEs]) associated with unbridled T cell activity remains a concern.
Patients and methods
A retrospective review of the electronic medical records of 290 patients with advanced cancer treated on an immunotherapy-based clinical trial in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center between February 2010 and September 2015 was performed. Clinical and laboratory parameters were collected to determine the incidence of irAEs, risk factors, and their association with treatment outcomes.
Results
Ninety eight of 290 patients (34%) experienced any grade irAEs. Among the 15 (5.2%) patients with grade ≥3 irAEs, the most common irAEs were dermatitis and enterocolitis. Although 80% of the patients with grade ≥3 irAEs required systemic corticosteroids, all the 15 patients recovered from the irAEs. On re-challenge, 4 of the 5 patients who had received systemic corticosteroids for irAE continued to respond. There were no irAE-related deaths. Importantly, patients with grade ≥3 irAEs had improved overall response rate (25 vs. 6%; p=0.039) and longer median time to progression (30 weeks vs. 10 weeks; p=0.0040) when compared to those without grade ≥3 irAEs.
Conclusion
Incidence of irAEs with immunotherapeutic agents indicates an active immune status, suggestive of potential clinical benefit to the patient. Further validation of this association in a large prospective study is warranted.
Keywords: checkpoint inhibitors, immune-related adverse event, immunotherapy, response, systemic steroids, time to progression
INTRODUCTION
The coming of age of immunotherapy as a treatment paradigm for oncology has opened of intriguing avenues for further research [1]. Notably, modulation of immune inhibitory pathways using checkpoint inhibitors has produced sustained clinical responses in a subset of patients, leading to their accelerated approval by the U.S. Food and Drug Administration for treatment of cancers as diverse as melanoma, renal cell carcinoma, non-small cell lung cancer, Hodgkin lymphoma, and squamous cell carcinoma of head and neck [2]. Remarkable responses have also been reported in other tumor types [3–6]. However, despite the dramatic rise in clinical utility of immunotherapeutic agents that include immune checkpoint inhibitors and cytokine- and vaccine-based treatments [1], disruption in immune homeostasis produces a unique spectrum of side effects collectively termed as immune-related adverse events (irAEs) [7]. The most common irAEs are dermatitis, enterocolitis, hepatitis, transaminitis, hypophysitis, thyroiditis, pneumonitis, and uveitis [8]. These irAEs are very different from the side effects produced by chemotherapeutic agents in that they may rapidly progress to life-threatening events if not treated promptly and vigorously.
The incidence and type of irAEs vary with the immunotherapeutic agent and duration of therapy [7]. For example, there are considerable differences in the safety profiles of cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) inhibitors and programmed death-1 (PD-1) pathway inhibitors. The reported incidence of any grade irAEs ranges from 60–70% in patients treated with ipilimumab [9] as compared to 39–41 % on PD-1 inhibitors [10,11], and the incidence of grade 3–4 irAEs is higher with ipilimumab (15%) [9] compared to non-CTLA-4 checkpoint inhibitors (5–6%) [10,11] (p<0.001) [12]. In addition, certain irAEs may be more common with specific agents. For example, gastrointestinal disorders such as diarrhea and colitis are more frequent with ipilimumab [13], while vitiligo is frequently reported with pembrolizumab [14–16] and symptomatic pneumonitis with nivolumab [17–20]. Cytokine therapies such as interferon produce constitutional symptoms such as fever, fatigue, headache, and myalgia, though less frequently than irAEs seen with checkpoint inhibitors [21]. And, cancer vaccines are the least toxic with minimal irAEs [7]. In addition, the median time to onset of irAEs may vary with different immunotherapeutic agents (e.g. ipilimumab: 40 days vs. pembrolizumab: 64 days) [22]. In contrast, the adverse events (AEs) associated with vaccine therapy occur within 24 hours of infusion and last for 1–2 days [23]. Furthermore, the incidence of irAEs increases with increasing dose of ipilimumab [24] whereas; they are independent of the dose with PD-1 inhibitors [25,26]. Thus, irAEs vary widely in clinical presentation depending on the immunotherapeutic agent.
Enhanced awareness to irAEs is critical as prompt recognition and initiation of treatment for irAEs have important clinical implications on treatment outcomes. Most irAEs are low-grade and can be treated with administration of corticosteroids and/or interruption of therapy [27]. However, severe irAEs may produce substantial morbidities requiring other immunosuppressive measures and prolonged hospitalization. Furthermore, rapid deterioration and irAE-related deaths have been reported with immunotherapeutic agents [28,9,11,29]. Therefore, early detection and judicious management of irAEs is critical for improved treatment outcomes in patients on immunotherapy-based clinical trials.
With increasing use of immunotherapeutic agents in the clinic, the challenges associated with irAEs cannot be ignored. Therefore, it is extremely important for physicians to have awareness and a high index of suspicion for early recognition and prompt treatment of irAEs. Thus, dissemination of this knowledge among health care professionals is a priority. In this article, we report the incidence of irAEs in patients with advanced cancer treated with immunotherapeutic agents, associated risk factors, and treatment outcomes in the context of irAEs.
PATIENTS AND METHODS
Patients and Samples
We conducted a retrospective analysis of patients enrolled in early clinical trials that included at least 1 immunotherapy drug conducted by the Department of Investigational Cancer Therapeutics (phase I clinical trials program) at The University of Texas MD Anderson Cancer Center (MD Anderson) between February 2010 and September 2015. The retrospective study was approved by the Institutional Review Board (IRB) at MD Anderson and the requirement to obtain informed consent was waived. However, all patients provided written informed consent before enrollment on an IRB-approved clinical trial during this period. Patients who provided written informed consent but did not start the clinical trials were excluded.
Two investigators (TF and AMB) independently reviewed the electronic medical record to collect patient-specific information, including age at the time of trial enrollment, gender, tumor type, number of metastatic sites, Eastern Cooperative Oncology Group performance status, therapeutic regimen, duration of treatment, drug-related AEs with grades, irAEs with grades, imaging, and laboratory results. Any disagreements in data extraction were resolved through discussion with a third investigator (AN).
irAEs
irAEs were defined as unique AEs typically not seen with chemotherapeutic or targeted agents that had a potential immunologic basis that required more frequent monitoring and potential intervention with immune suppression or endocrine therapy. This includes dermatitis, diarrhea, enterocolitis, pneumonitis, hypothyroidism, thyroiditis/hyperthyroidism, hypophysitis, vitiligo, autoimmune hepatitis, elevated liver function tests, adrenal insufficiency, reactivation of hepatitis B and/or C, reactivation of HIV, uveitis, pancreatitis, nephritis, Guillain-Barre syndrome, myasthenia gravis, Stevens-Johnson syndrome, pleuritis, and myositis, all of which were attributed as related to immunotherapy by the treating physician. Injection site reaction and fever were not considered as irAEs.
Statistical Analysis
Standard descriptive statistics were used to summarize all variables. All AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 [30]. Investigators specified whether an AE was considered to be treatment-related and/or immune-related. Responses to treatment were categorized per Response Evaluation Criteria in Solid Tumors 1.0 criteria [31] and were reported as best response. Time to progression (TTP) was defined as the interval from the date of cycle 1 day 1 of the clinical trial until the date of disease progression or censoring. Patients who did not have disease progression were censored at the time of last follow-up, initiation of the next treatment, or death, whichever came first. Overall Survival (OS) was defined as the interval from the date of cycle 1 day 1 of the clinical trial until death. Patients still alive were censored at the time of last follow-up. The Fisher exact test was used to determine the association between categorical variables. Receiver operating characteristic curve analysis was used to assess the predictive ability of baseline biomarkers to identify patients at risk for irAEs. Kaplan-Meier method was used to analyze TTP and OS and between groups comparison was done using log-rank test. All tests were 2-sided, and P <0.05 was considered statistically significant. All statistical analyses were carried out using TIBCO Spotfire S+ Version 8.2 for Windows.
RESULTS
Patient Characteristics and Treatment
A total of 290 patients with advanced cancer participated in a clinical trial that included at least one immunotherapeutic agent. The patients’ baseline characteristics are summarized in Table 1. Seventy percent (n=204) of patients received monotherapy that included checkpoint inhibitors (n=64), cytokine therapies (n= 87), and cancer vaccines (n=53), while 86 patients (30%) received combination therapies. Of the 86 patients who received combination therapies, 63 (73%) received checkpoint inhibitor–based combination treatment with targeted therapy (n=35), immunomodulating agents (n=18), cytokines (n=5), radiation (n=4), or chemotherapy (n=1). Of the remaining 23 patients (27%), 15 received a combination of cytokine and chemotherapy and 8 received a combination of cytokine and targeted therapy.
Table 1.
Patients’ baseline characteristics
| Characteristics | Total n = 290 | irAE ≥ Grade 3 | |
|---|---|---|---|
| No (n=275) | Yes (n=15) | ||
| Age in years median, (range) | 58.5 (19–86) | 58 (19–86) | 60 (48–75) |
| Sex | |||
| Male | 136 (46.9) | 125 (45.5) | 11 (73.3) |
| Female | 154 (53.1) | 150 (54.5) | 4 (26.7) |
| ECOG performance status | |||
| 0 | 43 (14.8) | 43 (15.6) | 0 |
| 1 | 237 (81.7) | 222 (80.7) | 15 (100.0) |
| 2 | 10 (3.4) | 10 (3.6) | 0 |
| No. of metastatic sites | |||
| ≤ 2 | 152 | 144 (52.4) | 8 (53.3) |
| > 2 | 138 | 131 (47.6) | 7 (46.7) |
| Tumor Type | |||
| Breast | 16 (5.5) | 16 (5.8) | 0 |
| Colorectal | 31 (10.7) | 29 (10.5) | 2 (13.3) |
| CCC or HCC | 6 (2.1) | 6 (2.2) | 0 |
| Pancreatic | 15 (5.2) | 15 (5.5) | 0 |
| Gastric or GE junction | 10 (3.4) | 9 (3.3) | 1 (6.7) |
| Gynecologic | 26 (9.0) | 25 (9.1) | 1 (6.7) |
| NSCLC | 35 (12.1) | 33 (12.0) | 2 (13.3) |
| Renal cell carcinoma | 33 (11.4) | 30 (10.9) | 3 (20.0) |
| Head and neck | 16 (5.5) | 15 (5.5) | 1 (6.7) |
| Melanoma | 22 (7.6) | 22 (8.0) | 0 |
| Sarcoma or GIST | 28 (9.7) | 27 (9.8) | 1 (6.7) |
| Other rare tumors* | 52 (17.9) | 48 (17.5) | 4 (26.7) |
Note: All data are no. of patients (%) unless otherwise indicated.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; CCC, cholangiocarcinoma; HCC, hepatocellular carcinoma; GE, gastroesophageal; NSCLC, non-small cell lung cancer; GIST, gastrointestinal stromal tumor; irAE, immune-related adverse event.
Not listed owing to sparseness of data.
Incidence of irAEs
Of the 290 patients who were on an immunotherapy-based clinical trial, 98 patients (34%) reported an irAE, mostly grade 1 or 2. The most common among them were dermatitis (n=57), hypophysitis (n=18), elevated liver function tests (n=17), and diarrhea (n=15). There were no irAE-related deaths in our study.
Grade 3 or 4 irAEs
Fifteen (5.2%) patients reported grade 3 or 4 irAEs (Table 2), which included dermatitis (n=4), enterocolitis (n=3), autoimmune hepatitis, myositis, myasthenia gravis (n=2 each), and, elevated liver function tests, pneumonitis, pleuritic, and pancreatitis (n=1 each). One patient with renal cell carcinoma who received a checkpoint inhibitor had 3 different grade ≥3 irAEs. Grade ≥3 irAEs occurred in 10 of 122 patients who were on a clinical trial that included a checkpoint inhibitor (8%), 4 of 110 (4%) on a clinical trial that included a cytokine therapy, and 1 of 53 (2%) on a cancer vaccine clinical trial.
Table 2.
Characteristics of 15 patients with grade 3 or 4 immune-related adverse events
| Case No. | irAE | CTCAE Grade | Sex | Age, years | Cancer | Treatment | Systemic Steroid Use | Best Response |
|---|---|---|---|---|---|---|---|---|
| 1 | Dermatitis | 3 | Male | 53 | Colorectal | Cytokine | No | PD |
|
| ||||||||
| 2 | Enterocolitis | 3 | Female | 49 | Colorectal | Checkpoint | Yes | SD |
|
| ||||||||
| 3 | LFT elevation | 3 | Male | 60 | GE junction | Combination | Yes | SD |
|
| ||||||||
| 4 | Dermatitis | 3 | Female | 48 | Cervical | Checkpoint | Yes | PD |
|
| ||||||||
| 5 | Enterocolitis | 4 | Male | 73 | Thyroid | Checkpoint | Yes | NE |
|
| ||||||||
| 6 | Autoimmune hepatitis | 3 | Male | 56 | NSCLC | Combination | Yes | PR |
|
| ||||||||
| 7 | Pleuritis (serositis) | 3 | Male | 66 | NSCLC | Cytokine | Yes | SD |
|
| ||||||||
| 8 | Myositis | 4 | Male | 66 | RCC | Checkpoint | Yes | NE |
| Autoimmune hepatitis | 3 | Yes | ||||||
| Myasthenia gravis | 3 | * IV Ig | ||||||
|
| ||||||||
| 9 | Myositis | 4 | Male | 59 | RCC | Checkpoint | Yes | PR |
|
| ||||||||
| 10 | Enterocolitis | 3 | Male | 70 | RCC | Combination | Yes | NE |
|
| ||||||||
| 11 | Pneumonitis | 3 | Female | 71 | Leiomyosarcoma | Vaccine | No | SD |
|
| ||||||||
| 12 | Dermatitis | 3 | Male | 58 | Adenoid cystic carcinoma | Cytokine | No | SD |
|
| ||||||||
| 13 | Dermatitis | 3 | Female | 75 | Hurthle cell carcinoma | Combination | Yes | PD |
|
| ||||||||
| 14 | Pancreatitis | 4 | Male | 59 | Neuroendocrine tumor | Combination | No | PD |
|
| ||||||||
| 15 | Myasthenia gravis | 3 | Male | 71 | Prostate | Checkpoint | Yes | CR |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; GE, gastroesophageal; IV Ig, intravenous immunoglobulin; LFT, liver function test; NE, Not evaluable; NSCLC, non-small cell lung carcinoma; RCC, renal cell carcinoma.
In addition to systemic corticosteroid, patient received intravenous immunoglobulin for myasthenia gravis.
All grade ≥3 irAEs resolved with appropriate intervention. Of the 15 patients with grade ≥3 irAEs, 1 discontinued therapy following grade 4 pancreatitis. Of the remaining 14 patients, 12 (86%) received systemic steroid therapy including a patient who required intravenous immunoglobulin for myasthenia gravis, and 2 received topical steroids for grade 3 dermatitis (Table 2). In addition, the immunotherapeutic agent was discontinued in 4 of the 14 patients. Of the 10 patients in whom the immunotherapeutic drug was temporarily held, 7 were re-challenged with the immunotherapeutic agent (two with dose modification), and no irAEs were reported. Three patients were not re-challenged as they came off the study for disease progression (n=2) and due to personal reasons (n=1).
Treatment Outcomes Associated With irAEs
Of the 290 patients, 264 were evaluable for response at the time of data cut-off. Patients with grade ≥3 irAEs had significantly improved overall response rate (ORR; CR + PR), disease control rate (DCR; CR + PR + SD), and TTP than patients without. The ORR was 25% (3 of 12 evaluable) in patients with grade ≥3 irAEs compared to 6% (15 of 252 evaluable) in patients without (p=0.039). The DCR was also higher in patients who had grade ≥3 irAEs compared to those without (67 vs. 21%; p=0.001). Of the 12 patients with grade ≥3 irAEs evaluable for response, 1 had CR, 2 had PR and additional 5 had SD. Majority of these patients had progressed on a prior therapy. Importantly, despite the use of systemic corticosteroids for management of irAEs, patients continued to have clinical benefit. Of 5 patients re-challenged after receiving systemic corticosteroid for irAE, all 4 evaluable for response continued to respond (CR=1, PR=1, SD=2).
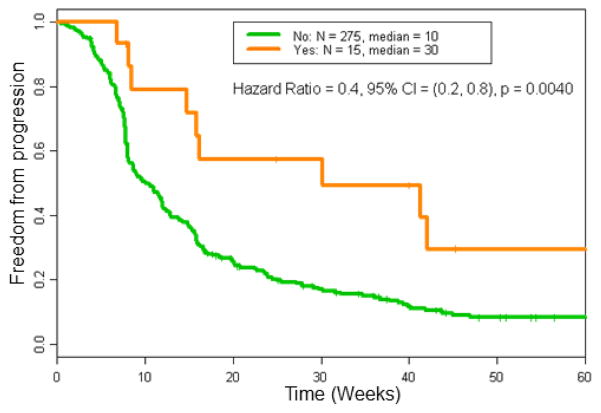
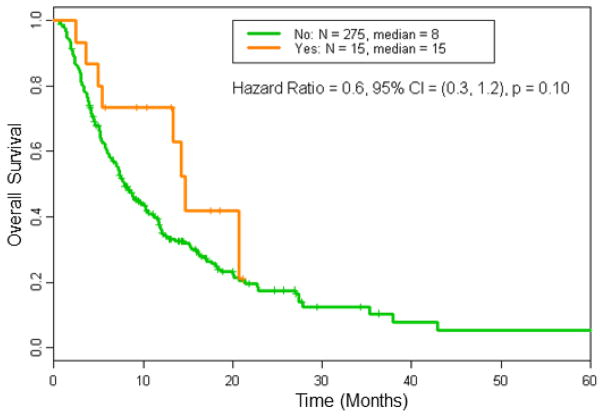
Further, Kaplan-Meier estimates of TTP were significantly longer in patients who had grade ≥3 irAEs (Figure 1). The estimates of median TTP was 30 weeks for patients with grade ≥3 irAEs compared to 10 weeks for those without (Hazard ratio [HR] = 0.4, 95% confidence interval [CI] = [0.2, 0.8], p = 0.0040). The median OS for patients with grade ≥3 irAEs was 15 months as compared to 8 months for those without (Figure 2, HR = 0.6, 95% CI = [0.3, 1.2], p = 0.10).
Figure 1. Time to progression (TTP) in patients treated on immunotherapy-based regimens by incidence of grade 3 or 4 irAEs.
The estimates of median TTP was longer in patients who developed grade ≥3 irAEs (30 weeks) compared to patients who did not (10 weeks); p=0.0040.
Figure 2. Overall survival (OS) in patients treated on immunotherapy-based regimens by incidence of grade 3 or 4 irAEs.
The estimates of median OS was longer in patients who developed grade ≥3 irAEs (15 months) compared to patients who did not (8 months); p=0.10
Factors Associated with Grade ≥3 irAE
The following baseline variables were evaluated for association with grade ≥3 irAEs: total white blood cells, absolute neutrophil count, absolute lymphocyte count, hemoglobin, platelet count, lactate dehydrogenase, albumin, total bilirubin, alanine transaminase, aspartate transaminase, sodium, potassium and calcium. But, none were associated with grade ≥3 irAEs.
DISCUSSION
Despite these promising results with immunotherapy, significant morbidity due to irAEs may be a limiting factor [7]. As irAEs quickly progress to life-threatening condition if not treated promptly [32], early detection of irAEs is critical to maximize the clinical benefit associated with these agents. This emphasizes the need for increased awareness among physicians and patients alike.
Overall, in our study, the immunotherapy-based regimens were well tolerated. Of the 290 patients treated on an immunotherapy-based clinical trial, 34% had irAEs of any-grade. Fifteen patients experienced grade 3–4 irAEs. Consistent with published data [7], most of the irAEs were seen in patients treated with immunotherapy regimens that included a checkpoint inhibitor while patients treated with cancer cell-derived vaccine experienced the least irAEs. Enhanced understanding of irAEs, anticipation, and strict adherence to toxicity management algorithms by treating physicians in our institution may possibly explain the low number of patients with grade ≥3 irAEs.
Most irAEs are reversible with immediate and appropriate intervention [33,13]. In our study, approximately 80% of patients with grade ≥3 irAEs required the use of systemic steroids (Table 2). Only one patient with RCC treated with a checkpoint inhibitor, received intravenous immunoglobulin for management of grade 3 myasthenia gravis. Nevertheless, all 15 patients with grade ≥3 recovered with supportive treatment and/or systemic corticosteroids.
Interestingly, emerging data suggest that use of systemic corticosteroids or additional immunosuppressive agent such as anti-tumor necrosis factor α (anti-TNFα) for management of irAEs does not negate the clinical activity or the survival benefits derived from the immunotherapeutic agents [34–36]. In a retrospective study of 298 melanoma patients treated with standard dose of ipilimumab, 85 % experienced an irAE of any grade [34]. Though one-third of the patients required systemic corticosteroid treatment and 10% required anti-TNFα immunosuppression, no difference in OS or time-to treatment failure was observed between patients who received corticosteroid or anti-TNFα immunosuppression for irAEs and those who did not. In our study, 80% of the patients with grade ≥3 irAEs required systemic corticosteroids. However, all the patients evaluable for response on re-challenge continued to respond. Thus, judicious use of immunosuppressive agents along with supportive measures not only helps to resolve the irAEs, but also helps the patient to stay longer on the therapy and experience clinical benefit.
The most striking finding in our study is the association between irAEs and improved treatment outcomes. The ORR and DCR were significantly higher in patients with patients with grade ≥3 irAEs, most of who had progressed on a prior therapy. Furthermore, based on Kaplan-Meier estimates we observed that patients with grade ≥3 irAEs had significantly prolonged median TTP than those without. Our results are concordant with those from some early phase clinical trials that suggest an association between irAEs and treatment outcome [35,37–39,36,15,40,41]. Attia et al reported that clinical response to anti–CTLA-4 antibody plus peptide vaccination was seen in 36% of patients with grade ≥3 irAEs compared to 5% of those without irAEs (p = 0.008) [35]. In a retrospective study of 148 melanoma patients treated with nivolumab, the median OS was significantly longer in patients who had any irAE versus those who did not (p ≤ 0.001) [38]. Subset analysis showed that OS was significantly longer in patients who developed rash (HR, 0.423; 95% CI, 0.243 to 0.735; p=0.001) and vitiligo (HR, 0.184; 95% CI, 0.036 to 0.94; p=0.012). ORR was also significantly higher in patients with rash (p=0.03) or vitiligo (p=0.009). Similarly, in another prospective study evaluating pembrolizumab in treatment of melanoma patients, objective response was higher in patients who developed vitiligo than those who did not (71 vs 28%, p=0.002) [15]. Further, in a meta-analysis of 27 studies [40] in melanoma patients treated with various immunotherapeutic agents, vitiligo was significantly associated with both improved progression-free survival (PFS) (HR, 0.51; 95% CI, 0.32 to 0.82; P=0.005) and OS (HR, 0.25; 95% CI, 0.10 to 0.61; P=0.003). Though most studies are limited to melanoma patients, similar improvement in response rate was reported in patients with renal cell cancer treated with ipilimumab [41]. The response rate was higher in patients who had significant toxicities, predominantly enteritis and hypophysitis, than the response rate in those without toxicities (30 vs 0%; p=0.0007). These findings from large clinical development programs suggest that irAEs may be an indicator of the immune status of the patients and may serve as a surrogate marker of response to treatment with immunotherapeutic agents.
Though the majority of irAEs resolve with established algorithms, they are increasingly recognized as a cause for dose modification or discontinuation of an otherwise beneficial therapy. For example, the combined administration of ipilimumab plus nivolumab produced significantly improved response rates, higher DCR, longer PFS, and more durable responses than either agents alone, irrespective of programmed death-ligand 1 (PD-L1) expression or BRAF status, in treatment-naïve melanoma patients [42]. However, 36.4% of patients on combination therapy, 7.7% on nivolumab and 14.8% on ipilimumab monotherapy discontinued the treatment due to treatment-related AEs. Though biomarkers to delineate patients who are likely to experience irAEs may be beneficial, there are currently no well-defined metrics that would allow for risk-stratification of patients receiving immunotherapeutic agents. Therefore, we sought to investigate the clinical and laboratory variables to find out if readily available parameters were predictive of toxicity to immunotherapeutic agents. However, none of the 13 parameters investigated were predictive for the development of irAEs. In earlier studies, an association between on-treatment increase in interleukin 17 levels and absolute eosinophil count from baseline and the occurrence of irAEs have been reported [43,44]. However, their reproducibility in large, multi-center trials is unknown. Recently, in a retrospective study in patients with prostate cancer treated with ipilimumab, CD8 T cell clonal expansion in peripheral blood samples was found to precede the development of grade 2–3 irAEs [45]. But, this finding has not been validated in a large prospective study yet. With recent increase in the use of checkpoint inhibitors in the clinic, the absolute burden of irAEs undoubtedly will rise. Thus, there is a critical need to develop sensitive and robust markers to identify patients who are at an increased risk for irAEs with immunotherapeutic agents.
Our study has some potential limitations. First, it is a retrospective study, and although it included 290 patients, the results have to be interpreted with caution due to the heterogeneous nature of the study population and different treatment regimens. Second, all patients were treated in early phase clinical trials, and some of these patients were in the dose-escalation phase of the trial and received a low dose of drug. Third, in the absence of an objective definition for irAE, the incidence may vary from one institution to another.
In conclusion, the unique class of toxicities seen with immunotherapies, checkpoint inhibitors in particular, is like a double-edged sword. If not treated promptly and vigorously, irAEs may be life threatening. Paradoxically, irAEs have been associated with improved treatment outcomes, suggestive of an active immune status. Thus, management of irAEs presents a challenge to the treating physician. With increasing use of immunotherapeutic agents in the clinic in recent years, increased awareness and early recognition is necessary for effective management of irAEs and improved treatment outcomes. Emerging data suggest that changes in symptoms burden often serve as an early indicator of toxicity [46]. Therefore, incorporation of patient-reported symptoms, development of biomarkers to identify patient at risk for irAEs, and validation of the association between irAEs and improved treatment outcomes with immunotherapeutic agents in a large prospective study is urgently needed.
Acknowledgments
Funding: This work was supported in part by The University of Texas MD Anderson Cancer Center support grant (P30 CA016672, FMB, KRH) and a K23 Career Development Award (K23 AI117024; AS) from the National Institutes of Health.
Footnotes
Previous Presentation: This study was presented in part at the 28th EORTC – NCI – AACR Molecular Targets and Cancer Therapeutics Symposium in November 2016.
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest: The authors declare no competing financial interests.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: For this type of study formal consent is not required.
Authors’ contributions: TF contributed to study design, collected and assembled the data, and was a major contributor in writing the manuscript; RRC contributed to data analysis and interpretation; AMB collected and assembled the data; KRH contributed to study design, performed statistical analysis, and contributed to interpretation of results; JH, MES-A, JG, PS, TM, AP, ST, AS contributed to interpretation of results; AA collected and assembled the data; DSH, AT, VS, SAP-P, SF, FMB contributed to patient enrollment, assembly and interpretation of data; BS contributed to data analysis, interpretation, and was a major contributor in writing the manuscript; AN designed the study, provided administrative support, contributed to patient enrollment, assembly and interpretation of data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
- 1.Rosenberg SA. Decade in review[mdash]cancer immunotherapy: Entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11(11):630–632. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Hematology/Oncology (Cancer) approvals & safety notifications 2016 [Google Scholar]
- 3.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu CN, Kim TY, Choo SP, Trojan J, Welling TH, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, dela Cruz C, Lang LX, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, Di Gravio D, Huang B, Gambhire D, Chen Y, Pathan N, Wang K, Schmidt EV, Chow LQM. Phase Ib study of PF-05082566 in combination with pembrolizumab in patients with advanced solid tumors. J Clin Oncol. 2016;34(15) doi: 10.1200/JCO.2016.34.15_suppl.3002. [DOI] [PubMed] [Google Scholar]
- 7.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen TW, Razak AR, Bedard PL, Siu LL, Hansen AR. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol. 2015;26(9):1824–1829. doi: 10.1093/annonc/mdv182. [DOI] [PubMed] [Google Scholar]
- 13.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 14.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, Tomasic G, Soria JC, Champiat S, Texier M, Lanoy E, Robert C. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA dermatology. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 16.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA dermatology. 2015;151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0160221. ARTN e0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–U2032. doi: 10.1200/Jco.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss M, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 22.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A investigators K. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 23.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 24.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 25.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 28.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/jco.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L Investigators K. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services. [Accessed 07/06/2015];Common Terminology Criteria for Adverse Events v4.03 (CTCAE) 2010 http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 31.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. S0959-8049(08)00873-3 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 33.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Horvat TZ, Adel NG, Dung TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM, Panageas KS, Wolchok JD, Chapman PB. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193. doi: 10.1200/Jco.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 38.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.Ccr-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 41.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AMM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24(6):1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 44.Schindler K, Harmankaya K, Kuk D, Mangana J, Michielin O, Hoeller C, Dummer R, Pehamberger H, Wolchok JD, Postow MA. Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab. J Clin Oncol. 2014;32(15_suppl):9096–9096. doi: 10.1200/jco.2014.32.15_suppl.9096. [DOI] [Google Scholar]
- 45.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L, Emerson RO, Yusko E, Vignali M, Robins HS, Sun J, Allison JP, Sharma P. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919–11924. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendoza TR, Zhao F, Cleeland CS, Wagner LI, Patrick-Miller LJ, Fisch MJ. The validity and utility of the M. D. Anderson Symptom Inventory in patients with breast cancer: evidence from the symptom outcomes and practice patterns data from the Eastern Cooperative Oncology Group. Clin Breast Cancer. 2013;13(5):325–334. doi: 10.1016/j.clbc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]