Abstract
Our previous studies used tibial compression overload to induce anterior cruciate ligament (ACL) rupture in mice, while others have applied similar or greater compressive magnitudes without injury. The causes of these differences in injury threshold are not known. In this study, we compared knee injury thresholds using a “prone configuration” and a “supine configuration” that differed with respect to hip, knee, and ankle flexion, and utilized different fixtures to stabilize the knee. Right limbs of female and male C57BL/6 mice were loaded using the prone configuration, while left limbs were loaded using the supine configuration. Mice underwent progressive loading from 2–20 N, or cyclic loading at 9 N or 14 N (n=9–11/sex/loading method). Progressive loading with the prone configuration resulted in ACL rupture at an average of 10.2±0.9 N for females and 11.4±0.7 N for males. In contrast, progressive loading with the supine configuration resulted in ACL rupture in only 36% of female mice and 50% of male mice. Cyclic loading with the prone configuration resulted in ACL rupture after 15±8 cycles for females and 24±27 cycles for males at 9 N, and always during the first cycle for both sexes at 14 N. In contrast, cyclic loading with the supine configuration was able to complete 1,200 cycles at 9 N without injury for both sexes, and an average of 45±41 cycles for females and 49±25 cycles for males at 14 N before ACL rupture. These results show that tibial compression configurations can strongly affect knee injury thresholds during loading.
Keywords: knee injury, tibial compression, osteoarthritis, bone adaptation, mechanical loading
Introduction
Tibial compression loading of mice is widely used in musculoskeletal research to investigate bone adaptation to increased mechanical loading (Brodt and Silva, 2010; De Souza et al., 2005a; De Souza et al., 2005b; Lynch et al., 2010; Zaman et al., 2006). Mouse tibial compression has also been used to investigate osteoarthritis (OA) development after mechanical loading (Christiansen et al., 2012; Christiansen et al., 2015; Ko et al., 2013; Onur et al., 2014; Poulet et al., 2015; Poulet et al., 2011; Rai et al., 2017; Wu et al., 2014). Some of these studies use multiple cycles of tibial compression without acute injury, while other studies use “tibial compression overload” to acutely injure soft tissue structures in the joint, often in a single compressive load.
We previously described using tibial compression overload in mice to examine development of PTOA after joint injury (Anderson et al., 2016; Christiansen et al., 2012; Hsia et al., 2016; Khorasani et al., 2015; Lockwood et al., 2014; Satkunananthan et al., 2014). This method consistently injures the anterior cruciate ligament (ACL) of a mouse knee using a single compressive load at magnitudes of approximately 8–10 N (Christiansen et al., 2012; Lockwood et al., 2014). Other groups have applied similar or even greater tibial compression loading magnitudes (12 N or more) for multiple cycles without acute injury (Berman et al., 2015; Govey et al., 2016; Kelly et al., 2016; Shirazi-Fard et al., 2015). The factors contributing to variable knee injury thresholds during tibial compression are currently unclear, but likely include the position of the mouse limb within the loading system and the fixtures (or “cups”) used to contact the knee and ankle joints. Identifying factors that affect knee injury threshold would inform studies of both bone adaptation and OA development, allowing researchers to design tibial compression systems to either induce or avoid soft tissue injuries during loading.
In this study, we compared two tibial compression configurations: our previously described “prone configuration” that reproducibly induces ACL rupture, and a “supine configuration” that is more representative of those used by other groups for non-injury tibial compression loading. We sought to identify factors contributing to the disparate knee injury thresholds reported during tibial compression in mice. We hypothesized that the supine configuration would be able to apply greater compressive loads for a greater number of cycles before inducing ACL rupture.
Methods
Animals
A total of 29 female and 29 male 10 week-old C57Bl/6 mice were obtained from Envigo (Indianapolis, IN). Mice were randomly assigned to experimental groups, and each mouse was subjected to tibial compression using both the prone and supine configurations (described below). Mice were anesthetized via isoflurane inhalation, and tibial compression loading was performed using an electromagnetic materials testing machine (ElectroForce 3200, TA Instruments, New Castle, DE). All mice were euthanized via carbon dioxide inhalation immediately following tibial compression. Mice were maintained and used in accordance with National Institutes of Health guidelines on the care and use of laboratory animals. All procedures were approved by the UC Davis Institutional Animal Care and Use Committee.
Prone Loading Configuration
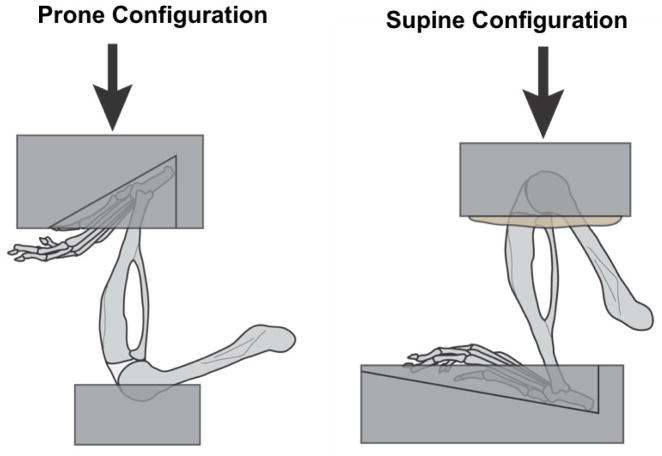
The right hindlimb of each mouse was loaded using the prone configuration (Fig. 1). The hip joint was fully extended so that the femur was approximately parallel to the plane of the body, and the knee joint was held at nearly 90°. The ankle joint was constrained by the top loading platen in 30° of flexion, and the knee was supported by a shallow aluminum cup on the bottom platen.
Figure 1.
In the prone configuration (left), the hip is fully extended, the knee joint is held at nearly 90° of flexion, and the ankle joint is held at 30° of flexion. In the supine configuration (right), the hip is flexed, the knee joint is fully flexed, and the ankle is held at 10° of flexion. The prone configuration uses a shallow metal cup to hold the knee, while the supine configuration uses a deeper metal cup with a molded PMMA insert. Arrows indicate the direction of loading.
Supine Loading Configurations
The left limb of each mouse was loaded using the supine configuration (Fig. 1). The hip joint was flexed so that the femur is approximately perpendicular to the plane of the body, and the knee was constrained in full flexion with the tibia held vertical. The knee joint was supported by a deeper aluminum cup that included a molded polymethylmethacrylate insert. The ankle joint was supported by the bottom platen in 10° of flexion.
Progressive Magnitude Loading
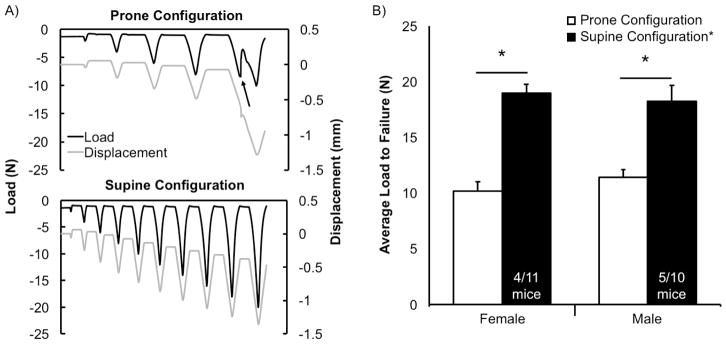
Mice were subjected to progressive loading (n=11 females, n=10 males) to identify the injury load. Tibial compression was applied at 1 mm/s, with magnitudes increasing from 2 to 20 N in increments of 2 N (Fig. 2A). Force-displacement curves were monitored for ACL rupture, identified as a characteristic sharp drop in recorded force with a subsequent translation in resting (zero-force) displacement (Fig. 2A). Compressive loads greater than 20 N were not investigated because this approaches the compressive force needed to induce tibial fracture.
Figure 2.
(A) Representative load (black) and displacement (grey) curves during progressive loading for prone and supine configurations. Arrow indicates ACL rupture. (B) Average load to failure in the prone configuration (white) and supine configuration (black) for females and males. All knees loaded with the prone configuration had ACL ruptures, compared to only 36% (4/11) and 50% (5/10) of knees loaded in the supine configuration for females and males, respectively. The supine configuration was able to withstand a significantly greater load before failure than the prone configuration. The supine configuration led to a significant increase in average load to failure for both females (+86.8%) and males (+59.8%), compared to the prone configuration. *p<0.001
Cyclic Loading
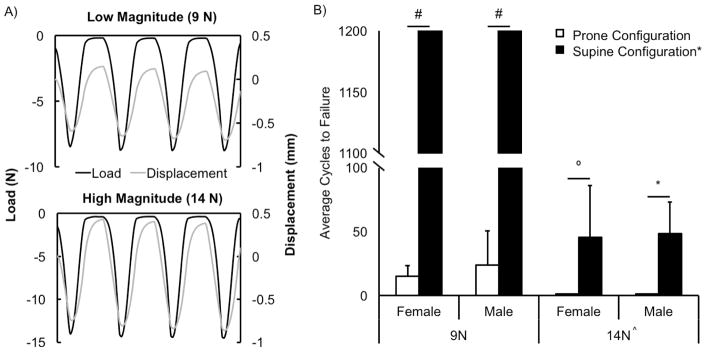
Mice were subjected to cyclic tibial compression loading at 4 Hz, previously determined as the average mouse stride frequency (Lee et al., 2002), for 1200 cycles or until ACL rupture. A sawtooth loading protocol was used, with a 0.1 s dwell between each load cycle at the pre-load level of 0.5 N, similar to commonly used tibial compression loading protocols (Berman et al., 2015; Govey et al., 2016; Kelly et al., 2016; Shirazi-Fard et al., 2015) (Fig. 3A). Peak compressive loads were 9 N in the low magnitude group (n=9 females, n=10 males), and 14 N in the high magnitude group (n=9 females, n=9 males).
Figure 3.
(A) Representative load (black) and displacement (grey) curves during 1 second of low (9 N) and high (14 N) magnitude cyclic loading. (B) Average cycles to failure with low and high magnitude cyclic loading using prone (white) and supine (black) configurations for females and males. At 9 N, loading with the prone configuration resulted in ACL rupture in all mice at an average of 15 cycles for females and 23 cycles for males, while loading with the supine configuration did not lead to any ACL ruptures within 1,200 cycles (#) for either sex. Cyclic loading at 14 N resulted in ACL rupture in all mice after a single cycle with the prone configuration, and after an average of 45 cycles in 8/9 (89%) female mice and 49 cycles in 8/9 (89%) male mice with the supine configuration. The supine configuration led to significantly greater cycles to failure regardless of sex or load magnitude. At high magnitude loading, the supine configuration led to significantly greater cycles to failure for females (45.4-fold) and males (48.5-fold). High magnitude cyclic loading led to significantly fewer cycles to failure compared to low magnitude. *p<0.001 °p<0.01 ^p<0.05
Qualitative Analysis of Joint Injury
Following tibial compression, joints were qualitatively assessed to characterize typical damage to joint structures created by each of the loading configurations. The knee joints of a total of 30 randomly selected mice (n=5/sex/loading method) were examined by an orthopaedic surgeon (TJS) immediately following euthanasia. Knees were dissected and evaluated under microscope for gross tissue damage, swelling, and hemarthrosis. Cruciate and collateral ligaments were specifically inspected for injury. Ligamentous injury was assessed by both physical exam to assess ligamentous laxity and by gross dissection to assess ligamentous rupture or tear. All observable trauma and the number of mice displaying each instance of damage were recorded.
Statistics
3-way ANOVA stratified by loading configuration, sex, and loading magnitude was used to compare cyclic loading results (JMP 11, SAS Institute, Inc., Cary, NC). 2-way ANOVA stratified by loading configuration and sex was used to compare progressive loading results. Paired t-tests were used to determine differences between loading configurations for each sex. The primary outcome of the progressive loading protocol was failure load; the primary outcome of the cyclic loading protocol was number of cycles to ACL rupture. Mean ± standard deviation is presented for all data. Significance was defined as p < 0.05.
Results
Progressive Magnitude Loading
As expected, tibial compression using the prone configuration induced ACL injury in all mice at an average magnitude of 10.2±0.9 N for females and 11.4±0.7 N for males (Fig. 2B). In contrast, tibial compression with the supine configuration induced ACL injury in only 36% of female mice (4/11) at an average force of 19.0±0.8 N, and 50% of male mice (5/10) at an average force of 18.2±1.4 N (Fig. 2B). Average load to failure in the supine configuration was 86.8% greater for females (p=0.0001) and 59.8% greater for males (p=0.0004) compared to the prone configuration. No significant differences between males and females were observed.
Cyclic Loading
Cyclic tibial compression using the prone configuration resulted in ACL rupture in all female mice after 15±8 cycles and in all male mice after 24±27 cycles with 9 N loading, and consistently on the first cycle with 14 N loading for both sexes (Fig. 3B). In contrast, tibial compression using the supine configuration did not induce ACL rupture in any mice within 1,200 cycles with 9 N loading, but induced ACL rupture in 89% (8/9) of both female and male mice after an average of 45±41 cycles for females and 49±25 cycles for males with 14 N loading. No significant differences between males and females were observed.
Qualitative Analysis of Joint Injury
Overall, tibial compression using the supine configuration resulted in less joint damage than with the prone configuration. For progressive loading, all mice injured in the prone configuration displayed ACL rupture, and 4/5 females and 2/5 males displayed joint swelling. Loading with the supine configuration induced ACL rupture in only 1 female and 2 males, with joint swelling in 2/5 females and 1/5 male, and tibial fracture in 1 female mouse at approximately 20 N. For 9 N cyclic loading in the prone configuration, all knees had ACL rupture, 3/5 knees exhibited joint swelling for both females and males, and 1 male mouse had a medial collateral ligament (MCL) tear. Mice loaded at 9 N with the supine configuration did not display any macroscopic damage except for 1 female mouse with a torn MCL. Cyclic loading at 14 N with the prone configuration led to ACL rupture in all mice, joint swelling in 3/5 females and 4/5 males, and a ruptured posterior cruciate ligament (PCL) in 1 male mouse. Using the supine configuration, 5/5 female mice and 3/5 male mice exhibited ACL rupture, 5/5 female mice and 2/5 male mice displayed joint swelling, and 3/5 female mice exhibited hemarthrosis.
Discussion
In this study, we examined ACL injury thresholds using two different tibial compression configurations. These configurations differed in mouse body position (prone vs. supine), hindlimb joint angles, and stabilizing fixtures for the knee and ankle joints. We found that tibial compression using the supine configuration allowed for higher compressive loads and more loading cycles without injury. These results provide insight into why some tibial compression setups lead to acute injury at relatively low compressive forces, while others can load at higher compressive forces without injury.
Mouse hindlimb position and passive muscle forces may have influenced ACL injury thresholds in this study. The primary muscles that span the knee joint are the quadriceps, hamstrings, and gastrocnemius. Quadriceps and gastrocnemius muscle contractions strain the ACL, while hamstring contractions do not (Beynnon et al., 1992; Draganich and Vahey, 1990; Durselen et al., 1995; Fleming et al., 2001). Hip extension in the prone configuration may have created tension in the quadriceps muscle, pulling the tibia anteriorly and straining the ACL. Similarly, holding the ankle in flexion may have created tension in the calf muscles, causing further tibial anterior translation and ACL strain. The supine configuration, on the other hand, may have prevented anterior tibial translation through hip flexion and hamstring muscle tension. However, we did not specifically examine joint kinematics during loading, therefore we are unable to draw definitive conclusions regarding the role of hindlimb positioning. Differences in knee fixture design may have also contributed to ACL rupture threshold. The prone configuration used a shallow metal cup to stabilize the knee, while the supine configuration used a deeper metal cup with a PMMA insert molded to a mouse knee. This cup may have prevented translation of the proximal tibia.
These results must be interpreted with several limitations in mind. Firstly, only two tibial compression configurations and one cyclic loading protocol were investigated; these are not necessarily representative of all tibial compression configurations and protocols used by various research groups. Further validation may be needed to examine the effect of compressive force, number of cycles, loading rate, and rest insertion on knee injury threshold. This study was also conducted exclusively with 10 week old C57Bl/6 mice; generalizability of these results to other genetic strains and ages is unclear.
Noninvasive tibial compression mouse models are effective, clinically relevant tools for studying bone adaptation (Melville et al., 2015). However, inadvertent joint injury during tibial compression could be an important confounding factor for bone adaptation studies. Soft tissue injury induces an inflammatory response that can affect bone remodeling and adaptation. In our previous study, we found that tibial compression overload injury of the ACL resulted in approximately 40% loss of trabecular bone volume from the tibial epiphysis within one week of injury (Christiansen et al., 2012). Furthermore, disruption of the soft tissue structures of the joint could change kinematics, potentially further influencing the bone adaptation response.
In conclusion, this study demonstrates that configuration of the tibial compression setup can significantly affect knee injury threshold during loading. This information is crucial for studies of bone adaptation, in which knee injury should be avoided, and studies of post-traumatic OA, in which acute ACL injury may be desirable.
Acknowledgments
We would like to thank Chrisoula Toupadakis Skouritakis, PhD for contributing Figure 1. We would also like to thank Dr. Andrew Pitsillides and Dr. Sue Grimston for their input on tibial compression configurations. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR062603. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding body was not involved with design, collection, analysis, or interpretation of data; or in the writing of the manuscript. The authors have no conflicts of interest to disclose.
Footnotes
Author contributions:
AWH contributed to study design, data collection and analysis, and was primarily responsible for manuscript writing. FDT contributed to study design, data collection and analysis. TJS examined tissues for macroscopic damage and provided qualitative analysis of the joint after loading. PMT contributed to data analysis. BAC designed the supine tibial compression model, coordinated all analyses, assisted with study design, and contributed to manuscript writing. All authors approved the final version of the manuscript.
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MJ, Diko S, Baehr LM, Baar K, Bodine SC, Christiansen BA. Contribution of mechanical unloading to trabecular bone loss following non-invasive knee injury in mice. J Orthop Res. 2016;34:1680–1687. doi: 10.1002/jor.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AG, Clauser CA, Wunderlin C, Hammond MA, Wallace JM. Structural and Mechanical Improvements to Bone Are Strain Dependent with Axial Compression of the Tibia in Female C57BL/6 Mice. PLoS One. 2015;10:e0130504. doi: 10.1371/journal.pone.0130504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16:1–12. doi: 10.1007/BF00182976. [DOI] [PubMed] [Google Scholar]
- Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared to young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010 doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, Silva MJ, van der Meulen MC, Haudenschild DR. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1627–1638. doi: 10.1016/j.joca.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005a;37:810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- De Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005b;20:2159–2168. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- Draganich LF, Vahey JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res. 1990;8:57–63. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- Durselen L, Claes L, Kiefer H. The influence of muscle forces and external loads on cruciate ligament strain. Am J Sports Med. 1995;23:129–136. doi: 10.1177/036354659502300122. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Renstrom PA, Ohlen G, Johnson RJ, Peura GD, Beynnon BD, Badger GJ. The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. J Orthop Res. 2001;19:1178–1184. doi: 10.1016/S0736-0266(01)00057-2. [DOI] [PubMed] [Google Scholar]
- Govey PM, Zhang Y, Donahue HJ. Mechanical Loading Attenuates Radiation-Induced Bone Loss in Bone Marrow Transplanted Mice. PLoS One. 2016;11:e0167673. doi: 10.1371/journal.pone.0167673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AW, Anderson MJ, Heffner MA, Lagmay EP, Zavodovskaya R, Christiansen BA. Osteophyte formation after ACL rupture in mice is associated with joint restabilization and loss of range of motion. J Orthop Res. 2016 doi: 10.1002/jor.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NH, Schimenti JC, Ross FP, van der Meulen MC. Transcriptional profiling of cortical versus cancellous bone from mechanically-loaded murine tibiae reveals differential gene expression. Bone. 2016;86:22–29. doi: 10.1016/j.bone.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasani MS, Diko S, Hsia AW, Anderson MJ, Genetos DC, Haudenschild DR, Christiansen BA. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res Ther. 2015;17:30. doi: 10.1186/s13075-015-0546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, van der Meulen MC. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FC, Dragomir CL, Plumb DA, Hsia AW, Adebayo OO, Goldring SR, Wright TM, Goldring MB, van der Meulen MC. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J Orthop Res. 2016;34:1941–1949. doi: 10.1002/jor.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–412. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32:79–88. doi: 10.1002/jor.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, van der Meulen MC. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–691. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville KM, Robling AG, van der Meulen MC. In vivo axial loading of the mouse tibia. Methods Mol Biol. 2015;1226:99–115. doi: 10.1007/978-1-4939-1619-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onur TS, Wu R, Chu S, Chang W, Kim HT, Dang AB. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J Orthop Res. 2014;32:318–323. doi: 10.1002/jor.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet B, de Souza R, Kent AV, Saxon L, Barker O, Wilson A, Chang YM, Cake M, Pitsillides AA. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterising a novel and adjustable non-invasive murine knee joint loading model. Arthritis Rheum. 2011;63:137–147. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- Rai MF, Duan X, Quirk JD, Holguin N, Schmidt EJ, Chinzei N, Silva MJ, Sandell LJ. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci Rep. 2017;7:45223. doi: 10.1038/srep45223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satkunananthan PB, Anderson MJ, De Jesus NM, Haudenschild DR, Ripplinger CM, Christiansen BA. In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2014;22:1461–1469. doi: 10.1016/j.joca.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi-Fard Y, Alwood JS, Schreurs AS, Castillo AB, Globus RK. Mechanical loading causes site-specific anabolic effects on bone following exposure to ionizing radiation. Bone. 2015;81:260–269. doi: 10.1016/j.bone.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissues to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol. 2014 doi: 10.1002/art.38375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006;21:1297–1306. doi: 10.1359/jbmr.060504. [DOI] [PubMed] [Google Scholar]