Abstract
Past research has found menstrual-cycle-related changes in functional immune response; we examined if sexual activity also changed markers of immune defense. We followed 32 naturally cycling women (15 sexually active with a partner ≥ 1 time/week, 17 sexually abstinent for the last four months) over one menstrual cycle. Participants provided serum and saliva samples at menses and ovulation, and additional saliva samples at midfollicular and midluteal phases. At each phase, participants also self-reported symptoms associated with colds, flu, pain, menstrual discomfort, and premenstrual syndrome. We tested saliva and serum for ability to kill Escherichia coli or Candida albicans, and serum for complement protein activity. For serum-mediated pathogen killing, among sexually active women only, there was a significant midcycle decrease in killing of E. coli. For saliva-mediated pathogen killing, among abstinent women only, there was a significant midcycle decrease in killing of E. coli, and midcycle increase in killing of C. albicans. Sexually active women had significantly lower complement activity than abstinent women overall. Finally, both groups reported lower physical symptoms at midcycle and higher symptoms at menses. There may be important differences in immune function between healthy women who are sexually active versus abstinent. Further replication is warranted.
Although it is accepted that sexual behavior can impact health by increasing exposure to sexually transmitted infections (STIs), the potential impact of sexual activity on other aspects of health is less well known. Several recent studies have suggested that sexual activity may moderate immune responses in healthy individuals (Aronoff et al., 2014; Charnetski & Brennan, 2004; Fortenberry et al., 2014; Lorenz & van Anders, 2014). These effects appear to be particularly relevant in women’s immune responses, as there are trade-offs between immunity and reproduction: Although the female immune system must defend against pathogens, it cannot risk accidentally attacking or rejecting sperm or conceptus. Sexual activity may thus serve as a trigger to modulate immune response, promoting defense during nonfertile times but allowing a more permissive environment around ovulation, during maximal fertility. Although beneficial for conception, this “window of opportunity” may increase infection risk (Wira & Fahey, 2008). Indeed, several studies have suggested that healthy sexually active women show significant cycle-related variation in immune responses while sexually abstinent women do not (Brown, Morrison, Calibuso, & Christiansen, 2008; Lorenz, Demas, & Heiman, 2017; Lorenz, Heiman, & Demas, 2015a, 2015b, 2017); these findings have been replicated in White and Latino samples (Lorenz, Worthman, & Vitzthum, 2015).
However, most of these studies have relied on markers of immune response such as cytokines or antibody levels. Enumerative markers such as these count the number of immune actors present at a given time and offer clues as to how the immune system communicates, when it is stimulated or inhibited, and what sorts of immune actors are mobilized for different kinds of challenges. In sum, enumerative markers are excellent sources of information on mechanisms of immune response. However, such markers are limited as indicators of immune function—that is, the actual observed capacity to carry out the core functions of the immune system, namely tissue repair and defense against pathogens. Immune markers such as cytokines or antibodies reflect how many or what kinds of players are on the field but not how well the team is working together, or if they are winning (for reviews of the various methods associated with enumerative versus functional assay of immunity, see Demas & Carlton, 2015; Vedhara, Wang, Fox, & Irwin, 2013).
How does the effect of sexual activity on immune markers translate to changes in the ability of the immune system to defend against pathogens? To date, there has been only one such study, which examined the effects of sexual activity on the antiviral activity on vaginal secretions from healthy women. This study found that relative to vaginal secretions collected following nonsexual activities (such as exercise), postintercourse vaginal secretions were significantly less effective at inhibiting viral infection (Aronoff et al., 2014; Fortenberry et al., 2014). However, this study examined only sexually active women and did not report on effects in nonvaginal immune response.
The present study added to this literature by investigating cycle-related changes in functional immunity in sexually active versus abstinent women; specifically, we assessed the ability to kill pathogens, activation of complement proteins (the ability to break open foreign cells), and self-reported symptoms associated with immune function. In our pathogen-killing assays, we assessed response to a common bacterial pathogen (Escherichia coli) as well as a fungus responsible for vaginal yeast infections (Candida albicans).
Method
These data are drawn from a larger study of menstrual-cycle-related changes in health-related variables (Lorenz, Demas, & Heiman, 2017; Lorenz, Heiman, & Demas, 2015a, 2015b).
Participants
Participants were recruited from the community via printed flyers and postings on online classifieds sites such as Craigslist and community boards, as well as from the student participant pool in the Department of Psychology at Indiana University. This study was exploratory in nature, and there were limited data on which to base an estimated effect size for a priori power analyses; nevertheless, we estimated that we would need at least 16 participants per group to have ~80% power to detect a medium-sized repeated-measures effect. A total of 135 potential participants contacted the lab; of these, 74 were unable to be reached after their initial interest e-mail, 19 were screened but did not qualify (taking hormonal contraceptives, n = 8; history of health conditions, n = 2; sexually active less than once a week but not sexually abstinent, n = 3; other, n = 6) and seven were scheduled but did not come to their first session.
We enrolled 35 healthy women who reported very regular cycles (every 26 to 34 days with no more than three days variance between cycles), not taking any medications on a regular basis (including hormonal contraceptives), and no history of health conditions, pregnancy, lactation, or STI in the past year. Three women dropped out after their first lab session, leaving a final N = 32; of these, 17 were sexually abstinent (no partnered genital sexual contact in the previous two months) and 15 were sexually active (intercourse with one and only one male partner at least once a week). All sexually active women reported using either a nonhormonal intrauterine device (IUD) or condoms.
The period of abstinence from intercourse (two months prior to the study) that defined the abstinent group was based on the perspective that the immune system generally creates its products (antibodies, cytokines, acute-phase proteins) in response to cues from the environment (internal or external)—that is, it constantly assesses and reassesses the need for new products and acts accordingly. The longest-lasting immune factor (an antibody termed immunoglobulin G) has an active viability of approximately six to eight weeks (Brekke & Sandlie, 2003). If there is an environmental or behavioral factor that alters the immune system’s production of antibodies (or other immune factors), we should expect to see differences in immune function about two months after the removal of this factor.
Prior research on the effects of different sexual activities on immune function is limited, but one study (Brown et al., 2008) suggested that women who engaged in any partnered sexual activity had different levels of antibody production than women who had no partnered sexual activity. We defined “sex” as “any genital contact with a partner, either theirs or yours,” thus including manual stimulation, oral sex, and penetrative sex. However, due to concerns that heterosexual and nonheterosexual women may differ significantly in other potentially important immune factors (such as lifetime exposure to STIs), we restricted the analyses to heterosexual women only for this first exploratory study. Neither abstinent nor sexually active women were excluded on the basis of masturbation, and most of the participants reported a history of masturbation (see Table 1); however, we did not collect data on actual frequency of masturbation during the study period and were thus unable to conclude any specific effect.
Table 1.
Demographics.
| Sexually Active (N = 15)
|
Sexually Abstinent (N = 17)
|
Total (N = 32)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | N | % | M | SD | N | % | M | SD | N | % | |
| Age | 24.96 | 7.05 | 22.16 | 2.85 | 23.56 | 5.54 | ||||||
| Years of education | 15.96 | 4.12 | 15.53 | 2.42 | 15.73 | 3.24 | ||||||
| Body fat percentage | 27.69 | 5.65 | 26.02 | 8.67 | 26.88 | 7.21 | ||||||
| Sexual orientationa | 1.86 | 0.86 | 1.53 | 1.23 | 1.68 | 1.08 | ||||||
| Age of menarche | 12.29 | 1.38 | 12.51 | 1.38 | 12.41 | 1.49 | ||||||
| Age of first vaginal intercourseb | 17.64 | 1.98 | 19.00 | 2.58 | 17.94 | 2.13 | ||||||
| Race | ||||||||||||
| White | 12 | 80 | 9 | 53 | 21 | 66 | ||||||
| Asian | 0 | 0 | 5 | 29 | 5 | 16 | ||||||
| Mixed race/other | 3 | 20 | 3 | 18 | 6 | 18 | ||||||
| Ethnicity | ||||||||||||
| Hispanic/Latina | 1 | 7 | 0 | 0 | 1 | 3 | ||||||
| Not Hispanic/Latina | 14 | 93 | 17 | 100 | 31 | 97 | ||||||
| Sexual history | ||||||||||||
| Ever masturbated | 13 | 87 | 13 | 76 | 26 | 81 | ||||||
| Ever performed oral sex | 13 | 87 | 5 | 29 | 18 | 56 | ||||||
| Ever received oral sex | 13 | 87 | 7 | 41 | 20 | 62 | ||||||
| Ever had vaginal intercourse | 15 | 100 | 4 | 24 | 19 | 59 | ||||||
| Ever had anal sex | 7 | 47 | 2 | 12 | 9 | 28 | ||||||
| Relationship status | ||||||||||||
| Married/long-term relationship | 7 | 47 | 0 | 0 | 6 | 19 | ||||||
| Dating | 8 | 53 | 3 | 18 | 12 | 38 | ||||||
| Single | 0 | 0 | 14 | 82 | 14 | 44 | ||||||
Sexual orientation was assessed on a continuous scale (0–7), with 0 Exclusively heterosexual and 7 Exclusively homosexual.
Calculated only for participants indicating lifetime history of intercourse.
There were no significant group differences in any demographic variable (Table 1); however, currently sexually active participants were significantly more sexually experienced (more likely to have engaged in oral, vaginal, and/or anal sex at some point in their lifetimes) than women who were abstinent. All participants provided informed consent. Participants received either $30 or four hours of credit toward their course requirements for completing the study. All procedures were approved by the Indiana University Institutional Review Board (IRB).
Salivary and Serum Sampling
The participants came to the lab within the first two days of menstrual bleeding and again at ovulation. Date of ovulation was confirmed via urine test for luteinizing hormone (LH) (OneStep Urine Ovulation Test, BlueCross Biomedical, Beijing, China). We calculated an expected date of ovulation using backward counting from participants typical cycle length and onset of their last period (van Anders, Goldey, & Bell, 2014). Participants were given a pack of test strips during the first lab session to test for an LH surge in the four days prior to and after their expected date of ovulation. They were instructed to contact the lab as soon as they saw a positive test strip; all participants had their second lab session within 48 hours of a positive test for the ovulation-associated LH surge. On average, ovulation occurred on forward cycle day 14.50 (SD = 1.92); however, it should be noted that these participants were selected on the basis of very regular cycling patterns and thus the variance in time to ovulation was artificially low.
During lab sessions, participants were measured for body composition with a bioimpedance scale (FitScale 585F, Tanita Corporation, Illinois, USA). They also provided samples of whole blood (using standard venipuncture procedures) and saliva, which were frozen immediately after collection. Participants also provided saliva samples from their follicular phase (7 to 10 days following the onset of menses) and luteal phase (7 to 10 days following ovulation), which they collected at home. Self-collected samples were kept in participants’ home freezer until transported to the lab in Styrofoam containers lined with deep-freeze packs. Samples were stored at −80°C until analysis, and no sample underwent more than two freeze-thaw cycles.
Assays
Pathogen killing was determined with a validated ex vivo bactericidal assay, modified for use with human serum and saliva (French & Neuman-Lee, 2012; Muehlenbein, Prall, & Chester, 2011). In short, each sample was incubated overnight on an agar plate with a standard concentration of the pathogen (Escherichia coli ATCC #8739 or Candida albicans ATCC #10231). The colonies formed on sample plates were then compared to plates in which there was unimpeded growth, creating a relative measure of the percent killing. Higher percent killing indicates the immune cells in the sample were more successful in impeding the growth of the pathogen, and this is thought to reflect greater resistance to infection. Complement activity of serum was assessed via validated CH50 assay (Costabile, 2010), which assesses the capacity of complement proteins to lyse a standardized foreign cell (sheep red blood cells). Higher lysis is thought to reflect the functional capacity of the innate immune system to destroy foreign cells (Demas, Zysling, Beechler, Muehlenbein, & French, 2011).
Self-Reported Symptoms
Once per phase (approximately once per week, corresponding to menstrual, follicular, ovulation, and luteal phases), participants completed an online survey. This survey assessed the degree to which participants had been bothered by commonly experienced physical symptoms in the previous week. Symptoms were chosen due to their relevance to acute immune response (e.g., fever), as well as inflammation-mediated symptoms associated with menstrual cycling (e.g., diarrhea, bloating; Bertone-Johnson et al., 2014); see Table 2 for a complete list). Scores ranged from 0 (Not at all) to 3 (Nearly every day). Items that were endorsed in < 5% of surveys (e.g., “cold sore”) were dropped from further analysis. Principal components and reliability analyses in this sample supported a five-factor solution (all eigenvalues > 1, explaining 59.8% of scale variance), composed of premenstrual symptoms (fluid retention, bloating, constipation, vaginal irritation, and diarrhea; Cronbach’s α = 0.73); menstrual symptoms (tender breasts, acne, abdominal cramps, and dizziness; Cronbach’s α = 0.58); pain (joint pain, muscle aches, backache; Cronbach’s α = 0.67), cold/allergy symptoms (coughing, sneezing, runny nose; Cronbach’s α = 0.66), and flu symptoms (fever, headache, fatigue, nausea, and skin rash; Cronbach’s α = 0.71). We averaged the constituent items to create subscale scores.
Table 2.
Self-report symptom measure.
| Over the last week, how often have you been bothered by the following problems? | Not at all | Several days | More than half the days | Nearly every day |
|---|---|---|---|---|
| Headache | □ | □ | □ | □ |
| Dizziness | □ | □ | □ | □ |
| Muscle aches | □ | □ | □ | □ |
| Skin rash | □ | □ | □ | □ |
| Cold sore | □ | □ | □ | □ |
| Coughing | □ | □ | □ | □ |
| Sneezing | □ | □ | □ | □ |
| Runny nose | □ | □ | □ | □ |
| Fever/chills | □ | □ | □ | □ |
| Painful urination | □ | □ | □ | □ |
| Vaginal irritation/itching | □ | □ | □ | □ |
| Backache | □ | □ | □ | □ |
| Abdominal cramps | □ | □ | □ | □ |
| Physical fatigue | □ | □ | □ | □ |
| Abdominal bloating | □ | □ | □ | □ |
| Breast tenderness/soreness | □ | □ | □ | □ |
| Acne flare-up | □ | □ | □ | □ |
| Constipation | □ | □ | □ | □ |
| Diarrhea | □ | □ | □ | □ |
| Fluid retention (“water weight”) | □ | □ | □ | □ |
| Nausea | □ | □ | □ | □ |
| Joint pain | □ | □ | □ | □ |
Analytic Plan
We conducted repeated-measures mixed general linear models with cycle phase, group (sexually active versus abstinent), and their interaction, and subject-level intercepts to control for individual differences at baseline. We also controlled for age and percent body fat because these are strongly linked to immune function (Nieman et al., 1995). For measures in which we had > 2 time points (salivary pathogen killing; self-reported symptoms), we additionally conducted post hoc tests of the hypothesized midcycle decreases using polynomial contrast tests (as specified at https://ibm.co/2wTsceb). That is, we conducted tests of how well the repeated-measures effects can be modeled as a one-bended (quadratic) curve with a nadir at midcycle (Cnaan, Laird, & Slasor, 1997).
Results
Serum-Mediated Microbiocidal Activity
There was a significant midcycle decrease in serum-mediated killing of E. coli among sexually active women (t (14) = 2.65, p = 0.02, d = 0.96) but not abstinent women (t (16) = 0.11, p = 0.91, d = 0.02), reflecting a significant group-by-time interaction (F (1, 30.52) = 4.63, p = 0.04, R2 = 0.13; Figure 1). For serum-mediated killing of C. albicans, although directions of change were opposite between groups, within-group change was not significant (sexually active: t (14) = 0.75, p = 0.46, d = 0.13; abstinent: t (16) = 1.10, p = 0.30, d = 0.25); the group-by-time interaction trended toward significance (F (1, 25.83) = 2.49, p = 0.13, R2 = 0.06).
Figure 1.
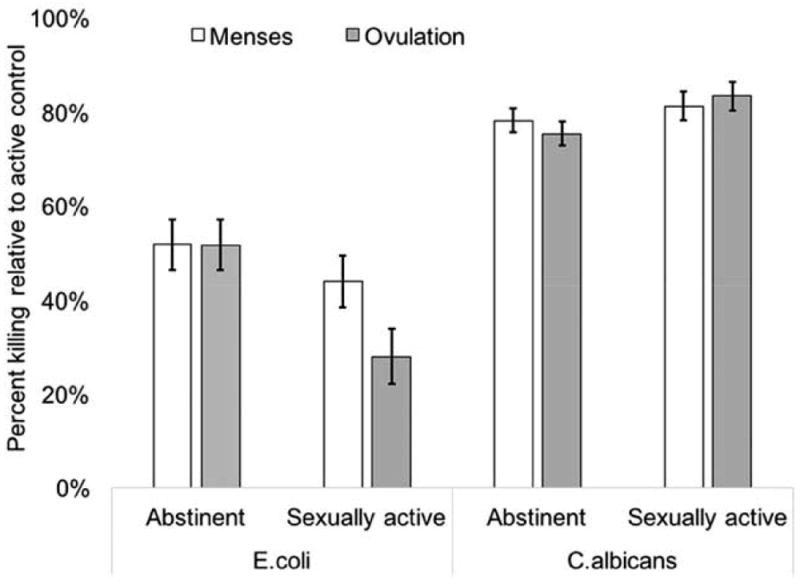
Estimated marginal means of model predicting cycle-related changes in serum-mediated pathogen killing in sexually active versus abstinent women. Error bars indicate ±1 standard error of the mean. Model controlled for age and body fat percentage. Although presented on the same axis, results of the pathogen-killing assays are relative to active control for that species; that is, percent killing of E. coli and C. albicans cannot be compared except within assay (that is, over time or between groups, within species).
Saliva-Mediated Microbiocidal Activity
Among abstinent women, the saliva-mediated killing of E. coli followed a quadratic pattern, with a significant midcycle decrease followed by a luteal-phase increase (t (75.44) = 2.37, p = 0.02, d = 0.51). The pattern of change among the sexually active women did not fit a quadratic pattern (t (80.41) = −0.49, p = 0.62, d = 0.11). The group-by-time interaction for saliva-mediated killing of E. coli was significant (F (3, 30.77) = 3.30, p = 0.03, R2 = 0.10; Figure 2).
Figure 2.
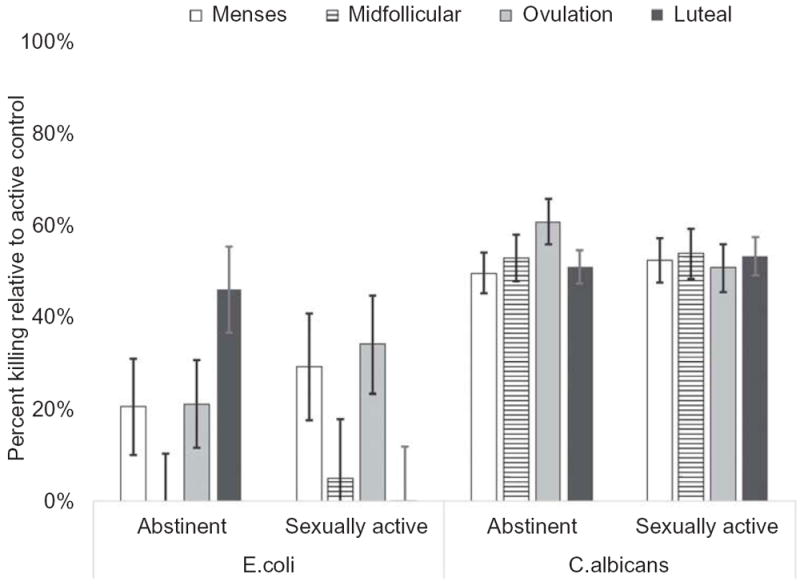
Estimated marginal means of model predicting cycle-related changes in saliva-mediated pathogen killing in sexually active versus abstinent women. Error bars indicate ±1 standard error of the mean. Model controlled for age and body fat percentage. Although presented on the same axis, results of the pathogen-killing assays are relative to active control for that species; that is, percent killing of E. coli and C. albicans cannot be compared except within assay (over time or between groups but within species).
For saliva-mediated killing of C. albicans, the data from abstinent women followed a quadratic pattern; however, this was due to a significant midcycle increase/luteal-phase decline (t (54.88) = −2.37, p = 0.02, d = 0.55). Among sexually active women, there was no significant change over time (t (60.17) = 0.18, p = 0.86, d = 0.13). The group-by-time interaction of saliva-mediated killing of C. albicans trended toward significance (F (1, 34.07) = 2.04, p = 0.13, R2 = 0.06).
Complement Protein Activity
There was a significant effect of group in activation of hemolytic complement (F (1, 33.88) = 4.92, p = 0.03, R2 = 0.14), with abstinent women showing higher activation (M = 176.66, SE = 103.57) than sexually active women (M = 16.49, SE = 68.63). The effect of time and the interaction between group and time were nonsignificant (Figure 3).
Figure 3.
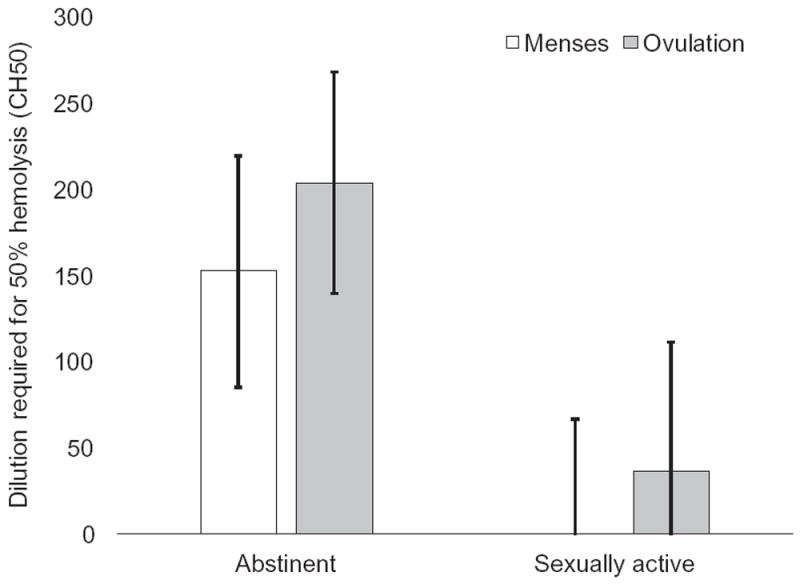
Estimated marginal means of model predicting cycle-related changes in complement protein activity in sexually active versus abstinent women. Error bars indicate ±1 standard error of the mean. Model controlled for age and body fat percentage.
Self-Reported Symptoms
The effect of group and the interaction of group and time were nonsignificant across all symptoms. Participants reported significantly lower flu symptoms at ovulation (F (3, 28.68) = 3.30, p = 0.04, R2 = 0.10) and lower pain in the luteal phase (F (3, 24.92) = 3.82, p = 0.02, R2 = 0.11, Figure 4). Participants reported significantly higher premenstrual and menstrual symptoms at menses (F (3, 27.04) = 3.36, p = 0.03, R2 = 0.10; and F (3, 29.49) = 9.83, p < 0.001, R2 = 0.25, respectively). There was no significant change in cold/allergy symptoms (F (3, 25.31) = 0.18, p = 0.91, R2 = 0.01).
Figure 4.
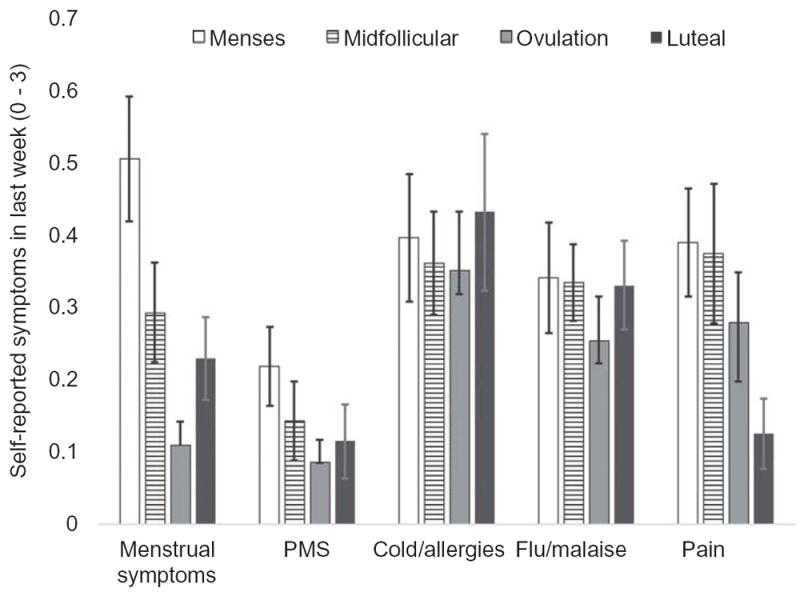
Estimated marginal means of model predicting cycle-related changes in self-reported physical symptoms, considering across groups. Error bars indicate ±1 standard error of the mean. Model controlled for age and body fat percentage.
Discussion
Although there is much interest in determining the health implications of sexual activity, remarkably little is known (Diamond & Huebner, 2012). We examined menstrual-cycle-related change in markers of immune function in healthy women who were sexually active versus abstinent. There were significant changes across the cycle, potentially reflecting differential capacity to defend against common pathogens. However, there was no consistent pattern across all markers of immune function in either group, suggesting that sexual activity does not have either a universally positive or negative effect on women’s immunity.
There were subtle signs of shifts in defenses against bacteria versus fungi: when defense against E. coli decreased, it was accompanied by a corresponding increase in defense against C. albicans. This may reflect immune redistribution, a phenomenon in which the immune system changes how it directs its resources to help respond to changing goals (Dhabhar & McEwen, 1999). This redistribution may be rapid (e.g., recruitment of leukocytes from the circulating blood into the tissues) or longer term (e.g., biasing development of new immune cells; Dhabhar, 2014). Both C. albicans and E. coli are commensal organisms; that is, they are typically present in most humans and they can be easily cleared by a healthy immune system. However, for each, this clearance uses different aspects of immune function, which may require commitment of immune resources. For example, E. coli (a bacterial pathogen) is mitigated via activity of T helper type 1 and type 2 cells, but C. albicans (a fungal pathogen) is more readily handled by T helper type 17 cells (Conti et al., 2009). By biasing the development of naive T helper cells to a particular subtype, the immune system can address the unique challenges of fighting bacterial versus fungal pathogens; however, any naive cell directed to specialize in one form of defense is not then available to develop in another direction. The immune system thus must constantly gather information about how to best distribute (and redistribute) resources to address competing demands. Much research has shown ways in which psychological and behavioral factors (such as stress) contribute to immune redistribution (Dhabhar, 2014); our findings suggest that sexual activity may similarly direct immune priorities. Speculatively, it is possible that there is an increased need for defense against C. albicans and other pathogens associated with vaginal infections around the time of ovulation. However, an increased defense against one pathogen type may come at the expense of defense against others, as reflected in our findings.
There were also differences between saliva-mediated and serum-mediated pathogen killing. For example, among sexually active women, there was a significant midcycle decline in E. coli killing by serum but not saliva. These differences potentially reflect differences in mucosal (salivary) versus humoral (peripheral blood) immune factors. Although they are deeply intertwined, humoral and mucosal immunity may be differentially responsive to behavioral factors: for example, both emotional and physical stress are associated with increased production of immunoglobulin A in serum but not saliva (Kiecolt-Glaser et al., 1984; Martins et al., 2009). Sexual behavior, too, may have relatively greater impact on humoral than mucosal immune factors; however, further replication is warranted.
Abstinent women showed higher complement activity than sexually active women, irrespective of cycle phase. The assay used here (CH50) quantifies the classical complement activation pathway, indicating the effectiveness of the adaptive immune system in harnessing the resources of the innate immune system to promote lysis and phagocytosis of foreign cells (Costabile, 2010). Thus, our findings suggest that the adaptive immune system of sexually active women may rely less on activating the innate immune system than do abstinent women. Insofar as the observed differences in immune function reflect the need to avoid interference with sperm (or conceptus), it is possible that sexual activity would be associated with lower innate—that is, nonspecific—immune response.
In terms of self-reported symptoms associated with immune activity (e.g., sneezing, fever, pain), both groups of women generally reported lower symptoms at midcycle and higher symptoms at menses. Because many of these symptoms are linked to inflammation, these midcycle decreases may reflect the lower levels of inflammation commonly noted at midcycle in both sexually active and abstinent women (Gaskins et al., 2012; Lorenz, Demas, & Heiman, 2017; Lorenz, Worthman, & Vitzthum, 2015). Alternatively, these findings may reflect changes in women’s self-monitoring of and attention to physical symptoms, which has been shown to increase in the days surrounding menses, particularly when women are primed to think of cultural norms of cycle-related changes in bodily sensation (Ruble, 1977). Given this study was advertised as “examining changes in women’s health across the menstrual cycle,” such priming was possible. Of note, in contrast to much research finding lower pain to experimental stimuli in the follicular versus luteal phase (Riley, Robinson, Wise, & Price, 1999), women in the present study reported lower pain symptoms in the luteal phase relative to the follicular phase, either a reflection of differences in experimentally induced versus self-reported everyday pain or a product of our small sample.
There are a variety of possible mechanisms for the observed effect of sexual activity on immune function. Hormonal influences are very likely, both because of the important interactions between endocrine and immune systems (Haddad, Saadé, & Safieh-Garabedian, 2002) and because of differences in hormonal profiles of menstrual cycles in sexually active versus abstinent women. In particular, sexually active women have higher ovulatory estradiol and luteal phase progesterone, but lower testosterone overall, than do abstinent women (Prasad et al., 2014; van Anders & Watson, 2007). Estradiol and progesterone are particularly important for regulation of mucosal immunity in the female reproductive tract and have been implicated in the “window of opportunity” associated with ovulation (Wira, Fahey, Sentman, Pioli, & Shen, 2005). Another likely mechanism is autonomic nervous system input, particularly activity of the sympathetic nervous system; sympathetic activity is particularly important for moderation of systemic humoral immunity (Herkenham & Kigar, 2017). Notably, vaginal blood flow during sexual arousal is facilitated by sympathetic activation (Lorenz, Harte, Hamilton, & Meston, 2012), and lack of sympathetic activation during sexual arousal is associated with female sexual dysfunction (Stanton, Pulverman, & Meston, 2017). It is possible that sexual arousal constitutes a unique state, in which there is activation of the sympathetic nervous system without significant coactivation of other aspects of the stress system (e.g., limited increases in cortisol; Hamilton & Meston, 2013), and that this unique state may in turn influence immune function.
Results from this exploratory study should be interpreted in context with its limitations. Our sample was small: The post hoc observed power across tests was 43% to 79%, indicating it is possible that we missed some small but potentially important effects. Of note, our sample did not include women taking hormonal contraceptives or women who were sexually active with multiple partners or with nonmale partners. Replication of these results is warranted, as is extension to a wider population of women representing greater diversity of sexual orientations and those taking commonly used medications such as contraceptives and antidepressants. These data were collected from women carefully screened for health status and thus may not reflect women with chronic health issues. In particular, it is unknown if these findings would extend to women with autoimmune conditions or fertility concerns. Also, we selected participants on the basis of menstrual cycle regularity; however, even among healthy samples, approximately one-quarter of women report some form of cycle irregularity (e.g., bleeding between menses; Rowland et al., 2002). Our immune assays offer only a snapshot of overall immunity; for example, our ex vivo pathogen-killing assays can index only the activity of immune cells that were present in the sample at the time of sampling and not the ongoing recruitment of other immune cells. Also, these tests reflect only the response to very common pathogens that have been previously encountered and do not reflect response to the first exposure. Nevertheless, the present study also had some strengths, including recruitment of a healthy sample not using immunoactive medications, careful assessment of menstrual phase (including ovulation), and testing multiple aspects of immune function.
In summary, we found that sexually active and abstinent women showed different patterns of change in immune function across the menstrual cycle. These data point to the need for further study of the ways in which sexual activity may influence women’s immune function and, more broadly, the dynamic interactions arising from tradeoffs between reproduction and immunity in healthy individuals.
Acknowledgments
We wish to thank Drs. Emily Chester and Michael Muehlenbein for assistance in adapting the bactericidal assay protocol for use in human saliva. We also wish to thank Chloe Nelson, Nichelle Whitney, and Kat O’Hara for their assistance in data collection and preparing samples for analysis.
Funding
This work was partly funded by the Office of the Vice Provost of Research at Indiana University, Bloomington, through the Collaborative Research and Creative Activity Funding Award, and partly by the American Psychological Foundation’s Henry P. David Award for Research in Human Reproductive Behavior and Population Studies. Dr. Lorenz was supported by grant T32HD049336 from the National Institute of Child Health and Human Development.
Contributor Information
Tierney K. Lorenz, Department of Psychological Science, University of North Carolina at Charlotte, The Kinsey Institute, Indiana University; and Center for Integrative Study for Animal Behavior, Indiana University
Julia R. Heiman, The Kinsey Institute, Indiana University; and Department of Psychological and Brain Sciences, Indiana University
Gregory E. Demas, Center for Integrative Study for Animal Behavior, Indiana University; and Department of Biology, Indiana University
References
- Aronoff DM, Fordyce K, Salzman E, Fortenberry JD, Rogers M, King S, van Anders S. Antiviral activity of vaginal secretions is menstrual cycle phase-dependent; 2014, March; Paper presented at the Society for Gynecologic Investigation; Florence, Italy. [Google Scholar]
- Bertone-Johnson E, Ronnenberg A, Houghton S, Nobles C, Zagarins S, Takashima-Uebelhoer B, Whitcomb B, et al. Association of inflammation markers with menstrual symptom severity and pre-menstrual syndrome in young women. Human Reproduction. 2014;29:1987–1994. doi: 10.1093/humrep/deu170. [DOI] [PubMed] [Google Scholar]
- Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nature Reviews: Drug Discovery. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- Brown SG, Morrison L, Calibuso MJ, Christiansen BA. The menstrual cycle and sexual behavior: Relationship to eating, exercise, sleep, and health patterns. Women and Health. 2008;48:429–444. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetski CJ, Brennan FX. Sexual frequency and Immunoglobulin A (IgA) Psychological Reports. 2004;94:839–844. doi: 10.2466/pr0.94.3.839-844. [DOI] [PubMed] [Google Scholar]
- Cnaan A, Laird N, Slasor P. Tutorial in biostatistics: Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine. 1997;16:2349–2380. doi: 10.1002/(ISSN)1097-0258. [DOI] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. Journal of Experimental Medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costabile M. Measuring the 50% haemolytic complement (CH50) activity of serum. Journal of Visualized Experiments. 2010;37:e1923–e1923. doi: 10.3791/1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Carlton ED. Ecoimmunology for psychoneuroimmunologists: Considering context in neuroendocrine–immune–behavior interactions. Brain, Behavior, and Immunity. 2015;44:9–16. doi: 10.1016/j.bbi.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. Journal of Animal Ecology. 2011;80:710–730. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Effects of stress on immune function: The good, the bad, and the beautiful. Immunologic Research. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proceedings of the National Academy of Sciences. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM, Huebner DM. Is good sex good for you? Rethinking sexuality and health. Social and Personality Psychology Compass. 2012;6:54–69. doi: 10.1111/spco.2011.6.issue-1. [DOI] [Google Scholar]
- Fortenberry JD, Rogers M, Fordyce K, King S, Aronoff DM, van Anders S. HIV inhibition and variation in antimicrobial peptides associated with intercourse; 2014, March; Paper presented at the Conference on Retroviruses and Opportunistic Infections; Boston, MA. [Google Scholar]
- French SS, Neuman-Lee LA. Improved ex vivo method for microbiocidal activity across vertebrate species. Biology Open. 2012;1(5):482–487. doi: 10.1242/bio.2012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, Schisterman EF, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle. American Journal of Epidemiology. 2012;175:423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuro–immune–endocrine interactions: A role for the hypothalamic–pituitary–adrenal revolving axis. Journal of Neuroimmunology. 2002;133:1–19. doi: 10.1016/S0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hamilton LD, Meston CM. Chronic stress and sexual function in women. Journal of Sexual Medicine. 2013;10:2443–2454. doi: 10.1111/jsm.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Kigar SL. Contributions of the adaptive immune system to mood regulation: Mechanisms and pathways of neuroimmune interactions. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;79:49–57. doi: 10.1016/j.pnpbp.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Garner W, Speicher C, Penn GM, Holliday J, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosomatic Medicine. 1984;46:7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- Lorenz T, Demas GE, Heiman JR. Partnered sexual activity moderates menstrual cycle–related changes in inflammation markers in healthy women: An exploratory observational study. Fertility and Sterility. 2017;107:763–773. doi: 10.1016/j.fertnstert.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology. 2012;49:111–117. doi: 10.1111/psyp.2012.49.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Heiman JR, Demas GE. Interaction of menstrual phase and sexual activity predicts mucosal and systemic humoral immunity in healthy women. Physiology and Behavior. 2015a;152:92–98. doi: 10.1016/j.physbeh.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Heiman JR, Demas GE. Sexual activity modulates shifts in Th1/Th2 cytokine profile across the menstrual cycle: An observational study. Fertility and Sterility. 2015b;104:1513–1521. doi: 10.1016/j.fertnstert.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Heiman JR, Demas GE. Testosterone and immune-reproductive tradeoffs in healthy women. Hormones and Behavior. 2017;88:122–130. doi: 10.1016/j.yhbeh.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Van Anders S. Interactions of sexual activity, gender, and depression with immunity. Journal of Sexual Medicine. 2014;11:966–979. doi: 10.1111/jsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Worthman C, Vitzthum VJ. Links between inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health. 2015;1:304–324. doi: 10.1093/emph/eov029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RA, Cunha MR, Neves AP, Martins M, Teixeira-Verissimo M, Teixeira AM. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG, and IgM in older men and women. International Journal of Sports Medicine. 2009;30:906–912. doi: 10.1055/s-0029-1237389. [DOI] [PubMed] [Google Scholar]
- Muehlenbein M, Prall S, Chester E. Development of a noninvasive salivary measure of functional immunity in humans; 2011, April; Paper presented at the 35th annual meeting of the Human Biology Association; Albuquerque, NM. [Google Scholar]
- Nieman DC, Buckley KS, Henson DA, Warren BJ, Suttles J, Ahle JC, Nehlsen-Cannarella SL, et al. Immune function in marathon runners versus sedentary controls. Medicine and Science in Sports and Exercise. 1995;27:986–992. doi: 10.1249/00005768-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Prasad A, Mumford SL, Buck Louis GM, Ahrens KA, Sjaarda LA, Schliep KC, Schisterman EF, et al. Sexual activity, endogenous reproductive hormones, and ovulation in premenopausal women. Hormones and Behavior. 2014;66:330–338. doi: 10.1016/j.yhbeh.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Price D. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, Sandler DP. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Ruble DN. Premenstrual symptoms: A reinterpretation. Science. 1977;197:291–292. doi: 10.1126/science.560058. [DOI] [PubMed] [Google Scholar]
- Stanton AM, Pulverman CS, Meston CM. Vagal activity during physiological sexual arousal in women with and without sexual dysfunction. Journal of Sex and Marital Therapy. 2017;43:78–89. doi: 10.1080/0092623X.2015.1115793. [DOI] [PubMed] [Google Scholar]
- van Anders S, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: Methodological considerations. Archives of Sexual Behavior. 2014;43:231–250. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- van Anders S, Watson NV. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Hormones and Behavior. 2007;51:286–291. doi: 10.1016/j.yhbeh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Wang EC, Fox JD, Irwin M. The measurement of stress-related immune dysfunction in humans: An introduction to psychoneuroimmunology. In: Vingerhoets A, editor. Assessment in behavioral medicine. New York, NY: Routledge; 2013. pp. 441–479. [Google Scholar]
- Wira CR, Fahey JV. A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunological Reviews. 2005;206:306–335. doi: 10.1111/imr.2005.206.issue-1. [DOI] [PubMed] [Google Scholar]


