Abstract
Although IL-10 is known to be an important cytokine in the immune response to TB, very little is known about the role of IL-20 subfamily of cytokines in the host response to TB. To identify the role of CD4+T and CD8+ T cells producing IL-20 subfamily of cytokines in human TB, we enumerated the frequencies of IL-10, IL-19 and IL-24 expressing CD4+ and CD8+ T cells following Mtb-specific antigen(s) stimulation of cells from individuals with pulmonary TB (PTB) and latent TB (LTB). We first demonstrated that Mtb-specific antigen(s) induce an expansion of CD4+ and CD8+ T cells expressing IL-10, IL-19 and IL-24 in PTB and LTB individuals, with frequencies being significantly higher in the PTB. Next, we demonstrated that IL-10, IL-19 and IL-24 play an important role in the regulation of CD4+ and CD8+ T cells expressing Th1/Tc1 and Th17/Tc17 cytokines in PTB but not LTB individuals. Thus, active PTB is characterized by an IL-10, IL-19 and IL-24 mediated down modulation of Th1/Tc1 and/or Th17/Tc17 cytokines in CD4+ and CD8+ T cell subsets. This suggests that the IL-20 subfamily of cytokines, similar to IL-10 might play a potentially crucial role in the modulation of T cell responses in active TB disease.
Keywords: Tuberculosis, latent tuberculosis, IL-20 subfamily cytokines, T cell responses
Introduction
IL-10 is an immunosuppressive cytokine crucial for dampening the immune response and limiting host immune pathology to numerous intracellular pathogens, including Mycobacterium tuberculosis (Mtb) (1). Thus, during acute infections, IL-10 is crucial in limiting host tissue damage as a consequence of excessive inflammation (2, 3). IL-10 is a regulatory cytokine that inhibits cytokine production and proliferation of CD4+ T cells and APC function (4, 5). IL-10 directly affects the function of T cells and inhibits IL-2, TNF, and IL-5 production depending on activation conditions (6, 7). IL-10 is produced by almost all innate and adaptive immune cells, including B cells, dendritic cells, macrophages, CD4+, and CD8+ T cells (2, 8). However, recent reports confirm that effector CD4+ T cell production from IL-10 plays an important role in limiting host pathology and fostering chronicity of infection (9). Effector function in CD4+ T cells is mainly facilitated by Th1, Th2 and Th17 cytokines (10). In addition, regulatory T cells, are also known to be key producers of IL-10 in immunity to infections (11).
IL-20 subfamily of cytokines comprises IL-19, IL-20, IL-22, IL-24 and IL-26, these cytokines are members of larger IL-10 family of cytokines, all together they are grouped to form the IL-20 subfamily based on their usage of common receptor subunit (8). IL-10 shares the receptor complexes of IL-10R1/IL-10R2, whereas IL-19 and IL-24 share common receptor complexes of IL-20R1/IL-20R2 heterodimer (12, 13). The regulated expression pattern of their receptors drives the unique biology of IL-20 subfamily cytokines and distinguishes them from other IL-10 family members (8, 13). These IL-20 subfamily cytokines contribute to tissue healing processes in injury or infection and promote innate immune responses to limit damage associated with viral and bacterial infections (1, 8). IL-20 subfamily cytokines are largely produced by leukocytes and act on epithelial tissues to augment epithelial innate immunity (8). IL-20 subfamily cytokines are chiefly produced by immune cells, such as myeloid cells and lymphocyte and T cells are the primary cellular source of IL-20 subfamily of cytokines including IL-22, IL-24 and IL-26.
Although, IL-10 plays a substantial role in the immune response to TB, the role of the IL-20 subfamily of cytokines is still not completely clear in TB infection and disease. By using flow cytometry to examine the expression pattern of IL-10, IL-19 and IL-24 in T cells of active pulmonary TB (PTB) and latent TB (LTB) individuals, we determine PTB is associated with expanded IL-10, IL-19 and IL-24 expressing CD4+ and CD8+ helper T cell subsets following Mtb-specific antigen(s) stimulation. Finally, we show that IL-10, IL-19 and IL-24 can each down modulate the Th1/Tc1 and/or Th17/Tc17 cytokine profile of TB antigen stimulated CD4+ and CD8+ T cells.
Materials and Methods
Ethics statement
This study was approved by the Ethics Committees of the National Institutes for Research in Tuberculosis. Informed written consent was obtained from all participants. All the methods were performed in accordance with the relevant to institutional ethical committee guidelines
Study population
We studied a group of 30 individuals with pulmonary TB (PTB) and 30 individuals with latent TB (LTB). Individuals with pulmonary TB were diagnosed by positive solid cultures in Lowenstein–Jensen medium. Smear grades were determined by sputum microscopy and culture was graded as 0, 1+, 2+, and 3+ with zero being no bacteria in microscopy and 3+ the highest number of bacteria. The extent of chest disease was assessed by Chest X-ray and was classified as unilateral or bilateral disease for the purposes of this study. Individuals were diagnosed as having LTB on the basis of being positive in the Quantiferon-TB Gold in Tube (Cellestis) assay but having an absence of pulmonary symptoms concurrent with a normal chest radiograph. The two groups of individuals were age and sex matched. All subjects had been bacillus Calmette–Guerin (BCG) vaccinated at birth. All the individuals were HIV negative, not diabetic or suffering from autoimmune disorders and blood was collected prior to commencement of anti-TB treatment. This study was approved by the Ethics Committees of the National Institute for Research in Tuberculosis, Chennai, India (NIRT-IRB No:2010002). Informed written consent was obtained from all participants. All the methods were performed in accordance with the relevant to institutional ethical committee guidelines.
Antigens
Mycobacterial antigens—PPD (Statens Serum Institute, Copenhagen, Denmark), ESAT-6 and CFP—10 (both from NIAID TB antigen repository at BEI resources) were used as antigenic stimuli, and anti-CD3 antibody was used as positive control. Final concentrations were 10 mg/ml for PPD, ESAT-6 and CFP-10, and 5 mg/ml for anti-CD3.
In vitro culture
Whole blood cell cultures were performed to determine the in vitro responses to antigens. Briefly, whole blood was diluted 1:1 with RPMI1640 medium supplemented with penicillin/streptomycin (100 U/100 mg/ml), L-glutamine (2 mM), and HEPES (10 mM; all from Invitrogen) and distributed in 12-well tissue culture plates (Costar). The cultures were then stimulated with PPD, ESAT-6+CFP-10, or anti-CD3 or with medium alone in the presence of CD49d/CD28 at 37° C for 18 h. Brefeldin A (10 mg/mL) was added after 12 h. After 18 h, centrifugation, washing, and red blood cell lysis was performed. The cells were fixed using cytofix/ cytoperm buffer (BD Biosciences, San Jose, CA) and stored at 80° C. For neutralization experiments, whole blood was cultured in the presence of anti-IL-10 (5 mg/ml), anti-IL-19 (5 mg/ml) and anti-IL-24 (5 mg/ml) (R&D Systems, Minneapolis, MN), at 37 °C for 6 h following which PPD was added and Brefeldin A (10 mg/ml) was added after 1 h. The cells were then cultured for a further 16 h.
Intracellular cytokine staining
The cells were thawed, washed with cold PBS and permeabilized with 1x permeabilization buffer (Foxp3 staining buffer from eBiosciences). Cells were then stained with surface antibodies for 30 min and washed twice. The cells were then stained with intracellular antibodies and incubated overnight for 4°C. Antibodies used were as follows: CD3, CD4, CD8, IL-10, IL-19 and IL-24; Th1 panel: IFNγ, IL-2 and TNFα; Th2 panel: IL-4, IL-5 and IL-13; Th17 panel: IL-17A, IL-17F and IL-22. To determine the T cell subsets, lymphocyte population was first gated and then selected cells were gated for CD3+ cells. CD4+ T cells were defined as CD3+ CD4+ T cells expressing IL-20 sub family (IL-10, IL-19 and IL-24) of cytokines, Th1 cytokines: IFNγ, IL-2 and TNFα; Th2 cytokines: IL-4, IL-5 and IL-13; Th17 cytokines: IL-17A, IL-17F and IL-22. CD8+ T cells were defined as CD3+ CD8+ T cells expressing IL-20 sub family (IL-10, IL-19 and IL-24) of cytokines, Tc1 cytokines: IFNγ, IL-2 and TNFα; Tc2 cytokines: IL-4, IL-5 and IL-13; Tc17 cytokines: IL-17A, IL-17F and IL-22. Eight-color flow cytometry was performed on a FACSCanto II flow cytometer with FACSDiva software, version 6 (Becton Dickinson, Franklin Lakes, NJ). The lymphocyte gating was set by forward and side scatter, and 100,000 lymphocytes events were acquired. Data were collected and analyzed using Flow Jo software (TreeStar, Ashland, OR). Frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted).
Statistical analysis
Geometric mean was used as the measure of central tendency. Comparisons were made using either the Mann–Whitney test (unpaired comparisons) or the Wilcoxon signed rank test (paired comparisons). All statistics were performed using GraphPad Prism version 6 for Windows (GraphPad Software, Inc).
Results
PTB is associated with increased frequencies of antigen- stimulated CD4+ T cells expressing IL-20 subfamily of cytokines
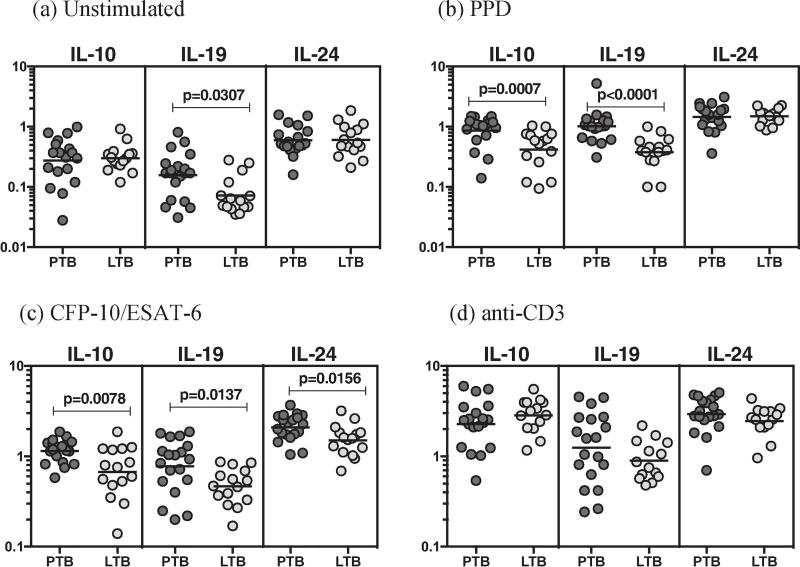
To determine the association between the frequencies of CD4+ T cell subsets and PTB, we used multi-parameter flow cytometry to define the frequencies CD4+ T cells expressing IL-20 sub family (IL-10, IL-19 and IL-24) of cytokines at baseline and following stimulation with either mycobacterial antigens or anti-CD3 (Figure 1). As shown in Figure 1A, there were significantly increased frequencies of CD4+ T cells expressing IL-19 at baseline in PTB compared to LTB. In response to PPD (Figure 1B), CFP-10+ESAT-6 (Figure 1C), we observed significantly elevated frequencies of CD4+ T cells expressing either IL-10 or IL-19 or IL-24 in PTB compared to LTB individuals.
Figure 1.
PTB is associated with increased antigen-induced frequency of CD4+ T cells expressing IL-10, IL-19 and IL-24. Whole blood from PTB (n=20) and LTB (n=20) individuals was stimulated with PPD, CFP-10+ESAT-6, and anti-CD3 for 24 h, and the frequencies of IL-20 subfamily of cytokines in PTB and LTB individuals (A) at baseline as well as in response to stimulation with (B) PPD, (C) CFP-10+ESAT-6, (D) anti-CD3 were estimated by flow cytometry. Stimulated frequencies are shown as scatter plots with the line representing the geometric means. Frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted). P values were calculated using the Mann–Whitney U test.
PTB is associated with increased frequencies of antigen- stimulated CD8+ T cells expressing IL-20 subfamily of cytokines
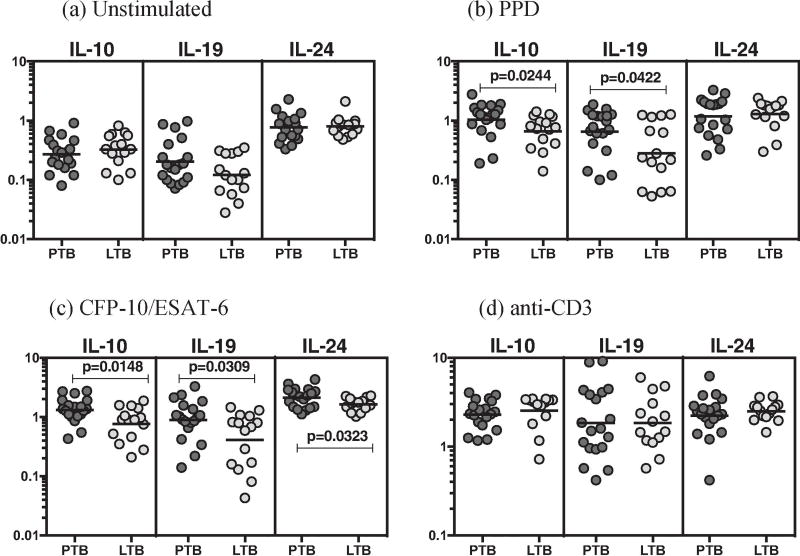
To determine the association between the frequencies of CD8+ T cell subsets and PTB, we used multi-parameter flow cytometry to define the frequencies CD8+ T cells expressing IL-20 sub family (IL-10, IL-19 and IL-24) of cytokines at baseline and following stimulation with either mycobacterial antigens or anti-CD3 (Figure 2). As shown in Figure 2, there were significantly increased frequencies of CD8+ T cells expressing IL-10 and IL-19 in response to PPD (Figure 2B), CFP-10+ESAT-6 (Figure 2C) in PTB compared to LTB individuals.
Figure 2.
PTB is associated with increased antigen-induced frequency of CD8+ T cells expressing IL-10, IL-19 and IL-24. Whole blood from PTB (n=20) and LTB (n=20) individuals was stimulated with PPD, CFP-10+ESAT-6, and anti-CD3 for 24 h, and the frequencies of IL-20 subfamily of cytokines in PTB and LTB individuals (A) at baseline as well as in response to stimulation with (B) PPD, (C) CFP-10+ESAT-6, (D) anti-CD3 were estimated by flow cytometry. Stimulated frequencies are shown as scatter plots with the line representing the geometric means. Frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted). P values were calculated using the Mann–Whitney U test.
IL-10, IL-19 and IL-24 regulate the antigen-stimulated frequencies of Th1 and Th17 cells in PTB
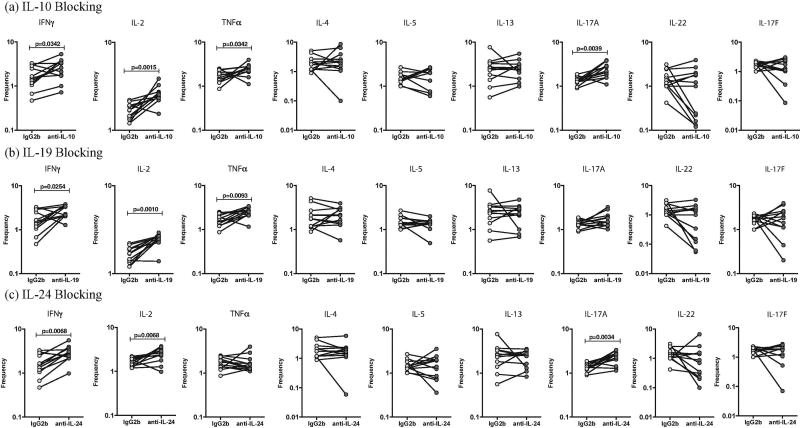
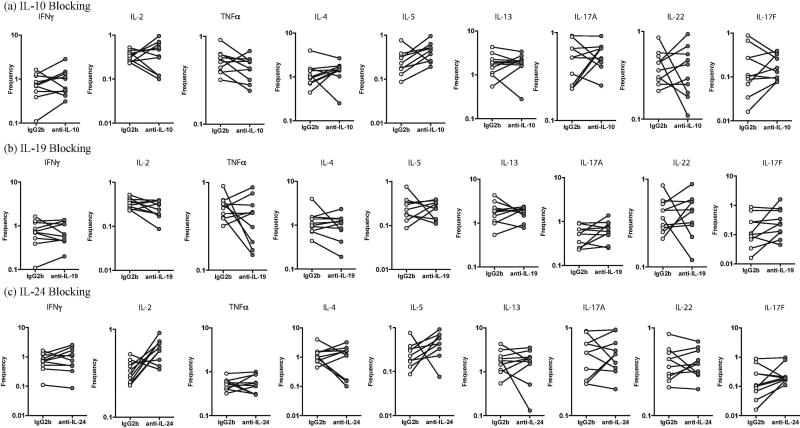
We sought to determine the role of IL-10, IL-19 and IL-24 in regulating Th1, Th2 and Th17 cytokines in PTB. Thus, we stimulated whole blood from a subset of PTB individuals (n=10) with PPD in the presence of neutralizing antibodies to IL-10 or IL-19 or IL-24 and measured the frequencies of Th1 (IFNγ, IL-2 and TNFα), Th2 (IL-4, IL-5 and IL-13) and Th17 (IL-17A, IL-17F and IL-22) cells (Fig. 3). As shown in Figure 3A, blockade of IL-10 resulted in a significant increase in the frequencies of PPD-stimulated Th1 (IFNγ, IL-2 and TNFα) and Th17 (IL-17A) but not Th2 cells. As shown in Figure 3B, blockade of IL-19 resulted in a significant increase in the frequencies of PPD-stimulated Th1 (IFNγ, IL-2 and TNFα) but not Th17 and Th2 cells. As shown in Figure 3C, blockade of IL-24 resulted in a significant increase in the frequencies of PPD-stimulated Th1 (IFNγ and IL-2) and Th17 (IL-17A) but not Th2 cells.
Figure 3.
Altered frequencies of CD4+ T-helper type 1 (Th1), Th2 and Th17 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24) in PTB. Frequencies of CD4+ Th1, Th2 and Th17 cells stimulated by PPD were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 10 pulmonary TB infected individuals. The data are represented as line diagrams, with each line representing a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction.
IL-10, IL-19 and IL-24 regulate the antigen-stimulated frequencies of Tc1 and Tc17 cells in PTB
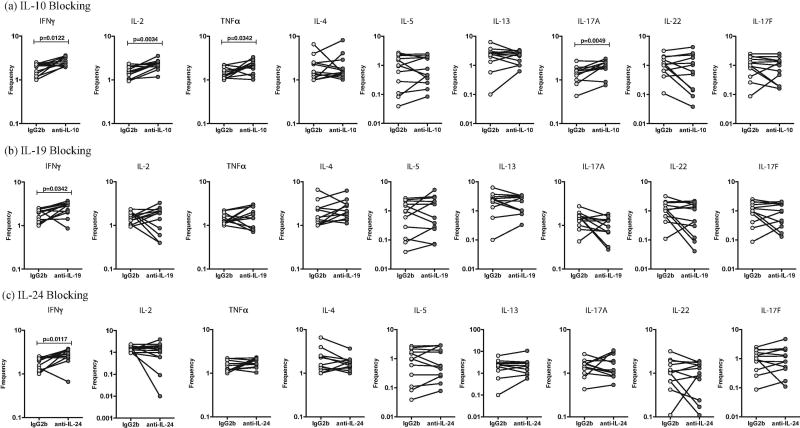
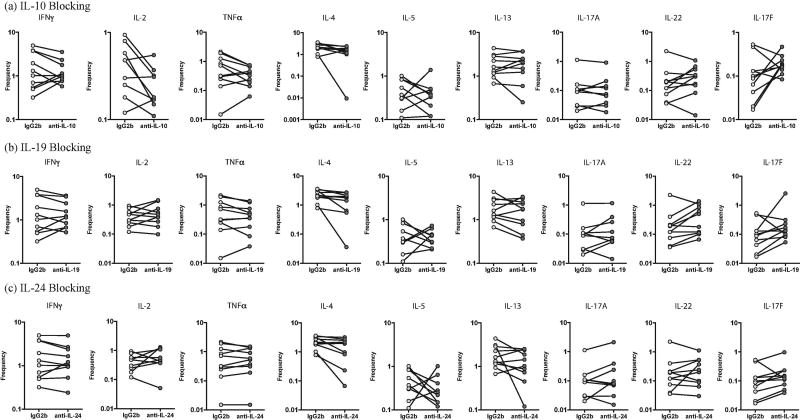
We sought to determine the role of IL-10, IL-19 and IL-24 in regulating Tc1, Tc2 and Tc17 cytokines in PTB. Thus, we stimulated whole blood from a subset of PTB individuals (n=10) with PPD in the presence of neutralizing antibodies to IL-10 or IL-19 or IL-24 and measured the frequencies of Tc1 (IFNγ, IL-2 and TNFα), Tc2 (IL-4, IL-5 and IL-13) and Tc17 (IL-17A, IL-17F and IL-22) cells (Fig. 4). As shown in Figure 4A, blockade of IL-10 resulted in a significant increase in the frequencies of PPD-stimulated Tc1 (IFNγ, IL-2 and TNFα) and Tc17 (IL-17A) but not Tc2 cells. As shown in Figure 4B, blockade of IL-19 resulted in a significant increase in the frequencies of PPD-stimulated Tc1 (IFNγ) but not Tc17 and Tc2 cells. As shown in Figure 4C, blockade of IL-24 resulted in a significant increase in the frequencies of PPD-stimulated Tc1 (IFNγ) but not Tc17 and Tc2 cells.
Figure 4.
Altered frequencies of CD8+ T-helper type 1 (Tc1), Tc2 and Tc17 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24) in PTB. Frequencies of CD4+ Tc1, Tc2 and Tc17 cells stimulated by PPD were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 10 pulmonary TB infected individuals. The data are represented as line diagrams, with each line representing a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction.
IL-10, IL-19 and IL-24 do not regulate the antigen-stimulated frequencies of Th1 and Th17 cells in LTB
We sought to determine the role of IL-10, IL-19 and IL-24 in regulating Th1, Th2 and Th17 cytokines in LTB. Thus, we stimulated whole blood from a subset of LTB individuals (n=10) with PPD in the presence of neutralizing antibodies to IL-10 or IL-19 or IL-24 and measured the frequencies of Th1 (IFNγ, IL-2 and TNFα), Th2 (IL-4, IL-5 and IL-13) and Th17 (IL-17A, IL-17F and IL-22) cells (Fig. 5). As shown in Figure 5, blockade of IL-10 (Figure 5A), IL-19 (Figure 5B) and IL-24 (Figure 5C) resulted in a no significant alteration in the frequencies of PPD-stimulated Th1, Th2 and Th17 cells.
Figure 5.
No alteration in frequencies of CD4+ T-helper type 1 (Th1), Th2 and Th17 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24) in LTB. Frequencies of CD4+ Th1, Th2 and Th17 cells stimulated by PPD were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 10 latent TB infected individuals. The data are represented as line diagrams, with each line representing a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction.
IL-10, IL-19 and IL-24 do not regulate the antigen-stimulated frequencies of Tc1 and Tc17 cells in LTB
We sought to determine the role of IL-10, IL-19 and IL-24 in regulating Tc1, Tc2 and Tc17 cytokines in LTB. Thus, we stimulated whole blood from a subset of LTB individuals (n=10) with PPD in the presence of neutralizing antibodies to IL-10 or IL-19 or IL-24 and measured the frequencies of Tc1 (IFNγ, IL-2 and TNFα), Tc2 (IL-4, IL-5 and IL-13) and Tc17 (IL-17A, IL-17F and IL-22) cells (Fig. 6). As shown in Figure 6, blockade of IL-10 (Figure 6A), IL-19 (Figure 6B) and IL-24 (Figure 6C) resulted in a no significant alteration in the frequencies of PPD-stimulated Tc1, Tc2 and Tc17 cells.
Figure 6.
No alterations in frequencies of CD8+ T-helper type 1 (Tc1), Tc2 and Tc17 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24) in LTB. Frequencies of CD4+ Th1, Th2 and Th17 cells stimulated by PPD were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 10 latent TB infected individuals. The data are represented as line diagrams, with each line representing a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction.
Discussion
The role of the IL-20 subfamily of cytokines is still not completely understood in the context of tuberculosis disease. Predominantly, IL-20 subfamily of cytokines acts on numerous epithelial cells and protect the cells from invasion by extracellular pathogens such as bacteria and yeast. IL-20 subfamily cytokines and IL-10 also prevent tissue damage caused by infections and inflammation (1). IL-10, on the other hand, controls and represses the expression of pro-inflammatory cytokines during the recovery phase of infections and reduces the tissue damage caused by these cytokines (2, 14). In addition, these cytokines boost tissue remodelling and wound-healing activities, which help to maintain tissue integrity and restore homeostasis of epithelial layers during infection and inflammatory responses (15, 16). It has been proposed that IL-20 subfamily members have similar function in the protection of epithelial tissue against viral and bacterial infections respectively (17). But, how these alterations impact the immune response to infections or bystander antigens remains unclear. We have previously shown that systemic levels IL-20 subfamily of cytokines like IL-19 IL-20 and IL-24 were significantly increased in PTB and LTB individuals compare to PTB and LTB individuals with coincident diabetes mellitus (18) and we also reported that PTB is associated with expanded IL-10 expression by CD4+ helper T cell subsets following TB antigen stimulation (19). Thus, IL-10 might play a main role in promoting susceptibility to TB disease by altering the cytokine milieu. Therefore, we examined the association of IL-20 subfamily of cytokines in TB infection and disease.
IL-10 plays a very vital role in the regulation of host immune response against Mycobacterium tuberculosis infection (20). IL-10 impedes the protective immune response to pathogens by blocking the production of proinflammatory cytokines, such as TNFα and the Th1-polarizing cytokine IL-12, by directly acting on antigen-presenting cells such as macrophages and dendritic cell (DC) (21, 22). IL-10 is also known to cause inhibition of macrophage effector functions, with reduced bacterial killing and diminished cytokine/chemokine secretion (23, 24), block the chemotactic factors that control DC trafficking to the lymph nodes (24), inhibit the differentiation of naive CD4+ T cells to Th1 cells (25) and finally suppress Th1, Th2, and Th17 cytokine production (26, 27). Although IL-10 production from myeloid cells is documented, T cells are also the major source of IL-10 in a variety of infections including tuberculosis (9). Previous published human TB studies report that IL-10 levels were significantly elevated in the lungs (28) and serum (29) of active pulmonary TB patients. Subsequently, it is also reported that IL-10 levels are significantly increased in individuals with active TB and a higher capacity to produce IL-10 is associated with an increase in the disease incidence (20). In addition, we have previously reported that upon TB antigen stimulus IL-10 expressing Th1 (IFNγ), Th2 (IL-4) and Th17 (IL-17A) cytokines were significantly enhanced in PTB compared to LTB individuals (19). In agreement with these reports, our data also show that IL-10 exhibits increased levels in PTB compared to LTB upon TB antigen stimulation. Since IL-10 is known to be a key cytokine in the immune response to TB (20), it is not surprising to find that CD4+ and CD8+ T cell subsets exhibit the ability to produce IL-10. In addition, our data report that neutralization of IL-10 resulted in the enhanced frequencies of CD4+ and CD8+ T cell subsets expressing Th1/Tc1 and Th17/Tc17 cytokines in only PTB individuals but not in the LTB individuals indicating that altered cytokines responses are seen only in the active disease state but not in the latent TB infection. Our study, therefore, confirms an important role for Th1 cells in the pathogenesis of tuberculosis and suggests that heightened frequencies of Th1 cytokines might actually reflect an increased severity of disease or may reflect a higher antigen load (30). Traditionally, IL-10 from T cells was shown to be mainly produced by the Th2 subsets, however in our study we didn’t see any alterations in Th2 cytokines upon IL-10 neutralisation. Our data on the examination of Th17 cytokines reveal that increased frequencies of IL-17A but not IL-17F or IL-22, in patients with tuberculosis upon IL-10 neutralisation. Previous published studies have reported that IL-17A play an important for responses to the fungal pathogen Candida albicans, whereas Th17 cells co-expressing IL-17A and IL-10 are important for responses to the bacterial pathogen Staphylococcus aureus (31). Collectively, previously published studies (32, 33) and our data confirm that IL-10 functions to limit the immune response to Mtb and may contribute to TB pathogenesis.
IL-19 has been shown to increase Th2 cytokine expression in activated T cells and activated monocytes and its expression has been shown to be increased in certain Th2-mediated diseases such as atopic dermatitis and asthma (15, 16, 34). IL-24 is specially expressed by Th2 cells (35) but it is unknown how IL-24 expression in regulated by Th2 cells. Apparently, transcription factors that drive the expression of other Th2- cytokines such as STAT6 and GATA3 is also involved in the regulation of IL-24 (36). IL-24, has been implicated to play an important role in host defence against tumours (36). Previous published data from mouse model of S. aureus, however, clearly reveals a vital immuno-modulatory role for IL-19 and IL-24 in suppressing IL-1β and IL-17 dependent effector pathways and promoting susceptibility to infection (34). Similar to our finding from PTB patients, studies from helminth infections reported an expansion in CD4+ T cells expressing IL-19 and IL-24 levels compared to uninfected group (37). Subsequently another helminth study reported that upon IL-10, IL-19 and IL-24 neutralisation resulted in the increased CD4+ Th1, Th2 and Th17 cytokines and CD8+ Tc1, Tc2 and Tc17 cytokines (38). Published data from our lab also reports that systemic levels of IL-19 and IL-24 were significantly enhanced in PTB and LTB individuals compared to PTB or LTB individuals with coincident diabetes mellitus (18). Thus, existing results indicates that IL-20 subfamily of cytokines resemble IL-10 in functioning as down modulatory or regulatory cytokines in dampening inflammatory response during chronic inflammation which in turn may contribute to amelioration of TB pathology.
In this study, we provide complete evidence for the regulatory role for the two of the IL-20 subfamily of cytokines, IL-19 and IL-24. Our findings confirm the potent role played by IL-10 in TB infection by determining its effect on both CD4+ and CD8+ T cells responding to TB antigen. Thus, IL-10 clearly down-modulates the Th1/Tc1 and Th17/Tc17 cytokines of T cell subsets responding to TB antigens. We consistently detected a significantly higher proportion of CD4+ and CD8+ T cells expressing IL-10, IL-19 and IL-24 in the PTB group. Finally, to study the role IL-20 subfamily cytokines in regulating T-cell responses, we examined the frequency of Th1/Tc1, Th2/Tc2 and Th17/Tc17 cells following IL-10, IL-19 or IL-24 neutralisation, which resulted in significantly enhanced frequencies of Th1 and Th17 cytokines in PTB individuals. Our study discloses a novel role for the IL-20 subfamily cytokines in human TB infection and disease. In summary, the present study identifies the cellular origins of IL-20 subfamily cytokine to elucidate the factors regulating T cell subsets. Our data clearly highlight an important role for IL-20 subfamily cytokine in response to M. tuberculosis infection but also in the modulation of cell responses in tuberculous disease. To our knowledge, this is the first study describing the novel role of the cytokines – IL-19 and IL-24 in modulating T cell function in TB disease.
Supplementary Material
(A) The gating strategy for determining the frequencies of CD4+ and CD8+ T cell from whole blood. (B) A representative whole-blood intracellular cytokine assay flow data from a PTB individual showing expression of CD4+ T cell expressing IL-10, IL-19 and IL-24 cytokines (C) A representative whole-blood intracellular cytokine assay flow data from a PTB individual showing expression of CD8+ T cell expressing IL-10, IL-19 and IL-24 cytokines.
Table 1.
Demographics of study groups
| Study Demographics | Pulmonary TB | Latent TB |
|---|---|---|
| No. of subjects recruited | 30 | 30 |
| Gender (M/F) | 16/14 | 17/13 |
| Median Age (Range) | 35 (18 – 64) | 33 (21 – 60) |
| Smear Grade (1+ /2+ /3+) | 11/11/8 | Not applicable |
| Quantiferon TB Gold ELISA in Tube | Not done | Positive |
| Tuberculin skin test, mm | Not done | >12mm |
Highlights.
In this study, we provide complete evidence for the regulatory role for the two of the IL-20 subfamily of cytokines, IL-19 and IL-24
Our findings confirm the potent role played by IL-10 in TB infection by determining its effect on both CD4+ and CD8+ T cells responding to TB antigen
Our study discloses a novel role for the IL-20 subfamily cytokines in human TB infection and disease
To our knowledge, this is the first study describing the novel role of the cytokines – IL-19 and IL-24 in modulating T cell function in TB disease
This study suggests that the IL-20 subfamily of cytokines, similar to IL-10 might play a potentially crucial role in the modulation of T cell responses in active TB disease
Acknowledgments
We thank the staff of Department of Clinical Research, Department of Epidemiology and the Department of social work, NIRT and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study, R. Anuradha of the NIH-ICER for technical assistance.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- PTB
pulmonary TB
- LTB
latent TB
- Mtb
Mycobacterium tuberculosis
- BCG
bacillus Calmette–Guerin
- DC
dendritic cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Conceived and designed the experiments: NPK SB. Performed the experiments: NPK KM Analysed the data: NPK SB. Contributed reagents/materials/analysis tools: VVB DN. Wrote the paper: NPK SB
References
- 1.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 2.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 4.de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147(11):3815–22. [PubMed] [Google Scholar]
- 6.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81(11):2964–71. [PubMed] [Google Scholar]
- 7.Schandene L, Alonso-Vega C, Willems F, Gerard C, Delvaux A, Velu T, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152(9):4368–74. [PubMed] [Google Scholar]
- 8.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14(12):783–95. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3(3):239–46. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leng RX, Pan HF, Tao JH, Ye DQ. IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets. 2011;15(2):119–26. doi: 10.1517/14728222.2011.534461. [DOI] [PubMed] [Google Scholar]
- 13.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21(5):315–24. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 15.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178(4):2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 16.Uze G, Monneron D. IL-28 and IL-29: newcomers to the interferon family. Biochimie. 2007;89(6–7):729–34. doi: 10.1016/j.biochi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Gad HH, Dellgren C, Hamming OJ, Vends S, Paludan SR, Hartmann R. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J Biol Chem. 2009;284(31):20869–75. doi: 10.1074/jbc.M109.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar NP, Banurekha VV, Nair D, Kumaran P, Dolla CK, Babu S. Type 2 diabetes - Tuberculosis co-morbidity is associated with diminished circulating levels of IL-20 subfamily of cytokines. Tuberculosis (Edinb) 2015;95(6):707–12. doi: 10.1016/j.tube.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar NP, Moideen K, Banurekha VV, Nair D, Sridhar R, Nutman TB, et al. IL-27 and TGFbeta mediated expansion of Th1 and adaptive regulatory T cells expressing IL-10 correlates with bacterial burden and disease severity in pulmonary tuberculosis. Immun Inflamm Dis. 2015;3(3):289–99. doi: 10.1002/iid3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. Pillars Article: IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 1991;147:3815–3822. J Immunol. 2016;197(5):1539-46. [PubMed] [Google Scholar]
- 22.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. Pillars Article: IL-10 Acts on the Antigen-presenting Cell to Inhibit Cytokine Production by Thl Cells. J. Immunol. 1991;146:3444–3451. J Immunol. 2016;197(5):1531-8. [PubMed] [Google Scholar]
- 23.O'Leary S, O'Sullivan MP, Keane J. IL-10 blocks phagosome maturation in mycobacterium tuberculosis-infected human macrophages. Am J Respir Cell Mol Biol. 2011;45(1):172–80. doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183(2):1301–12. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–51. [PubMed] [Google Scholar]
- 26.Kumar NP, Gopinath V, Sridhar R, Hanna LE, Banurekha VV, Jawahar MS, et al. IL-10 dependent suppression of type 1, type 2 and type 17 cytokines in active pulmonary tuberculosis. PLoS One. 2013;8(3):e59572. doi: 10.1371/journal.pone.0059572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189(8):4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huard RC, Chitale S, Leung M, Lazzarini LC, Zhu H, Shashkina E, et al. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect Immun. 2003;71(12):6871–83. doi: 10.1128/IAI.71.12.6871-6883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115(1):110–3. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12(7):597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 32.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64(3):913–8. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10- producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105(9):1317–25. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14(8):804–11. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer G, Venkataraman C, Schindler U. Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells. J Immunol. 2001;166(10):5859–63. doi: 10.4049/jimmunol.166.10.5859. [DOI] [PubMed] [Google Scholar]
- 36.Stevens L, Htut TM, White D, Li X, Hanidu A, Stearns C, et al. Involvement of GATA3 in protein kinase C theta-induced Th2 cytokine expression. Eur J Immunol. 2006;36(12):3305–14. doi: 10.1002/eji.200636400. [DOI] [PubMed] [Google Scholar]
- 37.Anuradha R, George PJ, Hanna LE, Kumaran P, Chandrasekaran V, Nutman TB, et al. Expansion of parasite-specific CD4+ and CD8+ T cells expressing IL-10 superfamily cytokine members and their regulation in human lymphatic filariasis. PLoS Negl Trop Dis. 2014;8(4):e2762. doi: 10.1371/journal.pntd.0002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, Babu S. Modulation of CD4+ and CD8+ T-Cell Function by Interleukin 19 and Interleukin 24 During Filarial Infections. J Infect Dis. 2016;213(5):811–5. doi: 10.1093/infdis/jiv497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The gating strategy for determining the frequencies of CD4+ and CD8+ T cell from whole blood. (B) A representative whole-blood intracellular cytokine assay flow data from a PTB individual showing expression of CD4+ T cell expressing IL-10, IL-19 and IL-24 cytokines (C) A representative whole-blood intracellular cytokine assay flow data from a PTB individual showing expression of CD8+ T cell expressing IL-10, IL-19 and IL-24 cytokines.