Abstract
Objective
This report reflects a meta-analysis that systematically reviewed the literature on intravenous self-administration (IVSA) of nicotine in female and male rats. The goal was to determine if sex differences in nicotine IVSA exist, estimate the magnitude of the effect, and identify potential moderators of the relationship between sex differences and nicotine consumption.
Methods
Extensive search procedures identified 20 studies that met the inclusion criteria of employing both female and male rats in nicotine IVSA procedures. The meta-analysis was conducted on effect size values that were calculated from mean total intake or nicotine deliveries using the Hedges’ unbiased gu statistic.
Results
A random effects analysis revealed that overall females self-administered more nicotine than males (weighted gu = 0.18, 95% CI [0.003, 0.34]). Subsequent moderator variable analyses revealed that certain procedural conditions influenced the magnitude of sex differences in nicotine IVSA. Specifically, higher reinforcement requirements (> FR1) and extended-access sessions (23 h) were associated with greater nicotine IVSA in females versus males. Females also displayed higher nicotine intake than males when the experiment included a light cue that signaled nicotine delivery. Sex differences were not influenced by the diurnal phase of testing, dose of nicotine, or prior operant training.
Conclusion
Overall, the results revealed that female rats display higher levels of nicotine IVSA than males, suggesting that the strong reinforcing effects of nicotine promote tobacco use in women.
Keywords: Reward, Addiction, Pre-clinical, IVSA, Meta-analysis, Rat
1. Introduction
The addictive nature of tobacco products is largely due to the presence of the major alkaloid compound, nicotine. Clinical studies have revealed that nicotine self-administration induces positive subjective ratings of pleasure and drug liking in human subjects [26,42,52]. Some of the early pre-clinical studies also demonstrated reliable intravenous self-administration (IVSA) of nicotine in non-human primates [15,20] and rodents [13,51]. Nicotine IVSA is based on reinforcement principles that involve strengthening a behavioral response, such as a lever press, nose poke, or licking behavior for the delivery of nicotine infusions. The frequency of self-administered infusions and the quantity of intake are used as indices of the reinforcing effects of nicotine. This review focuses on behavioral studies involving IVSA because it is the most common route of administration used in rodent studies, and it mimics the rapid distribution of nicotine to the brain via inhalation methods [4].
The National Institutes of Health currently mandate that sex be included as a biological variable in biomedical studies [37]. Indeed, epidemiological studies have shown that women are more likely to use tobacco products, and are more susceptible to the long-term negative health consequences of smoking [34,54]. In order to reduce the health disparities produced by tobacco use in women, there is a critical need to understand the biological basis for sex-based differences in nicotine addiction [22]. One possible factor that promotes tobacco use in women is the strong reinforcing effects of nicotine. This claim is based on the finding that women rate nicotine as more pleasurable [43] and report greater positive subjective effects following presentation of smoking-related stimuli [41,42] as compared to men.
To understand the biological basis of sex differences in tobacco use, pre-clinical studies have compared nicotine IVSA in female and male rats. However, these reports have yielded mixed results. Some studies report that females display higher rates of nicotine IVSA than males [21,49,59], whereas other studies report that males display higher rates of nicotine IVSA than females [25,30]. There are also studies that report no sex differences in nicotine IVSA [18,31,44,55,56]. These conflicting findings may be due to methodological differences that influence the magnitude of sex differences, such as the presence of cues [6] or differences in social context [40]. The absence of sex differences in some studies may also be due to small sample sizes that reduce statistical power, thereby decreasing the likelihood of detecting sex differences in IVSA when such differences exist in the population.
Several narrative reviews of pre-clinical studies have shed light on the various factors that may promote tobacco use in females [3,14,35,58]. These reviews have been useful in suggesting patterns of sex differences in the litany of pre-clinical studies, and thereby generating hypotheses for further scientific investigation. Recently, Pogun et al. [45] presented an extensive narrative review of sex differences in the behavioral effects of nicotine. These authors suggested that sex is an important factor that influences nicotine IVSA, a hypothesis that is statistically tested in the current meta-analytical review. Despite the strengths of narrative reviews, they cannot statistically integrate findings from a large body of conflicting evidence, such as the existing studies of sex differences in nicotine IVSA. Also, narrative reviews cannot estimate the magnitude of sex differences in IVSA, identify moderator variables, or overcome problems arising from low statistical power among individual studies [5].
Meta-analytic reviews offer an alternative approach for summarizing IVSA findings, allowing for an empirical synthesis of effect sizes to help resolve uncertainties among a large pool of mixed reports [17,60]. Specifically, meta-analysis combines studies in a manner that increases statistical power and the likelihood of detecting sex differences in a set of studies even when the individual studies themselves may fail to reveal an effect [12]. Meta-analytical approaches can also be useful towards identifying the degree to which certain procedural variables influence sex differences in nicotine IVSA. The results of these moderator variable analyses are useful for generating hypotheses regarding the parameters under which sex differences are more likely to be detected, and these parametric variables need to be tested in future empirical studies. For example, Bardo et al. [1] published a meta-analysis of rodent studies that assessed the magnitude of conditioned place preference (CPP) produced by stimulant and opiate drugs. Their analysis revealed that certain experimental features, such as dose, housing conditions, and route of administration influenced the magnitude of drug-induced CPP. Based on their analysis, the authors provided recommendations regarding the optimal experimental features for studying CPP produced by drugs of abuse in rodents. Indeed, a subsequent empirical study found that the magnitude of CPP produced by cocaine was influenced by dose and route of administration [39], as suggested by Bardo and colleagues. A similar approach may help to advance our understanding of sex differences in nicotine IVSA. Thus, the current review presents a meta-analytic review of studies investigating sex differences in nicotine IVSA in female and male rats. The overall goal of this review was to estimate the magnitude of sex differences in nicotine IVSA and identify potential moderator variables.
2. Methods
2.1. Literature search
A comprehensive literature review was conducted via a computer search of the following databases: Web of Science, PubMed, JSTOR, and Google Scholar. A search for unpublished findings was also conducted via Proquest. The following terms were used for all searches: nicotine, reward, reinforcement, sex difference(s), gender difference(s), male(s), female(s), rat(s), rodent(s), intravenous self-administration, IVSA, SA, and operant procedure(s). The title and abstract of each paper were both searched for these terms. The search was limited to documents in English. The search period included January 1, 1955 to June 22, 2017. An unpublished study was also added from our laboratory. Additional studies were identified by searching the reference lists of an empirical report [44], and a recent review paper [45].
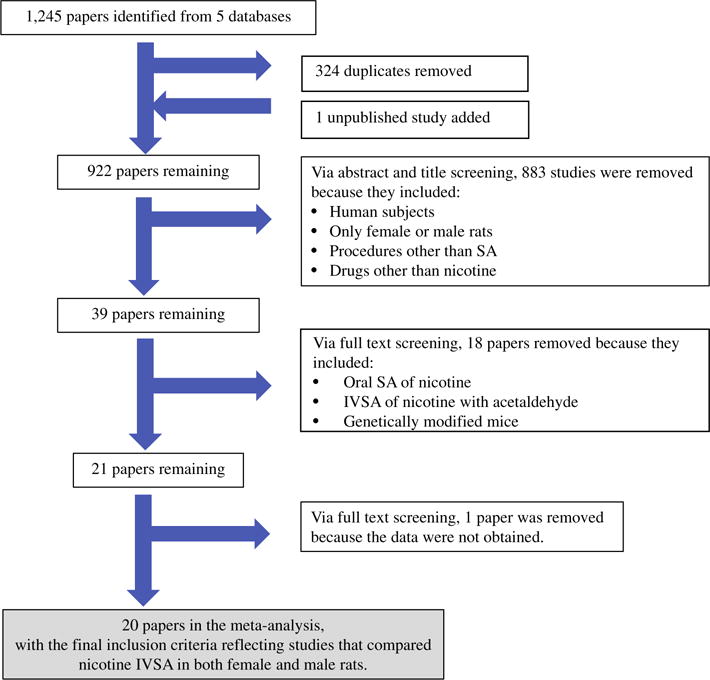
The PRISMA diagram above depicts the outcome of the literature search. The search procedure yielded a pool of 1,245 potentially relevant papers to include in the meta-analysis. The title and abstract of each study were carefully screened by an investigator (RF) that eliminated 324 duplicate studies and added a data set from our laboratory (Uribe et al., unpublished). Two investigators (RF and KU) then performed abstract and title screening, and they removed 883 studies on the basis of including: 1) human subjects, 2) only female or male rats, 3) procedures other than SA, and 4) drugs other than nicotine. The full text of the remaining 39 studies were screened, and 18 studies were removed because they included: 1) oral SA procedures, 2) IVSA of nicotine and acetaldehyde, and 3) genetically modified mice. Of the 21 remaining papers, 1 was removed because the information needed for the meta-analysis was not available and the statistics did not indicate if there was a main effect of sex [7]. The above criteria yielded 20 studies for inclusion in the meta-analysis. The study characteristics are presented above in Fig. 1
Fig. 1.
2.2. Effect size (ES) extraction and calculation
The process of extracting comparable ES values across IVSA studies presented unique challenges that were handled as follows. First, IVSA studies do not result in a single test day value given the longitudinal nature of this procedure. This was handled by calculating an ES value from total nicotine intake or drug deliveries across IVSA sessions, depending on which of these values were reported. Both intake and drug deliveries provide an assessment of nicotine consumption, since intake is a function of deliveries multiplied by the concentration of nicotine. Second, for some studies, multiple ES values could have been derived from the same set of animals during the acquisition and maintenance phases of IVSA. Since meta-analysis requires that ES values be derived from independent samples, some studies only contributed ES values from one phase of IVSA.
The index of ES used in this meta-analysis was the Hedges’ unbiased gu statistic, where gu represents the mean difference between female and male IVSA divided by the pooled within-group standard deviation. Subsequent ES values were corrected for small sample bias, which yielded an unbiased estimate of the population ES value [23]. The use of Hedges’ unbiased gu was preferred over other approaches, such as Cohen’s d, which yields a biased estimate of the population ES for small samples (n < 20; [24]).
When possible, sample ES values were computed directly from information provided in the reports using standard formulas for converting means, standard deviations, and test-statistic values (e.g., t, F) into gu[32]. When papers omitted the information needed to compute ES values, the authors were contacted for the missing information. Four published reports [8,44,46,49] provided the results of F-tests that were used to compute ES values. The corresponding authors of 13 published reports were contacted because their reports did not include the information needed to calculate ES values. Twelve of these authors provided the mean nicotine intake, infusions, standard deviations, and group sizes. Two studies were from our laboratory ([19]; Uribe et al., unpublished data). Two studies lacked ES information, but the authors indicated an absence of sex differences in nicotine IVSA [6,50]. In these cases, the ES values were set to zero, reflecting a conservative approach that made detecting sex differences in nicotine IVSA more difficult, if they exist [53].
2.3. Procedural variable coding
The present meta-analysis compared sex differences under different procedural variable conditions described in Table 1. A subsequent moderator analysis was performed on the following procedural variables (Age, Cue, Diurnal Phase, Dose, Operant Training, Schedule of Reinforcement, and Session Length). Age was categorized as adolescent (postnatal day; PND 28-55) or adult (> PND 60). Cue was categorized as the presence of only a light cue or both a light and tone cue that signaled nicotine delivery. Diurnal Phase was categorized by IVSA testing during the light or dark phase of the diurnal cycle. Dose was categorized as either < 0.03, 0.03, or > 0.03 mg/kg of nicotine. Operant Training was categorized as ‘present’ or ‘absent,’ with ‘present’ indicating training on the operant lever or lickometer with food or sucrose reinforcement prior to nicotine IVSA. Schedule of Reinforcement was categorized as either fixed-ratio 1 (FR1) or > FR1, with FR10 being the largest ratio requirement included in our analysis. A simple dichotomy of FR1 versus > FR1 allowed us to compare studies with a single operant requirement to those that exceeded a one-to-one response contingency. This approach also allowed for the inclusion of sufficient studies to compare the influence of a single versus multiple reinforcement contingencies on sex differences in nicotine IVSA. Session Length was categorized as either short (≤ 3 h) or extended (23 h) access to nicotine IVSA. Forty-five minutes was the minimum session length that was included in the short-access condition. Several experimental conditions were excluded from the analysis due to the low number of studies within each category or challenges in coding the relevant information, such as rat strain, the number of IVSA sessions, or days off between sessions.
Table 1.
Characteristics of studies examining sex differences in nicotine IVSA included in the meta-analysis (NA = not available; n = group size; F = females; M = males; Adol = adolescent; OG = olfactogustatory; L = Light; L + T = Light + Tone; FR = fixed ratio; VR = variable ratio).
| Reference | ES# | ES | Figure in Study | n = | Age | Cue | Diurnal Phase | Dose (mg/kg) | Operant Training | Schedule of Reinforcement | Session Length (hours) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | |||||||||||
| Donny et al., 2000 | 1 | 0.78 | 1 | 14 | 13 | Adult | L | Dark | 0.02 | Present | FR1, FR2, FR5 | 1 |
| “ | 2 | 0.45 | “ | 12 | 12 | “ | “ | “ | 0.03 | “ | “ | “ |
| “ | 3 | 0.15 | “ | 15 | 12 | “ | “ | “ | 0.06 | “ | “ | “ |
| “ | 4 | 0.33 | “ | 13 | 14 | “ | “ | “ | 0.09 | “ | “ | “ |
| Chaudhri et al., 2005 | 5 | 0.00 | 3a | 6 | 6 | Adult | None | Dark | 0.03 | Present | FR1, FR2, FR5 | 1 |
| “ | 6 | “ | “ | “ | “ | “ | “ | “ | 0.06 | “ | “ | “ |
| “ | 7 | “ | “ | “ | “ | “ | “ | “ | 0.15 | “ | “ | “ |
| Rezvani et al., 2008 | 8 | 1.54 | 4 | 6 | 6 | Adult | L+T | Dark | 0.03 | Present | FR1 | 0.75 |
| Lynch et al., 2009 | 9 | −0.35 | 3 | 9 | 6 | Adol | L | 23-hr | 0.005 | Present | FR1 | 23 |
| “ | 10 | 0.17 | “ | 14 | 11 | “ | “ | “ | 0.01 | “ | “ | “ |
| Chen et al., 2011 | 11 | 0.43 | 3c (F) 9 (M) |
6 | 7 | Adol | L+T | Dark | 0.03 | Absent | FR10 | 3 |
| Levin et al., 2011 | 12 | −0.22 | 2 | 9 | 10 | Adol | L+T | Dark | 0.03 | Present | FR1 | 0.75 |
| “ | 13 | −0.49 | “ | 9 | 9 | “ | “ | “ | “ | “ | “ | “ |
| “ | 14 | 0.05 | “ | 10 | 7 | “ | “ | “ | “ | “ | “ | “ |
| “ | 15 | −0.07 | “ | 9 | 7 | “ | “ | “ | “ | “ | “ | “ |
| “ | 16 | −1.71 | “ | 9 | 10 | Adult | “ | “ | “ | “ | “ | “ |
| Feltenstein et al., 2012 | 17 | −0.02 | 2 | 31 | 33 | Adult | L+T | Dark | 0.03 | Absent | FR1 | 2 |
| “ | 18 | −0.04 | “ | 29 | 27 | “ | “ | “ | 0.05 | “ | “ | “ |
| Johnson et al., 2012 | 19 | −0.79 | 2 | 8 | 10 | Adult | L+T | Dark | 0.03 | Present | FR1 | 3 |
| Li et al., 2012 | 20 | 0.11 | 2 | 13 | 10 | Adol | L+T | Dark | 0.0075 | Present | FR1, FR2 | 1 |
| “ | 21 | 0.37 | “ | 12 | 9 | “ | “ | “ | 0.015 | “ | “ | “ |
| “ | 22 | 0.71 | “ | 11 | 9 | “ | “ | “ | 0.03 | “ | “ | “ |
| Grebenstein et al., 2013 | 23 | 1.38 | NA | 7 | 7 | Adult | L | 23-hr | 0.06 | Absent | FR1, FR3 | 23 |
| Sanchez et al., 2014 | 24 | 0.89 | la | 17 | 20 | Adol | L+T | 23-hr | 0.005 | Absent | FR1 | 23 |
| Wang et al., 2014 | 25 | 1.18 | 3a (F) 3b (M) |
45 | 52 | Adult | OG | Dark | 0.03 | Absent | FR10 | 3 |
| Schassburger et al., 2016 | 26 | 0 | 1 | 4 | 3 | Adol | L | Dark | 0.003 | Absent | FR2 | 1 |
| “ | 27 | “ | “ | 14 | 9 | “ | “ | “ | 0.01 | “ | “ | “ |
| “ | 28 | “ | “ | 10 | 11 | “ | “ | “ | 0.03 | “ | “ | “ |
| “ | 29 | 0.27 | “ | 4 | 4 | “ | “ | “ | 0.1 | “ | “ | “ |
| “ | 30 | “ | “ | 4 | 6 | Adult | “ | “ | 0.003 | “ | “ | “ |
| “ | 31 | “ | “ | 8 | 8 | “ | “ | “ | 0.01 | “ | “ | “ |
| “ | 32 | “ | “ | “ | “ | “ | “ | “ | 0.03 | “ | “ | “ |
| Flores et al., 2016 | 33 | 0.84 | 1 | 14 | 10 | Adult | L | 23-hr | 0.015, 0.03,0.06 | Absent | FR1 | 23 |
| Pittenger et al., 2016 | 34 | 0.69 | 2 | 12 | 10 | Adult | L | Light | 0.03 | Present | VR3 | 1 |
| Swalve et al., 2016 (a) | 35 | 0.33 | 1 | 46 | 48 | Adult | L | Light | 0.03 | Absent | FR1 | 1 |
| Swalve et al., 2016 (b) | 36 | 0.42 | 4 | 42 | 19 | Adult | L | Light | 0.03 | Absent | FR1 | 2 |
| Larraga et al., 2017 | 37 | −0.15 | NA | 10 | 12 | Adol | “ | Light | 0.0075, 0.015,0.03 | Absent | FR1 | 2 |
| “ | 38 | −0.61 | “ | 8 | 10 | Adult | “ | “ | “ | “ | “ | “ |
| Peartree et al., 2017 | 39 | −0.35 | 2b(M) 2c(F) |
34 | 36 | Adult | None | Dark | 0.015 | Absent | FR1 | 2 |
| “ | 40 | −0.34 | 8b (M) | 20 | 21 | “ | “ | “ | “ | “ | FR1, FR2, FR3 | 2 |
| “ | 41 | 0.11 | 8c (F) | 20 | 22 | “ | 0.03 | “ | “ | “ | ||
| Uribe et al., unpublished | 42 | 0.41 | NA | 14 | 9 | Adult | L | 23-hr | 0.015, 0.03,0.06 | Absent | FR1 | 23 |
2.4. Statistical analyses
For the overall meta-analysis, both fixed effects (FE) and random effects (RE) analyses were conducted. A weighted average ES value (weighted gu) was computed for the FE and RE analyses, along with a 95% confidence interval (CI) to determine if the ES values were significantly different from zero. The FE analysis assumes that all sample ES values estimate the same population ES. Within the FE statistical model, the variability among sample ES values is presumed to reflect sampling error. This assumption is rarely justified, but many previous meta-analytic reviews have employed FE analyses, which are reported here for comparison purposes. RE analyses were also conducted, as recommended by the National Research Council [38]. RE analyses are considered to be more conservative when dealing with heterogeneity across studies, which reduces the likelihood of Type-I errors [27]. Metaanalyses can be subject to ‘publication bias’ because significant findings are more likely to be published than non-significant findings [33]. To address this issue, Rosenthal’s [47] Fail-Safe N was computed to provide an estimate of the number of additional studies with null effects that would need to be included in the meta-analysis to render the weighted average ES non-significant (p > 0.05).
For the moderator variable analyses, the goal was to assess the influence of various procedural variables on sex differences in nicotine IVSA. One common approach to confirm the presence of a moderator variable is to compare subgroup means across levels within an experimental condition [5]. For example, the magnitude of sex differences was compared between studies that employed short- versus extended- access sessions in order to assess the moderator effect of Session Length on sex differences in nicotine IVSA. The sub-group analysis first separated the ES values from each study into subgroup categories. Then, Hedge’s analog to ANOVA compared subgroup means for each procedural variable [24]. If the ANOVA was significant, follow up contrasts were conducted for procedural variables with more than two levels. The moderator variable analyses were conducted using both the FE and RE models. The RE model incorporated a pooled estimate of the random variance component, as recommended by Borenstein et al. [5].
3. Results
3.1. Overall meta-analysis
The results of the overall meta-analysis are presented in Table 2. Twenty studies yielded 42 independent ES values derived from n = 1,169 rats. Positive ES values indicate a sex difference that favors female rats, and negative ES values indicate a sex difference that favors males. ES values ranged from −1.71 to 1.54. The FE analysis yielded a weighted average ES value of 0.21, with a 95% CI of [0.09 to 0.33], indicating that female rats displayed an average of 0.21 standard deviations more nicotine IVSA than males. There was significant heterogeneity among sample ES values (Hedge’s Q statistic = 78, p ≤ 0.05), suggesting the presence of at least one moderator variable, as described below. The RE analysis yielded a weighted average ES value of 0.18, with a 95% CI of [0.003 to 0.35], indicating that nicotine IVSA in female rats was almost one-fifth of a standard deviation higher than males. Rosenthal’s Fail-Safe N test revealed that > 88 null results would need to be added to the current meta-analysis to render the present effects non-significant.
Table 2.
Effect sizes (ES) and results of the meta-analysis (CI = confidence interval).
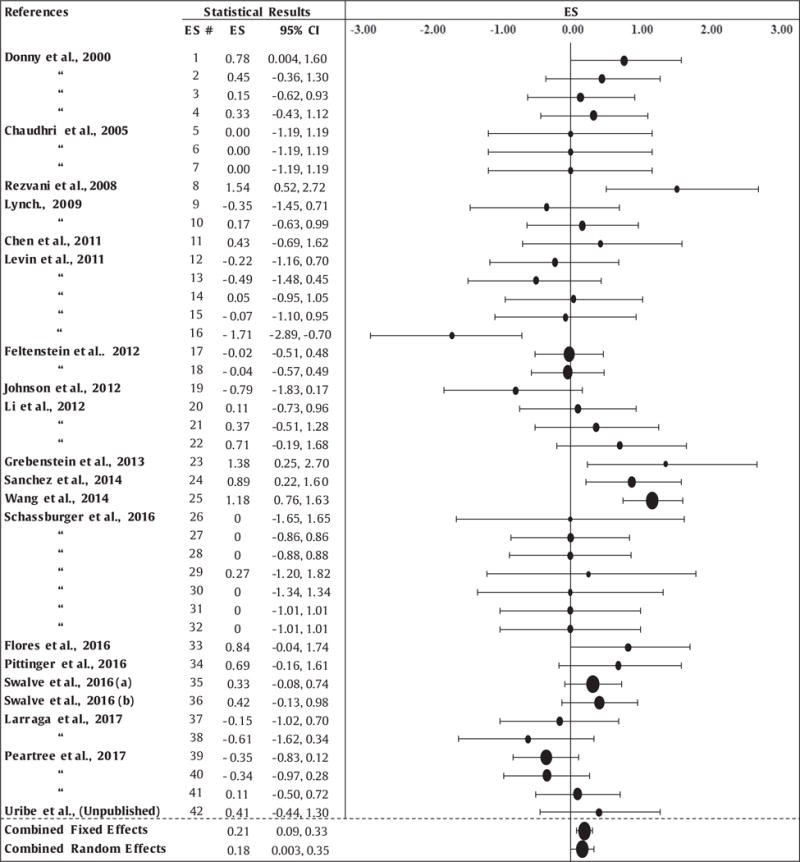
|
The size of the bubble in the Forest plot represents the relative contribution of each study to the meta-analysis.
3.2. Moderator variable analyses
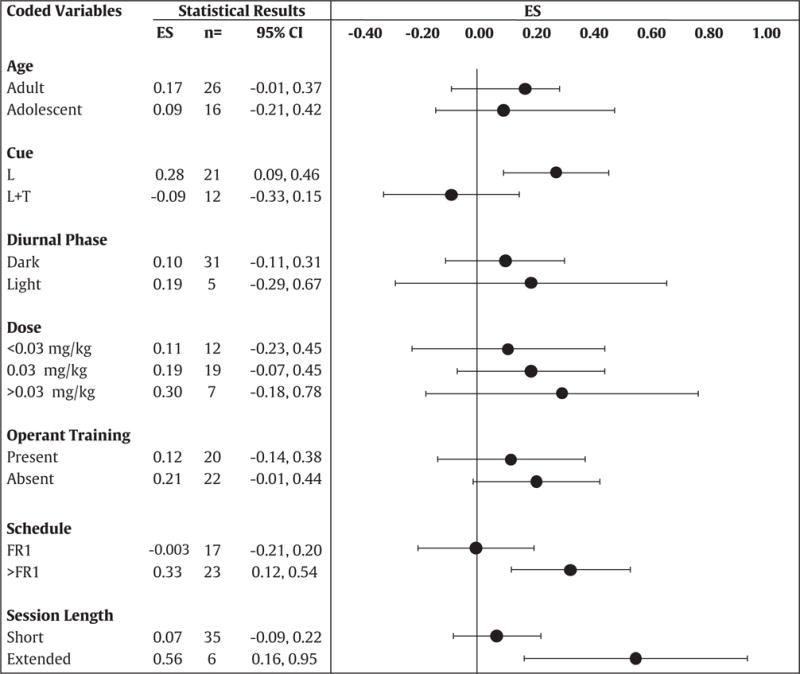
The results of the moderator variable analyses are presented in Table 3. The significant results from the Hedge’s Q test above signals the presence of variables that moderate sex differences in nicotine IVSA. With regard to Age, the FE model yielded an average ES value of 0.11 with a 95% CI of [−0.11, 0.34] for adolescents, and an ES value of 0.23 with a 95% CI of [0.09, 0.36] for adults. Sixteen ES values were included for adolescents (ES# 9-15, 20-22, 24, 26-29, and 37), and 26 for adults (ES# 1-8, 16-19, 23, 25, 30-36, and 38-42). There was no significant heterogeneity detected in the adolescent group (Q = 12.8, p = 0.61). However, significant heterogeneity was detected in the adult group (Q = 66.9, p ≤ 0.05). The RE model yielded an average ES value of 0.09 with a 95% CI of [−0.22, 0.39] for adolescents, and an ES value of 0.17 with a 95% CI of [−0.03, 0.37] for adults. The moderator variable analysis revealed that Age did not influence sex differences in nicotine IVSA (RE model; Hb = 1.65, df = 1, p = 0.19).
Table 3.
Results of the moderator variable analyses (ES = effect size; n = number of values; CI = confidence interval; L-light; L+ T = light + tone; FR = fixed ratio).
|
Positive values reflect an ES in favor of the females and the negative values reflect an ES in favor of the males. Values were derived from the RE model.
With regard to Cue, the FE model yielded an average ES value of 0.28 with a 95% CI of [0.10, 0.45] for studies that included a light cue, and an ES value of −0.08 with a 95% CI of [−0.31, 0.15] for studies that included a light + tone cue. Twenty-one ES values were derived from studies that included a light cue (ES# 1-4, 9-10, 23, 26-38, and 42), and 12 ES values were derived from studies that included a light + tone cue (ES# 11-22). There was no significant heterogeneity detected in studies that included a light cue (Q = 15.73, p = 0.73) or in studies that included a light + tone cue (Q = 17.30, p = 0.10). The RE model yielded an average ES value of 0.28 with an 95% CI [0.09, 0.46] for studies that included a light cue, and an ES value of −0.09 with a 95% CI of [−0.33, 0.15] for studies that included a light + tone cue. The moderator variable analysis revealed that Cue did influence sex differences in nicotine IVSA (RE model; Hb = 5.50, df = 1, p ≤ 0.01). Specifically, studies that employed a light cue only produced larger sex differences as compared to studies that included a light + tone cue.
With regard to Diurnal Phase, the FE model yielded an average ES value of 0.13 with a 95% CI of [−0.004, 0.27] for studies that tested in the dark phase, and an ES value of 0.25 with a 95% CI of [−0.01, 0.53] for studies that tested in the light phase of the diurnal cycle. Thirty-one ES values were derived from studies that tested in the dark phase (ES# 1-8, 11-22, 25-32, and 39-41), and 5 ES values from studies that tested in the light phase (ES# 34-38). Heterogeneity was detected in the dark phase group (Q = 60.0, p < 0.05). However, there was no significant heterogeneity detected in the light phase group (Q = 5.50, p = 0.23). The RE model yielded an ES value of 0.10 with a 95% CI of [−0.11, 0.31] for studies that tested in the dark phase, and an ES value of 0.19 with a 95% CI of [−0.29, 0.67] for studies that tested in the light phase of the diurnal cycle. The moderator variable analysis revealed that Diurnal Phase did not influence sex differences in nicotine IVSA (RE model; Hb = 0.12, df = 1, p = 0.72).
With regard to Dose, the FE model yielded an average ES value of 0.06 with a 95% CI of [−0.15, 0.29] for studies that used < 0.03, an ES value of 0.29 with a 95% CI of [0.13, 0.45] for studies that used 0.03, and an ES value of 0.18 with a 95% CI of [−0.13, 0.50] for studies that used > 0.03 mg/kg of nicotine. Twelve ES values were derived from studies using < 0.03 (ES# 1, 9, 10, 20-21, 24, 26-27, 30-31, 39, and 40), 19 ES values from studies using 0.03 (ES# 2, 5, 8, 11-17, 19, 22, 25, 28, 32, 34-36, and 41), and 7 ES values from studies using > 0.03 (ES# 3-4, 6-7, 18, 23, and 29) mg/kg of nicotine. There was no significant heterogeneity detected in the < 0.03 (Q = 14.68, p = 0.19) or > 0.03 mg/kg (Q = 5.10, p = 0.52) groups. However, heterogeneity was detected in the 0.03 mg/kg group (Q = 50.20, p ≤ 0.05). The RE model yielded an ES value of 0.11, with a 95% CI of [−0.23, 0.45] for studies that used < 0.03, an ES value of 0.19, with a 95% CI of [−0.07, 0.45] for studies that used 0.03, and an ES value of 0.30, with a 95% CI of [−0.18, 0.78] for studies that used > 0.03 mg/kg of nicotine. The moderator variable analysis revealed that Dose did not influence sex differences in nicotine IVSA (RE model; Hb = 2.54, df = 2, p = 0.28).
With regard to Operant Training, the FE model yielded an average ES value of 0.13 with a 95% CI of [−0.06, 0.34] for studies that included operant training, and an ES value of 0.24 with a 95% CI of [0.09, 0.38] for studies that did not. Twenty ES values were derived from studies that included operant training (ES# 1-10, 12-16, 19-22, and 34), and 22 ES values were derived from studies that did not include operant training (ES# 11, 17-18, 23-33, and 35-42). Heterogeneity was detected in the studies that included operant training (Q = 32.56, p ≤ 0.05) and in studies that did not include operant training (Q = 45.03, p ≤ 0.05). The RE model yielded an average ES value of 0.12 with a 95% CI of [−0.14, 0.38] for studies that included operant training, and an ES value of 0.21 with a 95% CI of [−0.01, 0.44] for studies that did not include operant training. The moderator variable analysis revealed that Operant Training did not influence sex differences in nicotine IVSA (RE model; Hb = 0.32, df = 1, p = 0.56).
With regard to Schedule of Reinforcement, the FE model yielded an average ES value of 0.07 with a 95% CI of [−0.09, 0.23] for studies that used an FR1, and an ES value of 0.37 with a 95% CI of [0.20, 0.55] for studies that employed a schedule of reinforcement > FR1. Seventeen ES values were derived from studies employing an FR1 schedule (ES# 9-10, 12-18, 24, 33, 35-39, and 42), and 23 ES values were derived from studies that used a schedule > FR1 (ES# 1-7, 11, 20-23, 25-32, 34, and 40-41). There was significant heterogeneity detected in the FR1 schedule group (Q = 41.0, p ≤ 0.05). However, heterogeneity was not detected in the > FR1 schedule group (Q = 29.56, p = 0.13). The RE model yielded an average ES value of −0.003 with a 95% CI of [−0.21, 0.20] for studies that used an FR1, and an ES value of 0.33 with a 95% CI of [0.12, 0.54] for studies that employed a schedule > FR1. The moderator variable analysis revealed that Schedule of Reinforcement did influence sex differences in nicotine IVSA (RE model; Hb = 4.99, df = 1, p ≤ 0.02). Specifically, studies that employed a schedule of reinforcement > FR1 produced larger sex differences as compared to studies that employed an FR1 schedule of reinforcement.
With regard to Session Length, the FE model yielded an average ES value of 0.07 with a 95% CI of [−0.06, 0.20] for studies that employed short sessions, and an ES value of 0.56 with a 95% CI of [0.21, 0.91] for studies that used extended sessions. Thirty-five ES values were derived from studies that used short sessions (ES# 1-8, 11-22, 26-32, and 34-41), and 6 ES values were derived from studies that used extended sessions (ES# 9-10, 23-24, 33, and 42). There was no significant heterogeneity detected in the short (Q = 43.22, p = 0.13) or extended access (Q = 7.12, p = 0.21) groups. The RE model yielded an average ES value of 0.07 with a 95% CI of [−0.09, 0.22] for studies that included short sessions, and an ES value of 0.56 with a 95% CI of [0.16, 0.95] for studies that used extended sessions. The moderator variable analysis revealed that Session Length did influence sex differences in nicotine IVSA (RE model; Hb = 5.16, df = 1, p ≤ 0.05). Specifically, studies that employed extended sessions resulted in larger sex differences as compared to studies that employed short sessions.
4. Discussion
4.1. Overall meta-analysis of sex differences in nicotine IVSA
The present meta-analysis assessed the presence and magnitude of sex differences in nicotine IVSA based on data derived from 20 studies and 42 independent ES values. The major finding was that there are sex differences in nicotine IVSA, with female rats displaying higher levels of nicotine IVSA compared to males. The finding that female rats self-administer more nicotine than males is consistent with several published reports [6,16,19,21,36,46,49,59]. The pattern of results is also consistent with several studies showing that female rodents display higher rates of oral nicotine SA (see [45]). Moreover, females display a larger preference for a chamber paired with nicotine than male rats [57] and mice [28]. These pre-clinical studies suggest that the reinforcing effects of nicotine likely promote tobacco use in women. Importantly, the present results appear to generalize to other drugs of abuse, given that the reinforcing effects of cocaine, opiates, and alcohol are also magnified in female versus male rodents (see [2,35,48]). Together, these studies suggest that strong reinforcing effects of drugs of abuse contribute to the rapid development of drug dependence in females.
The unique contribution of this meta-analysis is that it provides an estimate of the magnitude of sex differences in nicotine IVSA. Specifically, female rats self-administer on average 0.18 standard deviations more nicotine than males. This numerical index of sex differences in nicotine IVSA can be useful to the field in several ways. First, future studies assessing sex differences in nicotine IVSA can compare their ES values to the present meta-analysis, which provides an estimate of the population ES based on a collection of reports. Second, future studies can compare sex differences in IVSA to other behavioral effects of nicotine, such as the aversive effects of withdrawal or locomotor behavior. These types of comparisons may provide insight into the degree to which the different behavioral effects of nicotine contribute to tobacco use in women. Meta-analytic reviews may also be useful to compare sex differences in IVSA of nicotine to other drugs of abuse, such as cocaine or methamphetamine. Lastly, the present meta-analysis provides an ES estimate to conduct power analyses to calculate adequate group sizes. This will help advance future studies examining the mechanisms that modulate sex differences in nicotine IVSA.
4.2. Influence of moderator variables on sex differences in nicotine IVSA
The moderator variable analyses assessed the influence of various experimental conditions on the magnitude of sex differences in nicotine IVSA. To summarize, female rats displayed higher levels of nicotine IVSA than males in experimental conditions involving a light cue to signal nicotine delivery, schedules of reinforcement > FR1, and extended-access sessions. The influence of each of these procedural variables is considered in the following text below in the context of the existing literature.
The present study revealed that sex differences in nicotine IVSA were larger in studies that included a light cue to signal nicotine delivery. Specifically, female rats displayed higher levels of nicotine IVSA than males in studies that included a light cue. The latter finding is consistent with a previous report showing that female rats display greater nicotine IVSA than males in an experimental condition that included a cue light that signaled nicotine delivery as compared to a condition where there were no light cues [6]. Surprisingly, the present study revealed that there were no sex differences in nicotine IVSA in studies that included both a light and tone cue. Future studies are needed to examine the contribution of compound stimuli to sex differences in nicotine IVSA.
Sex differences in nicotine IVSA were also larger in studies involving reinforcement schedules > FR1, with females displaying higher levels of nicotine IVSA when the reinforcement requirements were > 1 operant response. This finding might reflect sex differences in motivation, with females responding more for nicotine under schedules with high reinforcement demands. Indeed, a previous report revealed that female rats display higher levels of nicotine IVSA than males when the schedule of reinforcement was steadily increased from an FR1 to a progressive ratio [16].
Sex differences in nicotine IVSA were also larger in studies that employed extended- versus short-access sessions. One possibility is that the latter effect is related to a more rapid development of nicotine dependence in females as compared to males. This is based on previous work showing that extended access to nicotine IVSA produces an escalation of nicotine intake and physical signs of withdrawal that are believed to reflect the development of dependence [9–11]. Thus, sex differences may be more readily observed in procedures involving extended access to nicotine IVSA as compared to short-access procedures that do not induce nicotine dependence. An alternative explanation is that females are able to sustain higher levels of nicotine IVSA in extended access procedures because they experience reduced aversive effects of nicotine as compared to males [57]. Indeed, female rats display more nicotine intake than males at higher doses of an escalating dose regimen of extended access to nicotine IVSA [19].
Lastly, there were several procedural variables that did not appear to influence the magnitude of sex differences in nicotine IVSA. With regard to age, sex differences in nicotine IVSA were similar in adolescent versus adult rats. This finding is consistent with previous reports showing a lack of sex differences in IVSA procedures that included both female and male adolescent and adult rats [29,50]. With regard to diurnal phase of testing, there were no sex differences in nicotine IVSA. This is consistent with a previous report showing that female and male rats display similar levels of nicotine intake in the light versus dark phase of the diurnal cycle [7]. Lastly, sex differences were not influenced by operant training prior to nicotine IVSA. Future studies are needed to empirically test the unique contribution of different procedural variables that may influence the magnitude of sex differences in nicotine IVSA.
4.3. Limitations of the present meta-analysis
There are some limitations to consider with the present meta-analysis. First, one report met the inclusion criteria, but was not included in the final analysis because the data were not available. One might consider that this study may limit the current estimate of the true population ES. However, this study did not detect sex differences in nicotine IVSA and Rosenthal’s Fail-Safe N test revealed that 88 additional ES values of zero would be needed to nullify the present findings. Second, the moderator variable analysis included an array of experimental conditions that were dichotomized based on our experience with IVSA procedures. Although some groupings may appear to be arbitrary, the ranges allowed for sufficient ES values in each subgroup, and an attempt was made to include reasonable minimal and maximal extremes. For example, the categories for the moderator variables that were significant included minimum (FR1 and short-access sessions) versus maximum (> FR1 and 23 h) values. The information provided in this meta-analysis is an important first step towards understanding the mechanisms that promote sex differences in the reinforcing effects of nicotine.
Acknowledgments
The authors would like to thank Dr. Larry Cohn for sharing his expertise in meta-analytic approaches and for providing copious and thoughtful feedback on this review. The authors are also grateful to Drs. Hao Chen, Eric Donny, Marilyn Carroll, Matt Feltenstein, Mark LeSage, Anh D Le, Frances Leslie, Ed Levin, Wendy Lynch, Janet Neisewander, and Alan Sved for providing data for this meta-analysis. We also thank Drs. Luis Carcoba and Victor Correa, as well as Bryan Cruz and Miriam Alvarez for their input. This research was supported by the National Institute on Drug Abuse (R01-DA021274 and R25-DA033613).
References
- 1.Bardo M, Rowlett J, Harris M. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19(1):39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- 2.Becker JB, Hu M. Sex differences in drug abuse, Front. Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95(1-2):136–147. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35(4):499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- 5.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to MetaAnalysis. 2009 [Google Scholar]
- 6.Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine selfadministration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36(13):2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens KJ, Lay BP, Holmes NM. Extended nicotine self-administration increases sensitivity to nicotine, motivation to seek nicotine and the reinforcing properties of nicotine-paired cues. Addict Biol. 2015;22(2):400–410. doi: 10.1111/adb.12336. [DOI] [PubMed] [Google Scholar]
- 10.Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37(9):2153–2160. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen A, Treweek J, Edwards S, Leão RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF1receptor dependent increase in anxietylike behavior and hyperalgesia in rats. Addict Biol. 2015;20(1):56–68. doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn LD, Becker BJ. How meta-analysis increases statistical power. Psychol Methods. 2003;8(3):243–253. doi: 10.1037/1082-989X.8.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 14.Cross SJ, Linker KE, Leslie FM. Sex-dependent effects of nicotine on the developing brain. J Neurosci Res. 2017;95(1-2):422–436. doi: 10.1002/jnr.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deneau GA, Inoki R. Nicotine self-administration in monkeys. Ann N Y Acad Sci. 1967;142:277–279. 1 The effects o. [Google Scholar]
- 16.Donny E, Caggiula A, Rowell P, Gharib M, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Ebrahim S, Smith GD. Where now for meta-analysis? Int J Epidemiol. 2002;31(1):1–5. doi: 10.1093/ije/31.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE. Estradiol promotes the rewarding effects of nicotine in female rats. Behav Brain Res. 2016;307:258–263. doi: 10.1016/j.bbr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214(4520):573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- 21.Grebenstein P, Burroughs D, Zhang Y, Lesage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy, Pharmacol. Biochem Behav. 2013;114-115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes SN, Redberg RF. Dispelling the myths: calling for sex-specific reporting of trial results. Mayo Clin Proc. 2008;83(5):523–525. doi: 10.4065/83.5.523. [DOI] [PubMed] [Google Scholar]
- 23.Hedges LV. Statistical Methodology in Meta-Analysis, ERIC Clearinghouse on Tests, Measurement, and Evaluation. Educational Testing Service. 1982 [Google Scholar]
- 24.Hedges LV, Olkin I. Statistical Method for Meta-Analysis. Academic Press; Orlando, FL: 1985. [Google Scholar]
- 25.Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige PA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology. 2012;222(2):269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalman D, Smith S. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tob Res. 2005;7(3):317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- 27.Kisamore JL, Brannick MT. An illustration of the consequences of meta-analysis model choice. Organ Res Methods. 2007;11(1):35–53. [Google Scholar]
- 28.Kota D, Martin B, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198(2):201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- 29.Lárraga A, Belluzzi JD, Leslie FM. Nicotine increases alcohol intake in adolescent male rats. Front Behav Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K, Rezvani AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res. 2011;225(2):473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zou S, Coen K, Funk D, Shram MJ, Lê A. Sex differences in yohimbine- induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2012;19(2):156–164. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- 32.Lipsey MW, Wilson DB. Practical Meta-analysis. SAGE Publications Inc; 2000. [Google Scholar]
- 33.Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: confirmation from meta-analysis. Am Psychol. 1993;48(12):1181–1209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi EMS, Prado GF, de Paula Santos U, Fernandes FLA. Women and smoking: risks, impacts, and challenges. Braz J Pulmonol. 2011;37(1):118–128. doi: 10.1590/s1806-37132011000100017. [DOI] [PubMed] [Google Scholar]
- 35.Lynch WJ, Roth M, Carroll M. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 36.Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough LD, Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014;5(1) doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Research Council. Combining Information: Statistical Issues and Opportunities for Research. Vol. 1. National Academies; 1992. [Google Scholar]
- 39.O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology. 1996;123(2):144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- 40.Peartree NA, Hatch KN, Goenaga JG, Dado NR, Molla H, Dufwenberg MA, Neisewander JL. Social context has differential effects on acquisition of nicotine self-administration in male and female rats. Psychopharmacology. 2017;234(12):1815–1828. doi: 10.1007/s00213-017-4590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins KA, Donny E, Caggiula A. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 42.Perkins KA. Smoking cessation in women. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 43.Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184(3-4):600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- 44.Pittenger ST, Swalve N, Chou S, Smith MD, Hoonakker AJ, Pudiak CM, Fleckenstein AE, Hanson GR, Bevins RA. Sex differences in neurotensin and substance P following nicotine self-administration in rats. Synapse. 2016;70(8):336–346. doi: 10.1002/syn.21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pogun S, Yararbas G, Nesil T, Kanit L. Sex differences in nicotine preference. J Neurosci Res. 2016;95(1-2):148–162. doi: 10.1002/jnr.23858. [DOI] [PubMed] [Google Scholar]
- 46.Rezvani A, Eddins D, Slade S, Hampton D, Christopher N, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154(3):885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 48.Roth M, Cosgrove K, Carroll M. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28(6):533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology. 2014;231(8):1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC, Sved AF. Adolescent rats self-administer less nicotine than adults at low doses. Nicotine Tob Res. 2016;18(9):1861–1868. doi: 10.1093/ntr/ntw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stauffer HP, Riedwyl H. Interaction and pH dependence of effects of nicotine and carbon monoxide in cigarette smoke inhalation experiments with rats. Agents Actions. 1977;7(5-6):579–588. doi: 10.1007/BF02111133. [DOI] [PubMed] [Google Scholar]
- 52.Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117(1):2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- 53.Strube MJ, Hartmann DP. A critical appraisal of meta-analysis. Br J Clin Psychol. 1983;21(2):129–139. doi: 10.1111/j.2044-8260.1982.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 54.Surgeon General. The Health Consequences of Smoking — 50 Years of progress: A Report of the Surgeon General. PsycEXTRA Dataset. 2014 [Google Scholar]
- 55.Swalve N, Smethells JR, Carroll ME. Sex differences in attenuation of nicotine reinstatement after individual and combined treatments of progesterone and varenicline. Behav Brain Res. 2016;308:46–52. doi: 10.1016/j.bbr.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swalve N, Smethells JR, Carroll ME. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology. 2016;233(6):1005–1013. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres OV, Natividad LA, Tejeda HA, Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-and sex-dependent manner. Psychopharmacology. 2009;206(2):303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres OV, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;65:260–268. doi: 10.1016/j.pnpbp.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, Palmer AA, Chen H. Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav. 2014;13(2):202–212. doi: 10.1111/gbb.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf FM. Meta-analysis: Quantitative methods for research synthesis. Vol. 59. Sage; 1986. [Google Scholar]


