Abstract
Despite its acute efficacy for the treatment of panic disorder, benzodiazepines (BZs) are associated with a withdrawal syndrome that closely mimics anxiety sensations, leading to difficulty with treatment discontinuation and often disorder relapse. An exposure-based cognitive-behavioral treatment for BZ discontinuation, Panic Control Treatment for BZ Discontinuation (PCT-BD) targets the fear of these sensations and has demonstrated efficacy in preventing disorder relapse and facilitating successful BZ discontinuation among patients with panic disorder. In this randomized controlled trial, PCT-BD was compared to taper-alone and a taper plus a relaxation condition to control for the effect of therapist contact and support among 47 patients with panic disorder seeking taper from BZs. Based on the primary outcome of successful discontinuation of BZ use, results indicate that adjunctive CBT provided additive benefits above both taper alone and taper plus relaxation, with consistently medium and large effect sizes over time that reached significance at the six month follow-up evaluation. The efficacy of CBT relative to either of the other taper conditions reflected very large and significant effect sizes at that time. These findings suggest that PCT-BD provides specific efficacy for the successful discontinuation from BZs, even when controlling for therapist contact and relaxation training.
Keywords: Benzodiazepines, Panic Disorder, Cognitive Behavior Therapy, Drug Withdrawal
Benzodiazepine (BZ) treatment has demonstrated efficacy across a number of anxiety disorders, including panic disorder (for review see Gould, Buckminster, Pollack, Otto, & Yap, 1997; Gould, Otto, & Pollack, 1995; Gould, Otto, Pollack, & Yap, 1997). However, the benefits associated with BZ treatment for panic disorder are attenuated by the difficulties associated with treatment discontinuation (Lader & Petursson, 1983). The abrupt withdrawal of BZ treatment is associated with moderate to severe symptoms of anxiety (Fontaine, Chouinard, & Annable, 1984), and even with careful, gradual tapering, a significant proportion of patients experience symptoms of BZ withdrawal, rebound anxiety, and relapse of the disorder (Mellman & Uhde, 1986; Pecknold, Swinson, Kuch, & Lewis, 1988; Schweizer, Rickels, Case, & Greenblatt, 1991). For example, Noyes et al. (1991) in a study of the efficacy of BZ treatment for panic disorder reported a relapse rate of greater than 70% following medication discontinuation. Indeed, 60% of patients reported post-treatment anxiety symptoms similar to or greater than those at pre-treatment. Many patients will not complete discontinuation attempts given the severity of symptoms associated with discontinuation (Fyer et al., 1987; Noyes, Garvey, Cook, & Suelzer, 1991). Slower taper attenuates, but does not eliminate, these difficulties (for review see Michelini, Cassano, & Perugi, 1996; Salzman, 1993).
The BZ withdrawal syndrome is characterized by anxiety-like symptoms much like those that motivate treatment initiation (Roy-Byrne & Hommer, 1988; Tyrer, Murphy, & Riley, 1990). There is some evidence to suggest that panic patients treated with BZs may be particularly attentive to, and fearful of, these anxiety sensations (Stewart, Westra, Thompson, & Conrad, 2000). Vigilance to such sensations may further intensify during BZ discontinuation, when patients fear the return of their disorder. Thus, discontinuation of BZs may provoke the very symptoms to which such patients are particularly vulnerable (Otto, Pollack, Meltzer-Brody, & Rosenbaum, 1992). Despite the acute anxiolytic efficacy of BZ treatment, it is unlikely to change core fears of panic disorder (fears of anxiety symptoms), and hence may leave them at risk for relapse following treatment discontinuation. Therefore, a focus on treating the fear of somatic sensations – regardless of whether they arise from the discontinuation attempt, panic disorder, or some other source – may be a useful strategy for treating the panic disorder and facilitating BZ discontinuation.
To date, studies in this area have been supportive of this model. In our initial investigation (Otto, Pollack, Sachs, Reiter, Meltzer-Brody, & Rosenbaum, 1993), we studied 33 outpatients with panic disorder who were seeking help in discontinuing their treatment with high-potency BZs (alprazolam or clonazepam). All patients had been treated for a minimum of six months, and were randomized to a supportive taper condition or the supportive taper plus 10 sessions of cognitive-behavioral therapy (see Otto and Pollack, 2009). Treatment components included: (1) informational interventions to demystify the cascade of symptoms and avoidance that characterizes panic disorder, and provide a model for the interventions to follow; (2) cognitive restructuring interventions targeting the elimination of catastrophic beliefs about the meaning and consequences of anxiety and panic sensations as well as symptoms of withdrawal; (3) exposure to feared somatic sensations (interoceptive exposure) to extinguish fear of these sensations regardless of their source, but with specific preparation for sensations that would be experienced as part of the BZ discontinuation; (4) when agoraphobic avoidance was present, exposure to feared situations to help patients eliminate panic-provoking responses to both internal and external situations; and (5), limited use of arousal management strategies including training in diaphragmatic-breathing and muscle-relaxation techniques to attenuate some of the symptoms of the taper process. At study endpoint, dramatic differences in discontinuation success were evident, with only 25% of the slow-taper alone group achieving successful BZ discontinuation, as compared to a 76% success rate among patients who received PCT-BD. In addition, patients successfully discontinuing their BZs had lower levels of distress than prior to discontinuation, supporting the notion that we were treating panic disorder as well as discontinuation difficulties.
Two additional studies provided similar results. In an open trial, 22 patients received 12-weeks of CBT (Panic Control Treatment; Barlow & Craske, 1989) and alprazolam for panic disorder (Hegel, Ravaris, & Ahles, 1994). The CBT intervention included components of psychoeducation, relaxation, cognitive restructuring, and interoceptive exposure; following the fourth session an alprazolam taper was initiated. At one year follow-up, 76% of patients remained off of alprazolam and 85% were panic free (defined as no panic attacks in the past 2 weeks). Spiegel and colleagues (1994) randomized 20 patients with panic disorder to a slow alprazolam taper with or without CBT following successful treatment with the medication. No differences were found post-treatment in BZ discontinuation (80% discontinuation with taper alone vs. 90% with taper plus CBT); however, at six-months post-treatment, the group receiving CBT demonstrated significantly better maintenance of drug discontinuation relative to the taper only group, among whom only 40% successfully discontinued. In a follow-up study of this and another similar study, long-term (2–5 year) follow-up assessments suggested that greater than 75% of patients receiving adjunctive CBT were able to maintain medication discontinuation (Bruce, Spiegel, & Hegel, 1999).
Together, these studies provide consistent support for the application of CBT to facilitate discontinuation of BZ medications. However, these investigations did not control for the additional therapy contact and support received by patients in CBT conditions compared to other groups. In the absence of this control, questions about the active and unique elements of CBT in facilitating BZ discontinuation remain unanswered.
The present study was designed to control for the influence of therapy contact and support by utilizing an additional treatment group that (1) matched the amount of therapy contact of our adjunctive CBT, and (2) trained patients to use relaxation techniques, rather than exposure-based fear reduction techniques, to lessen withdrawal and anxiety symptoms. Although relaxation training may well be useful for attenuating anxiety symptoms when applied, we did not hypothesize that it would alter fears of anxiety symptoms. The use of a relaxation condition allowed for the isolation of the modification of emotional responses to internal cues (in this case, fears of anxiety-related sensations), which is the component of CBT that we believe to be the crucial mechanism for helping patients tolerate BZ withdrawal symptoms (Otto, Safren, & Pollack, 2004). Hence, we hypothesized that CBT would offer superior outcome to both the slow taper condition alone, and the slow-taper condition plus the relaxation-based treatment. In addition, we hypothesized that because of the efficacy of CBT for treating panic disorder as well as aiding BZ discontinuation, the benefits of CBT for the goal of remaining BZ-free would become increasingly evident over the follow-up period, consistent with other studies supporting the durability of treatment gains over time with CBT (Barlow et al., 2000).
Methods
Design Overview
This randomized controlled trial was designed to establish and compare the efficacy of three strategies for the discontinuation of benzodiazepine treatment. Patients with panic disorder were randomized (based on a randomization table created for this study) to one of the following interventions: a conservative taper program alone, a taper program in conjunction with individual relaxation treatment, or a taper program in conjunction with an individual, exposure-based CBT. The conservative taper program was designed to represent the standard of care for BZ discontinuation as it now exists, hence it is referred to as the Taper as Usual condition (TAU). The TAU is the core taper program to which the other conditions are added and compared. The individual relaxation treatment (IRT) provides the additional element of weekly individual treatment that includes training in progressive muscle relaxation skills to be applied to withdrawal and anxiety symptoms. The exposure-based CBT represents the application of cognitive and behavioral methods most strongly associated with reducing the fears of anxiety symptoms which may underlie BZ discontinuation difficulties and subsequent relapse (Otto, Hong, & Safren, 2002). Typical interoceptive exposure exercises included hyperventilation, head rolling, and stair running.
The outcome measure was the successful discontinuation of BZ treatment and maintenance of BZ-free functioning during the follow-up period. Because of the limited sample size (greatly restricting power), differences between conditions were examined with effect-size analyses as well as traditional significance testing.
Participants
Participants were outpatients seeking treatment for help with BZ discontinuation. Individuals who contacted the clinic were screened by telephone for general medical, diagnostic, and treatment eligibility and interest in research participation. Patients were excluded from participation if they had previously undergone behavioral treatment for panic disorder; concurrently used psychoactive medications other than alprazolam, clonazepam, or a single antidepressant medication; were pregnant or lactating; had significant abnormal laboratory values or uncontrolled renal, hepatic, cardiac, pulmonary, endocrinological, CNS, or collagen vascular disease as determined by physical examination and laboratory determinations; were psychotic or currently suicidal; met criteria for a substance use disorder in the last six months (other than nicotine); or had a history of bipolar disorder, schizophrenia, or seizure disorder. Eligible participants were between 18 and 65 years old and met Diagnostic and Statistical Manual, 4th Edition (DSM-IV; American Psychological Association, 1994) criteria for a primary diagnosis of panic disorder with or without agoraphobia according to the Anxiety Disorders Interview Schedule, 4th Edition (ADIS-IV; DiNardo, Brown, & Barlow, 1994). However, we excluded patients who were judged to require additional treatment interventions for panic disorder, generalized anxiety disorder, posttraumatic stress disorder, specific phobia, social phobia, or obsessive-compulsive disorder, as indicated by a score of 6 (“severe”) or greater on the ADIS-IV for these disorders. This was done to differentiate this medication discontinuation trial from treatment trials that include patients who have failed to respond adequately to medication interventions. Concurrent psychotherapeutic treatment for an Axis I anxiety disorder was not allowed, and patients wishing to participate in the study had to discontinue such treatment prior to the baseline assessment. Eligible participants had been receiving treatment for panic disorder with alprazolam or clonazepam for a minimum of 6 months, and during the last month of this treatment were taking between 1.0 to 10.0 mg/day of alprazolam or 0.5 to 5.0 mg/day of clonazepam. Patients with DSM-IV depressive disorders were not excluded from participation unless their 23-item Hamilton Rating Scale for Depression (HRSD; 24-item; Hamilton, 1959) score was greater than 18.
All patients provided written informed consent after the study procedures had been fully explained to them, and all procedures in this study were conducted in accordance with the standards of the Massachusetts General Hospital’s Subcommittee on Human Subjects.
Clinicians
The therapists for the IRT and PCT-BD conditions were highly trained licensed and unlicensed post-doctoral clinical staff in a specialty clinic of a large teaching hospital with experience in the administration of PCT and relaxation interventions. The TAU interventions common to all three taper conditions were performed by psychiatrists (the “monitoring physician”) experienced in the psychopharmacological treatment of panic disorder with benzodiazepines.
Procedures
Patients were block randomized to one of three taper conditions – TAU, IRT, and PCT-BD – on the basis of the following dichotomous variables: benzodiazepine type (alprazolam vs. clonazepam), and concurrent use of an antidepressant medication. Study assessments were conducted by monitoring physicians (who were blind to treatment condition) at baseline, post-medication discontinuation, and follow-up assessments at 2 weeks (posttreatment) and 3 and 6 months post-discontinuation.
Measures
The primary outcome measure for this study was successful discontinuation of BZs (described in detail below). Additionally, the following measures were administered.
The Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV; DiNardo et al., 1994) is a semi-structured diagnostic instrument that assesses for common Axis I disorders including anxiety, mood, somatoform and substance use disorders and includes a screen for psychotic disorders. This clinician-administered measure was used to evaluate clinical diagnoses for consideration of the study inclusion/exclusion criteria.
The Physician Withdrawal Checklist (PWC; Rickels, Schweizer, Case, & Greenblatt, 1990) is a clinician-rated measure that assesses 34 potential symptoms of BZ withdrawal on a severity scale ranging from 0–3. It has demonstrated strong inter-rater (r = 0.91) and test-retest (r = 0.87) reliability (Rickels et al., 1990). In this study, the PWC was administered weekly to assess symptoms of BZ withdrawal.
Additionally, the following self-report symptom measures were included: the Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986), a measure of fear of symptoms related to anxiety; the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) a measure of general symptoms of anxiety; and the Beck Depression Inventory-II (BDI-II; Beck & Steer, 1984), a measure of symptoms of depression. These measures are widely used in the study of anxiety and depression and have demonstrated high internal consistency reliability (see Beck, Epstein, Brown, & Steer, 1988; Beck & Steer, 1984; Peterson & Reiss, 1992). Weekly panic attack frequency was determined through review of a panic diary completed on a daily basis by the patient.
Taper and Treatment Conditions
All taper conditions included the TAU component, which was designed to represent the type of clinical care that patients would receive using standard recommended discontinuation procedures for psychiatric practice (e.g., Colvin, 1983; Dupont, 1990). Elements of this treatment included information on discontinuation effects, a slow taper schedule, weekly clinical monitoring, and encouragement and support regarding discontinuation difficulties. Patients were scheduled to meet with their monitoring physician on a weekly basis during medication discontinuation. Postponement or discontinuation of scheduled dose reductions was decided by individual patients in conjunction with their monitoring physician.
The withdrawal schedule for all patients taking alprazolam was a reduction of the daily dose by 0.25 mg every 2 days for doses above 2.0 mg. Patients who started at 2.0 mg or below, or who reached this level during their taper, underwent a reduction of the daily dose by 0.125 mg every 2 days. Accordingly, the taper lasted approximately 5 weeks for patients starting daily dose of 2 mg, 7 weeks for patients taking 4 mg, and 9 weeks for patients taking 6 mg of alprazolam at baseline. Alprazolam was prescribed on a four-times-per-day (q.i.d.) basis, with the first morning dose being the last to be discontinued. Patients taking clonazepam followed a similar taper schedule adjusted for the approximate 2:1 difference in potency relative to alprazolam and the smallest pill size (0.5 mg) available at the time for clonazepam. Hence, patients taking clonazepam had their daily dose reduced by 0.25 mg every four days for daily doses above 1.0 mg, or by 0.25 mg every eight days for daily doses of 1.0 mg or less. Patients taking clonazepam were prescribed on a twice-per-day (b.i.d.) basis.
Patients recorded the actual number of doses they took in the space provided on their written taper schedule, which was collected at each visit. This written withdrawal schedule served as a guide for dose reduction and was complemented by take-home panic diaries. Monitoring physicians uniformly instructed patients to attempt to use no more than one p.r.n. dose each week.
Exposure-based CBT
Participants receiving taper plus cognitive-behavioral treatment received all of the elements of TAU, including identical taper schedules, but also received eight weekly, individual exposure-based CBT sessions, followed by three booster sessions scheduled at intervals of two weeks, four weeks, and six weeks, respectively. Patients met independently with their TAU and CBT clinicians. Patients in the CBT initiated their TAU taper after the third CBT session. All sessions lasted 60 minutes, except the initial 90-minute session.
The CBT under study (Otto & Pollack, 2009) combined four primary treatment components: an informational component, interoceptive exposure, somatic coping skills, and cognitive restructuring. The informational component comprised a review of behavioral patterns in panic disorder and a rationale of the alternative responses to be learned in treatment, discussion of the role of medications in blocking panic and review of behavioral alternatives, and identification of symptoms of both withdrawal and panic and maladaptive interpretations of symptoms. Interoceptive exposure procedures repeatedly exposed patients to somatic sensations associated with their panic attacks and, potentially, their medication discontinuation, to weaken the exaggerated emotional responses and anticipatory fear of these sensations. The somatic skills component included breathing retraining designed to reduce the intensity of physical symptoms of hyperventilation and training in muscle relaxation procedures to help reduce anticipatory anxiety and to help patients cope with withdrawal-associated increases in muscle tension, anxiety, and agitation. In the cognitive component of CBT, patients were instructed in strategies to modify maladaptive cognitions related to their disorder- and BZ discontinuation-induced anxiety and panic sensations through interventions targeted at decreasing catastrophic misinterpretations of symptoms and increasing cognitive self-control skills.
Individual Relaxation Therapy (IRT)
In addition to the nonspecific support provided by therapist contact, IRT involves two treatment components: an informational component and progressive muscle relaxation training. The informational component includes a review of the time course and nature of withdrawal symptoms and discussion of these symptoms in an individual setting as they occur. Relaxation training includes training and review of progressive muscle relaxation procedures in session and home assignment of these skills. Progressive muscle relaxation is based on procedures outlined by Bernstein and Borkovec (1973) and represents a modification of the application of these procedures to panic disorder (Barlow, Craske, Cerny, & Klosko, 1989; Craske, Brown, & Barlow, 1991). In the treatment, the number of muscle groups targeted for relaxation is reduced across treatment sessions from 16, to 8, to 4. Discrimination training, the induction of relaxation by recall, and the use of cue-controlled relaxation is also included. The number, timing, and length of the IRT sessions were identical to the PCT-BD sessions. Patients in the IRT condition also initiated their TAU taper after the third session.
Outcome Criteria
To be included in the primary outcome analyses, patients must have completed the baseline assessment and been scheduled for at least one dose reduction at the time of dropout. Patients were asked to continue their daily and weekly assessments for four weeks post-withdrawal. The final two of these weeks constituted the post-discontinuation assessment period.
The principal dependent measure was the proportion of patients successfully achieving discontinuation for each of the treatment conditions. Successful discontinuation was defined as completion of the taper schedule without significant deviation and no use of benzodiazepine medications beyond “minimal p.r.n. use” during the month following the zero-dose date. Significant deviation from the taper schedule included (1) failure to make a scheduled dose decrease for a 10 day period, or (2) falling more than 14 days behind the allowed taper schedule dose for any consecutive three day period, or (3) continued BZ use beyond 14 days from the scheduled zero-dose date. These deviations from the taper schedule and all other results beyond the BZ use criteria were considered treatment failures for the purpose of data analyses. Minimal BZ use was defined as use of no more than two p.r.n. doses of medication (each not exceeding 0.5 mg alprazolam or clonazepam) during the four week period starting at the zero-dose date. This a priori criterion insured that patients taking a minimal p.r.n. dose for extraordinary circumstances or relatively rare phobic events (e.g., trip to the dentist or a yearly plane trip) were not considered discontinuation failures.
The credibility of the treatment conditions as judged by patients was evaluated by three questions, each rated on a Likert scale, similar to the format utilized by Borkovec and Mathews (1988). Treatment credibility was administered after the second session by the research assistant. The three questions assessed perceptions about how logical treatment was, expected outcome (i.e., success), and whether the patient would recommend the treatment to a friend.
Results
Differences between groups in continuous variables were examined with one-way ANOVAs, with follow-up pairwise t-tests. Group differences in categorical outcomes were evaluated with chi-square tests, with follow-up evaluation of pairwise differences with two-tailed Fisher’s Exact Tests. Logistic regression was used for predictor analysis utilizing continuous variables and dichotomous outcomes. Given the limited sample size, traditional significance testing was complemented by evaluation of effect sizes (d, Cohen, 1988); for categorical outcomes, effect sizes were computed from proportions as per Glass and colleagues (Glass, McGaw & Smith, 1981, p. 139).
Baseline Patient Characteristics
Forty-seven patients (31 women) provided informed consent, underwent screening, and were randomized to treatment groups: taper plus CBT (n = 16), taper plus IRT (n = 16), and taper as usual (n = 15). Table 1 presents baseline data on age, sex, medication status, psychiatric comorbidity, and symptom levels in randomized participants. Four participants in each group were taking antidepressant medications at baseline and continued their stable doses of these medications throughout the taper and post-discontinuation periods.
Table 1.
Demographics, medication, psychiatric comorbidity, and severity of anxiety symptoms in randomized participants at baseline
| Taper Group | |||
|---|---|---|---|
| PCT-BD | IRT | TAU | |
| n | 16 | 16 | 15 |
| Age (yrs) | 44.8 ± 13.9 | 35.8 ± 9.2 | 39.7 ± 9.1 |
| Female (n) | 9 (56%) | 10 (63%) | 12 (80%) |
| Benzodiazepine | |||
| alprazolam (n) | 4 (25%) | 3 (19%) | 3 (20%) |
| dose (mg) | 2.8 ± 3.2 | 2.8 ± 1.3 | 1.7 ± 0.3 |
| duration (yrs) | 5.6 ± 3.5 | 6.4 ± 6.6 | 2.7 ± 1.2 |
| clonazepam (n) | 12 (75%) | 13 (81%) | 12 (80%) |
| dose (mg) | 1.4 ± 0.5 | 1.1 ± 0.5 | 1.1 ± 0.7 |
| duration (yrs)* | 4.9 ± 4.3 | 5.2 ± 4.0 | 3.3 ± 3.9 |
| Number on an antidepressant (n) | 4 (25%) | 4 (25%) | 4 (27%) |
| Comorbid anxiety disorder (n) | 7 (44%) | 9 (56%) | 9 (60%) |
| Comorbid depressive disorder (n) | 9 (56%) | 6 (38%) | 8 (53%) |
| Symptom Measures | |||
| ASI | 29.0 ± 10.7 | 27.8 ± 12.0 | 25.7 ± 13.6 |
| BAI | 13.3 ± 8.0 | 18.0 ± 8.2 | 12.2 ± 8.8 |
| PWC | 12.6 ± 5.9 | 17.5 ± 20.8 | 13.8 ± 10.7 |
| Number of Panic Attacks (sq. root) | .6± .7 | 1.0 ± 1.2 | .5 ± .7 |
Duration values missing for one patient in each group
ASI = Anxiety Sensitivity Index, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory, PWC = Physician’s Withdrawal Checklist
Therapy Credibility
Significant differences between groups emerged only for the second item assessing expected outcome (F [2, 33] = 9.46, p < .001). When pairwise contrasts were examined, both CBT and IRT were found to be more credible for expected outcomes (both p-values < .01), but the two active treatments did not differ from each other; no treatment differences were evident for the other items (p-values > .11).
BZ-Free Status
Two patient in the CBT program prematurely discontinued treatment and their final BZ status was unknown. To provide a conservative analysis of the data, both of these patients were considered to have failed BZ discontinuation. At the post-discontinuation visit, 56.3% of patients treated with exposure-based CBT had achieved BZ-free status, as compared to 31.3% in the IRT condition, and 40% in the TAU condition. Differences between the CBT and IRT conditions reflected a medium to large effect size (d = 0.65), whereas differences between CBT and TAU reflected a small to medium effect size (d = 0.38), but in this small-sample study none of the differences between groups reached statistical significance (X2 = 2.10, df = 2, n = 47, p = .35). The difference between IRT and TAU reflected a small effect size (d = 0.24)
We examined a number of baseline covariates in logistic regression analyses, seeking to predict discontinuation success at the end of the post-discontinuation period. The results were notable for how little diagnostic or treatment characteristics were linked to discontinuation success. Of the variables examined—sex, BZ type (alprazolam or clonazepam), antidepressant medication use, depression comorbidity, anxiety comorbidity, dose of BZ, and years of use of BZs—none were statistically significant (all p values > .19). There was some indication, by effect size, that patients taking antidepressants were less likely to have successfully discontinued their BZ use by the post-discontinuation visit (25% success rate) as compared to those not taking antidepressants (48% success rate); however this was not statistically significant (p = .19, d = 0.62). Because antidepressant use was a block randomization factor, it did not confound evaluation of treatment effects, with identical numbers of patients (n = 4) taking antidepressant in each treatment cell (see Table 1).
At three month follow-up, a number of patients missed evaluation appointments. If patients had a BZ-free status at both the previous visit (acute outcome visit) and the subsequent visit (6-month visit), a BZ-free status was assigned; otherwise missing values were assumed to be treatment failures, ensuring a conservative analysis of discontinuation success rates. At this evaluation, similar large effect-sizes were evident for the advantage of CBT over the other two groups, with 43.7% of patients in PCT-BD in BZ-free status compared to 26.7% for TAU patients (d = 0.47) 12.5% for IRT patients (d = 1.05), with a trend toward an advantage for CBT (χ2 = 3.92; df = 2, n = 47, p = .141). The trend advantage for CBT reached significance over IRT (p < .045) only when years of benzodiazepine use was treated as a covariate in a logistic regression analysis (see below).
The advantage for PCT-BD became more evident by the 6-month follow up with 62.5% BZ-free in PCT-BD, compared to only 12.5% in IRT and 26.7% in TAU (χ2 = 9.44, df = 2, n = 47, p < .01). Follow-up tests of this significant overall effect indicated that PCT-BD had significantly higher BZ-free rates than the IRT (Fisher’s exact test p = .009, d = 1.53) with a trend toward a similar advantage over TAU (Fisher’s exact test p = .073, d = 0.95). We examined this effect in more complex, logistic regression models. In addition to the treatment group, the first model included benzodiazepine characteristics—type of BZ, BZ dose, years of BZ use, and antidepressant use—as covariates, and the second model included symptoms characteristics—anxiety and depression comorbidity, current ASI, BAI, BDI, and panic frequency scores—as covariates. Of these covariates, only years of BZ use emerged as a significant predictor (p < .02); the mean years (+SD) taking BZs was 2.4±2.4 for those remaining BZ-free and 5.7±4.2 for those failing to remain BZ-free. In the context of this single covariate, the PCT-BD treatment demonstrated significantly better outcome for BZ-free status than both the IRT (p < .006) and TAU (p < .008) conditions at the six month evaluation period.
Distress at Post-treatment and Follow-up
PWC scores were included only for participants continuing with the taper. These scores indicated no differences between groups in BZ withdrawal symptoms as patients progressed through taper at the point of 75%, 50%, and 25% reductions from their original dose (all p-values > .41), but a significant difference emerged at the zero dose assessment (F [2, 23] = 3.51, p < .047), with those subjects in the TAU condition who successfully discontinued reporting less withdrawal distress relative to the other groups (PWC mean±SD, 7.1±8.0 for TAU, 17.8±13.3 for IRT, and 14.5±17.7 for CBT). As shown in Figure 1, which shows scores for patients reaching each assessment point, as well as scores for the last visit carried forward, this effect is reflective of the attrition in the sample. The last-assessment-carried-forward scores shows different pattern reflecting more equal levels of distress across the taper groups when data from patients who did not complete the taper were included in the analysis (F [2,44] = 1.82, p < .18). This distress did not translate into differential panic frequency once the post-taper period was reached. For all participants who completed this assessment, regardless of whether they returned to benzodiazepine treatment, no significant differences were evident for panic frequency (F [2,39] =.143, p < .87).
Figure 1.
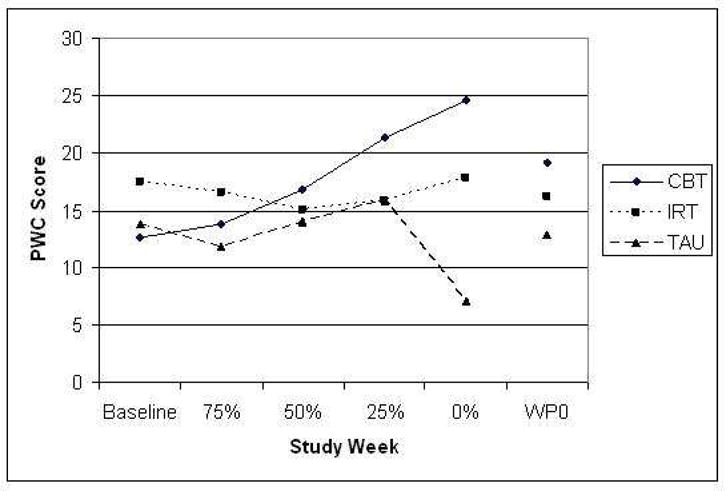
Withdrawal checklist scores across the designated weeks of taper for each taper condition. Sample sizes decrease across the study weeks (from n = 16 to n = 11 for the CBT group, from n = 16 to n = 7 for the IRT group, from n = 15 to n = 8 for the TAU group), and WP0 represents the study week prior to reaching the lowest dose obtained. PWC is the Physician Withdrawal Checklist.
Therapist Adherence and CBT Outcome
Adherence as evaluated by taped session material was rated according to a scale with critical items defined for each session, with rating of adherence to each of these treatment elements on a 7-point Likert scale (and examined as a percent of total potential score) by an independent rater (not otherwise connected with the study) who was trained by the first author in identifying study elements. Adherence overall was strong (mean = 83.1%, SD = 11.8%). Blind ratings of these therapist behaviors for the 14 patients assigned to the CBT condition revealed a trend toward greater efficacy (acute BZ-free status) when there was greater adherence to the treatment manual as defined by the total adherence scores for each patient (p < .09).
Discussion
This randomized controlled trial evaluated the relative efficacy of three interventions for benzodiazepine discontinuation among panic disorder patients: taper alone (TAU), taper plus relaxation (IRT), and taper plus exposure-based CBT (Otto & Pollack, 2009) in 47 patients with panic disorder seeking BZ discontinuation. Given the small sample size, we focused on effect sizes as well as significance levels and utilized a conservative approach to missing data. Consistent with previous findings, adjunctive CBT significantly increased rates of successful BZ discontinuation relative to taper alone by the 6-month follow-up evaluation. Also consistent with previous findings, the type of BZ and other baseline characteristics had little effect on outcome (see Otto et al., 1994), although years of benzodiazepine use did emerge as a significant predictor of longer term outcomes; those who had been using BZs longer had a more difficult time remaining BZ free at the three and six month evaluation periods.
One interpretation of the profile of withdrawal symptoms across the taper period is that CBT helped patients continue taper despite the presence of withdrawal symptoms, whereas patients in the other two groups completed the taper only if they were at a lower level of symptomatic distress. Also, as reflected by panic frequency, despite the return of more patients in the other two groups to BZ use, there was no evidence of a worsening of panic in the CBT group at the post-taper evaluations.
This study of patients with panic disorder is unique in controlling for the additive effects of therapist time and support as well as relaxation training during BZ discontinuation. Patients who received CBT had significantly higher rates of discontinuation success than those who received relaxation training (IRT). We found no evidence that IRT offered an advantage over slow taper alone, with all effects indicating a disadvantage for IRT on the order of a small effect size. This finding is consistent with recent data suggesting that attempts to manage symptoms with arousal reduction strategies (e.g., relaxation training or breathing retraining) offers little additional benefit to packages of CBT treatment for panic disorder (see Schmidt et al., 2000). Given evidence for the deleterious effect of safety behaviors on outcomes in panic disorder (Salkovskis Clark, Hackmann, Wells, & Gelder, 1999), relaxation presumably could have resulted in worse outcome relative to TAU if this strategy served as safety behavior for patients. Also, the failure of IRT to offer beneficial outcome suggests that non-specific effects are not likely to account for effects of our program of exposure-based CBT.
We found CBT was superior on the order of large effect sizes to IRT for BZ discontinuation success, and tended to have an advantage on the order of medium to large effect size for TAU depending on the evaluation period; effect sizes increased over the follow-up interval, presumably due to changes in the core patterns underlying panic (e.g., anxiety sensitivity, avoidance and safety behaviors) in the CBT condition. Although power to detect significant differences was limited by the small sample sizes, these treatment effects grew over time, consistent with the notion that patients learned skills for responding differently to somatic sensations of both BZ withdrawal and panic. Thus, CBT may offer both a more durable treatment relative to medication and one that buffers the return of panic in response to symptoms associated with medication discontinuation. This success stands in contrast to the limited success reported for pharmacologic strategies to aid benzodiazepine discontinuation (see Oude Voshaar, Couvée, van Balkom, Mulder, & Zitman, 2006; Parr, Kavanagh, Cahill, Mitchell & Yount, 2009).
We also found a trend association between adherence to the CBT manual and acute outcome for CBT treatment. Because this analysis is only applicable to patients in the CBT condition, it is necessarily limited by the small sample size available for analysis, and should be interpreted cautiously. Nonetheless, this finding suggest that active elements of treatment may indeed be captured well by the manual and suggest that fidelity to the manual may be important for the additive benefits of CBT over nonspecific treatment elements.
BZs remain a frequently prescribed intervention, and the ability of exposure-based CBT to facilitate successful discontinuation while attending to core patterns maintaining the panic disorder provides clinicians with an effective alternative to long term medication management. Regardless of the source of fear of somatic sensations (e.g., the taper, panic disorder, etc.) this approach may aid in successful transition to being both medication-free and panic-free. Indeed, decreases are also seen in distress following discontinuation, suggesting that panic disorder symptoms are also changing with the intervention. This treatment strategy also appears to apply to discontinuation of antidepressant medications (Whittal, Otto, & Hong, 2001). Furthermore, principles from this treatment may have applications more generally to other drug discontinuation issues. Specifically, this conceptualization has been expanded to the treatment of substance abuse, in which altering the response to interoceptive cues (both physical and emotional) may aid in discontinuing harmful substance use patterns (Otto, Safren, & Pollack, 2004). Successful BZ discontinuation programs using disorder-specific CBT interventions (vs. general programs, see Oude-Voshaar et al., 2006; p. 503) have also been reported for generalized anxiety disorder (Gosselin, Ladouceur, Morin, Dugas, & Baillargeon, 2006) and insomnia (Morin et al., 2004).
In addition, the availability of an efficacious treatment strategy for BZ discontinuation has broader public health implications. For example, the accrual of costs over time from the use of pharmacotherapy for panic disorder results in less favorable cost-efficacy ratios relative to CBT (see McHugh et al., 2007; Otto, Pollack, & Maki, 2000). Thus, the application of an intervention to aid in successful medication discontinuation while maintaining treatment gains has the potential for substantial cost savings.
This study has several limitations, the most prominent of which is a limited sample size. Given this limitation, we have provided effect sizes to complement traditional significance testing. As is common in studies of this kind, our results are also limited by the reliance on self-report of benzodiazepine use without biological verification of benzodiazepine levels. Also, we did not assess for allegiance effects in our study.
In summary, the results from this randomized controlled trial support findings from previous studies suggesting that adjunctive CBT facilitates discontinuation from BZs among those with panic disorder and prevents the return of panic symptoms often seen with discontinuation. This study expands upon previous investigations to control for the amount of therapist contact provided in an adjunctive CBT intervention through comparing it with an individual relaxation intervention. Results suggest that CBT has a specific effect on BZ discontinuation beyond that accounted for by therapist contact alone. Given the reported high rates of unsuccessful BZ discontinuation and return of panic symptoms upon medication discontinuation, adjunctive CBT provides a particularly promising strategy for aiding with the discontinuation of BZ treatment in panic patients.
Acknowledgments
Work on this project was supported by NIDA grant R10 DA09692 to Dr. Otto. The authors are aware of no conflicts with the content of this manuscript, nonetheless Dr. Otto would like to report current consultant and research support from Schering-Plough, and royalties received in the last year for use of the SIGH-A from Lilly. Dr. Pollack would like to report advisory board and/or consultation from Brain Cells, Eli Lilly, Medavante, Mindsite, Targia Pharmaceuticals, and Pfizer; research grant support from Bristol Myers Squibb, Forest Laboratories, GlaxoSmithKline, Eli Lilly, NCCAM, NIDA, NIMH, and Sepracor; CME supported activities from Astra-Zeneca, Sepracor, and Pfizer; equity interests in Medavante, Mensante Corporation, Mindsite, and Targia Pharmaceuticals; and royalty or patent payments regarding the SIGH-A and SAFER interviews. Dr. Pollack would like to report advisory board and/or consultation from Astra Zeneca, Cephalon, Forest Laboratories, Glaxo SmithKline, Janssen, Lilly, NARSAD, NIMH, Pfizer, UCB-Pharma, Sepracor; and speaking/CME supported activities from MGH Psychiatry Academy, Astra Zeneca, and Pfizer. Dr. Worthington would like to report grant-research support from Eli Lilly & Company, Pfizer Inc, and Sepracor; and speaker support from Pfizer Inc. The remaining authors have no conflicts to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Barlow DH, Craske MG. Mastery of your anxiety and panic (MAP) Albany, NY: Graywind; 1989. [Google Scholar]
- Barlow DH, Craske MG, Cerny JA, Klosko JS. Behavioral treatment of panic disorder. Behavior Therapy. 1989;20:261–282. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. Journal of Clinical Psychology. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Bernstein DA, Borkovec TD. Progressive relaxation training. Champaign, IL: Research Press; 1973. [Google Scholar]
- Borkovec TD, Mathews AM. Treatment of nonphobic anxiety disorders: A comparison of nondirective, cognitive, and coping desensitization therapy. Journal of Clinical and Consulting Psychology. 1988;56:877–884. doi: 10.1037//0022-006x.56.6.877. [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Spiegel DA, Hegel MT. Cognitive-behavioral therapy helps prevent relapse and recurrence of panic disorder following alprazolam discontinuation: A long-term follow-up of the Peoria and Dartmouth studies. American Journal of Psychiatry. 1999;67:151–156. doi: 10.1037//0022-006x.67.1.151. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Colvin M. A counseling approach to outpatient benzodiazepine detoxification. Journal of Psychoactive Drugs. 1983;15:105–108. doi: 10.1080/02791072.1983.10472130. [DOI] [PubMed] [Google Scholar]
- Craske MG, Brown TA, Barlow DH. Behavioral treatment of panic: A two year follow-up. Behavior Therapy. 1991;22:289–304. [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime Version (ADIS-IV-L) San Antonia, TX: Psychological Corporation; 1994. [Google Scholar]
- Dupont RL. Thinking about stopping treatment for panic disorder. Journal of Clinical Psychiatry. 1990;51:38–45. [PubMed] [Google Scholar]
- Fontaine R, Chouinard G, Annable L. Rebound anxiety in anxious patients after abrupt withdrawal of benzodiazepine treatment. American Journal of Psychiatry. 1984;141:848–852. doi: 10.1176/ajp.141.7.848. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Liebowitz MR, Gorman JM, Campeas R, Levin A, Davies SO, Goetz D, Klein DF. Discontinuation of alprazolam treatment in panic patients. American Journal of Psychiatry. 1987;144:303–308. doi: 10.1176/ajp.144.3.303. [DOI] [PubMed] [Google Scholar]
- Glass GV, McGaw B, Smith ML. Meta-analysis in social research. Beverly Hills, CA: Sage; 1981. [Google Scholar]
- Gosselin P, Ladouceur R, Morin CM, Dugas MJ, Baillargeon L. Benzodiazepine discontinuation among adults with GAD: A randomized trial of cognitive-behavioral therapy. Journal of Consulting and Clinical Psychology. 2006;74:908–919. doi: 10.1037/0022-006X.74.5.908. [DOI] [PubMed] [Google Scholar]
- Gould RA, Buckminster D, Pollack MH, Otto MW, Yap L. Cognitive-behavioral and pharmacological treatment for social phobia: A meta-analysis. Clinical Psychology: Science and Practice. 1997;4:291–306. [Google Scholar]
- Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Clinical Psychology Review. 1995;15:819–844. [Google Scholar]
- Gould RA, Otto MW, Pollack MH, Yap L. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: A preliminary meta-analysis. Behavior Therapy. 1997;28:285–305. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegel MT, Ravaris CL, Ahles TA. Combined cognitive-behavioral and time-limited alprazolam treatment of panic disorder. Behavior Therapy. 1994;25:183–195. [Google Scholar]
- Lader MH, Ron M, Petursson H. Computed axial brain tomography in long-term benzodiazepine users. Psychological Medicine. 1984;14:203–206. doi: 10.1017/s0033291700003214. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Otto MW, Barlow DH, Gorman JM, Shear MK, Woods SW. Cost-efficacy of individual and combined treatments for panic disorder. Journal of Clinical Psychiatry. 2007;68:1038–1044. doi: 10.4088/jcp.v68n0710. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Uhde TW. Withdrawal syndrome with gradual tapering of alprazolam. American Journal of Psychiatry. 1986;143:1464–1466. doi: 10.1176/ajp.143.11.1464. [DOI] [PubMed] [Google Scholar]
- Michelini S, Cassano GB, Frare F, Perugi G. Long-term use of benzodiazepines: Tolerance, dependence and clinical problems in anxiety and mood disorders. Pharmacopsychiatry. 1996;29:127–134. doi: 10.1055/s-2007-979558. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallières A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. American Journal of Psychiatry. 2004;161:332–342. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- Noyes R, Garvey MJ, Cook B, Suelzer M. Controlled discontinuation of benzodiazepine treatment for patients with panic disorder. American Journal of Psychiatry. 1991;148:517–523. doi: 10.1176/ajp.148.4.517. [DOI] [PubMed] [Google Scholar]
- Otto MW, Hong JJ, Safren SA. Benzodiazepine discontinuation difficulties in panic disorder: Conceptual model and outcome for cognitive-behavior therapy. Current Pharmaceutical Design. 2002;8:75–80. doi: 10.2174/1381612023396726. [DOI] [PubMed] [Google Scholar]
- Otto MW, Pollack MH. Stopping anxiety medication (Therapist guide, 2nd Edition) New York: Oxford University Press; 2009. [Google Scholar]
- Otto MW, Pollack MH, Maki KM. Empirically supported treatments for panic disorder: costs, benefits, and stepped care. Journal of Consulting and Clinical Psychology. 2000;68:556–563. [PubMed] [Google Scholar]
- Otto MW, Pollack MD, Meltzer-Brody S, Rosenbaum JF. Cognitive-behavioral therapy for benzodiazepine discontinuation in panic disorder patient. Psychopharmacology Bulletin. 1992;28:123–130. [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Sachs GS, Reiter SR, Meltzer-Brody S, Rosenbaum JF. Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavior therapy for patients with panic disorder. American Journal of Psychiatry. 1993;150:1485–1490. doi: 10.1176/ajp.150.10.1485. [DOI] [PubMed] [Google Scholar]
- Otto MW, Safren SA, Pollack MH. Internal cue exposure and the treatment of substance use disorders: lessons from the treatment of panic disorder. Journal of Anxiety Disorders. 2004;18:69–87. doi: 10.1016/j.janxdis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Oude Voshaar RC, Couvée JE, van Balkom AJ, Mulder PG, Zitman FG. Strategies for discontinuing long-term benzodiazepine use: meta-analysis. British Journal of Psychiatry. 2006;189:213–220. doi: 10.1192/bjp.189.3.213. [DOI] [PubMed] [Google Scholar]
- Oude-Voshaar RC, Gorgels WJ, Mol AJ, van Balkom AJ, Van de Lisdonk EH, Breteler MH, van den Hoogen HJ, Zitman FG. Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: Three-conditioned, randomized controlled trial. British Journal of Psychiatry. 2006;182:498–504. doi: 10.1192/bjp.182.6.498. [DOI] [PubMed] [Google Scholar]
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, Young RMcD. Effectiveness of current treatment approaches for benzodiazepine discontinuation: A meta-analysis. Addiction. 2009;104:13–24. doi: 10.1111/j.1360-0443.2008.02364.x. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, Swinson RP, Kuck K, Lewis CP. Alprazolam in panic disorder and agoraphobia: Results from a multicenter trial, III: Discontinuation effects. Archives of General Psychiatry. 1988;45:429–436. doi: 10.1001/archpsyc.1988.01800290043006. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Revised manual. Worthington, OH: International Diagnostic Systems Publishing Corportation; 1992. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines: I. Effects of abrupt discontinuation. Archives of General Psychiatry. 1990;47:899–907. doi: 10.1001/archpsyc.1990.01810220015002. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Hommer D. Benzodiazepine withdrawal: overview and implications for the treatment of anxiety. American Journal of Medicine. 1988;84:1041–1052. doi: 10.1016/0002-9343(88)90309-9. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Clark DM, Hackmann A, Wells A, Gelder MG. An experimental investigation of the role of safety-seeking behaviours in the maintenance of panic disorder with agoraphobia. Behaviour Research and Therapy. 1999;37:559–574. doi: 10.1016/s0005-7967(98)00153-3. [DOI] [PubMed] [Google Scholar]
- Salzman C. Benzodiazepine treatment of panic and agoraphobic symptoms: Use, dependence, toxicity, abuse. Journal of Psychiatric Research. 1993;27:97–110. doi: 10.1016/0022-3956(93)90021-s. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J. Dismantling cognitive-behavioral treatment for panic disorder: Questioning the utility of breathing retaining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Rickels K, Case WG, Greenblatt DJ. Carbamazepine treatment in patients discontinuing long-term benzodiazepine therapy. Archives of General Psychiatry. 1991;48:448–452. doi: 10.1001/archpsyc.1991.01810290060012. [DOI] [PubMed] [Google Scholar]
- Spiegel DA, Bruce TJ, Gregg SF, Nuzzarello A. Does cognitive behavior therapy assist slow-taper alprazolam discontinuation in panic disorder? American Journal of Psychiatry. 1994;151:876–881. doi: 10.1176/ajp.151.6.876. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Westra HA, Thompson CE, Conrad BE. Effects of naturalistic benzodiazepine use on the selective processing of threat cues among anxiety disorder patients. Cognitive Therapy and Research. 2000;24:67–85. [Google Scholar]
- Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. Journal of Affective Disorders. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- Whittal ML, Otto MW, Hong JJ. Cognitive-behavior therapy for discontinuation of SSRI treatment of panic disorder: A case series. Behaviour Research and Therapy. 2001;39:939–945. doi: 10.1016/s0005-7967(00)00067-x. [DOI] [PubMed] [Google Scholar]


