Abstract
Purpose
Osteoarthritis (OA) and diabetes mellitus (DM) share common risk factors with a potential underlying relationship between both diseases. The purpose of this study was to investigate the longitudinal effects of DM on cartilage deterioration over 24-months with MR-based T2 relaxation time measurements.
Methods
From the Osteoarthritis Initiative cohort 196 diabetics were matched in small sets for age, sex, BMI and Kellgren-Lawrence score with 196 non-diabetic controls. Knee cartilage semi-automatic segmentation was performed on 2D multi-slice multi-echo spin-echo sequences. Texture of cartilage T2 maps was obtained via grey level co-occurrence matrix analysis. Linear regression analysis was used to compare cross-sectional and changes in T2 and texture parameters between the groups.
Results
Both study groups were similar in age (63.3 vs 63.0 years, p=0.70), BMI (30.9 vs 31.2 kg/m2, p=0.52), sex (female 53.6% vs 54.1%, p=0.92) and KL score distribution (p=0.97). In diabetics, except for the patella, all compartments showed a significantly higher increase in mean T2 values when compared to non-diabetic controls. Global T2 values increased almost twice as much; 1.77ms vs 0.98ms (0.79ms [CI: 0.39,1.19]) (p < 0.001). Additionally, global T2 values showed a significantly higher increase in the bone layer (p=0.006), and in a separate analysis of the texture parameters, diabetics also showed consistently higher texture values (p<0.05), indicating a more disordered cartilage composition.
Conclusion
Cartilage T2 values in diabetics show a faster increase with a consistently more heterogeneous cartilage texture composition. DM seems to be a risk factor for developing early OA with an accelerated degeneration of the articular cartilage in the knee.
Keywords: Knee osteoarthritis, diabetes mellitus, cartilage imaging, magnetic resonance imaging, biomarkers
INTRODUCTION
Osteoarthritis (OA) and diabetes mellitus (DM) are both common and progressive disorders with rising incidence 1,2. OA is the leading cause of chronic disability in the field of musculoskeletal diseases and the primary cause of disability in the elderly 3. The main characteristics of OA, gradual irreversible loss of articular cartilage accompanied by degeneration of other joint tissues, interfere with quality of life 4 and result in pain and motion restriction. DM is a metabolic disorder characterized by high blood glucose levels that originates either from peripheral insulin resistance with subsequent failure of the pancreatic β-cell to adequately compensate for the insulin resistance (type 2) 5 or the T-cell mediated destruction of insulin-producing cells in the pancreas (type 1) 6. The incidence and prevalence of the more common DM type (DM type 2) has nearly doubled within the last two decades 7, and its presence is reported in a high proportion of knee OA cases 8,9. Both diseases share many risk factors 10,11, which may explain the increased prevalence of musculoskeletal diseases in diabetics 12; however, the underlying pathophysiology and biologic relationship between these two diseases is not yet completely understood.
Until recently, only a few studies focused on the effects of DM on OA. Initial experimental work has suggested that diabetes-induced impaired glucose metabolism may impact cartilage matrix microanatomy by influencing protein folding 13, and thus, induce cartilage matrix degradation 14. Another study showed that the articular cartilage of diabetics was significantly softer and more permeable, suggesting a compromised structural integrity of the articular cartilage 15. Additional studies have suggested that DM both doubles the risk for a total joint replacement in patients with more advanced OA and increases the risk of postoperative complications 16,17. Furthermore, through several biochemical abnormalities related to impaired glucose metabolism, hyperglycemia may also affect the ligaments via altered collagen synthesis 12,18. Ultimately, identifying the relationship of these two diseases will further the understanding of OA and will contribute to developing effective therapies to treat this “metabolic” subtype of OA.
MR-based T2 relaxation time measurements and grey-level co-occurrence matrix (GLCM) texture analysis are relatively novel quantitative compositional imaging techniques that can be used to assess early compositional changes in the cartilage matrix as they reflect the change of hydration and organization of collagen fibrils in the extracellular matrix 19. Several studies have demonstrated that not only individuals with existing OA, but also those with developing OA, show increased T2 relaxation times, demonstrating its potential as a biomarker for early and developing OA 20–22. Thus, this imaging technique may help us to better understand if there is an altered cartilage matrix in diabetics, which may be potentially caused by an early deterioration of the collagen architecture.
The purpose of our study was to assess longitudinal changes in the articular cartilage composition in diabetics using cartilage T2 relaxation time measurements and texture T2 maps and to compare these changes with a sex-, age- and body mass index (BMI)-matched non-diabetic control cohort.
METHODS
Participants
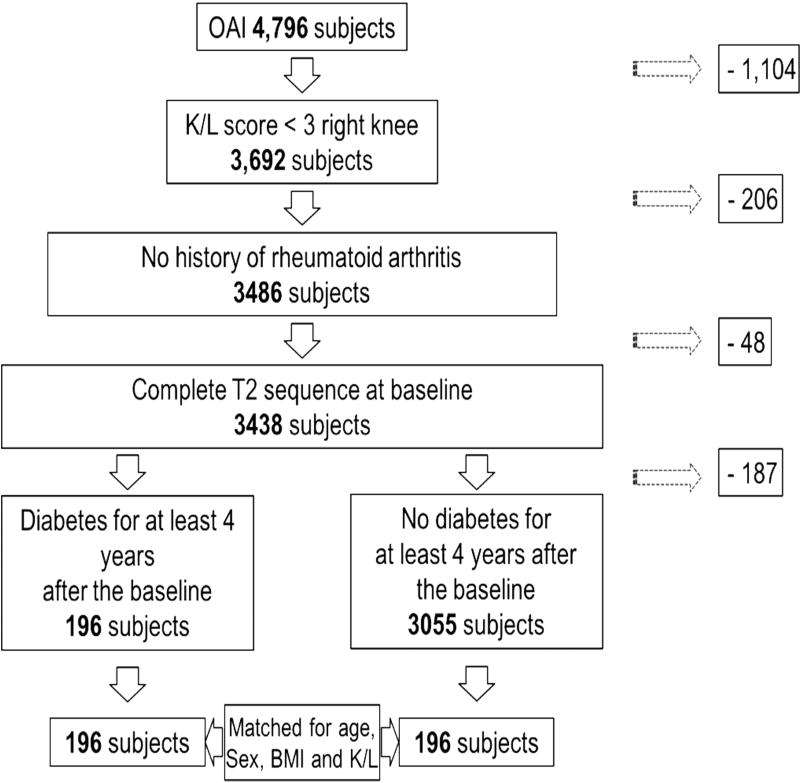
For this study, 392 participants with (n=196) and without DM (n=196) were selected from the Osteoarthritis Initiative (OAI) cohort. The OAI is a longitudinal, prospective, multi-center cohort study of knee OA that enrolled 4796 participants and is sponsored by the U.S. National Institutes of Health (NIH) for investigating diagnosis, treatment, and prevention of OA. The OAI study protocol contains an ethnically diverse cohort of women and men ages 45 – 79 years including participants with symptomatic knee OA at baseline and participants with risk factors for OA but without presenting knee OA symptoms yet. Purpose of the OAI is to develop a public domain research resource to investigate the role of MRI-based imaging biomarkers in an attempt to better understand the disease onset and ultimately prevent its progression 23.
For our study, we selected diabetics based on a self-administered questionnaire and non-DM participants as a control group (Figure 1). To ensure a consistent study cohort, we only included diabetic participants who maintained their diabetic status (using the self-administered questionnaire) for at least 4 years after the time point of enrollment and excluded controls who developed DM during the first 4 years of the OAI. Additionally, participants were only selected if sex, BMI, and age were documented. Further inclusion criteria were a Kellgren-Lawrence (KL) score of 0 – 2 to focus on participants with sufficient cartilage 24 and complete MR imaging and T2 maps. Exclusion criteria were a past history of rheumatoid arthritis or another inflammatory arthropathy. A total of 196 diabetics and 3151 non-diabetic controls met the inclusion criteria. The diabetics were matched in small sets of size 2 to 8 with the controls based on sex, KL score, age and BMI, resulting in 392 study participants for the baseline. Categories for the matching were defined by combination of: sex (male or female), KL score (0–1 or 2), age (45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79) and BMI (17.5–19.9, 20.0–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–32.4, 32.5–34.9, 35.0–37.4, 37.5–39.9, 40.0–42.4, 42.5–44.9 and > 45) resulting in 102 sets in total. During the course of the study 40 diabetics and 31 non-diabetics dropped out (no data available for the 24-month follow up time point). Furthermore, a small number of follow-up scans showed severe motion artifacts and could therefore not be included in the data analysis (5 and 6 participants, respectively). In total, MRI studies of only 310 (151 diabetics and 159 non-diabetics) knees were available for analysis at the 24-month follow-up time point.
Figure 1. Patient selection from the OAI.
Flow-chart illustrating patient selection from the OAI cohort.
Diabetes related complications
Based on a self-administered questionnaire, the diabetics were at all time points asked if DM had already caused problems with kidneys (diabetic nephropathy), problems with eyes (diabetic retinopathy), or whether participants had no problems related to DM. Diabetes related complications are summarized in Table 2.
Table 2.
Diabetes related complications of all diabetics at both time points.
| Baseline (n=196) | 24-months (n=151) | |
|---|---|---|
|
| ||
| Diabetes related complications | ||
|
| ||
| No secondary diabetic complications [n (%)] | 169 (86.2%) | 130 (86.1%) |
| Diabetics with complications [n (%)] | 27 (13.8%) | 21 (13.9%) |
|
| ||
| Diabetic nephropathy [n (%)] | 5 (18.5%) | 5 (23.8%) |
| Diabetic retinopathy [n (%)] | 18 (66.7%) | 14 (66.7%) |
| Diabetic nephropathy and retinopathy [n (%)] | 4 (14.8%) | 2 (9.5%) |
|
| ||
| Diabetics treated with insulin | 35 (17.9%) | 25 (16.6%) |
Categorical data are presented in numbers of participants with percentage in parentheses.
MR Imaging
All MRI scans were performed using 3.0T scanners (Siemens Magnetom Trio; Siemens Healthcare, Erlangen, Germany) with quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at the four OAI clinical sites (University of Maryland, Baltimore, Maryland; Memorial Hospital of Rhode Island / Brown University, Pawtucket, Rhode Island; Ohio State University, Columbus, Ohio; University of Pittsburgh, Pittsburgh, Pennsylvania).
For the T2 relaxation time measurements, a sagittal 2D multi-slice multi-echo (MSME) spin-echo sequence with total of seven echo times (TEs 10ms, 20ms, 30ms, 40ms, 50ms, 60ms, 70ms), a repetition time (TR) of 2700ms, a field of view (FOV) of 120mm, a slice thickness of 3mm (with 0.5mm gap) and an in-plane spatial resolution of 0.313×0.446mm2 was used.
Quantitative Image Analysis
To measure T2 using the 2D MSME SE image stack, cartilage was segmented in five compartments: patella (PAT), lateral femur (LF), medial femur (MF), lateral tibia (LT), and medial tibia (MT). Due to strong interfering flow artifacts from the popliteal artery, the trochlear region was excluded from the analysis. The cartilage of each compartment was independently segmented by two trained researchers (F.H. and W.A.), both blinded to the clinical information and the DM status, under the supervision of an experienced radiologist (T.M.L.). The software used for the T2 analysis was an in-house, spline-based algorithm written in MATLAB (the Mathworks, Natick, Massachusetts) that allows semi-automatic segmentation of each compartment by analyzing the T2 values in a mono-exponential decay model as a fitting function for the signal intensity using 6 echoes (TE 20–70 ms) after excluding the first echo in order to minimize errors and improve signal-to-noise ratio 25,26. T2 parameters were calculated in each compartment, and the mean was calculated across all compartments for the whole knee joint.
Laminar and GLCM Texture Analysis
For laminar analysis, the cartilage was divided into two layers with equal thickness. Each compartment consisted of a deep layer adjacent to the bone surface and the articular superficial layer 27. In addition, GLCM parameters were used to assess the grey level distributions of pixels, respectively cartilage T2 values of the T2 maps. Contrast (i), variance (ii), and entropy (iii) were calculated which characterize the heterogeneity of T2 values throughout the cartilage matrix 28,29. In detail, contrast (i) describes the local grey level variation by comparing each pixel to its neighboring horizontal or vertical pixel. Larger differences of grey levels in neighboring pixels result in a higher contrast, indicating inhomogeneous pixel pairs in the cartilage matrix. Variance (ii) compares the disparity of each single pixel to the compartment mean, indicating how many pixels vary from the average compartment grey level. Whereas contrast represents the grey level variation of single neighboring pixel pairs, entropy (iii) expands on the grey level equation by representing the probability of finding another pixel pair with the same value in the whole texture image, illustrating the disorder of the image. A higher entropy suggests a more random distribution of the pixel pairs.
Statistical Analysis
Statistical analysis was performed using STATA software (Version 14, College Station, TX: StataCorp LP), and p<0.05 was considered statistically significant. Differences in the group characteristics were assessed using either independent t-tests for continuous variables or chi-square tests for categorical variables.
Linear regression models were used to assess the differences in cartilage T2 parameters (mean, laminar analysis, and GLCM texture analysis) between diabetics and controls at baseline, 24-months, and changes over 24-months (delta 24-months-baseline). Cluster robust standard errors were used to account for the matching by sets. Beta values and their 95% Cis were calculated and added in the tables. All analyses were adjusted for the common risk factors of knee OA (age, sex, and BMI) to minimize bias due to confounding by these main risk factors. Furthermore, we also matched and adjusted for K/L scores to minimize a possible confounding since differences in K/L scores can cause variations of T2 relaxation times 30. The underlying assumptions for each statistical test were fulfilled. To address missing data due to attrition of our study participants we conducted a sensitivity analysis using multiple imputation. We used multivariate normal imputation with 20 imputations to impute the missing T2 values at the compartment level on the basis T2 values in other compartments, age, sex, BMI, diabetes status, race, and KL grade.
Studies have shown racial differences in the biochemical knee cartilage composition 31. Due to the racial differences in our study groups with a higher percentage of African Americans in the diabetic cohort, adjustments for race were performed.
Longitudinal change of mean T2 values were recorded as primary outcome measures (compartments: global knee compartment, patella, lateral tibia, medial tibia, lateral femur, and medial femur). Based on previous results 24,28,32,33 the laminar [superficial/bone layer] and texture analysis [contrast, variance, entropy], as well as the cross-sectional mean T2 analysis were considered as secondary outcome measures (compartments: global knee compartment, patella, and lateral tibia).
Inter-/Intrareader Reproducibility
To assess inter- and intra-reader reproducibilities, coefficients of variation (CV) were calculated on a percentage basis as the root mean square average 34. For the interreader reproducibility, two readers analyzed images from 10 identical participants and CVs were calculated as above. For the intrareader reproducibility each of the two readers was given 10 randomized image datasets to read and reread the same images at two different time points, with a minimum interval of at least 4 weeks between the readings. For the interreader reproducibility CVs ranged from 1.59% in the lateral femur to 2.36% in in the medial tibia with an overall average of 1.93%. For the intrareader reproducibility the CVs ranged from 0.64% in the lateral femur to 2.85% in the medial tibia with an overall average of 1.57%; reproducibilities were similar to those reported previously 32.
RESULTS
Participant characteristics
The participant characteristics are summarized in Table 1. The average age of all study participants was 63.1years (SD ± 9.1), with slightly more women (53.8%), and an average BMI of 31.05 (SD ± 4.5). Both subcohorts groups were well matched, with no significant differences (p>0.05) in age, sex, BMI, and K/L. However, there were a higher percentage of African American among diabetics versus the non-diabetic controls (36.2% and 14.8% respectively (p<0.001)). Osteoarthritic risk factors, such as history of knee injury, history of knee surgery, and family history of knee replacement surgery, showed no significant differences (p>0.05). Although patient attrition occurred throughout the course of the study, the overall group characteristics did not change (p>0.05). Despite the different racial composition, all other study participant characteristics showed no significant differences at 24-months follow-up (p>0.05).
Table 1.
Demographic characteristics, PASE, KL-scores and OA risk factors in all diabetics and controls at baseline and 24-months.
| Baseline | Non-diabetics (n=196) | Diabetics (n=196) | p-values ɫ |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age (years) | 63.31 ± 9.17 | 62.96 ± 8.99 | 0.701 |
| Body mass index (kg/m2) | 30.91 ± 4.50 | 31.20 ± 4.51 | 0.523 |
| Height (m) | 1.68 ± 0.09 | 1.69 ± 0.09 | 0.749 |
| Females [n (%)] / Males [n (%)] | 105 (53.6%) / 91 (46.4%) | 106 (54.1%) / 90 (45.9%) | 0.919 |
| Physical Activity Score for the Elderly | 154.19 ± 85.92 | 144.20 ± 78.99 | 0.232 |
| Right knee Kellgren-Lawrence | 0.965 | ||
| Grade 0 [n (%)] | 79 (40.3%) | 79 (40.3%) | |
| Grade 1 [n (%)] | 48 (24.5%) | 50 (25.5%) | |
| Grade 2 [n (%)] | 69 (35.2%) | 67 (34.2%) | |
|
| |||
| Racial composition | <0.001 | ||
|
| |||
| Caucasian [n (%)] | 162 (82.7%) | 115 (58.7%) | |
| African American [n (%)] | 29 (14.8%) | 71 (36.2%) | |
| Asian [n (%)] | 1 (0.5%) | 4 (2.0%) | |
| Other Non-white [n (%)] | 4 (2.0%) | 6 (3.1%) | |
|
| |||
| OA risk factors | |||
|
| |||
| History of knee injury [n (%)] | 70 (35.7%) | 73 (37.2%) | 0.568 |
| History of knee surgery [n (%)] | 30 (15.3%) | 36 (18.4%) | 0.510 |
| Family history of knee replacement surgery [n (%)] | 26 (13.3%) | 15 (7.7%) | 0.233 |
|
| |||
| 24-months | Non-diabetics (n=159) | Diabetics (n=151) | p-values ɫ |
|
| |||
| Demographics | |||
|
| |||
| Age (years) | 64.99 ± 8.96 | 65.40 ± 8.83 | 0.685 |
| Body mass index (kg/m2) | 30.48 ± 4.37 | 31.00 ± 4.48 | 0.303 |
| Height (m) | 1.68 ± 0.09 | 1.68 ± 0.09 | 0.798 |
| Females [n (%)] / Males [n (%)] | 84 (52.8%) / 75 (47.2%) | 83 (55.0%) / 68 (45.0%) | 0.706 |
| Physical Activity Score for the Elderly | 151.10 ± 84.13 | 141.45 ± 76.63 | 0.295 |
| Right knee Kellgren-Lawrence | 0.678 | ||
| Grade 0 [n (%)] | 57 (35.8%) | 60 (39.7%) | |
| Grade 1 [n (%)] | 41 (25.8%) | 40 (26.5%) | |
| Grade 2 [n (%)] | 61 (38.4%) | 51 (33.8%) | |
|
| |||
| Racial composition | <0.001 | ||
|
| |||
| Caucasian [n (%)] | 133 (83.6%) | 92 (60.9%) | |
| African American [n (%)] | 23 (14.5%) | 51 (33.8%) | |
| Asian [n (%)] | 0 (0.0%) | 3 (2.0%) | |
| Other Non-white [n (%)] | 3 (1.9%) | 5 (3.3%) | |
|
| |||
| OA risk factors | |||
|
| |||
| New history of knee injury [n (%)] | 6 (3.8%) | 6 (4.0%) | 1.000 |
| New history of knee surgery [n (%)] | 5 (3.1%) | 3 (2.0%) | 0.520 |
Continues data are expressed as mean ± SD. Categorical data are presented in numbers of participants with percentage in parentheses.
p-values listed in the right column were calculated using either Pearson’s χ2-test or independent t-test as appropriate.
At baseline, no complications related to DM were reported for 169 (86.2%) diabetics. However, 27 participants (13.8%) reported complications. In detail, 5 reported renal complications, 18 reported ophthalmological complications, and 4 participants reported both renal and ophthalmological complications. Furthermore, 35 (17.9%) diabetics were treated with insulin injections (Table 2).
Longitudinal change in mean T2 relaxation
In the analysis of our primary outcome measures, comparing the longitudinal change of mean T2 values, diabetics showed a significantly faster increase compared to non-diabetic controls. As demonstrated in Table 3, global T2 values increased almost twice as much in diabetics compared with the control group (p<0.001).
Table 3.
Longitudinal change in mean T2 values in diabetics and non-diabetics.
| Absolute change in T2 values over 2 years
| ||||||
|---|---|---|---|---|---|---|
| Diabetics (n=151) | Non-diabetics (n=159) | |||||
|
|
||||||
| Adjusted means |
[95% CI] | Adjusted means |
[95% CI] | Effect size [95% CI]* | p-value | |
| Absolute change in mean T2 values | ||||||
|
| ||||||
| Global knee T2 | 1.77 | [1.51,2.03] | 0.98 | [0.68,1.28] | 0.79 [0.39,1.19] | <0.001 |
| PAT T2 | 1.33 | [0.82,1.84] | 1.38 | [0.87,1.89] | −0.05 [−0.79,0.68] | 0.885 |
| MT T2 | 1.85 | [1.50,2.20] | 1.27 | [0.90,1.64] | 0.58 [0.05,1.11] | 0.033 |
| LT T2 | 1.92 | [1.59,2.25] | 1.36 | [0.99,1.74] | 0.56 [0.04,1.07] | 0.034 |
| MF T2 | 1.08 | [0.76,1.40] | 0.52 | [0.14,0.91] | 0.56 [0.07,1.05] | 0.026 |
| LF T2 | 2.03 | [1.69,2.36] | 1.47 | [1.12,1.82] | 0.56 [0.09,1.03] | 0.020 |
|
| ||||||
| Absolute change in laminar (superficial) T2 values over 2 years | ||||||
|
| ||||||
| Global knee T2 | 1.82 | [1.51,2.13] | 1.37 | [1.02,1.71] | 0.45 [−.021,0.93] | 0.061 |
| PAT T2 | 1.41 | [0.81,2.00] | 1.65 | [0.96,2.34] | −0.24 [−1.15,0.66] | 0.592 |
| LT T2 | 2.27 | [1.85,2.69] | 1.62 | [1.14,2.10] | 0.65 [0.003,1.30] | 0.049 |
|
| ||||||
| Absolute change in laminar (bone) T2 values over 2 years | ||||||
|
| ||||||
| Global knee T2 | 1.51 | [1.28,1.75] | 0.98 | [0.71,1.25] | 0.53 [0.15,0.91] | 0.006 |
| PAT T2 | 1.21 | [0.73,1.69] | 1.21 | [0.78,1.64] | 0.004 [−0.63,0.64] | 0.991 |
| LT T2 | 1.31 | [0.97,1.65] | 1.06 | [0.73,1.38] | 0.25 [−0.22,0.73] | 0.292 |
|
| ||||||
| Absolute change in texture parameters | ||||||
|
| ||||||
| Global knee contrast | 93.75 | [73.01,114.48] | 56.09 | [38.09,74.08] | 37.66 [9.77,65.56] | 0.009 |
| PAT contrast | 78.25 | [59.30,97.20] | 53.89 | [33.11,74.66] | 24.36 [−05.79,54.51] | 0.112 |
| LT contrast | 44.72 | [28.02,61.41] | 32.42 | [20.37,44.46] | 12.30 [−9.06,33.66] | 0.256 |
|
| ||||||
| Global knee variance | 44.50 | [34.35,54.65] | 35.18 | [25.81,44.55] | 9.32[−4.69,23.32] | 0.190 |
| PAT variance | 35.21 | [24.13,46.30] | 33.88 | [22.28,45.48] | 1.33 [−15.06,17.73] | 0.872 |
| LT variance | 24.70 | [15.39,34.01] | 25.70 | [17.74,33.66] | −1.00[−13.12,11.12] | 0.870 |
|
| ||||||
| Global knee entropy | 0.01 | [−0.02.0.04] | 0.04 | [0.01,0.07] | −0.03 [−0.07,0.02] | 0.255 |
| PAT entropy | −0.04 | [−0.09,0.01] | −0.08 | [−0.14,−0.03] | 0.04 [−0.03,0.12] | 0.260 |
| LT entropy | 0.12 | [0.07,0.17] | 0.12 | [0.07,0.18] | −0.002 [−0.09,0.08] | 0.954 |
Data are given as adjusted means, corrected for race, age, sex, baseline BMI, baseline KL score, with [95% confidence intervals] and computed as the absolute change between the 24-months and the baseline.
Effect size for difference in T2 and texture parameters between groups; P-values <0.05 are in bold with the significant higher mean T2 value in underlined characters. PAT = patella, MT = medial tibia, LT = lateral tibia, MF = medial femur, LF = lateral femur.
When analyzing the compartments separately, every single compartment, except for the patella, also showed a significantly faster increase in mean T2 values (p<0.05). Sensitivity analyses using multiple imputation to deal with attrition did not significantly change our results; the effect sizes for the single compartments with missing-data imputation (vs without missing-data imputation) were as follows: patella 0.03 [-0.72,0.77], p=0.944 (vs −0.05 [-0.79,0.68], p=0.885), medial tibia 0.60 [0.08,1.11] p=0.025 (vs 0.58 [0.05,1.11], p=0.033), lateral tibia 0.59 [0.05,1.12], p=0.031 (vs 0.56 [0.04,1.07]), p=0.034), medial femur 0.55 [0.05,1.06], p=0.031 (vs 0.56 [0.07,1.05], p=0.026), and lateral femur 0.66 [0.20,1.11], p=0.005 (vs 0.56 [0.09,1.03], p=0.020).
Regarding the longitudinal change in the articular and bone layer, similar results were obtained: The global T2 values were higher for both, the superficial and bone layers with statistical significance reached in the bone layer (p=0.006). Significantly higher results were also reached for the superficial layer in the lateral tibia (p=0.049).
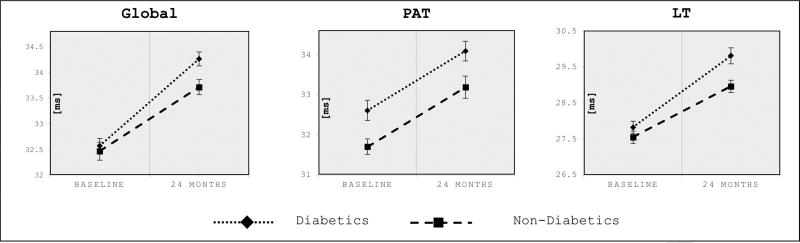
Our secondary outcome measures, cross-sectional mean T2 analysis, showed similar global T2 values at baseline: 32.65ms (non-diabetics) vs 32.39ms (diabetics), 27.55ms (non-diabetics) vs 27.82ms (diabetics) for the lateral tibia and 31.70ms (non-diabetics) vs 32.61ms (diabetics) for the region of the patella (Figure 3), whereas, statistically difference was only reached in the patella, showing higher mean T2 values for diabetics (p=0.003).
Figure 3. Cross-sectional cartilage mean T2 values in diabetics and non-diabetics at baseline and 24-months.
Cross-sectional mean cartilage T2 values in diabetics and non-diabetics at baseline and 24-months. Data are given as adjusted means (ms), corrected for race, age, sex, baseline BMI and baseline KL score. Error bars indicate standard errors. P-values in bold refer to significance of differences between diabetics vs non-diabetics at baseline and 24-months, respectively: global knee (p=0.669, baseline; p=0.009, 24-months), patella (p=0.003, baseline; p=0.012, 24-months), lateral tibia (p=0.314, baseline; p=0.006, 24-months). Global = global T2 values/mean of all compartments, PAT = patella, LT = lateral tibia.
However, with the faster increase of mean T2 values, the cross-sectional differences in mean T2 values were significantly higher in diabetics for the 24-month time point: Cross-sectional T2 values for the 24-month time point were significantly higher in the global knee compartment (p=0.009), the patella (p=0.012), and the lateral tibia (p=0.006) (Figure 3).
Diabetics with severe disease (presence of diabetes-related renal, ophthalmological complications, or treated with insulin) also showed a faster increase in mean T2 values when compared to non-diabetic controls. However, these results did not translate into statistical significance (Table 4).
Table 4.
Longitudinal change in mean T2 values in diabetics with severe disease and non-diabetics.
| Absolute change in T2 values over 2 years – Severe-Diabetics
| ||||||
|---|---|---|---|---|---|---|
| Severe-Diabetics (n=38) | Non-diabetics (n=159) | |||||
|
|
||||||
| Adjusted means |
[95% CI] | Adjusted means |
[95% CI] | Effect size [95% CI]* | p-value | |
| Absolute change in mean T2 values | ||||||
|
| ||||||
| Global knee T2 | 1.29 | [0.76,1.83] | 1.07 | [0.80,1.35] | 0.22 [−0.40,0.83] | 0.482 |
| PAT T2 | 1.55 | [0.55,2.54] | 1.34 | [0.84,1.84] | 0.21 [−0.82,1.24] | 0.682 |
| MT T2 | 1.29 | [0.48,2.09] | 1.26 | [0.90,1.63] | 0.03 [−0.90,0.95] | 0.960 |
| LT T2 | 1.52 | [0.86,2.17] | 1.33 | [0.96,1.70] | 0.19 [−0.64,1.01] | 0.652 |
| MF T2 | 0.71 | [0.01,1.41] | 0.46 | [0.09,0.83] | 0.25 [−0.54,1.04] | 0.536 |
| LF T2 | 1.43 | [0.61,2.25] | 1.47 | [1.13,1.81] | −0.04 [−0.94,0.85] | 0.920 |
Data are given as adjusted means, corrected for race, age, sex, baseline BMI, baseline KL score, with [95% confidence intervals] and computed as the absolute change between the 24-months and the baseline
Effect size for difference in T2 and texture parameters between groups; P-values <0.05 are in bold. PAT = patella, MT = medial tibia, LT = lateral tibia, MF = medial femur, LF = lateral femur.
Performing a sensitivity analysis by only including the KL2 participants (mild radiographic OA), we noticed that diabetics with radiographic OA (KL2) showed a slightly higher increase of mean T2 values than diabetics with no radiographic OA (KL0–1). However, we did not find significant differences in results between this subcohort and the overall cohort.
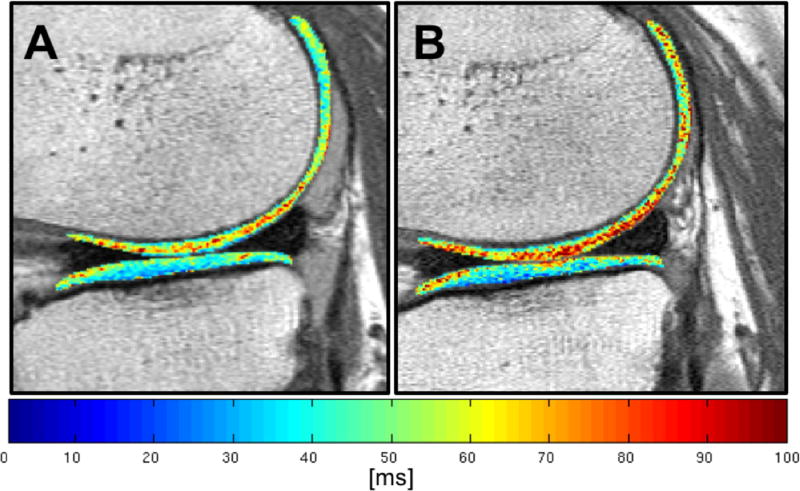
Figure 2 demonstrates the increase in mean T2 values in one of our diabetics using a color-scale T2 map with change in the color of the cartilage from baseline with predominantly lower T2 values (blue and green) to higher T2 values at 24-months areas of (yellow and red).
Figure 2. Longitudinal change of T2 color map of a diabetic patient over 24-months.
T2 color maps of the same diabetic patient at baseline (A) and after 24-months (B), both showing the region of the lateral femoral condyle and the lateral tibial plateau. At baseline (A), the tibial plateau shows predominantly lower T2 values (blue and green), whereas, especially the weight-bearing portion of the lateral condyle already shows areas of higher T2 values (yellow and red). After 24-months (B) the cartilage of the femoral condyle shows overall increased T2 values, including the posterior aspect of the femoral condyle. The tibial plateau also shows several areas of higher T2 values, with particular emphasis at the anterior and posterior aspect of the tibial plateau, consistent with an accelerated rate of cartilage matrix degradation.
Change in cartilage T2 texture parameters
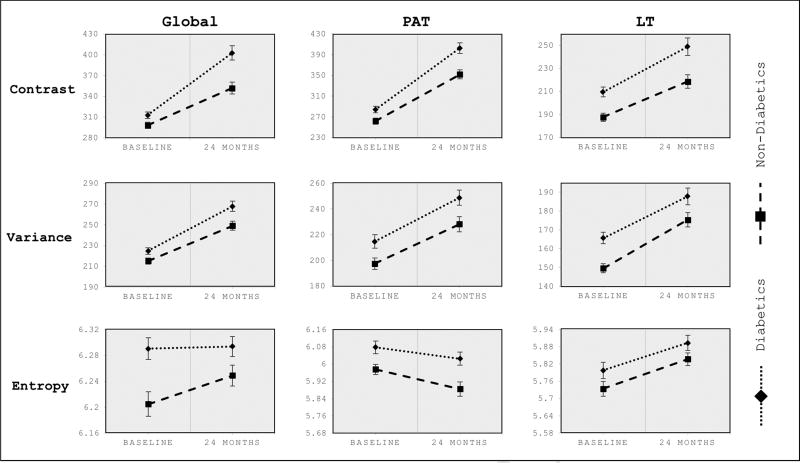
Although, longitudinal changes in texture parameters were only significantly higher for diabetics in the global knee contrast (p=0.009; Table 3), in the cross-sectional analysis diabetics exhibited a more heterogeneous and disordered cartilage composition at both time points. Figure 4 shows the texture composition for both groups and time points.
Figure 4. Cross-sectional GLCM texture analysis in diabetics and non-diabetics at baseline and 24-months.
Texture parameters (contrast, variance, and entropy) in diabetics and non-diabetics at baseline and 24-months. Data are given as adjusted means, corrected for age, sex, baseline BMI and baseline KL score. Error bars indicate standard errors. P-values in bold refer to significance of differences between diabetics vs non-diabetics at baseline and 24-months, respectively: Global knee contrast (p=0.021, baseline; p<0.001, 24-months), patella contrast (p=0.010, baseline; p=0.001, 24-months), lateral tibia contrast (p<0.001, baseline; p=0.004, 24-months). Global knee variance (p=0.029, baseline; p=0.007, 24-months), patella variance (p=0.009, baseline; p=0.009, 24-months), lateral tibia variance (p<0.001, baseline; p=0.041, 24-months). Global knee entropy (p=0.001, baseline; p=0.058, 24-months), patella entropy (p=0.009, baseline; p=0.002, 24-months), lateral tibia entropy (p=0.144, baseline; p=0.121, 24-months). Global = global T2 values/mean of all compartments, PAT = patella, LT, lateral tibia.
At 24-months, the diabetic status was associated with overall more heterogeneous texture parameters. With respect to mean T2 variance and mean T2 contrast, diabetics showed significantly higher texture measures for the global knee (p<0.001, contrast; p=0.007, variance), the patella (p=0.001, contrast; p=0.009, variance), and the lateral tibia (p=0.004, contrast; p=0.041, variance). Also, significantly higher texture parameters were found in the patella for the mean T2 entropy (p=0.002). In contrast to all other texture parameters, showing an increase over time, T2 entropy in the patella showed a decrease for both groups. This unexpected decrease might be explained by the thicker cartilage of the patella, with a higher probability of finding similar pixel pairs, and therefore, a less disordered cartilage composition.
DISCUSSION
We examined the impact of DM on biochemical cartilage matrix composition and spatial distribution over 24-months in a double cohort study using T2 relaxation times and GLCM texture as surrogate markers of cartilage integrity and heterogeneity. We found that diabetics showed a significantly higher increase in mean T2 values compared to non-diabetic controls with consecutive also higher mean T2 values at 24-months. Furthermore, in a separate longitudinal analysis of cartilage GLCM texture parameters, diabetics showed a significantly more heterogeneous and disordered cartilage composition at both time points.
To date, only a small number of studies have focused on analyzing the direct relationship of OA and DM, although several previous studies have already suggested potentially underlying interactions between these diseases 16,35–38. Berenbaum proposed that an independent hyperglycemia-induced systemic inflammation could be a risk factor with severe impact on the progression of OA 39. King et al found that diabetics not only received more joint arthroplasties, but also that they were performed at a younger age than in the non-diabetic control group 16. Our study showed a faster deterioration of the cartilage matrix in the knee of diabetics, indicating a higher loss of collagen content with a disruption of the collagen network in the extracellular matrix of diabetic knees, possibly causing accelerated OA. We also found, that the difference of deterioration is significantly higher in the diabetics bone cartilage layer (generally referred to as the deep cartilage layer). In general, the bone layer shows lower T2 values relative to the articular layer as it includes the calcified cartilage layer and the tidemark 40. Our results indicate a more advanced cartilage deterioration, with more water influx, in the deeper layers of the cartilage in the diabetics, findings which are different from non-diabetics and also different from the normal evolution of cartilage degenerative disease, which starts at the superficial layer.
Nielen et al also evaluated the risk of knee replacement in diabetics and demonstrated a decreased rate of total joint replacements with increasing DM severity 41. While an advantage of his study was the large study population with over 400,000 diabetics, the participants were of older age than ours and there was limited data on risk factors for OA and DM, such as BMI. Simply by their high prevalence and shared risk factors, such as BMI 10,11,32, OA and DM frequently co-exist in the elderly population, which leads to a greater challenge when studying the independent impact of DM and OA. Schett et al considered DM to be a predictor of severe OA for joint arthroplasty while also controlling for established risk factors, including age, sex, and BMI 37. Nieves-Plaza et al also adjusted for age, sex, and BMI, and showed that diabetics had a greater risk of hand or knee OA when compared to non-diabetic controls 38.
Our study also matched and adjusted for BMI and showed a faster increase of mean T2 values and consecutive higher mean T2 values at the 24-month time point, indicating that diabetics are at higher risk for developing OA. Our results have also revealed, that diabetics with severe case status show a faster increase of mean T2 values as well. However, these results did not translate into statistical significance, most likely due to the small subcohort of participants with severe disease.
Interestingly, our diabetics showed a significantly higher percentage of African American than the control group. This is in accordance with other studies that have showed a higher prevalence for DM in African American than in Caucasians 42. However, Signorello et al discovered that this difference is rather related to differences in modifiable risk factors, such as socioeconomic status, than to the genetic background 43. With respect to the cartilage composition, a longitudinal study of cartilage T2 relaxation times also revealed differences in the cartilage composition of African American. Kretzschmar et al show that cartilage T2 values in African American increased faster over 72 months than Caucasian American 31. With still limited knowledge on the ethical and racial differences in cartilage composition we strictly controlled for race differences in our study. Further studies are needed to assess the cartilage composition in African American while also controlling for the economic and sociological exposure.
Overall, our results confirm and expand on studies reporting DM to be a risk factor for OA 44–50, whereas, in contrast to our work, the majority of previous studies used either arthroplasty 44,45, plain radiographs 46–48, or questionnaires 49,50 to determine the outcome of OA. However, these outcomes measures are not able to show early cartilage changes as detected by quantitative MR-imaging.
Quantitative imaging does not only provide detailed information about early changes in the articular matrix but also reflects clinical changes in patients with higher risk of developing OA. Previous studies demonstrated that increased cartilage T2 values are associated with higher pain levels in the knee and can predict the development of radiographic confirmed OA over a 4-year period 20,51. The differences found in T2 values in these studies were in the same range as in our study, in fact indicating that our increased T2 values are also clinically significant. Therefore, the use of quantitative imaging seems to be a promising biomarker in the setting of OA and OA-related risk factors. To the best of our knowledge, only one previous study 52 used MR-imaging as an outcome parameter for degenerative joint disease in diabetics. In this study, diabetics also had higher T2 values with a more heterogeneous cartilage composition but the longitudinal impact of DM on articular cartilage was not considered. Additionally, with respect to the fact that both articular cartilage and DM are dependent upon a number of metabolic changes, studies that focus on biochemical and biomechanical changes in the articular cartilage are useful to understand the relationship between DM and OA. Rosa et al demonstrated that chondrocytes in a hyperglycemic environment show a higher expression of matrix metalloproteinases and suggested that this promotes articular cartilage degradation. Furthermore, hyperglycemia leads to an overproduction and accumulation of advanced glycation products (AGEs) in articular cartilage 53. In this context, Steenvoorden et al showed that higher levels of AGEs induced degradation of cartilage and a release of cartilage fragments 35. The observation that accumulation of AGEs in cartilage results in inferior mechanical properties 54,55 may explain the findings in our study as our diabetics showed a more inhomogeneous and disordered cartilage composition, which is likely an indicator for the disrupted architecture and suggests decreased biomechanical properties.
We acknowledge that our study has several limitations. Firstly, the definition of DM is based on a self-administered questionnaire, provided by the OAI database, with no data available to specify the type of DM. However, in adults, type 2 DM accounts for about 90% of all diagnosed cases of DM 56. Therefore, the overall majority of our cases have T2DM. Secondly, no information on the age at diagnosis and duration of DM among our diabetics is available. However, we ensured that all of our diabetics maintained their diabetic status for at least 4 years after the time point of enrollment and we also ensured that controls were free of self-reported DM over the entire follow-up period. Thirdly, we are aware of the fact that the initial antidiabetic therapy might change during the study. Further investigation will be necessary to assess how alterations in the baseline antidiabetic treatment algorithm might affect the outcome of OA in diabetics. Fourthly, changes as seen in MR-based T2 relaxation time measurements are dominated by the anisotropic motion of water molecules and the orientation of the collagen fibers in the extracellular matrix. However, quantitative T2 imaging is not a specific intrinsic imaging biomarker to assess glycosaminoglycan concentration and other techniques such as gagCEST and sodium MRI may be better suited for this purpose. Finally, a relatively short time interval of 2 years was chosen for this study to better monitor early and potentially accelerated degenerative disease in our diabetic cohort versus the controls, who both had no or only mild OA (KL0–2). A previous study has shown that T2 values have limitations in measuring cartilage loss in advanced disease with significant cartilage loss 24, a longer time interval could have potentially resulted in more significant cartilage loss in the diabetic cohort, where T2 values would not have been useful any more to measure the cartilage matrix.
In conclusion, our study shows that DM is associated with an accelerated degeneration of the cartilage matrix as demonstrated by a faster rate of mean T2 increase in the articular cartilage of the knee. Furthermore, diabetics exhibited both a faster increase of mean T2 values and a more heterogeneous cartilage T2 texture composition at both time points, suggesting an increasingly disrupted collagen architecture.
Acknowledgments
We would like to thank the participants and staff of the Coordinating Center of the OAI for their invaluable assistance with patient selection, statistical analysis, and technical support.
Role of Funding Source:
The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the Osteoarthritis Initiative Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Jan Neumann, M.D. (Jan.Neumann@ucsf.edu) and Thomas M. Link, Ph.D., M.D. (Thomas.Link@ucsf.edu) take responsibility for the integrity of the work as a whole, from inception to finished article.
Conception and design of the study: Neumann, Hofmann, Heilmeier, Joseph, Nevitt, Lane, McCulloch, Link
Acquisition of data: Neumann, Hofmann, Heilmeier, Ashmeik, Tang, Gersing, Schwaiger, Link
Analysis and interpretation of data: Neumann, Hofmann, Heilmeier, Joseph, McCulloch, Nevitt, Link
Drafting of article or revising it critically for important intellectual content: Neumann, Hofmann, Heilmeier, Gersing, Joseph, McCulloch, Nevitt, Link
Final approval of the version of the article to be published: Neumann, Hofmann, Heilmeier, Ashmeik, Tang, Gersing, Schwaiger, Nevitt, Joseph, Lane, McCulloch, Link
Competing interest statement
None of the authors have any financial or other interests related to the manuscript submitted to Osteoarthritis and Cartilage that might constitute a potential conflict of interest.
References
- 1.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet Lond Engl. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. https://doi.org/10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. https://doi.org/10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS) Third. Rosemont, IL: 2014. [Accessed on December 8, 2017]. Available at http://www.boneandjointburden.org. [Google Scholar]
- 4.Bay-Jensen AC, Hoegh-Madsen S, Dam E, Henriksen K, Sondergaard BC, Pastoureau P, et al. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol Int. 2010;30:435–42. doi: 10.1007/s00296-009-1183-1. https://doi.org/10.1007/s00296-009-1183-1. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. 1999
- 6.Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primer. 2017;3:17016. doi: 10.1038/nrdp.2017.16. https://doi.org/10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Incidence of Diagnosed Diabetes per 1,000 Population Aged 18–79 Years, by Age, United States, 1980–2014. 2016 [Google Scholar]
- 8.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121:9–20. doi: 10.3810/pgm.2009.11.2073. https://doi.org/10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 9.Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8:S383–91. [PubMed] [Google Scholar]
- 10.Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp J Intern Med. 2011;2:205–12. [PMC free article] [PubMed] [Google Scholar]
- 12.Arkkila PE, Gautier JF. Musculoskeletal disorders in diabetes mellitus: an update. Best Pr Res Clin Rheumatol. 2003;17:945–70. doi: 10.1016/j.berh.2003.11.001. https://doi.org/10.1016/j.berh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. https://doi.org/10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardin JA, Cobelli N, Santambrogio L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat Rev Rheumatol. 2015;11:521–9. doi: 10.1038/nrrheum.2015.70. https://doi.org/10.1038/nrrheum.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athanasiou KA, Fleischli JG, Bosma J, Laughlin TJ, Zhu CF, Agrawal CM, et al. Effects of diabetes mellitus on the biomechanical properties of human ankle cartilage. Clin Orthop Relat Res. 1999:182–9. [PubMed] [Google Scholar]
- 16.King KB, Findley TW, Williams AE, Bucknell AL. Veterans with diabetes receive arthroplasty more frequently and at a younger age. Clin Orthop Relat Res. 2013;471:3049–54. doi: 10.1007/s11999-013-3026-3. https://doi.org/10.1007/s11999-013-3026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oren TW, Botolin S, Williams A, Bucknell A, King KB. Arthroplasty in veterans: analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. J Rehabil Res Dev. 2011;48:1195–210. doi: 10.1682/jrrd.2010.09.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira RR, Lemos A, de Castro Silveira PV, da Silva RJ, de Moraes SR. Alterations of tendons in patients with diabetes mellitus: a systematic review. Diabet Med. 2011;28:886–95. doi: 10.1111/j.1464-5491.2010.03197.x. https://doi.org/10.1111/j.1464-5491.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- 19.Link TM, Neumann J, Li X. Prestructural cartilage assessment using MRI. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25554. https://doi.org/10.1002/jmri.25554. [DOI] [PubMed]
- 20.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74:1353–9. doi: 10.1136/annrheumdis-2013-204157. https://doi.org/10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:69–76. doi: 10.1016/j.joca.2012.09.011. https://doi.org/10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, et al. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:997–1005. doi: 10.1016/j.joca.2004.09.001. https://doi.org/10.1016/j.joca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Clin North Am. 2004;30:783–97. vii. doi: 10.1016/j.rdc.2004.07.005. https://doi.org/10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur. J Magn Reson Imaging JMRI. 2013;38:1415–24. doi: 10.1002/jmri.24137. https://doi.org/10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquardt D. An Algorithm for Least-Squares Estimation of Nonlinear Parameter. J Soc Ind Appl Math. 1963;11:431–41. [Google Scholar]
- 26.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63:181–93. doi: 10.1002/mrm.22178. https://doi.org/10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 27.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63:465–72. doi: 10.1002/mrm.22201. https://doi.org/10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. https://doi.org/10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–66. doi: 10.1148/radiology.214.1.r00ja15259. https://doi.org/10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 30.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. https://doi.org/10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kretzschmar M, Heilmeier U, Yu A, Joseph GB, Liu F, Solka M, et al. Longitudinal analysis of cartilage T2 relaxation times and joint degeneration in African American and Caucasian American women over an observation period of 6 years - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24:1384–91. doi: 10.1016/j.joca.2016.03.002. https://doi.org/10.1016/j.joca.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, et al. Is Weight Loss Associated with Less Progression of Changes in Knee Articular Cartilage among Obese and Overweight Patients as Assessed with MR Imaging over 48 Months? Data from the Osteoarthritis Initiative. Radiology. 2017:161005. doi: 10.1148/radiol.2017161005. https://doi.org/10.1148/radiol.2017161005. [DOI] [PMC free article] [PubMed]
- 33.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Müller-Höcker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline - data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2012;35:370–8. doi: 10.1002/jmri.22834. https://doi.org/10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–9. doi: 10.1016/j.joca.2011.04.002. https://doi.org/10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steenvoorden MMC, Huizinga TWJ, Verzijl N, Bank RA, Ronday HK, Luning HAF, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–63. doi: 10.1002/art.21523. https://doi.org/10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 36.Rosa SC, Rufino AT, Judas FM, Tenreiro CM, Lopes MC, Mendes AF. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J Cell Biochem. 2011;112:2813–24. doi: 10.1002/jcb.23196. https://doi.org/10.1002/jcb.23196. [DOI] [PubMed] [Google Scholar]
- 37.Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36:403–9. doi: 10.2337/dc12-0924. https://doi.org/10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vilá LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2013;19:1–6. doi: 10.1097/RHU.0b013e31827cd578. https://doi.org/10.1097/RHU.0b013e31827cd578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J. 2012;88:240–2. doi: 10.1136/pgmj.2010.146399rep. https://doi.org/10.1136/pgmj.2010.146399rep. [DOI] [PubMed] [Google Scholar]
- 40.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–68. doi: 10.1055/s-2004-861764. https://doi.org/10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 41.Nielen JTH, Emans PJ, Dagnelie PC, Boonen A, Lalmohamed A, de Boer A, et al. Severity of Diabetes Mellitus and Total Hip or Knee Replacement. Medicine (Baltimore) 2016:95. doi: 10.1097/MD.0000000000003739. https://doi.org/10.1097/MD.0000000000003739. [DOI] [PMC free article] [PubMed]
- 42.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 43.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing Diabetes Prevalence Between African Americans and Whites of Similar Socioeconomic Status. Am J Public Health. 2007;97:2260–7. doi: 10.2105/AJPH.2006.094482. https://doi.org/10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stürmer T, Brenner H, Brenner RE, Günther KP. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis.The Ulm osteoarthritis study. Scand J Rheumatol. 2001;30:169–71. doi: 10.1080/030097401300162969. [DOI] [PubMed] [Google Scholar]
- 45.Engström G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17:168–73. doi: 10.1016/j.joca.2008.07.003. https://doi.org/10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster J-Y, Bruyère O, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23:851–9. doi: 10.1016/j.joca.2015.01.013. https://doi.org/10.1016/j.joca.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J Rheumatol. 2011;38:921–30. doi: 10.3899/jrheum.100569. https://doi.org/10.3899/jrheum.100569. [DOI] [PubMed] [Google Scholar]
- 48.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang Y, Ashton-Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61:1328–36. doi: 10.1002/art.24739. https://doi.org/10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philbin EF, Ries MD, Groff GD, Sheesley KA, French TS, Pearson TA. Osteoarthritis as a determinant of an adverse coronary heart disease risk profile. J Cardiovasc Risk. 1996;3:529–33. [PubMed] [Google Scholar]
- 50.Rahman MM, Kopec JA, Cibere J, Goldsmith CH, Anis AH. The relationship between osteoarthritis and cardiovascular disease in a population health survey: a cross-sectional study. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2013-002624. https://doi.org/10.1136/bmjopen-2013-002624. [DOI] [PMC free article] [PubMed]
- 51.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res. 2012;64:248–55. doi: 10.1002/acr.20672. https://doi.org/10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB, et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: Data from the osteoarthritis initiative. J Magn Reson Imaging JMRI. 2017 doi: 10.1002/jmri.25766. https://doi.org/10.1002/jmri.25766. [DOI] [PMC free article] [PubMed]
- 53.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. https://doi.org/10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 54.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–23. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. https://doi.org/10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]; National Centre for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. Centers for Disease Control and Prevention. 2017. [Google Scholar]