Abstract
In the last decade, the advent of microfabrication and microfluidics and an increased interest in cellular mechanobiology have triggered the development of novel microfluidic-based platforms. They aim to incorporate the mechanical strain environment that acts upon tissues and in-vivo barriers of the human body. This article reviews those platforms, highlighting the different strains applied, and the actuation mechanisms and provides representative applications. A focus is placed on the skin and the lung barriers as examples, with a section that discusses the signaling pathways involved in the epithelium and the connective tissues.
I. INTRODUCTION
The recent emergence of organs-on-a-chip opens new opportunities in cell biology by uniquely reproducing key aspects of the in-vivo cellular microenvironment. One of these parameters is mechanical force, which imparts strain on cells and tissues and is an integral part of the environment that modulates the cellular phenotype. There is an extensive body of literature that describes the mechanisms whereby physical forces are transduced into biochemical signals that lead to responses at a single cell level.1–3 These responses, in turn, affect the function of multicellular systems (tissues), which is critical in health and disease. Physiological forces provide cues that superpose with biochemical signals that significantly impact morphogenesis4 during organ development, tissue homeostasis,5 and wound healing.6 Disease processes of fibrosis and cancer metastasis are also intimately linked to abnormal tissue mechanical properties.5,7,8
Mechanotransduction mechanisms operate through many of the familiar pathways that are involved in more “traditional” signaling triggered by biochemical factors. Yet, there have been few studies that examine the combined effects of physical and biochemical factors to ask questions about potential synergistic or antagonistic interactions. There is a need to fill this gap, as recent data have emerged suggesting that modulation of the mechanical environment can be purposely used to improve the wound healing response,9 either by promoting faster wound closure or by decreasing fibrosis.10 More generally, better characterization of the influence of mechanical forces is central to improving our understanding of pathophysiology and pathogenesis.11
Recent developments in the design of microphysiological “organ-on-a-chip” systems that recapitulate actual tissues on a small scale provide new opportunities for probing interactions between mechanical and biochemical signals. In this review, we describe the latest approaches used to recreate the in vivo biomechanical environment, in particular, mechanical strain, and how they can then be used to explore fundamental biological events, such as the wound healing response, as well as optimize potential drug screening tools. In contrast to the review by Schmitt et al.12 that focuses on the applicability of stretched systems for drug discovery, the present review focuses on microfluidic platforms able to create mechanical strain in in-vitro barriers. For the sake of conciseness, we focus primarily on the lung and skin systems as illustrative examples. After reviewing the types of microfluidic platforms reported and the associated type of mechanical strain, the last paragraph describes how the mechanical strain affects biological events via mechanotransduction signaling pathways.
II. SKIN AND LUNG: EXAMPLES OF IN-VIVO BARRIERS EXPOSED TO MECHANICAL STRAIN
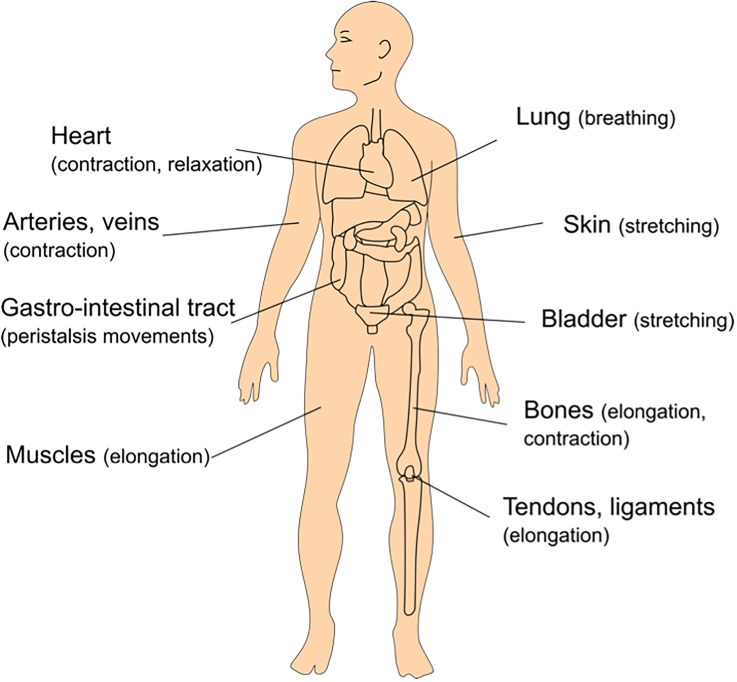
A number of tissues and in-vivo barriers in the human body are exposed to mechanical strain. Mechanically active tissues, such as the heart or the muscles, are stretched continuously during daily activities. They in turn generate strain in other organs, such as the lungs, the skin, the tendons, the bones, and others. In-vivo barriers are particularly susceptible to such forces by their specific location at the interface with the outer world. As examples, the skin is exposed to stretching, the lung air-blood barrier to breathing movements, the gastro-intestinal tract to peristalsis motion, and the urothelium to stretching due to hydrostatic pressure. Figure 1 illustrates some examples of mechanical strain taking place in the human body.
FIG. 1.
Examples of tissues exposed to mechanical strain in the human body.
The level of mechanical strain is very variable; it is a function of the tissue or barrier type and age, as well as other factors, such as the mechanical properties of the surrounding tissue [amount of extracellular matrix (ECM)]. It ranges between a few percent in the tendons and ligaments (2%–5%),13 and in the lung alveoli (4%–12%, see Sec. II B), and can reach up to tens of percent when muscles14 or skin is stretched (see Sec. II A). Pathological conditions can generate strain on tissues, for example, following hyperplasia,15 wound-healing, or tumor growth.
A. Skin
Skin consists of three major layers: the epidermis, the dermis, and the hypodermis. The epidermis consists of multiple layers of epidermal cells (keratinocytes) forming tight junctions among themselves. At the dermal-epidermal junction, the first layer of keratinocytes functions as stem cells that self-renew and continuously generate new keratinocytes. The latter are pushed towards the skin surface and, in this process, undergo terminal differentiation into a cornified layer (the stratum corneum). This stratified organization underlies the main function of the epidermis, which is to form a barrier. Underneath is the dermis, which consists of a dense extracellular matrix network that harbors fibroblasts, blood vessels, and skin appendages (such as sweat glands, nerves, etc.). From a physical standpoint, the main function of the dermis is to provide mechanical integrity to the skin. In normal non-injured skin, the majority of compressive and tensile forces imparted onto the skin are borne by the extracellular matrix network in the skin dermis, with little force directly applied onto the resident fibroblasts or other structures.16 The hypodermis mainly consists of subcutaneous fat and serves as a shock absorber.
The mechanical stiffness of normal human dermis varies depending on the body location and depth inside the skin and has been reported to range between 0.1 and 10 kPa.17 Furthermore, the dermis is mechanically anisotropic due to preferential orientation of the collagen fibers along “Langer's lines,” topographical skin lines, which are generally parallel to the orientation of the underlying muscle fibers, and therefore the direction of stretch during joint movement. For example, Maiti et al. showed that forearm extension causes a tensile strain of 20 to 30% in the forearm skin18 but results in a relatively minimal level of stress. When skin is damaged deep enough to disrupt the underlying extracellular matrix, the wound fills with a fibrin clot with a stiffness between 0.01 and 1 kPa; because the force-bearing structure of the ECM is disrupted, part of the mechanical load is shifted onto the cellular network.16 Migrating fibroblasts form a network of differentiated myofibroblasts that promote wound contraction. These cells also secrete and restore the ECM. As this occurs, the load is shifted from the cellular network back to the ECM and hemostasis is restored. The resulting healed wound is however not identical to uninjured skin, as it consists of a scar which typically exhibits 10× more stiffness than normal skin.19
The local mechanical environment plays a critical role in whether healing results in a normal functioning scar or abnormal fibrotic scar (also known as hypertrophic scar and keloid). It is a well-known clinical observation that areas where skin is often under tension are more prone to develop a pathological process characterized by excessive fibrosis and wound contraction, which eventually limits joint movement.20 To minimize scarring, surgical incisions are usually performed along Langer's lines so as to minimally disturb the natural mechanical load distribution. Wounds that cut across those lines are subjected to greater mechanical tension and are more likely to result in excessive scarring.
Externally applied mechanical forces have been used as a therapeutic tool to improve the wound healing response. For example, recent studies have shown decreased hypertrophic scar formation when devices as simple as tape or silicone gel sheeting are applied over the wound area because they divert some of the mechanical load away from the fibroblast.21,22 Silicone balloon expanders have been used for decades to promote skin regeneration in cases where not enough skin is available before plastic and reconstructive procedures.23 In this case, the application of tensile force promotes the formation of new tissues. In cases of impaired wound healing (such as in diabetes), a popular method to enhance the healing response is the application of vacuum over the wound site (in a procedure called vacuum-assisted wound closure or negative pressure wound therapy). Although the mechanism of action is likely multifaceted, there are strong suggestions that mechanical deformation and stretching play an important role.24
The general consensus is that in skin, mechanical tension promotes tissue formation. As will be discussed later (see Sec. IV), epidermal keratinocytes and dermal fibroblasts can sense these mechanical effects, which, together with soluble and immobilized signal cues, are responsible for the observed wound healing response. Mechanically competent skin-on-a-chip platforms make it possible to investigate these interactions and provide a controlled environment to better understand the conditions that provide better wound healing responses.
B. Lung
The human lung is an organ with a complex architecture that comprises two tree-like structures, the airway and the vascular trees. The airways divide into a succession of 23 generations of airways that terminate with tiny alveoli, where the gas exchange between air and blood takes place. The arterial pulmonary vasculature25 splits into smaller vessels that end in a large network of capillaries enrobing the alveoli. Oxygen diffuses through the ultra-thin alveolar barrier and is taken up by red blood cells, while carbon dioxide is simultaneously released. The alveolar epithelium consists of type I and type II lung alveolar epithelial cells. Type I epithelial cells are squamous and constitute 95% of the lung alveolar surface area. These ultra-thin and broad cells facilitate the rapid diffusion of oxygen and carbon dioxide26 from the air to the capillaries and vice-versa. In contrast, type II alveolar epithelial cells are small and cuboidal and only make up a small portion of the alveolar surface. They secrete a lipoprotein, called surfactant, that reduces the surface tension of the alveoli and prevents atelectasis, the collapse of the lung alveoli. The lung alveolar epithelial barrier results from tight junctions created by intercellular proteins, mostly from the claudins27 and occludens families.28 Epithelial cells are polarized with the apical side in contact with air and the basal side in contact with the ultra-thin basement membrane made of collagen and elastin. It is sandwiched between the epithelium and the blood capillaries. The endothelial barrier shows nearly identical intercellular junctions like the epithelial counterpart and controls the exchange of fluid and solutes with the surrounding tissue.29 Adjacent alveoli are separated by the alveolar septum that consists of the basement membrane and of the connective tissue. Immune cells, mostly macrophages and dendritic cells, are located in this environment in close proximity to the air-blood barrier. They communicate with epithelial cells to respond to infection, epithelial barrier damage, and pathogen clearance.30
In addition to these physiological aspects, this complex environment is continuously exposed to a number of physical forces that are transmitted across length scales, from the organ-to-the microscale level.31 These forces include the mechanical strain induced by breathing motions, the shear stress created by the blood, and interstitial flow and surface tension, in particular, in the alveoli.32
At rest, a healthy human lung typically inflates at a respiratory rate of 10–12 breaths per minute. The question on how the alveoli deforms—like a pre-stressed balloon or like an accordion-like structure33—and thus whether the volume fluctuation translates to a stretching of the epithelial cells is still a matter of debate. Roan and Waters suggest that the alveoli expansion results from a combination of mechanisms involving isotropic expansion and shape change.34 Using optical sectioning microscopy, Perlman and Bhattacharya observed non-uniform alveoli expansion in rat lungs, with type I alveolar epithelial cells being stretched more than type II cells.35 During normal breathing, the basement membrane is distended to about 4% linear strain on average. During a deep inspiration, the distal tissues undergo a larger deformation of up to 12% linear strain.3 The distension level of the lung alveoli strongly depends on the mechanical properties of the lower airways. The stiffness of a healthy human lung is about 2 kPa. In diseases, such as lung fibrosis, the tissue stiffens due to a pathological accumulation of ECM proteins secreted by epithelial cells and fibroblasts. This results in stiffer fibrotic tissues, typically with stiffness around 16 kPa.36 This leads to an increase in airway resistance to inflation37 and thus to a decrease in the mechanical strain in the parenchymal area. The lungs can also be exposed to larger pathophysiological strains (>20% linear strain) during positive mechanical ventilation.38 The lung of patients suffering from acute respiratory distress syndrome (ARDS) can be partly obstructed, which causes overdistension of the lung. This has been reported to augment the disruption of the lung alveolar epithelial barrier leading to infiltration and even to fibrosis.39
Our knowledge on the impact of the mechanical strain on lung cells has increased importantly in the last two decades.3,12 However, relatively little has been done to assess the effects of mechanical forces on the pathophysiology of parenchymal lung diseases.40 In lung fibrosis, mechanical forces are known to affect the development and the progression of the disease,41 but recent findings regarding the effects of mechanical cues42,43 suggest that much remains to be done to understand and find therapeutic options for this illness. The advent of organs-on-a-chip technologies that make it possible for the first time to mimic mechanical strain on in-vitro barriers may help elucidate mechanistic pathways involved in such diseases (see Sec. III B).
III. MICROFLUIDIC PLATFORMS AIMED AT REPRODUCING THE IN-VIVO MECHANICAL STRAIN
In-vitro assays have been used early on to reproduce the mechanical strain that takes place in various tissues. Many research groups either produced their own stretching platform44 or used the Flexcell system (Flexcell International Corp.) that has been commercially available for over 30 years. With the advent of microfluidics and microfabricated systems and renewed interest in cellular mechanobiology, a number of microfluidic platforms have been reported to reproduce various types of mechanical strains. The introduction of poly-dimethylsiloxane (PDMS) soft lithography45 enabled the development of cost-efficient microsystems, with arrays of microwells using less cells and suitable for parallel experimentations. Furthermore, in addition to the mechanical strain, other aspects of the cellular environment were integrated in these platforms, such as shear stress (perfusion) or more recently in-vitro barriers with flexible and porous membranes.
In this section, we review two categories of microfluidic platforms aimed at mimicking the mechanical strain (Table I). The first involves devices equipped with microfabricated membranes, on which cells are cultured and mechanically stretched. The second group summarizes microfluidic devices that in addition to the mechanical strain integrate an ultra-thin, porous, and elastic membrane used as an in-vitro barrier. This sorting was chosen, as the potential of the platforms from the second category is much greater as discussed later (see Sec. III B). The type of mechanical strain is another important aspect that will be discussed in this section, as the diversity of the devices and of strain applied makes cross-comparison between studies difficult.
TABLE I.
Microfluidic platforms aimed at reproducing mechanical strain in-vitro. The first category summarizes devices aimed at stretching cells cultured on a substrate, while the second category enables emulating in-vivo barriers, based on a thin, elastic, and porous membrane.
| Device type | Type of strain | Cell culture support | Actuation | Applications | Year | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Direction | Level (in %) | Frequency | Membrane | Array | Coating | |||||
| Non barrier | Uni-axial | 10% (linear strain) | Cyclic (1 Hz) | 0.5–1 mm thick PDMS substrate | 2 | Gelatin | A precision linear motor applies cyclic stretch to the PDMS device. | Differentiation of murine embryonic stem cells in cardiomyocytes upon cyclic strain. | 2011 | Wan et al. (46) |
| 4% (linear strain) | Cyclic (0.5 Hz, 10 cycles) | Thin PDMS membrane with 10 μm grooves | 1 | ProNectin F | Two linear actuators operated with a syringe pump. | Ca2+ signaling of tenocytes in response to cyclic strain. | 2013 | Wall et al.62 | ||
| 3–7% (linear strain) | Cyclic (2 Hz) | 100 μm thick PDMS membrane | 1 | Fibronectin | Stretching by actuation of thin walls connected to adjacent channels with cyclic vacuum. | Provide mechanical, electrical, and biochemical stimulation to mesenchymal stem cells. | 2015 | Pavesi et al.86 | ||
| Bi-axial (xy) | 0 up to 60% (surface strain) | Cyclic (1.3 Hz) | 100 μm to 330 μm thick PDMS membrane | 1 | Fibronectin | Combination of hydrodynamic pressure & mechanical pressure with a post. | Hemodynamic stimulation of cardiomyocytes | 2010 | Giridharan et al.55 | |
| Bi-axial (xz) | 2–20% (circumferential strain) | Cyclic (1 Hz) | 35 μm thin PDMS membrane | 5 | Collagen, fibronectin, gelatin | Hydrodynamic actuation (microfluidic channel filled with liquid). | Mimic the circumferential strain to which small blood vessels are exposed | 2012 | Zhou et al.47 | |
| Equi-bi-axial (xy) | 2–15% (circumferential & radial strain) | Cyclic (1 Hz) | 15 μm thin PDMS membrane | 9 × 12 | Collagen | Positive pressure created by flat posts pushed against the culturing membrane. | Activation of the canonical Wnt/b-catenin signaling pathway in cardiac valve mesenchymal progenitor cells | 2010 | Moraes et al.50 | |
| 1, 2, 4, 6% (linear strain) | Cyclic (1 Hz) | 150 μm thick PDMS membrane | 5 × 5 | Fibronectin | The PDMS membrane is stretched with a vacuum around cylindrical, flat micropillars. | Strain of C2C12 skeletal myoblasts. | 2012 | Simmons et al.53 | ||
| Tri-axial | 17–20% (surface strain) | Cyclic (0.2, 1, 5 Hz) | 100 μm thick PDMS membrane | 3 × 8 | Fibronectin | Mechanical movements of small pins that deflect the membrane (Braille display). | Strain of human dermal microvascular endothelial cells | 2008 | Kamotani et al.48 | |
| 15–50% (linear strain) | Cyclic (0.2–0.3 Hz) | 100 μm thick PDMS membrane | 1 | Fibronectin | Fluidic pressure created by a syringe pump (negative pressure). | Combined effects of fluid and solid mechanical stress on alveolar cells (mimic pathophysiology of ventilator induced lung injury). | 2011 | Douville et al.54 | ||
| 3 and 12% (circumferential & radial strain) | Cyclic (1 Hz) | 45 μm and 100 μm thin membranes made of PDMS with PU coating | 12 × 9 | Collagen or fibronectin | Pneumatic positive pressure (microfluidic channel filled with air). | Investigation of mechanobiological response profiles of valvular interstitial cells. | 2013 | Moraes et al.49 | ||
| 6% (linear strain) | Cyclic (1 Hz) | 10 μm thin PDMS membrane | 1 | Collagen | Pneumatic negative pressure created below the thin membrane. | Study of cellular (MSC) responses to cyclical hypoxia and stretch. | 2016 | Campillo et al.56 | ||
| 2.2–3.5% (linear strain) | Cyclic (0.33 Hz) | 130 μm thick PDMS membrane | 5 × 6 | None | Pneumatic negative pressure (vacuum created in microchannels). | Effect of mechanical strain on proliferation and differentiation of mesenchymal stem cells. | 2014 | Gao et al.52 | ||
| 12–20% (circumferential & radial strain) | Cyclic (1 Hz) | 35, 55, 75 μm thin membrane in PDMS | 32 | Fibronectin | Pneumatic positive pressure to deflect the PDMS membrane. | Investigation on effect of cyclic stretch on membrane permeability of both healthy and dystrophic myotube. | 2015 | Michielin et al.51 | ||
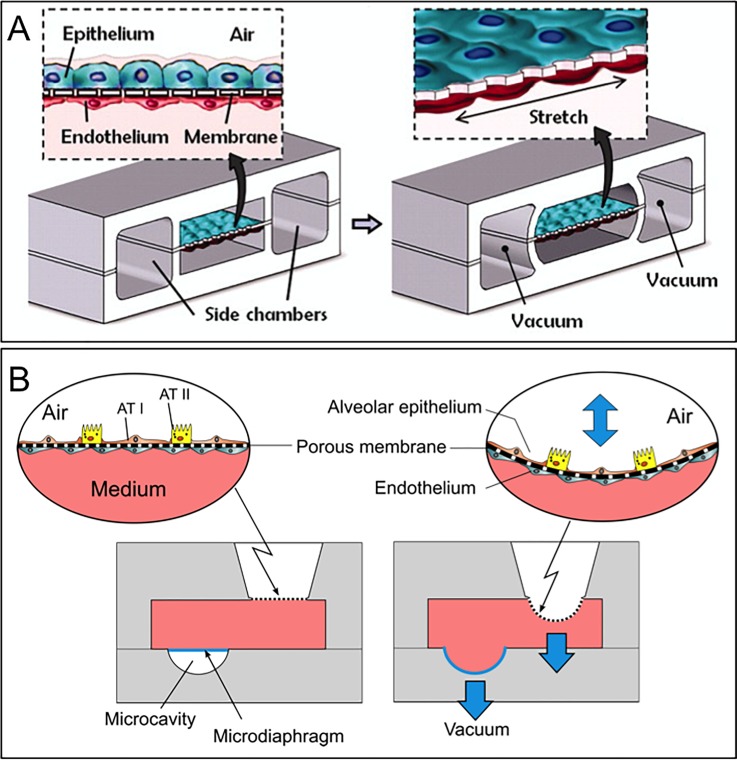
| In-vitro barrier | Uniaxial | 5–15% (linear strain) | Cyclic (0.2 Hz) | 10 μm thin, PDMS membrane with 10 μm wide pentagonal pores | 1 | Fibronectin or collagen | Stretching by actuation of thin walls connected to adjacent channels with cyclic vacuum [Fig. 2(a)]. | Lung-on-a-Chip: Mimic the lung alveolar barrier and investigate the effects of the mechanical strain on toxic and inflammatory response. | 2010 | Huh et al.58 |
| Tri-axial | 21% (surface strain) | Cyclic (0.2 Hz) | 3 μm thin, PDMS membrane with 3 or 8 μm pores | 3 | Fibronectin | Stretching by indirect actuation using a bio-inspired microdiaphragm [see Fig. 2(b)]. | Lung-on-a-Chip: Mimic the lung alveolar barrier and investigate the effects of the mechanical strain on primary lung alveolar cells. | 2015 | Stucki et al.59 | |
A. Stretching platforms without a porous membrane
A number of microfluidic-based platforms aimed at reproducing mechanical strain either in one, two, or three directions were reported in the last decade (Table I). The versatility of the technologies used to design and produce these systems makes it possible to easily create more complex structures, such as the coupling of a microfluidic channel with a stretchable membrane. An additional advantage of those systems is the ability to miniaturize the size of the culture well and to simultaneously create an array of such wells in order to increase the number of biological experiments. Arrays from a few parallel wells46,47 of up to tens of microwells48–51 were reported. The technologies used to produce these devices can be scaled up in view to integrate these systems in drug screening programs. As the number of wells increases, the stretching actuation needs to be kept as simple as possible to make the handling of such systems by non-experts as easy as possible. Takayama and colleagues were the first to report about a microfluidic stretching platform.48 They used an array of individually addressable pins to push upon a culturing elastic membrane to create a mechanical strain. Moraes et al. employed the same technique to generate a uniform equi-bi-dimensional strain using flat pillars.50 Pneumatic49,51–53 or hydrodynamic47,54 actuations are other popular techniques to create mechanical strain. Their simplicity allows filling a channel either with a gas or a liquid to inflate (positive pressure) or deflect (negative pressure) a thin membrane, on which cells are cultured. The culturing membranes were all based on PDMS, a material that among others exhibits great mechanical properties (its Young's modulus is typically around 1 MPa) and that can easily be deformed. To make the PDMS less oxygen permeable and reduce the absorption and adsorption of small molecules, PDMS was dip-coated with a thin layer of polyurethane to improve cell adhesion.49 Biological questions that have been investigated with these technologies cover a wide range: effect of the mechanical strain on the differentiation of embryonic stem cells; stimulation of cardiomyocytes46 or other cardiac cells;55 mimicking the rhythmic beating of small blood vessels;47 and emulating the breathing motions of the lung.54,56 Recently, based on the lung-on-a-chip system reported by Huh and colleagues,58 Kamm's group reported a system able to provide mechanical, biochemical, and electrical stimulation to both guide and monitor the morphological and genetic modifications of human bone marrow mesenchymal stem cells.86
B. Stretching platforms with a porous membrane
In sharp contrast to the devices presented above, platforms equipped with a porous membrane make it possible to evaluate transport across the cultured cell layers, thus more closely mimicking in-vivo barriers. Cells can be cultured on both sides of the membrane, through which they can touch each other and/or communicate via paracrine and endocrine signaling. Furthermore, those systems enable reproduction of the air-liquid interface by providing nutrients to the cells cultured on the apical side via the porous membrane. This is a key feature that is needed to promote epithelial differentiation in skin and lung systems. Furthermore, the cells on one or both sides of the porous membrane can be exposed to shear stress by perfusing cell culture medium, blood, or other biological solutions.
Micro-engineered systems with an integrated membrane in a microfluidic setting have been reported to model various in-vivo barriers, such as those of the lung alveoli, the brain, and the gut, to name a few.57 By implementing a flexible membrane in the microfluidic system, mechanical forces, such as those induced by the breathing motions, could be reproduced.58,59 Huh and colleagues reported an innovative microsystem made of a central chamber, itself divided by a thin, elastic, and porous membrane [Fig. 2(a)]. Lung epithelial cells and lung endothelial cells were cultured on both sides of the membrane to recreate the air-blood barrier at the air-liquid interface. The membrane can be stretched unidirectionally via the action by providing a vacuum in two adjacent chambers. The system was used to investigate the inflammatory response on lung epi- and endothelium upon exposure to nanoparticles and more recently to recreate drug toxicity-induced pulmonary edema.60 The system was further used to mimic the peristalsis movement of the gastro-intestinal barrier.61
FIG. 2.
Lung-on-chips with a thin, elastic, and porous membrane used to culture cells on both sides of the membrane. Type I (ATI) and type II (ATII) lung alveolar epithelial cells are cultured on the apical side of the membrane, whereas endothelial cells are cultured on its basolateral side. (a) 10 μm thin, membrane stretched with a uniaxial strain. Reproduced with permission from Huh et al., Science 328(5986), 1662–1668 (2010). Copyright 2010 American Association for the Advancement of Science. (b) The membrane is stretched with a three-dimensional strain induced by a microdiaphragm located at the bottom of the basolateral chamber.59
A second lung-on-a-chip reproducing an array of three thin, stretchable, alveolar barriers was recently described by our group58 [Fig. 2(b)]. In contrast to the lung-on-a-chip reported by Huh and colleagues, the mechanical strain created is a three-dimensional strain, such as that taking place in-vivo. The breathing movements of the alveolar barrier are generated by applying a very small cyclic pressure in the medium located on the basolateral side of the alveolar membrane. The pressure variation is generated by the inflation and deflation of an actuation membrane (called micro-diaphragm) located in this small compartment and connected to an external electro-pneumatic generator. The alveolar membrane is made of an ultra-thin (3 μm thin) polymeric membrane with an array of pores (either 3 or 8 μm in diameter) on which cells are cultured. The results obtained with the device demonstrated that the strain influences the metabolic activity and the cytokine secretion of primary human pulmonary alveolar epithelial cells obtained from patients.
C. Types of mechanical strain and their effects
The types of mechanical strain reproduced with the microfluidic platforms reported above cover a wide range: unidirectional, bi-directional (either in xy or in xz directions), and three-dimensional strain. It is either quantified in terms of linear (elongation) or surface deformation or in strain components: circumferential or radial. Most devices have been used to generate a cyclic mechanical strain of relatively small amplitude at a frequency comprised between 0.2 and 2 Hz. Some platforms produced a uniform and others a non-uniform strain (strain gradient). This large variety of strains makes cross-comparisons between experimental results from different laboratories very difficult. Several attempts to compare62 or to correlate34 the strains were made. Assuming an isotropic deformation, a uni-axial strain, εLIN, (linear elongation) can be correlated with a bi-axial strain, εSA, (surface expansion) using the following equation:34
| (1) |
with and , L0 and Lf being the length before and after elongation and SA0 and SAf, the surface area before and after expansion. Table II gives a numerical example of the correlation between these strains based on a 10% linear strain. In the cases of a two-dimensional deformation in x and z and of a three-dimensional deformation, the deflection variation, Δz, is negligible over the length scale of a cell (about 20 μm) compared to the typical dimensions (from 500 μm to several mm) of the substrate diameter. The surface expansion is thus similar to that taking place in a uni-axial and a bi-axial strain device, respectively.
TABLE II.
Types of mechanical strain generated with microfluidic-based stretching platforms. Cross-comparison between types of strain is often difficult. As an example, a 10% linear strain is correlated for each strain type with its associated surface strain.
| Type of mechanical strain (illustration of a stretched cell) | Type of mechanical strain | Linear strain (num. example) | Surface area strain |
|---|---|---|---|
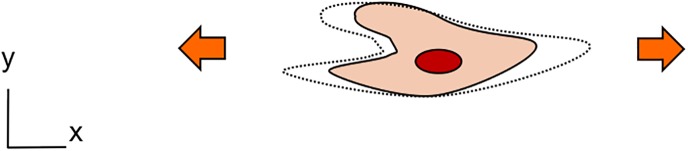 |
Uniaxial strain (x) | 10% | 10% |
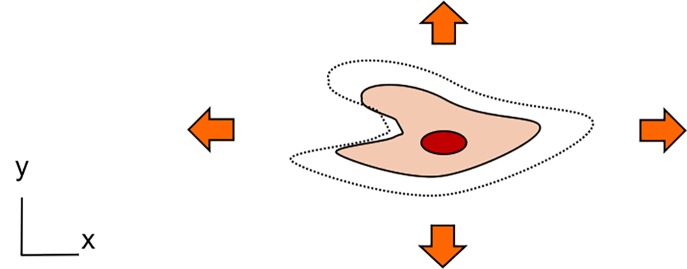 |
Bi-axial strain (xy) | 10% | 21% |
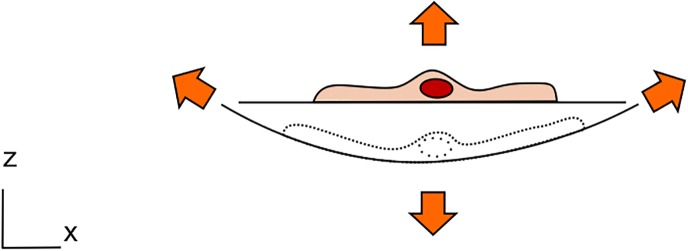 |
Bi-axial strain (xz) | 10% | ≈10% |
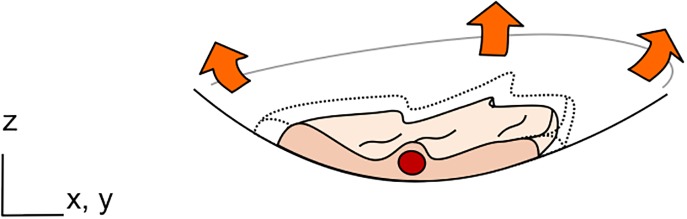 |
Tri-axial strain (xyz) | 10% | ≈21% |
The various types of mechanical strain add to the complexity of the heterogeneous biological experimental procedures (types of cells, primary cells or cell lines, physiological medium, etc.). The first question to be answered is: Do all types of strain have the same effects on the cells? The second one is: How accurately does the in-vivo mechanical strain need to be reproduced in-vitro to trigger an in-vivo-like mechanoresponse? No straightforward and definite answers are yet provided to those questions.
Only a few studies cross-compared the effects of various types of mechanical strain. The cells respond to strain by morphological, biochemical, and genetic changes. The cells “feel” mechanical forces via their focal adhesion that transmit it to the cytoskeleton. As a result, the cytoskeleton remodels, which alters cellular morphology. The strain profile affects differently the cell's response. In airway smooth muscle cells, a uniaxial strain, but not a biaxial strain is a procontractile and proliferative stimulus.63 In vascular smooth muscle cells, a uniaxial, but not an equiaxial strain induces a transient increase in collagen I expression.64 Berry and colleagues reported about alterations in cellular alignment and of metabolic activity on adult and neonatal human dermal fibroblasts.65 The young fibroblasts showed greater cell proliferation and collagen production than adult dermal fibroblasts under unstrained conditions. More recently, Gould and colleagues investigated the effect of increasing anisotropy of biaxial strain on aortic valve interstitial fibroblasts.66 Cyclic biaxial strain in contrast to equiaxial strain induced both fibroblast proliferation and apoptosis and resulted in ECM reorganization. They suggested that cyclic equiaxial strain promotes a tendency towards a quiescent fibroblastic phenotype, and increasing strain anisotropy supports a tendency towards an active myofibroblastic differentiation. These results suggest that the type of mechanical strain importantly affects cells and should be carefully considered during the experimental design.
IV. MECHANOTRANSDUCTION SIGNALING PATHWAYS INVOLVED IN MECHANICAL STRAIN
A. Epithelia
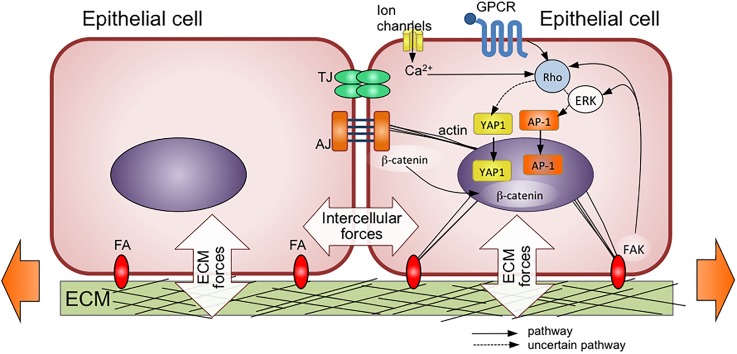
Several mechanosensitive pathways have been identified, which are summarized in Fig. 3. Some of the main inputs for mechanosignals include ion channels, G-protein coupled receptors, cell-ECM focal adhesions, and intercellular adherens junctions.67 Most of these pathways converge towards the cytoskeleton as a mediator of mechanical signals, although some bypass it altogether. Collectively, these inputs lead to the activation of three major transcription factors, namely, YAP, AP-1, and β-catenin.
FIG. 3.
Forces transmitted via cell-cell and cell-extracellular matrix (ECM) attachments to epithelial cells during mechanical stretching. TJ = tight junction, AJ = adherens junction, FA = focal adhesion complex. Mechanical forces then activate several pathways that converge towards the activation of the transcription factors YAP1, AP-1, and β-catenin. Some of these pathways, such as GPCR-mediated activation of Rho signaling, are also activated by biochemical signals. Ultimately, mechanical and biochemical signals interact to lead to the observed physiological response. Dotted lines refer to pathways that are not entirely elucidated. Adapted with permission from Wang et al., Cell Mol. Life Sci. 72, 2091–2106 (2015). Copyright 2015 Springer Nature.
YAP (Yes-associated protein) and its associated co-activator TAZ (transcriptional co-activator with PDZ-binding motif, also known as WWTR1) have received a great deal of attention due to their ability to regulate organ size and sense cell crowding.68,69 After tissue wounding, the spatial void allows cell spreading to occur, and epithelial cell spreading promotes nuclear translocation of YAP and its associated co-activator TAZ.70 In the particular case of the skin epidermis, YAP-mediated activation of transcription promotes the proliferation of keratinocytes as well as epidermal progenitors, while inhibiting terminal differentiation.70,71 Restoration of normal cell density and packing after reconstitution of the epithelial lining downregulate YAP signaling and enable return to homeostasis.
The AP-1 family of transcription factors is known to play a role in various skin diseases as well as during wound healing.72 AP-1 has been reported to be upregulated in stretched skin; however, the mechanism of activation is not yet understood. Another mechanosensitive pathway involves β-catenin, which is bound to the cytoplasmic domain of the intercellular adhesion molecule E-cadherin. β-catenin exhibits an armadillo repeat region that undergoes conformational changes upon mechanical stretching.73 Upon activation, β-catenin accumulates in the nucleus and causes transcription of target genes of the Wnt pathway, which promote cell proliferation and migration, both of which are inherently involved with the wound healing response.74
B. Connective tissue
Epithelial barriers are generally supported by underlying connective tissue rich in ECM, blood vessels, and fibroblasts. In the case of skin, the dermis is profoundly affected by mechanical forces, especially after injury during wound repair. In the case of the lung, few fibroblasts are present under normal healthy conditions, but their prevalence increases dramatically during fibrosis.
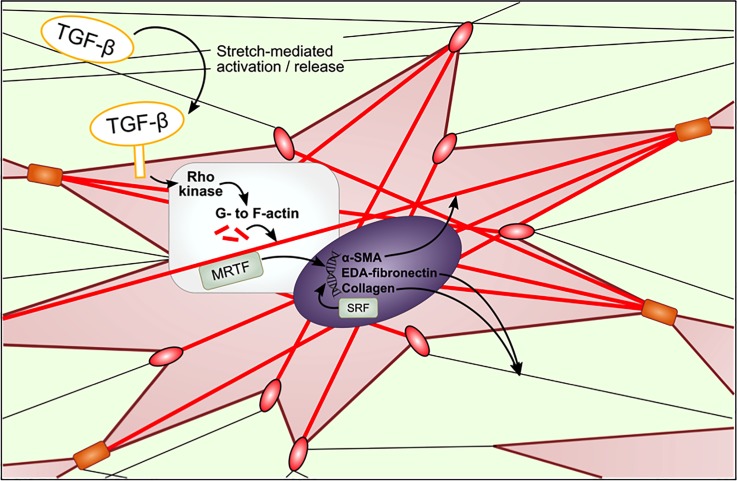
The Van de Water group summarized the mechano-stimulated pathways in fibroblasts, and their interaction with TGF-beta, the main pro-fibrogenic growth factor.75 Individual fibroblasts are part of a network of ECM comprising mainly of collagen but also fibronectin and other proteins. Transmembrane focal adhesions consisting of integrin clusters link the ECM network to the intracellular cytoskeleton. Fibroblasts also form intercellular adhesions with other fibroblasts via cadherins, from which also originate cytoskeleton fibers (Fig. 4).
FIG. 4.
Mechanotransduction pathways in fibroblasts embedded in ECM. Fibroblasts interact with ECM through focal adhesion complexes (red ovals) and other fibroblasts via adherens junctions (orange rectangle). Red lines represent cytoskeletal stress fibers, which are initially made of F-actin, but incorporate alpha-smooth muscle actin (α-SMA) as fibroblasts differentiate into myofibroblasts. The pathways shown here are discussed in the text in more detail.
Wong et al. distinguishes two types of mechanotransduction: extracellular and intracellular.76 In the former case, mechanical forces alter the way the ECM “presents” bioactive groups towards cells, by exposing hidden domains and/or physically altering their spatial density. Furthermore, a mechanism whereby surface-bound TGF-β can be released into solution by subjecting the ECM to stretch has been described.77 TGF-β signaling promotes the formation of F-actin from unpolymerized G-actin. In this intracellular process, the myocardin-related transcription factor (MRTF) is released from G-actin, which then enters the nucleus and joins with the constitutively expressed serum response factor (SRF), to trigger the expression of alpha-smooth muscle actin (α-SMA), EDA-fibronectin, and collagen.75 The appearance of α-SMA, which combines with the actin stress fibers, signals the differentiation of fibroblasts into myofibroblasts with enhanced contractile force. EDA-fibronectin, a splice variant of fibronectin specifically expressed after injury and in fibrotic disorders, enhances pro-fibrotic effects of TGF-β. Mechanical tension, via focal adhesions, also activates F-actin polymerization, and synergizes with TGF-β signaling intracellularly. Although not shown in the figure above, a recent report suggests that MRTF is a key player in the activation of TAZ in the YAP/TAZ pathway in fibroblasts.78 Activation of YAP/TAZ promotes the expression of TGF-β and the connective tissue growth factor (CTGF), which favor ECM deposition and crosslinking.79
Mechanical stress also activates YAP/TAZ, highlighting its role as a master regulator of mechanotransduction.79 NIH/3T3 cells exhibit increased YAP/TAZ activation when cultured in stiff ECM, but not soft ECM, which promotes pro-fibrotic pathways of ECM production and contraction.80 YAP/TAZ are highly expressed in fibrotic lesions but not normal healthy areas of the lung.81 An interplay between TGF-β and YAP/TAZ has also been implicated in pro-fibrotic pathways underlying diseases in lung82 as well as in other tissues.83 Fibrotic lesions, through activation of YAP/TAZ, may also promote epithelial to mesenchymal transition, as well as loss of contact inhibition, which are associated with carcinogenesis.68
V. CONCLUSION
The advent of microfluidics and the renewed interest in mechanobiology have inspired researchers to develop new types of microfluidic devices able to reproduce in-vitro the in vivo mechanical strain. Results from the literature suggest that the strain type (1-D, 2-D, or 3-D), magnitude, and frequency can greatly influence the morphology and the metabolism of cells. Therefore, the strain parameters should be given as much attention as the choice of culture medium, hormone and growth factor supplements, or any other experimental parameters of the cell culture.
From a technological point of view, more advanced microfluidic-based platforms incorporating mechanical strain will very likely be developed in the years to come. They will combine strain, shear forces, stiffness, and additional features in a high throughput format. Furthermore, sensors can be integrated in the devices to monitor the mechanical strain,84 or extract biochemical information from the system,85 and actuators can electrically and/or mechanically stimulate cells.86
The ability to implement in-vivo-like strain in cell culture environments will enable a precise and thorough investigation of the mechanobiology of tissues exposed to strain. Besides understanding the effect of mechanical forces on cells, such studies will also provide new information on how mechanical and biochemical signals interact within signal transduction pathways to elicit cellular responses. This may open the way to a new discipline, mechanopharmacology, which will target specific pathways associated with mechanical strain.87
ACKNOWLEDGMENTS
F. Berthiaume is a recipient of a Collaborative Research Travel Grant from the Burroughs Wellcome Fund (#1016230). O. Guenat is founder and one of the shareholders of the start-up AlveoliX that aims at bringing organs-on-a-chip on the market. Anne Morbach is kindly acknowledged for the illustration of Fig. 1.
References
- 1. Liu M. Y., Tanswell A. K., and Post M., “Mechanical force-induced signal transduction in lung cells,” Am. J. Physiol. Lung Cell Mol. Physiol. 277, L667–L683 (1999). 10.1152/ajplung.1999.277.4.L667 [DOI] [PubMed] [Google Scholar]
- 2. Mammoto T., Mammoto A., and Ingber D. E., “Mechanobiology and developmental control,” Annu. Rev. Cell Dev. Biol. 29, 27–61 (2013). 10.1146/annurev-cellbio-101512-122340 [DOI] [PubMed] [Google Scholar]
- 3. Waters C. M., Roan E., and Navajas D., “Mechanobiology in lung epithelial cells: Measurements, perturbations, and responses,” Compr. Physiol. 2, 1–29 (2012). 10.1002/cphy.c100090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varner V. D. and Nelson C. M., “Cellular and physical mechanisms of branching morphogenesis,” Development 141(14), 2750–2759 (2014). 10.1242/dev.104794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes J. M., Przybyla L., and Weaver V. M., “Tissue mechanics regulate brain development, homeostasis and disease,” J. Cell Sci. 130, 71–82 (2017). 10.1242/jcs.191742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosinczuk J., Taradaj J., Dymarek R., and Sopel M., “Mechanoregulation of wound healing and skin homeostasis,” Biomed. Res. Int. 2016, 1–14 (2016). 10.1155/2016/3943481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connor J. W. and Gomez E. W., “Biomechanics of TGFβ-induced epithelial-mesenchymal transition: Implications for fibrosis and cancer,” Clin. Transl. Med. 3(1), 23 (2014). 10.1186/2001-1326-3-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lampi M. C. and Reinhart-king C. A., “Targeting extracellular matrix stiffness to attenuate disease : From molecular mechanisms to clinical trials,” Sci. Transl. Med. 475, 1–14 (2018). 10.1126/scitranslmed.aao0475 [DOI] [PubMed] [Google Scholar]
- 9. Lancerotto L. and Orgill D. P., “Mechanoregulation of angiogenesis in wound healing,” Adv. Wound Care 3(10), 626–634 (2014). 10.1089/wound.2013.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duscher D., Maan Z. N., Wong V. W., Rennert R. C., Januszyk M., Rodrigues M. et al. , “Mechanotransduction and fibrosis,” J. Biomech. 47(9), 1997–2005 (2014). 10.1016/j.jbiomech.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panciera T., Azzolin L., Cordenonsi M., and Piccolo S., “Mechanobiology of YAP and TAZ in physiology and disease,” Rev. Mol. Cell Biol. 18(12), 758–770 (2017). 10.1038/nrm.2017.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitt S., Hendricks P., Weir J., Somasundaram R., and Sittampalam G. S., “Stretching mechanotransduction from the lung,” Assay Drug Dev. Technol. 10(2), 137–147 (2012). 10.1089/adt.2011.418 [DOI] [PubMed] [Google Scholar]
- 13. Carlstedt C. A. and Nordin M., “Biomechanics of tendons and ligaments,” in , 2nd ed, edited by Nordin M. and Frankel V. H. ( Lea & Febiger, Philadelphia, 1989), pp. 59–74. [Google Scholar]
- 14. Schleifenbaum S., Schmidt M., Möbius R., Wolfskämpf T., Schröder C., Grunert R. et al., “Load and failure behavior of human muscle samples in the context of proximal femur replacement,” BMC Musculoskeletal Disord. 17, 149 (2016). 10.1186/s12891-016-0998-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park J. M., Borer J. G., Freeman M. R., Peters C. A., John M., Borer J. G. et al. , “Stretch activates heparin-binding EGF-like growth factor expression in bladder smooth muscle cells,” Am. J. Physiol. 275, C1247–C1254 (1998). 10.1152/ajpcell.1998.275.5.C1247 [DOI] [PubMed] [Google Scholar]
- 16. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., and Brown R. A., “Myofibroblasts and mechano-regulation of connective tissue remodelling,” Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002). 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- 17. Achterberg V. F. et al. , “The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function,” J. Invest. Dermatol. 134, 1862–1872 (2014). 10.1038/jid.2014.90 [DOI] [PubMed] [Google Scholar]
- 18. Maiti R. et al. , “In vivo measurement of skin surface strain and sub-surface layer deformation induced by natural tissue stretching,” J. Mech. Behav. Biomed. Mater. 62, 556–569 (2016). 10.1016/j.jmbbm.2016.05.035 [DOI] [PubMed] [Google Scholar]
- 19. Grant C. A., Twigg P. C., and Tobin D. J., “Static and dynamic nanomechanical properties of human skin tissue using atomic force microscopy: Effect of scarring in the upper dermis,” Acta Biomater. 8, 4123–4129 (2012). 10.1016/j.actbio.2012.06.042 [DOI] [PubMed] [Google Scholar]
- 20. Wong V. W., Levi K., Akaishi S., Schultz G., and Dauskardt R. H., “Scar zones: Region-specific differences in skin tension may determine incisional scar formation,” Plast. Reconstr. Surg. 129, 1272–1276 (2012). 10.1097/PRS.0b013e31824eca79 [DOI] [PubMed] [Google Scholar]
- 21. Atkinson J. A., McKenna K. T., Barnett A. G., McGrath D. J., and Rudd M., “A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines,” Plast. Reconstr. Surg. 116, 1648–1656 (2005). Discussion 1657–1648. 10.1097/01.prs.0000187147.73963.a5 [DOI] [PubMed] [Google Scholar]
- 22. Akaishi S., Akimoto M., Hyakusoku H., and Ogawa R., “The tensile reduction effects of silicone gel sheeting,” Plast. Reconstr. Surg. 126, 109e–111e (2010). 10.1097/PRS.0b013e3181df7073 [DOI] [PubMed] [Google Scholar]
- 23. De Filippo R. E. and Atala A., “Stretch and growth: The molecular and physiologic influences of tissue expansion,” Plast. Reconstr. Surg. 109, 2450–2462 (2002). 10.1097/00006534-200206000-00043 [DOI] [PubMed] [Google Scholar]
- 24. Lancerotto L., Bayer L. R., and Orgill D. P., “Mechanisms of action of microdeformational wound therapy,” Semin. Cell. Dev. Biol. 23, 987–992 (2012). 10.1016/j.semcdb.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 25. Townsley M. I., “Structure and composition of pulmonary arteries, capillaries and veins,” Compr. Physiol. 2, 675–709 (2013). 10.1002/cphy.c100081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weibel E. R., “It takes more than cells to make a good lung,” Am. J. Respir. Crit. Care Med. 187(4), 342–346 (2013). 10.1164/rccm.201212-2260OE [DOI] [PubMed] [Google Scholar]
- 27. Frank J. A., “Claudins and alveolar epithelial barrier function in the lung,” Ann. N. Y. Acad. Sci. 1257, 175–183 (2012). 10.1111/j.1749-6632.2012.06533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei Q. and Huang H., “Insights into the role of cell–cell junctions in physiology and disease,” in , 1st ed ( Elsevier Inc., 2013), Vol. 306. [DOI] [PubMed] [Google Scholar]
- 29. Radeva M. Y. and Waschke J., “Mind the gap: Mechanisms regulating the endothelial barrier,” Acta Physiol. 222, 1–20 (2018). 10.1111/apha.12860 [DOI] [PubMed] [Google Scholar]
- 30. Kopf M., Schneider C., and Nobs S. P., “The development and function of lung- resident macrophages and dendritic cells,” Nat. Immunol. 16(1), 36–44 (2015). 10.1038/ni.3052 [DOI] [PubMed] [Google Scholar]
- 31. Fredberg J. J. and Kamm R. D., “Stress transmission in the lung: Pathways from organ to molecule,” Annu. Rev. Physiol 68, 507–541 (2006). 10.1146/annurev.physiol.68.072304.114110 [DOI] [PubMed] [Google Scholar]
- 32. Chatterjee S., Fujiwara K., Pérez N. G., Ushio-fukai M., and Fisher A. B., “Mechanosignaling in the vasculature: Emerging concepts in sensing, transduction and physiological responses,” Am. J. Physiol. Heart Circ. Physiol. 308(12), H1451–H1462 (2015). 10.1152/ajpheart.00105.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gil J., Bachofen H., Gehr P., and Weibel E. R., “Alveolar volume-surface area relation in air- and lungs fixed by vascular perfusion,” J. Appl. Physiol. 47, 990 (1979). 10.1152/jappl.1979.47.5.990 [DOI] [PubMed] [Google Scholar]
- 34. Roan E. and Waters C. M., “What do we know about mechanical strain in lung alveoli?,” Am. J. Physiol. Lung Cell Mol. Physiol. 301(5), L625–L635 (2011). 10.1152/ajplung.00105.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perlman C. E. and Bhattacharya J., “Alveolar expansion imaged by optical sectioning microscopy,” J. Appl. Physiol. 103(3), 1037–1044 (2007). 10.1152/japplphysiol.00160.2007 [DOI] [PubMed] [Google Scholar]
- 36. Booth A. J., Hadley R., Cornett A. M., Dreffs A. A., Matthes S. A., Tsui J. L. et al. , “Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation,” Am. J. Respir. Crit. Care Med. 186, 866–876 (2012). 10.1164/rccm.201204-0754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pini A., Viappiani S., Bolla M., Masini E., and Bani D., “Prevention of bleomycin-induced lung fibrosis in mice by a novel approach of parallel inhibition of cyclooxygenase and nitric-oxide donation using NCX 466, a prototype cyclooxygenase inhibitor and nitric-oxide donor,” J. Pharmacol. Exp. Ther. 341(2), 493–499 (2012). 10.1124/jpet.111.190660 [DOI] [PubMed] [Google Scholar]
- 38. Frank J. and Matthay M. A., “Science review: Mechanisms of ventilator-induced injury,” Crit. Care 7(3), 233–241 (2003). 10.1186/cc1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cabrera-Benitez N., Laffey J., Parotto M., Spieth P. M., Villar J., Zhang H., and Slutsky A. S., “Mechanical ventilation–associated lung fibrosis in acute respiratory distress syndrome,” Anesthesiology 121(1), 189–198 (2014). 10.1097/ALN.0000000000000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bates J. H. T., Davis G., Majumbar A., Butnor K., and Suki B., “Linking parenchymal disease progression to changes in lung mechanical function by percolation,” Am. J. Respir. Crit. Care Med. 176, 617–623 (2007). 10.1164/rccm.200611-1739OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wells R. G., “Tissue mechanics and fibrosis,” Biochim. Biophys. Acta 1832(7), 884–890 (2013). 10.1016/j.bbadis.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Froese A. R., Shimbori C., Bellaye P., Obex S., Fatima S., and Kolb M., “Stretch induced activation of Transforming Growth Factor-β1 in pulmonary fibrosis,” Am. J. Respir. Crit. Care Med. 94(1), 84–96 (2016). 10.1164/rccm.201508-1638OC [DOI] [PubMed] [Google Scholar]
- 43. Noguchi S., Saito A. et al. , “TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts,” Sci. Rep. 7, 42595 (2017). 10.1038/srep42595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waters C. M., Glucksberg M. R., Lautenschlager E. P., Lee C., Van Matre R. M., Warp R. J., et al. , “A system to impose prescribed homogenous strains on cultured cells,” J. Appl. Physiol. 91, 1600–1610 (2001). 10.1152/jappl.2001.91.4.1600 [DOI] [PubMed] [Google Scholar]
- 45. Xia Y. and Whitesides G. M., “Soft lithography,” Annu. Rev. Mater. Sci. 28, 153–184 (1998). 10.1146/annurev.matsci.28.1.153 [DOI] [Google Scholar]
- 46. Wan C.-R., Chung S., and Kamm R. D., “Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system,” Ann. Biomed. Eng. 39(6), 1840–1847 (2011). 10.1007/s10439-011-0275-8 [DOI] [PubMed] [Google Scholar]
- 47. Zhou J. and Niklason L. E., “Microfluidic artificial “vessels” for dynamic mechanical stimulation of mesenchymal stem cells,” Integr. Biol. 4, 1487–1497 (2012). 10.1039/c2ib00171c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamotani Y., Bersano-Begey T., Kato N., Tung Y.-C., Huh D., Song J. W., and Takayama S., “Individually programmable cell stretching microwell arrays actuated by a Braille display,” Biomaterials 29(17), 2646–2655 (2008). 10.1016/j.biomaterials.2008.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moraes C., Likhitpanichkul M., Lam C. J., Beca B. M., and Simmons C. A., Integr. Biol. 5, 673–680 (2013). 10.1039/c3ib20254b [DOI] [PubMed] [Google Scholar]
- 50. Moraes C., Chen J.-H., Sun Y., and Simmons C. A., “Microfabricated arrays for high-throughput screening of cellular response to cyclic substrate deformation,” Lab Chip 10(2), 227–234 (2010). 10.1039/B914460A [DOI] [PubMed] [Google Scholar]
- 51. Michielin F., Serena E., Pavan P., and Elvassore N., “Micro fluidic-assisted cyclic mechanical stimulation affects cellular membrane integrity in a human muscular dystrophy in vitro model,” RSC Adv. 5, 98429–98439 (2015). 10.1039/C5RA16957G [DOI] [Google Scholar]
- 52. Gao X., Zhang X., Tong H., Lin B., and Qin J., “A simple elastic membrane-based microfluidic chip for the proliferation and differentiation of mesenchymal stem cells under tensile stress,” Electrophoresis 32, 3431–3436 (2011). 10.1002/elps.201100237 [DOI] [PubMed] [Google Scholar]
- 53. Simmons C. S., Sim J. Y., Baechtold P., Gonzalez A., Chung C., Borghi N., and Pruitt B. L., “Integrated strain array for cellular mechanobiology studies,” J. Micromech. Microeng.: Struct., Devices, Syst. 21(5), 54016–54025 (2011). 10.1088/0960-1317/21/5/054016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Douville N. J., Zamankhan P., Tung Y.-C., Li R., Vaughan B. L., Tai C.-F. et al. , “Combination of fluid and solid mechanical stresses contribute to cell death and detachment in a microfluidic alveolar model,” Lab Chip 11(4), 609–619 (2011). 10.1039/C0LC00251H [DOI] [PubMed] [Google Scholar]
- 55. Giridharan G. A., Nguyen M.-D., Estrada R., Parichehreh V., Hamid T., Ismahil M. A. et al. , “Microfluidic cardiac cell culture model (μCCCM),” Anal. Chem. 82(18), 7581–7587 (2010). 10.1021/ac1012893 [DOI] [PubMed] [Google Scholar]
- 56. Campillo N., Jorba I., Schaedel L., Casals B., Gozal D., Farré R. et al., “A novel chip for cyclic stretch and intermittent hypoxia cell exposures mimicking obstructive sleep apnea,” Front. Physiol. 7, 319 (2016). 10.3389/fphys.2016.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhatia S. N. and Ingber D. E., “Microfluidic organs-on-chips,” Nat. Biotechnol. 32(8), 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 58. Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., and Ingber D. E., “Reconstituting organ-level lung functions on a chip,” Science 328(5986), 1662–1668 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stucki A. O., Stucki J. D., Hall S. R. R., Felder M., Mermoud Y., Schmid R. A., Geiser T., and Guenat O. T., “A lung-on-a-chip array with an integrated bio-inspired respiration mechanism,” Lab Chip 15(5), 1302–1310 (2015). 10.1039/C4LC01252F [DOI] [PubMed] [Google Scholar]
- 60. Fukumoto J. and Kolliputi N., “Human lung on a chip: Innovative approach for understanding disease processes and effective drug testing,” Front. Pharmacol. 3, 1–2 (2013). 10.3389/fphar.2012.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim H. J. and Ingber D. E., “Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation,” Integr. Biol.: Quant. Biosci. Nano Macro 5(9), 1130–1140 (2013). 10.1039/c3ib40126j [DOI] [PubMed] [Google Scholar]
- 62. Wall M. E., Weinhold P. S., Siu T., Brown T. D., and Banes A. J., “Comparison of cellular strain with applied substrate strain in vitro,” J. Biomech. 40, 173–181 (2007). 10.1016/j.jbiomech.2005.10.032 [DOI] [PubMed] [Google Scholar]
- 63. Deng L., Bosse Y., Brown N., Chin L. Y. M., Connolly S. C., Fairbank N. J. et al. , “Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle,” Pulm. Pharmacol. Ther. 22(5), 407–416 (2009). 10.1016/j.pupt.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 64. Park J. S., Chu J. S. F., Cheng C., Chen F., Chen D., and Li S., “Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells,” Biotechnol. Bioeng. 88(3), 359 (2004). 10.1002/bit.20250 [DOI] [PubMed] [Google Scholar]
- 65. Berry C. C., Cacou C., Lee D. A., Bader D. L., and Shelton J. C., “Dermal fibroblasts respond to mechanical conditioning a strain profile dependent manner,” Biorheology 40, 337–345 (2003), PMID: 12454424. [PubMed] [Google Scholar]
- 66. Gould R. A., Chin K., Santisakultarm T. P., Dropkin A., Richards J. M., Schaffer C. B., and Butcher J. T., “Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture,” Acta Biomater. 8(5), 1710–1719 (2012). 10.1016/j.actbio.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang J. et al. , “An updated review of mechanotransduction in skin disorders: Transcriptional regulators, ion channels, and microRNAs,” Cell Mol. Life Sci. 72, 2091–2106 (2015). 10.1007/s00018-015-1853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Halder G., Dupont S., and Piccolo S., “Transduction of mechanical and cytoskeletal cues by YAP and TAZ,” Nat. Rev. Mol. Cell Biol. 13, 591–600 (2012). 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- 69. Low B. C. et al. , “YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth,” FEBS Lett. 588, 2663–2670 (2014). 10.1016/j.febslet.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 70. Lee M. J., Byun M. R., Furutani-Seiki M., Hong J. H., and Jung H. S., “YAP and TAZ regulate skin wound healing,” J. Invest. Dermatol. 134, 518–525 (2014). 10.1038/jid.2013.339 [DOI] [PubMed] [Google Scholar]
- 71. Zhang H., Pasolli H. A., and Fuchs E., “Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin,” Proc. Natl. Acad. Sci. U. S. A. 108, 2270–2275 (2011). 10.1073/pnas.1019603108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang M. et al. , “A preliminary study of differentially expressed genes in expanded skin and normal skin: implications for adult skin regeneration,” Arch. Dermatol. Res. 303, 125–133 (2011). 10.1007/s00403-011-1123-2 [DOI] [PubMed] [Google Scholar]
- 73. Valbuena A., Vera A. M., Oroz J., Menendez M., and Carrion-Vazquez M., “Mechanical properties of beta-catenin revealed by single-molecule experiments,” Biophys. J. 103, 1744–1752 (2012). 10.1016/j.bpj.2012.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yonemura S., “A mechanism of mechanotransduction at the cell-cell interface: Emergence of alpha-catenin as the center of a force-balancing mechanism for morphogenesis in multicellular organisms,” Bioessays 33, 732–736 (2011). 10.1002/bies.201100064 [DOI] [PubMed] [Google Scholar]
- 75. Van De Water L., Varney S., and Tomasek J. J., “Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: Opportunities for new therapeutic intervention,” Adv. Wound Care (New Rochelle) 2, 122–141 (2013). 10.1089/wound.2012.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wong V. W., Longaker M. T., and Gurtner G. C., “Soft tissue mechanotransduction in wound healing and fibrosis,” Semin. Cell Dev. Biol. 23, 981–986 (2012). 10.1016/j.semcdb.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 77. Bochaton-Piallat M. L., Gabbiani G., and Hinz B., “The myofibroblast in wound healing and fibrosis: Answered and unanswered questions,” F1000Res 5, 1–8 (2016). 10.12688/f1000research.8190.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miranda M. Z. et al. , “TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism,” J. Biol. Chem. 292, 14902–14920 (2017). 10.1074/jbc.M117.780502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dupont S. et al. , “Role of YAP/TAZ in mechanotransduction,” Nature 474, 179–183 (2011). 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 80. Jorgenson A. J. et al. , “TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression,” Am. J. Physiol. Cell Physiol. 312, C277–C285 (2017). 10.1152/ajpcell.00205.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu F. et al. , “Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis,” Am. J. Physiol. Lung. Cell Mol. Physiol. 308, L344–L357 (2015). 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saito A. and Nagase T., “Hippo and TGF-beta interplay in the lung field,” Am. J. Physiol. Lung Cell Mol. Physiol. 309, L756–L767 (2015). 10.1152/ajplung.00238.2015 [DOI] [PubMed] [Google Scholar]
- 83. Liang M. et al. , “Yap/Taz deletion in Gli(+) cell-derived myofibroblasts attenuates fibrosis,” J. Am. Soc. Nephrol. 28, 3278–3290 (2017). 10.1681/ASN.2015121354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mermoud Y., Felder M., Stucki J. D., Stucki A. O., and Guenat O. T., “Sensors and actuators B: Chemical microimpedance tomography system to monitor cell activity and membrane movements in a breathing lung-on-chip,” Sens. Actuators: B Chem. 255, 3647–3653 (2018). 10.1016/j.snb.2017.09.192 [DOI] [Google Scholar]
- 85. Jakob B., Generelli S., Barbe L., and Guenat O. T., “Sensors and Actuators B: Chemical low-cost disposable ALT electrochemical microsensors for in-vitro hepatotoxic assessment,” Sens. Actuators: B Chem. 228, 360–365 (2016). 10.1016/j.snb.2016.01.008 [DOI] [Google Scholar]
- 86. Pavesi A., Adriani G., Rasponi M., Zervantonakis I. K., Fiore G. B., and Kamm R. D., “Controlled electromechanical cell stimulation on-a-chip,” Sci. Rep. 5, 11800 (2015). 10.1038/srep11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Krishnan R., Park J., Seow C. Y., Lee P. V., and Stewart A. G., “Cellular biomechanics in drug screening and evaluation: Mechanopharmacology,” Trends Pharmacol. Sci. 37(2), 87–100 (2016). 10.1016/j.tips.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]