Abstract
Objective
Microscopic residual disease following complete cytoreduction (R0) is associated with a significant survival benefit for patients with advanced epithelial ovarian cancer (EOC). Our objective was to develop a prediction model for R0 to support surgeons in their clinical care decisions.
Methods
Demographic, pathologic, surgical, and CA125 data were collected from GOG 182 records. Patients enrolled prior to September 1, 2003 were used for the training model while those enrolled after constituted the validation data set. Univariate analysis was performed to identify significant predictors of R0 and these variables were subsequently analyzed using multivariable regression. The regression model was reduced using backward selection and predictive accuracy was quantified using area under the receiver operating characteristic area under the curve (AUC) in both the training and the validation data sets.
Results
Of the 3882 patients enrolled in GOG 182, 1,480 had complete clinical data available for the analysis. The training data set consisted of 1,007 patients (234 with R0) while the validation set was comprised of 473 patients (122 with R0). The reduced multivariable regression model demonstrated several variables predictive of R0 at cytoreduction: Disease Score (DS) (p < 0.001), stage (p =0.009), CA125 (p < 0.001), ascites (p < 0.001), and stage-age interaction (p =0.01). Applying the prediction model to the validation data resulted in an AUC of 0.73 (0.67 to 0.78, 95% C.I.). Inclusion of DS enhanced the model performance to an AUC of 0.83 (0.79 to 0.88 95% CI)
Conclusions
We developed and validated a prediction model for R0 that offers improved performance over previously reported models for prediction of residual disease. The performance of the prediction model suggests additional factors (i.e. imaging, molecular profiling, etc) should be explored in the future for a more clinically actionable tool.
Keywords: Ovarian cancer, microscopic residual
INTRODUCTION
Ovarian cancer is the fourth most common gynecologic malignancy among women in the United States. By American Cancer Society estimates, 22,280 women will be newly diagnosed in 2016 and 14,240 women will die of their disease.1 Patients with ovarian cancer are typically treated with a combination of surgical cytoreduction and adjuvant chemotherapy. Although outcomes are adversely affected by the grade, histology, and stage of disease, anatomical distribution of disease and the volume of residual disease following surgical debulking may be the strongest determinants of survival.2 Upper abdominal surgery is often needed to achieve maximal cytoreduction of disease and institutional improvements in patient outcome have coincided from programmatic transition to more aggressive operative intervention.3 The Gynecologic Oncology Group (GOG) has previously defined “optimal” residual as <1cm, but multiple reports now support maximum cytoreduction to microscopic residual (R0) as being the new standard.4–7
The management of patients in whom R0 cannot be obtained surgically remains controversial. Some authors advocate for Neoadjuvant Chemotherapy (NACT) with Interval Debulking Surgery (IDS) in this scenario8,9 while others support aggressive Primary Cytoreductive Surgery (PDS) as long as the patients can be debulked to <1cm of disease.10 Some institutional studies show improved outcomes from cytoreduction to minimal gross residual disease (>R0<1cm).10 However, these improvements have not been reproduced using multi-institutional data.6,7 In a study by Horowitz et al11, multivariable modeling of 2,655 GOG patients with <1cm of disease affirmed that disease distribution and disease residual, not the surgical procedure, appear to be the most important determinants of patient prognosis. In addition, a prospective clinical trial performed by the European Organization for Research and Treatment of Cancer, investigators found that R0 patients with NACT and IDS had a median survival of 38 months and 28% 5-year survival compared to patients primarily debulked to minimal gross residual disease (>R0<1cm), who had a median survival of 32 months and a 23% 5-year survival.12 Although no statistical analysis was provided, these results do not demonstrate worse outcome for patients that receive NACT and have a higher chance for R0 at IDS compared to patients that undergo PDS with >R0<1cm. Based on these studies9–12, many feel that radical surgery should be reserved for those patients that can be debulked to R0, but this is not easy to predict prior to surgery. In cases in which R0 is feasible and safe for the patient, the full range of upper abdominal and conventional cytoreductive procedures should be deployed.13,14
Accurate pre-operative identification of patients who are likely to achieve R0 status at surgery could allow for more careful selection of patients for PDS while patients with a low likelihood of R0 could be considered for NACT and IDS, or a minimally invasive diagnostic assessment with laparoscopy to determine R0 feasibility. Previous attempts to develop prediction models of postoperative residual disease have predominantly used <1cm (instead of R0) as an endpoint for measuring accuracy. In addition, the models developed thus far have either not been validated in a set of patients independent from which the model was developed or failed external validation. We sought to develop a more robust validated model for prediction of R0 in women with ovarian cancer using data from a multi-institutional cooperative group randomized trial..
PATIENTS AND METHODS
All patient data were abstracted from GOG patients enrolled on the international collaborative trial GOG-182-ICON515 using existing case report forms. Patients were enrolled with FIGO stage III or IV histologically confirmed epithelial ovarian cancer (EOC) or primary peritoneal carcinoma (PPC). All patients underwent PDS to either ≤1 cm or >1cm residual disease before being randomized to one of five platinum and paclitaxel-based chemotherapy regimens. No statistically significant treatment effects on progression-free survival (PFS) or overall survival (OS) were found among the five chemotherapy regimens. Further details of eligibility criteria and results from the original study have been published.15
Patient demographics and tumor characteristics, including age, race, performance status, tumor grade, presence of ascites (any versus none), and histology were extracted from the GOG database. Information describing surgical procedures and preoperative extent of disease based on 56 anatomic locations was abstracted from GOG surgical reporting forms and diagrams. All operative and pathology reports were evaluated to obtain accurate descriptions of disease (distribution and residual) and surgical procedures. Preoperative CA125 was obtained from medical records submitted to the GOG as part of enrollment in GOG-182. Initial sites of disease identified during primary debulking surgery were used to develop the preoperative disease score (DS). The DS classes were defined as: (1) DS-low, with pelvic and retroperitoneal spread; (2) DS-moderate (DS-mod), with additional spread to the abdomen but sparing the upper abdomen; or (3) DS-high, with the presence of upper abdominal disease affecting the diaphragm, spleen, liver or pancreas. The current study included all patients with FIGO stage III or IV EOC or PPC. The residual disease outcome was scored as completely resected disease (R0), minimal gross residual disease (>R0<1cm), or suboptimal cytoreduction (>1cm) following PDS. The raw (unadjusted) proportion of patients with R0 outcomes was estimated for each of Institution in the dataset. Exploratory analysis showed considerable variability in the R0 estimates among Institutions supported by fewer patients. The 3-level R0 stratification factor was defined at the tertiles of R0 distribution. The R0 factor levels were low (0 to 16%), middle (16.5 to 31%) and high (31.5 to 100%).
Model training and validation subsets were defined based on the date of patient enrollment on the study. The patients enrolled between February 7, 2001 and August 31, 2003 were assigned to the training data set while the remaining patients enrolled from September 1, 2003 to September 1, 2004 were used for model validation. While the training and validation patients were similar by virtue of enrollment on the same study, this temporal allocation to training and validation sets provides a prospective assessment of model performance.16 Using the training data, exploratory univariate analysis was performed to identify predictors of R0 following PDS. Covariates for the full multivariable logistic regression model included patient characteristics believed to be both available to inform the surgery decision and related to the R0 outcome. The model specification included main effects and second-order interaction terms for age, stage, presence of ascites, CA125 (log transformed), and GOG performance score (similar to Karnofsky). This full model was then reduced using backward selection constrained to respect interaction term hierarchy, with a covariate retention threshold of p < 0.20. The reduction did not significantly degrade the model fit (p=0.7 by full and reduced model Chi square test). This process was repeated with DS included as a covariate. To explore institutional effects on predictive accuracy, the R0 model including DS was respecified to adjust for Institute (parent) or level R0 strata. This factor was included as a fixed effect in the multivariable model, with the middle tertile as the reference group.
R0 prediction scores for the patients in the validation data were obtained using coefficient estimates from the reduced model fit to the training data. The ability of the models to accurately distinguish between R0 vs >R0 was graphically presented with Receiver Operating Characteristic (ROC) curves. ROC curves display the classification accuracy of a predictive model score as the classification threshold is varied. For different cut-off points of the model score, the true positive fraction is plotted in function of the false positive fraction. The classification accuracy is summarized by the area under the ROC (AUC) and its 95% confidence limits. AUC values greater than 0.80 were considered to have some utility in predicting the response of individual patients.17 The Youden Index18 estimated from the training data was used to classify patients as having either a high or low propensity for R0. This index is the basis of other indicators of classification accuracy, including estimates for sensitivity, specificity, positive and negative predictive values.
All data analyses were generated using SAS/STAT software, Version 9.4. Copyright 2012, SAS Institute Inc. SAS is a registered trademark of SAS Institute Inc., Cary, NC, USA.
RESULTS
Of the 3,882 patients in the original dataset of GOG-182, 1,480 had complete preoperative data elements and were used to generate the training cohort (n=1007) and validation (n=473) cohort for this study. Although a significant number of patients were excluded because they did not have documentation of CA125 and ascites data (n=2,215), the proportion of R0 and >R0 was similar between the included and excluded cases (Supplementary Figure 1). A total of 356 patients (24%) had R0, 23% of the training cohort (n= 234) and 26% of the validation cohort (n=122).
There were 73 parent hospitals and 240 affiliates that contributed patients to this study. The Institutes had a median of 15 patients in this dataset and 75% of Institutes had 30 or fewer patients. The median R0 for the parent institution was 23% while the median R0 for affiliates was 17%. The distribution of R0 by anonymized institution and number of patients enrolled is shown in Supplementary Figures 2 and 3 and Supplementary Tables 1 and 2. Approximately 75% of affiliate hospitals in our sample were represented by less than 8 patients. Our sample was not strong enough to support hospital-level R0 estimates and so patients from affiliates were combined with patients from the parent program in providing an institution variable for modelling.
The demographics and disease characteristics for patients with R0 vs >R0 disease for the training cohort are presented in Table 1 and 2. Many of the clinical characteristics were similar between those with R0 and >R0 residual. However, the probability of having R0 disease was significantly associated with age, performance status, stage, extent of disease (DS) at exploration, and presence of ascites. Not surprisingly, older, symptomatic patients with advanced stage, large volume disease, and ascites were less likely to have R0 residual.
Table 1.
Demographics of the Training Cohort by Residual Disease Group
| >R0 | R0 | Overall | Pval | ||
|---|---|---|---|---|---|
| Overall | N | 773 (76.8) | 234 (23.2) | 1,007 (100%) | |
| Age(Y) | Mean/StdErr | 59.5/0.4 | 56.2/0.7 | 58.7/0.3 | <.001 |
| Median/Min/Max | 59.4/29.5/87.5 | 55.7/24.5/84.7 | 58.9/24.5/87.5 | ||
| Age(Cat) | 0-54 | 274 (35.4%) | 107 (45.7%) | 381 (37.8%) | 0.005 |
| 55-64 | 248 (32.1%) | 74 (31.6%) | 322 (32.0%) | ||
| 65+ | 251 (32.5%) | 53 (22.6%) | 304 (30.2%) | ||
| Race | Unknown | 13 (1.7%) | 4 (1.7%) | 17 (1.7%) | 0.708 |
| Asian | 15 (1.9%) | 3 (1.3%) | 18 (1.8%) | ||
| Black | 34 (4.4%) | 7 (3.0%) | 41 (4.1%) | ||
| White | 711 (92.0%) | 220 (94.0%) | 931 (92.5%) | ||
| Ethnicity | Hispanic | 13 (1.7%) | 4 (1.7%) | 17 (1.7%) | 0.682 |
| Non-Hispanic | 704 (91.1%) | 209 (89.3%) | 913 (90.7%) | ||
| Not Known | 56 (7.2%) | 21 (9.0%) | 77 (7.6%) | ||
| BMI | Under | 27 (3.5%) | 8 (3.4%) | 35 (3.5%) | 0.412 |
| Normal | 321 (41.5%) | 89 (38.0%) | 410 (40.7%) | ||
| Over | 213 (27.6%) | 69 (29.5%) | 282 (28.0%) | ||
| Obese | 186 (24.1%) | 54 (23.1%) | 240 (23.8%) | ||
| Missing | 26 (3.4%) | 14 (6.0%) | 40 (4.0%) | ||
| Performance | Asymptomatic | 320 (41.4%) | 127 (54.3%) | 447 (44.4%) | 0.001 |
| Ambulatory | 397 (51.4%) | 98 (41.9%) | 495 (49.2%) | ||
| In bed < 50% | 56 (7.2%) | 9 (3.8%) | 65 (6.5%) | ||
| Performance | Symptomatic | 453 (58.6%) | 107 (45.7%) | 560 (55.6%) | <.001 |
| Asymptomatic | 320 (41.4%) | 127 (54.3%) | 447 (44.4%) |
Table 2.
Pathologic and surgical characteristics of the Training Cohort by Residual Disease Group
| >R0 | R0 | Overall | P-value | ||
|---|---|---|---|---|---|
| Overall | N | 773 (76.8) | 234 (23.2) | 1,007 (100%) | |
| Site | Ovary | 662 (85.6%) | 220 (94.0%) | 882 (87.6%) | <.001 |
| Other | 111 (14.4%) | 14 (6.0%) | 125 (12.4%) | ||
| Histology | Adeno Unsp | 5 (0.6%) | 2 (0.9%) | 7 (0.7%) | <.001 |
| Clear Cell | 9 (1.2%) | 19 (8.1%) | 28 (2.8%) | ||
| Endomet | 41 (5.3%) | 28 (12.0%) | 69 (6.9%) | ||
| Mucinous | 7 (0.9%) | 1 (0.4%) | 8 (0.8%) | ||
| Mixed Epi | 41 (5.3%) | 13 (5.6%) | 54 (5.4%) | ||
| Undiffer | 7 (0.9%) | 4 (1.7%) | 11 (1.1%) | ||
| Transition | 3 (0.4%) | 2 (0.9%) | 5 (0.5%) | ||
| Serous | 660 (85.4%) | 165 (70.5%) | 825 (81.9%) | ||
| Grade | 1 | 20 (3.1%) | 11 (5.6%) | 31 (3.7%) | 0.267 |
| 2 | 130 (20.1%) | 40 (20.2%) | 170 (20.1%) | ||
| 3 | 497 (76.8%) | 147 (74.2%) | 644 (76.2%) | ||
| Stage | 3 | 638 (82.5%) | 214 (91.5%) | 852 (84.6%) | <.001 |
| 4 | 135 (17.5%) | 20 (8.5%) | 155 (15.4%) | ||
| Ascites | Yes | 681 (88.1%) | 117 (50.0%) | 798 (79.2%) | <.001 |
| No | 92 (11.9%) | 117 (50.0%) | 209 (20.8%) | ||
| Ext of Diseas | DS-Low | 14 (1.8%) | 37 (15.8%) | 51 (5.1%) | <.001 |
| DS-Mod | 100 (12.9%) | 131 (56.0%) | 231 (22.9%) | ||
| DS-High | 659 (85.3%) | 66 (28.2%) | 725 (72.0%) |
Adeno unsp- Adenocarcinoma unspecified; Endomet- Endometrioid, Epi – Epithelial; Undiff – Undifferentiated; Transition – Transitional ; Ext of Diseas – Extent of disease; DS- Disease Score; Mod- moderate;
CA125 values were significantly lower for R0 (median 345 u/mL) compared to >R0<1cm (median 839 u/mL) or >1cm, (median 1241 u/mL), while the values between >R0<1cm and >1cm did not differ significantly (Figure 1A). Indeed, patients in all three residual disease groups had the full range of CA125 values, making classification on the basis of CA125 alone problematic. An exploratory analysis considered log-transformed CA125 as a continuous predictor of the residual disease status (R0, >R0<1cm, and >1cm). As illustrated in Figure 1B, increasing CA125 was related to increasing the predicted probability of >R0<1cm and >1cm, and decreasing probability of R0. The pattern is intuitively satisfying, and the predicted probabilities become more compelling for CA125 value greater than 1000 u/mL. At this point, the predicted probably of R0 dropped to 0.18 (0.16 to 0.21), vs 0.51 (0.47 to 0.54) for >R0<1cm and 0.31 (0.28 to 0.34) for >1cm. Unfortunately, the CA125 model is least effective in lower ranges. For example, at CA125=35 u/mL, the predicted probably of R0 is 0.55 (0.47 to 0.63), providing no more information than a coin flip.
Figure 1.
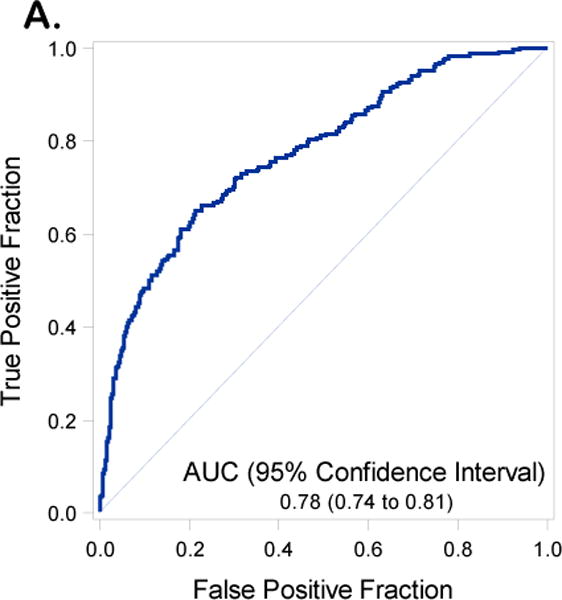
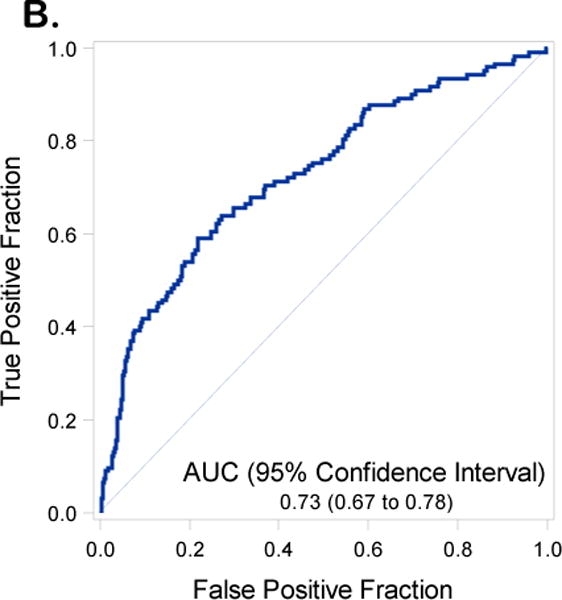
Receiver operating curves with Area Under the Curve (AUC) and 95% Confidence Intervals (CIs) for the training set inclusive of including Disease Distribution Score (DS) (1A) and excluding DS (1B)
A multivariable logistic regression model to predict the probability of R0 disease (reference: >R0) was specified to include factors believed to be both available to inform the surgery decision and related to the R0 outcome. In preliminary analysis, non-linear effects of age and CA125 were not statistically significant, and were thus excluded. We elected to control for institutions as a variable in our modelling which was inclusive of affiliates. Since 75% of affiliates were represented by <8 patients, we did not use hospital as a variable in the adjusted model. The full regression model was reduced by backward selection leaving age (linear) (p<0.001), log-transformed preoperative CA125 (linear) (p<0.001), DS (p<0.001), stage (p=0.009), presence of ascites (p<0.001), and the age-stage interaction term (p=0.01) as statistically significant factors. Final model specification and coefficient estimates are shown in Table 4.
Table 4.
Maximum likelihood coefficient estimates to predict R0 (ref:>R0) from the predictive models using the training dataset.
| Covariate | Log Odds Estimate | Standard Error | p value |
|---|---|---|---|
|
Model Excluding DS (Fig 1A and 1B) |
|||
| Intercept | 2.55 | 0.64 | <.001 |
| Log(Pre-op CA125) (continuous) |
−0.35 | 0.06 | <.001 |
| Age (continuous) | −0.03 | 0.01 | <.001 |
| Ascites | |||
| Yes | reference | ||
| No | 1.65 | 0.19 | <.001 |
| Stage | |||
| 3 | reference | ||
| 4 | −3.92 | 1.56 | 0.01 |
| Age*Stage Interaction | 0.06 | 0.03 | 0.03 |
|
Model Including DS (Fig 2A and 2B) |
|||
| Intercept | 1.54 | 0.71 | 0.03 |
| Log(Pre-op CA125) (continuous) |
−0.27 | 0.07 | <.001 |
| Age (continuous) | −0.04 | 0.01 | <.001 |
| Ascites | |||
| Yes | reference | ||
| No | 1.19 | 0.22 | <.001 |
| Stage | |||
| 3 | reference | ||
| 4 | −4.28 | 1.64 | 0.01 |
| Age*Stage Interaction | 0.07 | 0.03 | 0.01 |
| Disease Score | |||
| DS-High | reference | ||
| DS-Mod | 2.32 | 0.20 | <.001 |
| DS-Low | 2.53 | 0.37 | <.001 |
DS – Disease score
Log Odds estimates greater than zero indicate a greater propensity for microscopic residual disease after surgery, as compared to patients in the reference group, given the other covariates in the model.
We reproduced the full and reduced multivariable regression models including and excluding DS from the modeling. The performance of the model exclusive of DS revealed an AUC for the model in the training set of 0.78 (95% C.I. 0.74-0.81) (Figure 1A) and in the validation data set of 0.73 (95% C.I. 0.67-0.78) (Figure 1B). Inclusion of DS in the prediction model resulted in an AUC 0.87 (95% C.I 0.84 – 0.90) in the training set (Figure 2A) and an AUC of 0.83 (95%CI 0.79 to 0.88) in the validation data set (Figure 2B). An AUC of 0.87 was obtained in the validation set when both DS and institution were included in the final model (Supplementary Table 3 and Supplementary Figure 4). With the DS model scoring the validation dataset, the optimal Youden Index18 = 0.28 had corresponding sensitivity and specificity of 0.80 and 0.76 respectively. Additional indicators of classification accuracy are provided in Table 5.
Figure 2.
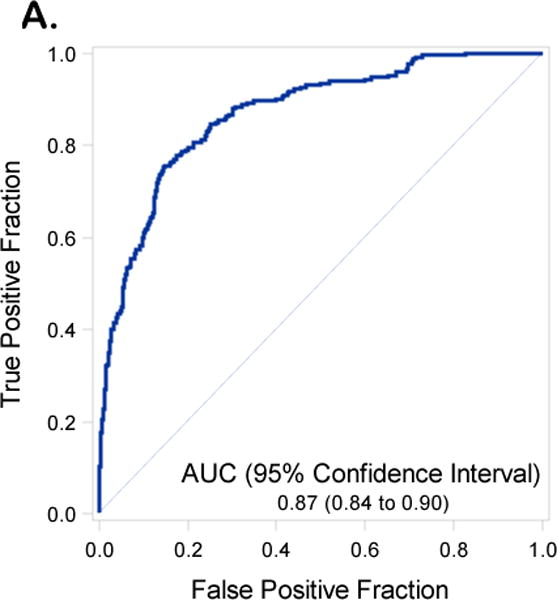
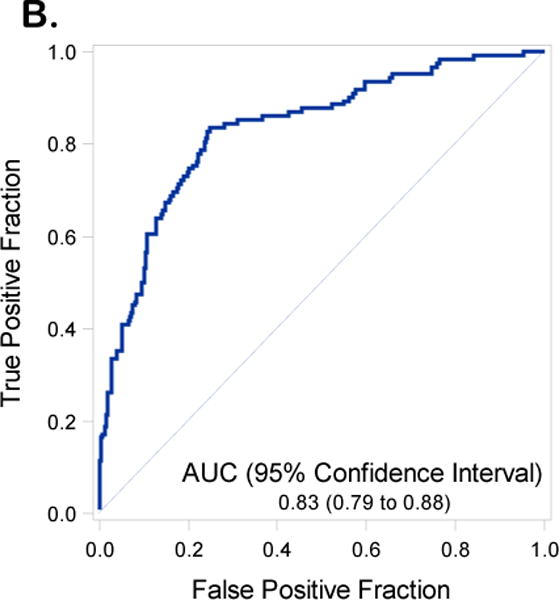
Receiver operating curves with Area Under the Curve (AUC) and 95% Confidence Intervals (CIs) for the validation set inclusive of including Disease Distribution Score (DS) (2A) and excluding DS (2B)
Table 5.
Classification accuracy of the predictive models
| Model | Accuracy | Training Data | Validation Data |
|---|---|---|---|
| Excluding DS | AUC | 0.78 (0.74 to 0.81) | 0.73 (0.67 to 0.78) |
| (Youden=0.23) | Sensitivity | 0.65 (0.58 to 0.70) | 0.61 (0.52 to 0.69) |
| Specificity | 0.79 (0.76 to 0.82) | 0.74 (0.70 to 0.79) | |
| PPV | 0.48 (0.42 to 0.53) | 0.45 (0.37 to 0.53) | |
| NPV | 0.88 (0.85 to 0.90) | 0.84 (0.80 to 0.88) | |
| Including DS | AUC | 0.87 (0.84 to 0.90) | 0.83 (0.79 to 0.88) |
| (Youden=0.28) | Sensitivity | 0.76 (0.70 to 0.81) | 0.80 (0.72 to 0.86) |
| Specificity | 0.85 (0.82 to 0.87) | 0.76 (0.72 to 0.81) | |
| PPV | 0.60 (0.55 to 0.66) | 0.54 (0.47 to 0.61) | |
| NPV | 0.92 (0.90 to 0.94) | 0.91 (0.88 to 0.94) |
DS- Disease score, AUC – area under curve, PPV- positive predictive value, NPV- negative predictive value
Measures of discrimination accuracy of the R0 prediction scores. Patients were classified as testing positive or negative using the Youden index estimated from the Training data. Accuracy indicators from the Training and Validation data are shown separately. Patients with predicted R0 probability greater or equal to the Youden index were classified as testing positive, or having a high likelihood of R0 after surgery. PPV is the proportion of patients with a positive test who actually attained R0. NPV is the proportion of patients with a negative test who actually failed to attain R0. Two-sided, α=0.05 Jeffrey’s confidence intervals indicate reliability of the estimates.
DISCUSSION
Large numbers of patients with clinical information are required to develop prediction models that control for multiple confounding influences. Typically, one predictor for every 10 events is recommended in order to avoid creation of an exploratory model that does not validate in subsequent independent testing.19,20 Although prior models using small numbers of patients and a regression model with multiple variables demonstrated impressive internal prediction accuracy,21 these initial findings were not substantiated in subsequent validation studies in independent cohorts of patients.22,23
Many surgeons have now adopted R0 as the most appropriate goal for primary or interval surgery, even though clinical outcomes are influenced by other factors, including the initial extent of disease. Our prediction model incorporating the clinical parameters of preoperative CA125 (linear), disease score, stage, and age-stage interaction is the first non-surgical, validated, and reproducible predictor for disease residual and is applicable to the contemporary endpoint of R0. Such a model and/or further refinements could complement clinical judgement when considering a patient for PDS versus NACT. This model was built on an extensive training set derived from multiple institutions avoiding the problem of overfitting while enhancing generalizability. Our current model included 1007 patients, 237 of which had the residual disease endpoint (R0) thereby mitigating the effect of overfitting. Further validation of our prediction model has shown consistency in performance. Prior prediction models of residual disease have used <1cm as an endpoint for defining “optimal” residual disease22–23,25–28 and we have chosen R0 as the more contemporary definition for “optimal” for the current modelling. Development of an accurate predictive model for achieving R0 status would have significant clinical utility for many surgeons in making decisions about primary cytoreduction verus NACT.
Establishment of an exploratory model and subsequent testing in an independent validation set also should be approached with caution in order to establish generalizability while avoiding selection bias.24 If one has 2 populations of patients (i.e. A and B), it is ideal to select patients from both A&B to create the prediction model and then test the model in an independent set of patients from A&B. Development of the model in one population (group A) and then validation in another population (group B) can lead to poor performance of the model in independent cohorts. In a study by Dowdy et al25, a prediction model incorporating ascites and diffuse peritoneal thickening was developed to identify 23 of 87 patients with suboptimal (>1 cm) cytoreduction achieving 79% accuracy. Although this prediction model showed promising performance characteristics, two subsequent studies used patients from other institutions to externally validate the model and noted that accuracy dropped to 48%21-65%.23 Similarly, the accuracy of two prediction models for patients with <1cm of residual disease developed by investigators from Italy26 demonstrated a drop in AUC from 0.78 and 0.81 to 0.56-0.59 and 0.55-0.60 respectively when assessed in an independent patient cohort by investigators in the Netherlands.27 In the current study, patients from 73 institutions participating in GOG-182 reflected a diversity of surgical skill levels while providing standardization of adjuvant therapy. Our training set and validation set of patients were similar in terms of the institutions from which they were enrolled avoiding effects of selection bias on the prediction model performance in validation while enhancing the generalizability of a model that might result from single institution reporting. Consecutive patients were used to create the training model and the subsequent validation test set so that the temporal relation allowed for prospective testing of the model thereby further enhancing its reliability.16
Investigators from the Mayo Clinic recently published a prediction model for R0.29 Although the model (including ascites, omental thickening, and diffuse peritoneal thickening) was not externally validated using an independent sample set, investigators noted an AUC of 0.748 when internally assessing the model using a bootstrap approach.29 Conversely, our model for R0 was developed using a large training set reflective of patients from multiple institutions and represents the only externally validated model reported to date. In the validation data, our model inclusive of DS had a AUC of 0.83 (0.79 to 0.88). Inclusion of institution as a variable in the model improved the AUC further to 0.87 in the validation dataset.
Despite its strengths, our current study has limitations. Distribution of disease (DS) was obtained from operative notes and not pre-operative CT scans. Previous reports have highlighted the limitations of pre-operative CT scans for identification of intraperitoneal disease. In a study by Ko et al30, 68 of 148 patients with stage III and IV EOC had false negative metastasis that were not detected on initial CT but identified during surgery. Re-examination of the CT scans a second time identified 63% of lesions that were missed on the initial examination. Most importantly, no case was found to be false positive for metastatic disease.30 Although we recognize that limitations in CT detection of metastasis might affect negative predictive value, patients unexpectedly found to have widespread disease intraoperatively could have aggressive cytoreduction postponed if radical debulking cannot render the patient R0. When we assessed the performance of the model for R0 prediction with and without inclusion of DS in the validation dataset, we identified a substantial improvement in the AUC from 0.73 in the model without DS to 0.83 in the model inclusive of DS in the validation cohort. One could assume that the accuracy of the model could fall somewhere in that range depending on the sensitivity of radiologic imaging in detection of disease distribution. Finally, although inclusion of institutions improved the accuracy of the prediction model to an AUV of 0.87, there are limitations in the accuracy of the institution rate of R0 which was limited to small numbers of patients enrolled in GOG 182 at each site. R0 rates of 100% or 0% from a few of the institutions enrolling low numbers of patients highlight this limitation.
Given the limitations of imaging and clinical prediction of R0 at cytoreduction, some surgeons have advocated use of diagnostic laparoscopy to evaluate disease distribution and assess resectability. Using intraoperative findings at diagnostic laparoscopy to develop a risk score, Fagotti and colleagues31 developed a model to increase their rates of achieving R0 at PDS up to 57% of patients. At a threshold score of 10, the likelihood of R0 was 0 and the risk of laparotomy was 32%. Using a predictive index value (PIV) ≥ 8 the probability of achieving R0 at cytoreduction was zero. The discriminating performance of this predictor for R0 revealed an AUC of 0.885. Several other prospective trials using laparoscopic disease assessment to triage patients are ongoing potentially allowing personalized surgical management.14, 32
Alternative to clinical or surgical parameters to predict R0 cytoreduction, molecular markers and gene expression signatures are likely to be informative for surgical outcome. Using data from 13 publicly available databases Reister and colleagues developed and validated a gene expression signature to predict suboptimal cytoreduction.33 Based on protein expression of POSTN, CXCL14, and pSmad 2/3 these authors report a 93% accuracy of determining a high risk and low risk group for suboptimal cytoreduction. Perhaps combining our model with this genomic tool will provide surgeons the opportunity to triage patients appropriately for primary cytoreduction or NACT.
In conclusion, our model is an independently reproducible model for prediction of disease residual and is applicable to the more contemporary endpoint of R0 as the goal of surgery. However, it is not meant to be used as the sole determinant of whether a surgeon should offer a patient PDS versus NACT and IDS. Nonetheless, we hope that model will provide surgeons with additional data to use in conjunction with the medical condition of the patient, clinical intuition, and their patient’s preferences in deciding on a course of action.
Supplementary Material
Table 3.
Treatment and Outcomes of the Training Cohort by Residual Disease Group
| >R0 | R0 | Overall | P-value | ||
|---|---|---|---|---|---|
| Overall | N | 773 (76.8) | 234 (23.2) | 1,007 (100%) | |
| Treatment | Pac+Car | 168 (21.7%) | 37 (15.8%) | 205 (20.4%) | 0.174 |
| Pac+Car+Gem | 148 (19.1%) | 46 (19.7%) | 194 (19.3%) | ||
| Pac+Dox+Car | 156 (20.2%) | 52 (22.2%) | 208 (20.7%) | ||
| Car+Top+Pac+Car | 138 (17.9%) | 54 (23.1%) | 192 (19.1%) | ||
| Car+Gem+Pac+Car | 163 (21.1%) | 45 (19.2%) | 208 (20.7%) | ||
| Alive/Dead | Alive | 113 (14.6%) | 91 (38.9%) | 204 (20.3%) | <.001 |
| Dead | 660 (85.4%) | 143 (61.1%) | 803 (79.7%) | ||
| Surg Aggr Scr | Mean/StdErr | 5.0/0.1 | 5.2/0.2 | 5.0/0.1 | 0.262 |
| Median/Min/Max | 4.0/0.0/15.0 | 4.0/1.0/15.0 | 4.0/0.0/15.0 | ||
| Score Group | CS-Low | 180 (23.3%) | 36 (15.4%) | 216 (21.4%) | 0.014 |
| CS-Mod | 465 (60.2%) | 164 (70.1%) | 629 (62.5%) | ||
| CS-High | 128 (16.6%) | 34 (14.5%) | 162 (16.1%) | ||
| Surgery Grp | NUAP | 643 (83.2%) | 190 (81.2%) | 833 (82.7%) | 0.481 |
| UAP | 130 (16.8%) | 44 (18.8%) | 174 (17.3%) | ||
| Resid Abstrtd | Microscopic | 234 (100.0%) | 234 (23.2%) | <.001 | |
| Opt (<=1) | 481 (62.2%) | 481 (47.8%) | |||
| Sub Opt | 292 (37.8%) | 292 (29.0%) |
Pac – Paclitaxel; Car- Carboplatin; Dox- Liposomal Doxorubicin; Top- Topotecan; Gem- Gemcitabine; ; Dis- Disease; Surg aggr Scr- Surgical aggressiveness score; CS- surgical complexity; Grp- Group; NUAP – no upper abdominal procedures; UAP- upper abdominal surgery; Resid Abstrd – Residual disease abstracted; Opt – optimal
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology SDMC (1U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, University of Kentucky, Eastern Virginia Medical School, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Southwestern Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women’s Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona Health Science Center, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Case Western Reserve University, and Tampa Bay Cancer Consortium.
Dr. David Mutch received a consulting fee or honorarium from AstraZeneca, as well as a consultancy fee from AstraZeneca and Clovis. Dr. Linda Van Le received royalties for being an Editor of Te Linde Operative Gynecology; publisher is Wolters Kluwer. Dr. Michael Bookman reports personal fees from McKesson Specialty Health and USOR, Genentech-Roche, Mateon, AstraZeneca, AbbVie, Tesaro, Endocyte, Clovis, and Pfizer, outside the submitted work.
Footnotes
Presented in part at the Society of Gynecologic Oncology (SGO) 2014 Annual Meeting on Women’s Cancer, March 28-1 April, 2014, Tampa FL.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense.
CONFLICTS OF INTEREST
All other co-authors have no conflicts of interest to declare.
References
- 1.http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/index
- 2.Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26:336–342. doi: 10.3802/jgo.2015.26.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J Gynecol Oncol. 2010;21:75–80. doi: 10.3802/jgo.2010.21.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;03:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic Factors for Advanced Epithelial Ovarian Cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez N, Miller A, Richard SD, Rungruang B, Hamilton CA, Bookman MA, et al. Upper Abdominal Procedures in Advanced Stage Ovarian or Primary Peritoneal Carcinoma Patients with Minimal or No Gross Residual Disease: An Analysis of GOG 182. Gynecol Oncol. 2013;130:487–492. doi: 10.1016/j.ygyno.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130:493–498. doi: 10.1016/j.ygyno.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 9.Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced stage ovarian cancer: redefining “optimal” residual disease. Gynecol Oncol. 2012;125:483–492. doi: 10.1016/j.ygyno.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Barlin JN, Long KC, Tanner EJ, Gardner GJ, Leitao MM, Jr, Levine DA, et al. Optimal (≤1 cm) but visible residual disease: is extensive debulking warranted? Gynecol Oncol. 2013;130:284–288. doi: 10.1016/j.ygyno.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz NS, Miller A, Rungruang B, Richard DS, Rodriguez N, Bookman MA, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937–943. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz NS, Miller A, Rungruang B, Richard S, Rodriguez N, Bookman MA, et al. Miles of Agreement and Millimeters of Controversy. J Clin Oncol. 2015;33:3521–3522. doi: 10.1200/JCO.2015.62.5707. [DOI] [PubMed] [Google Scholar]
- 14.Nick AM, Coleman RL, Ramirez PT, Sood AK. A framework for a personalized surgical approach to ovarian cancer. Nat Rev Clin Oncol. 2015;12:239–245. doi: 10.1038/nrclinonc.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and Prognostic Research: Validating a Prognostic Model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE. Springer Series in Statistics: Regression Modeling Strategies. New York, NY: Springer New York; 2010. p. 247. (Corrected ed.) [Google Scholar]
- 18.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;13:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modeling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions, and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Bristow RE, Duska LR, Lambrou NC, Fishman EK, O’Neill MJ, Trimble EL, et al. A model for predicting surgical outcome in patients with advanced epithelial ovarian carcinoma using computed tomography. Cancer. 2000;89:1532–1540. doi: 10.1002/1097-0142(20001001)89:7<1532::aid-cncr17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Axtell AE, Lee MH, Bristow RE, Dowdy SC, Cliby WA, Raman S, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol. 2007;25:384–389. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 23.Gemer O, Gdalevich M, Ravid M, Piura B, Rabinovich A, Gasper T, et al. A multicenter validation of computerized tomography models as predictors of non- optimal primary cytoreduction of advanced epithelial ovarian cancer. Eur J Surg Oncol. 2009;35:1109–1112. doi: 10.1016/j.ejso.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Kang S, Park SY. To predict or not to predict? The dilemma of predicting the risk of suboptimal cytoreduction in ovarian cancer. Ann Oncol. 2011 Dec;22(Suppl 8):viii23–viii28. doi: 10.1093/annonc/mdr530. [DOI] [PubMed] [Google Scholar]
- 25.Dowdy SC, Mullany SA, Brandt KR, Huppert BJ, Cliby WA. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer. 2004;101:346–352. doi: 10.1002/cncr.20376. [DOI] [PubMed] [Google Scholar]
- 26.Ferrandina G, Sallustio G, Fagotti A, Vizzielli G, Paglia A, Cucci E, et al. Role of CT scan-based and clinical evaluation in the preoperative prediction of optimal cytoreduction in advanced ovarian cancer: a prospective trial. Br J Cancer. 2009;101:1066–1073. doi: 10.1038/sj.bjc.6605292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutten IJ, van de Laar R, Kruitwagen RF, Bakers FC, Ploegmakers MJ, Pappot TW, et al. Prediction of incomplete primary debulking surgery in patients with advanced ovarian cancer: An external validation study of three models using computed tomography. Gynecol Oncol. 2016;140:22–28. doi: 10.1016/j.ygyno.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Suidan RS, Ramirez PT, Sarasin DM, Teicher JB, Milonov S, Iyer RB, et al. A multi-center prospective trial evaluating the ability of preoperative computed tomography scan and serum CA125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134:455–461. doi: 10.1016/j.ygyno.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janco JM, Glaser G, Kim B, McGree ME, Weaver AL, Cliby WA, et al. Development of a prediction model for residual disease in newly diagnosed advanced ovarian cancer. Gynecol Oncol. 2015;138:70–77. doi: 10.1016/j.ygyno.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Ko SM, Jung DC, Oh YT, Kim SH, Kang S. Discrepancy between preoperative imaging and surgical findings in patients with advanced ovarian cancer: a study of initial false-negative lesions. J Comput Assist Tomogr. 2013;37:568–571. doi: 10.1097/RCT.0b013e31828b683b. [DOI] [PubMed] [Google Scholar]
- 31.Fagotti A, Vizzielli G, Fanfani F, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of a concept. Gynecol Oncol. 2015;139:5–9. doi: 10.1016/j.ygyno.2015.07.095. [DOI] [PubMed] [Google Scholar]
- 32.Rutten MJ, Garenstroom KN, Van Gorp T, et al. Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa-trial): a multicenter randomized controlled study. BMC Cancer. 2012;12:31. doi: 10.1186/1471-2407-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riester M, Wei W, Waldron L, Culhane AC, Trippa L, Oliva E, et al. Risk prediction for late-stage ovarian cancer by meta-analysis of 1525 patient samples. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


