Abstract
Background & Aims
Acetaminophen overdose is the leading cause of acute liver injury (ALI) and acute liver failure (ALF) in the developed world. Sex differences in acetaminophen-induced hepatotoxicity have not been described.
Methods
We collected data from the Acute Liver Failure Study Group cohort, a national registry of 32 academic medical centers in North America of adults with ALI or ALF, including 1162 patients with acetaminophen-induced ALI (n=250) or acetaminophen-induced ALF (n=912) from January 2000 through September 2016. We analyzed data on patient presentation, disease course, demographics, medical and psychiatric history, medication use, substance use, and details of acetaminophen ingestion. Sex differences in continuous and categorical variables were evaluated by Wilcoxon rank-sum and χ2 analysis or the Fisher exact test. Our primary aim was to evaluate sex differences in the presentation and clinical course of acetaminophen-induced acute liver injury or liver failure, and our secondary goal was to compare overall and transplant-free survival between sexes.
Results
Most patients with acetaminophen-induced ALI (68%) or ALF (76%) were women. Higher proportions of women than men had psychiatric disease (60% of women vs 48% of men, P<.01) and had co-ingestion with sedating agents (70% of women vs 52% of men, P<.01)—more than half of which were opioids. Higher proportions of women had severe hepatic encephalopathy (HE) (68% of women vs 58% of men), and required intubation (67% of women vs 59% of men, P values <.03). Higher proportions of women used vasopressors (26% of women vs 19% of men, P=.04) or mannitol (13% of women vs 6% of men, P<.01); proportions of male vs female patients with transplant-free survival were similar (68%). On adjusted analysis, women had higher risk of severe HE (adjusted odds ratio [AOR], 1.66; 95% CI, 1.17–2.35). We found a significant interaction between sex and co-ingestion of sedating agents (P<.01); co-ingestion increased odds of severe HE in women 2-fold (AOR, 1.86; 95% CI, 1.28–2.69; P<.01) but not in men (AOR; 0.62, 95% CI 0.34–1.13; P=.12).
Conclusion
In an analysis of the Acute Liver Failure Study Group cohort, we found acetaminophen-induced ALI and ALF to be more common among women. Women have greater critical care needs than men, and increased risk for severe HE, which could be due in part to increased use of sedatives. Future studies should investigate sex differences in acetaminophen metabolism and hepatotoxicity, particularly among users of opioids.
Keywords: APAP, ALFSG, Tylenol, paracetamol, gender, narcotics
Acetaminophen is one of the most commonly consumed medications worldwide, either alone or in combination with other over the counter or prescription products, including opioids (1). In the United States (US), 82,000 emergency room visits and 26,000 hospitalizations are attributed to acetaminophen overdose annually (2,3). Acetaminophen toxicity is also the leading cause of acute liver injury (ALI) and acute liver failure (ALF) in the US, accounting for approximately 50% of all ALF cases. Given the clinical implications of this disease, as well as the growing opioid epidemic in the United States, it is essential to identify those at highest risk.
Sex differences in the epidemiology and natural history of many diseases are evident, with growing adoption of sex-based models of care for medical conditions outside the realm of hepatology (4–6). Sex differences are also described in the presentation and clinical course of many liver diseases (7), including a higher risk of ALF in women for many etiologies (8–11). Prior data on sex and ALF outcomes have largely focused on idiopathic drug injury (DILI), where women are noted to be at increased risk of progression to ALF (12). The reasons for increased prevalence and severity of DILI in women are not clear, though may relate to greater use of hepatotoxic medications in women (1, 13), and differential drug metabolism by sex (14). Despite apparent sex differences in the prevalence and progression of other etiologies of ALF, less is known regarding the natural history of acetaminophen-induced ALF in men and women.
Using data from the prospective, multi-center Acute Liver Failure Study Group (ALFSG) cohort we aimed to evaluate sex differences in the presentation and clinical course of acetaminophen-induced ALI and ALF, and to secondarily compare overall and transplant-free survival by sex. Understanding the differential risk and clinical course of acetaminophen-induced ALI and ALF in men and women could help to reduce the burden of disease, while optimizing the management of those that progress to liver failure.
PATIENTS AND METHODS
Study Population
We evaluated differences in presentation and clinical course between men and women with acetaminophen-induced ALI and ALF within the ALFSG. The ALFSG is a national registry of 32 academic medical centers in North America designed to examine clinical data and outcomes in adults with ALI and ALF. Detailed study protocols are previously described (15). ALI was defined as acute hepatic illness <2 weeks with international normalized ratio (INR) ≥ 2.0, as well as alanine aminotransferase (ALT) >10 times the upper limit of normal and total serum bilirubin >3 mg/dL, without encephalopathy. ALF was defined by an INR of ≥ 1.5, disease duration of <26 weeks, the absence of prior chronic liver disease, with the presence of hepatic encephalopathy. Patients with acetaminophen overdose between January 1, 2000 and September 27, 2016 were included in the current study. Acetaminophen was determined to be the etiology of ALI or ALF if there was a history of potentially toxic acetaminophen ingestion within a week of presentation, detection of any level of acetaminophen in the serum, or ALT >1000 IU/L with any history of acetaminophen ingestion irrespective of acetaminophen level. Per institutional review board guidelines, informed consent was obtained from patients or next of kin prior to enrollment. Patients with missing or unknown 21-day follow-up status, or liver transplant prior to study enrollment, were excluded.
Data Collection
Patient demographics (age, sex, race, and ethnicity), medical and psychiatric history, medication use, substance use (alcohol and illicit drugs), and acetaminophen ingestion details (suicidal intent, dose, timing, co-ingestions), were obtained at study entry from the patient or primary contact, as history was limited by hepatic encephalopathy in most patients. Data were often corroborated with patient after recovery. The case report forms also allowed investigators to describe other medical or psychiatric illnesses. Check boxes were available on data collection forms for most major medical/psychiatric conditions. Endocrine disorders reflected a single check box for any endocrine disorder (such as diabetes). Psychiatric medications included antidepressants, antipsychotics, and non-benzodiazepine anxiolytics. Heavy alcohol use was defined as >14 drinks per week within the past 6 months. Co-ingestions were considered those combining acetaminophen with other medications with sedating properties including opioids, benzodiazepines, and diphenhydramine. Intentionality was defined as a probable suicide attempt if a large quantity of acetaminophen was ingested at single time point or if suicidal intent was acknowledged by the patient or surrogate. Physical exam, laboratory values, and details of hospital course were obtained by study coordinators daily for seven days, or until day of discharge if prior to seven days. “Pre-study” data reflects peak labs values or clinical interventions performed at outside hospitals prior to ALFSG enrollment. These data were captured when available (in approximately 80% of patients). Seven-day follow up reflects cumulative interventions through day 7 post-enrollment or through hospital discharge if patient discharged before day 7. Hepatic encephalopathy (HE) was graded 1 to 4 by the West Haven criteria; with grade 1 defined as mild changes in sensorium to grade 4 defined as coma (16). The King’s College criteria for acetaminophen-induced ALF were defined as having an arterial pH <7.30 or all 3 of the following: (1) grade 3 or 4 encephalopathy; (2) prothrombin time >100 seconds (INR >6.5); and (3) serum creatinine >3.4 mg/dL (17). Standard 21-day outcome data captured by the ALFSG includes death, listing for liver transplant, transplant-free and overall patient survival.
Statistical Analysis
Descriptive statistics were used to describe demographics, clinical characteristics, and outcomes. Continuous variables were reported as medians with interquartile ranges (IQR) due to non-normal distributions. Sex differences in continuous and categorical variables were evaluated by Wilcoxon rank-sum and Chi Square or Fisher exact test, respectively. All tests were 2-sided and p values <0.05 were considered significant. Multivariate models were performed using logistic regression with backward covariate selection. Variables were also excluded from the multivariable model if there was substantial missing data. We evaluated for interactions between sex and relevant clinical characteristics, with interaction p values of ≤ 0.10 considered statistically significant. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics and Co-Morbidities
Among the 1162 patients with acetaminophen toxicity enrolled between January 2000 and September 2016, 250 (22%) had acetaminophen-induced ALI and 912 (78%) had acetaminophen-induced ALF. There were 17 patients in the cohort that presented with ALI and progressed to ALF who were analyzed only within the ALF group. Of patients in the ALFSG cohort with either acetaminophen-induced ALI or ALF, 864 (74%) were women, including 170 (68%) of the ALI patients and 694 (76%) of the ALF patients.
Table 1 displays the baseline demographics, medical comorbidities, and ingestion characteristics for patients with acetaminophen-induced ALF stratified by sex. Women were older than men, with a median age of 37 (IQR 29–47) versus 33 (IQR 25–45) years (p<0.01). The racial/ethnic distribution was similar by sex, with the majority (85%) of the cohort being white, and approximately 7% of all racial groups reporting Hispanic ethnicity. A greater proportion of women than men reported over 12 years of formal education (42% vs 28%, p=0.01).
Table 1.
Baseline Demographics, Comorbidities, and Ingestion Characteristics for Acetaminophen-Induced ALF By Sex
| Women (n = 694) |
Men (n = 218) |
p value | |
|---|---|---|---|
|
| |||
| Median age (IQR), years | 37 (29–47) | 33 (25–45) | 0.0015 |
|
| |||
| Race | |||
| White | 589 (84.9) | 182 (83.5) | 0.84 |
| Black | 58 (8.4) | 21 (9.6) | |
| Other | 47 (6.8) | 15 (6.9) | |
|
| |||
| Hispanic ethnicity | 42 (6.1) | 18 (8.3) | 0.25 |
|
| |||
| Years of education1 | |||
| 0–7 | 5 (1.2) | 3 (2.4) | 0.014 |
| 8–12 | 234 (56.4) | 88 (69.3) | |
| >12 | 176 (42.4) | 36 (28.4) | |
|
| |||
| US census region | |||
| Northeast | 102 (14.7) | 38 (17.4) | 0.34 |
| South | 184 (26.5) | 54 (24.8) | |
| Midwest | 162 (23.3) | 61 (28.0) | |
| West | 233 (33.6) | 60 (27.5) | |
| Canada | 13 (1.9) | 5 (2.3) | |
|
| |||
| Comorbidities (ever) | |||
| Psychiatric disease | 415 (60.0) | 104 (47.7) | 0.0014 |
| Intravenous drug use | 49 (7.1) | 31 (14.5) | 0.0010 |
| Hypertension | 93 (13.4) | 26 (11.9) | 0.56 |
| Endocrine | 102 (14.7) | 16 (7.3) | 0.0046 |
| Heart disease | 41 (5.9) | 11 (5.1) | 0.63 |
| BMI ≥30 (Obesity)1 | 139 (24.3) | 44 (24.4) | 0.9687 |
|
| |||
| Alcohol intake, drinks per week1 | |||
| 0–6 | 170 (72.3) | 36 (52.2) | 0.0017 |
| 7–14 | 21 (8.9) | 6 (8.7) | |
| >14 | 44 (18.7) | 27 (39.1) | |
|
| |||
| Psychiatric medication use (per patient report) | 289 (42.3) | 54 (24.9) | <0.001 |
|
| |||
| Oral contraceptive use | 38 (5.5) | – | – |
|
| |||
| Hormone replacement therapy use | 37 (5.4) | 1 (0.5) | 0.0016 |
|
| |||
| Intentionality | |||
| Suicide attempt | 254 (38.4) | 88 (43.6) | 0.15 |
| Unintentional | 350 (53.0) | 104 (51.5) | |
| Unknown | 57 (8.6) | 10 (4.9) | |
|
| |||
| Initiation of therapeutic acetaminophen use1 | |||
| Physician recommended | 69 (24.7) | 15 (17.9) | 0.19 |
| Self-initiated | 210 (75.3) | 69 (82.1) | |
|
| |||
| Therapeutic intent1,2 | |||
| Acute pain | 28 (26.7) | 10 (30.3) | 0.48 |
| Infectious process | 9 (8.6) | 1 (3.0) | |
| Sub-acute pain | 22 (21.0) | 10 (30.3) | |
| Chronic pain | 46 (43.8) | 12 (36.4) | |
|
| |||
| Median reported acetaminophen dose (IQR), mg | 14,950 (4,000–36,000) | 20,000 (5,000–50,000) | 0.095 |
|
| |||
| Median weight-adjusted acetaminophen dose (IQR), mg/kg | 226.3 (57.4–523.3) | 235.4 (59.2–595.2) | 0.57 |
|
| |||
| Median time from ingestion to acetaminophen level (IQR), hours | 48 (24–72) | 24 (24–72) | 0.088 |
|
| |||
| Median time from symptom onset to initial hospitalization (IQR), hours | 24 (0–72) | 24 (0–48) | 0.56 |
|
| |||
| Median time from symptom onset to maximum HE (IQR), hours | 168 (96–216) | 168.0 (96–216) | 0.79 |
|
| |||
| Median time from initial hospitalization to transfer to transplant center (IQR), days | 4.9 (4.5–5.3) | 4.8 (4.5–5.3) | 0.26 |
NOTE: data shown are number (percentage) unless otherwise indicated
ALF = acute liver failure; IQR = interquartile range; BMI = body mass index; HE = hepatic encephalopathy
Incomplete data for these variables: for education level n = 542, for BMI >30 n = 752, for Alcohol intake n = 304, for Initiation of APAP use n = 363, for Therapeutic intent n = 138
Acute pain = <1 week of symptoms, Sub-acute pain = 1–4 weeks of symptoms, Chronic pain = >4 weeks of symptoms
Regarding co-morbid conditions, women with acetaminophen-induced ALF were more likely to have psychiatric disease (60% vs 48%, p<0.01) and were more likely to be taking psychiatric medications (42% vs 25%, p<0.001). Half of patients with psychiatric comorbidities had depression (52% in men, 48% in women), and an additional 15% had anxiety or a combination of anxiety and depression. Distribution of psychiatric illnesses did not differ between men and women (p=0.62). Regarding substance use, men were more likely to have a history of intravenous drug use (15% vs 7%, p<0.01). Alcohol data were missing in 66% of the cohort, although a higher proportion of men than women reported recent heavy use (39% vs 19%, p<0.01). Endocrine disorders were more common in women (60% vs 48%, p<0.001), and a similar proportion (~ 25%) of men and women were obese (BMI >30).
Characteristics of Acetaminophen Use
Approximately half of patients with acetaminophen-induced ALF had unintentional acetaminophen overdose (53%), while 40% were suicide attempts (unknown intentionality in remaining 7%). Rates of suicide attempt were similar between men and women (44% and 38%, respectively, p=0.15). In those using acetaminophen for therapeutic purposes, acetaminophen use was more often self-initiated (77%) than recommended by physicians (23%); this was similar for men and women p=0.19). Only 15% of patients provided indications for acetaminophen use, of whom 42% reported use for chronic pain and 28% for acute pain, which was also similar by sex (p=0.5). There was no sex difference in reported total acetaminophen dose, even when adjusted for body weight (p=0.57). Interestingly, in women first acetaminophen level was performed a median of 48 hours (IQR 24–72) after acetaminophen ingestion, compared to 24 hours (IQR 24–72) in men (p=0.088), though median time to transfer to transplant center, and median time from symptom onset to either initial hospitalization or maximum HE were similar (p values >0.2). At the time of study enrollment, 90% of all patients had been started on N-acetyl cysteine.
Co-Ingestions
A greater proportion of women had co-ingestions with either opioids, benzodiazepines or diphenhydramine (70% vs 52%, p<0.001). This was true for patients with and without suicide attempts. Interestingly, the prevalence of co-ingestions was lower in patients with documented suicide attempts, compared to those without (43% vs 65%, p<0.001). Just over half of co-ingestions in women reflected opioid use, and all individual co-ingestion categories were more common in women than men (Figure 1). Approximately 25% of patients used a combination of sedating medications.
Figure 1.
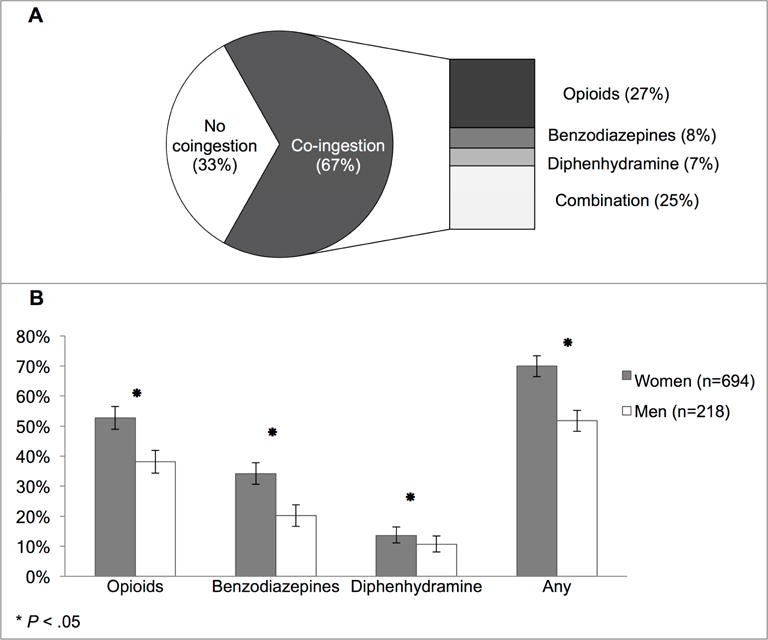
Co-ingestion with sedating medications in acetaminophen-induced ALF (A) Proportion with co-ingestion, by type. (B) Proportion with any sedating medication use, by sex.
Clinical Characteristics by Sex
Pre-study peak, study entry, and peak clinical characteristics by sex are shown in Table 2. At all time points, men had higher median ALT, bilirubin, creatinine, albumin and model for end-stage liver disease (MELD) scores, and lower sodium levels than women (p values ≤ 0.02). However, weight adjust ALT was similar between men and women (55.2 vs 51.8, p=0.23). Women had lower phosphorus levels at study entry (p<0.01) and follow-up (p=0.06), and lower study entry pH levels (p=0.04). INR, ammonia, acetaminophen, glucose, and lactate levels were not significantly different by sex (p values ≥0.06). A higher proportion of men met Kings College Criteria at study entry, although this did not reach statistical significance (p=0.09).
Table 2.
Clinical Parameters for Acetaminophen-Induced ALF By Sex
| Women (n = 694) |
Men (n = 218) |
p value | |
|---|---|---|---|
|
| |||
| ALT, IU/L | |||
| Pre-study peak | 4556 (2261–7595) | 5374 (2545–9374) | 0.017 |
| Study entry | 3406 (1845–5605) | 4506 (2572–7115) | <0.001 |
| Study peak | 3865 (2113–6210) | 4999 (2764–7825) | <0.001 |
|
| |||
| AST, IU/L | |||
| Pre-study peak | 6125 (2663–10,314) | 6364 (3156–11,044) | 0.41 |
| Study entry | 3473 (1302–7578) | 4165 (1968–7971) | 0.077 |
| Study peak | 4029 (1480–8403) | 4748 (2394–8360) | 0.078 |
|
| |||
| INR | |||
| Pre-study peak | 4.2 (2.8–6.2) | 4.2 (3.1–6.6) | 0.45 |
| Study entry | 2.9 (2.0–4.4) | 3.0 (2.2–4.6) | 0.14 |
| Study peak | 3.2 (2.1–4.8) | 3.2 (2.3–5.5) | 0.087 |
|
| |||
| Bilirubin, mg/dL | |||
| Pre-study peak | 3.9 (2.5–5.7) | 5.8 (3.4–8.0) | <0.001 |
| Study entry | 4.1 (2.6–5.9) | 5.4 (3.3–7.7) | <0.001 |
| Study peak | 6.3 (3.7–11.1) | 8.6 (4.4–14.2) | <0.001 |
|
| |||
| Creatinine, mg/dL | |||
| Pre-study peak | 1.8 (1.0–3.1) | 2.4 (1.4–3.6) | <0.001 |
| Study entry | 1.7 (0.9–3.0) | 2.4 (1.0–4.1) | <0.001 |
| Study peak | 2.3 (1.0–4.2) | 2.9 (1.1–5.7) | <0.001 |
|
| |||
| Arterial ammonia, umol/L1 | |||
| Study entry | 97 (58–162) | 109 (59–182) | 0.52 |
| Study peak | 102 (62–164) | 110 (59–175) | 0.94 |
|
| |||
| Venous ammonia, umol/L1 | |||
| Study entry | 102 (70–154) | 89 (59–159) | 0.28 |
| Study peak | 106 (76–163) | 104 (70–167) | 0.69 |
|
| |||
| Lactate, mmol/L | |||
| Study entry | 4.3 (2.3–8.4) | 3.5 (2.1–7.6) | 0.32 |
| Study peak | 4.4 (2.4–8.8) | 3.9 (2.2–7.7) | 0.31 |
|
| |||
| pH | |||
| Study entry | 7.41 (7.34–7.47) | 7.43 (7.37–7.47) | 0.041 |
| Study peak | 7.47 (7.42–7.52) | 7.47 (7.42–7.52) | 0.94 |
|
| |||
| Glucose, mg/dL | |||
| Study entry | 122 (98–158) | 119 (97–157) | 0.56 |
| Study peak | 133 (106–168) | 130 (107–171) | 0.71 |
|
| |||
| Albumin, g/dL | |||
| Study entry | 2.8 (2.5–3.2) | 3.0 (2.7–3.4) | <0.001 |
| Study peak | 3.0 (2.7–3.4) | 3.2 (2.9–3.6) | <0.001 |
|
| |||
| Sodium, mmol/L | |||
| Study entry | 140 (137–144) | 139 (136–142) | <0.001 |
| Study peak | 143 (139–147) | 141 (138–144) | <0.001 |
|
| |||
| Phosphate, mg/dL | |||
| Study entry | 2.5 (1.7–3.5) | 2.9 (1.8–4.0) | 0.0083 |
| Study peak | 3.5 (2.3–4.6) | 3.8 (2.7–5.0) | 0.059 |
|
| |||
| MELD | |||
| Study entry | 32.5 (24.1–39.2) | 34.9 (27.3–41.3) | 0.0041 |
| Study peak | 34.3 (25.4–41.0) | 36.3 (28.4–43.4) | 0.0082 |
|
| |||
| NAC Use, n (%) | |||
| Study entry | 621 (89.6) | 195 (89.5) | 0.95 |
| Study peak | 622 (89.8) | 197 (90.4) | 0.79 |
|
| |||
| Acetaminophen level, mg/L | |||
| Study entry | 42.7 (18.0–107.0) | 45.0 (20.5–97.0) | 0.85 |
|
| |||
| Met King’s College Criteria, n (%) | |||
| Study entry | 65 (9.5) | 29 (13.8) | 0.075 |
|
| |||
| Study entry HE grade, N (%) | |||
| No coma | 13 (2.0) | 8 (3.7) | <0.001 |
| I | 152 (22.8) | 70 (32.7) | |
| II | 114 (17.1) | 47 (22.0) | |
| III | 166 (24.9) | 28 (13.1) | |
| IV | 222 (33.3) | 61 (28.5) | |
|
| |||
| Maximum HE grade, N (%) | |||
| No coma | 14 (1.9) | 7 (3.3) | 0.023 |
| I | 104 (15.4) | 47 (22.0) | |
| II | 96 (14.2) | 37 (17.3) | |
| III | 136 (20.2) | 28 (13.1) | |
| IV | 325 (48.2) | 95 (44.4) | |
|
| |||
| Length of ICU stay, days | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 0.29 |
NOTE: Data shown are Median (IQR), unless otherwise indicated
ALF = acute liver failure; INR = international normalized ratio; IQR = interquartile range; MELD = Model for End-Stage Liver Disease; NAC = N-acetylcysteine; HE = hepatic encephalopathy; ICP = intracranial pressure; ICU = intensive care unit
For arterial ammonia, on study entry n = 248, at study peak n = 278; for venous ammonia, on study entry n = 364, at study peak, n = 418
Regarding complications of ALF, women were more likely to have high-grade hepatic encephalopathy (grade 3–4) at study entry (58% vs 42%) and during the 7-day follow-up period (68% vs 58%, p values <0.01) (Figure 2). Likewise, women were more likely to be intubated at study entry (60% vs 45%, p<0.001) and during follow-up (67% vs 59%, p=0.03). Vasopressor (26% vs 19%, p=0.04) and mannitol use (13% vs 6%, p<0.01) were also greater at study entry in women. Mannitol (22% vs 17%, p=0.09) and antibiotic use (14% vs 9%, p=0.06) were also more commonly used in women during follow-up. Despite these greater critical care needs in women, length of intensive care unit stay was similar by sex (p=0.29).
Figure 2.
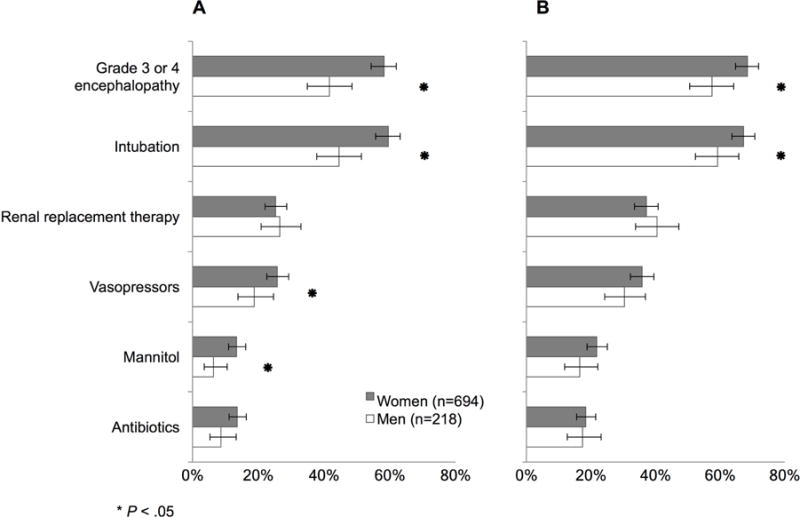
Clinical characteristics in acetaminophen-induced ALF by sex at (A) study entry (B) during cumulative 7-day follow up.
Risk of Severe Hepatic Encephalopathy
Given marked sex differences in prevalence of severe HE (grade 3–4), we evaluated whether female sex was an independent predictor of severe HE at study entry and follow-up. On univariate analysis, female sex was associated with increased risk of severe HE at both study entry (OR 1.95, 95% CI 1.43–2.67, p<0.01) and follow-up (OR 1.60, 95% CI 1.17–2.20, p<0.01). Covariates associated with severe HE are shown in Table 3, which included co-ingestion use at study entry (OR 1.64, 95% CI 1.24–2.18, p<0.01). On multivariate analysis the odds of severe HE remained significantly higher in women than men at both study entry (AOR 1.78, 95% CI 1.28–2.51, p<0.01) and follow-up (AOR 1.66, 95% CI 1.17–2.35, p<0.01), independent of age, co-ingestions, study entry MELD, and ALT levels. Importantly, we identified a significant interaction between sex and co-ingestions (study entry p=0.1; follow up p<0.01). On adjusted analysis, co-ingestions in women conferred a nearly two-fold higher odds of severe HE at both study entry (AOR 1.81, 95% CI 1.26–2.59, p<0.01) and follow-up (AOR 1.86, 95% CI 1.28–2.69, p<0.01). In contrast, there was no significant difference in risk of severe HE in men by co-ingestion history at either study entry (AOR 1.03, 95% CI 0.57–1.84, p=0.93) or follow-up (AOR 0.62, 95% CI 0.34–1.13, p=0.12).
Table 3.
Factors Associated with Severe Hepatic Encephalopathy in Acetaminophen-Induced ALF at Study Entry and During Cumulative 7-Day Follow-Up
| Study Entry | Cumulative 7-Day Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Covariates | OR (95% CI) | p value | AOR (95% CI) | p value | OR (95% CI) | p value | AOR (95% CI) | p value |
| Age | 1.016 (1.006–1.027) | 0.0026 | 1.010 (0.999–1.021) | 0.0820 | 1.101 (1.003–1.025) | 0.012 | 1.012 (1.001–1.024) | 0.0393 |
| Female sex | 1.953 (1.429–2.669) | <0.001 | 1.787 (1.275–2.506) | <0.001 | 1.601 (1.168–2.196) | 0.0035 | 1.659 (1.172–2.348) | 0.0043 |
| White race | 0.607 (0.351–1.050) | 0.36 | – | – | 0.722 (0.405–1.290) | 0.74 | – | – |
| Psychiatric disease (Yes) | 0.828 (0.633–1.083) | 0.17 | – | – | 0.868 (0.655–1.149) | 0.32 | – | – |
| Alcohol (Yes)1 | 1.164 (0.706–1.918) | 0.55 | – | – | 1.378 (0.822–2.312) | 0.22 | – | – |
| Co-ingestion | 1.644 (1.242–2.177) | <0.001 | 1.552 (1.140–2.112) | 0.0052 | 1.220 (0.921–1.615) | 0.17 | 1.122 (0.707–1.781) | 0.6254 |
| Acetaminophen level1 | 1.002 (1.000–1.003) | 0.024 | 1.002 (1.000–1.004) | 0.020 | ||||
| ALT (per 100 units increase) | 0.991 (0.987–0.995) | <0.001 | 0.989 (0.984–0.993) | <0.001 | 0.995 (0.991–0.999) | 0.0097 | 0.990 (0.986–0.995) | <0.001 |
| AST (per 100 units increase) | 0.998 (0.996–1.001) | 0.21 | – | – | 1.003 (1.00–1.006) | 0.066 | – | – |
| INR | 0.975 (0.922–1.031) | 0.38 | – | – | 1.067 (1.002–1.137) | 0.043 | – | – |
| Bilirubin | 1.021 (0.992–1.051) | 0.16 | – | – | 1.028 (0.995–1.062) | 0.099 | – | – |
| Creatinine | 1.112 (1.031–1.199) | 0.0059 | – | – | 1.187 (1.088–1.294) | <0.001 | – | – |
| MELD | 1.027 (1.013–1.042) | 0.0001 | 1.047 (1.031–1.063) | <0.001 | 1.056 (1.041–1.073) | <0.001 | 1.072 (1.055–1.090) | <0.001 |
| Sodium | 1.067 (1.042–1.092) | <0.001 | 1.063 (1.037–1.089) | <0.001 | ||||
| Phosphate | 1.084 (1.008–1.167) | 0.031 | – | – | 1.192 (1.088–1.306) | <0.001 | – | – |
| Lactate | 0.996 (0.971–1.022) | 0.75 | – | – | 0.990 (0.965–1.017) | 0.47 | – | – |
| Glucose | 1.000 (0.998–1.001) | 0.73 | – | – | 0.999 (0.998–1.001) | 0.48 | – | – |
| PH | 1.580 (0.552–4.525) | 0.39 | – | – | 1.461 (0.498–4.283) | 0.49 | – | – |
| NAC Use | 0.939 (0.593–1.488) | 0.79 | – | – | 0.938 (0.579–1.520) | 0.80 | – | – |
NOTE: laboratory values reflect study entry data
ALF = acute liver failure; HE = hepatic encephalopathy; OR = odds ratio; AOR = adjusted odds ratio; INR = international normalized ratio; NAC = N-acetylcysteine; MELD = model for end-stage liver disease
66% of Alcohol intake values missing; 34% of APAP level values missing
Overall and Transplant Free Survival
Clinical outcomes at day 21, stratified by sex, are shown in Figure 3. The majority of patients with acetaminophen-induced ALF (75%) were alive at 21 days. Approximately 20% of patients were listed for liver transplant, 35% of which received a transplant. The median time to transplantation was 2.0 days. There were no significant sex differences in overall or transplant-free survival at 21 days (p values ≥0.57).
Figure 3.
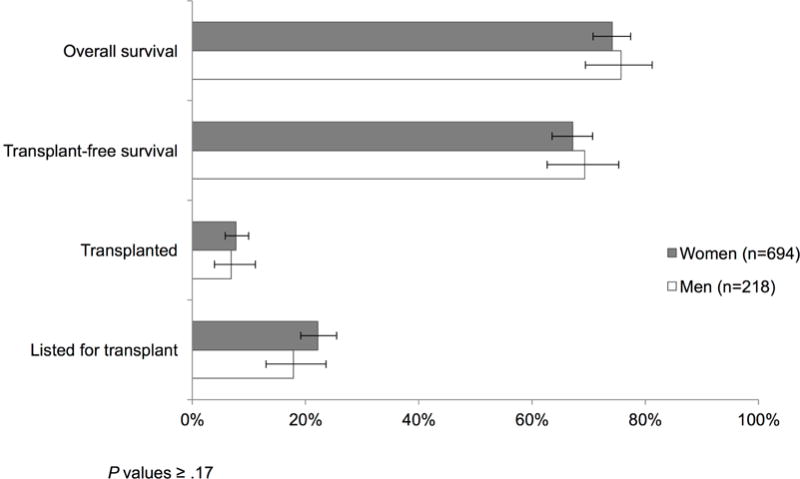
Clinical outcomes for acetaminophen-induced ALF at 21 days, by sex.
DISCUSSION
In this large U.S. cohort, we identified novel sex differences in the presentation and clinical course of acetaminophen-induced ALI and ALF. Importantly, nearly three quarters of patients with acetaminophen overdose were women. Moreover, women with acetaminophen-induced ALF were more likely to present with high-grade hepatic encephalopathy and to have critical care needs at both study entry and throughout hospitalization. Co-ingestions with sedating agents were not only more common in women, but also contributed to their greater risk for severe HE.
Prior data on sex differences in ALF have largely focused on idiosyncratic (non-acetaminophen) drug-induced liver injury (DILI), suggesting that women may be more sensitive to hepatotoxicity. Women are more likely to present with DILI-induced ALF (12) and to require subsequent liver transplant (18). While recent studies have questioned whether women are systematically at greater risk of hepatotoxicity (11, 19), it is clear that women are more likely to progress to DILI-induced ALF, and to have increased susceptibility to hepatotoxicity caused by specific medications (11, 20–22).
A striking finding in the current study was the higher prevalence of co-ingestions in women, most notably with combination opioid-acetaminophen products. These results have important public health implications in light of the current opioid epidemic in the US (23–25), particularly among younger Americans (23, 26). According to NHANES, 2 million Americans adults report using acetaminophen plus an opioid combination with acetaminophen (13). In a 2015 study of patients with new acetaminophen-containing prescriptions, less than one third were aware that their medication contained acetaminophen. Moreover, acetaminophen was identified on the primary container label by the full name “acetaminophen” on only one in 14 prescriptions (27). In a US-based study of patients interviewed about their knowledge of over the counter acetaminophen-containing products, less than half were aware of potential risks of overdose by combining products (28). More transparency in medication labeling as well as greater patient education regarding combination drug ingredients may help to reduce the morbidity and mortality associated with acetaminophen hepatotoxicity.
In the current study, women were not only more likely to ingest sedating agents but the risk of severe HE was nearly 2-fold higher among women with co-ingestions as compared to those without. This increased risk by co-ingestion history was not evident in men. Prior data from the ALFSG have shown that acetaminophen in combination with opioids resulted in higher-grade encephalopathy than acetaminophen/diphenhydramine or acetaminophen alone (29). However, despite greater co-ingestion use in the current study, female sex remained independently associated with severe HE at both study enrollment and follow-up. This suggests that factors beyond their greater co-ingestion use are at play, and could potentially reflect factors such as differences in acetaminophen metabolism by sex or differential patterns of acetaminophen use (14, 30, 31).
In humans, acetaminophen clearance has been shown to be 22% greater in men than women due to increased glucuronidation activity in men (30), a key enzyme in acetaminophen metabolism (31). These sex differences in glucuronidation activity are mitigated by oral contraceptive use, suggesting a potential hormonal contribution (30). Studies from both the US and Europe have also shown that women across age groups are more likely to use prescription and over-the-counter analgesics than men (1, 13, 32, 33). Data from the US-based National Health and Nutrition Examination Survey (NHANES) from 1988–2004 found that women in the US were more likely to use acetaminophen for pain, while men were more likely to use aspirin. In this study women were also more likely to use OTC analgesics on a regular basis (13).
Women in the current study were more likely to have psychiatric disease, including anxiety and depression. Prior data from this cohort evaluating detailed psychiatric history found that rates of psychiatric illness in acetaminophen-induced ALF patients were higher than in the general population, in both intentional and unintentional overdoses (34). In our cohort, suicidal intent was approximately 40% overall, and similar by sex. Acetaminophen dosage adjusted by weight and acetaminophen levels were also similar. Timing from ingestion to first acetaminophen measurement did vary by sex, with women having first levels drawn a median of 48 hours after ingestion, compared to 24 hours in men. Hepatotoxicity in women may have been recognized later than in men, leading to later and more severe illness on presentation or referral to tertiary care centers. Although there were no reported differences in time from symptom onset to initial hospitalization or in time to transfer to transplant center, data from outside hospitals prior to study enrollment are limited.
There are several notable limitations of the current study, including variable timing from outside hospital presentation to study enrollment. Details on outside hospital clinical parameters and interventions are not always recorded, such as timing from initial presentation to NAC administration, although approximately 90% of all patients were on NAC at the time of study enrollment. Though NAC use was not reported in 10% of suspected acetaminophen-induced ALF cases, this is likely due to underreporting of NAC in certain centers, particularly prior to 2010, which has been identified in prior ALFSG analyses (data not shown). Underlying hepatic encephalopathy may also affect patient recall, including amount, timing, and co-ingestions details, although this information was extracted from medication records and/or patient families, then later verified with patients in most cases. Acetaminophen levels were missing on approximately one third of patients, and alcohol history was missing in 66% of the cohort, the latter of which is a known P450 inducer that increases production of the toxic acetaminophen metabolite N-acetyl-p-benzoquinone imine (NAPQI) (35). Despite these limitations, the ALFSG is the largest cohort of patients with acetaminophen-induced ALI and ALF to date, providing daily serologic and clinical measures, which allowed us to capture novel differences in the clinical course of acetaminophen-induced ALF between men and women. These findings can help to inform risk in women with combination acetaminophen and sedative use, and support the need for more restrictive use of opiates, particularly when used in combination with acetaminophen.
In summary, women comprise near three-quarters of patients presenting with acetaminophen-induced ALI and ALF in the United States. Women with acetaminophen-induced ALF have greater critical care needs than men, including more severe encephalopathy. These findings may help to guide inpatient management of women with acetaminophen hepatotoxicity, including consideration for earlier transfer to intensive care units, and aggressive monitoring of neurologic status. Importantly, co-ingestions are more common in women with acetaminophen-induced ALF, which differentially increases their risk of high-grade encephalopathy. In light of the current opioid epidemic, there is a greater need for awareness of the risk of combination acetaminophen-opioid agents in the outpatient setting, which differentially affects the morbidity of women with acetaminophen-induced ALF.
LAY SUMMARY.
Women are more likely than men to have liver failure from acetaminophen (Tylenol, paracetamol) overdose. Women are also more likely to develop changes in mental status requiring more intensive medical care, particularly in those with concurrent opioid use.
Acknowledgments
Grant Support: The Acute Liver Failure Study Group is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U-01 58369). The UCSF Liver Center is supported by P30 DK026743.
Abbreviations
- ALF
acute liver failure
- ALFSG
Acute Liver Failure Study Group
- ALI
acute liver injury
- ALT
alanine aminotransferase
- AOR
adjusted odds ratio
- CI
confidence interval
- DILI
drug-induced liver injury
- HE
hepatic encephalopathy
- INR
international normalized ratio
- IQR
interquartile range
- MELD
model for end-stage liver disease
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
Author contributions:
Rubin: study concept and design, analysis and interpretation of data, drafting and revision of the manuscript, critical revision of the manuscript for important intellectual content
Hameed: study concept and design, acquisition of data, analysis and interpretation of data, study supervision
Gottfried: statistical analysis
Lee: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision
Sarkar: study concept and design, analysis and interpretation of data, drafting and revision of the manuscript, critical revision of the manuscript for important intellectual content, study supervision
References
- 1.Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone Survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Altyar A, Kordi L, Skrepnek G. Clinical and economic characteristics of emergency department visits due to acetaminophen toxicity in the USA. BMJ Open. 2015;5:e007368. doi: 10.1136/bmjopen-2014-007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nourjah P, Ahmad SR, Karwoski C, et al. Estimates of acetaminophen (paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 4.Paulus JK, Lai LYH, Lundquist C, et al. Field synopsis of the role of sex in stroke prediction models. J Am Heart Assoc. 2016;5(5):e002809. doi: 10.1161/JAHA.115.002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulus JK, Wessler BS, Lundquist C, et al. Field synopsis of sex in clinical prediction models for cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2016;9(2 Suppl 1):8. doi: 10.1161/CIRCOUTCOMES.115.002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy J, Peters MG. Liver disease in women: The influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol. 2013;9(10):633. [PMC free article] [PubMed] [Google Scholar]
- 8.Rakela J, Lange SM, Ludwig J, et al. Fulminant hepatitis: Mayo clinic experience with 34 cases. Mayo Clin Proc. 1985;60(5):289–292. doi: 10.1016/s0025-6196(12)60534-5. [DOI] [PubMed] [Google Scholar]
- 9.Wei G, Bergquist A, Broomé U, et al. Acute liver failure in Sweden: Etiology and outcome. J Intern Med. 2007;262(3):393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 10.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761–794. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalasani N, Björnsson E. Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology. 2010;138(7):2246–2259. doi: 10.1053/j.gastro.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Paulose-Ram R, Hirsch R, Dillon C, et al. Prescription and non prescription analgesic use among the US adult population: Results from the Third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiology and Drug Safety. 2003;12(4):315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 14.Amacher DE. Female gender as a susceptibility factor for drug-induced liver injury. Hum Exp Toxicol. 2014;33(9):928–939. doi: 10.1177/0960327113512860. [DOI] [PubMed] [Google Scholar]
- 15.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SHB, McCashland TM, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 17.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 18.Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10(8):1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro MA, Lewis JH. Causality assessment of drug-induced hepatotoxicity: Promises and pitfalls. Clinics in Liver Disease. 2007;11(3):477–505. doi: 10.1016/j.cld.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):20. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the united states. Gastroenterology. 2008;135(6):4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, Barnhart H, Gu J, et al. Associations of gender and a proxy of female menopausal status with histological features of drug-induced liver injury. Liver Int. 2017 doi: 10.1111/liv.13380. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United states: A retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 24.Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3 Suppl):38. [PubMed] [Google Scholar]
- 25.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 26.Ihongbe TO, Masho SW. Prevalence, correlates and patterns of heroin use among young adults in the united states. Addict Behav. 2016;63:74–81. doi: 10.1016/j.addbeh.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 27.King J, McCarthy D, Serper M, et al. Variability in acetaminophen labeling practices: A missed opportunity to enhance patient safety. J Med Toxicol. 2015;11(4):410–414. doi: 10.1007/s13181-015-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf M, King J, Jacobson K, et al. Risk of unintentional overdose with nonprescription acetaminophen products. J Gen Intern Med. 2012;27(12):1587–1593. doi: 10.1007/s11606-012-2096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serper M, Wolf M, Parikh N, et al. Risk factors, clinical presentation, and outcomes in overdose with acetaminophen alone or with combination products: Results from the Acute Liver Failure Study Group. J Clin Gastroenterol. 2016;50(1):85–91. doi: 10.1097/MCG.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miners J, Attwood J, Birkett D. Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol. 1983;16(5):503–509. doi: 10.1111/j.1365-2125.1983.tb02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Court MH. Interindividual variability in hepatic drug glucuronidation: Studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metab Rev. 2010;42(1):209–224. doi: 10.3109/03602530903209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonov KI, Isacson DG. Prescription and nonprescription analgesic use in Sweden. Ann Pharmacother. 1998;32(4):485–494. doi: 10.1345/aph.16409. [DOI] [PubMed] [Google Scholar]
- 33.Sarganas G, Buttery AK, Zhuang W, et al. Prevalence, trends, patterns and associations of analgesic use in Germany. BMC Pharmacol Toxicol. 2015;16(1):28. doi: 10.1186/s40360-015-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pezzia C, Sanders C, Welch S, et al. Psychosocial and behavioral factors in acetaminophen-related acute liver failure and liver injury. J Psychosom Res. 2017;101:51–57. doi: 10.1016/j.jpsychores.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thummel KE, Slattery JT, Ro H, et al. Ethanol and production of hepatotoxic metabolite of acetaminophen in healthy adults. Clin Pharmacol Ther. 2000;67(6):591–599. doi: 10.1067/mcp.2000.106574. [DOI] [PubMed] [Google Scholar]


