Abstract
Methamphetamine (METH) abuse is a major public health issue around the world, yet there are currently no effective pharmacotherapies for the treatment of METH addiction. METH is potent psychostimulant that increases extracellular dopamine levels by targeting the dopamine transporter (DAT) and alters neuronal activity in the reward centers of the brain. One promising therapeutic target for the treatment of METH addiction is the sigma-1 receptor (σ1R). The σ1R is an endoplasmic reticulum-localized chaperone protein that is activated by cellular stress, and, unique to this chaperone, its function can also be induced or inhibited by different ligands. Upon activation of this unique “chaperone receptor”, the σ1R regulates a variety of cellular functions and possesses neuroprotective activity in the brain. Interestingly, a variety of σ1R ligands modulate dopamine neurotransmission and reduce the behavioral effects of METH in animal models of addictive behavior, suggesting that the σ1R may be a viable therapeutic target for the treatment of METH addiction. In this review, we provide background on METH and the σ1R as well as a literature review regarding the role of σ1Rs in modulating both dopamine neurotransmission and the effects of METH. We aim to highlight the complexities of σ1R pharmacology and function as well as the therapeutic potential of the σ1R as a target for the treatment of METH addiction.
Keywords: sigma-1 receptor, dopamine, dopamine transporter, methamphetamine
1. INTRODUCTION
Methamphetamine (METH) is one of the most commonly used illicit drugs in the world (Krasnova and Cadet, 2009), with reports that up to 35 million people use amphetamine-type stimulants (ATSs) worldwide (Salamanca et al., 2014). Although there have been clinical trials for drugs targeting the dopaminergic, serotonergic, and opioid systems (Karila et al., 2010), there are currently no Food and Drug Administration (FDA) approved pharmacotherapies for the treatment of METH addiction (Napier et al., 2013). While it is well established that METH elicits its addictive effects primarily through interactions with the dopamine transporter (DAT) (Sulzer et al., 1993), growing evidence indicates that sigma receptors (σRs) may be involved in METH addiction. Two isoforms of σRs are known to exist, σ1R and σ2R; however, the σ1R has been more thoroughly characterized in the literature (Bowen, 2000; Quirion et al., 1992). In addition to responding to cellular stress, the σ1R is a ligand-operated chaperone protein that can be activated or inhibited by different ligands in an agonist-antagonist manner (Hayashi and Su, 2003b; Tsai et al., 2009). Several σ1R-targeting drugs are proposed as possible treatments for human diseases including neurodegeneration, psychiatric disorders, neuropathic pain, and drug abuse (Hayashi, 2015). Importantly, multiple FDA-approved drugs that are widely used for the treatment of schizophrenia and depression have high affinity for the σ1R, supporting the therapeutic potential of drugs targeting σ1Rs. While several studies have reported that σR ligands attenuate some of the behavioral effects of cocaine and METH in rodent models, the mechanisms by which σR ligands produce these effects are largely unknown. With the ongoing synthesis of novel, highly selective σ1R ligands every year and the increased understanding of the protein’s function, the σ1R remains a highly attractive therapeutic target for METH addiction.
2. METHAMPHETAMINE
Patterns of Methamphetamine Use
Methamphetamine (METH) is a widely abused, highly addictive psychostimulant that belongs to a class of synthetic drugs called amphetamine-type stimulants (ATSs). This class includes amphetamine (AMPH), METH, methylenedioxy-methamphetamine (MDMA), and other designer drugs (Chomchai and Chomchai, 2015). AMPH and METH were once widely distributed as over-the-counter drugs for the myriad of “positive” effects that quickly became causative for their use (increased wakefulness, appetite suppression, etc.) (Vearrier et al., 2012). The intended beneficial use of these drugs, however, was offset by their highly addictive potential, ultimately leading to ATSs becoming among the most abused drugs in the world. The 2016 United Nations Office on Drugs and Crime World Drug Report estimated that over 35 million people use AMPHs and prescription stimulants across the world (UNODC, 2016). METH specifically has surpassed the other ATSs in popularity, production, and trafficking. While AMPH and METH share similar mechanisms of action as well as behavioral effects, the drugs have differing molecular structures and cellular effects (Goodwin et al., 2009; Saha et al., 2014), and METH is more commonly associated with recreational use and drug addiction. METH can be synthesized by the reduction of everyday over-the-counter nasal decongestants that contain ephedrine or pseudoephedrine, and the relative accessibility of these starting materials led to the expansion of small scale METH laboratories across the United States (Ciccarone, 2011; Panenka et al., 2013). Despite attempts to limit sales of these products with the 2005 Combat Methamphetamine Epidemic Act, larger-scale illicit METH manufacturers emerged (Maxwell and Brecht, 2011; SAMHSA, 2006). In 2014, there was a global peak in ATS law enforcement seizures worldwide, with METH accounting for the largest share of ATS seizures increasing an estimated 21% from the previous year (UNODC, 2016). Despite widespread efforts to decrease METH production, METH use continues to be a major public health problem worldwide.
Effects of Methamphetamine Use
Upon administration, there are several acute physiological effects associated with METH use. By promoting the release of epinephrine and norepinephrine, METH acts as a potent stimulant by activating the sympathetic nervous system (Schneider, 1972). This provokes an array of responses including increased blood pressure, hyperthermia, tachycardia, increased breathing, pupil dilatation, peripheral hypertension, and reduced appetite (Courtney and Ray, 2014; Rawson and Condon, 2007). Non-essential physiological activities, such as gastrointestinal function, are inhibited (Panenka et al., 2013), and levels of stress hormones including cortisol and adrenocorticotrophic hormone can increase up to 200% (Harris et al., 2003). Most notably, METH administration also elicits a suite of reinforcing effects including euphoria, arousal, heightened awareness, reduced fatigue, behavioral disinhibition, positive mood, increased self-confidence, and acute cognitive improvement (Courtney and Ray, 2014). Conversely, METH can also induce acute negative psychological effects including anxiety, insomnia, aggressive behavior, paranoia, and psychosis (Rawson and Condon, 2007). At high doses, METH can elevate the body temperate to potentially lethal levels, resulting in convulsions, coma, stroke, or even death (Rawson and Condon, 2007).
Like other drugs of abuse, prolonged METH use often results in drug tolerance, typically leading to increased dosage and frequency of use (Rawson and Condon, 2007). Long-term chronic METH use often leads to the development of symptoms including violent behavior, anxiety, cognitive impairment, and insomnia (Rawson and Condon, 2007). Additionally, many chronic METH users report psychotic symptoms similar to those of schizophrenia, namely paranoia, auditory hallucinations, mood disturbances, delusions, and abnormal speech (Hsieh et al., 2014). Additional adverse physiological consequences of prolonged METH use include cardiovascular problems, pulmonary disease, and infections from repeated intravenous injections (such as HIV) (Rawson and Condon, 2007). Patterns of METH abuse may also lead to other negative repercussions including disrupted personal relationships, unemployment, and incarceration (Hsieh et al., 2014).
Methamphetamine Regulation of Extracellular Dopamine
The administration of ATSs results in an acute increase in the monoamines dopamine (DA), norepinephrine, and serotonin in the brain (Azzaro and Rutledge, 1973; Halpin et al., 2014). Both the rewarding and addictive properties of ATSs are primarily attributed to their ability to increase extracellular DA levels (Sonders et al., 1997). In both humans and animal models, blockade of DA receptors decreases the euphoric effects of AMPH, demonstrating the importance of DA in the rewarding effects of the drug (Davis and Smith, 1975; Gunne et al., 1972; Jonsson et al., 1971; Yokel and Wise, 1975). In 1988, Di Chiara and Imperato reported that while administration of cocaine, morphine, methadone, ethanol, and nicotine in rats increased extracellular DA levels in the striatum up to 400%, AMPH treatment increased extracellular DA levels up to 1000% (Di Chiara and Imperato, 1988), demonstrating the profound capacity of ATSs to activate the DA system. Human imaging studies have similarly reported increased DA levels after the administration of amphetamines (Volkow et al., 2007; Volkow et al., 1999). The mechanism by which METH increases DA levels in the brain – a direct interaction with its primary target the dopamine transporter (DAT) - has been well characterized.
Methamphetamine Interactions with DAT
Dopamine (DA) is important for regulating processes including reward, motivation, movement, working memory, and cognition (Chinta and Andersen, 2005). The main source of DA in the brain is midbrain dopaminergic neurons, which includes the substantia nigra (SN) and ventral tegmental area (VTA). The dopamine transporter (DAT) is a transmembrane protein located on dopaminergic neurons that primarily functions to take up DA released into the extracellular space. Upon reuptake, dopamine can then be sequestered into synaptic vesicles via the vesicular monoamine transporter-2 (VMAT-2) for storage until the next exocytosis event (Giros et al., 1996). DAT knockout mice display hyperactivity, cognitive deficits, altered psychostimulant responses, and persistently high levels of extracellular DA with low tissue DA content (Giros et al., 1996), highlighting the requirement of DAT for regulating DA homeostasis as well as the actions of psychostimulants.
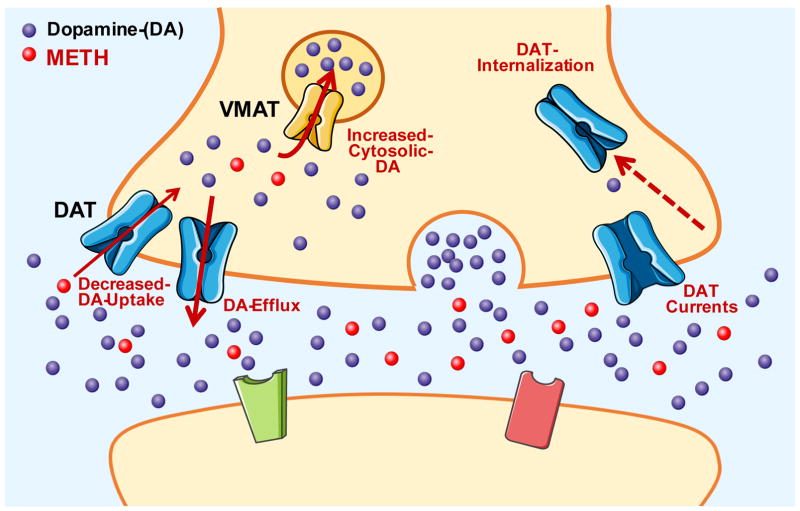
Both cocaine and ATSs increase extracellular DA levels through direct interactions with DAT. While cocaine increases extracellular DA levels by blocking DAT and preventing DA reuptake, METH interacts with DAT to increase extracellular DA levels through a variety of mechanisms (Figure 1). As a substrate for the transporter, METH enters DA neurons directly through DAT and consequently decreases DA uptake via competitive inhibition (Fleckenstein et al., 1997). Once inside the neuron, METH disrupts vesicular stores of DA, resulting in the release of DA into the cytoplasm of the neuron (Sulzer and Rayport, 1990). Importantly, METH also induces the reverse transport of DA from the cytosol to the extracellular space via DAT-mediated, action potential independent DA efflux (Sulzer et al., 1995). This is thought to occur via the facilitated exchange diffusion model in which forward transport of AMPHs is followed by a counter movement of DA outside the cell (Fischer and Cho, 1979; Khoshbouei et al., 2003). This model is supported by evidence showing that compounds that release DA from vesicular stores without affecting DAT activity do not produce DA efflux, demonstrating the importance of the inward transport of AMPH (Jones et al., 1998). This process is voltage-dependent and highly regulated by AMPH-induced increases in intracellular Na+ and Ca2+ as well as proteins interacting with DAT (Khoshbouei et al., 2003).
Figure 1.
METH-stimulated, DAT-mediated increase in extracellular dopamine. Molecule and protein images are courtesy of Servier Medical Art, licensed under CC BY 3.0.
Beyond competitive inhibition of DA uptake and reverse DA transport, ATSs also impact the electrophysiological properties of DA neurons through a DAT-dependent mechanism. AMPH-mediated DA release activates D2 autoreceptors which inhibit the firing activity of DA neurons (Bunney et al., 1973). Pharmacological blockade of these autoreceptors, however, reveals AMPH-mediated increases in firing activity above baseline (Shi et al., 2000). This increase in firing activity is inhibited by DAT blockers, suggesting it occurs via a DAT-dependent mechanism (Ingram et al., 2002; Lin et al., 2016; Saha et al., 2014). DAT also carries a channel-like current uncoupled to DA transport (DeFelice and Blakely, 1996; Kahlig et al., 2005; Lester et al., 1994). AMPHs increase this DAT-dependent inward positive current (Fischer and Cho, 1979; Saha et al., 2014) leading to membrane depolarization and increased firing activity of dopaminergic neurons. Importantly, previous studies revealed that repeated METH exposure in rodents decreases the sensitivity of D2 autoreceptors, which in turn could increase METH-mediated, DAT-dependent firing activity (White and Wang, 1984; Wolf et al., 1993).
AMPHs are also known to internalize the transporter in a protein kinase C (PKC)-dependent manner. This is presumably via AMPH-induced increases in intracellular Ca2+ (Gnegy et al., 2004; Goodwin et al., 2009) that in turn activate PKC (Giambalvo, 1992), or AMPH-induced membrane depolarization (Richardson et al., 2016). By promoting DAT internalization, AMPHs decrease DA transport by limiting available DAT at the membrane for uptake (Cowell et al., 2000; Melikian and Buckley, 1999). Over the past several years, other signaling pathways involved in DAT trafficking have also been identified, including a role for Ca2+/calmodulin-dependent protein kinase II, MAP/ERK, and membrane depolarization (Fog et al., 2006; Moron et al., 2003; Richardson et al., 2016). Collectively, these effects of AMPHs on DAT activity result in a robust increase in extracellular DA levels that contribute to the highly addictive properties of the drug.
Other Mechanisms
In addition to DAT-mediated increases in extracellular DA through disrupting DAT activity, METH also decreases the activity of monoamine oxidase, resulting in decreased dopamine metabolism and thus prolonged elevated extracellular DA levels (Sulzer et al., 2005). Importantly, METH also affects others neurotransmitter systems outside of the monoamines. ATSs have been shown to activate the endogenous opioid system, also contributing to the rewarding effects of the drugs (Chiu et al., 2005; Jones and Holtzman, 1994; Shen et al., 2010). Additionally, studies have demonstrated that METH increases levels of glutamate, acetylcholine, and the neuropeptide neurotensin (Courtney and Ray, 2014).
Chronic Effects on the Dopaminergic System
High doses and persistent use of METH can lead to neurotoxic effects on dopaminergic neurons. Human positron emission tomography (PET) imaging in METH abusers demonstrates decreases in striatal DAT density, D2 dopamine receptor (D2R) availability, and VMAT-2 density compared to non-METH users (Johanson et al., 2006; McCann et al., 1998; Volkow et al., 2001a; Volkow et al., 2001c). The mechanisms by which these dopaminergic markers are down-regulated are not understood, however evidence suggests that METH-mediated oxidative stress may be responsible for neuronal toxicity (Berman et al., 2008). Interestingly, human imaging studies have revealed that prolonged METH abstinence (more than one year) can improve these deficits in dopaminergic markers (Curtin et al., 2015; Volkow et al., 2001b). Despite the potential for improvement after abstinence, the connection between METH use and Parkinson’s disease (PD) is an ongoing concern. Individuals with a history of METH use were reported to have nearly a three-fold increased risk for developing PD (Callaghan et al., 2010; Callaghan et al., 2012), however whether or not METH use directly causes PD remains unresolved (Guilarte, 2001; Kish et al., 2017).
Treatment Strategies
Currently, there are few effective options for the treatment for METH addiction. Psychosocial interventions are often employed consisting of either Cognitive-Behavioral Treatment (CBT) or Contingency Management (CM) (Courtney and Ray, 2014). CBT involves teaching individuals how to reduce or discontinue drug use, whereas CM involves using positive reinforcement, such as money vouchers, to promote decreased drug use (Lee and Rawson, 2008). Unfortunately, these psychosocial treatments have low rates of treatment induction as well as patient retention (Shearer, 2007). Currently, there is no FDA-approved drug treatment for METH addiction, but several drugs are currently under clinical trial. These drugs mainly target dopaminergic, serotonergic, GABAergic, and/or glutamatergic systems in the brain (Courtney and Ray, 2014). In addition to these known pathways involved in psychostimulant addiction, there is growing interest in a unique target in the brain for the treatment of METH addiction called the sigma-1 receptor (σ1R), which will be the focus for the remainder of this review.
3. SIGMA-1 RECEPTOR
The sigma-1 receptor (σ1R) is a ubiquitously expressed protein implicated for the treatment of a number of neurological conditions including stroke, neurodegenerative disease, psychiatric disorders, and neuropathic pain (Rousseaux and Greene, 2015). The σ1R is widely expressed throughout the central nervous system and peripheral organs. Within the brain, σ1Rs are highly concentrated within the limbic system and brainstem (Maurice et al., 2002). At the cellular level, the σ1R is located at the endoplasmic reticulum (ER) membrane where it possesses chaperone activity in response to misfolded proteins. A unique property of this chaperone, however, is that it can also be regulated by different ligands in an agonist-antagonist manner. Upon activation, the σ1R acts as intracellular signaling modulator, regulating a variety of cellular functions. Despite the σ1R’s wide expression and numerous functions, σ1R knockout mice are viable and do not display any overt phenotype. In part, this could be due to compensatory mechanisms occurring during development. Interestingly, a depressive-like phenotype has been observed in σ1R knockout mice, supporting a role for σ1Rs in psychiatric disease (Rousseaux and Greene, 2015; Sabino et al., 2009). Although there are many unanswered mechanistic questions, the past 20 years of research suggests that the σ1R is a viable target for the treatment of numerous pathological conditions.
Protein Characterization
Because of their affinity for the opioid-receptor targeting benzomorphan N-allylnormetazocine (SKF-10,047), sigma receptors (σR) were initially classified as the “σ opioid” receptor subtype in 1976 (Martin et al., 1976). A later study revealed that the classic opioid receptor antagonist naltrexone failed to antagonize the effects of SKF-10,047, leading researchers to distinguish the σR as a non-opioid receptor (Vaupel, 1983). SKF-10,047 was later shown to have a similar behavioral profile as the dissociative drug phencyclidine (PCP) as well as share binding to the N-methyl-D-aspartate (NMDA) receptor, creating further uncertainty about the σR’s identity (Hayashi and Su, 2004; Quirion et al., 1992). Eventually, the development of more selective ligands revealing a distinct binding profile led to the recognition of σRs as a separate class of proteins (de Costa et al., 1989).
At least two subtypes of σRs have been identified, sigma-1 (σ1) and sigma-2 (σ2), initially characterized based on their differential ligand affinities (Maurice et al., 2002; Quirion et al., 1992). In general, σ1Rs have higher affinity and stereoselectivity for (+)-isomers of benzomorphans whereas σ2Rs have more affinity for (−)isomers (Hellewell et al., 1994). Additionally, the subtypes display different molecular weights; the σ1R is identified at 25–30 kDa and the σ2R at 18–21 kDa (Maurice et al., 2002). The σ1R was first cloned from guinea pigs in 1996 (Hanner et al., 1996) and later identified as a 223-amino acid protein with 90% homology across mammalian species (Su et al., 2010). Interestingly, the σ1R has 33% identity and 66% homology with a yeast sterol C8-C7 isomerase involved in sterol biosynthesis (Hanner et al., 1996; Su and Hayashi, 2003). Although the σ1R was later shown to bind cholesterol and to be involved in subcellular lipid distribution (Hayashi and Su, 2005; Palmer et al., 2007), it lacks sterol or cholesterol isomerase activity (Labit-Le Bouteiller et al., 1998). Furthermore, the σ1R lacks enzymatic activity and shares no sequence homology with any other known mammalian proteins (Hayashi and Su, 2003b; Su et al., 2010), further complicating efforts to categorize the protein. In 2016, the crystal structure of the human σ1R was identified and shown to have a trimeric organization with a single transmembrane domain for each protomer (Schmidt et al., 2016). Because of these advances in characterizing the σ1R, the σ1R has been more widely investigated throughout the field compared to the σ2R. Only recently was the σ2R cloned and identified as TMEM97 (Alon et al., 2017), an ER membrane protein known to contribute to cholesterol homeostasis (Alonso et al., 2000; Bartz et al., 2009) and potentially other cellular functions (Derbez et al., 2002; Sahn et al., 2017; Walker et al., 1993). It is unclear how this recent development might alter the interpretation of previous σ2R studies. It should be noted that although several of the σR ligands discussed in this report and used in many other studies have affinity for both the σ1R and σ2R, the focus of this review is on σ1Rs. This is important to consider as the two subtypes may differ in cellular activity and thus overall function.
Pharmacology
A variety of drugs bind to σ1Rs, including benzomorphans and PCP as described above. Although there is no known explicit endogenous ligand, neurosteroids including progesterone and dehydroepianderosterone (DHEA) have affinity for the σ1R (Su et al., 1988a). Additionally, N,N-dimethyltryptamine (DMT), an endogenous compound also used recreationally as a hallucinogen, has been shown to bind to the σ1R (Fontanilla et al., 2009). DMT is generally recognized as targeting serotonin receptors, but DMT’s affinity for the σ1R suggests a potential role for σ1Rs in its psychedelic effects and further implicates the σ1R in psychiatric disease (Rousseaux and Greene, 2015).
In addition to these compounds, a wide range of unrelated and structurally diverse ligands used both recreationally and therapeutically in humans have high affinity for the σ1R. This includes the drugs of abuse cocaine (Sharkey et al., 1988) and METH (Nguyen et al., 2005), the antipsychotic drug haloperidol (Su, 1982), and antidepressants such as fluoxetine (Prozac®) (Safrany and Brimson, 2016) and sertraline (Zoloft®) (Narita et al., 1996). Importantly, fluoxetine has been reported to reach serum concentrations at clinically relevant doses that can bind σ1Rs (Di Rosso et al., 2016; Safrany and Brimson, 2016), thus the actions of these drugs and potentially many other clinically used drugs could be in part due to their interactions with the σ1R. Additionally, anticonvulsants, cytochrome P450 inhibitors, and monoamine oxidase inhibitors also have reported σ1R affinity (Maurice et al., 2002). It is still unclear if and how the σ1R contributes to the actions of many of these drugs, but these findings have generated great interest in the potential clinical role of the σ1R and has also led to the generation of several novel drugs selectively targeting σ1Rs. Table 1 summarizes drugs mentioned in this review with their affinities for σ1R, σ1R, and DAT.
Table 1.
Ligand Affinities for σ1R, σ2R, and DAT (Ki, nM)
| Stimulants and Psychiatric Drugs | |||||
|---|---|---|---|---|---|
|
| |||||
| Ligand | Activity | σ1R | σ2R | DAT | Reference |
| Cocaine | Agonist | n.d. | n.d. | 164 | Izenwasser et al., 1999 |
| 5,190 | 19,300 | 77 | Garcés-Ramírez et al., 2012 | ||
| 1,347 | 48,170 | n.d. | Lever et al., 2016 | ||
|
| |||||
| Fluoxetine | Agonist | 240 | 16,100 | n.d. | Narita et al., 1996 |
|
| |||||
| Haloperidol | Antagonist | 7.0 | n.d. | n.d. | Su, 1982 |
| 1.9 | 79.8 | n.d. | Bowen et al., 1993 | ||
| n.d. | n.d. | 5,232 | Izenwasser et al., 1993 | ||
| 3.3 | 270.0 | n.d. | Takahashi et al., 1999 | ||
| 2.2 | 16.0 | 9,578 | Moison et al., 2003 | ||
| 0.9 | 7.9 | n.d. | Lever et al., 2006 | ||
| 1.2 | 18.1 | n.d. | Lever et al., 2014 | ||
| 1.4 | 72.8 | n.d. | Lever et al., 2016 | ||
|
| |||||
| Methamphetamine | ??? | †2,160 | †46,670 | n.d. | Nguyen et al., 2005 |
| 4,390 | 15,900 | n.d. | Hiranita et al., 2013 | ||
|
| |||||
| MDMA | ??? | 3,057 | 8,889 | 10,320 | Brammer et al., 2006 |
|
| |||||
| Sertraline | Agonist | 57 | 5,297 | n.d. | Narita et al., 1996 |
| Selective σ Ligands | |||||
|---|---|---|---|---|---|
|
| |||||
| Ligand | Activity | σ1R | σ2R | DAT | Reference |
| (+)-3-PPP | Agonist | 49.3 | 120.0 | n.d. | Bowen et al., 1993 |
| n.d. | n.d. | 1,784 | Izenwasser et al., 1993 | ||
|
| |||||
| AC927 | Antagonist | 30.0 | 138.0 | 2,939 | Matsumoto et al., 2008 |
|
| |||||
| AZ66 | Antagonist | 2.4 | 0.5 | 872 | Seminerio et al., 2012 |
| 4.7 | 1.4 | 1,950 | Katz et al., 2016 | ||
|
| |||||
| BD1008 | Antagonist | 2.0 | 8.0 | n.d. | McCrackena et al., 1999 |
| 2.1 | 16.6 | 2,510 | Garcés-Ramírez et al., 2012 | ||
|
| |||||
| BD1047 | Antagonist | 0.9 | 47.0 | n.d. | Matsumoto et al., 1995 |
| 3.0 | 48.0 | 3,220 | Garcés-Ramírez et al., 2012 | ||
|
| |||||
| BD1063 | Antagonist | 9.2 | 449.0 | n/a | Matsumoto et al., 1995 |
| 6.0 | 440.0 | 16,820 | Brammer et al., 2006 | ||
| 9.0 | 625.0 | 8,020 | Garcés-Ramírez et al., 2012 | ||
| 8.8 | 626.0 | 8,020 | Katz et al., 2016 | ||
| 9.7 | 762.0 | n.d. | Lever et al., 2016 | ||
|
| |||||
| BMY-14802 | Antagonist | 265.0 | 391.0 | n.d. | Weigl et al., 2002 |
|
| |||||
| CM156 | Antagonist | 1.3 | 0.6 | 1,175 | Xu et al., 2010 |
|
| |||||
| DTG | Agonist | 74.3 | 61.2 | n.d. | Bowen et al., 1993 |
| 69.0 | 21.0 | >10,000 | Moison et al., 2003 | ||
| 35.5 | 39.9 | n.d. | Lever et al., 2006 | ||
| 57.0 | 22.0 | 93,500 | Garcés-Ramírez et al., 2012 | ||
| 111.8 | 39.1 | n.d. | Lever et al., 2016 | ||
|
| |||||
| MR200 | Agonist | 1.5 | 21.9 | >10,000 | Moison et al., 2003 |
|
| |||||
| MS-377 | Antagonist | 73.0 | 6,900.0 | n.d. | Takahashi et al., 1999 |
|
| |||||
| NE100 | Antagonist | *1.5 | *633.0 | n.d. | Chaki et al., 1994 |
| 2.5 | 121.0 | 3,590 | Hiranita et al., 2011 | ||
|
| |||||
| (+)-Pentazocine | Agonist | 6.7 | 1,361 | n.d. | Bowen et al., 1993 |
| 1.6 | 728.4 | n.d. | Lever et al, 2006 | ||
| 4.6 | 224.0 | n.d. | Hiranita et al., 2013 | ||
| 5.1 | 2,060.0 | n.d. | Lever et al., 2016 | ||
|
| |||||
| PRE-084 | Agonist | *44.0 | n.d. | n.d. | Su et al., 1991 |
| n.d. | n.d. | 5,344 | Izenwasser et al., 1993 | ||
| 46.5 | n.d. | n.d. | Cao et al., 2003 | ||
| 48.5 | 12,700.0 | n.d. | Hiranita et al., 2013 | ||
| 53.0 | 32,100.0 | 19,600 | Garcés-Ramírez et al., 2012 | ||
|
| |||||
| SA4503 | Agonist | *17.4 | *1,784.0 | n/a | Matsuno et al., 1996 |
| 4.6 | 63.1 | n.d. | Lever et al., 2006 | ||
|
| |||||
| (+)-SKF-10,047 | Agonist | 28.7 | 33,654.0 | n.d. | Bowen et al., 1993 |
| Other Drugs | |||||
|---|---|---|---|---|---|
|
| |||||
| Ligand | Activity | σ1R | σ2R | DAT | Reference |
| DHEA-sulfate | Agonist | 15,129 | n.d. | n.d. | Maurice et al., 1996 |
|
| |||||
| DMT | ??? | †14,750 | †21,710 | n.d. | Fontanilla et al., 2009 |
|
| |||||
| PCP | ??? | *2,541 | n.d. | n.d. | Su, 1982 |
|
| |||||
| Progesterone | Antagonist | 175 | n.d. | n.d. | Maurice et al., 1996 |
| 2.7 | n.d. | n.d. | Su et al., 1988 | ||
|
| |||||
| Rimacozole | Antagonist | 908.0 | 302.0 | 224 | Husbands et al., 1999 |
| 96.6 | 883.0 | 238 | Hiranita et al., 2011 | ||
n.d.: not determined,
KD,
IC50
Defining σ1R ligands as agonists or antagonists has been complicated for various reasons. Several ligands with σ1R affinity remain uncharacterized as either agonists or antagonists (Gonzalez-Alvear and Werling, 1994). One widely accepted metric for determining σ1R agonist activity is the ability of the ligand to dissociate the σ1R from its constitutive binding partner protein, Binding immunoglobulin Protein (BiP). Agonists dissociate σ1R from BiP whereas antagonists either increase the association or block the effect of agonists (Hayashi and Su, 2007). Interestingly, several selective σ1R ligands show no effect when administered alone and only modulate stimulated cellular responses (Hayashi and Su, 2003a), limiting the feasibility of classifying ligands as agonists versus antagonists based cellular effects. Additionally, modulation of these responses is often dose-dependent as a number of studies have shown opposing effects depending on whether high or low doses were used (Rousseaux and Greene, 2015); this makes it challenging to compare diverse studies using different doses of the same σ1R ligands. Another proposed method of determining agonist versus antagonist activity is that σ1R agonists mimic the effects of σ1R overexpression while σ1R antagonists mimic σ1R knockdown (Mei and Pasternak, 2002), although this requires further validation. Additionally, the possibility that some σ1R antagonists may act as partial or inverse agonists is a developing concept within the field. It is important to consider that many σ ligands to-date have dual or partial affinity for both σ receptor subtypes making it difficult to distinguish which target is primarily contributing to the effects of the drugs. Furthermore, ligands may act as an agonist of one subtype but an antagonist of the other, and the cellular responses to agonism and antagonism of the σ2R are not defined. Adding additional complexity, it was recently shown that drugs thought to be highly selective for the σ1R can also directly influence the activity of voltage-gated K+ channels, potentially explaining the effects on neuronal physiology without a direct role of the σ1R (Liu et al., 2017). Lastly, the in vitro pharmacokinetics of many of these selective σR ligands is undetermined, with the half-life and brain concentrations of these drugs after administration unknown. Given this complexity, further investigation is required to properly characterize and categorize σR ligands and allow for proper interpretation across studies utilizing these drugs.
Cellular Localization
The cellular localization of the σ1R is dynamic in nature. σ1Rs exist predominantly in the endoplasmic reticulum (ER) membrane (Hayashi et al., 2000) where they are highly localized in cholesterol-rich areas of the ER adjacent to the mitochondria called the mitochondrial-associated membrane (MAM). Additionally, σ1Rs have also been localized to the plasma membrane, nuclear envelope, and post-synaptic thickenings of neurons (Alonso et al., 2000; Su et al., 2010). As described above, the σ1R is constitutively bound to BiP, and agonists dissociate σ1R from BiP allowing σ1R translocation within the cell. In addition to agonist activation, ER Ca2+ depletion has also been shown to dissociate the σ1R from BiP, suggesting a role of the σ1R as a sensor of ER Ca2+ levels (Hayashi and Su, 2007). A recent study showed that mutations in the σ1R gene linked to neurodegenerative diseases resulted in aberrant cellular localization of the σ1R, supporting the importance of proper σ1R localization in its physiological function (Wong et al., 2016).
While the precise localization of σ1Rs is poorly understood, multiple independent studies have demonstrated that σ1Rs exist within or adjacent to the plasma membrane. Subcellular fractionation in rat brain homogenates revealed (+)[3H]SKF-10,047 binding sites within non-synaptic plasma membrane fractions (Hayashi and Su, 2003a; McCann and Su, 1990). Treatment with selective σ1R ligands increased the detection of σ1Rs in the plasma membrane fraction using this method, supporting the hypothesis that σ1R activation promotes its translocation from the ER to other cellular compartments such as the membrane (Hayashi et al., 2000). A 2013 study by Kourrich et al. suggested that the σ1R is directly incorporated within the plasma membrane via an assay detecting C- and N- terminal tags on the σ1R putatively at the membrane (Kourrich et al., 2013). In contrast, Mavlyutov et al. used electron microscopy in motor neurons as well as ganglion cells to identify σ1R localization to subsurface cisternae of the ER near but not integral to the plasma membrane (Mavlyutov et al., 2015; Mavlyutov et al., 2010). These ER subsurface cisternae, also referred to as cortical ER, are areas of the ER that come in close contact with the plasma membrane and provide direct communication between ER and membrane proteins (Berridge, 1998; Kosaka, 1980; Rosenbluth, 1962; Spacek and Harris, 1997). Importantly, the σ1R has been shown to interact with and modulate the activity of various membrane proteins (Pabba, 2013; Rousseaux and Greene, 2015), supporting the notion that a population of σ1Rs are capable of translocating to the area at or near the plasma membrane where they can directly or indirectly modulate the activity of transmembrane proteins.
Cellular Functions
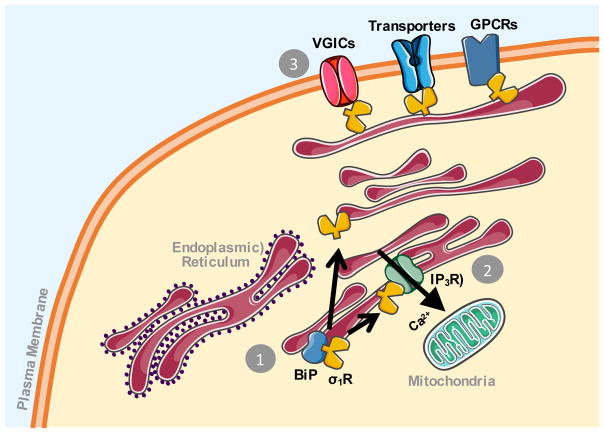
σRs regulate a variety of second messenger signaling pathways, including cyclic guanosine monophosphate (cGMP), inositol phosphates, kinases, and Ca2+ (Matsumoto et al., 2003). σ1Rs modulate these effects in a manner distinct from traditional ionotropic or metabotropic receptors by translocating between cellular compartments. Figure 2 broadly summarizes the existing theories for the cellular function of σ1R. Overall, the σ1R is considered pro-survival under cellular stress (Hayashi and Su, 2007; Pabba et al., 2014). As an ER chaperone protein, it attenuates protein misfolding and stabilizes other ER proteins (Hayashi and Su, 2007). σ1R interactions with the IP3R type 3 at the MAM have been well characterized and are hypothesized to regulate IP3R-mediated Ca2+ mobilization from the ER to the mitochondria. This in turn promotes cell survival by upregulating Ca2+-dependent enzymes involved in the tricarboxylic acid (TCA) cycle (Hajnoczky et al., 1995; Hayashi and Su, 2007). Thus, by promoting cellular metabolism, the σ1R is believed to promote cell survival.
Figure 2.
Schematic of sigma-1 receptor cellular activity. (1) The sigma-1 receptor (σ1R) is constitutively bound to another endoplasmic reticulum protein Binding Immunoglobulin Protein (BiP). Upon activation, the σ1R dissociates from BiP where it can then translocate to other cellular compartments. (2) The σ1R has been shown to stabilize IP3 type 3 receptors at the mitochondrial associated membrane and promote calcium (Ca2+) influx into the mitochondria. This promotes cellular metabolism and contributes to the pro-survival effects of the σ1R. (3) The σ1R has also been shown to associate with and regulate the activity of different membrane proteins, including voltage-gated ion channels (VGICs), transporter proteins, and G-protein coupled receptors (GPCRs). The ER, mitochondria, and protein images are courtesy of Servier Medical Art, licensed under CC BY 3.0.
Numerous reports indicate that the σ1R also regulates Ca2+ homeostasis outside of the MAM. A study utilizing different chemical classes of selective σ1R agonists, pregnenolone sulfate, (+)-pentazocine, and PRE-084, revealed no effect of these drugs alone but reported a potentiation of bradykinin-induced Ca2+ release from the ER. Interestingly, the different σ1R agonists differentially affected depolarization-induced Ca2+ increases, with PRE-084 potentiating the response and pregnenolone sulfate and (+)-pentazocine attenuating it (Hayashi et al., 2000). This study highlights a number of important concepts within σ1R biology, namely the modulatory nature of selective σ1R ligands (i.e. the lack of effect when administered in the absence of a stimulated response), the different modulatory responses of the different σ1R ligands despite all drugs used being classified as σ1R agonists, and the ability of σ1Rs to regulate both ER Ca2+ release as well as extracellular Ca2+ influx supporting diversity of cellular functions. The latter is supported by reports showing σ1R-regulation of L-type Ca2+ channel activity in hippocampal slices as well as retinal ganglion cells (Sabeti et al., 2007; Tchedre et al., 2008). Additionally, σ1R ligands were reported to differentially influence both NMDA receptor and nicotinic acetylcholine receptor mediated Ca2+ signaling (Hayashi et al., 1995; Paul et al., 1993). More recently, activation of the σ1R was shown to inhibit store operated Ca2+ entry (SOCE), a ubiquitously expressed pathway that facilitates Ca2+ influx into the cell in response to ER Ca2+ depletion (Srivats et al., 2016). This attenuation by the σ1R was proposed to prevent excess Ca2+ influx that may in turn lead to apoptosis. Overall, these studies suggest that the σ1R can influence multiple intracellular Ca2+ signaling pathways through a variety of mechanisms, potentially acting in parallel.
Protein Interactions
The σ1R associates with and regulates the activity of diverse classes of proteins both intracellularly and at the plasma membrane. This includes several different voltage-gated ion channels (VGICs) where interactions with these channels can have either inhibitory or enhancing effects on channel activity (Kourrich et al., 2012; Pabba, 2013). Some examples include σ1R associations with the Kv1.4 channel in posterior pituitary of rats (Aydar et al., 2002), L-type Ca2+ channels in retinal ganglion cells (Tchedre et al., 2008), Kv1.3 in a human embryonic kidney cell (HEK) line (Kinoshita et al., 2012), and Kv1.2 channels in medium spiny neurons of nucleus accumbens (Kourrich et al., 2013). Interactions between the σ1R and different ion channels, particularly K+ channels, support the hypothesis that the σ1R may act as a regulatory subunit for these channels (Aydar et al., 2002) and implicates the σ1R as an indirect regulatory of neuronal excitability.
In addition to VGICs, the σ1R has also been shown to interact with and modulate the activity of the NMDA receptor in the rat hippocampus (Balasuriya et al., 2013; Pabba et al., 2014) as well as the μ opioid receptor expressed in HEK cells (Kim et al., 2010). In addition to interactions with BiP, IP3R, and STIM1 described above, the σ1R forms a complex with other ER-localized proteins including ankyrin B. σ1R agonist treatment dissociated ankyrin from the IP3R, potentiating IP3-mediated intracellular Ca2+ signaling (Hayashi and Su, 2001). The σ1R also associates with one of the ER stress-sensing proteins involved in the unfolded protein response, inositol-requiring enzyme 1 (IRE1). Mori et al. found that like the σ1R, IRE1 is also enriched at the mitochondrial-associated membrane, and σ1R stabilizes IRE1 thereby prevent its degradation under cellular stress (Mori et al., 2013).
Relevant to its potential role in psychostimulant addition, the σ1R also associates with proteins directly involved in dopaminergic signaling. This includes the D1R in HEK cells co-expressing σ1R/D1R, where researchers reported that σ1R ligands attenuated cocaine-induced D1R-mediated increases in cAMP levels and ERK1/2 phosphorylation in cells as well as striatal tissue (Navarro et al., 2010). The same group later found that the σ1R interacts with the D2R, and cocaine binding to this complex inhibits downstream D2R-mediated signaling pathways (Navarro et al., 2013). More recently, the σ1R/DAT association was revealed in HEK cells co-expressing σ1R/DAT (Hong et al., 2017b). This association was shown to increase DA uptake at high concentration of σ1R agonist and enhanced cocaine binding to the transporter. In an independent study, our laboratory also confirmed that the σ1R associates with DAT at or near the plasma membrane and that this association is potentiated by combined treatment with a σ1R agonist and METH (Sambo et al., 2017). This study is further described in a later section of the review. Overall, σ1R interactions with D1R, D2R, and DAT provide further support for the role of σ1R in modulating the dopaminergic neurotransmission and the effects of psychostimulants and also provide potential molecular mechanisms by which the σ1R mediates its effects.
4. SIGMA-1 RECEPTOR AND THE DOPAMINERGIC SYSTEM
The role of σRs in dopaminergic neurotransmission has been of interest since early findings that antipsychotics and other drugs involved in dopaminergic signaling have affinity for σRs (Iyengar et al., 1990; Wachtel and White, 1988). σRs are expressed in dopaminergic regions including the striatum, substantia nigra (SN), and ventral tegmental area (VTA) (Gundlach et al., 1986; Hayashi et al., 2010; McLean and Weber, 1988). Using immunohistochemistry, σ1Rs were identified in dopaminergic neurons (Francardo et al., 2014). Despite several reports, well-defined functional implications of σRs in the regulation of dopamine neurotransmission remain unclear.
Effects on Dopamine Release
Reports on the effects of σRs on DA release have yielded varying results over the past several years. In 1990, Iyengar et al. first investigated the effect of σR ligands on DA release in the striatum and olfactory tubercles and found that local administration of the benzomorphan σR agonists (+)-pentazocine and (+)-SKF 10,047 increased the levels of DA metabolites dihydrophenylacetic acid (DOPAC) and homovanillic acid (HVA) with no change in DA steady state levels (Iyengar et al., 1990). Benzomorphans (+)-N-allylnormetazocine and (+)-pentazocine were later shown to increase the activity of tyrosine hydroxylase, the rate limiting enzyme for DA synthesis, in ex vivo rat striatal tissue (Booth and Baldessarini, 1991). While this implied a role for σRs in DA metabolism and synthesis, there was no distinction between the effect of σ1R and σ2R because the antagonist used to block these effects, BMY-14802, is not selective between the two subtypes. In 1993, Patrick et al. showed that systemic administration of σR agonists (+)-pentazocine and 1,3-Di-(2-tolyl)guanidine (DTG) increased striatal DA levels in freely moving rats. In this study, only higher doses of the σ1R selective ligand (+)-pentazocine had an effect whereas lower doses of the non-discriminant σ1/2R agonist DTG were sufficient to increase DA levels. This suggested that activation of the σ2R, rather than the σ1R, induced DA release in these rats (Patrick et al., 1993). This conclusion was further substantiated in later studies revealing systemic injection of only higher doses of (+)-pentazocine increased striatal DA levels (Gudelsky, 1995) and intra-nigral injections of σ1/2R agonist DTG increased extracellular levels of dopamine metabolites DOPAC and HVA in the striatum (Bastianetto et al., 1995). The effect of σR ligands on DA release was later shown to be biphasic in a study where intra-striatal administration of (+)-pentazocine, (−)-pentazocine, or DTG all initially increased DA levels followed by a prolonged decrease (Gudelsky, 1999). Supporting the inhibitory effect of σR on DA release, intra-striatal application of the σ1/2R agonist MR200 as well as DTG decreased DA levels in the striatum (Moison et al., 2003).
Later efforts to clarify the role of the σR subtype on DA release were made possible with the development of more selective σ1R ligands. In 1997, Kobayashi et al., showed that acute oral administration of the selective σ1R agonist SA4503 increased DA levels in the rat frontal cortex, but interestingly not the striatum or midbrain (Kobayashi et al., 1997). This was further supported by a more recent study showing a range of doses of SA4503 as well as the σ1R antagonists BD1047 and BD1063 failed to evoke [3H]DA overflow in preloaded rat striatal slices (Rodvelt et al., 2011a), however the frontal cortex was not examined in this report. In 2011, Garcés-Ramírez et al. recapitulated the dose-dependent effects of σR ligands demonstrating again that DTG increased dopamine levels in the nucleus accumbens at lower concentrations whereas the selective σ1R agonist PRE-084 only increased dopamine levels at higher doses. Furthermore, the PRE-084-mediated increase in dopamine levels was not blocked by σ1R antagonists BD1063, suggesting potential off-target effects PRE-084 at the dose used in this study (Garces-Ramirez et al., 2011). PRE-084 was later tested at 1, 3.2, and 10 mg/kg and shown to have no effect on extracellular DA levels in the nucleus accumbens shell in saline-treated animals as well as animals with a history of cocaine exposure (Hiranita et al., 2013a).
Taken together, these studies suggest that σ2R activation, but not σ1R activation, may regulate DA neurotransmission within the striatum. The potential role of σ1R in cortical DA neurotransmission was observed in at least one report, suggesting potential brain region differences in the actions of σRs. Furthermore, the time-dependency of these effects may also contribute to whether potentiation or inhibition of DA release is observed. The mechanisms by which the σRs promote dopamine release are currently unknown, although (+)-pentazocine-mediated dopamine release in the striatum was shown to occur in a DAT-independent manner (Gudelsky, 1995). Additionally, treatment with the σ1R agonist (+)-pentazocine increased tyrosine hydroxylase activity (Booth and Baldessarini, 1991) and the σ1R agonist SA4503 increased in L-DOPA levels in the presence of DOPA decarboxylase inhibitors (Kobayashi et al., 1997), suggesting a role of σRs in increasing DA synthesis. Currently, the molecular mechanisms by which σR ligands promote DA release or synthesis are unknown.
Electrophysiological Effects on Dopamine Neurons
In addition to dopamine release, the effects of σR ligands on the firing activity of dopaminergic neurons has also been examined. Dopaminergic neurons display spontaneous baseline firing activity (Grace and Bunney, 1984). Consistent with its ability to increase locomotor activity alone, the σ1R agonist (+)SKF-10,047 also increased the basal firing activity of VTA DA neurons which was blocked by the σ1R antagonist rimcazole (Ceci et al., 1988). Proposed as a potential antipsychotic drug, the σ1/2R antagonist BMY 14802 reversed the effect of apomorphine (a D2R agonist) on inhibiting the firing activity of SN and VTA dopaminergic neurons (Wachtel and White, 1988). In vivo extracellular recordings in the SN revealed intravenous administration of the σ1/2R agonist (+)-3-PPP decreased neuronal firing rate which was reversed by treatment with BMY 14802 (Steinfels and Tam, 1989). These early studies suggest a role for the σR in dopaminergic neuron firing activity or D2R-mediated inhibition of firing activity, however relatively non-specific ligands were utilized. In vivo recordings in the SN and VTA dopaminergic neurons of rats intravenously injected with the selective σ1R agonist SA4503 showed no change on the spontaneous firing activity but significantly decreased the number of spontaneously active neurons in the SN and increased the number of spontaneously active neurons in the VTA (Minabe et al., 1999). Further studies are required to more conclusively determine the role of the σ1R in the firing activity of dopamine neurons. Additionally, the mechanisms involved in this regulation are not characterized, but σ1R associations with different ion channels may serve as potential mechanisms that have yet to be investigated.
Role in Parkinson’s Disease
The established role of the σ1Rs in dopaminergic physiology has also led to the investigation of the therapeutic potential of the σ1R in Parkinson’s disease (PD). Human PET imaging revealed lower binding of a radiolabeled σ1R tracer ([11C]SA4503) on the side of the anterior putamen that exhibited lower radiolabeled DAT substrate binding (Mishina et al., 2005). While this meant that the σ1R could be a plausible marker of dopaminergic degeneration, there was no difference in the binding measured between PD patients and controls, ruling out any potential of σ1R imaging as a biomarker for early PD.
Studies in animal models reported paradoxical results in regard to the role of σ1R as a neuroprotective target. In 6-hydroxydopamine (6-OHDA) lesioned mice, five weeks of treatment with the σ1R agonist PRE-084 (0.3 mg/kg) produced a profound recovery of motor function as well as DA neuronal protection (Francardo et al., 2014). The treatment also increased levels of BDNF, GDNF, and pERK and decreased microglial activation. In an alternative model utilizing the neurotoxin MPTP, σ1R hetero- and homozygous knockout mice had reduced DA cell loss and recovery of motor function subsequent to MPTP injection (Hong et al., 2015). This effect was attributed to σ1R deficiency induced suppression of NMDA receptors. The observed neuroprotective σ1R knockout effect was recapitulated in wild-type mice using the σ1R antagonist NE-100. Further complicating these paradoxical findings, the σ1R knockout mouse was shown to undergo age-dependent dopaminergic neurodegeneration, suggesting that the σ1R knockout mouse may be suitable to serve as a unique animal model of PD (Hong et al., 2017a). The aged σ1R KO mice exhibited multiple pathological characteristics that mimic the hypothesized cascade leading to cell death in PD, and also exhibited measurable motor defects. Taken together, these studies underscore the complexity of the σ1R’s role in neuronal function and disease, yet still provide rationale for the therapeutic targeting of the σ1R in PD.
5. SIGMA-1 RECEPTOR AND METHAMPHETAMINE
Interest in the role of σRs in METH addiction stems from the early observation that the σR agonist (+)SKF-10,047 induces psychotomimetic effects in dogs (Martin et al., 1976). Additionally, the discovery that antipsychotics such as haloperidol have high affinity for the σ1R and the known parallels between schizophrenia and METH-induced psychosis further provoked the investigation of the potential role of σRs in psychostimulant addiction. At least one Japanese study found no link between known mutations in the σ1R gene and METH abuse, concluding that σ1Rs are unlikely to play a major role in the susceptibility for METH addiction (Inada et al., 2004). Although no genetic link was observed in humans, a number of studies have revealed restorative effects of σR ligands in animal models of METH addiction. It should be noted that the effects of σR ligands have also been widely studied in models of cocaine addiction. Although both METH and cocaine are widely abused psychostimulants, the different cellular mechanisms, pharmacokinetics, and potential long-term effects are worth considering these two drugs of abuse as distinct addiction targets. This is briefly discussed in the Conclusions section.
Regulation of Sigma-1 Receptor Expression
The upregulation of σ1R expression after METH administration was first demonstrated in 1993 in rats exposed to 4 mg/kg of METH for 10 days. Increased binding of [3H](+)-pentazocine was observed in the SN, frontal cortex, and cerebellum, suggesting a upregulation of σ1Rs in response to METH (Itzhak, 1993). In 2004, Stefanski et al. investigated the effect of both self-administration and passive administration of METH on σ1R levels in the brain (Stefanski et al., 2004). An increase in σ1R protein levels, as measured via Western blot, was found in the midbrain of rats self-administering METH 5-days-per-week for 5 weeks but not in rats that passively received the same amount of METH over the same period (Stefanski et al., 2004). In 2009, Hayashi et al. similarly investigated the effect of METH administration on σ1R levels, as well as other chaperone proteins, in the brain of rats either self-administered METH or passively received the drug. They found via Western blot that ER chaperone proteins σ1R, BiP, and calreticulin were all significantly elevated in the VTA and SN of rats under both treatment paradigms (Hayashi et al., 2010). This is consistent with studies reporting ER chaperone protein upregulation by METH due to ER stress (Jayanthi et al., 2004). The differences between the Stefanski study where σ1R levels were only upregulated in the midbrain of self-administering rats compared to the Hayashi study where the σ1R was upregulated in the VTA and SN of both rats self-administering and passively receiving METH was attributed to the detection of proteins in the VTA and SN as opposed to the entire midbrain. Because a global upregulation of σ1R levels in many different brain regions was not observed, this upregulation appears to selectively occur in dopaminergic structures. Currently, the functional consequence of the upregulation of σ1R levels in response to METH is unknown. σ1R upregulation may be a contributing factor to the development of METH addiction, or conversely could act as a compensatory mechanism to counteract the untoward effects of METH.
Interactions with the Sigma-1 Receptor
In addition to METH-induced increases in σ1R levels in the rodent VTA and SN, METH interacts with the σ1R at physiologically relevant concentrations. METH’s affinity for σRs was first demonstrated in 1993 in whole rat brain membrane preparations showing a concentration-dependent inhibition of [3H](+)-pentazocine binding by METH (Itzhak, 1993). In 2005, Nguyen et al. determined the binding affinity of METH for the σ1R and σ2R in rat brains labeled with [3H](+)-pentazocine for σ1R selectivity or [3H]DTG in the presence of (+)-pentazocine for σ2R selectivity. METH displayed over 20-fold selectivity for the σ1R over the σ2R (Ki 2.16 ± 0.25 μM vs. 46.67 ± 10.34 μM, respectively) (Nguyen et al., 2005). The consequences of METH binding to the σ1R are currently unknown; however, Hayashi et al. demonstrated that METH treatment increased the association between the σ1R and BiP suggesting METH may have antagonist activity (Hayashi and Su, 2007). A recent review by Yasui and Su posits that METH may act as an inverse agonist (Yasui and Su, 2016). In addition to METH, both cocaine and MDMA also have affinity for the σ1R (Brammer et al., 2006; Matsumoto et al., 2002; Sharkey et al., 1988).
Locomotor Activity
Before σRs were identified as a unique class of proteins in the brain, the “σ opioid receptor” agonist (+)SKF-10,047 was shown to induce psychotomimetic effects alone (Fujiwara et al., 1990; Martin et al., 1976). This finding, in addition to studies described above suggesting a role of σRs in the regulation of dopamine homeostasis, predicted early on a potential role of σRs in basal locomotion as well as METH-stimulated locomotor activity. Although (+)SKF-10,047, as well as pentazocine, promote hyperactivity, more selective σ1R ligands do not induce locomotion when administered alone. When investigating the effects of METH-induced (3 mg/kg) acute locomotor activity in σ1R knockout mice, no difference was observed between the knockout and wild-type mice (Fontanilla et al., 2009). The lack of effect of selective σ1R ligands alone as well as METH-stimulated locomotor activity being unaffected in σ1R knockout mice suggests that the σ1R is not required to regulate motor activity at baseline or in response to METH. In 1992, Ujike et al. showed that while the nonselective σ1/2R antagonist BMY 14802 had no effect on acute METH-induced (2 mg/kg) locomotor activity, it blocked locomotor sensitization after repeated METH exposure (Ujike et al., 1992a). Similarly, the selective σ1R ligand MS-377 also attenuated the development of METH-induced (2 mg/kg) locomotor sensitization; however, it was not distinguished whether MS-377 acted as a σ1R agonist or antagonist in this study (Takahashi et al., 2000). In the same report that determined the affinity of METH for the σRs, treatment with σ1R antagonists BD1063 and BD1047 as well as administration of an antisense oligodeoxynucleotide against the σ1R into the ventricles of rats attenuated acute METH-induced locomotor activity at METH doses between 0.1 and 3 mg/kg (Nguyen et al., 2005). The nonselective σ1/2R antagonist AC927 also decreased acute locomotor activity induced by 0.5 and 1 mg/kg METH in a separate study (Matsumoto et al., 2008). Both intraperitoneal injection and oral administration of the σ1/2R ligand AZ66, which putatively acts as an σ1/2R antagonist, dose-dependently decreased METH-induced (1 mg/kg) acute locomotor activity as well as METH-induced locomotor sensitization (Seminerio et al., 2012). It is currently unclear why some σR antagonists attenuate acute locomotor activity and some are only effective on locomotor sensitization; nevertheless, these findings suggest specific σR antagonists may have different effects.
In addition to σR antagonists and knockdown, the effect of σ1R agonists on METH-induced locomotion is investigated. Administration of the selective σ1R agonist SA4503 dose dependently affected METH-induced (0.5 mg/kg) locomotor activity with lower doses enhancing hyperactivity and higher doses inhibiting it (Rodvelt et al., 2011b). More recently, Miller et al., 2016 investigated the effect of different N-phenylpropyl-N′-substituted piperazine ligands, including SA4503, on METH-induced hyperactivity (Miller et al., 2016). All ligands tested had high affinity for the σ1R and no appreciable affinity for DAT. They found that higher doses of the compounds attenuated METH-induced (0.5 mg/kg) locomotor activity, whereas lower doses potentiated it (Miller et al., 2016). Utilizing a different selective σ1R agonist, our laboratory recently revealed that 8 mg/kg PRE-084 attenuates acute METH-stimulated (2 mg/kg) locomotor activity. Interestingly, 30 mg/kg of the σ1R antagonist BD1063, but not 10 mg/kg, also significantly reduced METH-induced locomotor activity. When analyzing locomotion during the pretreatment period, we found that 30 mg/kg BD1063 alone decreased locomotion, an effect that appears to be acute as no difference was detected after a subsequent one hour of saline treatment. This effect of 30 mg/kg of BD1063 on locomotor activity alone suggests this dose of the antagonist may have some sedative effects (Sambo et al., 2017). Collectively, these reports indicate that the type and dose of σ1R ligand used may present varying results but support the potential ability of σ1R ligands to reduce the hyperactive effects of METH, both acutely and after sensitization. A comprehensive study using multiple σR ligands at different doses would potentially provide a clearer picture of how σR ligands influence METH-stimulated locomotion.
Stereotypic Behavior
Another behavioral consequence of METH exposure is the induction of repetitive and compulsive behaviors called stereotypies (Canales and Graybiel, 2000). In rodents, this includes repetitive grooming, sniffing, biting, licking, head-bobbing, and circling (Kitanaka et al., 2009). An early study by Ujike et al. showed that treatment with 3 mg/kg of the σ1/2R agonist (+)-3-PPP acutely decreased rearing, locomotion, sniffing, and head movement but potentiated these behaviors when higher doses were utilized. In rats that received repeated 4 mg/kg METH treatment followed by 5 to 8 days of abstinence, (+)-3-PPP enhanced sensitization to locomotor behavior but decreased sensitization to rearing, sniffing, and head movement (Ujike et al., 1992b). Similar to this study, the σ1/2R antagonist BMY 14802 also had no effect on acute stereotypic behavior but decreased stereotypy sensitization (Akiyama et al., 1994). While MS-377 did not affect acute METH-induced stereotypic behavior in rats, pretreatment significantly attenuated the behavioral sensitization of stereotypic behavior in rats receiving METH for 10 days (Takahashi et al., 2000). Investigating the effect of different σR ligands with dual affinity as well as selective affinity for σ1R and σ2R, Kitanaka et al. found that different σR ligands differentially affected the pattern and the type of stereotypies expressed but produced no change in the overall frequency of METH-induced stereotypy behavior (Kitanaka et al., 2009). While many of these studies only reported effects on stereotypy sensitization and not acute stereotypic behavior, several of the METH locomotor studies showed acute effects of σ1R ligands, this suggesting the potential involvement of different mechanisms across different METH-induced behaviors.
Reinforcing Behavior
In addition to the acute behavioral effects of psychomotor activation and stereotypy behavior, the role of σ1R in animal models of addictive-like behavior has also been investigated. While the σ1R agonist SA4503 did not substitute for METH at any dose tested, pretreatment with SA4503 augmented drug discrimination for METH such that lower dose of METH elicited responding compared to animals pretreated with saline (Rodvelt et al., 2011b). In contrast, in 2013 Rahmadi et al. found that the antidepressant and σ1R agonist fluoxetine decreases the rewarding effects of METH as measured by conditioned place preference (CPP). The effect of fluoxetine was blocked by the σ1R antagonist NE-100, suggesting the role of σ1Rs in attenuating METH-induced reinforcing behaviors (Rahmadi et al., 2013). Similar to this study, in 2014 Mori et al. reported that the σ1R agonist SA4503 but not (+)-pentazocine reduced CPP to morphine, cocaine, and METH (Mori et al., 2014). This study supports the ability of the σ1R agonist SA4503 to attenuate the rewarding effects of drugs of abuse in general and highlights the phenomenon that not all σ1R agonists produce the same outcomes. Similar to SA4503, the σ1R agonist PRE-084 also reduced the acquisition of METH-induced CPP in rodents (Sambo et al., 2017). Considering fluoxetine, SA4503, and PRE-084 are all more selective for the σ1R compared to the σ2R and no studies have shown effects of σ2R ligands, this suggests that agonist activity at the σ1R is effective for reducing METH-induced CPP. The effect of pretreatment with σ1R ligands on METH self-administration has not been reported, although Hiranita et al. showed that rats first trained to self-administer METH, but not heroin or ketamine, subsequently self-administered the σ1R agonists PRE-084 and (+)-pentazocine. The effect of PRE-084 was blocked by treatment with σ1R antagonist BD1008 but not the dopamine receptor antagonist (+)-butaclamol or opioid antagonist (−)-naltrexone (Hiranita et al., 2013b), suggesting that neither dopaminergic nor opioid mechanisms were involved. A recent report from our laboratory showed that PRE-084 treatment reduced the ability of METH to potentiate brain reward function as measured by intracranial self-stimulation (ICSS) (Sambo et al., 2017). It was previously shown that like other drugs of abuse, METH reduces the stimulation threshold for ICSS in rats (Harris et al., 2015). Our study showed that PRE-084 pretreatment decreased the ability of METH to reduce the ICSS threshold, with no effect of PRE-084 by itself (Sambo et al., 2017). Although reinforcing behavior has been less examined compared to locomotor activity, few studies similarly support a lack of effect of σ1R ligands alone, with no reports showing aversive or rewarding effects of the σ1R drugs. The effect of σ1R antagonist on reinforcing behaviors seems to be under-investigated. Furthermore, the effect of σ1R on more protracted addictive processes such as drug extinction and reinstatement, to our knowledge, has not been examined.
Toxicity
An important consequence of METH use is resultant dopaminergic neurodegeneration that is at least in part due to direct neurotoxic actions of METH on DA neurons (Bowyer et al., 1994; Broening et al., 1997; Hotchkiss and Gibb, 1980). Hyperthermia and excitotoxic NMDA receptor activation have been identified as critical mechanistic components of METH-induced neurotoxicity (Bowyer et al., 1994; Sonsalla et al., 1989). As described above, the METH-mediated dopaminergic insult is hypothesized to increase the risk for PD in individuals with a history of METH, but not cocaine, abuse (Callaghan et al., 2012).
σ1Rs have been investigated extensively in the context of METH-induced DA neurotoxicity due to their described neuroprotective potential and ability to modulate METH’s behavioral responses (Nguyen et al., 2005). One of the first compounds examined for neuroprotection against METH-induced neurotoxicity in vitro and in vivo was the σ1/2R antagonist AC927 (Matsumoto et al., 2008). Pretreatment with AC927 prior to METH (1 mg/kg) exposure not only blocked the METH-induced increases in locomotor activity but similarly blocked METH-induced (5 mg/kg) decreases in DA levels and dopaminergic immunoreactivity as well as METH-induced hyperthermia (Matsumoto et al., 2008). These results were replicated in further studies, and the protective effects of AC927 were shown to extend to the serotonergic system (Seminerio et al., 2011). Similarly, the high-affinity σ1/2R antagonist CM156 was also shown to be protective against METH toxicity (Kaushal et al., 2011; Kaushal et al., 2013). Additional mechanistic insight revealed that inhibition of σ1R with BD1047 prevented NMDA receptor induced neurotoxicity in the hippocampi of mice injected with METH (Smith et al., 2010). Furthermore, AC927 pretreatment attenuated the generation of reactive oxygen species triggered by METH in differentiated NG108-15 cells (Seminerio et al., 2011). The mechanisms by which σRs are neuroprotective specifically in the dopaminergic or serotonergic systems requires further investigation, but taken together, the ability of σ1Rs to attenuate METH-induced hyperthermia, NMDA receptor activation in the hippocampus, and reactive oxygen species generation in vitro constitute the therapeutic potential of σ1Rs against METH-induced neurotoxicity in DA neurons.
Ligands with Dual Affinity for the Sigma-1 Receptor and DAT
Several studies have investigated the role of non-selective σR binding drugs in the rewarding effects of psychostimulants. An early study using rimacozole, a benztropine compound that acts as an atypical DAT blocker with σR antagonist activity, decreased (+)SKF-10,047-induced hyperactivity but had no effect on AMPH-induced hyperactivity (Ceci et al., 1988). Similarly, a variety of benztropines as well as the combined treatment with the DA-uptake inhibitor WIN35,428 and the σR antagonist BD1008 attenuated METH self-administration with no effect on self-administration of heroin or ketamine (Hiranita et al., 2014). These studies suggest that σR antagonists capable of DAT blockade may be a viable target for METH addiction, and further support the idea that σRs may be involved in the clinical efficacy of commonly prescribed drugs with dual affinity for σRs and other targets (such as haloperidol and fluoxetine).
Potential Mechanisms
To date, there is no clear mechanistic evidence as to how the σ1R modulates METH-mediated behavioral responses, although σ1R-regulation of the dopaminergic system remains a potential candidate. In addition to investigating the effects of σ1R on basal dopamine release, σ1R ligands have also been examined for their effects on METH-stimulated dopamine release. A 2011 study by Rodvelt et al. revealed that while the σ1R antagonists BD1047 and BD1063 did not alter METH-induced [3H]DA overflow in preloaded rat striatal slices, higher doses of the σ1R agonist SA4503 attenuated evoked [3H]DA release by METH but not nicotine (Rodvelt et al., 2011b). Recently, our laboratory similarly revealed both in vitro in DAT-expressing HEK cells as well in vivo in the mouse striatum using amperometry that the σ1R agonist PRE-084 also attenuated METH-stimulated dopamine release, while the σ1R antagonist BD1063 had no effect (Sambo et al., 2017). In both studies, neither the σ1R agonists nor antagonists affected dopamine release at baseline, consistent with reports described above that ligands selective for the σ1R, but not the σ2R, do not influence dopamine release alone.
The molecular mechanisms by which σ1R agonists attenuate METH-mediated dopamine neurotransmission is poorly understood. σ1R interactions with the D2R and DAT regulating the actions of these proteins could in turn regulate METH-stimulated dopamine release. Although the σ1R/D2R interaction has not been investigated in the context of METH, our laboratory showed that METH treatment alone had no effect on the interaction between σ1R and DAT as measured by Foster Resonance Energy Transfer; however, METH exposure after treatment with the σ1R agonist PRE-084 potentiated the σ1R/DAT interaction. This suggests that σ1R agonism alone does not affect its interaction with DAT but the presence of METH produces the cellular environment for the σ1R to then association with DAT. In this study, we also revealed that σ1R activation by the σ1R agonist PRE-084 decreased the METH-stimulated increase in intracellular calcium, which is a crucial cellular event for METH-stimulated dopamine efflux (Sambo et al., 2017). Interactions with DAT or regulation of intracellular calcium represent potential mechanisms by which the σ1R may reduce METH-stimulated dopamine release, which would in turn reduce METH-mediated behavioral responses. Further investigation is required to properly link these effects.
6. DISCUSSION
Overall, while the studies described in this review implicate the σ1R in the effects of METH, results are varied regarding whether the σ1R plays a positive or negative role in the regulation of METH-mediated responses. This variability across the field may be due to several factors. By virtue of having a wide variety of structurally and functionally different ligands targeting σRs as well as the ongoing development of novel σR ligands that have not been widely characterized across different laboratories, comparing studies using different σR drugs may not allow for accurate conclusions. Furthermore, the bioavailability and pharmacokinetics of many of the selective σ1R ligands used have yet to be determined, further complicating the interpretation of studies regarding whether the doses or incubation times utilized are physiologically relevant to effect σ1Rs. As such, it is difficult, if not impossible, to compare doses used in vitro to those used in vivo without knowing if the drug effectively reaches the brain at concentrations high enough to activate σ1Rs, making in vitro mechanistic studies difficult to compare to functions measured in in vivo studies. As σ1R agonism is canonically defined as the dissociation of σ1R from BiP, studies showing whether σ1R ligands can dissociate σ1R from BiP in vivo would better allow researchers to understand the nature of these ligands and whether the doses used reach physiological levels that replicate in vitro cellular findings.
Importantly, the focus of this review was to summarize studies regarding the effects of σ1R ligands on METH addiction, although σ1R ligands have been investigated against other drugs of abuse. In particular, several studies have examined the effects of different σ1R ligands in different models of cocaine addiction. It should be noted that while METH and cocaine are both psychostimulants with similar behavioral profiles, as a DAT substrate and not a DAT blocker, METH acts via different cellular mechanisms including promoting DAT-mediated reverse transport of DA, increasing the firing activity of DA neurons, stimulating DAT internalization, and increasing intracellular calcium levels, all of which are blocked by cocaine. These molecular differences should be considered as these different mechanisms may require different pharmacological treatment strategies. For example, it was recently shown that a single infusion of AMPH reversed cocaine-induced deficits in DAT function (Ferris et al., 2015). This highlights the importance in considering these classes of drugs separately as AMPHs can potentially attenuate the effects of cocaine, and vice versa. Although studies show similar effectiveness for some σR ligands across different drugs of abuse (Hiranita et al., 2013b; Mori et al., 2014), it is important to not necessarily generalize the effectiveness of σR drugs, or other drugs for that matter, across different addictive drugs.
Although the actions of σ1R-targeting ligands remain complex, the potential therapeutic potential for σ1R drugs is promising. One attractive feature of selective σ1R ligands is the lack of baseline activity, suggesting minimal off-target effects. This may seem contradictory given the wide distribution of σ1Rs as well as the large number of proteins and pathways it has been shown to modulate, but importantly the σ1R seems to selectively elicit responses under stimulated or pathological conditions. This, in theory, would suggest that in cells at physiological homeostasis, ligand activation or inhibition of σ1Rs would not provoke a response. Along these lines, many selective σ1R ligands also do not show rewarding effects on their own, which is an important feature when considering pharmacotherapies for the treatment of drug abuse. Several clinically used drugs have appreciable affinity for the σ1R, including the antipsychotic haloperidol and the antidepressants fluoxetine and sertraline, supporting the efficacy of σ1R-targeting drugs for the treatment of psychiatric disorders. Selective σ1R ligands have been investigated in clinical trials for the treatment of neuropathic pain (Abadias et al., 2013; Collina et al., 2013), neurodegenerative disease (Collina et al., 2013), anxiety (Moller et al., 2001), depression (Moller et al., 2003; Volz et al., 2000), and schizophrenia (Frieboes et al., 1999; Frieboes et al., 1997; Gewirtz et al., 1994; Huber et al., 1999; Niitsu et al., 2012). Although these studies overall indicate the safety and tolerability of the drugs tested, the efficacy of these drugs remains inconclusive. To our knowledge, clinical trials investigating the effect of σ1R ligands for psychostimulant abuse are not reported; however, the efficacy of different σ1R drugs in reducing the effects of METH in different preclinical models of METH addiction and reported safety of σ1R ligands in humans support further investigation of the σ1R as a target for METH abuse.
ABBREVIATIONS
- AC927
1-(2-phenylethyl)piperidine
- AMPH
amphetamine
- ATS
amphetamine-type stimulants
- AZ66
3-(4-(4-cyclohexylpiperazin-1-yl)pentyl)-6-flourobenzo(Abadias et al., 2013)thiazol-2(3H)-one
- BD1008
N-(Brammer et al., 2006)-N-methyl-1-pyrrolidineethanamine
- BD1047
N-(Brammer et al., 2006)-N-methyl-2-(dimethylamino)ethylamine
- BD1063
1-(Jones et al., 1998)-4-methylpiperazine
- BiP
binding immunoglobulin protein
- BMY 14802
alpha-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazine-butanol
- CM156
3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione
- CPP
conditioned place preference
- DA
dopamine
- DAT
dopamine transporter
- DMT
N,N-dimethyltryptamine
- DTG
1,3-di-O-tolylguanidine
- MDMA
Methylenedioxy-methamphetamine
- METH
methamphetamine
- MS-377
(R)-(+)-1-(4-chlorophenyl)-3-[4-(2-methoxyethyl)piperazin-1-yl]me thyl 2- pyrrolidinone L-tartrate
- NE-100
4-methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine
- NMDA
N-methyl-D-aspartate
- PCP
phencyclidine
- PD
Parkinson’s disease
- PKC
protein kinase C
- PRE-084
2-(4-Morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride
- SA4503
1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride
- SKF-10,047
N-allylnormetazocine ((−)-ANMC)
- SN
substantia nigra
- VMAT2
vesicular monoamine transporter-2
- VTA
ventral tegmental area
- σ1R
sigma-1 receptor
- σ2R
sigma-2 receptor
Footnotes
7. CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadias M, Escriche M, Vaque A, Sust M, Encina G. Safety, tolerability and pharmacokinetics of single and multiple doses of a novel sigma-1 receptor antagonist in three randomized phase I studies. Br J Clin Pharmacol. 2013;75:103–117. doi: 10.1111/j.1365-2125.2012.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr Res. 1994;12:251–257. doi: 10.1016/0920-9964(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Kruse AC. Identification of the gene that codes for the sigma2 receptor. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Azzaro AJ, Rutledge CO. Selectivity of release of norepinephrine, dopamine and 5-hydroxytryptamine by amphetamine in various regions of rat brain. Biochem Pharmacol. 1973;22:2801–2813. doi: 10.1016/0006-2952(73)90147-0. [DOI] [PubMed] [Google Scholar]
- Balasuriya D, Stewart AP, Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci. 2013;33:18219–18224. doi: 10.1523/JNEUROSCI.3360-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz F, Kern L, Erz D, Zhu M, Gilbert D, Meinhof T, Wirkner U, Erfle H, Muckenthaler M, Pepperkok R, Runz H. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab. 2009;10:63–75. doi: 10.1016/j.cmet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Rouquier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between sigma sites and nigro-striatal dopaminergic pathways. Neuropharmacology. 1995;34:281–287. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Booth RG, Baldessarini RJ. (+)-6,7-benzomorphan sigma ligands stimulate dopamine synthesis in rat corpus striatum tissue. Brain Res. 1991;557:349–352. doi: 10.1016/0006-8993(91)90159-s. [DOI] [PubMed] [Google Scholar]
- Bowen WD. Sigma receptors: recent advances and new clinical potentials. Pharm Acta Helv. 2000;74:211–218. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Bowen WDdCBR, Hellewell SB, Michael Walker, Rice KC. [3H]-(+)-Pentazocine: A potent and highly selective benzomorphan-based probe for sigma-1 receptors. Molecular Neuropharmacology. 1993;3:117–126. [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T, Katz JL, George C, Newman AH. Dual probes for the dopamine transporter and sigma1 receptors: novel piperazinyl alkyl-bis(4′-fluorophenyl)amine analogues as potential cocaine-abuse therapeutic agents. J Med Chem. 2003;46:2589–2598. doi: 10.1021/jm030008u. [DOI] [PubMed] [Google Scholar]
- Ceci A, Smith M, French ED. Activation of the A10 mesolimbic system by the sigma-receptor agonist (+)SKF 10,047 can be blocked by rimcazole, a novel putative antipsychotic. Eur J Pharmacol. 1988;154:53–57. doi: 10.1016/0014-2999(88)90362-7. [DOI] [PubMed] [Google Scholar]
- Chaki S, Tanaka M, Muramatsu M, Otomo S. NE-100, a novel potent sigma ligand, preferentially binds to sigma 1 binding sites in guinea pig brain. Eur J Pharmacol. 1994;251:R1–2. doi: 10.1016/0014-2999(94)90453-7. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67:100–109. doi: 10.1016/j.brainresbull.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomchai C, Chomchai S. Global patterns of methamphetamine use. Curr Opin Psychiatry. 2015;28:269–274. doi: 10.1097/YCO.0000000000000168. [DOI] [PubMed] [Google Scholar]
- Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38:41–58. v–vi. doi: 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collina S, Gaggeri R, Marra A, Bassi A, Negrinotti S, Negri F, Rossi D. Sigma receptor modulators: a patent review. Expert Opin Ther Pat. 2013;23:597–613. doi: 10.1517/13543776.2013.769522. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend. 2015;146:30–38. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Effect of haloperidol on (+)-amphetamine self-administration. J Pharm Pharmacol. 1975;27:540–542. doi: 10.1111/j.2042-7158.1975.tb09502.x. [DOI] [PubMed] [Google Scholar]
- de Costa BR, Bowen WD, Hellewell SB, Walker JM, Thurkauf A, Jacobson AE, Rice KC. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–58. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ, Blakely RD. Pore models for transporters? Biophys J. 1996;70:579–580. doi: 10.1016/S0006-3495(96)79604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbez AE, Mody RM, Werling LL. Sigma(2)-receptor regulation of dopamine transporter via activation of protein kinase C. J Pharmacol Exp Ther. 2002;301:306–314. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosso ME, Palumbo ML, Genaro AM. Immunomodulatory effects of fluoxetine: A new potential pharmacological action for a classic antidepressant drug? Pharmacol Res. 2016;109:101–107. doi: 10.1016/j.phrs.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. A Single Amphetamine Infusion Reverses Deficits in Dopamine Nerve-Terminal Function Caused by a History of Cocaine Self-Administration. Neuropsychopharmacology. 2015;40:1826–1836. doi: 10.1038/npp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Gibb JW, Hanson GR. A rapid and reversible change in dopamine transporters induced by methamphetamine. Eur J Pharmacol. 1997;323:R9–10. doi: 10.1016/s0014-2999(97)00148-9. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- Frieboes RM, Murck H, Antonijevic I, Kraus T, Hinze-Selch D, Pollmacher T, Steiger A. Characterization of the sigma ligand panamesine, a potential antipsychotic, by immune response in patients with schizophrenia and by sleep-EEG changes in normal controls. Psychopharmacology (Berl) 1999;141:107–110. doi: 10.1007/s002130050813. [DOI] [PubMed] [Google Scholar]
- Frieboes RM, Murck H, Wiedemann K, Holsboer F, Steiger A. Open clinical trial on the sigma ligand panamesine in patients with schizophrenia. Psychopharmacology (Berl) 1997;132:82–88. doi: 10.1007/s002130050323. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Kuribara H, Tadokoro S. Effects of repeated administration of pentazocine on ambulatory activity in mice: comparison with the effects of morphine and methamphetamine. Jpn J Pharmacol. 1990;54:61–67. doi: 10.1254/jjp.54.61. [DOI] [PubMed] [Google Scholar]
- Garces-Ramirez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, Tanda G. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz GR, Gorman JM, Volavka J, Macaluso J, Gribkoff G, Taylor DP, Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT. Protein kinase C and dopamine transport--2. Effects of amphetamine in vitro. Neuropharmacology. 1992;31:1211–1222. doi: 10.1016/0028-3908(92)90049-u. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, Galli A. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA. Effects of sigma receptor ligands on the extracellular concentration of dopamine in the striatum and prefrontal cortex of the rat. Eur J Pharmacol. 1995;286:223–228. doi: 10.1016/0014-2999(95)00415-8. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Biphasic effect of sigma receptor ligands on the extracellular concentration of dopamine in the striatum of the rat. J Neural Transm (Vienna) 1999;106:849–856. doi: 10.1007/s007020050205. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Is methamphetamine abuse a risk factor in parkinsonism? Neurotoxicology. 2001;22:725–731. doi: 10.1016/s0161-813x(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunne LM, Anggard E, Jonsson LE. Clinical trials with amphetamine-blocking drugs. Psychiatr Neurol Neurochir. 1972;75:225–226. [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014;97:37–44. doi: 10.1016/j.lfs.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, LeSage MG, Shelley D, Perry JL, Pentel PR, Owens SM. The anti-(+)-methamphetamine monoclonal antibody mAb7F9 attenuates acute (+)-methamphetamine effects on intracranial self-stimulation in rats. PLoS One. 2015;10:e0118787. doi: 10.1371/journal.pone.0118787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Reus VI, Wolkowitz OM, Mendelson JE, Jones RT. Altering cortisol level does not change the pleasurable effects of methamphetamine in humans. Neuropsychopharmacology. 2003;28:1677–1684. doi: 10.1038/sj.npp.1300223. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Sigma-1 receptor: The novel intracellular target of neuropsychotherapeutic drugs. J Pharmacol Sci. 2015;127:2–5. doi: 10.1016/j.jphs.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kagaya A, Takebayashi M, Shimizu M, Uchitomi Y, Motohashi N, Yamawaki S. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-D-aspartate in primary culture of rat frontal cortical neurons. J Pharmacol Exp Ther. 1995;275:207–214. [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003a;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003b;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL. Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2014;348:174–191. doi: 10.1124/jpet.113.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, Tanda G, Katz JL. Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology. 2013a;38:605–615. doi: 10.1038/npp.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. J Pharmacol Exp Ther. 2013b;347:20–29. doi: 10.1124/jpet.113.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Sha S, Zhou L, Wang C, Yin J, Chen L. Sigma-1 receptor deficiency reduces MPTP-induced parkinsonism and death of dopaminergic neurons. Cell Death Dis. 2015;6:e1832. doi: 10.1038/cddis.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Wang L, Zhang T, Zhang B, Chen L. Sigma-1 receptor knockout increases alpha-synuclein aggregation and phosphorylation with loss of dopaminergic neurons in substantia nigra. Neurobiol Aging. 2017a;59:171–183. doi: 10.1016/j.neurobiolaging.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Hong WC, Yano H, Hiranita T, Chin FT, McCurdy CR, Su TP, Amara SG, Katz JL. The sigma-1 receptor modulates dopamine transporter conformation and cocaine binding and may thereby potentiate cocaine self-administration in rats. J Biol Chem. 2017b;292:11250–11261. doi: 10.1074/jbc.M116.774075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi: 10.3389/fnhum.2014.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MT, Gotthardt U, Schreiber W, Krieg JC. Efficacy and safety of the sigma receptor ligand EMD 57445 (panamesine) in patients with schizophrenia: an open clinical trial. Pharmacopsychiatry. 1999;32:68–72. doi: 10.1055/s-2007-979194. [DOI] [PubMed] [Google Scholar]
- Husbands SM, Izenwasser S, Kopajtic T, Bowen WD, Vilner BJ, Katz JL, Newman AH. Structure-activity relationships at the monoamine transporters and sigma receptors for a novel series of 9-[3-(cis-3, 5-dimethyl-1-piperazinyl)propyl]carbazole (rimcazole) analogues. J Med Chem. 1999;42:4446–4455. doi: 10.1021/jm9902943. [DOI] [PubMed] [Google Scholar]
- Inada T, Iijima Y, Uchida N, Maeda T, Iwashita S, Ozaki N, Harano M, Komiyama T, Yamada M, Sekine Y, Iyo M, Sora I, Ujikec H. No association found between the type 1 sigma receptor gene polymorphisms and methamphetamine abuse in the Japanese population: a collaborative study by the Japanese Genetics Initiative for Drug Abuse. Ann N Y Acad Sci. 2004;1025:27–33. doi: 10.1196/annals.1316.003. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Repeated methamphetamine-treatment alters brain sigma receptors. European journal of pharmacology. 1993;230:243–244. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Dilworth VM, Mick SJ, Contreras PC, Monahan JB, Rao TS, Wood PL. Sigma receptors modulate both A9 and A10 dopaminergic neurons in the rat brain: functional interaction with NMDA receptors. Brain Res. 1990;524:322–326. doi: 10.1016/0006-8993(90)90709-k. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Newman AH, Katz JL. Cocaine and several sigma receptor ligands inhibit dopamine uptake in rat caudate-putamen. Eur J Pharmacol. 1993;243:201–205. doi: 10.1016/0014-2999(93)90381-q. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones DN, Holtzman SG. Influence of naloxone upon motor activity induced by psychomotor stimulant drugs. Psychopharmacology (Berl) 1994;114:215–224. doi: 10.1007/BF02244839. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson LE, Anggard E, Gunne LM. Blockade of intravenous amphetamine euphoria in man. Clin Pharmacol Ther. 1971;12:889–896. doi: 10.1002/cpt1971126889. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. British journal of clinical pharmacology. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Hiranita T, Kopajtic TA, Rice KC, Mesangeau C, Narayanan S, Abdelazeem AH, McCurdy CR. Blockade of Cocaine or sigma Receptor Agonist Self Administration by Subtype-Selective sigma Receptor Antagonists. J Pharmacol Exp Ther. 2016;358:109–124. doi: 10.1124/jpet.116.232728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Robson MJ, Vinnakota H, Narayanan S, Avery BA, McCurdy CR, Matsumoto RR. Synthesis and pharmacological evaluation of 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79), a cocaine antagonist, in rodents. AAPS J. 2011;13:336–346. doi: 10.1208/s12248-011-9274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR. Pharmacological evaluation of SN79, a sigma (sigma) receptor ligand, against methamphetamine-induced neurotoxicity in vivo. Eur Neuropsychopharmacol. 2013;23:960–971. doi: 10.1016/j.euroneuro.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278:12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien CC, Pasternak GW. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsuoka Y, Suzuki T, Mirrielees J, Yang J. Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res. 2012;1452:1–9. doi: 10.1016/j.brainres.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Boileau I, Callaghan RC, Tong J. Brain dopamine neurone ‘damage’: methamphetamine users vs. Parkinson’s disease - a critical assessment of the evidence. Eur J Neurosci. 2017;45:58–66. doi: 10.1111/ejn.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Hall FS, Uhl GR, Tanaka K, Nishiyama N, Morita Y, Takemura M. Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology (Berl) 2009;203:781–792. doi: 10.1007/s00213-008-1425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsuno K, Murai M, Mita S. Sigma 1 receptor subtype is involved in the facilitation of cortical dopaminergic transmission in the rat brain. Neurochem Res. 1997;22:1105–1109. doi: 10.1023/a:1027361101419. [DOI] [PubMed] [Google Scholar]
- Kosaka T. The axon initial segment as a synaptic site: ultrastructure and synaptology of the initial segment of the pyramidal cell in the rat hippocampus (CA3 region) Journal of neurocytology. 1980;9:861–882. doi: 10.1007/BF01205024. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labit-Le Bouteiller C, Jamme MF, David M, Silve S, Lanau C, Dhers C, Picard C, Rahier A, Taton M, Loison G, Caput D, Ferrara P, Lupker J. Antiproliferative effects of SR31747A in animal cell lines are mediated by inhibition of cholesterol biosynthesis at the sterol isomerase step. Eur J Biochem. 1998;256:342–349. doi: 10.1046/j.1432-1327.1998.2560342.x. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Mager S, Quick MW, Corey JL. Permeation properties of neurotransmitter transporters. Annu Rev Pharmacol Toxicol. 1994;34:219–249. doi: 10.1146/annurev.pa.34.040194.001251. [DOI] [PubMed] [Google Scholar]
- Lever JR, Fergason-Cantrell EA, Watkinson LD, Carmack TL, Lord SA, Xu R, Miller DK, Lever SZ. Cocaine occupancy of sigma1 receptors and dopamine transporters in mice. Synapse. 2016;70:98–111. doi: 10.1002/syn.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Lever JR, Miller DK, Fergason-Cantrell EA, Green CL, Watkinson LD, Carmack TL, Lever SZ. Relationship between cerebral sigma-1 receptor occupancy and attenuation of cocaine’s motor stimulatory effects in mice by PD144418. J Pharmacol Exp Ther. 2014;351:153–163. doi: 10.1124/jpet.114.216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Sambo D, Khoshbouei H. Methamphetamine Regulation of Firing Activity of Dopamine Neurons. J Neurosci. 2016;36:10376–10391. doi: 10.1523/JNEUROSCI.1392-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fu Y, Yang H, Mavlyutov T, Li J, McCurdy CR, Guo LW, Pattnaik BR. Potential independent action of sigma receptor ligands through inhibition of the Kv2.1 channel. Oncotarget. 2017;8:59345–59358. doi: 10.18632/oncotarget.19581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: Evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S. Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur J Pharmacol. 1996;306:271–279. doi: 10.1016/0014-2999(96)00201-4. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to sigma 1 receptors in the mouse forebrain. J Neurosci Res. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein M, Guo LW. Subcellular localization of the sigma-1 receptor in retinal neurons - an electron microscopy study. Sci Rep. 2015;5:10689. doi: 10.1038/srep10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JC, Brecht ML. Methamphetamine: here we go again? Addict Behav. 2011;36:1168–1173. doi: 10.1016/j.addbeh.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Su TP. Haloperidol-sensitive (+)[3H]SKF-10,047 binding sites (sigma sites) exhibit a unique distribution in rat brain subcellular fractions. Eur J Pharmacol. 1990;188:211–218. doi: 10.1016/0922-4106(90)90004-h. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Weber E. Autoradiographic visualization of haloperidol-sensitive sigma receptors in guinea-pig brain. Neuroscience. 1988;25:259–269. doi: 10.1016/0306-4522(88)90024-3. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Sigma1 receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Park ES, Lever SZ, Lever JR. N-phenylpropyl-N′-substituted piperazines occupy sigma receptors and alter methamphetamine-induced hyperactivity in mice. Pharmacol Biochem Behav. 2016;150–151:198–206. doi: 10.1016/j.pbb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minabe Y, Matsuno K, Ashby CR., Jr Acute and chronic administration of the selective sigma1 receptor agonist SA4503 significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 1999;33:129–140. doi: 10.1002/(SICI)1098-2396(199908)33:2<129::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, Oda K, Sasaki T, Sakayori O, Hamamoto M, Kobayashi S, Katayama Y. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol Scand. 2005;112:103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- Moison D, De Deurwaerdere P, Cagnotto A, Marrazzo A, Prezzavento O, Ronsisvalle G, Mennini T, Spampinato U. Intrastriatal administration of sigma ligands inhibits basal dopamine release in vivo. Neuropharmacology. 2003;45:945–953. doi: 10.1016/s0028-3908(03)00253-3. [DOI] [PubMed] [Google Scholar]
- Moller HJ, Volz HP, Reimann IW, Stoll KD. Opipramol for the treatment of generalized anxiety disorder: a placebo-controlled trial including an alprazolam-treated group. J Clin Psychopharmacol. 2001;21:59–65. doi: 10.1097/00004714-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Moller HJ, Volz HP, Stoll KD. Psychopharmacotherapy of somatoform disorders: effects of opipramol on symptoms of somatization, anxiety, and depression. Acta Neuropsychiatr. 2003;15:217–226. doi: 10.1034/j.1601-5215.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Hayashi T, Hayashi E, Su TP. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8:e76941. doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Rahmadi M, Yoshizawa K, Itoh T, Shibasaki M, Suzuki T. Inhibitory effects of SA4503 on the rewarding effects of abused drugs. Addict Biol. 2014;19:362–369. doi: 10.1111/j.1369-1600.2012.00488.x. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H. Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev. 2013;37:2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortes A, Casado V, Canela EI, Ortiz J, Fuxe K, Lluis C, Ferre S, Franco R. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farre D, Aguinaga D, Mallol J, Cortes A, Casado V, Lluis C, Ferre S, Franco R, Canela E, McCormick PJ. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8:e61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Niitsu T, Fujisaki M, Shiina A, Yoshida T, Hasegawa T, Kanahara N, Hashimoto T, Shiraishi T, Fukami G, Nakazato M, Shirayama Y, Hashimoto K, Iyo M. A randomized, double-blind, placebo-controlled trial of fluvoxamine in patients with schizophrenia: a preliminary study. J Clin Psychopharmacol. 2012;32:593–601. doi: 10.1097/JCP.0b013e3182664cfc. [DOI] [PubMed] [Google Scholar]
- Pabba M. The essential roles of protein-protein interaction in sigma-1 receptor functions. Front Cell Neurosci. 2013;7:50. doi: 10.3389/fncel.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabba M, Wong AY, Ahlskog N, Hristova E, Biscaro D, Nassrallah W, Ngsee JK, Snyder M, Beique JC, Bergeron R. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci. 2014;34:11325–11338. doi: 10.1523/JNEUROSCI.0458-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Patrick SL, Walker JM, Perkel JM, Lockwood M, Patrick RL. Increases in rat striatal extracellular dopamine and vacuous chewing produced by two sigma receptor ligands. Eur J Pharmacol. 1993;231:243–249. doi: 10.1016/0014-2999(93)90456-r. [DOI] [PubMed] [Google Scholar]
- Paul IA, Basile AS, Rojas E, Youdim MB, De Costa B, Skolnick P, Pollard HB, Kuijpers GA. Sigma receptors modulate nicotinic receptor function in adrenal chromaffin cells. FASEB J. 1993;7:1171–1178. doi: 10.1096/fasebj.7.12.8375616. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Rahmadi M, Mori T, Kanazawa M, Kubota H, Shibasaki M, Suzuki T. Involvement of sigma 1 receptor in the SSRI-induced suppression of the methamphetamine-induced behavioral sensitization and rewarding effects in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 2013;33:49–56. [PubMed] [Google Scholar]
- Rawson RA, Condon TP. Why do we need an Addiction supplement focused on methamphetamine? Addiction. 2007;102(Suppl 1):1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, Swant J, Zou MF, Newman AH, Khoshbouei H. Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat Commun. 2016;7:10423. doi: 10.1038/ncomms10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvelt KR, Lever SZ, Lever JR, Blount LR, Fan KH, Miller DK. SA 4503 attenuates cocaine-induced hyperactivity and enhances methamphetamine substitution for a cocaine discriminative stimulus. Pharmacol Biochem Behav. 2011a;97:676–682. doi: 10.1016/j.pbb.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvelt KR, Oelrichs CE, Blount LR, Fan KH, Lever SZ, Lever JR, Miller DK. The sigma receptor agonist SA4503 both attenuates and enhances the effects of methamphetamine. Drug Alcohol Depend. 2011b;116:203–210. doi: 10.1016/j.drugalcdep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. The Journal of cell biology. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux CG, Greene SF. Sigma receptors [sigmaRs]: biology in normal and diseased states. J Recept Signal Transduct Res. 2015:1–62. doi: 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Nelson TE, Purdy RH, Gruol DL. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. doi: 10.1002/hipo.20273. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrany ST, Brimson JM. Are fluoxetine’s effects due to sigma-1 receptor agonism? Pharmacol Res. 2016;113:707–708. doi: 10.1016/j.phrs.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Saha K, Sambo D, Richardson BD, Lin LM, Butler B, Villarroel L, Khoshbouei H. Intracellular methamphetamine prevents the dopamine-induced enhancement of neuronal firing. J Biol Chem. 2014;289:22246–22257. doi: 10.1074/jbc.M114.563056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn JJ, Mejia GL, Ray PR, Martin SF, Price TJ. Sigma 2 receptor/Tmem97 agonists produce long lasting anti-neuropathic pain effects in mice. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.7b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca SA, Sorrentino EE, Nosanchuk JD, Martinez LR. Impact of methamphetamine on infection and immunity. Front Neurosci. 2014;8:445. doi: 10.3389/fnins.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo DO, Lin M, Owens A, Lebowitz JJ, Richardson B, Jagnarine DA, Shetty M, Rodriquez M, Alonge T, Ali M, Katz J, Yan L, Febo M, Henry LK, Bruijnzeel AW, Daws L, Khoshbouei H. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat Commun. 2017;8:2228. doi: 10.1038/s41467-017-02087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration (SAMHSA) U.S. Department of Health and Human Services; Rockville, MD: 2006. National Survey on Drug Use. [Google Scholar]
- Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, Kruse AC. Crystal structure of the human sigma1 receptor. Nature. 2016;532:527–530. doi: 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider FH. Amphetamine-induced exocytosis of catecholamines from the cow adrenal medulla. J Pharmacol Exp Ther. 1972;183:80–89. [PubMed] [Google Scholar]
- Seminerio MJ, Kaushal N, Shaikh J, Huber JD, Coop A, Matsumoto RR. Sigma (sigma) receptor ligand, AC927 (N-phenethylpiperidine oxalate), attenuates methamphetamine-induced hyperthermia and serotonin damage in mice. Pharmacol Biochem Behav. 2011;98:12–20. doi: 10.1016/j.pbb.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminerio MJ, Robson MJ, Abdelazeem AH, Mesangeau C, Jamalapuram S, Avery BA, McCurdy CR, Matsumoto RR. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14:43–51. doi: 10.1208/s12248-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Shearer J. Psychosocial approaches to psychostimulant dependence: a systematic review. J Subst Abuse Treat. 2007;32:41–52. doi: 10.1016/j.jsat.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Shen X, Purser C, Tien LT, Chiu CT, Paul IA, Baker R, Loh HH, Ho IK, Ma T. mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010;88:2294–2302. doi: 10.1002/jnr.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Butler TR, Prendergast MA. Inhibition of sigma-1 receptor reduces N-methyl-D-aspartate induced neuronal injury in methamphetamine-exposed and -naive hippocampi. Neurosci Lett. 2010;481:144–148. doi: 10.1016/j.neulet.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivats S, Balasuriya D, Pasche M, Vistal G, Edwardson JM, Taylor CW, Murrell-Lagnado RD. Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J Cell Biol. 2016;213:65–79. doi: 10.1083/jcb.201506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl) 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Tam SW. Selective sigma receptor agonist and antagonist affect dopamine neuronal activity. Eur J Pharmacol. 1989;163:167–170. doi: 10.1016/0014-2999(89)90413-5. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988a;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Su TP, Schell SE, Ford-Rice FY, London ED. Correlation of inhibitory potencies of putative antagonists for sigma receptors in brain and spleen. Eur J Pharmacol. 1988b;148:467–470. doi: 10.1016/0014-2999(88)90130-6. [DOI] [PubMed] [Google Scholar]
- Su TP, Wu XZ, Cone EJ, Shukla K, Gund TM, Dodge AL, Parish DW. Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. J Pharmacol Exp Ther. 1991;259:543–550. [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sonehara K, Takagi K, Miwa T, Horikomi K, Mita N, Nagase H, Iizuka K, Sakai K. Pharmacological profile of MS-377, a novel antipsychotic agent with selective affinity for sigma receptors. Psychopharmacology (Berl) 1999;145:295–302. doi: 10.1007/s002130051061. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9:184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (sigma) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci. 1992a;50:PL129–134. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- Ujike H, Okumura K, Zushi Y, Akiyama K, Otsuki S. Persistent supersensitivity of sigma receptors develops during repeated methamphetamine treatment. Eur J Pharmacol. 1992b;211:323–328. doi: 10.1016/0014-2999(92)90388-k. [DOI] [PubMed] [Google Scholar]
- UNODC. United Nations Office on Drug and Crime (UNODC) United Nations; New York: 2016. World Drug Report. [Google Scholar]
- Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur J Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon. 2012;58:38–89. doi: 10.1016/j.disamonth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- Volz HP, Moller HJ, Reimann I, Stoll KD. Opipramol for the treatment of somatoform disorders results from a placebo-controlled trial. Eur Neuropsychopharmacol. 2000;10:211–217. doi: 10.1016/s0924-977x(00)00074-2. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, White FJ. Electrophysiological effects of BMY 14802, a new potential antipsychotic drug, on midbrain dopamine neurons in the rat: acute and chronic studies. J Pharmacol Exp Ther. 1988;244:410–416. [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Patrick SL, Williams WE, Mascarella SW, Bai X, Carroll FI. A comparison of (−)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of sigma 2 receptors in motor function. Eur J Pharmacol. 1993;231:61–68. doi: 10.1016/0014-2999(93)90684-a. [DOI] [PubMed] [Google Scholar]
- Weigl M, Bedurftig S, Maier CA, Wunsch B. Conformationally constrained ethylenediamines: synthesis and receptor binding of 6,8-diazabicyclo[3.2.2]nonanes. Bioorg Med Chem. 2002;10:2245–2257. doi: 10.1016/s0968-0896(02)00043-3. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309:283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–255. [PubMed] [Google Scholar]
- Wong AY, Hristova E, Ahlskog N, Tasse LA, Ngsee JK, Chudalayandi P, Bergeron R. Aberrant Subcellular Dynamics of Sigma-1 Receptor Mutants Underlying Neuromuscular Diseases. Mol Pharmacol. 2016;90:238–253. doi: 10.1124/mol.116.104018. [DOI] [PubMed] [Google Scholar]
- Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, Matsumoto RR. A novel substituted piperazine, CM156, attenuates the stimulant and toxic effects of cocaine in mice. J Pharmacol Exp Ther. 2010;333:491–500. doi: 10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Su TP. Potential Molecular Mechanisms on the Role of the Sigma-1 Receptor in the Action of Cocaine and Methamphetamine. J Drug Alcohol Res. 2016:5. doi: 10.4303/jdar/235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]