Abstract
Objective
We sought to describe a large, international cohort of patients diagnosed with primary mucinous ovarian carcinoma (PMOC) across three tertiary medical centers to evaluate differences in patient characteristics, surgical/adjuvant treatment strategies, and oncologic outcomes.
Methods
This was a retrospective review spanning 1976–2014. All tumors were centrally reviewed by an expert gynecologic pathologist. Each center used a combination of clinical and histologic criteria to confirm a PMOC diagnosis. Data were abstracted from medical records, and a de-identified dataset was compiled and processed at a single institution. Appropriate statistical tests were performed.
Results
222 patients with PMOC were identified; all had undergone primary surgery. Disease stage distribution was as follows: stage I, 163 patients (74%); stage II, 8 (4%); stage III, 40 (18%); and stage IV, 10 (5%). Ninety-nine (45%) of 219 patients underwent lymphadenectomy; 41 (19%) of 215 underwent fertility-preserving surgery. Of the 145 patients (65%) with available treatment data, 68 (47%) had received chemotherapy—55 (81%) a gynecologic regimen and 13 (19%) a gastrointestinal regimen. The 5-year progression free survival (PFS) rates were 80% (95% CI, 73–85%) for patients with stage I–II disease and 17% (95% CI, 8–29%) for those with stage III–IV disease. The 5-year PFS rate was 73% (95% CI, 50–86%) for patients who underwent fertility-preserving surgery.
Conclusions
Most patients (74%) presented with stage I disease. Nearly 50% were treated with adjuvant chemotherapy using various regimens across institutions. PFS outcomes were favorable for those with early-stage disease and lower but acceptable for those who underwent fertility preservation.
Keywords: ovarian cancer, primary mucinous ovarian cancer, fertility preservation, survival
INTRODUCTION
Mucinous ovarian adenocarcinomas are rare, comprising 2–3% of primary epithelial ovarian malignancies (1, 2). These tumors are often misdiagnosed as primary ovarian tumors rather than non-ovarian metastases. Other sites of origin to consider in the differential diagnosis of these tumors include the upper and lower gastrointestinal (GI) tracts, the pancreaticobiliary tract, and the lung. In 2011, Zaino et al published sentinel findings that highlighted the true rarity of primary mucinous ovarian cancers (PMOCs). The authors comprehensively reviewed a primary mucinous adenocarcinoma subset of 3,400 epithelial ovarian malignancies and reclassified 29 (71%) of 41 cases as metastatic, rather than primary, to ovary (3).
Although most patients present with a dominant mass and stage I disease (2), some will present with bilateral, smaller-sized ovarian tumors or advanced-stage disease. In these settings, clinical correlation and review of imaging, with expert gynecologic pathology assessment, are essential to discern a primary from metastatic carcinoma. Upper and lower endoscopy, and focused imaging, may help preclude a diagnosis of a GI, pancreatic, biliary, or appendiceal malignancy. Immunohistochemistry may aid in the diagnosis, and many of the markers used to discern mucin-producing carcinomas, such as diffuse cytokeratin-7 (CK-7) staining and absent cytokeratin-20 (CK-20) staining, have been incorporated in the diagnosis of these tumors (1, 4).
The primary strategy for treating mucinous carcinomas of the ovary is surgical resection with staging. Unless fertility preservation is considered, surgery typically entails hysterectomy, bilateral salpingo-oophorectomy, and omentectomy; regional lymph node dissection can be included (5). Many gynecologic oncologists advocate an appendectomy only with obvious tumor involvement or if the appendix appears abnormal at the time of staging (6–8). Adjuvant treatment ranges from observation in early-stage disease to systemic chemotherapy using both GI and epithelial ovarian treatment regimens (9). PMOC is considered a relatively chemoresistant disease, with poor outcomes, compared stage for stage with the more common high-grade serous epithelial ovarian cancer (10). As with epithelial ovarian malignancies, surgical cytoreduction is recommended for advanced-stage PMOC, as this has been shown to improve outcomes (5, 6).
Considering the rarity, diagnostic challenges, and wide variability of surgical and adjuvant treatment approaches in PMOC, we sought to review an international experience of PMOCs across three tertiary medical centers with expert pathology assessment to confirm diagnoses. We hypothesized that there could be significant heterogeneity in patient characteristics and surgical treatment across the institutions. Here, we describe a large cohort of patients with PMOC to evaluate differences in patient characteristics, surgical and adjuvant treatment strategies, and outcomes.
METHODS
This was an institutional review board-approved retrospective review. We identified all cases of PMOC using institutional datasets of patients treated at Memorial Sloan Kettering Cancer Center (MSK) in the United States from 1998–2013, Rigshospitalet (DEN) in Denmark from 2005–2013, and Gustave Roussy Cancer Campus (GRCC) in France from 1976–2014. Cases of pure borderline pathology, intraepithelial carcinoma, or metastatic mucinous carcinoma from another site were excluded. All tumors were reviewed by an expert gynecologic pathologist within each respective center. Each center used identical, mutually agreed upon clinical and histologic criteria to confirm a PMOC diagnosis; patients who presented with a unilateral ovarian tumor >10 cm in size were included, and in patients with bilateral tumors or a tumor <10 cm in size, a GI work-up often was performed to rule out a GI primary. CK-7, CK-20, and caudal-type homeobox 2 (CDX2) staining, if available, were used to support the diagnosis. Clinical and pathologic data were abstracted from medical records, and a de-identified dataset was compiled and processed at a single institution. MSK and GRCC data were retrospectively collected from patients’ electronic medical records. DEN data were retrieved from a national clinical quality data registry that includes many clinical variables but has incomplete treatment data (11). Patients were staged according to the 2014 International Federation of Gynecology and Obstetrics (FIGO) classification system. Histologic grading was performed according to the Prat grading system for GRCC and DEN cases, and the Shimizu and Silverberg system (12) for MSK cases.
Statistical Analysis
Variable differences among the three centers or between defined cohorts were tested using the Fisher exact test for categorical variables and Kruskal-Wallis test/Wilcoxon rank sum test for continuous variables. P<0.05 was considered statistically significant. Progression-free survival (PFS) was defined as the time between the date of first surgery to the date of progression, death or last follow-up. Both progression and death were considered as events in our survival analysis. Median PFS and PFS rate were estimated using the Kaplan-Meier method. P values were obtained through a fitting marginal Cox proportional hazards model by incorporating centers as clusters (13). A log-rank p value was used to examine the PFS differences among centers. All analyses were performed by MSK’s Biostatistics Department using SAS9.4 and R3.2.
RESULTS
Of 537 patients initially evaluated for inclusion across the three institutions, 222 (41%) met the inclusion criteria. At MSK, 175 patients were initially evaluated for inclusion, of whom 34 met the inclusion criteria. Of the 141 remaining patients who were excluded, 62 had borderline tumors, 24 had microinvasive tumors, 12 had metastasis to ovary, 4 had tumor of mixed histology, 38 had advanced-stage disease at presentation, and 1 had a dual malignancy. At GRCC, 350 patients were initially evaluated for inclusion, of whom 111 met the inclusion criteria. The remaining 239 patients were excluded for the following reason(s): they had metastatic disease from another primary; they were seen at the institution as a second opinion, with no clinical follow-up; or they had tumor of mixed histology or a borderline mucinous tumor. At DEN, 95 patients were initially evaluated for inclusion, of whom 77 met the inclusion criteria. Of the remaining 18 patients who were excluded, 14 had borderline tumors and 4 had metastasis to ovary.
Overall cohort
The median age of the overall cohort was 49 years (range, 15–89 years). Fifty-one (49%) of 104 patients had an elevated CA-125 level (>35 U/mL) at diagnosis. The median tumor size was 15 cm (range, 5–36 cm). In the 141 patient records with laterality data available, 134 (95%) unilateral tumors were documented. Among unilateral tumors with tumor size data available, 107 (88%) of 122 were ≥10 cm. Of the 177 tumors that were histologically graded, 109 (62%) were grade 1 and 68 (38%) were grade 2 or 3. Thirty-seven (26%) and 41 (28%) of 145 patients underwent upper or lower endoscopy, respectively. Any endoscopic evaluation was performed in 48 (33%) of 145 patients.
Of the 221 patients with documented staging details, 163 had stage I (74%), 8 had stage II (4%), 40 had stage III (18%), and 10 had stage IV (5%) disease. Hysterectomy was performed in 164 (75%) of 220 patients, peritoneal cytology in 206 (93%) of 222, bilateral salpingo-oophorectomy in 173 (81%) of 214, appendectomy in 168 (80%) of 210, omentectomy in 196 (90%) of 217, pelvic lymphadenectomy in 92 (42%) of 219, and para-aortic lymphadenectomy in 83 (38%) of 219 patients. The median number of pelvic lymph nodes resected was 14 (range, 1–34), and the median number of para-aortic lymph nodes resected was 11 (range, 1–46) (Table 1).
Table 1.
Clinical and pathologic characteristics of the patient cohort (N=222)
| Variable | All (N=222) | GRCC (N=111) | DEN (N=77) | MSK (N=34) | p* |
|---|---|---|---|---|---|
|
| |||||
| N (%) | N (%) | N (%) | N (%) | ||
| Age at Diagnosis | |||||
| Median | 49 | 39 | 60 | 54 | <0.001 |
| Range | 15–89 | 15–83 | 21–89 | 20–86 | |
|
| |||||
| Smoker (9 missing) | |||||
| Yes | 78 (37%) | 20 (19%) | 44 (59%) | 14 (42%) | <0.001 |
|
| |||||
| Stage (1 missing) | |||||
| I | 163 (74%) | 85 (77%) | 52 (68%) | 26 (76%) | 0.454 |
|
| |||||
| IA | 100 (45%) | 45 (41%) | 38 (50%) | 17 (50%) | |
| IB | 1 (0.5%) | 0 (0%) | 1 (1%) | 0 (0%) | |
| IC | 22 (10%) | 0 (0%) | 13 (17%) | 9 (26%) | |
| ICI | 15 (7%) | 15 (14%) | 0 (0%) | 0 (0%) | |
| IC2 | 20 (9%) | 20 (18%) | 0 (0%) | 0 (0%) | |
| IC3 | 5 (2%) | 5 (5%) | 0 (0%) | 0 (0%) | |
| II/III/IV | 58 (26%) | 26 (23%) | 24 (32%) | 8 (24%) | |
|
| |||||
| IIA | 1 (0.5%) | 0 (0%) | 1 (1%) | 0 (0%) | |
| IIB | 4 (2%) | 1 (1%) | 3 (4%) | 0 (0%) | |
| IIC | 3 (1%) | 0 (0%) | 0 (0%) | 3 (9%) | |
| IIIA | 1 (0.5%) | 0 (0%) | 1 (1%) | 0 (0%) | |
| IIIA1 | 1 (0.5%) | 1 (1%) | 0 (0%) | 0 (0%) | |
| IIIA2 | 4 (2%) | 4 (4%) | 0 (0%) | 0 (0%) | |
| IIIB | 6 (3%) | 3 (3%) | 3 (4%) | 0 (0%) | |
| IIIC | 28 (13%) | 13 (12%) | 10 (13%) | 5 (15%) | |
| IV | 10 (5%) | 4 (4%) | 6 (8%) | 0 (0%) | |
|
| |||||
| Upper GI Endoscopy** | |||||
| Yes | 37 (26%) | 22 (20%) | 15 (44%) | 0.007 | |
| Lower GI Endoscopy** | |||||
| Yes | 41 (28%) | 27 (24%) | 14 (41%) | 0.08 | |
| Both Upper and Lower GI Endoscopy** | |||||
| Yes | 48 (33%) | 30 (27%) | 18 (53%) | 0.007 | |
|
| |||||
| CA125 >35 U/mL (41 missing)** | |||||
| Yes | 51 (49%) | 40 (49%) | 11 (48%) | 1 | |
|
| |||||
| Tumor Size, cm (17 missing)** | |||||
| Median | 15 | 14.5 | 15 | 0.271 | |
| Range | 4.5–36 | 4.5–30 | 7–36 | ||
|
| |||||
| Capsule Rupture (12 missing) | |||||
| Yes | 71 (34%) | 47 (43%) | 19 (28%) | 5 (15%) | 0.004 |
|
| |||||
| Cytology | |||||
| Yes | 206 (93%) | 105 (95%) | 69 (90%) | 32 (94%) | 0.431 |
| Positive Cytology | |||||
| Yes | 34 (17%) | 15 (14%) | 11 (16%) | 8 (25%) | 0.361 |
|
| |||||
| Laterality** (4 missing) | |||||
| Unilateral | 134 (95%) | 102 (95%) | 32 (94%) | ||
| Bilateral | 7 (5%) | 5 (5%) | 2 (6%) | ||
|
| |||||
| Hysterectomy (2 missing) | |||||
| Yes | 164 (75%) | 70 (63%) | 68 (91%) | 26 (76%) | <0.001 |
|
| |||||
| Salpingo-oophorectomy (8 missing) | |||||
| Unilateral | 41 (19%) | 32 (29%) | 1 (1%) | 8 (24%) | <0.001 |
| Bilateral | 173 (81%) | 79 (71%) | 68 (99%) | 26 (76%) | |
|
| |||||
| Appendectomy (12 missing) | |||||
| Yes | 168 (80%) | 82 (77%) | 57 (83%) | 29 (85%) | 0.506 |
|
| |||||
| Omentectomy (5 missing) | |||||
| Yes | 196 (90%) | 100 (90%) | 63 (87.5%) | 33 (97%) | 0.296 |
|
| |||||
| Pelvic Lymphadenectomy (3 missing) | |||||
| Yes | 92 (42%) | 37 (33%) | 26 (35%) | 29 (85%) | <0.001 |
| Median | 14 | 13 | 20 | 15 | <0.001 |
| Range | 1–34 | 3–27 | 1–33 | 1–34 | |
| Number of Positive Pelvic Lymph Nodes | |||||
| 0 | 87 (95%) | 33 (89%) | 25 (96%) | 29 (100%) | – |
| 1 | 4 (4%) | 3 (8%) | 1 (4%) | 0 (0%) | |
| 2 | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Para-aortic Lymphadenectomy (3 missing) | |||||
| Yes | 83 (38%) | 42 (38%) | 14 (19%) | 27 (79%) | <0.001 |
| Median | 11 | 16.5 | 6 | 7 | <0.001 |
| Range | 1–46 | 2–46 | 1–23 | 1–42 | |
| Number of Positive Para-aortic Lymph Nodes | |||||
| 0 | 77 (93%) | 37 (88%) | 14 (100%) | 26 (96%) | − |
| 1 | 3 (4%) | 2 (5%) | 0 (0%) | 1 (4%) | |
| 2 | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| 5 | 2 (2%) | 2 (5%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Lymphadenectomy Performed (3 missing) | |||||
| Total cohort | |||||
| Yes | 99 (45%) | 44 (40%) | 26 (35%) | 29 (85%) | |
| Stage I | |||||
| Yes | 76 (47%) | 30 (35%) | 22 (42%) | 24 (92%) | |
| Stage II – IV | |||||
| Yes | 23 (42%) | 14 (54%) | 4 (19%) | 5 (63%) | |
| Chemotherapy** | |||||
| Yes | 68 (47%) | 54 (49%) | 14 (41%) | 0.556 | |
| Number of Cycles** | |||||
| Median | 6 | 6 | 6 | 0.292 | |
| Range | 1–12 | 1–12 | 2–12 | ||
| Chemotherapy Regimen Type** | |||||
| Gynecologic | 55 (81%) | 44 (81%) | 11 (79%) | 1 | |
| Gastrointestinal | 13 (19%) | 10 (19%) | 3 (21%) | ||
GRCC, Gustave Roussy Cancer Campus; DEN, Rigshospitalet in Denmark; MSK = Memorial Sloan Kettering Cancer Center; GI = gastrointestinal
p values were obtained using the Kruskal-Wallis test (three centers)/Wilcoxon rank sum test (two centers) for continuous variables and the Fisher exact test for categorical variables
MSK and GRCC data available (N=145)
Comparisons across centers
Table 1 details demographic characteristics, staging procedures, and treatment strategies across the three centers. The median age was 39 years (range, 15–83) in the GRCC cohort, 60 years (range, 21–89 years) in the DEN cohort, and 54 years (range, 20–86 years) in the MSK cohort (p<0.001). In the overall cohort, 78 (37%) of 213 patients were smokers—20 (19%) of 105 in the GRCC group, 44 (59%) of 75 in the DEN group, and 14 (42%) of 33 in the MSK group (p<0.001). In the overall cohort, 41 (19%) of 215 patients were managed using a fertility-preservation approach—32 (29%) of 111 in the GRCC group, 1 (1%) of 70 in the DEN group, and 8 (24%) of 34 in the MSK group (p<0.001). Of the 220 patients with reported hysterectomy status, 70 (63%) of 111 in the GRCC group, 68 of (91%) 75 in the DEN group, and 26 (77%) of 34 in the MSK group underwent hysterectomy before or during the index surgery (p<0.001). Of the 214 patients with reported oophorectomy status, 79 (71%) of 111 in the GRCC group, 68 (99%) of 69 in the DEN group, and 26 (77%) of 34 in the MSK group underwent bilateral oophorectomy before or during the index surgery (p<0.001). We evaluated staging procedures while controlling for age that were statistically significant on univariate analysis across centers. Patients were grouped according to age younger than 45 years or 45 years and older, as this is the upper age limit for consideration of fertility-preservation procedures. In patients who underwent a hysterectomy, there was no association with either age group across each center. There was a difference across centers regarding the performance of pelvic and para-aortic lymph node dissection, with MSK performing more nodal dissections in both age groups compared with the other centers in this series (Table 2).
Table 2.
Staging procedures performed across centers based on age
| Variables | Age at diagnosis | GRCC (N=111) | DEN (N=77) | MSK (N=34) | p* |
|---|---|---|---|---|---|
|
| |||||
| N (%) | N (%) | N (%) | |||
| Hysterectomy (2 missing) | |||||
| Yes | Age <45 | 34 (31%) | 9 (12%) | 3 (9%) | 0.15 |
| Age ≥45 | 36 (32%) | 59 (79%) | 23 (68%) | ||
|
| |||||
| USO (8 missing) | |||||
| Yes | |||||
| Age <45 | 28 (25%) | 0 | 7 (20%) | 0.008 | |
| Age ≥45 | 4 (4%) | 1 (1%) | 1 (3%) | ||
| BSO | |||||
| Yes | Age <45 | 41 (37%) | 9 (13%) | 3 (9%) | |
| Age ≥45 | 38 (34%) | 59 (86%) | 23 (68%) | ||
|
| |||||
| Pelvic LN (3 missing) | |||||
| Yes | Age <45 | 22 (20%) | 7 (9%) | 8 (24%) | <0.001 |
| Age ≥45 | 15 (14%) | 19 (26%) | 21 (62%) | ||
|
| |||||
| Para-aortic LN (3 missing) | |||||
| Yes | Age <45 | 26 (23%) | 5 (7%) | 8 (24%) | <0.001 |
| Age ≥45 | 16 (14%) | 9 (12%) | 19 (56%) | ||
GRCC, Gustave Roussy Cancer Campus; DEN, Rigshospitalet in Denmark; MSK = Memorial Sloan Kettering Cancer Center; LN = lymph node; USO = unilateral salpingo-oophorectomy; BSO = bilateral salpingo-oophorectomy
p values were obtained using the Cochran-Mantel Haenszel test.
In the overall cohort, 99 (45%) of 219 patients underwent a pelvic and/or para-aortic lymphadenectomy. A pelvic lymphadenectomy was performed in 37 (35%) of 107 patients in the GRCC group, 26 (35%) of 74 in the DEN group, and 29 (85%) of 34 in the MSK group (p<0.001). A para-aortic lymphadenectomy was performed in 42 (38%) of 111, 14 (19%) of 74, and 27 (79%) of 34, respectively (p<0.001). In the overall cohort, 76 (47%) of 163 patients with stage I disease underwent lymphadenectomy. Twenty-three of (42%) 55 patients with stage II or higher disease underwent lymphadenectomy (Table 1).
Of the 221 patients in the overall cohort with reported FIGO stage status, 85 (77%) of 111 in the GRCC group, 52 (68%) of 76 in the DEN group, and 26 (81%) of 32 in the MSK group presented with stage I disease (p=0.454). Of the 177 tumors in the overall cohort that were histologically graded, 43 (59%) of 73, 46 (64%) of 72, and 20 (63%) of 32, respectively, were grade 1. There were no statistically significant differences in median tumor size, stage or grade distribution, rates of appendectomy or omentectomy, or use of adjuvant chemotherapy.
Fertility-preservation
Patients undergoing surgery for stage I PMOC who were less than 45 years of age were identified and analyzed separately. Fertility preservation in this group of patients was defined as the retention of one ovary and uterus at the time of primary surgery. Of the 76 patients under age 45 years, 32 (42%) underwent initial fertility-preserving surgery (Table 3). Of these 32 patients, 26 (81%) were from GRCC, 0 (0%) were from DEN, and 6 (19%) were from MSK. The median age at diagnosis of the fertility-preserved group was 26 years (range, 15–40 years); it was 38 years (range, 17–45 years) for the remaining 44 patients who did not undergo fertility-preserving surgery (p<0.001). Six (19%) of the 32 patients who underwent fertility preservation underwent a combined upper and lower GI endoscopic evaluation compared with 12 (33%) of the 36 remaining patients (p=0.271). Median CA-125 was elevated in 5 (24%) of 21 and 8 (35%) of 23 patients, respectively (p=0.518). Median tumor size was 15 cm (range, 5–36 cm) and 13 cm (range, 8–30 cm), respectively (p=0.533).
Table 3.
Comparison of women under 45 years of age with stage I disease who had or had not undergone fertility preservation (N=76)
| Variable | Fertility Preservation | p* | |
|---|---|---|---|
| No | Yes | ||
| 44 | 32 | ||
|
| |||
| Center | |||
| MSK | 3 (7%) | 6 (19%) | 0.013 |
| GRCC | 33 (75%) | 26 (81%) | |
| DEN | 8 (18%) | 0 (0%) | |
|
| |||
| Age at Diagnosis | |||
| Median | 38 | 26 | <0.001 |
| Range | 17–45 | 15–40 | |
|
| |||
| Smoker (6 missing) | |||
| Yes | 10 (25%) | 8 (27%) | 1 |
|
| |||
| Capsule Rupture (2 missing) | |||
| Yes | 13 (30%) | 16 (52%) | 0.091 |
|
| |||
| Cytology** | |||
| Yes | 41 (93%) | 32 (100%) | – |
| Positive Cytology (1 missing)** | |||
| Yes | 4 (10%) | 0 (0%) | – |
|
| |||
| Appendectomy (3 missing) | |||
| Yes | 35 (85%) | 27 (84%) | 1 |
|
| |||
| Omentectomy** | |||
| Yes | 41 (93%) | 31 (97%) | – |
|
| |||
| Pelvic Lymphadenectomy (1 missing) | |||
| Yes | 17 (39%) | 13 (41%) | 1 |
| Median | 13 | 14 | 0.967 |
| Range | 5–33 | 1–34 | |
| Para-aortic Lymphadenectomy (1 missing) | |||
| Yes | 15 (34%) | 16 (50%) | 0.237 |
| Median | 13 | 8.5 | 0.1 |
| Range | 2–42 | 2–29 | |
|
| |||
| Grade (20 missing)** | |||
| G1 | 22 (69%) | 18 (75%) | 0.767 |
| G2/G3 | 10 (31%) | 6 (25%) | |
|
| |||
| Chemotherapy*** | |||
| Yes | 14 (39%) | 6 (19%) | 0.109 |
| Median Number of Cycles | 6 | 5 | 0.175 |
| Range | 4–8 | 3–9 | |
| Chemotherapy Regimen Type*** | |||
| Gynecologic | 11 (79%) | 4 (67%) | 0.613 |
| Gastrointestinal | 3 (21%) | 2 (33%) | |
|
| |||
| Upper GI Endoscopy*** | |||
| Yes | 8 (22%) | 6 (19%) | 0.722 |
| Lower GI Endoscopy*** | |||
| Yes | 11 (31%) | 4 (12.5%) | 0.087 |
| Combined Upper and Lower GI Endoscopy*** | |||
| Yes | 12 (33%) | 6 (19%) | 0.271 |
|
| |||
| CA125 >35 U/mL*** (24 missing) | |||
| Yes | 8 (35%) | 5 (24%) | 0.518 |
|
| |||
| Tumor Size, cm*** (10 missing) | |||
| Median | 13 | 15 | 0.533 |
| Range | 8–30 | 4.5–36 | |
GCCR, Gustave Roussy Cancer Campus; DEN, Rigshospitalet in Denmark; MSK = Memorial Sloan Kettering Cancer Center; GI = gastrointestinal
p values were obtained using the Wilcoxon rank sum test for continuous variables and the Fisher exact test for categorical variables
A p value is not provided if there were less than 5 patients within a group.
MSK and GRCC data available (N=68)
None of the patients in the fertility-preservation group had positive cytology compared with 4 (10%) of 41 in the remaining patients. An appendectomy was performed in 27 (84%) of 32 and 35 (85%) of 41 patients, respectively, (p=1). A pelvic lymphadenectomy was performed in 13 (41%) of 32 and 17 (44%) of 44 patients, respectively (p=1). The median number of pelvic lymph nodes removed was 14 (range, 1–34 nodes) and 13 (range, 5–33 nodes), respectively. A para-aortic lymphadenectomy was performed in 16 (50%) of 32 and 15 (34%) of 44 patients, respectively (p=0. 237). The median number of para-aortic lymph nodes removed was 9 (range, 2–29 nodes) and 13 (range, 2–42 nodes), respectively (p=0.1). Six (25%) of 24 tumors in the fertility-preservation group were grade 2 or higher compared with 10 (31%) of 32 tumors in the remaining group (p=0.767). Pregnancy data were not collected.
Adjuvant treatment
GRCC and MSK had data regarding adjuvant treatment. Of the 145 patients (65%) who had treatment data available, 68 (47%) received systemic cytotoxic treatment, with a median of 6 cycles (range, 1–12). Chemotherapy treatments were categorized as gynecologic (GYN) or GI regimens. A GYN regimen entailed platinum plus taxane chemotherapy. Gastrointestinal (GI) treatment regimens contained combinations of cisplatin, 5-fluorouracil, capecitabine, gemcitabine, or oxaliplatin. Fifty-five (81%) of 68 patients were treated with a GYN regimen, 13 (19%) with a GI regimen (Table 1). Thirty (79%) of 38 patients with stage I/II disease were treated with a GYN regimen and 8 (21%) were treated with a GI regimen. Twenty-five (83%) of 30 patients with stage III/IV disease were treated with a GYN regimen and 5 (17%) were treated with a GI regimen. No association was noted between type of chemotherapy regimen and stage of disease (p=0.76).
Six (19%) of 32 patients who underwent fertility-preservation surgery received chemotherapy compared with 14 (39%) of the remaining 36 patients in the cohort (p=0.109). The median number of chemotherapy cycles was 5 (range, 3–9 cycles) and 6 (range, 4–8 cycles), respectively. A GYN chemotherapy regimen was used in 4 (67%) of 6 and 11 (79%) of 14 patients, respectively. A GI chemotherapy regimen was used in 2 (33%) of 6 and 3 (21%) of 14 patients, respectively (Table 3).
Survival
Univariate PFS analysis was performed for the total cohort. Within a median follow-up of 72.2 months (range, 6–365 months), there were 44 recurrences. The median PFS for the total cohort was 255 months (95% CI, 103–382), with a 5-year PFS rate of 65% (95% CI, 58–71%) (Figure 1).
Figure 1.
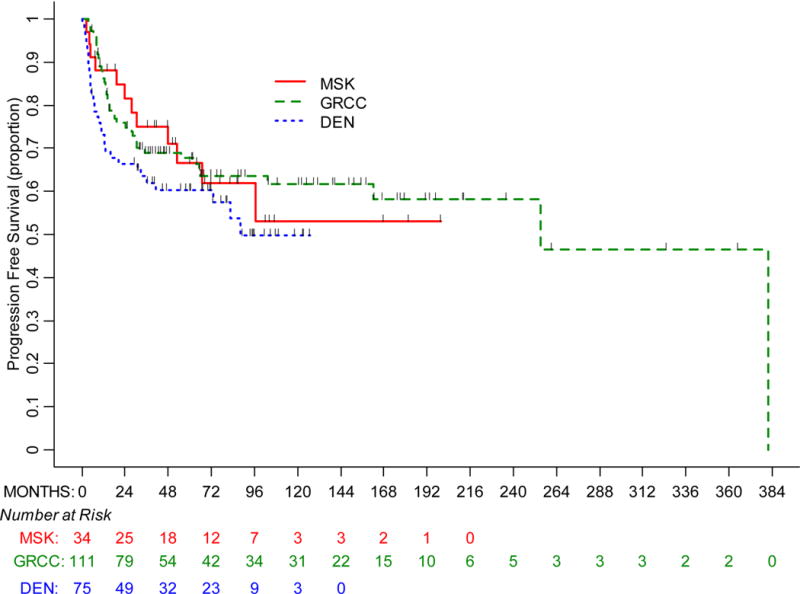
Progression-free survival by treatment center
Univariate PFS analysis was performed separately for the under age 45 cohort. There were 8 recurrences (25%) among the 32 patients who underwent fertility-preserving surgery compared with 9 recurrences (20%) among the 44 patients who did not undergo fertility-preserving surgery. The median PFS was not reached in the former group and was 382 months (95% CI, not estimable) in the latter group. The 5-year PFS rates were 73% (95% CI, 50–86%) and 84% (95% CI, 70–92%), respectively (p=0.043).
Univariate PFS analysis was performed for each center. The median follow-up was 82.4 months (range, 6–365 months) in the GRCC cohort, 70.3 months (range, 6–127 months) in the DEN cohort, and 70.4 months (range, 8–200 months) in the MSK cohort (Figure 1). The median PFS in the GRCC cohort was 255 months (95% CI, 162–382), with a 68% (95% CI, 58–76%) 5-year PFS rate. The median PFS in the DEN cohort was 88 months (95% CI, 36- not estimable), with a 60% (95% CI, 48–71%) 5-year PFS rate. The median PFS in the MSK cohort was not reached, with a 67% (95% CI, 46–81%) 5-year PFS rate (p=0.275).
Univariate PFS analysis was performed by stage. The median PFS for patients with stage I–II disease was 382 months (95% CI, not estimable), with an 80% (95% CI, 73–85%) 5-year PFS rate. The median PFS for patients with stage III–IV disease was 11 months (95% CI, 8–14%), with a 17% (95% CI, 8–29%) 5-year PFS rate.
DISCUSSION
Our series represents a substantial cohort of patients with PMOC across three international centers, each of which is a referral center for rare ovarian malignancies. The institutions share similar diagnostic criteria, and all cases had been reviewed by institutional gynecologic pathologists. Given the numerous histologic mimics and the diagnostic challenges associated with this uncommon malignancy, expert pathology review strengthens these data.
In a highly cited publication, Seidman et al. reviewed a consecutive series of 52 mucinous ovarian carcinomas and sought to develop an algorithm to distinguish primary from metastatic mucinous carcinomas of the ovary. By applying size and laterality criteria, they correctly distinguished 90% of these tumors. Metastatic tumors were defined as bilateral or <10 cm in size; primary tumors were defined as unilateral and >10 cm in size (2). Further investigation is needed to reliably distinguish PMOC in advanced-stage cases from an extra-ovarian primary malignancy. In patients who do not conform to the classic presentation of this disease, additional efforts to rule out a metastatic mucinous tumor, including a GI endoscopic evaluation, are warranted.
Our cooperative effort highlights the challenges associated with multi-institutional collaborations and affirms our initial hypothesis that there could be differences across institutions regarding patient characteristics and surgical staging strategy. We encountered specific population differences, which introduced heterogeneity to our data. For example, GRCC is a known referral site for fertility-preservation efforts in ovarian cancer, which is reflected in age at diagnosis and surgical procedures performed. At MSK, more lymph node dissections were performed. This heterogeneity also introduces referral bias, which can limit comparisons across centers.
Although our series is large and from three high-volume specialized centers, specific data capture and retrieval methods differ across institutions (12). Some data were recorded in a prospective and compulsory manner but were not all inclusive of the many data points of interest for this rare tumor type. Most data were captured retrospectively.
An unanswered question of our series is the impact of lymphadenectomy on oncologic outcome in clinically apparent stage I PMOC. This is an important question. Lymphadenectomy was not routinely performed across the institutions. Of the 99 patients who underwent any form of lymphadenectomy, 5 patients had positive pelvic nodes and 7 had positive para-aortic nodes. These small numbers limit meaningful analysis. Lymph nodes were removed in less than half of the DEN and GRCC cohorts, and although 85% of the patients in the MSK cohort underwent lymph node removal, no lymph node metastases were detected. This suggests that occult lymph node metastasis is a rare event in this malignancy. Furthermore, PFS was not significantly different across the institutions. This is a clinically relevant question that has been examined in prior literature. In a large series from MD Anderson, Schmeler et al. (14) reported no nodal metastasis in a cohort of 107 patients with PMOC, of whom 51 underwent lymphadenectomy. Although the series was retrospective, it was strengthened in that it was from a single institution, was composed of a large cohort, and a significant proportion of patients with clinical stage I disease underwent lymphadenectomy. Muyldermans et al. (15) looked at 44 patients diagnosed with PMOC, of whom 20 underwent lymphadenectomy, and reported that occult nodal disease was more likely to be found in tumors with infiltrative rather than expansile subtypes of invasion. More recently, Gouy et al. (16) looked at a cohort of 68 patients with apparent stage I PMOC, of whom 31 underwent lymphadenectomy, and reported occult nodal metastasis in infiltrative-type tumors but not in expansile-type tumors. The numbers of node-positive cases in these series are small, and the ability to apply these findings to guide surgical decisions remains limited. Histologic pattern of invasion, whether expansile or infiltrative, is not available when surgeons are deciding whether to pursue nodal evaluation at the time of primary surgery. Based on the available retrospective literature, the question of whether or not to perform lymphadenectomy in clinical stage I PMOC is not definitively answered. Although there may be a narrowly defined subset of patients who may benefit from this approach, the available literature suggests that more research is needed to identify such a population and to guide risk stratification at the time of primary surgery.
We conducted a univariate PFS analysis in the overall cohort and in young women with stage I disease. Analyzing survival in a cohort with different follow-up times, methods of data collection, and institutional patient selection bias should be interpreted in context. Given these limitations, we did not pursue a multivariate survival analysis. Eight patients, all from GRCC, had follow-up beyond 200 months, which widened the range, median follow-up, and median PFS. GRCC is well known for fertility-preserving surgical strategies in this cancer; thus, PFS outcomes must be interpreted in context. PFS outcomes were favorable in this largely stage I cohort of patients with PMOC, although there was one additional recurrence and a decrease in PFS in women treated with a fertility-preservation approach. These data are consistent with conclusions from a recently published, large cohort of patients with stage I ovarian cancers treated with typical staging surgery or a fertility-preserving strategy (17).
Work by Hess et al., which looked at the treatment of advanced PMOC, suggests mucinous ovarian cancer compared with other histologic subtypes does not respond as favorably to platinum-based GYN chemotherapy regimens (10). These tumors may require alternative chemotherapy regimens, such as those more commonly used for GI mucinous malignancies. In Gynecologic Oncology Group (GOG) study 241 (18), investigators sought to enroll 332 patients across four arms—every 3-week carboplatin plus paclitaxel with or without bevacizumab and every 3-week oxaliplatin plus capecitabine with or without bevacizumab. This trial closed early due to poor accrual—50 patients over 4 years. As presented at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting, 36 cases underwent pathologic review, of which 19 were considered metastatic disease rather than PMOC. This highlights two important points: PMOC requires expert pathology review given the diagnostic challenges, and the rarity of this tumor is an inherent limitation to its prospective study (18, 19).
The genomic analysis of PMOC may identify key driver mutations and therapeutic targets, aid in establishing an accurate diagnosis, and stratify patients to appropriate treatment (20). However, it is critical that expert pathologists carefully review all banked tumor specimens with well-annotated correlative clinical data, particularly in stage II and greater cases, to ensure the tumor studied is indeed a PMOC.
Tumor registries and institutional collaborations, in combination with a practical and cooperative effort to include the numerous more contemporary variables that allow for uniform and accurate data capture and analysis of rare tumors, are needed for the study of PMOC. Cooperative databases of clinical and immunohistochemistry data, scanned slide sets for review, and a GI/imaging work-up would further support recruitment of accurately diagnosed cases. With a diminishing likelihood of prospective study of PMOCs, the many questions investigators seek to ask at the trial level will need to be creatively addressed with collaborative, accurate, and comprehensive data sharing.
Acknowledgments
Funding: Supported in part by the MSK Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement: Outside this work, Dr. Gouy has consulted for Roche. The other authors have no conflicts of interest to disclose.
References
- 1.McCluggage GW. Immunohistochemistry in the distinction between primary and metastatic ovarian mucinous neoplasms. J Clin Pathol. 2012;65:596–600. doi: 10.1136/jcp.2010.085688. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries. Am J Surg Pathol. 2003;27:985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Zaino RJ, Brady MF, Subodh ML, et al. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal. Cancer. 2011;117:554–562. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231–247. doi: 10.1111/j.1365-2559.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Current Oncol Rep. 2014;16:389. doi: 10.1007/s11912-014-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng A, Li M, Kanis MJ, et al. Is it necessary to perform routine appendectomy for mucinous ovarian neoplasms? A retrospective study and meta-analysis. Gynecol Oncol. 2017;144:215–222. doi: 10.1016/j.ygyno.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Feigenberg T, Covens A, Ghorab Z, et al. Is routine appendectomy at the time of primary surgery for mucinous ovarian neoplasms beneficial? Int J Gynecol Cancer. 2013;23:1205–1209. doi: 10.1097/IGC.0b013e31829b7dca. [DOI] [PubMed] [Google Scholar]
- 8.Lin JE, Seo S, Kushner DM, Rose SL. The role of appendectomy for mucinous ovarian neoplasms. Am J Obstet Gynecol. 2013;208:46.e1–4. doi: 10.1016/j.ajog.2012.10.863. [DOI] [PubMed] [Google Scholar]
- 9.NCCN guidelines Version 1.2017 Mucinous Carcinoma of the Ovary. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf accessed on May 13, 2017.
- 10.Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–1044. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen SM, Bjorn SF, Jochumsen KM, et al. Danish Gynecological Cancer Database. Clin Epidemiol. 2016;8:485–490. doi: 10.2147/CLEP.S99479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y, Kamoi S, Amada S, et al. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by using the marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 14.Schmeler KM, Tao X, Frumovitz M, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol. 2010;116(2 Pt 1):269–273. doi: 10.1097/AOG.0b013e3181e7961d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muyldermans K, Moerman P, Amant F, Leunen K, Neven P, Vergote I. Primary invasive mucinous ovarian carcinoma of the intestinal type: importance of the expansile versus infiltrative type in predicting recurrence and lymph node metastases. Eur J Cancer. 2013;49:1600–1608. doi: 10.1016/j.ejca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Gouy S, Saidani M, Maulard A, et al. Staging surgery in early-stage ovarian mucinous tumors according to expansile and infiltrative types. Gynecol Oncol Rep. 2017;22:21–25. doi: 10.1016/j.gore.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melamed A, Rizzo AE, Nitecki R, et al. All-cause mortality after fertility sparing surgery for stage I epithelial ovarian cancer. Obstet Gynecol. 2017;130:71–79. doi: 10.1097/AOG.0000000000002102. [DOI] [PubMed] [Google Scholar]
- 18.GOG 241 Carboplatin and Paclitaxel or Oxaliplatin and Capecitabine With or Without Bevacizumab as First-Line Therapy in Treating Patients With Newly Diagnosed Stage II-IV or Recurrent Stage I Epithelial Ovarian or Fallopian Tube Cancer. https://clinicaltrials.gov/ct2/show/NCT01081262 accessed on May 13, 2017.
- 19.Gore ME, Hackshaw A, Brady WE, et al. Multicentre trial of carboplatin/paclitaxel versus oxaliplatin/capecitabine, each with/without bevacizumab, as first line chemotherapy for patients with mucinous epithelial ovarian cancer (mEOC) As presented at the 2015 ASCO Annual Meeting. http://meetinglibrary.asco.org/content/145864-156 accessed on May 13, 2017. (abstract 5528)
- 20.Matson DR, Xu J, Huffman L, et al. KRAS and GNAS co-mutation in metastatic low-grade appendiceal mucinous neoplasm (LAMN) to the ovaries: A practical role for next-generation sequencing. Am J Case Rep. 2017;18:558–562. doi: 10.12659/AJCR.903581. [DOI] [PMC free article] [PubMed] [Google Scholar]


