Abstract
Thyroid hormone (TH) is essential for normal brain development and may also promote recovery and neuronal regeneration after brain injury. TH acts predominantly through the nuclear receptors, TH receptor alpha (THRA) and beta (THRB). Additional factors that impact TH action in the brain include metabolism, activation of thyroxine (T4) to triiodothyronine (T3) by the enzyme 5′-deiodinase Type 2 (Dio2), inactivation by the enzyme 5-deiodinase Type 3 (Dio3) to reverse T3 (rT3), which occurs in glial cells, and uptake by the Mct8 transporter in neurons. Traumatic brain injury (TBI) is associated with inflammation, metabolic alterations and neural death. In clinical studies, central hypothyroidism, due to hypothalamic and pituitary dysfunction, has been found in some individuals after brain injury. TH has been shown, in animal models, to be protective for the damage incurred from brain injury and may have a role to limit injury and promote recovery. Although clinical trials have not yet been reported, findings from in vitro and in vivo models inform potential treatment strategies utilizing TH for protection and promotion of recovery after brain injury.
Keywords: traumatic brain injury, thyroid hormone, thyroid hormone receptor, deiodinase, thyroid hormone transport, neuronal protection
1. Introduction
Thyroid hormone (TH) is essential for normal brain development. Thyroxine (T4) must be converted to the active form, triiodothyronine (T3), to activate the nuclear TH receptors, THRA and THRB. TH action in the brain requires activation of T4 to T3 in glial cells, which express 5′-deiodinase Type 2 (Dio2), as well as the action of specific TH transporters. Brain injury is associated with reduced activation of T3 from T4 and increased degradation by 5-deiodinase Type 3 (Dio3), which inactivates T3 by conversion to reverse T3 (rT3). The pathogenesis of brain injury includes inflammation, altered metabolism, and neuronal death. TH has been shown, in cellular and animal models, to be protective after brain injury, limiting injury and promoting neuronal regeneration. TH analogs, some of which enter neurons independent of TH transporters, may differentially promote recovery. Thyronamines, TH-related compounds, have potent effects on the brain and have been shown to promote recovery after stroke, in part by inducing hypothermia.
2. Thyroid hormone action on neural development and regeneration
Thyroid hormone action
TH is essential for normal development, growth, neural differentiation, and metabolic regulation (Bernal, 2007; G.A. Brent, 2012; Cheng, Leonard, & Davis, 2010; Liu & Brent, 2010). TH regulation of gene expression is influenced by; local ligand availability (Gereben, Zeold, Dentice, Salvatore, & Bianco, 2008), TH transport into the cytoplasm (Visser, Friesema, & Visser, 2011), the relative expression and distribution of the TH receptor alpha (THRA) and thyroid hormone receptor beta (THRB) isoforms, the expression of nuclear receptor corepressors and coactivators, and the sequence and location of the DNA thyroid hormone response elements (TRE)(Cheng, et al., 2010). T3 acts predominantly at the nucleus, binding to a nuclear receptor and then influencing gene expression (Liu & Brent, 2010). Distinct roles for THRA and THRB have been demonstrated by, animal models with selective mutations and gene inactivation, selective agonists, and clinical findings in patients with THRA and THRB gene mutations (G.A. Brent, 2012). THRA and THRB are differentially expressed developmentally and in adult tissues. THR interacts with specific DNA sequences, TREs, that positively or negatively regulate gene expression (Cheng, et al., 2010). Unliganded THRA and THRB bind to the TRE and reduce expression from positively regulated genes (Cheng, et al., 2010). A large family of receptor co-factors have been identified that interact with TR and modify gene expression, co-activators that increase and co-repressors that reduce expression (Hsia, Goodson, Zou, Privalsky, & Chen, 2010).
Nongenomic actions of thyroid hormone in the brain
Nongenomic actions of T3 have been identified in the brain (Davis, Goglia, & Leonard, 2016). The primary nongenomic pathway stimulated by T3 is the PI3Kinase/Akt (Protein kinase B) pathway (Flamant, Gauthier, & Richard, 2017). Although some truncated thyroid receptor proteins have been identified that mediate these actions, such as p30, the majority of nongenomic T3 actions are thought to utilize full length THR. Truncated forms of THRA have been reported in the brain that mediate nongenomic actions, such as regulation of actin (Davis, et al., 2016). A recent study of a combined THRA and THRB gene knockout in cultured cortical neurons, however, showed no T3 responsiveness, as measured by alterations of gene expression (Alvarez-Mora, et al., 2015).
Thyroid hormone metabolism in the brain
Activation of T3 from the prohormone T4, at the tissue level, is recognized as an important mechanism of regulation of TH action (G.A. Brent, 2012; Gereben, Zavacki, et al., 2008)(Figure 1). The deiodinase enzymes have variable developmental and tissue-specific expression (Gereben, Zavacki, et al., 2008). Rodents derive circulating T3 primarily from the Type 1 5′-deiodinase (Dio1), but humans rely on conversion of T4 to T3, by Dio2, as the primary source of circulating T3 (Gereben, Zavacki, et al., 2008). Dio3 inactivates T4 by conversion to rT3, which has no known physiological actions. Knockout of the Dio3 gene in mice is associated with profound deficits of neural and sensory development, as well as impaired TH regulation and maturation of the thyroid-pituitary axis (Hernandez, et al., 2007). More recently, Dio3 has been shown to play a role in TH action in the hypothalmus, influencing circadian rhythms and the leptin-melanocortin system (Wu, Martinez, St Germain, & Hernandez, 2017). The regulation of the deiodinase enzymes is complex with both transcriptional and posttranscriptional mechanisms (Gereben, Zavacki, et al., 2008). T3 directly upregulates Dio1 (Gereben, Zavacki, et al., 2008), and Dio3 (Barca-Mayo, et al., 2011) transcription. Dio 3 transcription is regulated by THRA. Dio2 is downregulated when T4 is increased and upregulated when T4 levels are low, by a ubiquitination/deubiquitination system (Gereben, Zavacki, et al., 2008). Although Dio2 activity is regulated predominantly at the post-transcriptional level, T3 downregulates Dio2 mRNA expression (Kim, Harney, & Larsen, 1998).
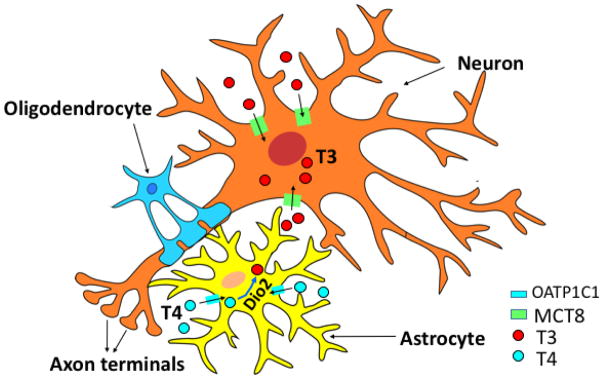
Figure 1. Model of Neurons and Glial Cells With Pathways of Thyroid Hormone Metabolism and Uptake.
Thyroid hormone action in the brain requires activation of thyroxine (T4) to the active triiodothyronine (T3), by the 5′-deiodinase 2 (Dio2), contained in glial cells. T3 uptake into neurons is mediated by specific transporters, Mct8 and Oatp1c1, present in both humans and mice, but humans appear most dependent on Mct8.
Thyroid hormone metabolism in neural development
Expression of Dio2 is essential for normal brain development and function (Gereben, Zavacki, et al., 2008). Dio3 also has an essential action in brain and sensory development, protecting brain areas from premature exposure to T3 (Ng, et al., 2009). This protective role of Dio3 has been demonstrated in the cortex, cerebellum, and in sensory development (Hernandez, Morte, Belinchon, Ceballos, & Bernal, 2012; Peeters, et al., 2013). Sonic hedgehog (Shh) promotes cell proliferation during neural development. Shh directly upregulates Dio3 expression and downregulates Dio2, by increasing Dio2 ubiquitination (Gereben, Zavacki, et al., 2008). Shh is upregulated by T3 in the embryonic and adult brain (Desouza, et al., 2011).
Thyroid hormone transport
TH is hydrophobic and was long thought to enter the cytoplasm by passive diffusion. TH transporters, such as the monocarboxylate family of organic ion transporters (MCT) and organic ion transporters, were first identified based on measurable in vitro activity (Visser, et al., 2011). The identification of Mct8 as an essential TH transporter (Figure 1), however, was based on the association of Mct8 mutations with a severe form of X-linked mental retardation, Allan-Herndon-Dudley Syndrome (AHDS) (Friesema, et al., 2004). Affected individuals have abnormal thyroid function studies, reduced serum T4, elevated serum T3, as well as severe spasticity, profound mental retardation, and hypermetabolism. It has been recognized that Mct8 is expressed in the thyroid and important for secretion of thyroxine, leading to the reduced serum T4 concentration in individuals with an Mct8 gene mutation (Di Cosmo, et al., 2010).
Thyroid hormone transporters in neural development
TH transporters in the developing brain are expressed in specific temporal and spatial patterns (Sharlin, Visser, & Forrest, 2011; Visser, et al., 2011). Individuals with an Mct8 mutation have myelination delays, thought to be due to impaired TH action on oligodendrocytes (Bernal, 2011). Mct8 is expressed in the hypothalamus, a major site of integration of TH signaling and metabolic pathways (Alkemade, et al., 2011). Studies in a zebra fish model show that inactivation of Mct8 was associated with significant defects in brain and spinal cord development (Vatine, et al., 2013). Exogenous T3 has reduced action on the fetal rat brain, due to the requirement for local production of T3 from T4 by the action of Dio2 (Grijota-Martinez, Diez, Morreale de Escobar, Bernal, & Morte, 2011)(Figure 1). The human brain is dependent on a functional Mct8 transporter for normal function and development, but in the mouse, Mct8 and Oatp1c1, both contribute to TH action in the brain. Neurologic deficit is only seen in the mouse when both transporters are inactivated (Mayerl, et al., 2014). A recent study utilized induced pleuripotent stem cells from AHDS patients, associated with Mct8 deficiency, compared to normal stem cells (Vatine, et al., 2017). Neurons derived from AHDS patient stem cells had a normal thyroid hormone response. Utilizing a model of the Blood Brain Barrier (BBB) from derived stem cells (Canfield, et al., 2017), a BBB derived with stem cells from AHDS patients did not transport TH normally (Vatine, et al., 2017). These findings suggest that reduced TH transport by the BBB may be a significant contributor to the abnormal neurologic phenotype in AHDS.
Thyroid hormone receptor isoforms and neural development
Models of TR isoform-selective inactivation have demonstrated specific roles for TR isoforms in brain and sensory development (Nunez, Celi, Ng, & Forrest, 2008). THRB isoforms are required for the development of the inner ear and the cone photoreceptors in the retina (Jones, Ng, Liu, & Forrest, 2007). THRA agonists have selective action in amphibian brain development, indicating that THRA mediates neuronal proliferation (Denver, Hu, Scanlan, & Furlow, 2009). However, a study of THRA-selective agonist treatment did not shown specificity of gene activation in the developing rat brain (Grijota-Martinez, Samarut, Scanlan, Morte, & Bernal, 2011). THRA is required for oligodendrocyte differentiation in the cerebellum (Picou, Fauquier, Chatonnet, & Flamant, 2012).
Integration of thyroid hormone pathways in neural development
THRA is expressed early in neurological development in Xenopus and mammalian models (Wang, Matsuda, & Shi, 2008). TH interfaces with other signaling pathways in its regulation of neural development (Table 1). The Sox2 transcription factor plays an important role in promoting neural progenitor growth, and when it is inhibited, neural differentiation occurs (Qu & Shi, 2009). TH and THRA promote the differentiation of neural stem cells to neuroblasts by repressing Sox2 (Lopez-Juarez, et al., 2012). The orphan nuclear receptor, Chicken Ovalbumin Upstream Transcription Factor 1 (COUP-TF1), is expressed before the developing brain is sensitive to TH (Oppenheimer & Schwartz, 1997). A number of TH gene targets have been identified with overlapping TR and COUP-TF1 response elements (Anderson, et al., 1998; Liu & Brent, 2002). The expression of COUP-TF1 blocks TH receptor from binding the TRE and correlates with developmental periods when it is important to blunt T3-stimulation. Calcium calmodulin-dependent Kinase IV (CamKIV), is a major TH target in the developing brain, contains a TRE and COUP-TF1 binding site (Liu & Brent, 2002; Morte, et al., 2010). T3 stimulates CamKIV expression in primary cultured fetal cortical neurons, which promotes the maturation and proliferation of GABAergic interneurons from their precursor cells (Manzano, Cuadrado, Morte, & Bernal, 2007). In a model of pyramidal cell differentiation from embryonic stem cells, COUP-TF1 expression was shown to modify the timing and magnitude of T3-responsive gene expression, as well as neural differentiation (Teng, Liu, Teng, & Brent, 2017).
Table 1.
Thyroid signaling cross-talk with other pathways in models of neural development
| Pathway/Nuclear Factor | Process or Tissue | Nature of Interaction | Reference |
|---|---|---|---|
| Retinoic Acid Receptor | Neural development | Inhibits T3 action | (Liu, et al., 2002) |
| Retinoic Acid | Brain development | Stimulates MCT8 expression and thyroid transport | (Kogai, et al., 2010) |
| COUP-TF1 | Expressed early in brain development | Inhibits T3-induction of gene expression | (Anderson, et al., 1998; Liu & Brent, 2002; Teng, et al., 2017) |
| SOX2 | Promotes renewal of neural stem cells | T3/THRA inhibits SOX2 expression (by downregulation of SSR1) and promotes differentiation | (Lopez-Juarez, et al., 2012) |
| LXR | Brain | Cortical layering | (Tan, et al., 2010) |
COUP-TF1-Chicken Ovalbumin Upstream Transcription Factor 1, MCT8-monocarboxylate transporter 8, LXR-Liver X Receptor, Sox2-SRY (Sex Determining Region Y)-box2, SSR1-Sox 2 Regulatory Region 1
Retinoic acid (RA) is an early morphogen in brain development and is linked to TH transport. Utilizing a neuronal cell model, it was shown that RA stimulates Mct8 mRNA expression and confers functional TH transport (Kogai, et al., 2010). TH, acting through THRA regulates adult hippocampal neurogenesis, important in learning, memory, and mood (Desouza, et al., 2005; Kapoor, et al., 2010). The developmental importance of THR isoforms is coupled with requirement for specific transporter expression, such as Mct8 expression in the mouse cochlea (Sharlin, et al., 2011), as well as a requirement for Dio2 and Dio3 expression to provide an appropriate level of T3 in a specific developmental sequence (Heuer, 2011). THRA protein is expressed in embryonic post-mitotic neurons and in most adult neurons in the mouse brain, indicating that TH has a role in the adult brain (Wallis, et al., 2010).
3. Alteration of thyroid hormones in Traumatic Brain Injury (TBI)
Acute changes in thyroid function tests
Reduced serum levels of T4 and T3, with a normal reference range thyrotropin (TSH), is seen in a range of severe illnesses and injuries, and is referred to as Nonthyroidal Illness (NTI) (Farwell, 2013). Thyroid function test abnormalities in NTI are not the result of primary thyroid disease, and normalizes weeks to months in patients that recover from their underlying illness (Farwell, 2013). The alterations in thyroid function tests in traumatic brain injury (TBI) were initially reported in a series of 66 patients with severe TBI (Woolf, Lee, Hamill, & McDonald, 1988). A reduced serum T4 and T3 were reported, which correlated with the severity of injury, as reflected in the Glasgow Coma Score (GCS) and with sympathetic nervous system activation. Serum TSH and reverse T3 levels were not altered as a result of TBI in this study. Those patients who died after TBI had the lowest serum T4 and T3 concentration, as has been shown in similar studies of patients with severe illnesses (Farwell, 2013). A prospective study of TH levels in patients with TBI showed reduced TH levels, including serum T3 and T4, and no change in serum TSH (Malekpour, Mehrafshan, Saki, Malekmohammadi, & Saki, 2012). Patients with lower serum T4 and T3 concentrations had worse GCS measures and increased mortality. Although a prospective thyroxine treatment trial has not been reported in acute TBI patients, routine treatment in other critically ill patients has not been associated with an improved outcome (G. A. Brent & Hershman, 1986). Most agree that patients with NTI do not benefit from routine treatment with levothyroxine (Farwell, 2013). A subset of patients with NTI, however, such as those with heart failure, cardiac surgery, and organ donors, may have some benefit from levothyroxine treatment.
Central hypothyroidism
In addition to thyroid changes associated with illness, present in most patients with severe TBI, a subset of patients may additionally have residual central hypothyroidism due to hypopituitarism or hypothalamic damage (Kokshoorn, et al., 2010) (Beck-Peccoz, Rodari, Giavoli, & Lania, 2017; Herrmann, et al., 2006). A recent study, which performed hormonal screening on a group of patients with TBI or subarachnoid hemorrhage, reported that 29% of patients had abnormality of at least one hormone, most commonly low testosterone in men (Kopczak, et al., 2014). Low serum thyroxine, however, was found in 6% of patients. These patients with low serum T4 need thyroxine supplementation, when identified, but may be missed. The usual approach to thyroid function testing relies on only a serum TSH measurement. Serum TSH is usually in the normal reference range, despite a reduced serum T4. Clinicians must be aware of the potential for central hypothyroidism in traumatic brain injury patients, and measure both a serum TSH and T4.
Local production of T3 in the brain
The importance of the role of local TH production in the brain was shown in a clinical study of individuals with a Dio2 polymorphism (Thr92Ala), associated in some studies with impaired T4 to T3 conversion (McAninch, et al., 2015). Subjects with the Dio2 polymorphism had increased brain expression of genes associated with brain abnormalities, including Alzheimer’s-associated genes and gene programs associated with apoptosis, inflammation and mitochondrial dysfunction. In the presence of the polymorphism, the Dio2 protein had a longer half-life and was retained in the Golgi. The association of altered thyroid hormone metabolism with the severity of Alzheimer’s disease was demonstrated in a recent clinical study. These Alzheimer’s patients had reduced Cerebral Spinal Fluid T3 and elevated rT3, consistent with reduced T4 to T3 conversion, and this correlated with disease severity (Accorroni, et al., 2017).
Thyronamines
Thyronamines (3-T1AM, T0AM) are endogenous compounds thought to be derived from levothyroxine or may be produced in the thyroid gland and then secreted into the blood stream (Hoefig, Zucchi, & Kohrle, 2016). A number of membrane receptors have been identified that mediate the response to thyronamines, including members of the trace amine associated receptor family G protein-coupled receptor (GPCR; now known as trace amine-associated receptor 1 [TAAR1]) and the adrenergic receptor ADRa2a. 3-T1AM has profound metabolic and cardiac effects, producing hypothermia and bradycardia in rodent models. The actions of 3-T1AM to slow metabolism have been used in an animal model of stroke to limit ischemic damage (Doyle, et al., 2007). Similar benefits of administration of 3-T1AM has been shown for protection of ischemic damage to the heart (Frascarelli, et al., 2011).
In studies of severely ill patients, serum levels of 3-T1AM were reduced and correlated with the reduction in serum T3 (Langouche, Lehmphul, Perre, Kohrle, & Van den Berghe, 2016). The significance of changes in serum thyronamine in the clinical course of NTI patients is not known.
4. Thyroid hormone and in vitro models of neuronal injury and hypoxia
There are a variety of animal models for TBI (Finnie & Blumbergs, 2002) and head trauma (Cernak, 2005). In vitro models are limited in providing a direct model of TBI, but can be used to map the pathways important in neuronal injury and the role of TH (Figure 2). Differentiation of embryonic stem cells to cortical neurons has been demonstrated to model the influence of TH on gene expression and differentiation, as well as the modulating effect of COUP-TF1 (Teng, et al., 2017).
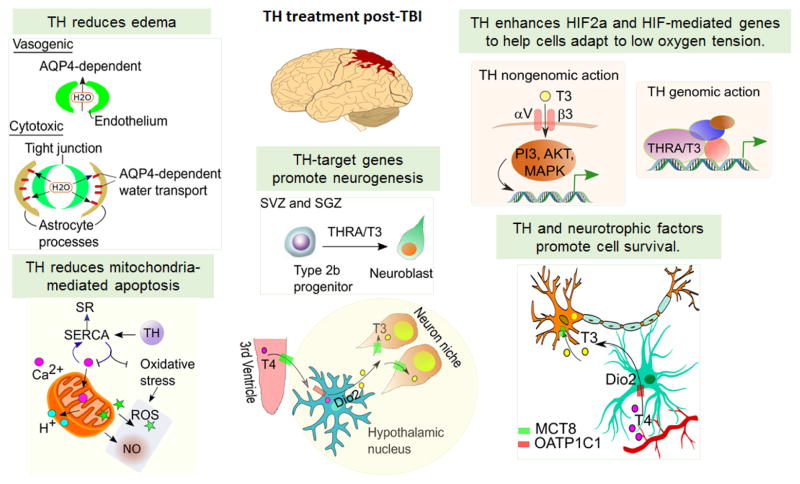
Figure 2. Model of the Pathways Activated by Brain Injury and Potential Sites of Thyroid Hormone Action.
Brain injury is associated with edema, mitochondrial-mediated apoptosis, and local hypoxia, and in response promotion of cell survival and neurogenesis. Thyroid hormone (TH) has demonstrated actions on these pathways with potential mechanisms, as are shown in this figure, and treatment may reduce edema, inflammation and enhance recovery. SERCA, Ca2+-ATPase; SR, Sarcoplasmic reticulum; ROS, Reactive oxygen species; NO, Nitric oxide; AQP4, Aquaporin 4; TH, Thyroid hormone; THRA, Thyroid hormone receptor alpha; SVZ, Subventricular zone; SGZ, Subgranular zone
Empty spiracles homolog 1 (Emx1) and a T box family gene, Tbr1, are regulated by TH and are critical for cortical neuronal differentiation and proliferation (Bishop, Garel, Nakagawa, Rubenstein, & O’Leary, 2003). Emx1 is homeobox-containing gene, first expressed on embryonic day 9.5 and continues expression into adulthood. Emx2 and Tbr1 are expressed in spatially and temporally overlapping patterns in the developing brain. Emx1 cooperates with Emx2 to regulate differentiation of cortical neurons and directs proper cortical lamination (Bishop, et al., 2003). Emx1 knockout mice display reductions in anxiety-related behaviors (Simeone, et al., 1992). Emx1 and COUP-TF1 are co-expressed in cortical neurons and important for cortical patterning. In COUP-TF1 knockout mice, Emx1 is significantly down regulated. Tbr1 is a transcription factor important for early cortical development that is highly expressed in glutamatergic neocortical neurons. In Tbr1-deficient mice, defects in cortical neuron migration was observed (Dwyer & O’Leary, 2001). Cortical pyramidal neurons, supplemented with T3, survive significantly longer than in conditions without T3. (Teng, et al., 2017). Emx1 and Tbr1 were significantly stimulated in T3 treated pyramidal neurons compared to control (Teng, et al., 2017)
In neurons, many genes activated by synaptically evoked Ca2+ signals require Ca2+ -dependent pathways to activate transcription factors for downstream action. CamKIV is sensitive to changes in intracellular Ca2+and is regulated by TH. THR binds to a TRE in the CamKIV 5′-flanking region (Liu & Brent, 2002; Liu, Tachiki, & Brent, 2002). CamKIV is activated by CamKIV kinase and translocated into the nucleus after phosphorylation. The nuclear form of CamKIV phosphorylates transcription factors including CREB and CBP/p300 (Mellstrom, Savignac, Gomez-Villafuertes, & Naranjo, 2008). This disrupts corepressor recruitment and enhances hormone receptor-mediated gene expression (McKenzie, et al., 2005). CamKIV exerts neuroprotective effect and inhibits apoptosis through phosphorylation of CREB and increased expression of pro-survival/anti-apoptosis genes (Bito & Takemoto-Kimura, 2003; Marshall, et al., 2003; See, Boutillier, Bito, & Loeffler, 2001; Tremper-Wells, Mathur, Beaman-Hall, & Vallano, 2002). A recent study demonstrated that a high level of CamKIV expression in the cortex boosted the learning response (Steenland, Wu, Fukushima, Kida, & Zhuo, 2010)..
In the adult brain, T3 is associated with accelerated differentiation of adult hippocampal progenitors (Kapoor, Desouza, Nanavaty, Kernie, & Vaidya, 2012). TH has a specific role in promoting the differentiation of adult hippocampal progenitors. In a mouse model, TH mediated its neurogenic effects by targeting Type 2b and Type 3 hippocampal progenitors (Kapoor, et al., 2012). This shows that induction of a specific transcription factor by TH contributed to the effects of TH on neural differentiation of adult hippocampal progenitors.
Dio3 is significantly upregulated in a variety of tissue types, including neurons, in response to injury, such as hypoxia (Simonides, et al., 2008). Hypoxia-Inducible Factor (Hif) directly stimulates expression of the Dio3 gene. In a rat model of unilateral brain hypoxia, Dio3 protein was increased in the nucleus, which was seen in pyramidal neurons and the hippocampus (Jo, et al., 2012). Hypoxia is often a feature of injury and illness and upregulation of Dio3 may explain the findings of reduced serum T3 and elevated reverse T3 in TBI.
5. Thyroid hormone action and in vivo models of neuronal injury
Studies in animal models suggest TH can induce developmental programs of neural proliferation and differentiation, after brain injury, and may improve recovery (Shulga, et al., 2009). Administration of T3 1 hour after injury in a mouse model of TBI significantly improved cognitive and motor recovery, compared to control (Crupi, et al., 2013). TBI is associated with deficits of cortical and hippocampal function, but damage has also been identified in the cerebellum (Cernak, 2005; Finnie & Blumbergs, 2002; Potts, Adwanikar, & Noble-Haeusslein, 2009). TH has significant developmental actions in the brain and TH has been associated with recovery after damage due to TBI (Table 2).
Table 2.
Studies of the action of thyroid hormone and thyroid hormone analogs targeting the brain
| Condition | Compound | TR isoform selectivity | Model | Results | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | T3 | THRB = THRA | Hypothyroid mIce and neural cell culture | T3 treatment downregulates beta amyloid precursor protein expression | (Contreras-Jurado & Pascual, 2012; O’Barr, et al., 2006) |
| AHDS (Mct8 mutation) | DITPA | THRB = THRA | Mouse model of MCT8 deficiency | Restore neuronal differentiation and brain function in mice | (Di Cosmo, et al., 2009) |
| AHDS (Mct8 mutation) | Tetrac | THRB>THRA (2-fold to 3-fold) | Mouse model of MCT8 deficiency | Restore neuronal differentiation and brain function in mice | (Horn, et al., 2013) |
| AHDS (Mct8 mutation) | DITPA | THRB = THRA | Humans with AHDS | Reduced serum T3, reduced hypermetabolism, no impact on neurologic manifestations | (Verge, et al., 2012) |
| X-linked adrenal-leukodystrophy (X-ALD) | Sobetirome (also referred to as GC1) | THRB>THRA (10-fold) | Mouse model of X-ALD and fibroblasts from X-ALD patients | Stimulation of ABCD2 expression, reduced very long chain fatty acid deposition in adrenals and some reduction in brain, Induction of ABCD2 in fibroblasts of X-ALD patients | (Hartley, et al., 2017; Milanesi & Brent, 2017) |
| Traumatic Brain Injury | T4 | THRB = THRA | Mouse hippocampal brain slice injury model | Promotes neurotrophins and neuronal survival | (Shulga, et al., 2009) |
| Traumatic Brain Injury | T3 | THRB = THRA | Mouse model of TBI, T3 treatment 1 hour after injury | Reduced brain edema, improved cognitive and motor function compared to control | (Crupi, et al., 2013) |
| Traumatic Brain Injury | T4 | THRB = THRA | CCI model in rat, T4 treatment 1 hour after injury | Reduced brain edema, restoration of some T3-responsive genes in the brain | (Li, et al., 2017) |
| Stroke | T3 | THRB = THRA | MCA occlusion mouse model, T3 before or after occlusion | Attenuation of ischemia and edema | (Sadana, et al., 2015) |
| Stoke | Thyronamine (3-T1AM) | Actions not mediated by THR | Given 1 hour after stroke in mouse model | Hypothermia, marked reduction in ischemic damage | (Doyle, et al., 2007) |
ADHD-Allan-Herndon-Dudley Syndrome, CCI-controlled cortical injury, THRA-thyroid hormone receptor alpha, THRB-thyroid hormone receptor beta, MCA-middle cerebral artery,
Animal model of TBI: thyroid hormone treatment Post-TBI
TBI models are typically described as modeling either diffuse or focal injury. The diffuse injury is a model for brain concussion, and is associated with axonal damage, ischemic injury, brain edema and diffused vascular injury. Focal injury is concentrated in a particular brain area with trauma at a specific site or cessation of blood flow to an area due to a blood clot. There are, however, similarities in the nature of the underlying injury in both categories. Both injuries produce brain edema, disruption of the vascular system, reduced oxygen supply, neural cell death and short-term or long-term deficits in cognition, memory and learning (Blennow, et al., 2016; Kurland, Hong, Aarabi, Gerzanich, & Simard, 2012)(Figure 2). In animal models, controlled cortical injury (CCI)-induced TBI models a focal injury, although the neuronal damage typically extends outside the focal area of induced injury. TH treatment of TBI induced by CCI have been shown to be beneficial. Here, we focus on TH treatment of CCI-induced TBI.
Thyroid hormone treatment post-TBI reduces brain edema
CCI is widely used to produce acute injury in animal models of TBI. The injury is induced by instrument-guided contusion. The depth of contusion varies, in mild injury 0.2 mm, moderate injury 0.5–1.0mm, and severe injury 1.2–2.0 mm (Romine, Gao, & Chen, 2014). The first stage of the injury is the result of the physical impact, which instantly disrupts blood flow resulting in brain hemorrhage, edema and necrosis at the epicenter. Studies from three laboratories have shown that treatment with TH, before or immediately post-TBI, reduces, or in some cases reverses, brain edema. In a study using a CCI-induced TBI rat model, a single dose of T4 (2.5g/100g body weight) was administrated to rats (i.p) one hour post-TBI. Edema was markedly reduced with T4 treatment, and the brain recovered to normal size 24 hours after the injury (Li, et al., 2017). In a CCI-induced TBI mouse model, a single dose of T3 (1.2ug/100 body weight), one hour post-TBI, reduced brain edema 60% compared to saline treatment (Crupi, Paterniti et al. 2013). In a stroke model induced by transient or permanent middle cerebral artery occlusion, T3 treatment (one dose, 25ug/kg body weight), either pre- or post-stroke, showed striking attenuation of brain ischemia and edema. Edema was reduced 55% after pre-stroke TH treatment (30 minutes), and 63% in post-injury treatment (Sadana, Coughlin, Burke, Woods, & Mdzinarishvili, 2015).
The mechanism of TH-mediated reduction in brain edema is not established, although it may be linked to regulation of the aquaporin water channels (Sadana, et al., 2015). In mammals, there are 13 aquaporin proteins. Aquaporin 4(AQP4) is the primary aquaporin expressed in brain and functions as water channel regulator of the osmotic gradient. TH represses aquaporin 4 (Aqp4) mRNA expression and TH treatment was associated with significantly reduced AQP4 protein in an ischemic brain model (Sadana, et al., 2015). The TH treatment reduction in post–TBI edema is likely an important pathway of the beneficial effects, leading to reduced intracranial pressure and reduce damage to nerve function.
T4 stimulates iNos after TBI
In the second stage of response to brain injury, neural cell survival pathways and apoptotic process are both active. Hypoxia, resulting from disruption of blood flow, leads to activation of hypoxia-inducible factors (HIFs). HIF-induced gene transcription is a critical component of cell survival as these HIF-induced transcription factors enhance the expression of the genes necessary for adaptation to conditions of low oxygen tension. These transcription factors include vascular endothelial growth factor A (VEGFA), JunB, c-Jun, MYC interactor 1 (Mxl1) and CREB (Beitner-Johnson & Millhorn, 1998; Culver, et al., 2010; Laderoute, et al., 2002; Lendahl, Lee, Yang, & Poellinger, 2009; Lonze & Ginty, 2002). Hypoxia increases the expression of pro-inflammatory factor NF-kB and rapidly releases NF-kB-mediated inducible nitric oxidase synthase (iNOS). iNOS deficient-mice have excessive hypoxia-induced oxidative stress in the post-TBI brain (Bayir, et al., 2005), which support the hypothesis that iNOS has neuroprotective properties in brain injury (Garry, Ezra, Rowland, Westbrook, & Pattinson, 2015). TH levels are directly associated with nitric oxide (NO) production in rat brain. Excess TH increases, and low TH decreases, NO production (McAllister, et al., 2005). T4 induction of iNOS has also been reported in postnatal cortical neurons (Serfozo, et al., 2008). In rat cortex post-TBI, T4 had no effect on hypoxia-induced Hif1α and NFκB expression, however, it significantly increased iNOS mRNA, 3.4-fold, compared to the TBI/saline treatment group (Li, et al., 2017).
Thyroid hormone-treatment post-TBI improves brain function
In response to hypoxia, neural cells switch from high-energy producing oxidative respiration to low energy producing anaerobic glycolysis. With prolonged hypoxia and low energy supply, neuron activity is reduced, leading to deficits of memory, cognition and learning. Behavioral analysis of animals after TBI show impairment in locomoter tasks. T3 treatment post-TBI resulted in improvement in latency in locomoter tests and reduced measures of anxiety (Crupi, et al., 2013).
Thyroid hormone post-TBI benefit in neural recovery in mice
Neuronal cell (neurons and neuroglia) death after injury is associated with anoxia and energy depletion. Apoptosis post-TBI involves multiple pathways, such as cell death induced by increasing intracellular calcium and free radicals, and activation of cell cycle-induced cell death (Raghupathi, 2004; Raghupathi, et al., 2002; Stoica & Faden, 2010). Mitochondrial-mediated apoptosis in the peri-lesion area is detectable in the acute post-TBI period. The mitochondrial pro-apoptotic markers (Bax, Apaf1, and caspase-3 and -9) and anti-apoptosis markers (Bcl-2, Bcl-xl and Mcl-1) along with neurogenesis (Sox2, Dcx, Gap43) and neurotrophic (BDNF, NGF, GDNF) factors expressed in the lesion area reflect the recovery potential. TH treatment post-TBI was associated with increased Bcl-2 mRNA and reduced Bax expression (Li, et al., 2017). In addition, T4 treatment partially restored the mRNA expression of Sox2, Dcx and Gap43, compared to the control group. These genes in the regions remote to the injury center, such as the hippocampus and cerebellum, were not influenced by TH treatment. Neural cell re-oxygenation after blood flow recovery may exacerbate hypoxia/reoxygenation-induced apoptosis via calcium overload, resulting in mitochondrial membrane depolarization and cell death. Apoptosis can be detected days or weeks post-TBI (Raghupathi, et al., 2002). TH treatment induces BCL-2 and neurogenesis-related genes and may promote neuronal injury recovery. In cardiac myocytes, TH influences calcium flux by enhancing calcium pump, sarcoplasmic reticulum (SERCa2) gene expression, and preventing intracellular calcium overload (Dillmann, 2010; Kahaly & Dillmann, 2005). Controlling calcium overload-induced apoptosis is a relevant target for TH treatment post-TBI.
Currently, only a few studies have directly tested TH in animal TBI models (Table 2). These few studies have demonstrated a beneficial effect of TH treatment post-TBI in reducing brain edema, apoptosis, and oxidative stress via iNOS, and increasing mRNA expression of neurogenesis-related genes.
6. Approaches to thyroid hormone treatment and protection from brain injury
TH therapy in acute severe illness and in chronic conditions associated with reduced serum T3, have not been shown to provide benefit (Farwell, 2013; Kaptein, Beale, & Chan, 2009). Although studies of acutely ill patients have not shown a benefit from T4, there may be subsets of patients that do benefit from treatment. The use of TH therapy in humans after TBI has not been reported, but studies of other brain conditions can provide insights into decisions about TH preparations and the potential impact of therapy (Table 2).
Thyroid hormone therapy in nontraumatic disorders of the brain
Alzheimer’s patients have altered Cerebral Spinal Fluid TH levels, and have an increased incidence of thyroid disease (Accorroni, et al., 2017). TH has been shown to influence APP (beta-amyloid precursor protein) gene expression in the brain and in neuronal culture (Contreras-Jurado & Pascual, 2012; O’Barr, Oh, Ma, Brent, & Schultz, 2006). A reliable marker of axonal injury after TBI is accumulation of amyloid protein (Potts, et al., 2009). Hypothyroidism is associated with increased beta amyloid precursor protein (APP) in the brain and TH downregulates expression (Contreras-Jurado & Pascual, 2012; O’Barr, et al., 2006)
TH analogs have been available and used for a variety of applications, especially the treatment of metabolic disorders (Milanesi & Brent, 2017). TH analogs differ from native TH in tissue distribution, metabolism, as well as selective affinity for the TH receptor isoforms. The selective THRB analog, sobetirome, has been used to treat adrenoleukodystrophy, a condition in which very long chain fatty acids are deposited in the brain and adrenals, due to a mutation in the membrane peroxisome lipid transporter ABCD1. Stimulation of ABCD2, a related transporter, by sobetirome provides an alternate path for the uptake of very long chain fatty acid. In an animal model of adrenoleukodystrophy, chronic sobetirome treatment reversed lipid deposition in the adrenal and brain (Hartley, Kirkemo, Banerji, & Scanlan, 2017).
An example of the challenges in treating brain disorders with TH come from experience with AHDS, individuals with mutations of the Mct8 neuronal T3 transporter. The TH metabolite 3,5-diiodothyropropionic acid (DITPA) does not require Mct8 to enter cells and is a potential therapy for AHDS patients (Di Cosmo, Liao, Dumitrescu, Weiss, & Refetoff, 2009). DITPA treatment in humans with Mct8 gene mutations is effective at correcting thyroid function tests and the hypermetabolism, with reduction in serum T3 and weight gain, although does not reverse the neurological deficits (Verge, et al., 2012). A report in animals with Mct8 gene knockout demonstrated that early treatment with the TH analog Tetrac was able to enter and act in the brain, independent of the Mct8 transporter, although it did not reach the hypothalamus (Horn, et al., 2013). The relative ability of DITPA and Tetrac to reverse the changes associated with Mct8 gene mutations in animal models and in clinical settings remains under investigation.
Factors influencing the selection of thyroid hormone preparation
In animal models of brain development, 80% of T3 in the brain is derived from local conversion of T4, rather than circulating T3. Administration of T4 to the mother in animal models of hypothyroidism normalizes T3 in embryonic tissues, but administration of T3 to the mother does not. Based on these studies, T4 is considered the best substrate for TH action in the brain. The importance of the Mct8 transporter for neurons and the BBB (Vatine, et al., 2017), and evidence that it may be downregulated after injury, raises the possibility of using one of the preparations, DITPA or Tetrac, for better brain penetration. Reduction in brain edema in animal models of TBI has been shown with both T4 and T3. A further consideration in acute injury is the reduction in T4 to T3 conversion and enhanced activity of Dio3 that occurs in hypoxia. In models of critical illness, Dio2 activity in the brain is generally preserved, although this has not been systematically examined in TBI.
Blood Brain Barrier
Administration of systemic TH in the setting of brain injury will need to account for transport across the BBB (Landers & Richard, 2017). The thyroid transporters Mct8 and Oatp1c1 are expressed in the blood brain barrier and play a significant role in transport (Vatine, et al., 2017). Many of the analogs studied, such as Tetrac and DITPA, have demonstrated brain penetration (Table 2).
Combination of thyroid hormone with other therapies
There is clear overlap of the actions of antioxidants and TH action. In an in vitro study, the actions of interleukin 6 were shown to block T4 activation to T3 and to promote TH inactivation to rT3 (Wajner, Goemann, Bueno, Larsen, & Maia, 2011). Treatment of the cells with an antioxidant, N-acetyl-cysteine, restored intracellular glutathione, and prevented the interleukin 6-mediated action on TH metabolism. A recent animal study administering N-acetylcysteine in a model of myocardial infarction, showed that the TH changes seen in NTI were reversed with antioxidant treatment (Lehnen, Santos, Lima, Maia, & Wajner, 2017). A clinical study had previously shown reversal of thyroid function test changes after myocardial infarction with N-acetylcysteine (Vidart, et al., 2014). This is likely due to enhanced expression of Dio2. It is not known if these actions are relevant for action in the brain. Antioxidant therapy has been shown to be beneficial in reducing damage from TBI and improving recovery (Hall, Vaishnav, & Mustafa, 2010). The improvement of TBI with antioxidants may be due, in part, to activation of TH.
Timing of administration
TBI is frequently associated with thyroid dysfunction. The in vitro and in vivo models of traumatic brain injury suggest a range of actions of TH in reducing the response to injury, especially reduced edema, as well as stimulation of neural regeneration. The animal models have used TH treatment shortly after, generally 1 hour, from the brain injury. This is a protocol that could be translated to clinical practice, administering TH after the injury and still experience the benefit. Levothyroxine is inexpensive, can be stored for a prolonged period of time under proper conditions, and adverse effects with short term treatment are minimal. Oral levothyroxine could be made available in the field in settings with high risk for traumatic brain injury. For injured individuals not able to take oral medications, parental thyroxine preparations are available, but are significantly more expensive and availability would be more restricted.
Conclusion
In vitro and in vivo models of brain injury show that the associated brain inflammation, altered metabolism and neuronal cell death, may be reduced through TH activated pathways. Limiting brain edema and cell death, and promoting neuronal regeneration, would provide a beneficial effect of TH in both acute and chronic injury. The appropriate TH preparation, dose and timing remains to be established, but benefits of treatment administered 1 hour after injury in animal models indicates that administering such treatment, alone, or with other agents such as antioxidants, may be of practical benefit.
Acknowledgments
Funding sources
This research was funded by the Veterans Affairs Merit Review and United States Department of Health and Human Services, National Institutes of Health, RO1-DK98576.
Abbreviations
- AHDS
Allan-Herndon-Dudley Syndrome
- AQP4
Aquaporin 4
- BBB
Blood Brain Barrier
- CamKIV
Calcium calmodulin-dependent Kinase IV
- CCI
controlled cortical injury
- COUP-TF1
Chicken Ovalbumin Upstream Transcription Factor 1
- Dio1-5′
deiodinase Type 1
- Dio2-5′
deiodinase Type 2
- Dio3-5
deiodinase Type 3
- DITPA-3
5, diiodothyropropionic acid
- Mct8
monocarboxylate transporter 8
- TH
thyroid hormone
- NOS
nitric oxidase synthase
- NTI
Nonthyroidal Illness
- RA
retinoic acid
- TBI
traumatic brain injury
- THRA
thyroid hormone receptor alpha
- THRB
thyroid hormone receptor beta
- TSH
thyrotropin
- TRE
thyroid hormone response element
- T3
triiodothyronine
- T4
thyroxine
- rT3
reverse T3
Footnotes
Disclaimer
This is an original review that has not been published and is not under consideration for publication elsewhere.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accorroni A, Giorgi FS, Donzelli R, Lorenzini L, Prontera C, Saba A, Vergallo A, Tognoni G, Siciliano G, Baldacci F, Bonuccelli U, Clerico A, Zucchi R. Thyroid hormone levels in the cerebrospinal fluid correlate with disease severity in euthyroid patients with Alzheimer7s disease. Endocrine. 2017;55:981–984. doi: 10.1007/s12020-016-0897-6. [DOI] [PubMed] [Google Scholar]
- Alkemade A, Friesema EC, Kalsbeek A, Swaab DF, Visser TJ, Fliers E. Expression of thyroid hormone transporters in the human hypothalamus. J Clin Endocrinol Metab. 2011;96:E967–971. doi: 10.1210/jc.2010-2750. [DOI] [PubMed] [Google Scholar]
- Alvarez-Mora MI, Rodriguez-Revenga L, Madrigal I, Garcia-Garcia F, Duran M, Dopazo J, Estivill X, Mila M. Deregulation of key signaling pathways involved in oocyte maturation in FMR1 premutation carriers with Fragile X-associated primary ovarian insufficiency. Gene. 2015;571:52–57. doi: 10.1016/j.gene.2015.06.039. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Larson RJ, Oas DR, Sandhofer CR, Schwartz HL, Mariash CN, Oppenheimer JH. Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) modulates expression of the Purkinje cell protein-2 gene. A potential role for COUP-TF in repressing premature thyroid hormone action in the developing brain. J Biol Chem. 1998;273:16391–16399. doi: 10.1074/jbc.273.26.16391. [DOI] [PubMed] [Google Scholar]
- Barca-Mayo O, Liao XH, Alonso M, Di Cosmo C, Hernandez A, Refetoff S, Weiss RE. Thyroid hormone receptor alpha and regulation of type 3 deiodinase. Mol Endocrinol. 2011;25:575–583. doi: 10.1210/me.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. 2005;25:673–684. doi: 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Rodari G, Giavoli C, Lania A. Central hypothyroidism - a neglected thyroid disorder. Nat Rev Endocrinol. 2017;13:588–598. doi: 10.1038/nrendo.2017.47. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Millhorn DE. Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. J Biol Chem. 1998;273:19834–19839. doi: 10.1074/jbc.273.31.19834. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone transport in developing brain. Curr Opin Endocrinol Diabetes Obes. 2011;18:295–299. doi: 10.1097/MED.0b013e32834a78b3. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- Canfield SG, Stebbins MJ, Morales BS, Asai SW, Vatine GD, Svendsen CN, Palecek SP, Shusta EV. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem. 2017;140:874–888. doi: 10.1111/jnc.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Jurado C, Pascual A. Thyroid hormone regulation of APP (beta-amyloid precursor protein) gene expression in brain and brain cultured cells. Neurochem Int. 2012;60:484–487. doi: 10.1016/j.neuint.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Crupi R, Paterniti I, Campolo M, Di Paola R, Cuzzocrea S, Esposito E. Exogenous T3 administration provides neuroprotection in a murine model of traumatic brain injury. Pharmacol Res. 2013;70:80–89. doi: 10.1016/j.phrs.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol. 2010;30:4901–4921. doi: 10.1128/MCB.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Hu F, Scanlan TS, Furlow JD. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol. 2009;326:155–168. doi: 10.1016/j.ydbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Sathanoori M, Kapoor R, Rajadhyaksha N, Gonzalez LE, Kottmann AH, Tole S, Vaidya VA. Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult Mammalian brain. Endocrinology. 2011;152:1989–2000. doi: 10.1210/en.2010-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. doi: 10.1172/JCI42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology. 2009;150:4450–4458. doi: 10.1210/en.2009-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Suchland KL, Ciesielski TM, Lessov NS, Grandy DK, Scanlan TS, Stenzel-Poore MP. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke. 2007;38:2569–2576. doi: 10.1161/STROKEAHA.106.480277. [DOI] [PubMed] [Google Scholar]
- Dwyer ND, O’Leary DD. Tbr1 conducts the orchestration of early cortical development. Neuron. 2001;29:309–311. doi: 10.1016/s0896-6273(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2013;20:478–484. doi: 10.1097/01.med.0000433069.09294.e8. [DOI] [PubMed] [Google Scholar]
- Finnie JW, Blumbergs PC. Traumatic brain injury. Vet Pathol. 2002;39:679–689. doi: 10.1354/vp.39-6-679. [DOI] [PubMed] [Google Scholar]
- Flamant F, Gauthier K, Richard S. Genetic Investigation of Thyroid Hormone Receptor Function in the Developing and Adult Brain. Curr Top Dev Biol. 2017;125:303–335. doi: 10.1016/bs.ctdb.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Frascarelli S, Ghelardoni S, Chiellini G, Galli E, Ronca F, Scanlan TS, Zucchi R. Cardioprotective effect of 3-iodothyronamine in perfused rat heart subjected to ischemia and reperfusion. Cardiovasc Drugs Ther. 2011;25:307–313. doi: 10.1007/s10557-011-6320-x. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Zeold A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65:570–590. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijota-Martinez C, Diez D, Morreale de Escobar G, Bernal J, Morte B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 2011;152:1713–1721. doi: 10.1210/en.2010-1014. [DOI] [PubMed] [Google Scholar]
- Grijota-Martinez C, Samarut E, Scanlan TS, Morte B, Bernal J. In vivo activity of the thyroid hormone receptor beta- and alpha-selective agonists GC-24 and CO23 on rat liver, heart, and brain. Endocrinology. 2011;152:1136–1142. doi: 10.1210/en.2010-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley MD, Kirkemo LL, Banerji T, Scanlan TS. A thyroid hormone based strategy for correcting the biochemical abnormality in X-linked adrenoleukodystrophy (X-ALD) Endocrinology. 2017 doi: 10.1210/en.2016-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148:5680–5687. doi: 10.1210/en.2007-0652. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Morte B, Belinchon MM, Ceballos A, Bernal J. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T(3)in the mouse cerebral cortex. Endocrinology. 2012;153:2919–2928. doi: 10.1210/en.2011-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann BL, Rehder J, Kahlke S, Wiedemayer H, Doerfler A, Ischebeck W, Laumer R, Forsting M, Stolke D, Mann K. Hypopituitarism following severe traumatic brain injury. Exp Clin Endocrinol Diabetes. 2006;114:316–321. doi: 10.1055/s-2006-924254. [DOI] [PubMed] [Google Scholar]
- Heuer H. Hear, hear! Thyroid hormone transporters in cochlear development. Endocrinology. 2011;152:4478–4480. doi: 10.1210/en.2011-1722. [DOI] [PubMed] [Google Scholar]
- Hoefig CS, Zucchi R, Kohrle J. Thyronamines and Derivatives: Physiological Relevance, Pharmacological Actions, and Future Research Directions. Thyroid. 2016;26:1656–1673. doi: 10.1089/thy.2016.0178. [DOI] [PubMed] [Google Scholar]
- Horn S, Kersseboom S, Mayerl S, Muller J, Groba C, Trajkovic-Arsic M, Ackermann T, Visser TJ, Heuer H. Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology. 2013;154:968–979. doi: 10.1210/en.2012-1628. [DOI] [PubMed] [Google Scholar]
- Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW. Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev. 2010;62:1227–1237. doi: 10.1016/j.addr.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Kallo I, Bardoczi Z, Arrojo e Drigo R, Zeold A, Liposits Z, Oliva A, Lemmon VP, Bixby JL, Gereben B, Bianco AC. Neuronal hypoxia induces Hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J Neurosci. 2012;32:8491–8500. doi: 10.1523/JNEUROSCI.6514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I, Ng L, Liu H, Forrest D. An intron control region differentially regulates expression of thyroid hormone receptor beta2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol. 2007;21:1108–1119. doi: 10.1210/me.2007-0037. [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Desouza LA, Nanavaty IN, Kernie SG, Vaidya VA. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J Neuroendocrinol. 2012;24:1259–1271. doi: 10.1111/j.1365-2826.2012.02329.x. [DOI] [PubMed] [Google Scholar]
- Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA. Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J. 2010;24:4793–4805. doi: 10.1096/fj.10-161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab. 2009;94:3663–3675. doi: 10.1210/jc.2009-0899. [DOI] [PubMed] [Google Scholar]
- Kim SW, Harney JW, Larsen PR. Studies of the hormonal regulation of type 2 5′-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998;139:4895–4905. doi: 10.1210/endo.139.12.6334. [DOI] [PubMed] [Google Scholar]
- Kogai T, Liu YY, Richter LL, Mody K, Kagechika H, Brent GA. Retinoic acid induces expression of the thyroid hormone transporter, monocarboxylate transporter 8 (Mct8) J Biol Chem. 2010;285:27279–27288. doi: 10.1074/jbc.M110.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokshoorn NE, Wassenaar MJ, Biermasz NR, Roelfsema F, Smit JW, Romijn JA, Pereira AM. Hypopituitarism following traumatic brain injury: prevalence is affected by the use of different dynamic tests and different normal values. Eur J Endocrinol. 2010;162:11–18. doi: 10.1530/EJE-09-0601. [DOI] [PubMed] [Google Scholar]
- Kopczak A, Kilimann I, von Rosen F, Krewer C, Schneider HJ, Stalla GK, Schneider M. Screening for hypopituitarism in 509 patients with traumatic brain injury or subarachnoid hemorrhage. J Neurotrauma. 2014;31:99–107. doi: 10.1089/neu.2013.3002. [DOI] [PubMed] [Google Scholar]
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012;29:19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Calaoagan JM, Gustafson-Brown C, Knapp AM, Li GC, Mendonca HL, Ryan HE, Wang Z, Johnson RS. The response of c-jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1 alpha dependent. Mol Cell Biol. 2002;22:2515–2523. doi: 10.1128/MCB.22.8.2515-2523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers K, Richard K. Traversing barriers - How thyroid hormones pass placental, blood-brain and blood-cerebrospinal fluid barriers. Mol Cell Endocrinol. 2017;458:22–28. doi: 10.1016/j.mce.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Langouche L, Lehmphul I, Perre SV, Kohrle J, Van den Berghe G. Circulating 3-T1AM and 3,5-T2 in Critically Ill Patients: A Cross-Sectional Observational Study. Thyroid. 2016;26:1674–1680. doi: 10.1089/thy.2016.0214. [DOI] [PubMed] [Google Scholar]
- Lehnen TE, Santos MV, Lima A, Maia AL, Wajner SM. N-Acetylcysteine Prevents Low T3 Syndrome and Attenuates Cardiac Dysfunction in a Male Rat Model of Myocardial Infarction. Endocrinology. 2017;158:1502–1510. doi: 10.1210/en.2016-1586. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- Li J, Donangelo I, Abe K, Scremin O, Ke S, Li F, Milanesi A, Liu YY, Brent GA. Thyroid hormone treatment activates protective pathways in both in vivo and in vitro models of neuronal injury. Mol Cell Endocrinol. 2017;452:120–130. doi: 10.1016/j.mce.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Liu YY, Brent GA. A complex deoxyribonucleic acid response element in the rat Ca(2+)/calmodulin-dependent protein kinase IV gene 5′-flanking region mediates thyroid hormone induction and chicken ovalbumin upstream promoter transcription factor 1 repression. Mol Endocrinol. 2002;16:2439–2451. doi: 10.1210/me.2001-0324. [DOI] [PubMed] [Google Scholar]
- Liu YY, Brent GA. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends Endocrinol Metab. 2010;21:166–173. doi: 10.1016/j.tem.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Tachiki KH, Brent GA. A targeted thyroid hormone receptor alpha gene dominant-negative mutation (P398H) selectively impairs gene expression in differentiated embryonic stem cells. Endocrinology. 2002;143:2664–2672. doi: 10.1210/endo.143.7.8906. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10:531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Malekpour B, Mehrafshan A, Saki F, Malekmohammadi Z, Saki N. Effect of posttraumatic serum thyroid hormone levels on severity and mortality of patients with severe traumatic brain injury. Acta Med Iran. 2012;50:113–116. [PubMed] [Google Scholar]
- Manzano J, Cuadrado M, Morte B, Bernal J. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar gamma-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology. 2007;148:5746–5751. doi: 10.1210/en.2007-0567. [DOI] [PubMed] [Google Scholar]
- Marshall J, Dolan BM, Garcia EP, Sathe S, Tang X, Mao Z, Blair LA. Calcium channel and NMDA receptor activities differentially regulate nuclear C/EBPbeta levels to control neuronal survival. Neuron. 2003;39:625–639. doi: 10.1016/s0896-6273(03)00496-3. [DOI] [PubMed] [Google Scholar]
- Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124:1987–1999. doi: 10.1172/JCI70324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RM, Albarracin I, Price EM, Smith TK, Turk JR, Wyatt KD. Thyroid status and nitric oxide in rat arterial vessels. J Endocrinol. 2005;185:111–119. doi: 10.1677/joe.1.06022. [DOI] [PubMed] [Google Scholar]
- McAninch EA, Jo S, Preite NZ, Farkas E, Mohacsik P, Fekete C, Egri P, Gereben B, Li Y, Deng Y, Patti ME, Zevenbergen C, Peeters RP, Mash DC, Bianco AC. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie GJ, Stevenson P, Ward G, Papadia S, Bading H, Chawla S, Privalsky M, Hardingham GE. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J Neurochem. 2005;93:171–185. doi: 10.1111/j.1471-4159.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Milanesi A, Brent GA. Beam Me In: Thyroid Hormone Analog Targets Alternative Transporter in Mouse Model of X-Linked Adrenoleukodystrophy. Endocrinology. 2017;158:1116–1119. doi: 10.1210/en.2017-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morte B, Diez D, Auso E, Belinchon MM, Gil-Ibanez P, Grijota-Martinez C, Navarro D, de Escobar GM, Berbel P, Bernal J. Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology. 2010;151:810–820. doi: 10.1210/en.2009-0958. [DOI] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150:1952–1960. doi: 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J, Celi FS, Ng L, Forrest D. Multigenic control of thyroid hormone functions in the nervous system. Mol Cell Endocrinol. 2008;287:1–12. doi: 10.1016/j.mce.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Barr SA, Oh JS, Ma C, Brent GA, Schultz JJ. Thyroid hormone regulates endogenous amyloid-beta precursor protein gene expression and processing in both in vitro and in vivo models. Thyroid. 2006;16:1207–1213. doi: 10.1089/thy.2006.16.1207. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev. 1997;18:462–475. doi: 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- Peeters RP, Hernandez A, Ng L, Ma M, Sharlin DS, Pandey M, Simonds WF, St Germain DL, Forrest D. Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor alpha1. Endocrinology. 2013;154:550–561. doi: 10.1210/en.2012-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picou F, Fauquier T, Chatonnet F, Flamant F. A bimodal influence of thyroid hormone on cerebellum oligodendrocyte differentiation. Mol Endocrinol. 2012;26:608–618. doi: 10.1210/me.2011-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts MB, Adwanikar H, Noble-Haeusslein LJ. Models of traumatic cerebellar injury. Cerebellum. 2009;8:211–221. doi: 10.1007/s12311-009-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Shi Y. Neural stem cells in the developing and adult brains. J Cell Physiol. 2009;221:5–9. doi: 10.1002/jcp.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R, Conti AC, Graham DI, Krajewski S, Reed JC, Grady MS, Trojanowski JQ, McIntosh TK. Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience. 2002;110:605–616. doi: 10.1016/s0306-4522(01)00461-4. [DOI] [PubMed] [Google Scholar]
- Romine J, Gao X, Chen J. Controlled cortical impact model for traumatic brain injury. J Vis Exp. 2014:e51781. doi: 10.3791/51781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana P, Coughlin L, Burke J, Woods R, Mdzinarishvili A. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J Neurol Sci. 2015;354:37–45. doi: 10.1016/j.jns.2015.04.042. [DOI] [PubMed] [Google Scholar]
- See V, Boutillier AL, Bito H, Loeffler JP. Calcium/calmodulin-dependent protein kinase type IV (CaMKIV) inhibits apoptosis induced by potassium deprivation in cerebellar granule neurons. FASEB J. 2001;15:134–144. doi: 10.1096/fj.00-0106com. [DOI] [PubMed] [Google Scholar]
- Serfozo Z, Kiss PB, Kukor Z, Lontay B, Palatka K, Varga V, Erdodi F, Elekes K. Thyroid hormones affect the level and activity of nitric oxide synthase in rat cerebral cortex during postnatal development. Neurochem Res. 2008;33:569–578. doi: 10.1007/s11064-007-9480-0. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. Endocrinology. 2011;152:5053–5064. doi: 10.1210/en.2011-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga A, Blaesse A, Kysenius K, Huttunen HJ, Tanhuanpaa K, Saarma M, Rivera C. Thyroxin regulates BDNF expression to promote survival of injured neurons. Mol Cell Neurosci. 2009;42:408–418. doi: 10.1016/j.mcn.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest. 2008;118:975–983. doi: 10.1172/JCI32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland HW, Wu V, Fukushima H, Kida S, Zhuo M. CaMKIV over-expression boosts cortical 4–7 Hz oscillations during learning and 1–4 Hz delta oscillations during sleep. Mol Brain. 2010;3:16. doi: 10.1186/1756-6606-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XJ, Fan XT, Kim HJ, Butler R, Webb P, Warner M, Gustafsson JA. Liver X receptor beta and thyroid hormone receptor alpha in brain cortical layering. Proc Natl Acad Sci U S A. 2010;107:12305–12310. doi: 10.1073/pnas.1006162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Liu YY, Teng W, Brent GA. COUP-TF1 Modulates Thyroid Hormone Action in an Embryonic Stem Cell Model of Cortical Pyramidal Neuronal Differentiation. Thyroid. 2017 doi: 10.1089/thy.2017.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremper-Wells B, Mathur A, Beaman-Hall CM, Vallano ML. Trophic agents that prevent neuronal apoptosis activate calpain and down-regulate CaMKIV. J Neurochem. 2002;81:314–324. doi: 10.1046/j.1471-4159.2002.00829.x. [DOI] [PubMed] [Google Scholar]
- Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, Garcia VJ, Ho R, Yucer N, Qian T, Lim RG, Wu J, Thompson LM, Spivia WR, Chen Z, Van Eyk J, Palecek SP, Refetoff S, Shusta EV, Svendsen CN. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell. 2017;20:831–843e835. doi: 10.1016/j.stem.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine GD, Zada D, Lerer-Goldshtein T, Tovin A, Malkinson G, Yaniv K, Appelbaum L. Zebrafish as a model for monocarboxyl transporter 8-deficiency. J Biol Chem. 2013;288:169–180. doi: 10.1074/jbc.M112.413831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge CF, Konrad D, Cohen M, Di Cosmo C, Dumitrescu AM, Marcinkowski T, Hameed S, Hamilton J, Weiss RE, Refetoff S. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97:4515–4523. doi: 10.1210/jc.2012-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidart J, Wajner SM, Leite RS, Manica A, Schaan BD, Larsen PR, Maia AL. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99:4537–4545. doi: 10.1210/jc.2014-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest. 2011;121:1834–1845. doi: 10.1172/JCI44678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis K, Dudazy S, van Hogerlinden M, Nordstrom K, Mittag J, Vennstrom B. The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24:1904–1916. doi: 10.1210/me.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Matsuda H, Shi YB. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology. 2008;149:5610–5618. doi: 10.1210/en.2008-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf PD, Lee LA, Hamill RW, McDonald JV. Thyroid test abnormalities in traumatic brain injury: correlation with neurologic impairment and sympathetic nervous system activation. Am J Med. 1988;84:201–208. doi: 10.1016/0002-9343(88)90414-7. [DOI] [PubMed] [Google Scholar]
- Wu Z, Martinez ME, St Germain DL, Hernandez A. Type 3 Deiodinase Role on Central Thyroid Hormone Action Affects the Leptin-Melanocortin System and Circadian Activity. Endocrinology. 2017;158:419–430. doi: 10.1210/en.2016-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]