Abstract
The vestibulo-ocular reflex (VOR) is the main vision-stabilising system during rapid head movements in humans. A visual-vestibular mismatch stimulus can be used to train or adapt the VOR response because it induces a retinal image slip error signal that drives VOR motor learning. The training context has been shown to affect VOR adaptation. We sought to determine whether active (self-generated) versus passive (externally imposed) head rotation vestibular training would differentially affect adaptation and short-term retention of the active and passive VOR responses. Ten subjects were tested, each over six separate 1.5-h sessions. We compared active versus passive head impulse (transient, rapid head rotations with peak velocity ~ 150 °/s) VOR adaptation training lasting 15 min with the VOR gain challenged to increment, starting at unity, by 0.1 every 90 s towards one side only (this adapting side was randomised to be either left or right). The VOR response was tested/measured in darkness at 10-min intervals, 20-min intervals, and two single 60-min interval sessions for 1 h post-training. The training was active or passive for the 10- and 20-min interval sessions, but only active for the two single 60-min interval sessions. The mean VOR response increase due to training was ~ 10 % towards the adapting side versus ~2 % towards the non-adapting side. There was no difference in VOR adaptation and retention between active and passive VOR training. The only factor to affect retention was exposure to a de-adaptation stimulus. These data suggest that active VOR adaptation training can be used to optimally adapt the passive VOR and that adaptation is completely retained over 1 h as long as there is no visual feedback signal driving de-adaptation.
Keywords: vestibulo-ocular reflex (VOR), VOR adaptation, active and passive VOR, retention of VOR adaptation, VOR training
INTRODUCTION
Classic studies of motor learning have shown that the vestibulo-ocular reflex (VOR) can be modified up or down using a visual-vestibular mismatch stimulus (Gauthier and Robinson 1975; Gonshor and Jones 1976a, b). The VOR maintains images stable on the retina during head movement by rotating the eyes in the opposite direction to the head. During far-viewing, the magnitude of eye velocity matches head velocity so that the gain (eye/head velocity) equals unity. Initially, during training, the VOR produces an eye movement that does not stabilise images on the fovea of the retina, which generates a retinal image slip error signal used to drive adaptive change within the VOR (Gauthier and Robinson 1975). Prior studies examining VOR adaptation have used lenses (e.g. Gauthier and Robinson 1975; Paige and Sargent 1991), reversing prisms (e.g. Gonshor and Jones 1976a, b), or moving visual displays (e.g. Shelhamer et al. 1992, 1994) paired with head movement to induce visual-vestibular mismatch. In addition to the visual signal, the head movement stimulus too has varied to include active/self-generated head motion during normal activities or having subjects undergo whole-body sinusoidal rotations in a rotary chair (e.g. Gauthier and Robinson 1975). A disadvantage with some of the prior adaptation studies is that the rotary chair training and testing stimuli have occurred at low frequencies of ~ 0.25 Hz (ranging from 0.025 to 4 Hz) with low peak velocities of ~ 60 °/s (ranging from 20 to 120 °/s) (e.g. Hattori et al. 2000; Solomon et al. 2003; Shelhamer et al. 1994; Paige and Sargent 1991), well below the frequency/velocity content of head motion encountered in daily life (Grossman et al. 1988). Recent studies have also used active transient head impulses as the VOR training stimulus (Schubert et al. 2008a; Migliaccio and Schubert 2013, 2014, Fadaee and Migliaccio 2016), but this is also a predictable stimulus. In summary, the majority of VOR training stimuli used in prior VOR adaptation studies have consisted of predictable head motion, either via self-generation or whole-body sinusoidal passive head rotation.
The passive head impulse is unpredictable in direction and timing (Halmagyi and Curthoys 1988) and is considered to be a more physiologically relevant stimulus than a single-frequency sinusoidal stimulus (as would be obtained from chair testing) due to its high-frequency content (up to 6 Hz). Additionally, the VOR must be responsive to unpredictable head motion, particularly at frequencies greater than 1 Hz (and velocities > 100 °/s) where the VOR becomes the main vision-stabilising mechanism. Below 1 Hz (and velocities < 100 °/s), other vision-stabilising systems such as smooth pursuit and the optokinetic reflex are likely to play the major role (Meyer et al. 1985). Finally, motor learning within the VOR is context-specific (human: Shelhamer et al. 1992; primate: Yakushin et al. 2003; Schubert et al. 2008b), which suggests that adaptation will be greatest when the VOR training and testing conditions are the same. The training context might therefore result in differences in retention of VOR adaptation depending on the similarity between training and testing conditions. We hypothesised that passive head impulse visual-vestibular mismatch training would result in greater magnitude VOR adaptation than active training, especially in response to rapid unpredictable passive head rotations, i.e. the mode in which the VOR is most essential.
The goal of this study was to determine whether active or passive VOR training affects how the active and passive VOR responses adapt and retain that adaptation. This study used the unilateral incremental VOR adaptation technique and exposed subjects to active or passive head impulses, while visually tracking a laser target that moves in the opposite direction to the head at an incrementally increasing percentage of head speed (Schubert et al. 2008a; Migliaccio and Schubert 2013, 2014; Fadaee and Migliaccio 2016). This method drives the VOR gain to increase significantly on one side after only 15 min of training. Short-term retention of the active and passive VOR gain increases was measured. To our knowledge, this is the first study examining the effects of passive versus active head movement training contexts on VOR adaptation.
METHODS
Subjects
Ten normal subjects (mean age 35 years, range 24–49 years) were recruited to participate in this study. Each subject participated in six separate 1.5-h long sessions, separated by at least 3 days with sessions not repeated. These subjects did not have any history or clinical signs of vestibular abnormality. Participation in this study was voluntary and subjects gave written informed consent before participating as approved by the University of New South Wales Human Ethics Committee.
Recording System
Head and eye rotations were measured using the EyeSeeCam system (Denmark), with the camera placed over the left eye (Bartl et al. 2009). The EyeSeeCam system consisted of a 220-Hz digital video camera, an infrared mirror to reflect the eye image to the camera, and an inertial measurement unit to measure 3D (yaw, pitch, and roll) angular head velocity. All components were rigidly mounted onto a lightweight swim goggle frame to minimise camera slippage relative to the head. In addition, silicon putty (Surgipack, Australia) was placed between the frame and face to further minimise slip and add some comfort to the tight fit, especially since subjects wore the goggles for the duration of each one and half hour session. The eye was illuminated via two on-board infrared LEDs. Horizontal and vertical eye positions were calibrated by having subjects fixate (goggle-mounted laser projected) visual targets at known angles with respect to the subject. The calibrated data were digitally filtered with a 50-tap zero-phase low-pass FIR filter with a bandwidth of 50 Hz.
Training System
An improved digital version of the analogue portable laser target system described in Migliaccio and Schubert (2014) controlled the position of a laser target directed onto a matte-white projection screen (2.4 × 2.4 m) 1 m in front of the subject. For more details of the device see Mahfuz et al. (2017). The device consists of a head unit (strapped securely to the forehead) and a base (or control) unit. The head unit consists of a laser mounted in a fixed position relative to, and aimed at the centre of, an electrostatic MEMS micromirror (Mirrorcle Technologies Inc., USA) and a 9D IMU (3D accelerometer, gyroscope, and magnetometer; STMicroelectronics, USA). Information from the IMU is processed so that 3D head orientation with respect to space can be calculated at 250 Hz to within 0.1 °. The head orientation is used to drive the mirror and hence laser target position with respect to the head. The peak-latency due to slippage between the head unit and EyeSeeCam goggles was 0.95 ± 1.29 ms during head impulses. Head impulses had peak velocities between 125 to 225 °/s, and for this range, there was no correlation between peak velocity and head unit latency. The base unit has a touch screen interface which allows the experimenter to set the training algorithm parameters, which can also be set via a Bluetooth or USB PC connection. The base unit provides auditory feedback when head impulse peak velocity is below 120 °/s or above 180 °/s. At 150 °/s head impulse amplitude is ~ 10 °.
Active and Passive VOR Adaptation Training Protocols
Each subject underwent six test sessions on separate days in pseudo-randomised order. For 4/6 test sessions, the unilateral incremental VOR adaptation training protocol was used as previously described (Migliaccio and Schubert 2013, 2014; Fadaee and Migliaccio 2016). In brief, using the head impulse test (Halmagyi and Curthoys 1988), the active and passive VOR gain was measured before and after active VOR adaptation training. Passive VOR gain testing prior to (and after) training required head impulses delivered manually in the horizontal canal plane, i.e. leftward and rightward. Subjects were trained to perform active head impulses similar in profile to the passive head impulses (per Fig. 1 in Migliaccio and Schubert 2013). For the remaining 2/6 test sessions, the unilateral incremental VOR adaptation training protocol was also performed, but used passive head impulses (i.e. the investigator delivered the head rotation). During VOR testing, a visual fixation target (laser target) located straight ahead and at eye level was provided. This target disappeared when the head rotated 0.6 ° away from neutral.
Unilateral VOR adaptation training consisted of a series of active only (4/6 test sessions) or passive only (2/6 test sessions) head impulses from a neutral starting position, i.e. only outward impulses were applied. For active VOR adaptation training, head impulses were alternated leftward and rightward, whereas for passive training, leftward and rightward impulses were presented randomly. The adapting side was pseudo-randomised, leftward, or rightward, across subjects (e.g. leftwards for 5/10 subjects). For each active or passive head impulse, subjects were instructed to maintain visual fixation of the laser target whose horizontal position was a function of horizontal head position, head impulse direction, and adaptation gain (eye/head angular speed) demand. After head peak velocity, other lower-latency vision-stabilising systems, such as smooth pursuit, begin contributing to the compensatory eye movement. In order to drive only VOR adaptation, the laser target was extinguished once head peak velocity was detected and reappeared only after the head returned slowly back to its neutral position. For rotations towards the non-adapting side, the gain demand was fixed to unity (i.e. driving no adaptation), whereas for rotations towards the adapting side, it increased from 1 (epoch 1) to 1.9 (epoch 10) in increments of 0.1 per 90-s epoch. The entire training period lasted 15 min. Apart from the laser target, all training and testing were performed in complete darkness.
Post-Adaptation Training VOR Retention Test Protocols
Both the active and passive VOR was measured immediately after training and then at regular time intervals. Prior human studies have suggested that loss of VOR gain retention follows a similar time course to VOR adaptation (e.g. Gonshor and Jones 1976a, b). Given that the present VOR adaptation training lasted 15 min and assuming an exponential delay in VOR gain retention, we postulated that tracking the VOR gain for 1 h after adaptation training would be sufficient to determine the time course of retention. In case the testing itself affected VOR gain retention, the number of times the VOR was stimulated and tested during this 1 h was varied (see Table 1 for description of the six sessions/protocols). For the three sessions after active adaptation training, the respective test time intervals were 10 (testing at 0, 10, 20, 30, 40, 50, and 60 min after training), 20 (0, 20, 40, and 60 min), and 60 (0 and 60 min) min. For the fourth session after active adaptation training, both the active and passive VOR was tested every 10 min, similar to the first session above, except that the visual fixation target at the start of each head impulse was only provided at test times 0 and 60 min after training. For the two sessions after passive adaptation training, the respective test time intervals were 10 and 20 min. A stop watch was used with pre-set auditory alarms to ensure VOR testing started on time. Testing both the active and passive VOR took a total of about 3 min to complete for each time point. Subjects were kept in complete darkness between testing while still wearing the video-oculography system and training device that controlled the laser target during testing. Subjects were kept alert throughout this 1-h period by listening to an engaging podcast (i.e. not music) of their choice, while the experimenter spoke and regularly asked them questions to ensure their alertness.
Table 1.
The six sessions shown were presented in pseudo-randomised order. Sessions 1–4 consisted of active head impulse training, whereas sessions 5 and 6 consisted of passive head impulse training. The “VOR stimulus times” refers to the times at which 90 s of active head impulses followed by 90 s of passive head impulses were delivered. The “VOR test times” refers to the “VOR stimulus times” where a fixation target was briefly provided immediately prior to each head impulse
| Session | Training type | VOR stimulus times (min after training) |
VOR test times (brief fixation light) |
|---|---|---|---|
| 1 | Active | 0, 10, 20, 30, 40, 50, 60 | 0, 10, 20, 30, 40, 50, 60 |
| 2 | Active | 0, 20, 40, 60 | 0, 20, 40, 60 |
| 3 | Active | 0, 60 | 0, 60 |
| 4 | Active | 0, 10, 20, 30, 40, 50, 60 | 0, 60 |
| 5 | Passive | 0, 10, 20, 30, 40, 50, 60 | 0, 10, 20, 30, 40, 50, 60 |
| 6 | Passive | 0, 20, 40, 60 | 0, 20, 40, 60 |
Data Analysis
Horizontal angular eye position was differentiated and the onset of each head impulse was calculated by fitting horizontal angular head velocity magnitude to a polynomial curve versus time. The point where the magnitude of the fitted curve was greater than 2 % of the curve’s peak magnitude (typically this threshold was 4 °/s) was defined as the impulse onset. Only head impulses with peak magnitude between 150 to 300 °/s were included in the analysis. Traces with saccades occurring inside a window starting at 100 ms before impulse onset and ending at impulse peak magnitude (typically 100 ms after onset) were also removed. Eye traces containing blinks and other artefacts affecting ~ 20 % of the data were removed, along with their corresponding head traces. The instantaneous VOR gain was calculated as the magnitude of eye velocity divided by head velocity. The impulse VOR gain was calculated as the median of the instantaneous VOR gains calculated during the 30-ms period (at 220 Hz, this corresponds to 6 to 7 instantaneous gain values) immediately prior to impulse peak magnitude. The percentage of VOR gain change for each side (adapting or non-adapting) was calculated by dividing the post-training by the pre-training VOR gain, subtracting by 1, and multiplying by 100. A positive percentage indicated an increase in VOR gain due to adaptation training.
Statistical Analysis
Statistical analysis was performed using SPSS version 23 (IBM, USA) and Excel 2013 (Microsoft, USA) software. Normal Q-Q plots of both the VOR gain and percentage gain change showed normal distributions, so a parametric multi-way analysis of variance (ANOVA) with three factor interactions was used to analyse the VOR gain data (Diggle et al. 1994). Independent variables included the following: subject ID, VOR training type (“active,” “passive”), VOR testing type (“active,” “passive”), head rotation side (“adapting,” “non-adapting”), and time (“pre-training,” “post-training”). To analyse VOR retention, the time variable was modified (“pre-training,” “0,” “10,” “20,” “30,” “40,” “50,” “60”) and the test interval protocol variable was added (“10 min,” “20 min,” “60 min,” “10/60 min”). The dependent variable was either gain or percentage gain change. All variables were included in the ANOVA initially and those found insignificant were subsequently removed. Only the interaction effects found to be significant are included in the results. Pooled data are described as mean ± 1 SD.
RESULTS
Pre-Adaptation Training VOR
There were differences in the pre-adaptation VOR gains between subjects (ANOVA: F9,189 = 7.1, P < 0.001). However, there were no differences in VOR gains between leftward and rightward (ANOVA: F1,189 = 0.8, P = 0.37) and between active and passive (ANOVA: F1,189 = 0.9, P = 0.35) head impulses.
Active VOR Training Retention in a Typical Subject
Figure 1 shows the active VOR responses immediately before (left column), immediately after (middle column), and 60 min after (right column) active VOR training across the three VOR testing protocols in a typical subject. For protocol one (top row), the VOR was tested once every 10 min after training for 60 min, i.e. the VOR was tested at a total of 7 time points after training. Similarly, for protocols two (middle row) and three (bottom row), the VOR was tested once every 20 (i.e. 4 time points) and 60 min (i.e. 2 time points), respectively. For all three testing protocols, the VOR gain immediately after training was ~ 10 % higher than before training (see Fig. 1, middle column). There was no difference between active and passive VOR gain increases (ANOVA: F1,23 = 0.002, P = 0.96) and no difference in gain increase between active and passive training (ANOVA: F1,23 = 0.4, P = 0.54) (not shown in Fig. 1). The VOR gain increase 60 min after training (see Fig. 1, right column) was only ~ 1 % (no retention) for testing protocol one, whereas it was ~ 5 % (half retention) and ~ 12 % (full retention) for testing protocols two and three, respectively.
Fig. 1.
Active VOR responses immediately before (left column), immediately after (middle column), and 60 min after (right column) active VOR training across the three VOR testing protocols in a typical subject. For protocol one (top row), the VOR was tested immediately after training and then every 10 min after training for 60 min. For protocol two (middle row), the VOR was tested immediately after training and then every 20 min after training for 60 min. For protocol 3 (bottom row), the VOR was tested immediately after training and once 60 min after training. Note, the panel in the third row and second column had a smaller number of valid traces (i.e. more traces removed) compared to the other panels due to artefact caused by blinking
Passive Versus Active VOR Training Across Subjects
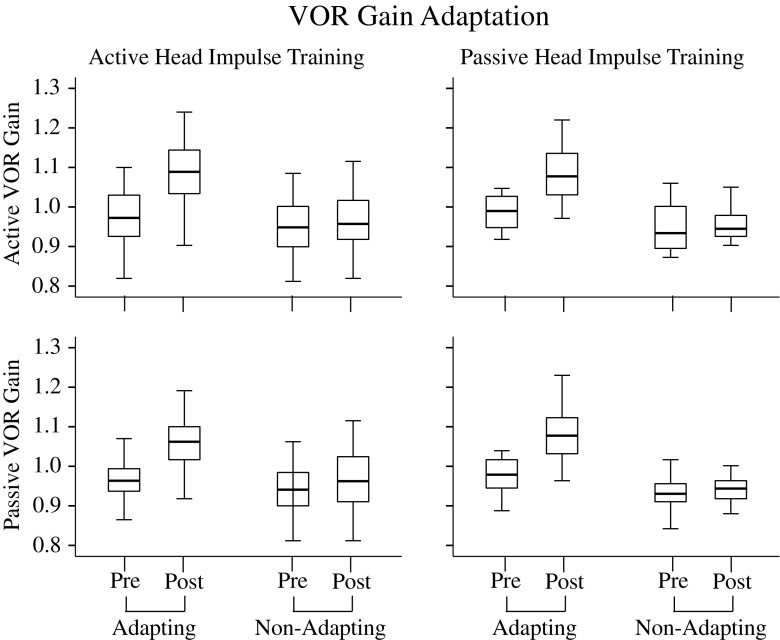
Figure 2 shows the active (top row) and passive (bottom row) VOR gains before and after active (left column) and passive (right column) VOR training. VOR gains towards both the adapting side (i.e. the side the training is designed to adapt) and non-adapting side are shown. The active and passive VOR gains measured before and immediately after active or passive VOR adaptation training were not affected by the type of training (active versus passive training, ANOVA: F1,374 = 1.2, P = 0.27). There was also no difference between active and passive VOR gains (ANOVA: F1,374 = 1.7, P = 0.19). The only significant factors to affect gain were time (ANOVA: F1,374 = 55.6, P < 0.001), i.e. post-training VOR gains were significantly higher than pre-training gains, and side (ANOVA: F1,374 = 112.2, P < 0.001), i.e. adapting side gains were significantly higher than non-adapting side gains. There was also a significant interaction between time and side (ANOVA: F1,374 = 26.2, P < 0.001), indicating that the adaptation training only affected the VOR gain on the adapting side.
Fig. 2.
Active (top row) and passive (bottom row) VOR gains before and after active (left column) and passive (right column) VOR training. The pre- and post-training VOR gains towards both the adapting side (i.e. the side the training is designed to adapt) and non-adapting side are shown. Each box shows the median and goes from the first to the third quartile with whiskers denoting the maximum and minimum values
Due to the variability of pre-adaptation gains across subjects, data were normalised for each subject/session by calculating the percentage VOR gain increase for each side (adapting, non-adapting). Using the normalised data, the effect of training type (active or passive) on VOR gain remained non-significant (ANOVA: F1,184 = 1.1, P = 0.29) and there was no difference between active and passive gains (ANOVA: F1,184 = 0.22, P = 0.64). The mean VOR gain increase after pooling active and passive VOR gains for active and passive VOR training was 10.1 ± 3.7 % towards the adapting side and 2.0 ± 3.8 % towards the non-adapting side.
Retention of VOR Training Across Subjects
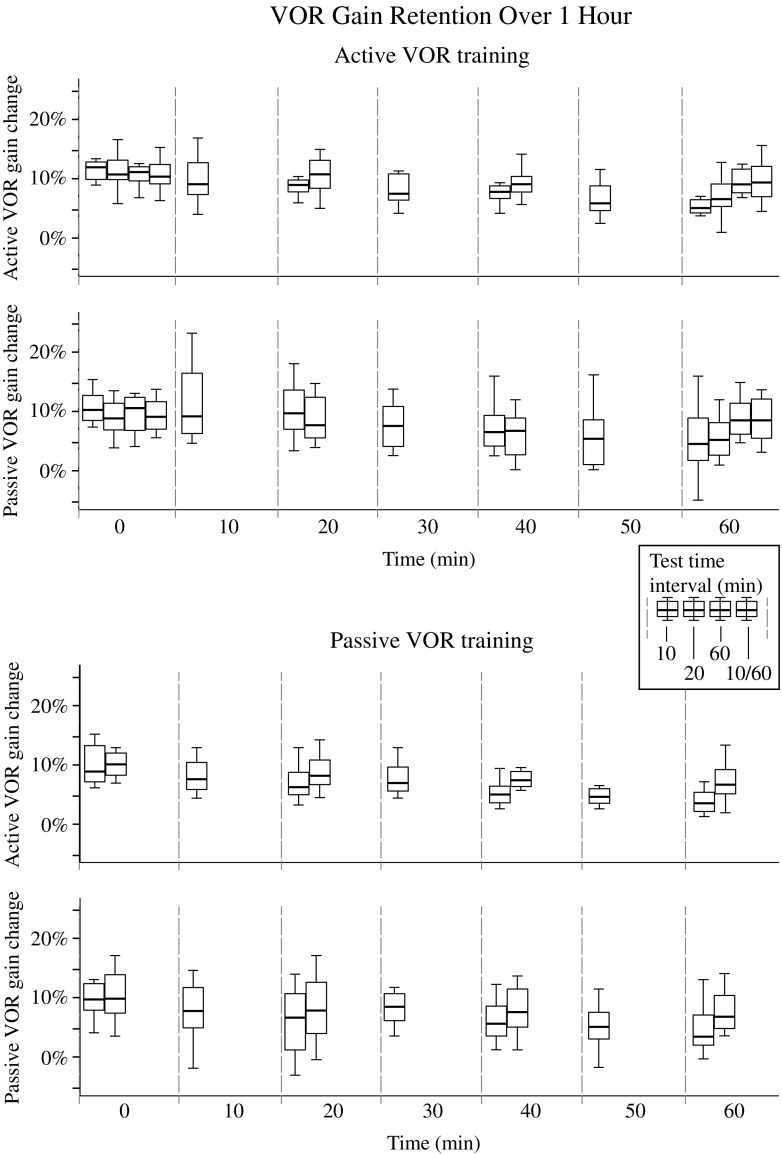
Figure 3 shows the normalised active (rows 1 and 3) and passive (rows 2 and 4) VOR gain percentage increases after active (top 2 rows) and passive (bottom 2 rows) VOR training towards the adapting side only. VOR gains were measured every 10 (first boxplot between dashed lines), 20 (second boxplot between dashed lines), or 60 min (third boxplot between dashed lines) for 60 min after adaptation training. In addition, the VOR gain was tested every 10 min, but the visual fixation light before the start of each impulse was only provided at testing times 0 and 60 min after adaptation training (fourth boxplot between dashed lines). The main factors that affected the VOR gain were type of training (active versus passive) (ANOVA: F1,849 = 10.1, P < 0.002), side (ANOVA: F1,849 = 203.5, P < 0.001), and time (ANOVA: F6,849 = 5.6, P < 0.001). The only significant interaction was between training, side, and test interval (ANOVA: F1,849 = 4.1, P < 0.05), indicating that retention of VOR gain on the adapting side after training depended on the test time interval. Across conditions, the active VOR gain increase was 8.5 ± 4.2 %, whereas for the passive VOR, it was 7.5 ± 4.5 %.
Fig. 3.
Normalised active (rows 1 and 3) and passive (rows 2 and 4) VOR gain percentage increases after active (top two rows) and passive (bottom two rows) VOR training towards the adapting side only. VOR gains were measured every 10 (first boxplot between dashed lines), 20 (second boxplot between dashed lines), or 60 min (third boxplot between dashed lines) for 60 min after adaptation training. In addition, the VOR gain was tested every 10 min, but the visual fixation light before the start of each impulse was only provided at testing times 0 and 60 min after adaptation training (fourth boxplot between dashed lines). Each box shows the median and goes from the first to the third quartile with whiskers denoting the maximum and minimum values
Analysis of only the VOR gains measured immediately after training (time = 0) and 60 min after training revealed a significant interaction between time and the testing time interval (ANOVA: F3,368 = 3.0, P < 0.05), indicating that VOR retention was affected by the testing time interval. When VOR gains were measured every 10 min, time significantly affected the VOR gain (ANOVA: F6,486 = 6.9, P < 0.001). However, when analysing only the data collected at 0, 10, and 20 min after training, the VOR gain was no longer affected by time (ANOVA: F2,211 = 2.5, P = 0.09). The VOR gain increase was significantly lower when tested at 30 min, i.e. the fourth 10-min interval test time, compared to immediately after testing (ANOVA: F1,135 = 86.0, P < 0.001). A similar pattern was observed when VOR gains were measured every 20 min; again, the test time significantly affected the VOR gain (ANOVA: F3,269 = 3.2, P < 0.05). However, when analysing only the data collected at 0, 20, and 40 min after training, the VOR gain was not affected by test time interval (ANOVA: F2,201 = 2.0, P = 0.14). The VOR gain increase was significantly lower when tested at 60 min, i.e. the fourth 20-min interval test time, compared to immediately after testing (ANOVA: F1,135 = 7.9, P < 0.01).
Taken together, these data suggest that the number of times the VOR is tested after training affects retention more so than the time lapsed after training. To further test the effect of time versus number of tests on retention, gains measured immediately after active training and 60 min later with no other testing between these times were compared. This showed time no longer affected the VOR gain (ANOVA: F1,47 = 0.4, P = 0.52).
To determine whether the number of head impulses versus increased exposure to the stationary visual fixation stimulus at the beginning of each head impulse affected VOR retention, the active and passive VOR gains were tested at 10-min intervals, except the fixation light was only provided at times 0 and 60 min after active adaptation training. These data showed that time did not affect the VOR gain (ANOVA: F1,47 = 0.1, P = 0.73), suggesting that increased exposure to the stationary visual fixation target, not vestibular stimuli, reduced VOR retention.
DISCUSSION
Data from this study suggest that in humans, the magnitude of unilateral VOR adaptation after active or passive head impulse training is the same for both the active and passive VOR. In other words, the active or passive head rotation context of the training had no effect on the VOR gain increase. Similarly, the training context did not affect retention of VOR adaptation. The only factor that significantly affected retention over the 1 h immediately after training was the brief exposure to a stationary fixation target before each head impulse during VOR testing. A significant decrease in VOR gain was only detected during the fourth time it was tested in the hour, corresponding to the 30th and 60th minute after training for the 10- and 20-min test interval protocols, respectively. Taken together, these data suggest that the number of times the VOR was tested after training affected retention, not the time lapsed after training, and that this was due to increased exposure to a fixation target. In support of this, the 60-min test interval data, where subjects were only tested after training at two time points: the first immediately after training and the second 60 min after training, showed no significant decrease in VOR gain. Similarly, the 60-min test interval data where subjects underwent active and passive VOR testing without a fixation target at 10, 20, 30, 40, and 50 min after training also showed no decrease in VOR gain, suggesting that time is unlikely to be affecting short-term retention. The present 60-min test interval data in humans is in agreement with a prior primate study showing that light deprivation (Miles and Lisberger 1981) is an efficient means for prolonging a newly acquired VOR gain.
Active Versus Passive Adaptation Training
Prior VOR studies have shown that the training context determines the adaptation characteristics. For example, the orientation of the head with respect to gravity during training is an important context for adaptation. If the horizontal or vertical VOR is trained left-ear-down, adaptation is maximal when the VOR is tested left-ear-down and minimal when tested right-ear-up, i.e. adaptation under these conditions is a cosine function of head position (Yakushin et al. 2003; Schubert et al. 2008b). Similarly, the kinematic characteristics (frequency, velocity, and acceleration) of the head movement during training determine the kinematics of the head movement where VOR adaptation is maximal. Stimulus frequency and velocity selectivity of VOR adaptation have been shown in monkeys (Lisberger et al. 1983; Raymond and Lisberger 1996), cats (Powell et al. 1991), rabbits (Collewijn and Grootendorst 1979; Angelaki and Hess 1998), and mice (Hübner et al. 2014). Acceleration is related to sinusoidal frequency and velocity, so it is not clear whether vestibular stimuli are encoded in three separate velocity, frequency, and acceleration channels or just two of these channels for selective adaptation (see discussion in Hübner et al. 2014). Nonetheless, by avoiding sinusoidal stimuli, we sought to train a broader frequency spectrum as has been shown to be the case for step impulses (Powell et al. 1991).
No prior study has examined the effects of passive versus active head movement training contexts on VOR adaptation. We hypothesised that because active VOR training involved efference copy, whereas passive training did not, that the training context would have differentially affected the active versus passive VOR gain increases. Data from the present study suggest that this was not the case and that most of the adaptation observed, regardless of whether the stimulus was active or passive, was occurring in the central vestibular pathways common to both the active and passive VOR, as opposed to the non-overlapping pathways. Animal studies suggest that changes in the central vestibular pathways can occur before changes at the level of the vestibular periphery (primates: Carriot et al. 2015; Mitchell et al. 2017) and that repeated exposure to a visual-vestibular mismatch stimulus results in changes in synaptic transmission and intrinsic properties of central vestibular neurons in the direct pathway of the VOR, albeit over a longer training time course than in the present study (mouse: Carcaud et al. 2017).
In a prior study with only active head impulse training using the incremental adaptation technique, a significant difference between active and passive VOR gain increases was detected (Migliaccio and Schubert 2013). In that study, the VOR gain to passive head impulses after active training increase was ~ 11 % compared to ~ 23 % increase in the VOR gain for active head impulses (after active training). The main differences between that study and the present study were twofold. First, that study did not train with passive head rotation. Second, the duration of the visual-vestibular mismatch stimulus during each head impulse was different. In the present study, the visual stimulus was extinguished once head impulse peak velocity was detected so that the training stimulus predominantly trained the VOR. In contrast, in the prior study, the visual stimulus was extinguished once head impulse velocity returned to zero, thus potentially adapting the response of the other non-vestibular vision-stabilising systems with latencies > 100 ms such as smooth pursuit (e.g. Krauzlis and Lisberger 1994). The results from these two studies suggest that passive VOR adaptation does not depend on whether the training is active or passive. In contrast, active VOR adaptation is improved if visual feedback during the second half of the head impulse is provided, presumably because this latter feedback is used by the longer-latency systems that also contribute to vision stabilisation.
Retention of VOR Adaptation
Exposure to the fixation target used during VOR testing is the likely explanation as to why the number of times the VOR gain was tested affected retention. A room-fixed visual fixation target was presented in between head impulses to help the subject (during active head impulses) and experimenter (during passive head impulses) bring the head and eyes back to neutral before the start of the next head impulse. The visual fixation target was only ON when the head was within 0.6 ° from neutral and was OFF once the head had moved away from this window, i.e. ~ 4 ms after head impulse onset. However, though short, when the target was ON, the VOR gain was being driven to unity, acting as a de-adapting stimulus. The typical exposure time to the visual target was ~ 1 s prior to each head impulse. A combined total of ~ 60 active and passive head impulses were delivered per test period, bringing the total exposure time to the fixation target to ~ 60 s per test period. Although the slow head movements during this period of exposure were a weak VOR stimulus, it seems to have been sufficient to de-adapt the VOR. The kinematic characteristics (frequency, velocity, and acceleration) of the head movement did not determine the characteristics of the head movement where VOR adaptation was most evident in this case. It is not clear why a de-adaptation stimulus during low-frequency, low-velocity head movements would lead to de-adaptation of the high-frequency, high-velocity VOR. Perhaps VOR de-adaptation relies on a less context-specific mechanism than adaptation.
Implications for VOR Rehabilitation
Findings from the present study show that active head impulse training is the same as passive head impulse training in terms of adapting the passive VOR response. Normalising the passive VOR response to rapid head rotations is critical for vestibular patients, i.e. those with damage to the peripheral vestibular end-organ or eight cranial nerve, because the latency of all other vision-stabilising mechanisms is several orders of magnitude longer than the VOR and therefore unreliable to stabilise vision under such circumstances. Additionally, the VOR must routinely stabilise the eyes during unpredictable head rotations, e.g. motion while driving a vehicle. Data from this study suggest that as long as active head impulses have similar velocity profiles to passive head impulses, then optimal VOR training can be performed with active only head rotations, eliminating the need for expensive and bulky equipment (e.g. a rotary chair) or human assistance to deliver passive head impulses. Evidence from this study supports recent Cochrane meta-analysis studies that provide strong recommendations for vestibular rehabilitation providers to prescribe gaze stability exercises using active head rotation (Hillier and McDonnell 2011, 2016; McDonnell and Hillier 2015). Additionally, recent evidence suggests that active head rotation training improves postural control (Matsugi et al. 2017). The only factor that affected short-term retention was the duration of exposure to a de-adaptation stimulus, which in this study drove the VOR gain down to unity. Presumably, retention would not be lost in vestibular patients whose ipsilesional VOR gains were increased due to training, but still below unity, because of the lack of a de-adaptation stimulus. In this case, real-world visual conditions would drive the VOR gain up to unity and reinforce the training, rather than down to unity and cancel the training as was the case in the healthy subjects used in this study.
It is possible that vestibular patients have developed other vision-stabilising strategies during the passive VOR, e.g. pre-programmed compensatory saccades (Schubert et al. 2006). Also, for rotations towards the ipsilesional ear, the active VOR response is often close to normal, sometimes indistinguishably so, whereas the passive VOR response is significantly less (Della Santina et al. 2002). It is not clear how these differences will affect VOR adaptation in vestibular patients. Further VOR adaptation studies are clearly needed in patients with peripheral-vestibular deficits, especially those with some residual VOR function.
Funding Information
A.A. Migliaccio was supported by the Garnett Passe and Rodney Williams Memorial Foundation Senior/Principal Research Fellowship in Otorhinolaryngology and Project Grant (2013-15) and NHMRC Development Grant APP105550.
References
- Angelaki DE, Hess BJM. Visually induced adaptation in three-dimensional organization of primate vestibuloocular reflex. J Neurophysiol. 1998;79:791–807. doi: 10.1152/jn.1998.79.2.791. [DOI] [PubMed] [Google Scholar]
- Bartl K, Lehnen N, Kohlbecher S, Schneider E. Head impulse testing using video-oculography. Ann N Y Acad Sci. 2009;1164:331–333. doi: 10.1111/j.1749-6632.2009.03850.x. [DOI] [PubMed] [Google Scholar]
- Carcaud J, França de Barros F, Idoux E, Eugène D, Reveret L, Moore LE, Vidal PP, Beraneck M. Long-lasting visuo-vestibular mismatch in freely-behaving mice reduces the vestibulo-ocular reflex and leads to neural changes in the direct vestibular pathway. eNeuro. 2017;4:1. doi: 10.1523/ENEURO.0290-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriot J, Jamali M, Brooks JX, Cullen KE. Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception. J Neurosci. 2015;35:3555–3565. doi: 10.1523/JNEUROSCI.3540-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Grootendorst AF. Adaptation of optokinetic and vestibulo-ocular reflexes to modified visual input in the rabbit. Prog Brain Res. 1979;50:771–781. doi: 10.1016/S0079-6123(08)60874-2. [DOI] [PubMed] [Google Scholar]
- Della Santina CC, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg. 2002;128:1044–1054. doi: 10.1001/archotol.128.9.1044. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- Fadaee SB, Migliaccio AA. The effect of retinal image error update rate on human vestibulo-ocular reflex gain adaptation. Exp Brain Res. 2016;234:1085–1094. doi: 10.1007/s00221-015-4535-y. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Robinson DA. Adaptation of human’s vestibulo-ocular reflex to magnifying glasses. Brain Res. 1975;92:331–335. doi: 10.1016/0006-8993(75)90279-6. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Jones GM. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976;256:361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A, Jones GM. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol. 1976;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Hattori K, Watanabe S, Nakamura T, Kato I. Flexibility in the adaptation of the vestibulo-ocular reflex to modified visual inputs in humans. Nihon Jibiinkoka Gakkai Kaiho. 2000;103:1186–1194. doi: 10.3950/jibiinkoka.103.1186. [DOI] [PubMed] [Google Scholar]
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2011;2:CD005397.pub3. doi: 10.1002/14651858.CD005397.pub3. [DOI] [PubMed] [Google Scholar]
- Hillier S, McDonnell M. Is vestibular rehabilitation effective in improving dizziness and function after unilateral peripheral vestibular hypofunction? An abridged version of a Cochrane review. Eur J Phys Rehabil Med. 2016;52:541–556. [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. Velocity-selective adaptation of the horizontal and cross-axis vestibulo-ocular reflex in the mouse. Exp Brain Res. 2014;232:3035–3046. doi: 10.1007/s00221-014-3988-8. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Temporal properties of visual motion signals for the initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1994;72:150–162. doi: 10.1152/jn.1994.72.1.150. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA, Optican LM. Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci. 1983;3:1234–1244. doi: 10.1523/JNEUROSCI.03-06-01234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz MM, Schubert MC, Todd CJ, Figtree WVC, Khan SI, Migliaccio AA. The effect of visual contrast on human vestibulo-ocular reflex training. J Assoc Res Otolaryngol, 2017 (In Press) [DOI] [PMC free article] [PubMed]
- Matsugi A, Ueta Y, Oku K, Okuno K, Tamaru Y, Nomura S, Tanaka H, Mori N. Effect of gaze-stabilization exercises on vestibular function during postural control. Neuroreport. 2017;28:439–443. doi: 10.1097/WNR.0000000000000776. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1:CD005397.pub4. doi: 10.1002/14651858.CD005397.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CH, Lasker AG, Robinson DA. The upper limit of human smooth pursuit velocity. Vis Res. 1985;25:561–563. doi: 10.1016/0042-6989(85)90160-9. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC. Unilateral adaptation of the human angular vestibulo-ocular reflex. J Assoc Res Otolaryngol. 2013;14:29–36. doi: 10.1007/s10162-012-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC. Pilot study of a new rehabilitation tool: improved unilateral short-term adaptation of the human angular vestibulo-ocular reflex. Otol Neurotol. 2014;35:310–316. doi: 10.1097/MAO.0000000000000539. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Della Santina CC, Cullen KE. Plasticity within excitatory and inhibitory pathways of the vestibulo-spinal circuitry guides changes in motor performance. Sci Rep. 2017;7:853. doi: 10.1038/s41598-017-00956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD, Sargent EW. Visually-induced adaptive plasticity in the human vestibulo-ocular reflex. Exp Brain Res. 1991;84:25–34. doi: 10.1007/BF00231759. [DOI] [PubMed] [Google Scholar]
- Powell KD, Quinn KJ, Rude SA, Peterson BW, Baker JF. Frequency dependence of cat vestibulo-ocular reflex direction adaptation: single frequency and multifrequency rotations. Brain Res. 1991;550:137–141. doi: 10.1016/0006-8993(91)90417-T. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J Neurosci. 1996;16:7791–7802. doi: 10.1523/JNEUROSCI.16-23-07791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Della Santina CC. Modification of compensatory saccades after aVOR gain recovery. J Vestib Res. 2006;16:285–291. [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Minor LB, Clendaniel RA. Retention of VOR gain following short-term VOR adaptation. Exp Brain Res. 2008;187:117–127. doi: 10.1007/s00221-008-1289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelhamer M, Robinson DA, Tan HS. Context-specific adaptation of the gain of the vestibulo-ocular reflex in humans. J Vestib Res. 1992;2:89–96. [PubMed] [Google Scholar]
- Shelhamer M, Tiliket C, Roberts D, Kramer PD, Zee DS. Short-term vestibulo-ocular reflex adaptation in humans. II. Error signals. Exp Brain Res. 1994;100:328–336. doi: 10.1007/BF00227202. [DOI] [PubMed] [Google Scholar]
- Solomon D, Zee DS, Straumann D. Torsional and horizontal vestibular ocular reflex adaptation: three-dimensional eye movement analysis. Exp Brain Res. 2003;152:150–155. doi: 10.1007/s00221-003-1460-2. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Gravity-specific adaptation of the angular vestibuloocular reflex: dependence on head orientation with regard to gravity. J Neurophysiol. 2003;89:571–586. doi: 10.1152/jn.00287.2002. [DOI] [PubMed] [Google Scholar]