Abstract
Background & Aims
Treatment with the combination of ledipasvir and sofosbuvir for 12 weeks has been approved by the Food and Drug Administration for patients with genotype 1 hepatitis C virus (HCV) infection; some patients can be treated with an 8-week course. Guidelines recommend a 12-week treatment course for black patients, but studies have not compared the effectiveness of 8 vs 12 weeks in black patients who are otherwise eligible for an 8-week treatment regimen.
Methods
We conducted an observational study of Kaiser Permanente Northern California members with HCV genotype 1 infection who were eligible for 8 weeks of treatment with ledipasvir and sofosbuvir (treatment-naive, no cirrhosis, no HIV infection, level of HCV RNA <6 million IU/mL) and were treated for 8 or 12 weeks from October 2014 through December 2016. We used χ2 analyses to compare sustained virologic response 12 weeks after the end of treatment (SVR12) among patients treated for 8 vs 12 weeks, and adjusted Poisson models to identify factors associated with receipt of 12 weeks of therapy among patients eligible for 8 weeks.
Results
Of 2653 patients eligible for 8 weeks of treatment with ledipasvir and sofosbuvir, 1958 (73.8%) received 8 weeks of treatment and 695 (26.2%) received 12 weeks; the proportions of patients with SVR12 were 96.3% and 96.3%, respectively (P=.94). Among 435 black patients eligible for the 8-week treatment regimen, there was no difference in the proportions who achieved an SVR12 following 8 vs 12 weeks’ treatment (95.6% vs 95.8%; P=.90). Male sex, higher transient elastography or FIB-4 scores, higher INR and level of bilirubin, lower level of albumin, obesity, diabetes, and ≥15 alcohol drinks consumed/week were independently associated with receiving 12 weeks of treatment among patients eligible for the 8-week treatment regimen, but were not associated with reduced SVR12 after 8 weeks of treatment.
Conclusion
In an observational study of patients who received ledipasvir and sofosbuvir treatment for HCV genotype 1 infection, we found that contrary to guidelines, 8-week and 12- week treatment regimens do not result in statistically significant differences in SVR12 in black patients. Patient characteristics were associated with receipt of 12-week regimens among patients eligible for 8 weeks, but were not associated with reduced SVR12 after 8 weeks. Shorter treatment courses might therefore be more widely used without compromising treatment effectiveness.
Keywords: direct-acting antiviral agents, sustained virologic response, race, effectiveness
BACKGROUND
The recent emergence of direct-acting antiviral agents for hepatitis C virus (HCV) infection, including the combination of ledipasvir and sofosbuvir (LDV/SOF), has dramatically increased the number of HCV-infected patients for whom treatment can be tolerated and successful.1–3 Labeling for LDV/SOF by the Food and Drug Administration, and clinical guidelines from the American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society for America (IDSA), recommend 12 weeks of treatment for genotype 1, treatment-naive patients, and state that an 8-week course can be considered in those who also have no cirrhosis, are human immunodeficiency virus (HIV)-uninfected, and have HCV RNA <6 million IU/mL.4,5 Recent revisions to the AASLD/IDSA guidelines further specify that black patients should not be treated with 8-week courses of LDV/SOF. However, the addition of race as a criterion for treatment duration is based on limited evidence and could exacerbate observed racial/ethnic disparities in HCV treatment initiation.6
The ideal evidence to support the AASLD/IDSA guidelines on race and treatment duration would be from a clinical trial in which patients who were eligible to receive 8 weeks were randomized to receive either 8 or 12 weeks. The ION-3 study randomized genotype 1, treatment-naive patients without cirrhosis to receive 8 or 12 weeks, but the study included few black patients and did not use HCV RNA <6 million IU/mL as an eligibility criterion for 8 weeks.1,7 Thus, observational data are needed to approximate a randomized comparison of treatment effectiveness between black patients receiving 8 and 12 weeks of therapy. Prior observational studies have suggested reduced response for black patients receiving 8 weeks of therapy,8–13 while a more recent pooled analysis of real-world cohorts found similar response by race for patients receiving 8 weeks.14 However, because prior studies did not limit analyses to black patients otherwise eligible for 8 weeks (i.e., treatment-naive, no cirrhosis, HIV-uninfected, and HCV RNA <6 million IU/mL), black patients receiving 8 and 12 weeks may have differed with respect to these key factors that affect treatment response. Furthermore, there may be other subgroups who are at higher risk of failure after 8 compared with 12 weeks of therapy, but prior studies have not restricted analyses of risk factors for treatment failure to those eligible for 8 weeks, thus limiting their clinical relevance. Finally, studies are needed to identify factors associated with the underuse of 8-week regimens, which could guide efforts to increase access to treatment.
We investigated outcomes of LDV/SOF for 8 compared with 12 weeks among HCV-infected patients within Kaiser Permanente Northern California (KPNC). Our primary objective was to compare the effectiveness of 8 and 12 weeks of LDV/SOF among patients eligible for 8 weeks, overall and by race/ethnicity and other subgroups. To evaluate potential underuse of 8-week regimens, we also evaluated factors associated with receipt of 12 weeks of LDV/SOF among patients eligible for 8 weeks.
METHODS
Study Setting, Population, and Design
KPNC is a large integrated healthcare system that provides comprehensive medical services to 4.1 million members, corresponding to approximately one-third of insured individuals in the surrounding population.15 We identified all adult KPNC members with confirmed HCV infection, defined as a positive HCV RNA test or a known HCV genotype, who initiated HCV treatment with direct-acting antiviral agents from October 2014 (month of approval of LDV/SOF and when direct-acting antiviral agent use became more widespread at KPNC) through December 2016. Our study population for this analysis was restricted to patients who were eligible to receive 8 weeks of LDV/SOF and received either 8 or 12 weeks without ribavirin, with eligibility for 8 weeks defined as follows: genotype 1, treatment-naive, no cirrhosis, HIV-uninfected, and HCV RNA <6 million IU/mL. Among otherwise eligible patients, we excluded 10 patients treated with durations <8 weeks, 2 patients treated with durations between 8 and 12 weeks, and 10 patients treated with durations >12 weeks. Black patients were considered eligible for 8 weeks of therapy in this analysis because the AASLD/IDSA guidelines were not updated to include the race criterion until after our study period ended, and because we aimed to assess the appropriateness of the added race criterion. KPNC follows AASLD/IDSA guidelines for HCV treatment, but final decisions about treatment duration were at the discretion of the treating clinicians.
The institutional review board at KPNC approved this study with a waiver of written informed consent.
Study Measurements
We extracted data from the clinical and administrative databases that comprise KPNC’s electronic health record, including age; sex; race/ethnicity; health plan enrollment periods; height and weight; pharmacy fills for HCV medications; medical center; laboratory tests and results (i.e., HCV genotype, HCV RNA, platelets, aspartate aminotransferase, alanine transaminase, creatinine, international normalized ratio, bilirubin, and albumin); number of alcoholic drinks per week, which has been systematically collected at KPNC since 2013;16 and inpatient and outpatient diagnoses of drug abuse (International Classification of Disease Codes, version 9 [ICD-9]: 305.2–305.5; ICD-10: F11.xx-F14.xx, F16.xx, F18.xx-F19.xx, where xx includes .10, .90, .120), smoking/tobacco use (ICD-9: 305.1, V15, V65, 649, internal social history codes; ICD-10: F17.200, .201, .210–211, .220–221, .290–291), and hepatitis B virus infection (ICD-9: 070.32; ICD-10: B18.1). HIV status was extracted from the KPNC HIV registry, which includes all known HIV cases since the early 1980s,17 and diabetes status was extracted from the KPNC diabetes registry.18
We estimated liver fibrosis using noninvasive measures that are routinely used in clinical practice, specifically transient elastography (i.e., FibroScanR; Echosens, Waltham, Massachusetts). Transient elastography scores were extracted from a clinician-maintained database. For patients without transient elastography measurements, we estimated liver fibrosis using the FIB-4 index, a serum biomarker comprised of age and routine liver function test results, specifically platelets, aspartate aminotransferase, and alanine transaminase.19 We defined cirrhosis as a transient elastography score ≥12 kPa,20 or as a FIB-4 score >5.88.21
We defined treatment effectiveness by the achievement of sustained virologic response at least 12 weeks after the end of treatment (SVR12). Because visit timing may vary in clinical practice, we assessed SVR12 using the first HCV RNA test conducted at least 11 weeks after the end of treatment. SVR12 was indicated by HCV RNA <20 IU/mL, while treatment failure was indicated by HCV RNA ≥20 IU/mL. Individuals without an HCV RNA test at least 11 weeks after the end of treatment were excluded from analyses of SVR12.
Statistical Analysis
The primary outcome of interest for this analysis was SVR12 after 8 compared with 12 weeks of therapy among individuals eligible for 8 weeks. Secondarily, we evaluated receipt of 12 weeks of therapy among individuals eligible for 8 weeks. Covariates of interest included race/ethnicity (white, black, Hispanic, Asian, other); age at treatment initiation (<50, 50–59, ≥60 years); sex; medical center; transient elastography score (<9.5, 9.5–11, 11.1–11.9 kPa), using the most recent measurement in the 6 months prior to treatment initiation; FIB-4 score (<1.45, 1.45–3.25, 3.26–5.88) for patients without transient elastography measurements, using the most recent laboratory test results in the 6 months prior to treatment initiation; creatinine (≤1.1, >1.1 mg/dL), international normalized ratio (≤1.1, >1.1), bilirubin (≤1.1, >1.1 g/dL), and albumin (<3.6, ≥3.6 g/dL), using the most recent test results in the 6 months prior to treatment initiation; diabetes (ever/never through 2016); body mass index >30 kg/m2, using the most recent height and weight measurements in the 6 months prior to treatment initiation; number of alcoholic drinks per week (0, 1–7, 8–14, ≥15), using the most recent measurement prior to treatment initiation; and drug abuse, smoking, and hepatitis B virus infection, all defined as ever/never through 2016.
We computed the proportion achieving SVR12 overall and within subgroups for patients receiving LDV/SOF for 8 or 12 weeks, using chi-square tests to compare SVR12 by treatment duration. We used Poisson models with robust variance to obtain adjusted risk ratios (RRs) comparing the effectiveness of 8 and 12 weeks overall and within subgroups, with models including terms for black race and any factors that were associated with SVR12 at P<0.1 in bivariable analysis, and using interaction terms to assess differences in the effect of treatment duration between subgroups. We then used chi-square tests to compare the characteristics of HCV-infected individuals who were eligible to receive LDV/SOF for 8 weeks and treated for 8 or 12 weeks. We used Poisson models with robust variance to identify factors independently associated with receipt of 12 weeks among those eligible for 8 weeks, including terms for black race and any variables that were associated with receipt of 12 weeks at P<0.1 in bivariable analysis. Finally, in addition to patient characteristics, we assessed whether medical center was associated with the proportion of patients treated for 12 weeks among those eligible for 8 weeks, and then used simple linear regression to assess whether variation in prescribing practices was associated with SVR12 at each medical center.
Analyses were conducted in SAS 9.4 (Cary, North Carolina). Statistical tests were two-sided and statistical significance was defined as P<0.05.
RESULTS
Study Population
We identified 6108 individuals with HCV infection who initiated treatment with direct-acting antiviral agents from October 2014 through December 2016. Of the 6108, 2653 (43.4%) were considered eligible to receive 8 weeks of LDV/SOF and treated for 8 (73.8%) or 12 weeks (26.2%; Table 1). Over half (57.9%) were white and 17.3% black, half (50.8%) were aged 60 years or older, and 42.5% were female. Of those with transient elastography measurements, 16.0% had scores of 9.5–11.9 kPa at baseline. Nearly one-third (32.1%) were obese and 15.1% had diabetes. Some alcohol use was reported among 15.7% of subjects, 18.5% had drug abuse diagnoses, and 39.2% ever smoked. Hepatitis B virus coinfection was present in 5.1% of the cohort.
Table 1.
Characteristics of 2653 HCV-infected individuals eligible for 8 weeks of ledipasvir/sofosbuvir and treated for 8 or 12 weeks
| Race/ethnicity, n (% among known) | |
| White | 1510 (57.9) |
| Black | 450 (17.3) |
| Hispanic | 331 (12.7) |
| Asian | 168 (6.5) |
| Other | 147 (5.6) |
| Unknown race/ethnicity, n (%) | 47 (1.8) |
|
| |
| Age in years, n (%) | |
| <50 | 392 (14.8) |
| 50–59 | 913 (34.4) |
| ≥60 | 1348 (50.8) |
|
| |
| Sex | |
| Female | 1127 (42.5) |
| Male | 1526 (57.5) |
|
| |
| Transient elastography kPa, n (%) | |
| <9.5 | 961 (84.0) |
| 9.5–11 | 135 (11.8) |
| 11.1–11.9 | 48 (4.2) |
| No transient elastography, n (%) | 1509 (56.9) |
|
| |
| FIB-4 (if no transient elastography), n (%) | |
| <1.45 | 575 (38.1) |
| 1.45–3.25 | 715 (47.4) |
| 3.26–5.88 | 219 (14.5) |
|
| |
| Creatinine, median (IQR) | 0.84 (0.73–0.97) |
|
| |
| International normalized ratio, median (IQR) | 1.0 (1.0–1.0) |
|
| |
| Bilirubin, median (IQR) | 0.6 (0.5–0.8) |
|
| |
| Albumin, median (IQR) | 4.2 (4.0–4.4) |
|
| |
| Diabetes, n (%) | 400 (15.1) |
|
| |
| BMI >30 kg/m2, n (%) | 819 (32.1) |
|
| |
| Alcoholic drinks per week, n (% among known) | |
| 0 | 2152 (84.3) |
| 1–7 | 312 (12.2) |
| 8–14 | 63 (2.5) |
| ≥15 | 27 (1.1) |
| Unknown alcohol use, n (%) | 99 (3.7) |
|
| |
| Drug abuse, n (%) | 490 (18.5) |
|
| |
| Smoking, n (%) | 1040 (39.2) |
|
| |
| Hepatitis B virus infection, n (%) | 136 (5.1) |
HCV, hepatitis C virus; IQR, interquartile range. Eligibility for 8 weeks was defined as genotype 1, treatment-naive, no cirrhosis (i.e., transient elastography <12 kPa if available, else FIB-4 ≤5.88), HIV-uninfected, and HCV RNA <6 million IU/mL.
Factors Associated with SVR12
Of the 2653 8-week-eligible patients, 2544 (95.9%) had an HCV RNA test at least 11 weeks after the end of treatment and were included in analyses of SVR12. Among the 109 excluded patients, 13.8% were black, compared with 17.1% of those with an HCV RNA test (P=0.36). The overall proportion with SVR12 was 96.3% among those treated for 8 weeks compared with 96.3% among those treated for 12 weeks (P=0.94; Table 2). Among black patients eligible to receive 8 weeks, there was no difference in SVR12 between those treated for 8 weeks and those treated for 12 weeks (95.6% vs. 95.8%, P=0.90).
Table 2.
Proportion with SVR12 after 8 and 12 weeks of ledipasvir/sofosbuvir among 2653 HCV-infected individuals eligible for 8 weeks
| 8 weeks | 12 weeks | P | |
|---|---|---|---|
| Overall | 96.3 (1807/1876) | 96.3 (643/668) | 0.94 |
|
| |||
| Race/ethnicity | |||
| White | 96.7 (1045/1081) | 96.9 (346/357) | 0.82 |
| Black | 95.6 (301/315) | 95.8 (115/120) | 0.90 |
| Hispanic | 95.4 (207/217) | 94.9 (93/98) | 0.78 |
| Asian | 96.7 (117/121) | 100.0 (43/43) | 0.57 |
| Other | 96.5 (109/113) | 88.2 (30/34) | 0.08 |
|
| |||
| Age in years | |||
| <50 | 99.0 (301/304) | 93.1 (54/58) | 0.014 |
| 50–59 | 96.2 (629/654) | 95.4 (207/217) | 0.61 |
| ≥60 | 95.5 (877/918) | 97.2 (382/393) | 0.16 |
|
| |||
| Sex | |||
| Female | 98.2 (854/870) | 97.2 (208/214) | 0.37 |
| Male | 94.7 (953/1006) | 95.8 (435/454) | 0.38 |
|
| |||
| Transient elastography kPa | |||
| <9.5 | 96.8 (788/814) | 98.1 (101/103) | 0.76 |
| 9.5–11 | 96.0 (71/74) | 100.0 (52/52) | 0.27 |
| 11.1–11.9 | 100.0 (8/8) | 100.0 (38/38) | ─ |
|
| |||
| FIB-4 (if no transient elastography) | |||
| <1.45 | 97.7 (468/479) | 95.8 (68/71) | 0.41 |
| 1.45–3.25 | 94.5 (433/458) | 96.2 (225/234) | 0.35 |
| 3.26–5.88 | 90.7 (39/43) | 93.5 (159/170) | 0.51 |
|
| |||
| Creatinine >1.1 mg/dL | |||
| No | 96.4 (1611/1671) | 96.6 (560/580) | 0.87 |
| Yes | 95.3 (183/192) | 93.9 (77/82) | 0.77 |
|
| |||
| International normalized ratio >1.1 | |||
| No | 96.4 (1744/1809) | 96.8 (569/588) | 0.68 |
| Yes | 93.2 (41/44) | 93.3 (70/75) | 1.00 |
|
| |||
| Bilirubin >1.1 g/dL | |||
| No | 96.5 (1734/1797) | 96.9 (568/586) | 0.61 |
| Yes | 91.9 (68/74) | 91.3 (73/80) | 0.89 |
|
| |||
| Albumin <3.6 g/dL | |||
| No | 96.2 (1707/1775) | 96.7 (556/575) | 0.56 |
| Yes | 97.6 (41/42) | 92.2 (71/77) | 0.42 |
|
| |||
| Diabetes | |||
| No | 96.4 (1595/1656) | 96.2 (477/496) | 0.88 |
| Yes | 96.4 (212/220) | 96.5 (166/172) | 0.94 |
|
| |||
| Body mass index >30 kg/m2 | |||
| No | 96.4 (1217/1262) | 97.0 (386/398) | 0.60 |
| Yes | 96.0 (506/527) | 95.0 (248/261) | 0.52 |
|
| |||
| Alcoholic drinks per week | |||
| 0 | 96.6 (1452/1503) | 96.3 (542/563) | 0.71 |
| 1–7 | 95.0 (230/242) | 98.2 (53/54) | 0.47 |
| 8–14 | 97.8 (44/45) | 92.9 (13/14) | 0.42 |
| ≥15 | 100.0 (12/12) | 85.7 (12/14) | 0.48 |
|
| |||
| Drug abuse | |||
| No | 96.2 (1476/1535) | 96.7 (531/549) | 0.55 |
| Yes | 97.1 (331/341) | 94.1 (112/119) | 0.16 |
|
| |||
| Smoking | |||
| No | 96.1 (1108/1153) | 95.5 (379/397) | 0.58 |
| Yes | 96.7 (699/723) | 97.4 (264/271) | 0.55 |
|
| |||
| Hepatitis B virus infection | |||
| No | 96.4 (1724/1788) | 96.0 (598/623) | 0.62 |
| Yes | 94.3 (83/88) | 100.0 (45/45) | 0.17 |
SVR, sustained virologic response; HCV, hepatitis C virus. Eligibility for 8 weeks was defined as genotype 1, treatment-naive, no cirrhosis (i.e., transient elastography <12 kPa if available, else FIB-4 ≤5.88), HIV-uninfected, and HCV RNA <6 million IU/mL. Excludes 109 with no HCV RNA test done after 11 weeks following end of treatment. P-values obtained from chi-square or Fisher's exact tests.
The percentage of patients achieving SVR12 exceeded 95% in most subgroups defined by patient characteristics and treatment duration. A higher proportion of patients aged <50 years achieved SVR12 after 8 weeks compared with 12 weeks (99.0% vs. 93.1%, P=0.003), but there were no other factors associated with differences in SVR12 by treatment duration, including sex, transient elastography or FIB-4 scores, creatinine, international normalized ratio, bilirubin, albumin, diabetes, body mass index, alcohol use, drug abuse, smoking, or hepatitis B virus infection.
After adjusting for black race and age, there was no difference in the likelihood of SVR12 after 8 compared with 12 weeks (RR 1.00, 95% confidence interval [CI]: 0.98–1.02; Table 3). There was also no difference in the effectiveness of 8 compared with 12 weeks among black individuals (RR 1.00, 95% CI: 0.95–1.04) or non-black individuals (RR 1.00, 95% CI: 0.98–1.02) after adjusting for age, or among individuals aged <50 (RR 1.06, 95% CI: 0.99–1.14) or ≥50 years (RR 0.99, 95% CI: 0.97–1.01) after adjusting for black race. There was a trend toward an interaction between age and treatment duration, suggesting a difference in the RRs for age <50 and ≥50 years, but this did not reach statistical significance (P interaction = 0.064).
Table 3.
Adjusted effect of 8 compared with 12 weeks of ledipasvir/sofosbuvir on SVR12 among 2653 HCV-infected individuals eligible for 8 weeks
| Adjusted RR (95% CI) | P | P interaction | |
|---|---|---|---|
| Overall | 1.00 (0.98–1.02) | 0.92 | |
| Race | 0.90 | ||
| Black | 1.00 (0.95–1.04) | 0.88 | |
| Non-black | 1.00 (0.98–1.02) | 0.96 | |
| Age in years | 0.064 | ||
| <50 | 1.06 (0.99–1.14) | 0.09 | |
| ≥50 | 0.99 (0.97–1.01) | 0.40 |
SVR, sustained virologic response; HCV, hepatitis C virus; RR, risk ratio; CI, confidence interval. Eligibility for 8 weeks was defined as genotype 1, treatment-naive, no cirrhosis (i.e., transient elastography <12 kPa if available, else FIB-4 ≤5.88), HIV-uninfected, and HCV RNA <6 million IU/mL. Excludes 109 with no HCV RNA test done after 11 weeks following end of treatment. RRs obtained from Poisson models with robust variance, with main terms for treatment duration, age, and race, and interaction terms to assess differences in the effect of treatment duration between subgroups.
Factors Associated with Treatment Duration
Among individuals eligible to receive 8 weeks of LDV/SOF, receipt of 12 weeks of treatment was more common among older patients; males; those with baseline transient elastography scores of 11.1–11.9 kPa or FIB-4 of 3.26–5.88; those with higher international normalized ratio, higher bilirubin, or lower albumin; patients with diabetes; those with higher body mass index; patients reporting ≥15 alcoholic drinks per week; and patients with hepatitis B virus coinfection (Table 4). Race/ethnicity, creatinine, drug abuse, and smoking were not associated with treatment duration among patients eligible for 8 weeks. In multivariable analysis, male sex (RR 1.39, 95% CI: 1.22–1.58), transient elastography ≥9.5 kPa or FIB-4 ≥1.45 (RR 3.29, 95% CI: 2.82–3.85), international normalized ratio >1.1 (RR 1.26, 95% CI: 1.09–1.47), bilirubin >1.1 g/dL (RR 1.37, 95% CI: 1.18–1.53), albumin <3.6 g/dL (RR 1.51, 95% CI: 1.31–1.75), body mass index >30 kg/m2 (RR 1.28, 95% CI: 1.14–1.44), diabetes (RR 1.40, 95% CI: 1.23–1.60), and reporting ≥15 alcoholic drinks per week compared with no alcohol use (RR 1.40, 95% CI: 1.06–1.84) were associated with a higher likelihood of receiving 12 weeks of therapy among those eligible for 8 weeks (Table 5). Reporting 1–7 alcoholic drinks per week compared with no alcohol use was associated with a reduced likelihood of receiving 12 weeks (RR 0.75, 95% CI: 0.60–0.93). Age and hepatitis B virus coinfection were no longer associated with receipt of 12 weeks after adjusting for covariates, and race/ethnicity, drug abuse, and smoking were not associated with treatment duration in unadjusted or adjusted analyses.
Table 4.
Factors associated with treatment duration among 2653 HCV-infected individuals eligible for 8 weeks of ledipasvir/sofosbuvir
| 8 weeks | 12 weeks | ||
|---|---|---|---|
| n (row %) | n (row %) | ||
| Overall | 1958 (73.8) | 695 (26.2) | P |
|
| |||
| Race/ethnicity | 0.08 | ||
| White | 1139 (75.4) | 371 (24.6) | |
| Black | 324 (72.0) | 126 (28.0) | |
| Hispanic | 227 (68.6) | 104 (31.4) | |
| Asian | 124 (73.8) | 44 (26.2) | |
| Other | 113 (76.9) | 34 (23.1) | |
|
| |||
| Age in years | <0.001 | ||
| <50 | 330 (84.2) | 62 (15.8) | |
| 50–59 | 686 (75.1) | 227 (24.9) | |
| ≥60 | 942 (69.9) | 406 (30.1) | |
|
| |||
| Sex | <0.001 | ||
| Female | 903 (80.1) | 224 (19.9) | |
| Male | 1055 (69.1) | 471 (30.9) | |
|
| |||
| Transient elastography kPa | <0.001 | ||
| <9.5 | 853 (88.8) | 108 (11.2) | |
| 9.5–11 | 78 (57.8) | 57 (42.2) | |
| 11.1–11.9 | 8 (16.7) | 40 (83.3) | |
|
| |||
| FIB-4 (if no transient elastography) | <0.001 | ||
| <1.45 | 502 (87.3) | 73 (12.7) | |
| 1.45–3.25 | 472 (66.0) | 243 (34.0) | |
| 3.26–5.88 | 45 (20.6) | 174 (79.5) | |
|
| |||
| Creatinine >1.1 mg/dL | 0.10 | ||
| No | 1746 (74.3) | 603 (25.7) | |
| Yes | 199 (69.8) | 86 (30.2) | |
|
| |||
| International normalized ratio >1.1 | <0.001 | ||
| No | 1889 (75.5) | 613 (24.5) | |
| Yes | 44 (36.4) | 77 (63.6) | |
|
| |||
| Bilirubin >1.1 g/dL | <0.001 | ||
| No | 1876 (75.5) | 610 (24.5) | |
| Yes | 77 (48.1) | 83 (51.9) | |
|
| |||
| Albumin <3.6 g/dL | <0.001 | ||
| No | 1852 (75.6) | 599 (24.4) | |
| Yes | 43 (35.3) | 79 (64.8) | |
|
| |||
| Diabetes | <0.001 | ||
| No | 1734 (77.0) | 519 (23.0) | |
| Yes | 224 (56.0) | 176 (44.0) | |
|
| |||
| Body mass index >30 kg/m2 | <0.001 | ||
| No | 1315 (76.1) | 414 (23.9) | |
| Yes | 547 (66.8) | 272 (33.2) | |
|
| |||
| Alcoholic drinks per week | 0.001 | ||
| 0 | 1569 (72.9) | 583 (27.1) | |
| 1–7 | 254 (81.4) | 58 (18.6) | |
| 8–14 | 48 (76.2) | 15 (23.8) | |
| ≥15 | 13 (48.2) | 14 (51.9) | |
|
| |||
| Drug abuse | 0.88 | ||
| No | 1595 (73.7) | 568 (26.3) | |
| Yes | 363 (74.1) | 127 (25.9) | |
|
| |||
| Smoking | 0.30 | ||
| No | 1202 (74.5) | 411 (25.5) | |
| Yes | 756 (72.7) | 284 (27.3) | |
|
| |||
| Hepatitis B virus infection | 0.023 | ||
| No | 1869 (74.3) | 648 (25.7) | |
| Yes | 89 (65.4) | 47 (34.6) | |
HCV, hepatitis C virus. Eligibility for 8 weeks was defined as genotype 1, treatment-naive, no cirrhosis (i.e., transient elastography <12 kPa if available, else FIB-4 ≤5.88), HIV-uninfected, and HCV RNA <6 million IU/mL.
Table 5.
Factors independently associated with receiving 12 compared with 8 weeks of ledipasvir/sofosbuvir among 2653 HCV-infected individuals eligible for 8 weeks
| Adjusted RR (95% CI) | P | |
|---|---|---|
| Black race (ref: non-Black) | 0.98 (0.84–1.14) | 0.81 |
| Age ≥50 years | 1.05 (0.85–1.31) | 0.65 |
| Male | 1.39 (1.22–1.58) | <0.001 |
| Transient elastography kPa ≥9.5 or FIB-4 ≥1.45 | 3.29 (2.82–3.85) | <0.001 |
| International normalized ratio >1.1 | 1.37 (1.18–1.59) | <0.001 |
| Bilirubin >1.1 g/dL | 1.51 (1.31–1.75) | <0.001 |
| Albumin <3.6 g/dL | 1.28 (1.14–1.44) | <0.001 |
| Body mass index >30 kg/m2 | 1.40 (1.23–1.60) | <0.001 |
| Diabetes | 0.98 (0.84–1.14) | 0.81 |
| Alcoholic drinks per week (ref: 0) | ||
| 1–7 | 0.75 (0.60–0.93) | 0.009 |
| 8–14 | 0.99 (0.69–1.43) | 0.96 |
| ≥15 | 1.40 (1.06–1.84) | 0.017 |
| Hepatitis B virus infection | 1.01 (0.81–1.26) | 0.90 |
HCV, hepatitis C virus; RR, risk ratio; CI, confidence interval. Eligibility for 8 weeks was defined as genotype 1, treatment-naive, no cirrhosis (i.e., transient elastography <12 kPa if available, else FIB-4 ≤5.88), HIV-uninfected, and HCV RNA <6 million IU/mL. RRs obtained from Poisson models with robust variance, including terms for all variables listed in the table.
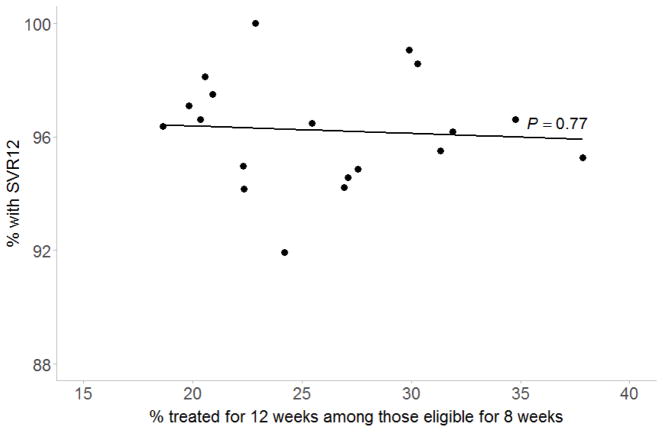
The proportion of patients receiving 12 weeks of treatment differed by medical center, ranging from 18.6% to 37.8% (P=0.002), but was not associated with the overall proportion achieving SVR12 among patients eligible for 8 weeks at each medical center (P=0.77; Figure 1).
Figure 1. Proportion receiving 12 weeks of ledipasvir and sofosbuvir and overall proportion with SVR12 among patients eligible for 8 weeks across medical centers.
SVR, sustained virologic response. Line and P-value obtained from simple linear regression.
DISCUSSION
In this cohort of HCV-infected adults treated with LDV/SOF in a large healthcare system, SVR12 rates were over 95% in most subgroups of patients who were eligible to receive LDV/SOF for 8 weeks and treated for 8 or 12 weeks. Among black patients eligible for 8 weeks, we found no difference in treatment response between those treated for 8 and 12 weeks, and black race was not associated with treatment failure regardless of treatment duration. We found that 8- week regimens were underused, with 26% of those eligible for 8 weeks receiving 12 weeks of therapy. Patient characteristics associated with receipt of 12 weeks (e.g., higher transient elastography scores, obesity, diabetes) were not associated with reduced treatment response after 8 weeks, suggesting room for improved efficiency in decisions about treatment duration. Furthermore, while receipt of 12 weeks varied across medical centers, there was no relationship between these prescribing practices and the overall proportion with SVR12 at each medical center. Our results indicate that 8-week regimens could be more widely used in clinical practice, and that, contrary to current AASLD/IDSA treatment guidelines, black patients who are otherwise eligible for 8-week regimens of LDV/SOF can be successfully treated with 8-week courses, providing a less costly alternative to patients who may not be able to afford 12 weeks of treatment.
In the absence of randomized trial data to support the AASLD/IDSA guidelines recommending against 8-week regimens of LDV/SOF in black patients, observational studies are needed that compare the effectiveness of 8 and 12 weeks among black patients who are eligible to receive either regimen. Several prior studies have suggested reduced treatment response7–13 among black patients receiving 8 weeks of LDV/SOF, but we are not aware of any published studies that have assessed whether black patients who are otherwise eligible for 8 weeks of treatment have reduced response after 8 compared with 12 weeks. Here, we found essentially equivalent SVR12 rates among 8-week-eligible black patients receiving 8 compared with 12 weeks (95.6% vs. 95.8%). In a study of HCV-infected patients treated within the Department of Veterans Affairs, Su et al. found a slightly lower proportion with SVR12 among black patients who were eligible for and treated for 8 weeks compared with those treated for 12 weeks (93.1% vs. 95.2%), but 95% CIs overlapped and the 12-week group was not restricted to those eligible for 8 weeks.8 In a re-analysis of published ION-3 data, O’Brien et al. found that black patients had lower SVR12 than non-black patients (91.3% vs. 96.2%), but the difference in the 8-week group was not statistically significant, SVR12 was not assessed in black patients by treatment duration, and HCV RNA was not considered.9 In contrast, in a pooled analysis of the ION trials, Wilder et al. found that black patients who had HCV RNA <6 million IU/mL had similar SVR12 rates after 8 compared with 12 weeks of therapy (96.3% vs. 98.3%), but SVR12 was not compared by treatment duration in the subgroup of black patients who met all 8-week eligibility criteria.7 Backus et al. found that 8-week treatment duration, but not black race, was independently associated with lower SVR12 in a multivariable model limited to treatment-naive patients with no cirrhosis and with HCV RNA <6 million IU/mL, but the authors did not directly assess whether SVR12 differed by treatment duration among black patients.12 A recent pooled analysis of real-world cohorts by Kowdley et al. found statistically similar SVR12 rates between black and Caucasian patients who were eligible for and treated for 8 weeks (95.8% vs. 98.8%), but did not compare to SVR12 rates among 8-week-eligible patients treated for 12 weeks.14 Our study extends the existing literature and informs clinical guidelines by demonstrating that black patients who are otherwise eligible for 8 weeks of therapy may not require 12 weeks to achieve SVR12.
We did not identify any demographic, clinical, or behavioral factors that were associated with reduced response after 8 compared with 12 weeks of LDV/SOF among individuals eligible for 8 weeks. We found that treatment with 8 compared with 12 weeks was associated with improved response among individuals aged <50 years in bivariable analysis, but the absolute difference in effectiveness was small (99.0% vs. 93.1%), few younger patients were treated for 12 weeks (n=58), and the association was not statistically significant in a Poisson model. Backus et al. found that 8-week duration was the only factor associated with a reduced likelihood of SVR12 among patients who were eligible for 8 weeks and treated for 8 or 12 weeks.12 However, we are not aware of prior studies that have compared the effectiveness of 8 and 12 weeks of therapy within subgroups of 8-week-eligible patients. Although our findings call into question the AASLD/IDSA guideline on race and treatment duration, they are consistent with recommendations for the use of 8-week regimens in patients who are treatment-naive, have no cirrhosis, are HIV-uninfected, and have HCV RNA <6 million IU/mL.
There are several limitations to our study. First, our sample size of black patients treated for 8 and 12 weeks was small, limiting our ability to detect a difference in SVR12 between the groups. Specifically, we had 80% power to detect a 9% difference in the proportion achieving SVR12 between black patients receiving 8 (n=315) and 12 weeks (n=120). However, the observed SVR12 proportions were essentially equal among 8-week-eligible black patients treated for 8 and 12 weeks (95.6% and 95.8%); thus, it is unlikely that the result was influenced by sample size. Furthermore, our sample size of black patients exceeds that of several studies on which the AASLD/IDSA race-based treatment guideline is based, two of which included only a total of 122 and 308 black patients treated for 8 and 12 weeks.7,9 Second, because our data were derived from a clinical practice setting, clinical decisions about treatment and the provision of care, including timing of laboratory measurements, may have varied across patients; however, our results reflect treatment effectiveness in a real-world setting, with SVR12 rates as high as those reported in clinical trials. Finally, there may have been some misclassification for behavioral factors such as drug abuse and smoking, and their timing with respect to treatment initiation could not be analyzed in detail.
Our study also has several strengths. First, to our knowledge, this is the first study to investigate whether black patients who are eligible for 8 weeks of LDV/SOF have similar response rates after treatment for 8 compared with 12 weeks, informing clinical guidelines regarding race and treatment duration. Second, in contrast to other large observational cohorts of HCV-infected patients that are almost entirely male,12 over 40% of our cohort was female, thus strengthening the generalizability of our results. Finally, the KPNC membership mirrors the age, sex, and race/ethnicity distributions of the surrounding population.15 Thus, our results are likely to be generalizable to other insured adults with HCV infection in California.
In summary, over one-third of HCV-infected patients initiated treatment with direct-acting antiviral agents during the initial two years of availability in this insured population, with high SVR12 rates across all subgroups of patients who were eligible for 8 weeks of LDV/SOF and treated for 8 or 12 weeks. Among black patients eligible for 8 weeks, there was no difference in response between those treated for 8 compared with 12 weeks. Demographic, clinical, and behavioral factors associated with receipt of 12 weeks were not associated with reduced treatment response after 8 weeks. Our findings do not support current AASLD/IDSA treatment guidelines that recommend against the use of 8-week courses of LDV/SOF in black patients, and instead suggest that 8-week courses could be more widely used in select patients in clinical practice, potentially resulting in increased treatment capacity without compromising treatment effectiveness.
Acknowledgments
Grant support: This work was supported by the Kaiser Permanente Delivery Science Research Program and the National Institute of Allergy and Infectious Diseases [K01 122853 to JLM].
Abbreviations
- LDV/SOF
ledipasvir/sofosbuvir
- AASLD
American Association for the Study of Liver Diseases
- IDSA
Infectious Diseases Society for America
- HIV
human immunodeficiency virus
- KPNC
Kaiser Permanente Northern California
- ICD
International Classification of Disease
- SVR
sustained virologic response
- CI
confidence interval
- RR
risk ratio
Footnotes
Disclosures: JLM reports research grant support from Merck. MJS and CPQ report research grant support from Pfizer and Merck. MPP reports research grant support from Merck and Gilead. All other authors report no potential conflicts.
Author contributions: JLM and MJS obtained funding for the study. LBH collected the data. JLM analyzed the data and drafted the manuscript. CPQ provided guidance on the statistical analysis. All authors contributed to study concept and design, interpretation of the data, and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. [Accessed December 13, 2017];Prescribing information for HARVONI. 2014 https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205834s000lbl.pdf.
- 5. [Accessed September 27, 2017];Recommendations for Testing, Managing, and Treating Hepatitis C. 2017 http://www.hcvguidelines.org.
- 6.Kanwal F, Kramer JR, El-Serag HB, et al. Race and Gender Differences in the Use of Direct Acting Antiviral Agents for Hepatitis C Virus. Clin Infect Dis. 2016;63(3):291–299. doi: 10.1093/cid/ciw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilder JM, Jeffers LJ, Ravendhran N, et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: A retrospective analysis of phase 3 data. Hepatology. 2016;63(2):437–444. doi: 10.1002/hep.28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65(2):426–438. doi: 10.1002/hep.28901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien TR, Lang Kuhs KA, Pfeiffer RM. Subgroup differences in response to 8 weeks of ledipasvir/sofosbuvir for chronic hepatitis C. Open Forum Infect Dis. 2014;1(3):ofu110. doi: 10.1093/ofid/ofu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457–471. e455. doi: 10.1053/j.gastro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther. 2017;22(6):481–493. doi: 10.3851/IMP3117. [DOI] [PubMed] [Google Scholar]
- 12.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405–414. doi: 10.1002/hep.28625. [DOI] [PubMed] [Google Scholar]
- 13.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Comparative effectiveness of ledipasvir/sofosbuvir +/− ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir +/− ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016;44(4):400–410. doi: 10.1111/apt.13696. [DOI] [PubMed] [Google Scholar]
- 14.Kowdley KV, Sundaram V, Jeon CY, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65(4):1094–1103. doi: 10.1002/hep.29005. [DOI] [PubMed] [Google Scholar]
- 15.Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey. [Accessed September 27, 2017];2015 https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf.
- 16. [Accessed August 11, 2017];Heading off and helping with unhealthy alcohol use. 2014 https://share.kaiserpermanente.org/article/heading-off-and-helping-with-unhealthy-alcohol-use/
- 17.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. Aids. 2009;23(17):2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. Jama. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg SD, Lu M, Rupp LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57(2):240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16(2):372. doi: 10.1007/s11894-014-0372-6. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21(12):930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]