Abstract
To battle adverse internal and external conditions and maintain homeostasis, diploid, organisms employ various cellular processes, such as proliferation and apoptosis. In, some tissues, an alternative mechanism, endoreplication, is employed toward similar, goals. Endoreplication is an evolutionarily conserved cell cycle program during which, cells replicate their genomes without division, resulting in polyploid cells. Importantly, endoreplication is reported to be indispensable for normal development and organ, formation across various organisms, from fungi to humans. In recent years, more, attention has been drawn to delineating its connections to wound healing and, tumorigenesis. In this review, we discuss mechanisms of endoreplication and, polyploidization, their essential and positive roles in normal development and tissue, homeostasis, and the relationship between polyploidy and cancer.
Keywords: Endoreplication, Polyploidy, Development, Compensatory Cellular Hypertrophy, Polyploid
Endoreplication, an alternative cell cycle program
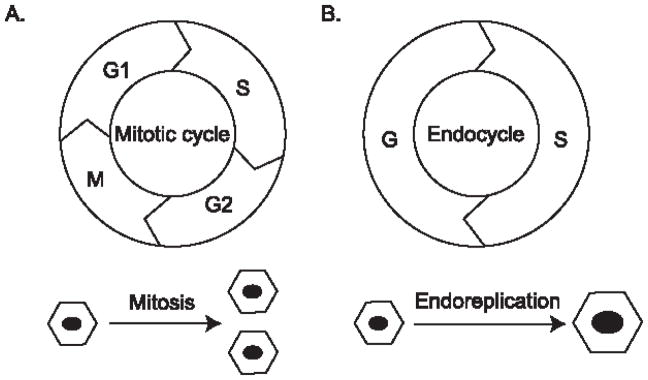
The cell cycle is spatiotemporally regulated in multicellular organisms, the precision of which ensures that the daughter cells maintain the same amount of genetic content as the mother cell. The most common cell cycle progression is the G1-S-G2-M cycle, or the ‘mitotic cycle’ (Figure 1A). The mother cell prepares itself during the G1 and G2 phases, replicates its DNA during the DNA synthesis (S) phase, and splits the DNA equally between two daughter cells during the mitosis (M) phase. In contrast, the endoreplication cycle (see Glossary) consists of only the G and S phases, and therefore generates polyploid cells (see Glossary) with multiple copies of the original DNA content (Figure 1B). The result is either a cell that maintains separate nuclei and remains multi-nucleated, or a cell with an enlarged, single nucleus containing all the DNA. The former process is called endomitosis (See Glossary), and the latter is called endoreplication or endocycling (1, 2).
Figure 1.
Comparison between the mitotic cycle and endocycle
A. The mitotic cycle comprises the G1, S, G2, ad M phases. A cell divides into two daughter cells. B. The endocycle only has the G and S phases. Through endoreplication, a cell increases its DNA content without cell division.
The endocycle employs a very similar set of regulators to the mitotic cycle for its transition and regulation, such as cyclin-dependent kinases (CDKs) and the transcriptional activator E2F (3, 4). However, inhibition of mitosis entry is key to the transition and maintenance of endoreplication in tissues. In essence, mitotic inhibition is achieved by the downregulation of mitotic CDK activity. In Drosophila ovarian follicle cells, upon activation by Fizzy-related (Fzr), the Drosophila homolog of Cdh1, the E3 ubiquitin ligase Anaphase-Promoting Complex/Cyclosome (APC/C) degrades mitotic cyclins to prevent the cell from entering mitosis (5). During endoreplication, APC/C oscillates to mediate Geminin for endocycle progression (6).
The alternating S and G phases of the endocycle are regulated in part by the key S-phase regulator Cyclin E (CycE)-Cdk2 kinase, whose accumulation is crucial for DNA synthesis; CycE is degraded during the G phase to ensure the pre-replication complex (pre-RC) forms for the next round of DNA replication. This oscillation is required for endoreplication and may be achieved through the destruction of the CDK inhibitor Dacapo, a member of the Cip/Kip family in mammals, during the S phase via a PIP degron (7). Dacapo is also reported to affect length of endoreplication and the extent of genome replication (8).
Interestingly, consistent high levels of CycE inhibit endoreplication (9). Recently, CycE was reported to be differentially up-regulated in different regions of the Drosophila wing imaginal disc by a growth regulatory pathway, Yki-Scalloped signaling, involving Yki and one of its transcription factors, Scalloped (10). This finding suggests that tissues may have different predispositions to endoreplication. Therefore, an understanding of the roles of endoreplication is key to deciphering direct and indirect links between endoreplication and tissue homeostasis. In this article, both the beneficial and detrimental roles of endoreplication are discussed.
Organismal benefits of endoreplication
Development
Polyploid cells are essential for achieving normal size and functionality of a range of tissues and organs (4). Endoreplication induced polyploidy plays a pivotal role in tissue development in various organisms, and is usually an irreversible process that is responsible for terminal cell differentiation. In mammals, endoreplication and polyploidy are observed in multiple tissues and organs during normal development, including the skin, placenta, liver, and blood (4). In placenta, trophoblast giant cells endoreplicate to provide a barrier between the maternal blood supply and that of the offspring embryo (12). Megakaryocytes become polyploid before fragmenting into platelets, a necessary type of blood cell for blood clotting. This polyploidy is achieved by induced endomitosis, resulting in aborted cytokinesis (11). Mammalian hepatocytes also undergo gradual polyploidization by endomitosis during postnatal growth, an indicator of terminal differentiation and senescence (13).
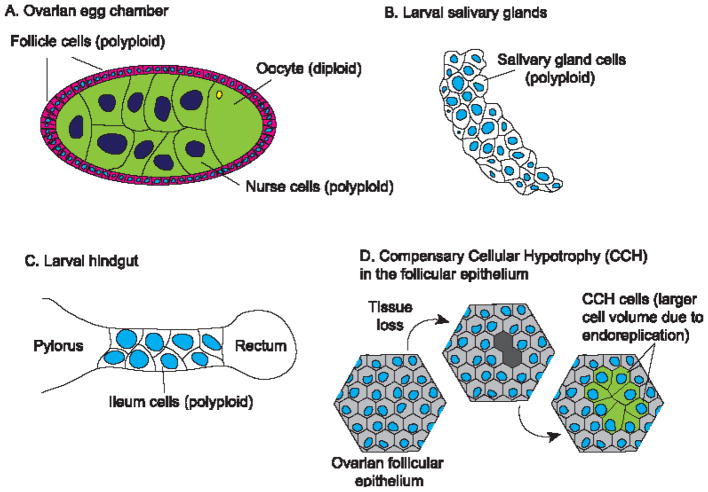
In insects like Drosophila melanogaster, many larval tissues including the salivary gland, fat body, gut, and trachea, and adult tissues including ovarian follicular epithelium, germ-line nurse cells, glia, and gut, experience polyploidy (4). Among these, development of the ovarian follicular epithelium has been well studied. This epithelial monolayer progresses through three continuous and distinct stages: mitotic, endoreplication and chorion gene amplification, for sufficient eggshell protein production (Figure 2A, 14, 15, 16). Endoreplication of the larval salivary gland cells ensures adequate synthesis of digestive enzymes and glue substances for secretion and storage (Figure 2B, 17). Endoreplication is also observed during Drosophila hindgut development (Figure 2C) and helps preserve its function in maintaining the water and ion balance of the hemolymph (18). Although polyploid cells tend to be terminally differentiated, in Drosophila rectal papillae and mosquito (Culex pipiens) ileum, polyploid cells undergo mitotic cycles during development, and this process is prone to errors such as extended anaphases, chromosome bridges, and lagging chromosomes (19). It is speculated that polyploid mitotic cycling is advantageous when only a small number of cells within a large polyploid population need to expand (19). This finding provides new perspectives on irreversibility of endoreplication and how the error-prone polyploid mitotic cycle may lead to aneuploidy and contribute to cancer development.
Figure 2.
Examples of endoreplication in Drosophila
A. During development, adult ovarian follicle cells and germline cells become polyploid. B. In Drosophila larva, salivary gland cells are polyploid. C. Polyploid cells are observed in the ileum region of hindgut in the larval Drosophila. D. In post-mitotic tissues like the follicular epithelium, compensatory cellular hypotrophy (CCH) takes place after tissue volume loss.
Endoreplication of the mammalian genome is generally uniform; in contrast, underreplicated genomic regions were observed in Drosophila (20). For example, in the salivary gland, the linker histone H1, directly interacting with the Suppressor of Underreplication (SUUR) to bind to chromatin, is required for the underreplication phenomenon during endoreplication (21). Interestingly, the localization of H1 in chromatin changes profoundly during the endocycle, which may play an important role in DNA replication timing (21). More recently, advances in genome-wide studies have revealed that somatic copy number variations (CNVs) are common in mammals. For example, underrepresented (UR) domains are found in the mouse polyploid placental genome (22, 23). Genetic variations in polyploid genomes may be a normal feature across different organisms, essential for development and homeostasis.
Endoreplication is more common in plants than animals, and plays a crucial role in plant development and to maintain genome and cell functions. For example, developing plant seeds depend on endosperm tissue, an endoreplicating tissue, as an energy source before becoming self-sufficient through photosynthesis and root formation (24). Endoreplication also increases plants’ tolerance to environmental stress and resource-limiting conditions. For example, in a high-temperature or water-deficit environment, a smaller endosperm is formed as endoreplication is negatively affected (25). Endoreplication also helps maintain cell functions. In Arabidopsis, endoreplication, which is promoted by the epidermal specification pathway, helps establish giant cell identity by regulating the cell cycle in the outer epidermis of the sepal (26). Plants can also regulate tissue-specific, context-dependent endoreplication via hormone pathways such as gibbererllin (GA) (27). Another critical plant-specific factor affecting endoreplication is light. For example, during plant seed development, the dark environment extinguishes DEL1, an atypical E2F that inhibits endoreplication, and therefore hypocotyl cells endoreplicate (28). Because endoreplication enables the plants to grow at a faster rate and achieve higher metabolism (29), polyploidy confers larger sizes and greater green mass than their diploid counterparts, providing a clear agronomical advantage to plants such as coffee, watermelon, maize and bananas, by endowing better fitness to the environment and consequently higher yields (30). It is, however, important to note that in plants, both somatic and germline cells can become polyploid and contribute to enlarged size. Germline polyploidy - alloployploidy - arises during crop breeding and selection, with multiple copies of certain chromosome sets. Discussed in the instances above is developmental polyploidy, in which specific somatic cell lineages become polyploid during plant growth and development (31).
Tissue homeostasis
Endoreplication is developmentally indispensable for many tissues across a diverse range of organisms. It has also been found to contribute significantly to maintaining genome integrity and tissue homeostasis. Some plants use endoreplication as a survival response to genome damage. For example, DNA damage in the Arabidopsis root tip and leaf cells elicits downregulation of mitotic factors, thus promoting endoreplication (32, 33). In animals, endoreplicating cells acquire resistance to DNA damage by lowering proapoptotic gene expression levels (34). Furthermore, endoreplication, in conjunction with endomitosis, has been found to play a critical role in regeneration. For example, after partial hepatectomy, mammalian livers have a remarkable capacity for regeneration, which occurs partially via CycE-mediated endoreplication (35, 36, 37). Endoreplication is also essential to maintain blood-brain barrier in Drosophila (38), a mechanism necessary for ionic homeostasis between the brain and the hemolymph. The Drosophila subperineurial glia (SPG) is polyploid and the increased cell size is required to maintain the SPG envelope surrounding the brain (38).
Over the past decade, several advances have been made in addressing the roles of endoreplication and polyploidization in wound healing and tissue repair mechanisms. Compensation for cell death in tissues is vital for normal development and maintaining tissue integrity. This can be achieved in two ways – by proliferation, or by growth of the remaining cells in the tissue to compensate for the resulting loss of tissue volume (39, 40). In post-mitotic tissues like the Drosophila follicular epithelium, ‘Compensatory Cellular Hypertrophy’ (CCH, see Glossary) is observed in response to a local loss of tissue volume from cell competition (Figure 2D). Cells experiencing CCH are characterized by greater than two-fold increase in DNA content and higher endoreplication rates (39, 40). In another set of experiments using the Drosophila adult epidermis, it was shown that after a tissue was wounded by puncturing, the remaining cells undergo polyploidization, through cell fusion and increased number of cells entering into endoreplication, a process called Wound-Induced Polyploidy (WIP), highly conserved in metazoans (41). Taken together, these studies highlight the essential roles of endoreplication in achieving tissue homeostasis.
Regulation of endoreplication
Inducing or regulating endoreplication during development involves signaling pathways. For example, InR/Pi3K/TOR signaling promote cell growth and endoreplication, while its reduction leads to endocycle inhibition and generation of small cells in Drosophila fat body and salivary glands (42). During oogenesis in Drosophila, Notch signaling plays a critical role in the mitotic-to-endocycle transition. After cystoblasts are generated by germline stem cells in the germarium, they undergo mitosis to produce a single layer of follicle cells that enclose the germline cells (43). The germline cells produce the ligand Delta that binds to the Notch receptor on the follicle cell membrane resulting in proteolytic cleavage of the receptor and release of the intracellular domain of Notch (NICD). In the nucleus, NICD, together with transcription factor Su(H), induces expression of downstream genes (44). One of these genes is Fizzy-related (Fzr), which induces the switch from the mitotic cycle to the endocycle (14, 16). During development, follicle cells undergo exactly three rounds of endocycle, as evidenced by their 2n, 4n, 8n, and 16n DNA peaks, observed using flow cytometry (45). Activation or suppression of the Notch pathway activity either induces premature endocycle entry or endocycle delay, respectively (14, 46). Endoreplication produces more copies of the genome, which increases the biosynthetic capability of cells. In these cells, endoreplication and the subsequent DNA amplification stages prepare them to produce sufficiently high levels of protein for eggshell biosynthesis (47). In Drosophila salivary glands, the sister chromatids in polyploid cells are arranged along their lengths, giving the “giant polytene” chromosomes their distinct banding pattern (48). Incidentally, it was in this system that the oscillating transcription factor E2F1 was found to be essential for endoreplication (49). This study also provided data in support of a model in which E2f1 accumulation during G phase activates CycE/Cdk2, which triggers S phase. CRL4CDT2 is then activated and it mediates the destruction of E2F1 (49, 50).
Endoreplication, when induced to maintain tissue integrity and homeostasis, involves a broader set of signaling pathways. The Insulin and Insulin-like growth factor signaling (IIS) pathway is required for CCH in Drosophila follicular epithelia, likely via mechanotransduction (39). The exact mechanism of how seemingly ‘random’ cells undergo CCH in the follicular epithelium is yet to be ascertained. The main hypothesis involves the link between the altered cortical tension within the remaining cells of the damaged tissue, and the induction of endoreplication, that together trigger the CCH response to maintain tissue integrity. Interestingly, CCH in the follicular epithelium does not involve Hippo signaling, while WIP in the adult Drosophila epidermis requires deregulation of Hippo signaling for polyploidization. Additionally, WIP also involves the activation of JNK signaling (41). The differences in tissue architecture and organization, and tissue-specific genetic constraints, could attribute to the different pathways leading to the same ‘phenotypes’ of wound-induced polyploidy and compensatory hypertrophy.
More recently, EGFR/RAS/MAPK signaling was found to play a fundamental role in Drosophila gut regeneration following epithelial damage, independent of Insulin/Pi3K/TOR signaling (51). This finding further suggests that distinct signaling pathways are used in post-mitotic homeostasis. Interestingly, in zebrafish, regeneration of the epicardium, a cardiac form of mesothelial tissue, involves endoreplication of leader cells, which is caused by tension anisotropy, i.e. greater velocities and mechanical tension within the epicardial tissue sheet (52).
Despite the tissue-specific responses observed in different tissues, the activators or inducers of the downstream pathways may well be the same – the ‘pulling’ of the surviving cells around or in close proximity to the damage site following either cell competition or tissue damage. It is known that Hippo signaling is activated in response to differences in cortical tension (53, 54); it is possible that the IIS and EGFR/RAS/MAPK pathways are also the downstream response of altered cortical tension. The detailed mechanism of how these pathways are induced in a tissue-specific manner by the same ‘stimulus’ for the maintenance of tissue integrity remains to be determined.
Cancer: An ugly consequence of endoreplication and polyploidization
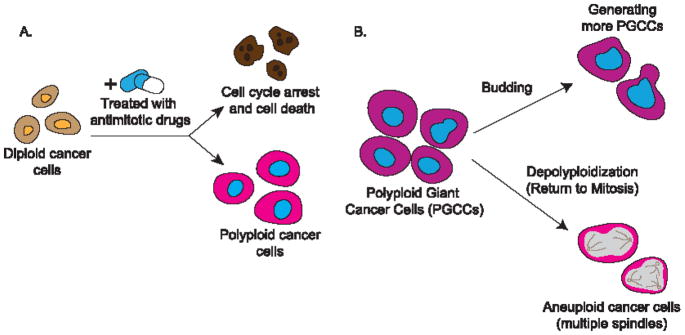
Although endoreplication and polyploidization play positive and sometimes essential roles in many developmental and homeostatic processes, they have been implicated in primary tumor formation, cancer progression and metastasis, and cancer relapse (Figure 3). Clinically, endoreplication and polyploidy have been observed in cancer tissues (55), with their occurrence ranging from 11% in stomach carcinoma to 54% in liver adenocarcinoma (56). Although it is not clear how endoreplicating cells overcome cell cycle regulation and escape homeostasis-induced apoptosis or senescence, polyploidy has been hypothesized to confer a survival advantage to tumor cells. It has been reported that tetraploid cancer cells gain enhanced viability over other diploid cancer cells in colon cancer (57).
Figure 3.
Endoreplication and cancer
A. When treated with antimitotic drugs, diploid cancer cells may undergo cell cycle arrest and cell death, leading to cancer remission; they may enter endoreplication and become polyploid, fueling cancer progression. B. Polyploid giant cancer cells (PGCC) may generate progeny cells through budding; they may also undergo depolyploidization and return to mitosis, which may result in aneuploid cancer cells.
Endoreplication and genome instability
Among these negative outcomes of endoreplication, its relevance to genome instability has the most evidence. As propagation of a stable genome is crucial for development and homeostasis, aberrant endoreplication incurs high risks of genome instability and the consequent disease states (66). It has been shown that in Drosophila follicle cells endoreplication induced by Fzr/Cdh1 expression likely results in genome instability (67). Even in developmentally programmed endoreplication, there are signs of chromosomal instability, such as chromosomal aberrations, anaphase bridging, and lagging chromosomes (19, 38, 68). Genome instability following endoreplication is also observed in transplanted mouse polyploid hepatocytes, in which aneuploid cells are produced (69).
One speculation regarding the roles of endoreplication in tumorigenesis is that it is tolerant to DNA replication errors, and these errors could contribute to tumor initiation and progression. Induced cytokinesis failure leads to tetraploidy in p53-null mouse mammary epithelial cells, and these cells give rise to malignant cancers in nude mice; these tumors all have numerous chromosomal translocations and several folds of amplification (58).
In endocycling cells, the S phase stops before the whole genome is replicated, causing under-replication of pericentric heterochromatin (42). After several rounds of endocycling, the DNA replication fork stalls and induces a DNA damage response. In mitotic cells, this triggers cell cycle arrest or apoptosis. In endocycling cells, however, this does not induce apoptosis, achieved by muting the response to p53 activation and downregulating proapoptotic genes (34, 59). Polyploid cells have also been found to help overcome DNA replication stress in tumor progression by rewiring the DNA damage response network (60). Additionally, it has been shown in human colon cancer lines that induced polyploidy, and not senescence, enables the cancer cells to persist and repopulate, potentially leading to cancer relapse (61). Because senescence is frequently coupled with polyploidy, and chemotherapy largely aims to induce cell cycle checkpoint dependent apoptosis, it is postulated that anticancer treatment may inadvertently give rise to endoreplication and cancer relapse. Polyploid cancer cells can also induce apoptosis of nearby diploid cells through cell competition, a fitness-sensing mechanism that removes less competitive cells (62).
An important question in the polyploidy and cancer puzzle is where these polyploid cells emerge. A recent study in Drosophila showed that activation of EGFR signaling and overexpression of microRNA-8 cause genome instability by targeting the Septin family protein Peanut, resulting in cytokinesis failure and polyploidy. These giant cells induce apoptosis and engulfment of nearby cells, and they become neoplastic and metastatic (63). A more recent study shows that in human hepatocytes dysregulated Hippo-Yap signaling promotes polyploidy and polyploid cell growth. Yap induces acetylation of the E3 ligase Skp2 via Akt signaling and causes accumulation of the cyclin-dependent kinase (CDK) inhibitor p27, which leads to mitotic arrest and subsequently cell polyploidy (64). The study suggests that these polyploid cells are associated with human hepatocellular carcinomas. Current cancer therapeutics can also lead to polyploidy. Exposure of p53-deficient solid tumor derived cell lines to ionizing radiation results in the development of multinucleated giant cells that are viable and exhibit DNA synthesis (65). This may represent a survival mechanism of cancer cells exposed to radiation therapies.
Polyploidy and aneuploidy
In addition to the reciprocal relationship between endoreplication and genome instability, the reversion from polyploidy to diploidy or aneuploidy, a process called depolyploidization (Figure 3B), provides another avenue for tumorigenesis. Although the mechanism is elusive, meiosis-specific genes are activated in depolyploidizing cancer cells and potentially re-initiate mitosis from the point where it was interrupted (70). When cells undergoing induced endoreplication returned to mitosis, their daughter cells exhibited aneuploidy, resulting from multipolar divisions, chromosome missegregation, and cytokinesis failure (71). It is therefore logical to postulate that endoreplication may be a drug resistance strategy employed by cancer cells, by which polyploid cancer cells persist despite chemotherapy and re-form tumors through depolyploidization. It has been shown that several cancer cell lines enter the endoreplication cycle when treated with a DNA topoisomerase II inhibitor, and the common anticancer agent etoposide (72, 73). In another set of studies, using cell sorting analysis, some diploid cells were found within original polyploid-only colonies, showing increased resistance to cytotoxic drugs (73, 75). Polyploid giant cancer cells (PGCCs, see Glossary) were reported to be highly resistant to oxygen deprivation, and presented spatiotemporal expression of embryonic stem cell markers, such as OCT4 and NANOG (75, 76). Interestingly, PGCCs, resembling the embryonic blastomere, can differentiate into all three germ layers in vitro. Tumors derived from PGCCs are also more resistant to cisplatin, a common cytotoxic chemotherapy drug. Furthermore, PGCCs can form regular-sized cancer cells through asymmetric budding or bursting, and tumors formed from PGCCs showed expression of the embryonic stem cell markers CD44 and CD133 (75, 76). Tumor budding, as a noticeable phenotype on the tumor periphery, is indicative of tumors with high growth rates, and correlates strongly to tumor recurrence and poor prognosis (77, 78). Together, these studies suggest that the endoreplication-depolyploidization cycle causes cancer relapse, and offer a model to explain tumor relapse following cancer therapy, involving the initiation, self-renewal, termination, and stability phases of the giant cell cycle (79).
With the advent of high-throughput sequencing techniques providing extensive genomic data, bioinformatic analyses can be effectively applied to cancer genomic data, revealing connections between tumorigenic phenotypes and their underlying genes, and gaining insight into the relationship between cancer and polyploidy. For example, using integrative functional genomics to study mechanisms of polyploid phenotypes in breast cancer, a study found GINS2, the highest ranking endoreplication-inducing gene, as a possible biomarker for aberrant cell proliferation, and therefore a potential therapeutic target (80). A high-throughput proteomic profiling study identified a set of proteins that are differentially expressed in PGCCs and regular cancer cells in human ovarian cancers (81). These proteins are involved in cell cycle regulation, invasion and metastasis, stem cell generation, chromatin remodeling, and hypoxia, suggesting possible mechanisms by which PGCCs are generated. Recently, a comparison between polyploid and diploid transcriptomes revealed that genes interacting with the oncogene myc are significantly overrepresented in polyploid cells when compared to diploid cells. This suggests that polyploidy is more conducive to myc-induced tumorigenesis (82).
Concluding Remarks
Endoreplication is widespread in animals and plants. It plays critical roles in the development of organisms, and is tightly regulated by intrinsic mechanisms that are unique to the context. It also has been regarded as a key mechanism to repair tissue damage and maintain homeostasis. Recent advances in these areas suggest that mechanical forces and related signaling pathways are linkers between polyploidization and tissue homeostasis; however, a mechanistic understanding of the entire process remains to be elucidated (See Outstanding Questions). Endoreplication and polyploidy also accompanies tumor progression. The seemingly complex relationships between cancer, endoreplication, and polyploidy are important concepts that remain to be uncovered, and may provide new perspectives on cancer mechanisms for improved cancer therapies. An emerging discussion on PGCCs will help broaden the perspective on the relationship between polyploidy and cancers. More insights into the underlying mechanisms of the generation and maintenance of PGCCs are needed for us to design novel therapeutic strategies for cancer relapse and cancers that refractory to traditional chemotherapy.
Outstanding Questions.
How do signaling pathways collaborate to induce endoreplication in various cell and tissue types?
How do tissues utilize endoreplication to maintain homeostasis while preventing genome instability and tumorigenesis?
How does mechanical tension initiate and affect endoreplication? In particular, what signaling pathways are affected in this process and how do they work together to achieve homeostasis?
What are the mechanisms of depolyploidization?
Whether and how do polyploid giant cancer cells (PGCCs) affect their neighboring cells to facilitate primary tumor formation?
How do PGCCs gain stemness? Specifically, what genes and signaling pathways are modulated in these PGCCs?
Trend Box.
As an alternative cell cycle program, endoreplication is crucial during development. Polyploid cells assume various pivotal functions in metazoans.
Besides its significant role in development, endoreplication is induced by various signaling pathways to maintain homeostasis and tissue integrity, and to assist the wound healing and tissue repair processes.
Polyploidy and aneuploidy are widespread across different types of cancer, contributing to cancer progression, surveillance escape, and relapse.
Polyploid Giant Cancer Cells (PGCCs) possess stem-cell characteristics, and can differentiate into three germ layers in vitro. PGCCs can form tumors through asymmetric budding or bursting.
Acknowledgments
We thank Sarah Ogden and Allison Jevitt for providing assistance in figure preparation, Yoichiro Tamori and Sheng-An Yang for inputs, and Jen Kennedy for proofreading the manuscript. This work was supported by grants from National Science Foundation IOS-1052333 and National Institutes of Health R01GM072562 to W.-M.D.
Glossary
- Endoreplication (endocycling)
The replication of DNA during the S phase of the cell cycle without the subsequent completion of mitosis and/or cytokinesis.
- Endomitosis
The genome is replicated and mitosis is initiated but not completed, resulting in mononucleated or binucleated polyploid cell.
- Polyploid cells
cells containing more than two sets of chromosomes. Most species are made of cells that are diploid, meaning they have two sets of chromosomes. Polyploidy could be achieved through endoreplication, endomitosis or cell fusion.
- Compensatory Cellular Hypertrophy (CCH)
A tissue homeostasis mechanism through which post-mitotic cells undergo hypertrophic growth to compensate for the lost tissue volume.
- Polyploid Giant Cancer Cells (PGCCs)
Large cancer cells with more than two sets of chromosomes, present in many solid tumors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edgar B, Orr-Weaver T. Endoreplication cell cycles: more for less. Cell. 2001;105(3):297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 2.Lilly M, Duronio R. New insights into cell cycle control from the Drosophila endocycle. Oncogene. 2005;24:2765–75. doi: 10.1038/sj.onc.1208610. [DOI] [PubMed] [Google Scholar]
- 3.Lee HO, et al. Endoreplication: polyploidy with purpose. Genes & development. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox D, Duronio R. Endoreplication and polyploidy: insight into development and disease. Development. 2013;140:3–12. doi: 10.1242/dev.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song L, Rape M. Substrate-specific regulation of ubiquitination by the anaphase-promoting complex. Cell Cycle. 2011;10(1):52–6. doi: 10.4161/cc.10.1.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielke N, et al. The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes & Development. 2008;22(12):1690–1703. doi: 10.1101/gad.469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson CI, et al. Expression of an S phase-stabilized version of the CDK inhibitor Dacapo can alter endoreplication. Development. 2015;142(24):4288–4298. doi: 10.1242/dev.115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong A, et al. The cyclin-dependent kinase inhibitor Dacapo promotes replication licensing during Drosophila endocycles. The EMBO Journal. 2007;26(8):2071–2082. doi: 10.1038/sj.emboj.7601648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss A, et al. Continuous Cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Current Biology. 1998;8(4):239–S1. doi: 10.1016/s0960-9822(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 10.Shu Z, Deng WM. Differential regulation of Cyclin E by Yorkie-Scalloped signaling in organ development. G3: Genes, Genomes, Genetics. 2017:g3–117. doi: 10.1534/g3.117.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravid K, et al. Roads to polyploidy: The megakaryocyte example. J Cell Physiol. 2002;190(1):7–20. doi: 10.1002/jcp.10035. [DOI] [PubMed] [Google Scholar]
- 12.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 13.Celton-Morizur S, Desdouets C. Polyploidization of liver cells. Adv Exp Med Biol. 2010;676:123–135. doi: 10.1007/978-1-4419-6199-0_8. [DOI] [PubMed] [Google Scholar]
- 14.Deng WM, et al. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–46. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 15.Lee L, Orr-Weaver T. Regulation of cell cycles in Drosophila development: intrinsic and extrinsic cues. Annu Rev Genet. 2003;37:545–78. doi: 10.1146/annurev.genet.37.110801.143149. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer V, et al. Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr Biol. 2004;14:630–36. doi: 10.1016/j.cub.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Berendes HD. Salivary gland function and chromosomal puffing patterns in Drosophila hydei. Chromosoma. 1965;17(1):35–77. doi: 10.1007/BF00285155. [DOI] [PubMed] [Google Scholar]
- 18.Bradley TJ. Physiology of osmoregulation in mosquitoes. Annual review of entomology. 1987;32(1):439–462. doi: 10.1146/annurev.en.32.010187.002255. [DOI] [PubMed] [Google Scholar]
- 19.Fox DT, et al. Error-prone polyploid mitosis during normal Drosophila development. Genes & development. 2010;24:2294–2302. doi: 10.1101/gad.1952710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sher N, et al. Fundamental differences in endoreplication in mammals and Drosophila revealed by analysis of endocycling and endomitotic cells. Proceedings of the National Academy of Sciences. 2013;110(23):9368–9373. doi: 10.1073/pnas.1304889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreyeva EN, et al. Regulatory functions and chromatin loading dynamics of linker histone H1 during endoreplication in Drosophila. Genes & Development. 2017;31(6):603–616. doi: 10.1101/gad.295717.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Huallachain M, et al. Extensive genetic variation in somatic human tissues. Proceedings of the National Academy of Sciences. 2012;109(44):18018–18023. doi: 10.1073/pnas.1213736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannibal RL, et al. Copy number variation is a fundamental aspect of the placental genome. PLoS genetics. 2014;10(5):e1004290. doi: 10.1371/journal.pgen.1004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabelli PA, et al. Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm. Proceedings of the National Academy of Sciences. 2013;110(19):E1827–E1836. doi: 10.1073/pnas.1304903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelen-Eigles G, et al. DNA endoreduplication in maize endosperm cells is reduced by high temperature during the mitotic phase. Crop Science. 2001;41(4):1114–1121. [Google Scholar]
- 26.Roeder A, et al. Cell cycle regulates cell type in the Arabidopsis sepal. Development. 2012;139:4416–27. doi: 10.1242/dev.082925. [DOI] [PubMed] [Google Scholar]
- 27.Chapman E, Estelle M. Mechanism of Auxin-regulated gene expression in plants. Annu Rev of Genet. 2009;43:265–85. doi: 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- 28.Berckmans, et al. Light-dependent regulation of DEL1 is determined by the antagonistic action of E2Fb and E2Fc. Plant Physiol. 2011;157(3):1440–51. doi: 10.1104/pp.111.183384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inzé D, De Veylder L. Cell cycle regulation in plant development. Annu Rev Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- 30.Dewey DR. Polyploidy. Springer US; 1980. Some applications and misapplications of induced polyploidy to plant breeding; pp. 445–470. [DOI] [PubMed] [Google Scholar]
- 31.Sattler MC, et al. The polyploidy and its key role in plant breeding. Planta. 2016;243:281–296. doi: 10.1007/s00425-015-2450-x. [DOI] [PubMed] [Google Scholar]
- 32.Adachi S, et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:10004–9. doi: 10.1073/pnas.1103584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radziejwoski A, et al. Atypical E2F activity coordinates PHR1 photolyase gene transcription with endoreplication onset. EMBO J. 2011;30(2):355–63. doi: 10.1038/emboj.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrotra S, et al. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev. 2008;22(22):3158–71. doi: 10.1101/gad.1710208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell division. 2013;8(1):8. doi: 10.1186/1747-1028-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevzorova YA, et al. Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin. Gastroenterology. 2009;137(2):691–703. doi: 10.1053/j.gastro.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denchi EL, et al. Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes & development. 2006;20(19):2648–2653. doi: 10.1101/gad.1453606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unhavaithaya Y, Orr-Weaver TL. Polyploidization of glia in neural development links tissue growth to blood–brain barrier integrity. Genes & development. 2012;26(1):31–36. doi: 10.1101/gad.177436.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamori Y, Deng WM. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Developmental cell. 2013;25(4):350–363. doi: 10.1016/j.devcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamori Y, Deng WM. Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Trends in cell biology. 2014;24(4):230–237. doi: 10.1016/j.tcb.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Losick VP, et al. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Current Biology. 2013;23(22):2224–2232. doi: 10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgar BA, et al. Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nature reviews Molecular cell biology. 2014;15(3):197. doi: 10.1038/nrm3756. [DOI] [PubMed] [Google Scholar]
- 43.Spradling AC. Germline cysts: communes that work. Cell. 1993;72(5):649–651. doi: 10.1016/0092-8674(93)90393-5. [DOI] [PubMed] [Google Scholar]
- 44.Artavanis-Tsakonas S, et al. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 45.Shcherbata HR, et al. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development. 2004;131(13):3169–3181. doi: 10.1242/dev.01172. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calvi BR, Spradling AC. Chorion gene amplification in Drosophila: A model for metazoan origins of DNA replication and S-phase control. Methods. 1999;18(3):407–417. doi: 10.1006/meth.1999.0799. [DOI] [PubMed] [Google Scholar]
- 48.Dej K, Spradling A. The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development. 1999;126(2):293–303. doi: 10.1242/dev.126.2.293. [DOI] [PubMed] [Google Scholar]
- 49.Zielke N, et al. Control of Drosophila endocycles by E2F and CRL4CDT2. Nature. 2011;480(7375):123. doi: 10.1038/nature10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibutani ST, et al. Intrinsic negative cell cycle regulation provided by PIP box-and Cul4Cdt2-mediated destruction of E2f1 during S phase. Developmental cell. 2008;15(6):890–900. doi: 10.1016/j.devcel.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiang J, et al. EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nature Communications. 2017:8. doi: 10.1038/ncomms15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao J, et al. Tension creates an endoreplication wavefront that leads regeneration of epicardial tissue. Developmental Cell. 2017;42(6):600–615. doi: 10.1016/j.devcel.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 54.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Current opinion in cell biology. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and caner. Nat Rev Mol Cell Biol. 2004;5(1):45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 56.Cancer Genome Anatomy Project. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. https://cgap.nci.nih.gov/Chromosomes/Mitelman.
- 57.Park S, et al. Effect of chromosomal polyploidy on survival of colon cancer cells. Korean J Gastroenterol. 2011;57(3):150–7. doi: 10.4166/2011.57.3.150. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara T, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437(7061):1043. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B, et al. Low levels of p53 protein and chromatin silencing of p53 target genes repress apoptosis in Drosophila endocycling cells. PLoS Genet. 2014;10:e1004581. doi: 10.1371/journal.pgen.1004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng L, et al. Polyploid cells rewire DNA damage response networks to overcome replication stress induced barrier for tumor progression. Nat Commun. 2012;3:815. doi: 10.1038/ncomms1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosieniak G, et al. Polyploidy formation in doxorubicin-treated cancer cells can favor escape from senescence. Neoplasia. 2015;17(12):882–893. doi: 10.1016/j.neo.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamori Y, Deng WM. Cell competition and its implications for development and cancer. Journal of genetics and genomics. 2011;38(10):483–495. doi: 10.1016/j.jgg.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eichenlaub T, et al. Cell competition drives the formation of metastatic tumors in a Drosophila model of epithelial tumor formation. Current Biology. 2016;26(4):419–427. doi: 10.1016/j.cub.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, et al. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell. 2017;31(5):669–684. doi: 10.1016/j.ccell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirzayans R, et al. Multinucleated giant cancer cells produced in response to ionizing radiation retain viability and replicate their genome. International journal of molecular sciences. 2017;18(2):360. doi: 10.3390/ijms18020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- 67.Hassel C, et al. Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development. 2014;141(1):112–123. doi: 10.1242/dev.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duncan AW, et al. Frequent aneuploidy among normal human hepatocytes. Gastroenterology. 2012;142:25–28. doi: 10.1053/j.gastro.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duncan AW, et al. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467(7316):707. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erenpreisa J, et al. Segregation of genomes in polyploid tumour cells following mitotic catastrophe. Cell Biol Int. 2005;29(12):1005–11. doi: 10.1016/j.cellbi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Chen S, et al. Transient endoreplication down-regulates the kinesin-14 HSET and contributes to genomic instability. Molecular Biology of the Cell. 2016;27(19):2911–2923. doi: 10.1091/mbc.E16-03-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen H, et al. Transient nutlin-3a treatment promotes endoreduplication and the generation of therapy-resistant tetraploid cells. Cancer research. 2008;68(20):8260–8268. doi: 10.1158/0008-5472.CAN-08-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakaue-Sawano A, et al. Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC Cell Biol. 2011;12:2. doi: 10.1186/1471-2121-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puig P, et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol Int. 2008;32(9):1031–43. doi: 10.1016/j.cellbi.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Niu N, et al. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36(34):4887. doi: 10.1038/onc.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, et al. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33(1):116–128. doi: 10.1038/onc.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai T. Growth patterns in human carcinoma: Their classification and relation to prognosis. Obstetrics & Gynecology. 1960;16(3):296. [PubMed] [Google Scholar]
- 78.Koelzer VH, et al. Tumor budding in colorectal cancer—ready for diagnostic practice? Human pathology. 2016;47(1):4–19. doi: 10.1016/j.humpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Niu N, et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis. 2016;5(12):e281. doi: 10.1038/oncsis.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rantala J, et al. A cell spot microarray method for production of high density siRNA transfection microarrays. BMC Genomics. 2011;12:162. doi: 10.1186/1471-2164-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang S, et al. iTRAQ-based proteomic analysis of polyploid giant cancer cells and budding progeny cells reveals several distinct pathways for ovarian cancer development. PloS one. 2013;8(11):e80120. doi: 10.1371/journal.pone.0080120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vazquez-Martin A, et al. Somatic polyploidy is associated with the upregulation of c-MYC interacting genes and EMT-like signature. Oncotarget. 2016;7(46):75235. doi: 10.18632/oncotarget.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]