Abstract
The ejaculatory bulb of Drosophila melanogaster males produces proteins and pheromones that play important roles in reproduction. This tissue is also the final mixing site for the ejaculate before transfer to the female. The ejaculatory bulb’s dynamics remain largely unstudied. By microscopy of the ejaculatory bulb in maturing adult males, we observed that the ejaculatory bulb expands in size as males age. Moreover, we document that when males mate, their ejaculatory bulb expands further as ejaculate transfer begins, and then contracts halfway through the course of mating as ejaculate transfer finishes. Although there is some male-to-male variation in the timing of these changes, ultimately the tissue changes in a predictable pattern that gives insight into the active mating process in Drosophila.
Keywords: Drosophila melanogaster, Ejaculatory bulb, Mating, Aging, Seminal fluid proteins, PEBme
Graphical abstract

1. Introduction
Seminal fluid molecules are essential for normal and successful reproduction, making it of interest to understand the biology of the tissues that produce or transmit them. For example, studies in Drosophila melanogaster have shown that the male’s accessory glands, which produce many peptides and proteins that cause critical post-mating changes in females (reviewed in (1)), contain two secretory cell types (around a lumen) with different structure, synthetic characteristics, and functions (2–7).
Another important secretory tissue in the male D. melanogaster reproductive tract is the ejaculatory bulb (EB) (reviewed in (8)). This tissue is at the most distal end of the male’s reproductive tract (closest to the outside of the male), a position at which a number of Diptera and other insects have broadenings or specializations of the ejaculatory duct (a few examples are: (9–14)). The EB is the last part of the tract through which other ejaculate components pass on their way into the female. Its secretions, added to the seminal fluid and transferred to females during mating, include proteins that coagulate to form the mating plug that retains the ejaculate in the female (and may facilitate sperm movement). One EB protein, the fluorescent protein PEBme (15, 16), has sequence features suggesting a structure that can mediate this coagulation, and its removal from males interferes with mating plug formation and thus retention of ejaculate in mated females (17). Another EB protein, PEB2, regulates female attractiveness shortly after mating (18). The EB also provides the hydrocarbon cis-vaccenyl acetate (cVA) which, after transfer to females, contributes to the pheromone blend that makes females unattractive to males for several hours post-mating (19). In addition to generating these important molecules, the EB is also thought to pump the ejaculate into females during mating.
In 1968, Bairati observed through structural and ultrastructural analysis that the EB is composed of a receptive apparatus that appears to control bulb compression (3). The ejaculatory bulb was described as “kidney bean shaped,” with four cylindrical “horns” that extend on all sides of the ejaculatory bulb’s central depression (Figure 1) (3). We wondered whether the accumulation and ejaculation of EB contents might impact the tissue’s shape during development and mating. Accordingly, we examined the morphology of the EB as male flies mature and mate. We report here that the EB consists of a lumen surrounded by cells, and further surrounded by muscles, and that the tissue expands as the males age and synthesize EB secretions. We also report that the EB expands further, and then contracts, during mating, presumably reflecting ejaculate accumulation and entry into, and then release from, this tissue.
Figure 1.

A normal ejaculatory bulb from an unmated 3-day-old Canton S male. Labels are based on Bairati (3). “A” points to 3 of the 4 “horns”; there is one horn at each of the four corners of the EB. B shows a point in the bulbar cavity, the rounded region in the center, which appears semicircular from the side. Bright autofluorescent particles are seen in this cavity, as discussed in the text. C marks the sclerite handle which, through a series of fibers, connects the two sides.
2. Materials and Methods
2.1 Fly strains
Unless otherwise noted, all flies used in this study were from the Canton S strain. Flies were raised on yeast-glucose agar medium at 23°C with a 12:12 light:dark photoperiod. Tubulin-Gal4; UAS-MHC-GFP flies (20) whose myosin heavy chain is labeled with green fluorescent protein, were used to visualize the muscles surrounding the ejaculatory bulb.
2.2 Mating
Matings were carried out with 3-day-old virgin males, and 3-5 day old virgin females, all previously aged in single-sex cultures. One fly of each sex was placed into a cylindrical glass vial. Pairs were observed to note the initiation of mating; mating typically lasted ~17 min. To measure changes in EB size during mating, flies were collected at the following time points: unmated, and at 2 min, 5 min, 10 min, 25 min, 1 h, and 3 h after the start of mating (ASM). For the 2 min, 5 min and 10 min ASM time-points, mating pairs that were separated by vigorous centrifugal motion of the vial; for the 3 latest time-points, the flies had uncoupled naturally, and the female was removed from the vial immediately after the uncoupling. Uncoupled males were aspirated individually into microcentrifuge tubes, flash frozen in liquid nitrogen, and stored at −80°C. Sample sizes ranged from 6-54 flies per timepoint, with an average of 20.
2.3 Dissection & Microscopy
Using stainless steel forceps, we dissected each male in 1× PBS, under a dissection microscope. The EB and one wing from each fly were mounted on a glass slide with two drops of PBS, placed under a glass coverslip, and examined using a Leica DM 500B fluorescence microscope. Under these conditions, the EB is slightly flattened, and its width (taken left-to-right across the vertical middle of EBs oriented as in Fig. 1) is 0.26mm. To measure EB size, we photographed the flattened tissue under 4× magnification, and used the freehand selections tool of Image J to trace the outline of one half of the EB (a line was drawn vertically through the center of the bilaterally symmetrical EB, and the most clearly visible half was traced, as both halves were not always fully visible or otherwise measurable). The area of the traced shape was determined with Image J, and measuring a straight line from the center of the anterior edge of the wing to the center of the posterior edge of the wing. For normalization, we measured the width of one wing of the same fly, using the straight lines tool in Image J. Results were analyzed statistically using ANOVA, in RStudio. For all experiments, dissections, microscopy, and measurements were performed blind as to treatment group. To visualize MHC-GFP staining, EB dissected from tub>MHC-GFP males were excited with 500nm wavelength light from a mercury lamp, and visualized with an I3 filter under a Leica DM 500B fluorescence microscope. Autofluorescence of PEBme in EBs or mated females was examined under UV illumination on the Leica DM 500B microscope, and EBs and wings were photographed under the UV filter.
2.5 DAPI Staining
DAPI (4′, 6-diamidine-2-phenylindole) staining was done as described in Wu and Luo (21), with minor modifications for dissection method, and the use of a final DAPI concentration of 1.75μg DAPI/μL PBS.
3. Results and Discussion
3.1 The Ejaculatory Bulb Contains a Lumen and is Surrounded by Muscle
Bairati (1968) posited that the ejaculatory bulb had a lumen full of seminal components and was able to compress during ejaculation, to pump the ejaculate into the female during mating. But the cellular and cell-type structure of this tissue had not been investigated. We stained EBs with DAPI to visualize the cellular composition. We saw that the center of the EB is a bulbar cavity devoid of cells (Fig. 2B), consistent with Bairati’s view that the EB has a lumen to hold ejaculate (3). To determine whether the EB is surrounded by muscles to facilitate contraction, as in other reproductive tract tissues (22), we examined MHC-GFP distribution on the EB of transgenic males. We observed that muscle surrounds the EB (Fig. 2A), with especially high density around the bulbar cavity.
Figure 2.

The ejaculatory bulb contains a lumen, and is surrounded by muscle. A) Muscles surrounding the EB are visualized with MHC-GFP in this EB of a mated 3-day-old tub> MHC-GFP male. B) A DAPI-stained EB, from a 3-day-old unmated Canton S male. The orientation of this EB is similar to that shown in panel A. Note the acellular lumen (unstained by DAPI) and the duct leading out of the EB.
Ejaculatory bulb secretions that are transferred to females include the autofluorescent protein PEBme (15–17). The EB lumen of mature unmated males contained autofluorescent particles that we believe fluoresce because of PEBme. In any case, these particles’ presence/absence can reveal accumulation or depletion of EB luminal contents. Fluorescent particle number in the lumen dropped drastically after mating (Fig. 3B, C), and particles appeared in the lumen of the female reproductive tract (Fig. S1), consistent with their transfer as part of the ejaculate.
Figure 3.
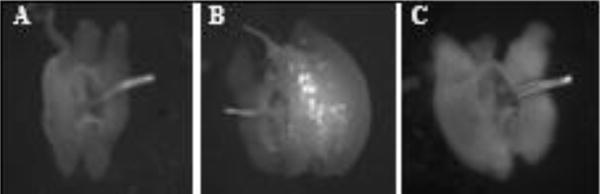
Autofluorescent particles accumulate in the EB lumen as males mature, and are lost during mating. Representative images of show A) the EB of a virgin one-day-old Canton S male showing no particles. B) the EB of a 3-day-old unmated Canton S male showing many autofluorescent particles. C) EB of a 3-day-old Canton S male 25 minutes ASM lacking autofluorescent particles.
Taken together, these data suggest that the EB has a lumen that can fill with secretions and is surrounded by muscles that can potentially contract during mating to facilitate ejaculate transfer.
3.2 Ejaculatory Bulb Size Increases with Age
The autofluorescent particles mentioned above are not detected in the EB until after the first day post-eclosion (Fig. 3A, B), but thereafter are always seen in the EB lumen if the male does not mate (Fig. S2). This suggests that, analogous to what is seen for the male accessory gland (2, 23–25), EB contents appear in young males, and accumulate further as an unmated male matures. As accumulation of lumenal contents might increase the size of the EB, we measured EB size (normalized to wing-size) as males matured. We found that, in the absence of mating, EB size increases in a nearly linear fashion for the first ~6 days post-eclosion. By day 7 post-eclosion in unmated males, EB size has plateaued (Fig. 4). Thus, as males mature they accumulate secretions in their EB, which grows in size.
Figure 4.
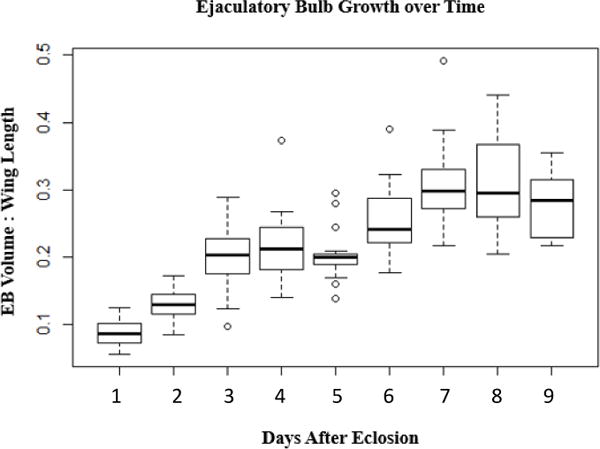
Ejaculatory bulb size increases with age. Area of ejaculatory bulb, normalized to wing size, of unmated Canton S males of the indicated age. Bar indicates mean, and box covers the two middle quartiles. The whiskers extend to the highest and lowest data points in the set. n = 19, 19, 19, 20, 17, 19, 17, 11, 20, respectively. p= 5.5×10−5 for ANOVA performed using R Studio. F= 38.769, df = 7. All pairwise comparisons were significant at p<0.05 except for day 6&7 (p= 0.061) and the following day-pairs: 3&4, 3&5, 4&5, 4&6, 6&8, 7&8, 7&9, and 8&9, whose pairwise p-values were all >0.1
3.3 The Ejaculatory Bulb Expands and Contracts Over the Course of Mating
Since EB contents are transferred to females during mating (15–17, 19, 26), and since sperm and contents of male reproductive glands must pass through the ejaculatory bulb during mating, we predicted that the EB’s size might change during mating and ejaculation. To determine whether this is the case, we compared EB sizes of virgin males to those of males during or after a single mating (Fig. 5 shows two representative experiments; three additional experiments are shown in Fig. S3). We examined EB size (normalized to wing-size) at the following time points: 2 min ASM (just before ejaculate transfer is reported to start; (27, 28); 5 min ASM (between the beginning and end of ejaculate transfer; (27, 28), 10 min ASM (transfer has typically finished and the mating plug has begun to form in the female; (17, 27); 25 min ASM (shortly after the end of mating), and 1 and 3h ASM, to test for recovery of EB size.
Figure 5.
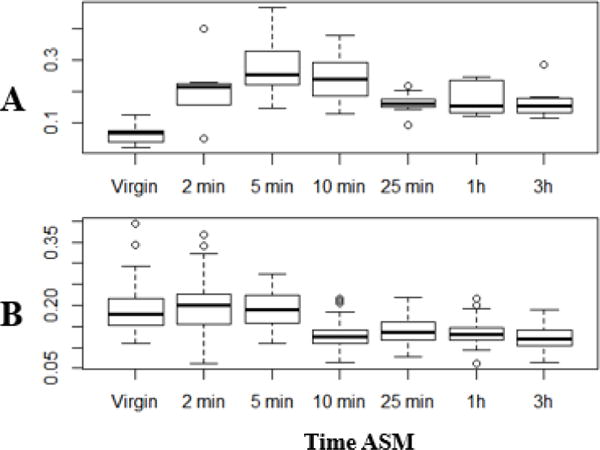
Ejaculatory bulb size changes during mating. Shown are two independent repeats of an experiment in which the area of the ejaculatory bulb size (normalized to wing size) was determined in Canton S males at different times after the start of mating (ASM); three additional repeats are shown in Fig. S3. Units are based on the units of area provided by ImageJ. Bars indicate means, and boxed cover the two middle quartiles. The whiskers extend to the highest and lowest data points in the set. A) n= 6, 7, 10, 10, 9, 8, 8, p=3.77×10−5 for ANOVA performed using R Studio. F= 6.517, df=6; pairwise p-values: v/2m: 0.018, v/5m: 0.000023, v/10m: 0.00025; 5m/25m: 0.025, 5m/3h: 0.049; v/1h: 0.087; all others had p>0.1. (B) n=47, 54, 51, 50, 50, 50, 46, p=2×10−16 for ANOVA performed using R Studio. F= 30.178, df= 6; pairwise p-values <0.01 for v (or 2m or 5m) vs. 10m, 25m, 1h, or 3h; all other pairwise p-values >0.1.
For the first 5 min ASM, EB size increases to varying extents, presumably as contents of other reproductive tissues enter the EB. After that, the EB’s size decreases significantly particularly by the end of mating. The EB remains small (close to virgin-sized) for at least three hours ASM.
To test whether the size changes correlated with ejaculate dynamics, we used the autofluorescent particles (Figs. 3, S1, S2) as a marker for EB contents. We examined the presence of these particles in EBs at 2 min, 5 min and 10 min ASM in flies from the experiment shown in Fig. 5B. We categorized each EB in terms of the presence (A (high levels), B (intermediate levels)) or absence (C (undetectable levels)) of these particles (Fig S4). Fig. S5 shows that the EBs without detectable particles at each time point, the EBs with the most particles (class A) were also the largest, and (class C) were the smallest. These results are consistent with EB size reflecting accumulation (vs. loss) of ejaculate, and allowed us to use the autofluorescence as a proxy for ejaculate amount in a time-course experiment. Fig. 6 shows that early in mating (2 min ASM), >90% of EBs contain particles (and most contain many particles (Class A). Three min later, when ejaculation has begun and “Acp” seminal proteins from the accessory gland have begun to enter the female (27, 28), the proportion of EBs containing high levels of ejaculate (class A) has dropped, while the proportion that is partially depleted in particles (class B) has risen. By 10 min ASM, with mating 2/3 over and ejaculation well in progress or nearing completion (27, 28), nearly half of EBs are devoid of particles (class C), and fewer than 5% show high numbers of particles (class A). By the end of mating, particle-devoid EBs predominate, with occasional cases of category B (few particles remaining after ejaculation). In addition to showing that during ejaculation particle number in the EB decreases as EB size decreases, we also saw significant variability among males in the rate of particle depletion (and thus ejaculate transfer). This suggests that at these timepoints after the start of mating, there is some variation in how far individual pairs have gotten in the mating process; males from a given timepoint can include ones who have not started to transfer ejaculate, others who are in mid-transfer, and still others who have finished. Moreover, D. melanogaster males have been reported to show differential allocation of ejaculate components (29–31), which could potentially also contribute to the variation we see in EB size. We believe that this variability also explains the variability in the extent of EB size changes at specific time points, between multiple repeats of mating experiments (Figs. 5A, 5B, and Fig. S3, S6).
Figure 6.
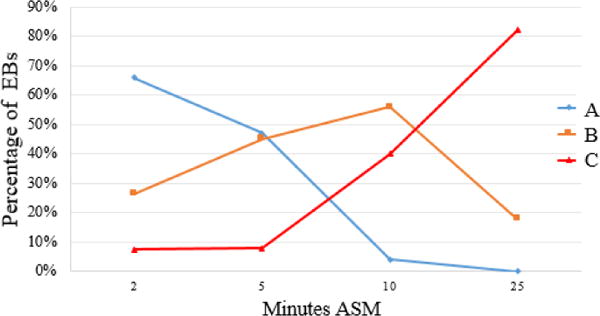
Proportion of EBs from the trials shown in Fig. 5 and Fig. S3 with many (A; blue), intermediate-amounts (B; yellow) or no detectable (C; red) autofluorescent particles in their lumens, over the course of a mating by 3-day-old Canton S males. Note that early in the mating, with little or no ejaculate transfer, most EBs contain particles, whereas by the end of mating almost all are devoid of particles.
Our results indicate that the lumen of the EB fills with secretions early in the mating process, thereby increasing the size of the EB. When ejaculation begins, the transfer of EB luminal contents to the female decreases the size of the EB. The tissue does not expand back during the first 3h post-mating.
3.4 Mating Multiply Deceases the Magnitude of EB Expansion
All the experiments described above involved flies mated singly. To determine whether subsequent matings cause the same type and magnitude of changes as the first mating, we measured the EBs of males that had been mated 1, 2 or 3 times, and flash-frozen at 2 min ASM of the last mating. Males were given up to one hour to re-mate, and the time between matings varied from about 2 minutes to an hour, with most re-mating within the first half hour. By measuring EB size (Fig. 7), we observed that the EBs of multiply-mated males appear less expanded than those virgin males, perhaps reflecting depletion of any residual contents.
Figure 7.

EB size at 2 min ASM differs in singly vs. multiply-mated males. The figure shows EB size (normalized to wing size) for Canton S males at 2 min ASM into their first, second, or third mating. Males were given up to one hour to re-mate between each mating; males that did not re-mate during this time were discarded. N =13, 12, and 11, respectively. P = 3×10−6 for ANOVA performed using RStudio. F= 18.601, df=2; pairwise p-values: virgin/1mating: ns (p=0.076), virgin/2matings: 0.000002, 1mating/2mating: 0.0017. Bars indicate means, and boxes cover the two middle quartiles. The whiskers extend to the highest and lowest data points in the set.
4. Conclusions
The ejaculatory bulb is a secretory organ wrapped in muscle, with an apparently cell-free lumen that acts as a storage site for the ejaculate before its transfer to the female. Changes in EB size are a part of a process of expansion and contraction of the tissue over at least the first 9 days of adult life (well past the onset of sexual maturity), with expansion as it accumulates and stores ejaculate, and contraction as the ejaculate is transferred to females. There are individual differences between males, even within a single genetic stock, in the timing of these changes over the course of mating.
Supplementary Material
Highlights.
The ejaculatory bulb expands in size as male Drosophila age.
The ejaculatory bulb expands and then contracts during mating.
The ejaculatory bulb remains contracted for several hours post-mating.
Based on autofluorescence, changes in ejaculatory bulb size reflect ejaculate transfer.
Ejaculatory bulb size-changes vary among males, and are greatest in a first-mating.
Acknowledgments
We thank two funding sources for supporting this research: the Rawlings Cornell Presidential Research Scholars Program for Undergraduates (ABC), and NIH grant R01 HD038921 (to MFW). We thank N. Buehner for assistance with the DAPI staining protocol, F. Avila for suggestions, guidance and comments on the manuscript, L. Sirot for advice on insect reproductive tract morphology, D. Barbash for use of his lab’s fluorescence microscope and D. Zinsteyn and S. Delbare for advice about its use, anonymous reviewers for helpful suggestions, and S. Parry, S. Delbare, and the Cornell Statistical Consulting Unit, for advice on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annual review of entomology. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertram M, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type specific gene expression in the Drosophila melanogaster male accessory gland. Mechs Dev. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- 3.Bairati A. Structure and ultrastructure of the male reproductive system of Drosophila melanogaster Meig. 2. The genital duct and accessory glands. Monit Zool Ital. 1968;2:105–82. [Google Scholar]
- 4.Gligorov D, Sitnik JL, Maeda RK, Wolfner MF, Karch F. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS genetics. 2013;9(3):e1003395. doi: 10.1371/journal.pgen.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitnik JL, Gligorov D, Maeda RK, Karch F, Wolfner MF. The Female Post-Mating Response Requires Genes Expressed in the Secondary Cells of the Male Accessory Gland in Drosophila melanogaster. Genetics. 2016;202(3):1029–41. doi: 10.1534/genetics.115.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiblich A, Marsden L, Gandy C, Corrigan L, Jenkins R, Hamdy F, Wilson C. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(47):19292–7. doi: 10.1073/pnas.1214517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minami R, Wakabayashi M, Sugimori S, Taniguchi K, Kokuryo A, Imano T, Adachi-Yamada T, Watanabe N, Nakagoshi H. The homeodomain protein defective proventriculus is essential for male accessory gland development to enhance fecundity in Drosophila. PloS one. 2012;7(3):e32302. doi: 10.1371/journal.pone.0032302. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avila FW, Wong A, Sitnik JL, Wolfner MF. Don’t pull the plug! the Drosophila mating plug preserves fertility. Fly. 2015;9(2):62–7. doi: 10.1080/19336934.2015.1120931. Epub 2015/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson JT. The Drosophilidae of the Southwest. University of Texas Publications; Austin: 1943. p. 4313. [Google Scholar]
- 10.Bjork A, Dallai R, Pitnick S. Adaptive modulation of sperm production rate in Drosophila bifurca, a species with giant sperm. Biol Lett. 2007;3(5):517–9. doi: 10.1098/rsbl.2007.0219. Epub 2007/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera-Cruz M, Abraham S, Nunez-Beverido N, Flores-Estevez N, Reyes-Hernandez M, Alvarado M, Perez-Staples D. Male age and strain affect ejaculate quality in the Mexican fruit fly. Insect Sci. 2017 doi: 10.1111/1744-7917.12446. Epub 2017/02/22. [DOI] [PubMed] [Google Scholar]
- 12.Chiang RG, Chiang JA. Reproductive physiology in the blood feeding insect, Rhodnius prolixus, from copulation to the control of egg production. Journal of insect physiology. 2017;97:27–37. doi: 10.1016/j.jinsphys.2016.06.001. Epub 2016/06/12. [DOI] [PubMed] [Google Scholar]
- 13.Ramamurthi BN. Studies on the male genital tube in the Dermaptera. Proc Roy Ent Soc Lond. 1958;A33(186–190) [Google Scholar]
- 14.Kamimura Y. Possible Removal of Rival Sperm by the Elongated Genitalia of the Earwig, Euborellia plebeja. Zoolog Sci. 2000;17(5):667–72. doi: 10.2108/zsj.15.667. doi: 10.2108/zsj.15.667. Epub 2008/06/04. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig MZ, Uspensky II, Ivanov AI, Kopantseva MR, Dianov CM, Tamarina NA, Korchin LI. Genetic control and expression of the major ejaculatory bulb protein PEB-me in Drosophila melanogaster. Bioch Genet. 1991;29:215–40. doi: 10.1007/BF00590103. [DOI] [PubMed] [Google Scholar]
- 16.Lung O, Wolfner MF. Identification of and characterization of the major D. melanogaster mating plug protein. Ins Bioch Mol Bio. 2001;31:543–51. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 17.Avila FW, Cohen AB, Ameerudeen FS, Duneau D, Suresh S, Mattei AL, Wolfner MF. Retention of Ejaculate by Drosophila melanogaster Females Requires the Male-Derived Mating Plug Protein PEBme. Genetics. 2015;200(4):1171–9. doi: 10.1534/genetics.115.176669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bretman A, Lawniczak MK, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. Journal of insect physiology. 2010;56(1):107–13. doi: 10.1016/j.jinsphys.2009.09.010. Epub 2009/10/06. [DOI] [PubMed] [Google Scholar]
- 19.Laturney M, Billeter JC. Drosophila melanogaster females restore their attractiveness after mating by removing male anti-aphrodisiac pheromones. Nat Commun. 2016;7:12322. doi: 10.1038/ncomms12322. Epub 2016/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35(2):383–96. doi: 10.1016/j.mcn.2007.04.001. Epub 2007/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JS, Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc. 2006;1(4):2110–5. doi: 10.1038/nprot.2006.336. Epub 2007/05/10. [DOI] [PubMed] [Google Scholar]
- 22.Norville K, Sweeney ST, Elliott CJ. Postmating change in physiology of male Drosophila mediated by serotonin (5-HT) J Neurogenet. 2010;24(1):27–32. doi: 10.3109/01677060903477601. Epub 2010/01/14. [DOI] [PubMed] [Google Scholar]
- 23.Monsma SA, Harada HA, Wolfner MF. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev Biol. 1990;142:465–75. doi: 10.1016/0012-1606(90)90368-s. [DOI] [PubMed] [Google Scholar]
- 24.DiBenedetto AJ, Harada HA, Wolfner MF. Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Dev Biol. 1990;139:134–48. doi: 10.1016/0012-1606(90)90284-p. [DOI] [PubMed] [Google Scholar]
- 25.Chapman KB, Wolfner MF. Determination of male-specific gene expression in Drosophila accessory glands. Dev Biol. 1988;126:195–202. doi: 10.1016/0012-1606(88)90253-9. [DOI] [PubMed] [Google Scholar]
- 26.Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS biology. 2008;6(7):e178. doi: 10.1371/journal.pbio.0060178. Epub 2008/08/01. doi: 08-PLBI-RA-1419 [pii] 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system through the walls of the posterior vagina. Insect Biochem Mol Biol. 1999;29:1043–52. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 28.Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution. 2000;54(2):534–42. doi: 10.1111/j.0014-3820.2000.tb00056.x. Epub 2000/08/11. [DOI] [PubMed] [Google Scholar]
- 29.Garbaczewska M, Billeter JC, Levine JD. Drosophila melanogaster males increase the number of sperm in their ejaculate when perceiving rival males. Journal of insect physiology. 2013;59(3):306–10. doi: 10.1016/j.jinsphys.2012.08.016. Epub 2012/11/28. [DOI] [PubMed] [Google Scholar]
- 30.Sirot LK, Wolfner MF, Wigby S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(24):9922–6. doi: 10.1073/pnas.1100905108. Epub 2011/06/02. doi: 1100905108 [pii] 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FC, Bretman A, Wolfner MF, Chapman T. Seminal fluid protein allocation and male reproductive success. Current biology: CB. 2009;19(9):751–7. doi: 10.1016/j.cub.2009.03.036. Epub 2009/04/14. doi: S0960-9822(09)00887-2 [pii] 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


