Abstract
Introduction
This study assessed longitudinal relationships between patient healthcare empowerment, engagement in care, and viral control in the Women's Interagency HIV Study, a prospective cohort study of U.S. women living with HIV.
Methods
From April 2014 to March 2016, four consecutive 6-month visits were analyzed among 973 women to assess the impact of Time 1 healthcare empowerment variables (tolerance for uncertainty, and the state of Informed Collaboration Committed Engagement) on Time 2 reports of ≥95% HIV medication adherence and not missing an HIV primary care appointment since last visit; and on HIV RNA viral control across Times 3 and 4, controlling for illicit drug use, heavy drinking, depression symptoms, age, and income. Data were analyzed in 2017.
Results
Adherence of ≥95% was reported by 83% of women, 90% reported not missing an appointment since the last study visit, and 80% were categorized as having viral control. Logistic regression analyses revealed a significant association between the Informed Collaboration Committed Engagement subscale and viral control, controlling for model covariates (AOR=1.08, p=0.04), but not for the tolerance for uncertainty subscale and viral control (AOR=0.99, p=0.68). In separate mediation analyses, the indirect effect of Informed Collaboration Committed Engagement on viral control through adherence (B=0.04, SE=0.02, 95% CI=0.02, 0.08), and the indirect effect of Informed Collaboration Committed Engagement on viral control through retention (B=0.01, SE=0.008, 95% CI=0.001, 0.030) were significant. Mediation analyses with tolerance for uncertainty as the predictor did not yield significant indirect effects.
Conclusions
The Informed Collaboration Committed Engagement healthcare empowerment component is a promising pathway through which to promote engagement in care among women living with HIV.
Introduction
Empowerment involves the transfer of power and mastery from one entity to another on issues of concern to that entity.1 Empowerment can occur at multiple levels, including psychological empowerment at the individual level, within specific subgroups (e.g., women and minority populations), and at the educational and policy levels.2-6 This transfer has emerged as an important health determinant, guiding intervention approaches that seek to build empowerment as a goal in its own right, but also as a means to promote health and reduce health inequities.6-9 Empowerment has also been applied to chronic disease management. Patient empowerment typically focuses on cognitive dimensions that indicate the motivation and perceived ability of a patient to make decisions about his or her own health care and an increased sense of responsibility for health outcomes.10-13 Although patient empowerment should lead to improved health behaviors, better health outcomes, and reduced healthcare costs,10,14-18 the advancement of research and theory in this area has been limited by diverse conceptual definitions.13,17,19-22 In an effort to synthesize this literature, Johnson19 and colleagues23,24 have advanced a unified construct of healthcare empowerment. In this model, healthcare empowerment is defined as “the process and state of being (1) engaged, (2) informed, (3) collaborative, (4) committed to one's health care, and (5) tolerant or resilient to uncertainties in treatment outcomes,” and is measured based on two factors; the first is termed Informed Collaboration Committed Engagement (ICCE).19 In ICCE, being informed refers to the importance to a patient of having information about health and treatment options, collaboration refers to the patient's perceived ability to be involved in clinical decision making, being committed involves the motivation to maintain and improve one's own health, and engagement refers to patient preferences to stay active in health care.23 The second component refers to tolerance of uncertainty (TU) and involves the capacity to manage expectations and the consequences of medical decisions with unknown outcomes.23 In the context of HIV management, for instance, TU may help patients stay involved in care when clinically recommended self-care behaviors do not result in desired changes in health. In the U.S., women are less likely than men to be virally suppressed, and it is estimated that only 44% of women with HIV have sustained viral suppression.24 This difference is explained, in part, by differences in adherence and retention in care.25,26 Given that lifelong HIV antiretroviral (ART) medication adherence and retention in HIV primary care are important predictors of viral control,27-30 healthcare empowerment could serve as an important organizing construct for improving HIV outcomes. Initial studies on healthcare empowerment conducted primarily with adult men and transgender women revealed positive correlations with medication adherence; relationships with HIV viral suppression and CD4 cell count have been less conclusive.23,31-33 Further work is required to gain clarity on these relationships in additional populations, including women.
The purpose of this analysis is to describe associations between women's healthcare empowerment and both HIV ART adherence and retention in HIV primary care. Relationships between healthcare empowerment and HIV viral suppression are described and assessed for mediating roles of adherence and retention. This analysis utilizes four waves of data collection, spanning 2 years, and controls for established predictors of care engagement, including substance use, heavy drinking, depression symptoms, age, and SES.34-44
Methods
Study Sample
Data were drawn from the Women's Interagency HIV Study (WIHS).45 Eligibility for HIV-seropositive women included a positive HIV antibody status confirmed by Western blot. WIHS sites are located in Brooklyn and Bronx, New York; Chicago, Illinois; Washington, District of Columbia; San Francisco, California; Chapel Hill, North Carolina; Atlanta, Georgia; Miami, Florida; Birmingham, Alabama; and Jackson, Mississippi.45,46
Study visits included standardized self-report interviews administered by centrally trained interviewers in English or Spanish, and a blood draw for assessment for HIV RNA viral load. Participants provided written informed consent, and study visits were spaced at 6-month intervals. Women were remunerated for time associated with study visits in the range of $50-$80, depending on study site activities conducted during that visit, and transportation costs were covered. All study activities were approved by the site's IRB, and data are protected by a federal Certificate of Confidentiality.
The healthcare empowerment model suggests that increases in empowerment should cause increases in self-care behaviors of adherence and retention in care, which in turn should lead to sustained control of HIV infection over time. A temporal order was therefore used for modeling the predictor variable (healthcare empowerment), proposed mediators (adherence and retention in care), and outcome (viral suppression), to reflect the hypothesized causal order of mediation. Healthcare empowerment subscales and all covariates were assessed at Time 1 (T1), completed between April and September 2014. Adherence and retention were assessed at the next study visit (T2) from October 2014 to March 2015. Viral control was assessed across the subsequent two study visits (T3/T4), first from April to September 2015 and again from October 2015 to March 2016. Data were analyzed in 2017.
Measures
Participants completed the Health Care Empowerment Inventory.23 The Health Care Empowerment Inventory ICCE and TU subscales each consist of four items with five response options (strongly disagree to strongly agree). The ICCE subscale includes items such as, “I try to get as much information as possible about treatment options,” and “I take my commitment to my treatment seriously.” The TU subscale includes items such as, “I have learned to live with the uncertainty of my condition.” For each subscale, items are summed, and higher scores indicate greater healthcare empowerment.
Self-reports on retention in care are provided at each study visit. Retention in care was defined as a self-report of having no missed HIV primary care appointments since the last study visit, versus at least one missed appointment. This definition is associated with viral control and mortality,47-49 and previous research demonstrates the validity of self-reported missed visits.50 An HIV primary care appointment was defined as a visit to a clinic or doctor's office, during which the patient would have met with a doctor, physician's assistant, or a nurse practitioner about her HIV. Excluded from this definition were sick visits, emergency services, hospital admissions for HIV/AIDS, and visits that were only for lab or blood work or x rays.
Retrospective 6-month self-report was used to assess the percentage of ART taken (none of the time, less than 75%, 75%–94%, 95%–99%, 100% of the time). In WIHS, self-reported adherence of >95% has been associated with HIV viral load measures, with quality of life, and with measures of ART exposure in hair.51-54 A report of 95%-100% adherence was categorized as optimal adherence; those below that threshhold were categorized as having suboptimal adherence.
Plasma HIV-1 RNA quantification is performed at each study visit. A cut off value for the assay of 200 copies/mL was used.55 To provide a stable estimate of viral suppression,56 two consecutive study visits were used; those with values <200 copies/mL across visits were categorized as having viral control, whereas those who exceeded this threshold at either visit were categorized as not having viral control.
Illicit substance use and heavy drinking were included given their established relationships with adherence and viral control among women with HIV infection.57,58 Illicit substance use was assessed with quantity and frequency measures of any substance since the last interview,59,60 and included assessement for any injection drug use, or other means of administration of crack/cocaine, heroin, methamphetamine, hallucinogens, club drugs, and non-prescribed methadone or other non-prescribed narcotics since the last interview. Marijuana use was excluded from this definition, as WIHS does not reliably distinguish between medicinal and recreational use. Substance use was defined as a report of use of any of the substances evaluated. Heavy drinking was defined as more than seven drinks per week, as per national guidelines established for women.61 To assess burden of depression symptoms, women completed the 20-item Centers for Epidemiologic Studies-Depression scale.62-67 A standard cut off of >16 differentiated women at greatest risk for depression.68 Income was dichotomized at the median sample value to reflect those who earn >$12,000 annually from all sources.
Statistical Analysis
Relationships between viral control and proposed covariates (i.e., depression symptoms, substance use, heavy drinking, age, income), healthcare empowerment, and both adherence and retention in care are described as a function of chi-square tests and logistic regression. Logistic regression was conducted to describe relationships between T1 ICCE subscale and T3/T4 viral control, controlling for all proposed covariates. A second logistic regression assessed the association between the TU subscale and viral control at the same timepoints and controlling for proposed covariates. For analyses that included medication adherence, women were excluded who were not on ART at T2.
Mediation analyses were conducted to test the indirect effects of adherence and missed visits on the relationship between healthcare empowerment and viral control; all tests of mediation included the following covariates: age, income, depression symptoms, heavy drinking, and substance use. Binary mediation models were conducted using Stata's binary_mediation command (Stata, version 14.2). This command accommodates mediation when the mediating variable (in this case, adherence and missed visits) is dichotomous, produces standardized path coefficients for all indirect effects, and provides a summary of total, indirect, and direct effects.69,70 Because the command does not produce bias-corrected 95% CIs for the indirect effect, Stata's bootstrapping command was used. A significant indirect effect is suggested when the bias-corrected CI does not include the value zero; this indirect effect indicates statistical mediation.
Results
At T1, a total of 1,197 women completed information on healthcare empowerment. From this group, women were excluded who did not have viral load values at both T3 and T4, resulting in an analytic sample of 973. There were no differences between those who did (n=973) and did not (n=224) have viral load values at follow-up as a function of TU healthcare empowerment scores at T1 (p=0.71). Women who had viral load data available for follow-up had somewhat higher T1 ICCE scores (mean, 17.96 [SD=2.10]), as compared with those who did not have viral load data available (mean, 17.63 [SD=2.28], p=0.03). Women ranged in age from 26 to 78 years, with 70% identifying primarily as both black/African American and non-Hispanic, 16% as Hispanic, 10% as white and non-Hispanic, and the remainder identifying with another racial or ethnic group. Among those women, 80.4% (782/973) had sustained viral suppression. A total of 886 women were on ART, and 83.2% of them (737/886) had ≥95% adherence. Most women (89.8%, [847/973]) reported not missing an appointment. Those who did not miss an appointment were more likely to have optimal adherence (85.7%) as compared with those who had missed an appointment (62%, OR=3.66, 95% CI=2.23, 6.02, p<0.001). Additional sample characteristics are provided in Table 1.
Table 1. Correlates of Sustained Viral Control Among Women Living With HIV Infection (N=973)a.
| Characteristic | Overall sample prevalence | Not virally suppressed at Time 3 or 4 (n=191) | Viral suppression at Time 3 and 4 (n=782) | OR (95% CI) | p-valueb |
|---|---|---|---|---|---|
| Mean age, years (SD)c | 49.3 (8.5) (n=973) | 47.0 (8.3) | 49.8 (8.5) | 1.04 (1.02, 1.06) | <0.001 |
| Annual income at least $12,000 (%) | 47.9 (n=452/944) | 39.3 | 49.9 | 1.54 (1.11, 2.14) | 0.01 |
| No substance use (%) | 93.4 (n=907/971) | 91.1 | 94.0 | 1.53 (0.86, 2.74) | 0.15 |
| No heavy drinking (%) | 89.1 (n=866/972) | 83.7 | 90.4 | 1.83 (1.17, 2.89) | 0.008 |
| Below depression symptom threshold (%) | 70.5 (n=680/965) | 65.1 | 71.8 | 1.37 (0.97, 1.91) | 0.07 |
| Adherent with HIV therapy (%) | 83.2 (n=737/886) | 60.1 | 87.6 | 4.69 (3.14, 6.99) | <0.001 |
| No missed HIV appointments (%) | 89.8 (n=847/943) | 80.3 | 92.1 | 2.86 (1.82, 4.48) | <0.001 |
| Mean ICCE healthcare empowerment (SD)c,d | 18.0 (2.1) (n=973) | 17.6 (2.3) | 18.0 (2.1) | 1.09 (1.02, 1.18) | 0.02 |
| Mean TU healthcare empowerment (SD)c,d | 16.0 (3.3) (n=973) | 16.0 (3.2) | 15.9 (3.3) | 1.00 (0.95, 1.05) | 0.95 |
Age, income, no substance use (no use of illicit drugs), no heavy drinking (seven or fewer drinks per week), depression symptoms threshold (score less than 16 on the Centers for Epidemiology Studies-Depression [CES-D] scale) and healthcare empowerment subscales assessed at Time 1; medication adherence (95% or greater adherence) and missed visits (not missing a scheduled HIV primary care appointment) assessed at Time 2.
Boldface indicates statistical significance (p<0.05).
ORs for 1-point increase; higher scores denote older age in years and greater healthcare empowerment.
Healthcare empowerment subscales include ICCE (Informed Collaboration Committed Engagement) and TU (Tolerance for Uncertainty); scale values for ICCE range from 8–20, (Cronbach's α=0.79, and for TU from 4–20 (Cronbach's α=0.79).
Viral control across T3/T4 was associated with higher age, higher income, and no heavy drinking at T1, and were included as covariates in the models (all p<0.05; Table 1). Although substance use and depression symptoms were not associated with viral control, they were related to T2 values of adherence (p<0.001 for substance use and p=0.02 for depression symptoms) and missed visits (p<0.001 for substance use and p=0.02 for depression symptoms), and were therefore retained as model covariates.
Logistic regression analyses revealed a significant association between the ICCE subscale at T1 and viral control at T3/T4, controlling for model covariates (AOR=1.08, 95% CI=1.004, 1.170, p=0.04). When adherence at T2 was added to the ICCE model, the association of adherence with viral control at T3/T4 was significant (AOR=4.59, 95% CI=3.00, 7.03, p<0.001); however, ICCE was no longer significantly associated with viral control at T3/T4 with this additional adjustment by adherence (AOR=1.04, 95% CI=0.95, 1.14, p=0.42). An analysis that repeated the covariate-controlled model, but which added retention at T2, maintained a significant effect of ICCE on viral control (AOR=1.09, 95% CI=1.00, 1.18, p=0.04). The effect of retention in care was also statistically significant (AOR=2.19, 95% CI=1.34, 3.57, p=0.002) in this model. These logistic regression analyses were repeated to examine associations between the TU subscale at T1 and viral control at T3/T4, controlling for model covariates. This analysis did not result in a statistically significant association between TU and viral control (AOR=0.99, p=0.68, 95% CI=0.94, 1.04). Adherence at T2 was significantly associated with viral control at T3/T4 when added to this model (AOR=4.87, p<0.001, 95% CI=3.18, 7.46), whereas the association between TU and viral control at T3/T4 remained nonsignificant (AOR=0.95, p=0.11, 95% CI=0.89, 1.01). A separate logistic regression that added retention at T2 to the model revealed a statistically significant relationship between retention and viral control (AOR=2.27, p=0.001, 95% CI=1.40, 3.69); TU was not associated with viral control at T3/T4 (AOR=0.99, p=0.78, 95% CI=0.94, 1.05) in this model.
As described in the preceding paragraph, the ICCE subscale of healthcare empowerment at T1 was significantly associated with sustained viral control at T3/T4, after covariate adjustment. Given that this direct effect was significant, a next step examined whether the association between the ICCE subscale at T1 and viral control at T3/T4 was mediated by (1) adherence at T2 and (2) by retention at T2. These indirect effects were also examined using the TU healthcare empowerment subscale. Separate mediation analyses for both ICCE and TU were conducted to test for these indirect effects, observing the hypothesized temporal order for the effects. Figure 1 presents the covariate-adjusted standardized path coefficients reflecting hypothesized relationships between ICCE at T1 and adherence at T2 (b=0.13, p=0.002), between T2 adherence and T3/T4 viral control (b=1.52, p<0.001), and between T1 ICCE and T3/T4 viral suppression without controlling for T2 adherence (b=0.07, p=0.10), and while controlling for T2 adherence (b=0.04, p=0.42). The indirect effect of ICCE at T1 on T3/T4 viral control indicates that T2 adherence mediates this relationship (B=0.04, SE=0.02, 95% CI=0.02, 0.08); the proportion of the total effect mediated was 52%.
Figure 1.
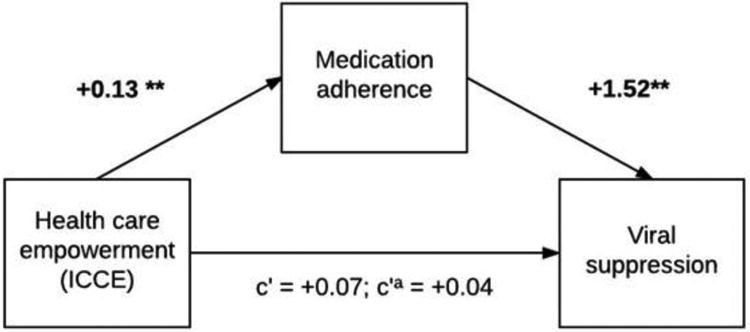
Mediation model depicting standardized path coefficients between healthcare empowerment (ICCE subscale), medication adherence, and sustained viral suppression, controlling for age, income, depression, alcohol use, and substance use (N=854). Notes: The path from ICCE to sustained viral suppression includes the coefficient without adjustment for adherence (c′) and with adjustment for adherence (c′a). The indirect effect of healthcare empowerment at Time 1 on viral suppression at Times 3 and 4 through adherence at Time 2 was statistically significant (B=0.04, SE=0.02, 95% CI=0.02, 0.08). For all paths, **p<0.01, *p<0.05.
Figure 2 presents the covariate-adjusted standardized path coefficients reflecting hypothesized relationships between ICCE at T1 and retention at T2 (b=1.0, p=0.06), between T2 retention and T3/T4 viral control (b=0.78, p<0.002), and between T1 ICCE and T3/T4 viral suppression without controlling for T2 retention (b=0.09, p=0.02), and while controlling for T2 retention (b=0.08, p=0.04). The indirect effect of ICCE at T1 on viral control at T3/T4 through retention at T2 indicates retention in care mediates the relationship between ICCE and viral control (B=0.01, SE=0.008, 95% CI=0.001, 0.030); the proportion of the total effect mediated was 13%. When the TU subscale was included as the predictor, the analyses did not yield significant indirect effects through adherence (B=0.03, SE=0.02, 95% CI= −0.002, 0.070) or through retention in care (B=0.009, SE=0.009, 95% CI= −0.006, 0.030). Finally, the mediation analyses were repeated while additionally controlling for T1 viral control. This covariate was included in order to control for baseline viral suppression, thereby increasing the stability of effects.52 In these analyses, significant indirect effects were maintained for the ICCE subscale when adherence at T2 was the mediator, (B=0.03, SE=0.01, 95% CI=0.006, 0.060), but not when retention at T2 was the mediator or when TU was the predictor (all p>0.05).
Figure 2.
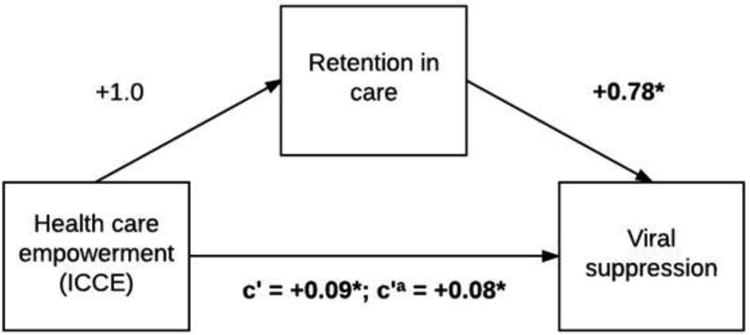
Mediation model depicting standardized path coefficients between healthcare empowerment (ICCE subscale), retention in care, and sustained viral suppression, controlling for age, income, depression, alcohol use, and substance use (N=908). Notes: The path from TU to sustained viral suppression includes the coefficient without adjustment for retention (c′) and with adjustment for retention (c′a). The indirect effect of healthcare empowerment at Time 1 on viral suppression at Times 3 and 4 through retention at was statistically significant (B=0.01, SE=0.008, 95% CI=0.001, 0.03). For all paths, **p<0.01, *p<0.05.
Discussion
The healthcare empowerment model posits that to maintain patient engagement in care, the patient should feel involved, committed, collaborative and engaged in health care, as well as tolerant of uncertainty regarding outcomes and complications associated with disease management. In this analysis, ICCE scores are associated with sustained viral suppression, and this relationship is mediated by HIV ART adherence and missed HIV primary care visits. Further, these mediating relationships are independent of burden of depression symptoms, substance use, heavy drinking, income, and age.
Support was not found for the role of TU on HIV outcomes. In this sample, many women have been living with HIV for some time, and often decades.45 It is possible that TU has a greater influence on behavior earlier in the course of disease, but has less impact over time. It could also be that in the era of highly effective therapy, a TU is not required to maintain behavioral activation.71 Further research is required to assess the relevance of TU across different chronic conditions and populations.
Limitations
Study findings should be interpreted in light of several considerations. First, behavioral measures were derived from self-report. Although these measures were related in expected directions to viral control, they are subject to errors in memory and biased responding. Given that WIHS study visits are separate from HIV primary care and that participants often receive their HIV care at separate clinical locations, it was not possible to use more objective measures, such as medical records. Second, WIHS visits occur at 6-month intervals, and in order to reasonably test mediating relationships, a longitudinal design was employed over four study visits. Only a single time period was used to assess behavioral variables of adherence and retention because it would not be reasonable to assume that empowerment would be associated with viral control over 2 years later. However, this design resulted in a high proportion of women reporting not having missed an appointment. Extending the assessment across an additional visit would result in 81% retention over a year, but this would overlap with viral load measures and compromise mediation assessment. Finally, generalizability may be limited given that women with lower ICCE scores were less likely to contribute outcome data and that participants are drawn from an ongoing prospective cohort study.
Conclusions
Intervention development and testing would be an important next step in applying the model, given that healthcare empowerment is modifiable.19,23,72 Initially promising empowerment-building approaches in other chronic diseases include training in communication skills with providers, providing access to and support for monitoring of clinical health markers, and improving coping skills for symptom and side-effect management73,74; these could be translated and tested in the context of HIV self-management. This analysis extends research linking healthcare empowerment to HIV continuum indices,31,32,75 and provides evidence of the importance of ensuring that patients remain engaged, informed, committed, and collaborative healthcare partners.
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of NIH.
WIHS Principal Investigators are as follows: UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Washington Metropolitan WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; and WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR-000004 (University of California, San Francisco Clinical and Translational Science Award), UL1-TR-000454 (Atlanta Clinical and Translational Science Award) and P30-AI-050410 (University of North Carolina Center for AIDS Research). This analysis was also supported by R01-MH104114 (Janet Turan, PI) and K24-DA037034 (Mallory Johnson, PI).
Dr. Adimora is on a Merck advisory board and has received research funds from Gilead.
Footnotes
No other financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rappaport J. Terms of empowerment/exemplars of prevention: toward a theory for community psychology. Am J Community Psychol. 1987;15(2):121–148. doi: 10.1007/BF00919275. https://doi.org/10.1007/BF00919275. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman MA. Psychological empowerment: issues and illustrations. Am J Community Psychol. 1995;23(5):581–599. doi: 10.1007/BF02506983. https://doi.org/10.1007/BF02506983. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman MA, Rappaport J. Citizen participation, perceived control, and psychological empowerment. Am J Community Psychol. 1988;16(5):725–750. doi: 10.1007/BF00930023. https://doi.org/10.1007/BF00930023. [DOI] [PubMed] [Google Scholar]
- 4.Israel BA, Checkoway B, Schulz A, Zimmerman M. Health education and community empowerment: conceptualizing and measuring perceptions of individual, organizational, and community control. Health Educ Behav. 1994;21(2):149–170. doi: 10.1177/109019819402100203. https://doi.org/10.1177/109019819402100203. [DOI] [PubMed] [Google Scholar]
- 5.Wallerstein N, Bernstein E. Introduction to community empowerment, participatory education, and health. Health Educ Q. 1994;21(2):141–148. doi: 10.1177/109019819402100202. https://doi.org/10.1177/109019819402100202. [DOI] [PubMed] [Google Scholar]
- 6.Becker AB, Israel BA, Schulz AJ, Parker EA, Klem L. Predictors of perceived control among African American women in Detroit: exploring empowerment as a multilevel construct. Health Educ Behav. 2002;29(6):699–715. doi: 10.1177/109019802237939. https://doi.org/10.1177/109019802237939. [DOI] [PubMed] [Google Scholar]
- 7.Tengland P. Empowerment: a goal or a means for health promotion? Med Health Care Philos. 2007;10(2):197–207. doi: 10.1007/s11019-006-9027-1. https://doi.org/10.1007/s11019-006-9027-1. [DOI] [PubMed] [Google Scholar]
- 8.Wallerstein N. Powerlessness, empowerment, and health: implications for health promotion programs. Am J Health Promot. 1992;6(3):197–205. doi: 10.4278/0890-1171-6.3.197. https://doi.org/10.4278/0890-1171-6.3.197. [DOI] [PubMed] [Google Scholar]
- 9.Wallerstein N, Duran B. Using community-based participatory research to address health disparities. Health Promot Pract. 2006;7(3):312–323. doi: 10.1177/1524839906289376. https://doi.org/10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Mullins CD, Novak P, Thomas SB. Personalized strategies to activate and empower patients in health care and reduce health disparities. Health Educ Behav. 2016;43(1):25–34. doi: 10.1177/1090198115579415. https://doi.org/10.1177/1090198115579415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carman KL, Dardess P, Maurer M, et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood) 2013;32(2):223–231. doi: 10.1377/hlthaff.2012.1133. https://doi.org/10.1377/hlthaff.2012.1133. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JM. Empowering patients: issues and strategies. Soc Sci Med. 1996;43(5):697–705. doi: 10.1016/0277-9536(96)00153-0. https://doi.org/10.1016/0277-9536(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 13.Aujoulat I, d'Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns. 2007;66(1):13–20. doi: 10.1016/j.pec.2006.09.008. https://doi.org/10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Graffigna G, Barello S, Bonanomi A, Menichetti J. The motivating function of healthcare professional in eHealth and mHealth interventions for Type 2 diabetes patients and the mediating role of patient engagement. J Diabetes Res. 2016;2016:2974521. doi: 10.1155/2016/2974521. https://doi.org/10.1155/2016/2974521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335(7609):24–27. doi: 10.1136/bmj.39246.581169.80. https://doi.org/10.1136/bmj.39246.581169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons L, Wolever RQ, Bechard EM, Snyderman R. Patient engagement as a risk factor in personalized health care: a systematic review of the literature on chronic disease. Genome Med. 2014;6:16. doi: 10.1186/gm533. https://doi.org/10.1186/gm533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurance J, Henderson S, Howitt PJ, et al. Patient engagement: four case studies that highlight the potential for improved health outcomes and reduced costs. Health Aff (Millwood) 2014;33(9):1627–1634. doi: 10.1377/hlthaff.2014.0375. https://doi.org/10.1377/hlthaff.2014.0375. [DOI] [PubMed] [Google Scholar]
- 18.Cene CW, Johnson BH, Wells N, Baker B, Davis R, Turchi R. A narrative review of patient and family engagement: the “foundation” of the medical “home”. Med Care. 2016;54(7):697–705. doi: 10.1097/MLR.0000000000000548. https://doi.org/10.1097/MLR.0000000000000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson MO. The shifting landscape of health care: toward a model of health care empowerment. Am J Public Health. 2011;101(2):265–270. doi: 10.2105/AJPH.2009.189829. https://doi.org/10.2105/AJPH.2009.189829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr PJ, Scholl I, Bravo P, Faber MJ, Elwyn G, McAllister M. Assessment of patient empowerment: a systematic review of measures. PLoS One. 2015;10(5):e0126553. doi: 10.1371/journal.pone.0126553. https://doi.org/10.1371/journal.pone.0126553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravo P, Edwards A, Barr PJ, Scholl I, Elwyn G, McAllister M. Conceptualising patient empowerment: a mixed methods study. BMC Health Serv Res. 2015;15:252. doi: 10.1186/s12913-015-0907-z. https://doi.org/10.1186/s12913-015-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister M, Dunn G, Payne K, Davies L, Todd C. Patient empowerment: the need to consider it as a measurable patient-reported outcome for chronic conditions. BMC Health Serv Res. 2012;12:157. doi: 10.1186/1472-6963-12-157. https://doi.org/10.1186/1472-6963-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MO, Dawson Rose CD, Dilworth SE, Neilands TB. Advances in the conceptualization and measurement of health care empowerment: development and validation of the Health Care Empowerment Inventory. PLoS One. 2012;7(9):e45692. doi: 10.1371/journal.pone.0045692. https://doi.org/10.1371/journal.pone.0045692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson MO, Sevelius JM, Dilworth SE, Saberi P, Neilands TB. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Prefer Adherence. 2012;6:395–404. doi: 10.2147/PPA.S30040. https://doi.org/10.2147/PPA.S30040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crepaz N, Tang T, Marks G, Hall HI. Viral suppression patterns among persons in the United States with diagnosed HIV infection in 2014. Ann Intern Med. 2017;167(6):446–447. doi: 10.7326/L17-0278. https://doi.org/10.7326/L17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beer L, Mattson CL, Bradley H, et al. Understanding cross-sectional racial, ethnic, and gender disparities in antiretroviral use and viral suppression among HIV patients in the United States. Medicine (Baltimore) 2016;95(13):e3171. doi: 10.1097/MD.0000000000003171. https://doi.org/10.1097/MD.0000000000003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yehia BR, Stephens-Shields AJ, Fleishman JA, et al. The HIV care continuum: changes over time in retention in care and viral suppression. PLoS One. 2015;10(6):e0129376. doi: 10.1371/journal.pone.0129376. https://doi.org/10.1371/journal.pone.0129376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah M, Risher K, Berry SA, Dowdy DW. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis. 2016;62(2):220–229. doi: 10.1093/cid/civ801. https://doi.org/10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;69(4):493–498. doi: 10.1097/QAI.0000000000000643. https://doi.org/10.1097/QAI.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. https://doi.org/10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. https://doi.org/10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Berg JJ, Neilands TB, Johnson MO, Chen B, Saberi P. Using path analysis to evaluate the healthcare empowerment model among persons living with HIV for antiretroviral therapy adherence. AIDS Patient Care STDS. 2016;30(11):497–505. doi: 10.1089/apc.2016.0159. https://doi.org/10.1089/apc.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2014;47(1):5–16. doi: 10.1007/s12160-013-9565-8. https://doi.org/10.1007/s12160-013-9565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. doi: 10.1097/QAI.0b013e31822d490a. https://doi.org/10.1097/QAI.0B013E31822D490A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blashill AJ, Perry N, Safren SA. Mental health: a focus on stress, coping, and mental illness as it relates to treatment retention, adherence, and other health outcomes. Curr HIV/AIDS Rep. 2011;8(4):215–222. doi: 10.1007/s11904-011-0089-1. https://doi.org/10.1007/s11904-011-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, Waldrop-Valverde D. The role of depression in retention in care for persons living with HIV. AIDS Patient Care STDS. 2016;30(1):34–38. doi: 10.1089/apc.2015.0214. https://doi.org/10.1089/apc.2015.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: results from the steps study. J Assoc Nurses AIDS Care. 2014;25(5):405–13. doi: 10.1016/j.jana.2013.12.002. https://doi.org/10.1016/jjana.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review. Curr HIV/AIDS Rep. 2015;12(4):421–436. doi: 10.1007/s11904-015-0285-5. https://doi.org/10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackstock OJ, Blank AE, Fletcher JJ, Verdecias N, Cunningham CO. Considering care-seeking behaviors reveals important differences among HIV-positive women not engaged in care: implications for intervention. AIDS Patient Care STDS. 2015;29(suppl 1):S20–26. doi: 10.1089/apc.2014.0271. https://doi.org/10.1089/apc.2014.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S, Jacobs EA, Moore RD, Beach MC. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS. 2010;24(7):415–420. doi: 10.1089/apc.2009.0288. https://doi.org/10.1089/apc.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horberg MA, Hurley LB, Klein DB, et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDS. 2015;29(11):582–590. doi: 10.1089/apc.2015.0139. https://doi.org/10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 42.Murphy K, Hoover D, Shi Q, et al. The association of race with death from AIDS in continuous HAART users in a cohort of HIV-infected women in the United States. AIDS. 2013;27(15):2413–2423. doi: 10.1097/01.aids.0000432537.92958.73. https://doi.org/10.1097/01.aids.0000432537.92958.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFall AM, Dowdy DW, Zelaya CE, et al. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. J Acquir Immune Defic Syndr. 2013;64(3):289–298. doi: 10.1097/QAI.0b013e3182a095e9. https://doi.org/10.1097/QAI.0b013e3182a095e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalichman SC, Hernandez D, Kegler C, Cherry C, Kalichman MO, Grebler T. Dimensions of poverty and health outcomes among people living with HIV infection: limited resources and competing needs. J Community Health. 2015;40(4):702–708. doi: 10.1007/s10900-014-9988-6. https://doi.org/10.1007/s10900-014-9988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. https://doi.org/10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9(2):117–125. https://doi.org/10.1097/00001648-199803000-00004. [PubMed] [Google Scholar]
- 47.Zinski A, Westfall AO, Gardner LI, et al. The contribution of missed clinic visits to disparities in HIV viral load outcomes. Am J Public Health. 2015;105(10):2068–2075. doi: 10.2105/AJPH.2015.302695. https://doi.org/10.2105/AJPH.2015.302695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS. 2013;27(8):442–449. doi: 10.1089/apc.2013.0073. https://doi.org/10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–1479. doi: 10.1093/cid/ciu603. https://doi.org/10.1093/cid/ciu603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. https://doi.org/10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 51.Kapadia F, Vlahov D, Wu Y, et al. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse. 2008;34(2):161–170. doi: 10.1080/00952990701877052. https://doi.org/10.1080/00952990701877052. [DOI] [PubMed] [Google Scholar]
- 52.Lazo M, Gange SJ, Wilson TE, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis. 2007;45(10):1377–1385. doi: 10.1086/522762. https://doi.org/10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 53.Wilson TE, Barron Y, Cohen M, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):529–534. doi: 10.1086/338397. https://doi.org/10.1086/338397. [DOI] [PubMed] [Google Scholar]
- 54.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–1275. doi: 10.1093/cid/cir131. https://doi.org/10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 56.Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr. 2016;73(2):205–212. doi: 10.1097/QAI.0000000000001036. https://doi.org/10.1097/QAI.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: Review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. https://doi.org/10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep. 2012;9(4):287–312. doi: 10.1007/s11904-012-0134-8. https://doi.org/10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLellan AT, Kushner H, Metzger D, et al. The 5th edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. https://doi.org/10.1016/0740-5472(92)90062-S. [DOI] [PubMed] [Google Scholar]
- 60.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. https://doi.org/10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among U.S. adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:1–11. doi: 10.5888/pcd11.140329. https://doi.org/10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270(21):2568–2573. https://doi.org/10.1001/jama.1993.03510210054027. [PubMed] [Google Scholar]
- 63.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. https://doi.org/10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 64.Lyketsos CG, Hoover DR, Guccione M. Depression and survival among HIV-infected persons. JAMA. 1996;275(1):35–36. doi: 10.1001/jama.1996.03530250039021. https://doi.org/10.1001/jama.1996.03530250039021. [DOI] [PubMed] [Google Scholar]
- 65.Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. Inter J Psychiatry Med. 2001;31(1):25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. https://doi.org/10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- 66.Cook JA, Cohen MH, Burke J, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30(4):401–409. doi: 10.1097/00042560-200208010-00005. https://doi.org/10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- 67.Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. doi: 10.2105/ajph.94.7.1133. https://doi.org/10.2105/AJPH.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. https://doi.org/10.1177/014662167700100306. [Google Scholar]
- 69.Iacobucci D. Mediation analysis and categorical variables: The final frontier. J Consum Psychol. 2012;22(4):582–594. doi: 10.1016/j.jcps.2012.03.009. https://doi.org/10.1016/j.jcps.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67(3):451–470. doi: 10.1111/bmsp.12028. https://doi.org/10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 71.Batey DS, Whitfield S, Mulla M, et al. Adaptation and implementation of an intervention to reduce HIV-related stigma among healthcare workers in the United States: piloting of the FRESH workshop. AIDS Patient Care STDS. 2016;30(11):519–527. doi: 10.1089/apc.2016.0223. https://doi.org/10.1089/apc.2016.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higa D, Marks G, Crepaz N, Liau A, Lyles C. Interventions to improve retention in HIV primary care: A systematic review of U.S. studies. Current HIV/AIDS Rep. 2012;9(4):313–325. doi: 10.1007/s11904-012-0136-6. https://doi.org/10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuijpers W, Groen WG, Aaronson NK, van Harten WH. A systematic review of web-based interventions for patient empowerment and physical activity in chronic diseases: relevance for cancer survivors. J Med Internet Res. 2013;15(2):e37. doi: 10.2196/jmir.2281. https://doi.org/10.2196/jmir.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greenberg AJ, Falisi AL, Finney Rutten LJ, et al. Access to electronic personal health records among patients with multiple chronic conditions: a secondary data analysis. J Med Internet Res. 2017;19(6):e188. doi: 10.2196/jmir.7417. https://doi.org/10.2196/jmir.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prigge JK, Dietz B, Homburg C, Hoyer WD, Burton JL. Patient empowerment: a cross-disease exploration of antecedents and consequences. Int J of Res Mark. 2015;32(4):375–386. https://doi.org/10.1016/j.ijresmar.2015.05.009. [Google Scholar]


