Abstract
While PD-1 blockade has revolutionized cancer immunotherapy, immune-related adverse events (irAEs) present life-threatening complications. Recent reports of aplastic anemia (AA) as irAEs implicate PD-1/PD-L1 as important in preventing immune-mediated destruction of the hematopoietic niche. Infusion of PD-1-deficient (PD-1 KO) lymph node (LN) cells into minor-antigen mismatched mice resulted in early mortality, as well as more severe BM hypoplasia, anemia, and BM microarchitecture disruption in PD-1 KO LN-infused mice relative to mice that received B6 LN cell infusion. Mice that received PD-1 KO LN cells had more CD8+ T cell infiltration of the BM and greater expansion of H60-specific CD8+ T cells than did their B6 LN-infused counterparts. In the spleen, CD8+ T cells were skewed to an effector memory phenotype, suggesting accelerated differentiation of PD-1 KO T cells. Our data suggest that PD-1 dysregulation has a role in murine BM failure, and vigilance in irAE monitoring may be desirable to treat early AA and related cytopenias.
Keywords: murine bone marrow failure, PD-1
Graphical Abstract

Introduction
Since the first clinical trial of the anti-PD-1 agent nivolumab in 2010 (1), PD-1 blockade has revolutionized the field of cancer immunotherapy. Despite the successes of PD-1-blocking agents in cancer treatment, a major and often life-threatening complication of their use is the development of immune-related adverse events (irAEs). The incidence of all irAEs in nivolumab-treated patients ranges from 30–45%, and more severe grade 3–4 irAEs occur in 3–7% of patients (summarized in (2)). Most common are diarrhea/colitis, pruritis, hepatitis, and hypophysitis (3), but a number of relatively rare autoimmune diseases, such as myasthenia gravis (4), autoimmune sarcoidosis (5, 6), and autoimmune cytopenias (7–9), have also arisen due to nivolumab treatment, implying that PD-1 plays an important role in the pathogenesis of these diseases. One such rare disease, aplastic anemia (AA), has been described in four patients treated with nivolumab (10, 11), despite an incidence in the general population of about 2 cases/million/year (12).
AA is severe bone marrow (BM) failure characterized by pancytopenia, BM hypoplasia, and loss of hematopoietic stem and progenitor cells due to immune-mediated BM destruction (13). While no universal auto-antigen in AA has been described to date, oligoclonal expansion of CD8+ T cells in the peripheral blood and marrow of AA patients (14) and the efficacy of anti-thymocyte globulin and cyclosporine A as a first-line therapy for AA (15) implicate CD8+ cytotoxic lymphocytes in the proximate pathogenesis and maintenance of T cell-mediated target cell destruction. Little is known of the role of costimulatory and coinhibitory molecules in the pathophysiology of AA, and the literature presents conflicting views on the role of immunomodulatory molecules such as TIM-3 (16, 17) and PD-1 (18–20) in T cell-mediated BM destruction.
Reports of four nivolumab-induced AA irAEs in a single year suggest that PD-1 might play a role in preventing AA in a non-oncological context. To dissect the effects of PD-1’s loss on BM failure, we assessed the impact of effector T cells without PD-1 in a well-characterized model of murine BM failure that resembles the pancytopenia and BM hypoplasia seen in AA patients (21). Our observations support a protective role for PD-1 in immune BM destruction and AA.
Materials and Methods
Mice and induction of BM failure
Inbred C57BL/6 (B6), congenic C.B10-H2b/LilMcd (C.B10), and mutant B6.Cg-Pdcd1tm1.1Shr/J (PD-1 knockout [KO]) mice, all from the Jackson Laboratory (Bar Harbor, ME), were bred and maintained in the National Institutes of Health animal facilities under standard nutrition and care. All animal studies were approved by the Animal Care and Use Committee at the National Heart, Lung, and Blood Institute.
Murine BM failure was induced as previously described (21). In brief, inguinal, axillary, and lateral axillary lymph nodes (LNs) from B6 or PD-1 KO mice were homogenized in RPMI 1640 medium using a tissue grinder (Daigger Scientific). After filtration through 90-μm nylon mesh (Small Parts), LN cells were counted using a Vi-Cell counter (Beckman Coulter, Miami, FL). B6 or PD-1 KO-derived LN cells (3–5 × 106) were injected through the lateral tail vein into sex-matched C.B10 recipients pre-irradiated with 5 Gy total body irradiation (TBI). Recipient mice were euthanized at 8 days following injection of LN cells (unless otherwise stated) for further cytometric and histological analyses.
Blood counts, tissue collection, cell staining, and flow cytometry
Blood was collected from the retro-orbital sinus into Eppendorf tubes containing EDTA. Blood cells were counted using a HemaVet 950 analyzer (Drew Scientific Inc., Waterbury, CT). After CO2-induced euthanasia, whole sternums, livers, and large intestines were fixed in 10% formalin solution (Sigma-Aldrich) and sent to VitroVivo Biotech, LLC (Rockville, MD) for decalcification of sternums, fixation, and hematoxylin and eosin (H&E) staining. BM cells were extracted from the tibiae and femurs in RPMI 1640 medium (Gibco Invitrogen) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) and 1% penicillin-streptomycin-glutamine (PSG; Invitrogen). Splenocytes were extracted from whole spleens using a DUALL® tissue grinder (Kimble Chase) and resuspended in 2 mL RPMI 1640 medium supplemented with 10% FBS and 1% PSG. After filtration through 95-μm nylon mesh, BM and spleen cells were counted and treated with ACK lysis buffer (Quality Biological) for 5 minutes on ice, followed by staining at room temperature for 15 minutes with antibody cocktails. After washing and filtration, stained cells were acquired on a BD LSRFortessa cell analyzer using FACSDiva software v.8.0.1 (Becton Dickinson), and data were analyzed using FlowJo software v.10.0 (Tree Star). Compensation was performed using single-color controls and unstained samples. A minimum of 10,000 events per sample were recorded.
Monoclonal antibodies (mAbs) for murine CD3 (clone 17A2), CD4 (clone RM4-5), CD8 (clone 53-6.7), CD44 (clone IM7), and CD62L (clone MEL-14) were purchased from BioLegend (San Diego, CA). mAbs were conjugated to phycoerythrin (PE), PE-cyanin 7 (PE-Cy7), allophycocyanin (APC), APC-cyanin 7 (APC-Cy7), brilliant violet 421 (BV421), and fluorescein isothiocyanate (FITC). H60 tetramer was produced by the MHC Tetramer Production Facility at the Dan L. Duncan Cancer Center, Baylor College of Medicine.
ELISpot
Isolated splenocytes (105) from TBI, PD-1 KO LN-infused, or B6 LN-infused recipient mice were resuspended in 200 μL RPMI-1640 + 10% FBS +1% PSG and plated in a 96-well enzyme-linked immunospot (ELISpot) microplate. After overnight incubation at 37°C and 5% CO2, quantification of IFN-γ-secreting cells was performed using the ELISpot kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The number of spots per well were counted using an ImmunoSpot analyzer (CTL, Cleveland, OH).
Statistics
Flow cytometric data were analyzed by unpaired t test, one-way or two-way ANOVA, and multiple comparisons using Prism statistical software (GraphPad Software). Data were expressed as mean ± SEM. Statistical significance was declared at p < 0.05.
Results and Discussion
PD-1 KO donor cell infusion results in anemia and widespread BM destruction
To determine the role(s) of PD-1 in the induction and maintenance of BM failure, we used LN cells from donor mice bearing a targeted deletion of exons 2 and 3 of PD-1 (22). We injected 5 × 106 B6 LN cells, or 3–5 × 106 PD-1 KO LN cells, into each pre-irradiated C.B10 recipient. We have shown previously that infusion of B6 LN cells into sublethally-irradiated C.B10 recipients results in a fatal BM failure syndrome due to expansion of CD8+ T-cells reactive to the immunodominant H60 minor antigen, for which the B6 mouse carries a null allele (21). All mice were bled and euthanized for tissue collection and analyses at day 8, upon the death of one mouse at day 7 that had received PD-1 KO LN cell infusion. This early mortality of mice infused with PD-1 deficient T cells was also reported by Blazar et al (23), as infusion of 1–3 × 106 B6 PD-1 KO CD4+ and CD8+ T cells induced lethality 19 or 11 days after infusion into B6(C)-H2-Ab1bm12/KhEgJ recipient mice, respectively. Modifications in immune response by PD-1 blocking agents may be effective to modulate anti-tumor activity by T cells(23–25). However, the absence of PD-1 signaling pathway can lead to increased IFN-γ production, Th1 differentiation of alloreactive T cells during graft-versus host disease, and resistance to apoptosis by modulating reactive oxygen species (23, 24). Therefore, patients treated with PD-1 inhibitors or PD-1-deficient mice are not only more sensitive to infections, but may also have exaggerated immune reactions against pathogens or autoantigens because of decreased self-tolerance(26, 27). The accelerated mortality of our C.B10 recipient mice infused by PD-1 KO LN cells may be due to a greater expansion and enhanced cytotoxicity of T cells against hematopoietic stem and progenitor cells in the BM. Indeed, while all mice infused with B6 or PD-1 KO LN cells had reduced BM cellularity, PD-1 KO LN-infused mice had fewer BM cells than B6 LN-infused controls (22.6±2.1 × 106 cells vs 33.4±5.2 × 106 cells per 2 legs, Fig. 1A). PD-1 KO LN-induced BM failure mice had lower red blood cell (4.70±0.17 × 1012 vs 6.19±0.49 × 1012/L, p < 0.01) and platelet (7.60±0.856 × 1010 vs 12.4±1.36 × 1010/L, p < 0.05) counts (Fig. 1B) as compared to their B6 LN cell-infused counterparts. By H&E staining, sternums of B6 or PD-1 KO LN-induced BM failure mice had reduced nucleated cell numbers and damaged BM structrue compared with those of TBI control, and sternums of BM failure mice infused with PD-1 KO LN cells displayed an even more dramatic reduction of nucleated cell numbers and more severely damaged BM structrue (Fig. 1C). Similar observations have also been reported in the case series by Michot et al., in which three advanced-stage lung adenocarcinoma patients developed severe acquired AA within 0.5–6.2 months after anti-PD-1 therapy, and biopsy shows a hypocellular BM (cellularity <10%) with activated CD8 T cells(11).
Figure 1.
PD-1 KO LN infusion induces severe BM failure. (A) Bone marrow (BM) nucleated cell number in TBI (n=2), B6 LN-induced BM failure (n=4), and PD-1 KO LN-induced BM failure (n=8). (B) Neutrophils (NE), white blood cells (WBC), red blood cells (RBC), and platelets (PLT) in the peripheral blood at day 8. (C) Representative H&E staining of the sternums of TBI, B6 LN-induced, and PD-1 KO LN-induced BM failure mice at day 8. Top row: 100× original magnification, with 50-μm scale bars (white bar). Bottom row: 200× original magnification. Capped lined indicate a p-value obtained through unpaired t test. *, p < 0.05; **, p < 0.01.
Antigen-specific T cells expand in the spleen and BM of PD-1 KO LN-induced BM failure mice
Because severity of BM failure was increased in PD-1 KO-LN infused mice, we evaluated T cells present in the BM and spleen. At day 8, we observed a greater percentage of CD4+ and CD8+ T cells in the BM of PD-1 KO LN-infused BM failure mice, as compared to B6 LN-induced BM failure and TBI controls (Fig. 2A). Notably, CD8+ T cells, the subset mainly responsible for BM destruction in this model (28), constituted a higher fraction of whole BM cells in PD-1 KO LN-induced BM failure mice than B6 LN-induced BM failure or TBI controls (1.79±0.26% vs 0.37±.09% vs 0.17±.07%, respectively; p < 0.05). In PD-1 KO LN-induced BM failure mice, H60 antigen-specific CD8+ T cells comprised 0.84±0.15% of the whole BM, as compared to 0.17±0.04% of the BM of B6 LN-induced BM failure mice, as measured by H60-specific tetramer staining (Fig. 2B). H60-specific CD8+ T cells were also increased in the spleens of PD-1 KO LN-induced BM failure mice (Fig. 2C).
Figure 2.
PD-1 KO LN-infused mice have increased T cell BM infiltration and antigen-specific T cell expansion. (A) Representative flow plots and statistical analyses of CD4+ and CD8+ T cells in the BM of PD-1 KO LN-infused mice. CD4+ and CD8+ T cells were gated on all live BM cells. (B) Representative flow plots and summarized results of H60-specific T cells in the BM of BM failure mice. H60 tetramer-positive cells were gated on live BM cells. (C) Representative flow plots and summarized results of H60-specific T cells in the spleens of BM failure mice. H60 tetramer-positive cells were gated on live splenocytes. Capped lines indicate a p-value obtained through unpaired t-test, while brackets indicate a p-value obtained through multiple comparisons. *, p < 0.05; **, p < 0.01.
PD-1 knockout in donor cells accelerates CD8 T cell differentiation in the BM and spleens of recipients
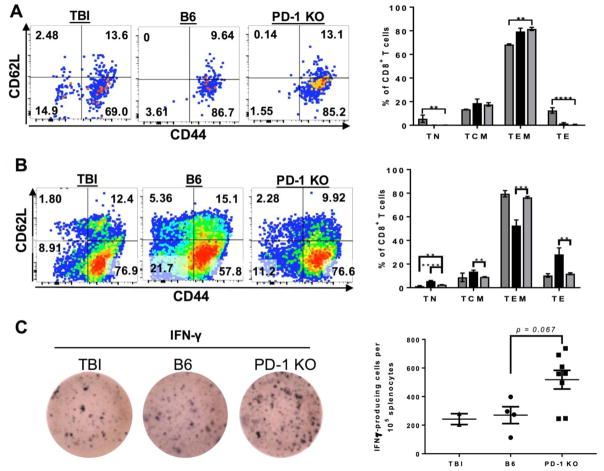
Because CD8+ T cells are the primary subset implicated in hematopoietic destruction during human AA and murine BM failure (28), we evaluated the differentiation of CD8+ T cells in the BM and spleen of BM failure mice. Of CD8+ T cells present in the BM of BM failure mice, the majority were effector memory T cells (TEMs), as defined by low expression of L-selectin (CD62L−) and high expression of CD44. While the TEM subset in TBI mice also constituted the majority of BM CD8+ T cells, the mean percentage of TEM cells was much lower than that of either BM failure group (68.4±0.65% TBI vs 79.5±2.73% B6 LN-induced BM failure and 81.6±1.39% PD-1 KO-induced BM failure; p < 0.01 for TBI vs PD-1 KO LN-induced BM failure, Fig. 3A). TBI mice had a higher percentage of central memory (TCM, CD44+CD62L+) and naïve CD8+ T cells (TN; CD44-CD62L+) than was present in any of the BM failure mice. There were no significant differences between B6 LN-induced and PD-1 KO LN-induced BM failure mice in composition of their CD8+ T cell phenotypes (p > 0.473 for all subsets), likely because T cells homing to the BM during BM failure differentiate rapidly there, compared to the blood and spleen, resulting in a “terminal” effector memory phenotype similar to the cytotoxic TEMs found in the BM of human AA patients (29).
Figure 3.
CD8+ T cells are terminally differentiated in the BM, while PD-1 KO T cells have accelerated terminal differentiation in the spleen. (A) Representative flow plots and statistical analyses of CD8+ T cell differentiation subsets in the BM of TBI (dark grey), B6 LN-induced BM failure (black), and PD-1 KO LN-induced (light grey) BM failure mice. Cells shown in the flow plots were gated on all live CD8+ T cells. (B) Representative flow plots and statistical analyses of CD8+ T cell differentiation subsets in the spleen. (C) Representative ELISpot wells (Right) showing IFN-γ secretion by splenocytes isolated from TBI only, B6 LN-infused, and PD-1 KO LN-infused mice. Cells were rested overnight at 37°C, 5% CO2 prior to ELISpot development and analysis. Quantification of IFN-γ-positive spots per 105 splenocytes plated (Left). Capped lines indicate a p-value obtained through unpaired t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
The majority of CD8+ T cells in the spleen of TBI mice were TCM and TEM, with similar proportions of both subsets in the spleen of B6 LN-induced BM failure mice (Fig. 3B). B6 LN-infused mice did have a higher proportion of naïve CD8+ T cells in the spleen than did TBI controls, with 5.61±0.48% of CD8+ T cells in the B6 LN-induced BM failure spleen expressing CD62L+ in the absence of CD44 as compared to 1.29±0.51% in TBI animals’ spleens. This increase in naïve CD8+ T cells in the spleens of BM failure mice is likely due to the B6 donor cells being LN-derived, as injected LN cells may not have differentiated into memory CD8+ T cells by day 8 of BM failure. In the spleens of PD-1 KO LN-induced BM failure mice, there were relatively few naïve or TCM cells, as the spleens of PD-1 KO-infused mice contained two-thirds as many TCM cells as the spleens of their B6-infused BM failure counterparts (Fig. 3B). Reduced TCM cells corresponded to an increase in TEM cells in the PD-1 KO LN-induced BM failure group, with TEM cells consisting of 76.5% of the splenic CD8+ T cell compartment. Since TEM cells are the pathogenic cell subset in human AA, and TEM cells tend to be more terminally differentiated than TCM cells (30), loss of PD-1 on donor T cells may accelerate terminal CD8+ T cell differentiation. Expansion of TEM cells also has been described in tumor-infiltrating lymphocytes from melanoma patients treated with PD-1 blockade therapy, and related to improved survival (31). However, during BM failure, TEM cells may be higher in patients who have a poor response to immunosuppressive therapies(32). Our findings confirm that loss of PD-1 is frequently associated with the expansion of TEM cells, supporting the key role of coinhibitory molecules such as PD-1 in T cell differentiation. Moreover, expanded TEM cells may play an important role in BM destruction during marrow failure. Accelerated CD8+ T cell differentiation in secondary lymphoid organs of mice infused with PD-1 KO LN cells may explain the accumulation of CD8+ T cells in the BM (Fig. 2A) and destruction of hematopoietic stem and progenitor cells without significant reduction in BM cellularity, as normal BM cells were replaced by pathogenic CD8+ TEM cells early in the BM destruction process (Fig. 1C & B, respectively). Splenic T cells also secreted more IFN-γ in mice infused with PD-1 KO LN cells (Figure 3C), suggesting that PD-1 deficiency in pathogenic T cells results in increased secretion of cytokines important in BM failure pathogenesis(23). Moreover, PD-1 blockade can cause apoptosis of central-memory T regulatory cells by interfering with IL-2 signaling, leading to the disruption of Treg-mediated tolerance(33).
In this study, we showed that induction of murine BM failure using PD-1 deficient LN cells resulted in severe BM hypoplasia, anemia, and destruction of BM. While the nucleated BM cellularity in PD-1 KO-infused BM failure mice was not significantly reduced as compared to B6 LN-induced BM failure controls, marked changes in BM histology imply that loss of PD-1 on pathogenic T cells results in a more severe anti-BM immune phenotype. In other experiments, we were unable to demonstrate alterations in the frequency or severity of immune BMF in the mouse when H2 antigen-mismatch models were tested (infusion of PD-1 KO LN cells into CByB6F1 recipients, for example; data not shown). Likely, modulation of T cell BM destruction via PD-1 is evident in a weaker allogeneic context, such as the minor histocompatibility antigen mismatch we report here.
As CD8+ T cells are believed to be the primary cell subset responsible for murine BM failure and human AA, we have characterized CD8+ T cells in irradiated control mice and mice with BM failure induced by infusion of B6 or PD-1 KO LN cells. CD8+ T cells from PD-1 KO LN-injected mice showed greater H60 antigen-specific expansion in the spleens and BM of recipient mice, with a greater proportion of TEM cells in the spleen as compared to B6 LN-induced BM failure mice. The increased proportion of TEMs in the spleens of PD-1 KO-infused mice suggests that PD-1-deficient T cells accelerate differentiation toward a terminal effector phenotype, allowing them to home to the BM and initiate hematopoietic stem cell destruction.
With the increasing use of costimulatory blockade in the clinic, monitoring of adverse reactions in patients will likely be important in preventing irAE-induced mortality. Close monitoring of peripheral blood counts in anti-PD-1 treated patients may serve to quickly detect developing cytopenic or AA-like syndromes and allow control of T cell dysregulation by halting treatment and administering immunosuppressive or immunomodulatory therapies effective in AA. Our findings, which support a role for PD-1 loss as an agonist of BM failure, support the need for careful consideration of PD-1 blockade’s off-target effects and highlights the importance of PD-1 as an internal control switch for autoimmune destruction.
Highlights.
Infusion of PD-1-deficient (PD-1 KO) lymph node (LN) cells into minor-antigen mismatched mice resulted in early mortality, as well as more severe BM hypoplasia, anemia, and BM microarchitecture disruption in PD-1 KO LN-infused mice relative to mice that received B6 LN cell infusion.
Mice that received PD-1 KO LN cells had more CD8+ T cell infiltration of the BM and greater expansion of H60-specific CD8+ T cells than did their B6 LN-infused counterparts.
In the spleen, CD8+ T cells were skewed to an effector memory phenotype, suggesting accelerated differentiation of PD-1 KO T cells.
Acknowledgments
This work was funded by the Intramural Research Program (IRP) of the National Heart, Lung, and Blood Institute.
Footnotes
Authorship Statement: M.K.H. and X.F. designed and performed the experiments, analyzed the data, and wrote the manuscript; V.G., N.A.C., G.R., H.Z., S.K., and J.C. performed the mouse experiments; K.K. contributed flow cytometric analyses; N.S.Y conceived the study and was involved in discussions, data analysis and manuscript preparation. All authors have critically reviewed the manuscript content and agree with the submission of the final manuscript.
Disclosures
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. Journal of Clinical Oncology. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Frontiers in Pharmacology. 2017:8. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 4.Makarious D, Horwood K, Coward JIG. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. European Journal of Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum MR, Ma MW, Fleisig S, Packer S, Amin BD, Jacobson M, McLellan BN. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Reports. 2017;3:208–211. doi: 10.1016/j.jdcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Gao J, Shannon VR, Siefker-Radtke A. Systemic sarcoidosis first manifesting in a tattoo in the setting of immune checkpoint inhibition. BMJ Case Reports. 2016 doi: 10.1136/bcr-2016-216217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turgeman I, Wollner M, Hassoun G, Bonstein L, Bar-Sela G. Severe complicated neutropenia in two patients with metastatic non-small-cell lung cancer treated with nivolumab. Anti-Cancer Drugs. 2017 doi: 10.1097/CAD.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 8.Bulbul A, Mustafa A, Chouial S, Rashad S. Idiopathic thrombocytopenic purpura and autoimmune neutropenia induced by prolonged use of nivolumab in Hodgkin’s lymphoma. Annals of Oncology. 2017;28:1675–1676. doi: 10.1093/annonc/mdx159. [DOI] [PubMed] [Google Scholar]
- 9.Inadomi K, Kumagai H, Arita S, Tsuruta N, Takayoshi K, Mishima K, Ota S, et al. Bi-cytopenia possibly induced by anti-PD-1 antibody for primary malignant melanoma of the esophagus: A case report. Medicine (Baltimore) 2016;95:e4283. doi: 10.1097/MD.0000000000004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helgadottir HKL, Ljungman P, Larkin J, Kefford R, Ascierto PA, Hansson J, GM Lethal aplastic anemia caused by dual immune checkpoint blockade in metastatic melanoma. Annals of Oncology. 2017;28:1672–1673. doi: 10.1093/annonc/mdx177. [DOI] [PubMed] [Google Scholar]
- 11.Michot JM, Vargaftig J, Leduc C, Quere G, Burroni B, Lazarovici J, Champiat S, et al. Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer. 2017;80:1–4. doi: 10.1016/j.ejca.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008;93:489–492. doi: 10.3324/haematol.12855. [DOI] [PubMed] [Google Scholar]
- 13.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. ASH Education Book. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-γ in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 16.Liu X, Cui X, Yuan D, Li Y, Shan N-n, Wang X, Hu Y. Altered expression of T cell Immunoglobulin-Mucin (Tim) molecules in peripheral blood mononuclear cells in aplastic anemia. Cancer Cell International. 2014;14:144. doi: 10.1186/s12935-014-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Gu Y, Xu C, Qu X. Increased T cell immunoglobulin mucin-3 and its ligand in acquired aplastic anemia. European Journal of Haematology. 2017;81:130–139. doi: 10.1111/j.1600-0609.2008.01095.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Miao M, Qiu Y, Qin Z, Wang J, Jiang Y, Ming Z, et al. Association between polymorphisms in PDCD1 gene and aplastic anemia in Chinese Han population. Leukemia & Lymphoma. 2013;54:2251–2254. doi: 10.3109/10428194.2013.772605. [DOI] [PubMed] [Google Scholar]
- 19.Ming ZJ, Hui H, Miao M, Qiu YH, Zhang XG. Polymorphisms in PDCD1 gene are not associated with aplastic anemia in Chinese Han population. Rheumatology International. 2012;32:3107–3112. doi: 10.1007/s00296-011-2127-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Zhang Y, Zhang P, Yang J, Zhang L, He A, Zhang W, et al. High programmed death 1 expression on T cells in aplastic anemia. Immunol Lett. 2017;183:44–51. doi: 10.1016/j.imlet.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young Neal S. Minor Antigen H60-Mediated Aplastic Anemia Is Ameliorated by Immunosuppression and the Infusion of Regulatory T Cells. The Journal of Immunology. 2007;178:4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 22.Keir ME, Freeman GJ, Sharpe AH. PD-1 Regulates Self-Reactive CD8+ T Cell Responses to Antigen in Lymph Nodes and Tissues. The Journal of Immunology. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 23.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 24.Tkachev V, Goodell S, Opipari AW, Hao LY, Franchi L, Glick GD, Ferrara JL, et al. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J Immunol. 2015;194:5789–5800. doi: 10.4049/jimmunol.1402180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Wu S, Guo G, Fei L, Guo S, Yang C, Fu X, et al. Programmed death (PD)-1-deficient mice are extremely sensitive to murine hepatitis virus strain-3 (MHV-3) infection. PLoS Pathog. 2011;7:e1001347. doi: 10.1371/journal.ppat.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thangavelu G, Parkman JC, Ewen CL, Uwiera RR, Baldwin TA, Anderson CC. Programmed death-1 is required for systemic self-tolerance in newly generated T cells during the establishment of immune homeostasis. J Autoimmun. 2011;36:301–312. doi: 10.1016/j.jaut.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Feng X, Desierto MJ, Keyvanfar K, Young NS. IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood. 2015;126:2621–2631. doi: 10.1182/blood-2015-06-652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Gu Y, Wang Y, Cong Y, Qu X, Xu C. Increased CD4+ and CD8+ effector memory T cells in patients with aplastic anemia. Haematologica. 2009;94:428–429. doi: 10.3324/haematol.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi NS, Kaech SM. Effector CD8 T Cell Development: A Balancing Act between Memory Cell Potential and Terminal Differentiation. The Journal of Immunology. 2008;180:1309. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 31.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119. e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29:1270–1277. doi: 10.1016/s0301-472x(01)00736-6. [DOI] [PubMed] [Google Scholar]
- 33.Asano T, Meguri Y, Yoshioka T, Kishi Y, Iwamoto M, Nakamura M, Sando Y, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129:2186–2197. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]