Abstract
The basal stem/progenitor cell maintains homeostasis of the epidermis. Progressive disturbance of this homeostasis has been implicated as a possible cause in the pathogenesis of epithelial disease, such as middle ear cholesteatoma. In many cases of stem/progenitor cell regulation, the importance of extracellular signals provided by the surrounding cells is well-recognized. Keratinocyte growth factor (KGF) is a mesenchymal-cell-derived paracrine growth factor that specifically participates in skin homeostasis; however, the overexpression of KGF induces middle ear cholesteatoma. In this study, two kinds of thymidine analogs were transferred at different time points and we investigated the effects of overexpressed KGF on the cell kinetics of stem/progenitor cells in vivo. As a result, BrdU(+)EdU(+) cells (stem/progenitor cells) were detected in the thickened epithelium of KGF-transfected specimens. The use of a high-resolution microscope enabled us to analyze the phosphorylated level of p63 in individual nuclei, and the results clearly demonstrated that BrdU(+)EdU(+) cells are regarded as progenitor cells. In the overexpression of KGF, the stimulation of progenitor cell proliferation was inhibited by SU5402, an inhibitor for tyrosine kinase of KGFR. These findings indicate that KGF overexpression may increase stem/progenitor cell proliferation and block terminal differentiation, resulting in epithelial hyperplasia, which is typical in middle ear cholesteatoma.
Keywords: keratinocyte growth factor (KGF), progenitor cell, p63 phosphorylation, cell tracing, middle ear cholesteatoma, high-resolution microscopy
Introduction
The epidermal basal stem and/or progenitor cells maintain homeostasis of the epithelium through its development, self-renewal, and differentiation (Mascré et al. 2012). In an adult mammal’s interfollicular epithelium, stem cells are localized in the basal epidermal layer. Dividing daughter cells either undergo self-renewal or remain as undifferentiated proliferative progenitor cells only 4–6 times per year. These progenitor cells not only replenish the basal layer, but also give rise to transcriptionally active spinous and granular layers, and finally the outer layers of terminally differentiated during homeostasis (Blanpain and Simons 2013; Hsu et al. 2014). The minimum transit time of basal stem cells to the cornified layer is estimated at about 7 to 9.5 days (Hoath and Leahy 2003; Potten et al. 1987). In many cases of adult basal stem and/or progenitor cell regulation, the extracellular signals provided by the surrounding cells that form the stem cell niche are well-recognized (Hsu et al. 2014). The soluble paracrine signals from stromal cells are also required for the maintenance of homeostasis of the epithelium. The progressive disturbance of these paracrine signals may break the homeostasis of the epithelium and contribute directly to the pathogenesis of epithelial disease (Turner and Grose 2010; Finch et al. 1997).
KGF, a unique member of the fibroblast growth factor (FGF) family, is a mesenchymal cell-derived paracrine mediator of epithelial cell growth (Finch et al. 1989). In fact, KGF was shown to be secreted from fibroblasts mainly in the stroma and to act on epithelial cells through KGFR in the normal skin of fetuses and adults (Finch et al. 1995; Werner et al. 1992). Moreover, the enhanced expression of KGF mRNA has been detected in fibroblasts during wound healing (Werner and Munz 2000), and KGFR mRNA was found to be localized only in the epithelial cells of wounded skin (Orr-Urtreger et al. 1993). In our previous study, we showed that KGF and KGFR played an important role in human middle ear cholesteatoma formation, as characterized by the hyper-proliferation of epithelial cells (Tanaka et al. 1999; Yamamoto-Fukuda et al. 2003). In addition, the electroporatic transfection of KGF gene-expressed vector induced middle ear cholesteatoma formation (Yamamoto-Fukuda et al. 2015).
Recently, it has been reported that the transcription factor p63 was overexpressed in a hyper-proliferative epithelium (Candi et al. 2008; Senoo et al. 2007). p63 is also known as a marker of epidermal stem cells, and phosphorylated p63 (pp63) plays an essential role in maintaining the proliferative potential of those cells (Senoo et al. 2007; Yang et al. 1999). Very recently, it has been shown that highly expressed KGF induced p63 expression and as a result, a hyper-proliferative epithelium was shown to develop in transgenic mice (Ogawa et al. 2008; Ramsey et al. 2013). Furthermore, another study revealed that KGF affected stem and/or progenitor cells’ proliferation (Greco et al. 2009). Based upon these observations, we hypothesized that the paracrine action of KGF might increase epidermal stem and/or progenitor cells through p63 activation and as a result, lead to the formation of cholesteatoma.
To address this hypothesis, first we examined the expression of KGF, KGFR, p63, and pp63 in human middle ear cholesteatoma tissues. We then investigated the role of KGF during epidermal stem and/or progenitor cell proliferation in vivo, which was induced by the transfection of KGF gene-expressed vector into the skin of the ear (Yamamoto-Fukuda et al. 2015). The proliferative activity of the epithelial cells was detected by immunihistochemical analysis for proliferating cell nuclear antigen (PCNA) (a marker of late G1 to S) and apoptotic cell death was addressed by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining. To evaluate the changes in epithelial cell differentiation, an immunohistochemical analysis of cell differentiation markers (cytokeratin (CK)14: undifferentiated epithelial cell marker, CK10: differentiated epithelial cell marker) was performed. The expression of p63 and pp63 in the KGF overexpressed epithelial cells was assessed by immunohistochemistry. To analyze the phosphorylated level of p63 in the nucleus of the epithelial cells in each tissue section, we applied Phos-tag, which was developed to capture phosphomonoester dianions in an aqueous solution (Kinoshita et al. 2004). The staining results were analyzed by super-resolution structured illumination microscopy (SR-SIM) to calculate pp63 spots in the nuclei. Finally, to monitor the levels of the progenitor cells under the influence of KGF, a cell tracing system using the thymidine analogues 5-bromo-2′-deoxyuridine (BrdU) and 5-ethynyl-2′-deoxyuridine (EdU) was performed. The paracrine actions of KGF are dependent upon KGFR, which is a transmembrane tyrosine kinase receptor (Miki et al. 1992). By binding to KGFR, KGF activates various mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK) (Portnnoy et al. 2004). SU5402 is an ATP mimetic known as a KGFR-selective inhibitor with 86 % homology to the KGFR tyrosine kinase domain and inhibits the tyrosine kinase activity of KGFR by interacting with the catalytic domain of KGFR with binding to the ATP-binding site (Mohammadi et al. 1997). Recently, the use of SU5402 in an in vitro experiment resulted in inhibition of differentiation and proliferation of epithelial cells through KGF secretion by fibroblasts isolated from cholesteatoma (Raffa et al. 2012). Combined with the results from an inhibitor for the tyrosine kinase of KGFR (SU5402) (Li et al. 2009), our objective was to demonstrate that the overexpression of KGF increased the population of epidermal progenitor cells through the activation of p63.
Materials and Methods
Subjects
Male ICR mice (8 weeks old, 33–37 g body weight) with normal ear skin tissues were used in this study. All experiments were conducted according to the principles and procedures outlined in the guidelines for animal experimentation of Nagasaki University with the approval of the Institutional Animal Care and Use Committee (Nos. 1209241015-2 and 1404011269).
Specimens from 29 ears with middle ear cholesteatoma, confirmed by histopathologic examination were obtained from 21 men and 8 women (average age 45 years; range 29–69 years). All of the patients were treated surgically at the Department of Otorhinolaryngology, Jikei University Hospital, between May 2016 and September 2017. The cholesteatoma tissues were harvested from the patients during surgery. In 22 of the ears of the study subjects with cholesteatoma (12 male and 10 female; average age 53 years; range 23–79 years), a small piece of normal skin was harvested during surgery. This study protocol was approved by the Human Ethics Review Committee of Jikei University School of Medicine, and signed informed consent was obtained from all the patients or their guardians for this study (approval number is 27-344 8229).
Experimental Design
hKGF cDNA-Transfected Mice
Flag-human KGF (hKGF) DNA plasmid driven by a CMV14 promoter (0.5 μg/ml) (KGF gene-transfected) or a null-plasmid driven by a CMV14 promoter (0.5 μg/ml) (vector alone-transfected) were transfected into the mouse ear skin using a Nepa21 Electroporator (Nepa Gene Co., Chiba, Japan), according to the protocol of a previous paper (Yamamoto-Fukuda et al. 2015). The hKGF cDNA expression vector was successfully transfected and KGF protein was expressed 4 days after vector transfection, the same as results described previously (Yamamoto-Fukuda et al. 2015). The animals were euthanized using an intraperitoneal injection of 200 mg/kg pentobarbital. Their ear skin tissues were removed at 1, 4, and 7 days after vector transfection, fixed with 4 % paraformaldehyde (PFA) in a phosphate-buffered saline (PBS) at 4 °C overnight, and then embedded in paraffin in the standard manner. The sections (5 μm to 6 μm thick) were prepared and some were stained with hematoxylin and eosin (H&E) for histological examination.
BrdU and EdU Uptake
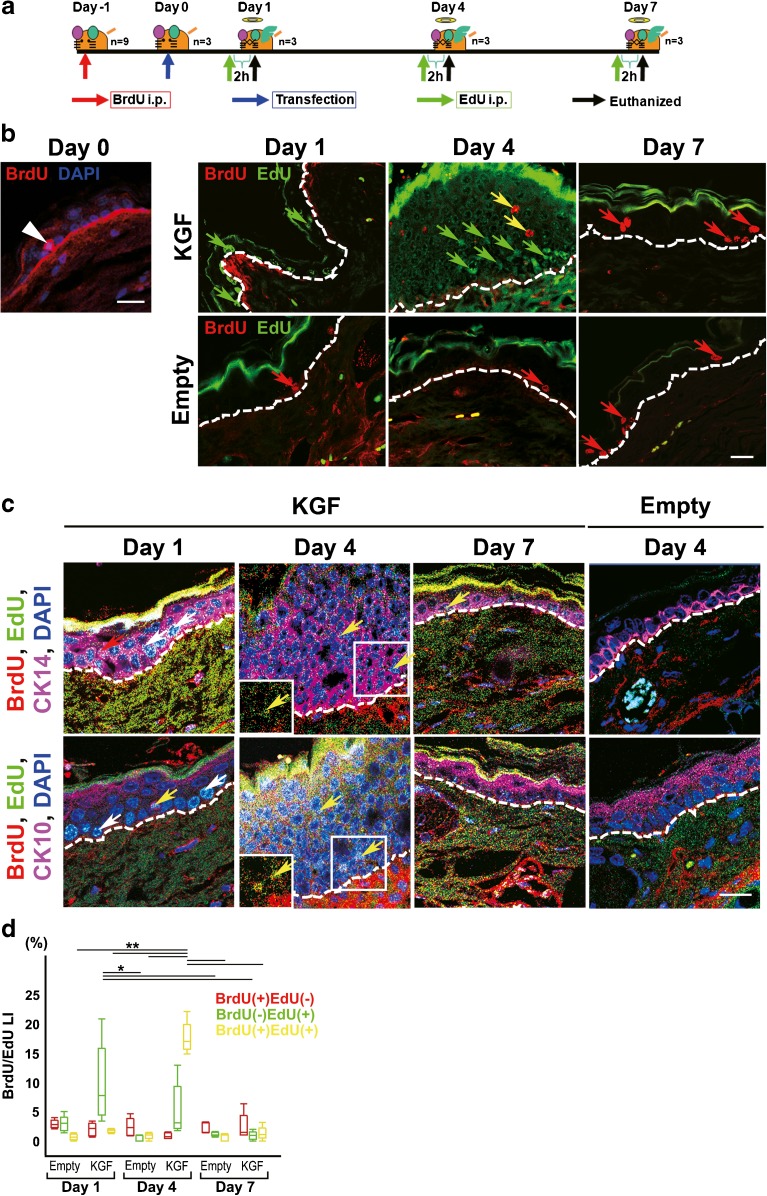
We labeled the newly synthesized DNA with BrdU and EdU, two analogues of thymidine, by successive intraperitoneal injections into the ICR mice to investigate the stem cells/progenitor cells after KGF transfection, as previously described (Salic and Mitchison 2008). We first injected thymidine analogues BrdU to trace stem/progenitor cells in mouse ear skin before KGF transfection (day −1). After KGF transfection (day 0), we injected another thymidine analogues EdU to monitor the levels of the progenitor cells under the influence of KGF at each time point (Fig. 5a). An intraperitoneal injection of BrdU (60 mg/kg body weight) dissolved in saline at 24 h prior to vector transfection and EdU (2.5 mg/kg body weight) dissolved in saline at 2 h prior to each euthanize was performed, as described previously (Yennek and Tajbakhsh 2013).
Fig. 5.
Analyzing the effect of overexpressed KGF on cell kinetics of stem cells, progenitor cells and more differentiated cells using a cell tracing system in vivo. a Schematic description of the method for injecting two thymidine analogues in KGF-transfected mouse ear skin. To investigate the stem cells or progenitor cells after KGF transfection, BdU and ErdU were injected at different time points. BrdU was injected intraperitoneally at 24 h prior to vector transfection and EdU was injected intraperitoneally at 2 h prior to each euthanization. b In the left panel, merged images consisting of BrdU (red) and nuclei stained with DAPI (blue) in the section at day 0. BrdU(+) cells (white arrow head) were detected in the basal layer at day 0. Double immunofluorescence detection of BrdU (red) and EdU (green) was performed in sections of KGF gene-transfected (KGF) and vector alone-transfected ear skin tissue (Empty) (day 1, day 4, and day 7). BrdU(+)EdU(−) cells (red arrow) were detected in the basal layer of KGF gene-transfected ear skin tissue at day 1 and day 7, and vector alone-transfected ear skin tissue. An increased number of BrdU(−) EdU(+) cells (green arrows) were detected in the basal layer at day 1 and upper layer at day 4 after KGF gene transfection. BrdU(+)EdU(+) cells (yellow arrows) were detected in the thickened epithelium in KGF gene-transfected ear skin tissue at day 4. Dashed lines: basement membrane. Scale bars, 20 μm. c Triple immunofluorescence detection of BrdU (red), EdU (green) and CK14 (magenta) or CK10 (magenta) in sections of KGF gene-transfected (KGF) and vector alone-transfected ear skin tissue (Empty) (day 1, day 4 and day 7). Nuclei were stained with DAPI (blue). BrdU(+)EdU(−) cells (red arrow) were detected in the CK14(+)CK10(−) basal layer of KGF gene-transfected ear skin tissue at day 1. An increased number of BrdU(−) EdU(+) cells (white arrows) were detected in the CK14(+)CK10(−) basal layer at day 1 after KGF gene transfection. BrdU(+)EdU(+) cells (yellow arrows) were detected in the CK14(+)CK10(−) thickened epithelium in KGF gene-transfected ear skin tissue at day 4. Inset, high-power view. Dashed lines: basement membrane. Scale bars, 20 μm. d Box plot showing the LI of BrdU(+)EdU(−) positive cells (red boxes), BrdU(−) EdU(+) positive cells (green boxes), or BrdU(+)EdU(+) positive cells (yellow boxes) of the epithelium in vector alone-transfected ear skin (n = 5 for each) and KGF gene-transfected ears (n = 5 for each). BrdU(−) EdU(+) LI (green boxes): *p < 0.01 as determined by a two-way ANOVA F(5, 24) = 5.41 with a Tukey’s post hoc test. BrdU(+)EdU(+) LI (yellow boxes): **p < 0.0001 as determined by a two-way ANOVA F(5, 24) = 139.39 with a Tukey’s post hoc test
Administration of Inhibitor for Tyrosine Kinase of KGFR In Vivo
Eighteen mice were used in the inhibition experiment. A tyrosine kinase of FGFR inhibitor (SU5402) administration was performed according to the previous method (Yamamoto-Fukuda et al. 2014). After each vector transfection, 2 mM SU5402 in 2 % DMSO in PBS was administered in the ear skin region by eardrops (20 μl per day every 24 h, from the day of vector transfection to the day before euthanization) in 18 of the right ears (KGF gene-transfected 9 ears; vector alone-transfected 9 ears). In the 18 left ears, 20 μl of 2 % DMSO in PBS was administered after vector transfection (KGF gene-transfected 9 ears; vector alone-transfected 9 ears). The ear tissues were then removed and the paraffin sections prepared.
Reagents
Chemicals and Biochemical
The SU5402 was purchased from Calbiochem (Darmstadt, Germany). The PFA was purchased from Merck (Darmstadt, Germany) and the 3 3′-diaminobenzidine 4HCl (DAB) and EDTA were purchased from Dojin Chemical Co. (Kumamoto, Japan). The dimethyl sulfoxide (DMSO), 3-aminopropyltriethoxysilane, proteinase K, Brij 35, Triton X-100, bovine serum albumin (BSA, essentially fatty acid- and globulin-free) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The Permount was from Thermo Fisher Scientific (Hudson, NH, USA). The horseradish peroxidase (HRP)-conjugated streptavidin was purchased from Amersham Biosciences (Piscataway, NJ, USA). The biotin-16-dUTP and terminal deoxynucleotidyl transferase (TdT) were purchased from Roche Diagnostics (Mannheim, Germany). The BrdU was from Zymed Laboratory (San Francisco, CA, USA). The 4,6′-Diamidino-2-phenylindole dihydrochloride (DAPI) was from Dako (Glostrup, Denmark). The EdU and Click-iT EdU Imaging Kits were purchased from Invitrogen/Molecular Probes (Carlsbad, CA, USA). All other reagents used in this study were purchased from Wako Pure Chemicals (Osaka, Japan) and were of analytical grade.
Antibodies
The primary antibodies used in this study were anti-KGF (goat polyclonal, Sigma-Aldrich #AB260376, 1:100); anti-FGFR2 (KGFR; rabbit polyclonal, Sigma-Aldrich#AB259379, 1:100); anti-Flag (M2) (mouse monoclonal, Sigma #AB259529, 1:250); anti-dephosphorylated ERK1&2 (p-ERK) (mouse monoclonal, Sigma #M8159, 1:50); anti-PCNA (PC10) (mouse monoclonal, Dako #AB2160651, 2.0 μg/ml); anti-p63 (4A4; TA and delta N p63; mouse monoclonal, Abcam #AB305870; 1:100); anti-pan-p63 (4A4; mouse monoclonal, BioGenex #MU418-UC, 1:200); anti-pp63 (Ser160/162, rabbit polyclonal, Cell Signaling #AB2286372, 1:150); anti-CK14 (CK14, rabbit polyclonal, BioLegend (formerly Covance Antibody Products) #PRB-155P, 1:4000); anti-CK10 (CK10; rabbit polyclonal, BioLegend (formerly Covance Antibody Products) # PRB-159P, 1:4000); and anti-BrdU (BrdU; mouse monoclonal, Leica Microsystems (formerly Novocastra Products)#AB563437, 1.0 μg/ml). The secondary antibodies used in this study were HRP-conjugated goat anti-mouse IgG F(ab)’ (Chemicon International #AP124P, 1:100); HRP-conjugated goat anti-rabbit IgG F(ab)’ (Thermo Fisher Scientific #31460, 1:200); FITC-labeled goat anti-biotin (Abcam #ab53469, 1:100); HRP-conjugated goat anti-biotin (Abcam # ab6651, 1:100); Alexa Fluor 546-goat anti-mouse IgG (Thermo Fisher Scientific #A-11030, 1:500); Alexa Fluor 546-goat anti-rabbit IgG (Thermo Fisher Scientific #A-11035, 1:500); and Alexa Fluor 647-goat anti-rabbit IgG (Thermo Fisher Scientific # A-21244, 1:500). The normal goat IgG and sheep IgG were from Sigma Chemical Co. The normal mouse and rabbit IgG were from Dako. Detailed information about the antibodies was shown in Table 1.
Table 1.
List of primary antibodies for immunohistochemistry
| Antibodies | Clone | Reactivity | Company | Cat. no. | Dilution or concentration |
|---|---|---|---|---|---|
| Goat polyclonal anti-KGF | NA | Human | Sigma | K4760 | 1:100 |
| Rabbit polyclonal anti-FGFR2 | PA5-29426 | Human (a region within amino acids 539 and 821 of human FGFR2) | Thermo Fisher Scientific | AB_2546902 | 1:100 |
| Mouse monoclonal anti-Flag M2 | M2 | Binding site: N-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-C | Sigma | F1804 | 1:250 |
| Mouse monoclonal anti-dephosphorylated ERK1&2 | ERK-YNP | Mouse, human, rat | Sigma | M3557 | 1:50 |
| Mouse monoclonal anti-PCNA | PC10 | Mouse, rat, zebrafish | Dako | M0879 | 2.0 μg/ml |
| Mouse monoclonal anti-p63 | 4A4 | Mouse, rat, human, turtle | Abcam | ab735 | 1:100 |
| Mouse monoclonal anti-pan-p63 | 4A4 | Human | BioGenex | B-AM2525M | 1:200 |
| Rabbit polyclonal anti-pp63 | NA | Human, mouse, rat, chicken, Xenopus | Cell Signaling | #4981 | 1:150 |
| Rabbit polyclonal anti-CK14 | NA | Human, mouse, rat, dog, primate | Covance | PRB-155P | 1:4000 |
| Rabbit polyclonal anti-CK10 | NA | Human, mouse, rat, dog, primate | Covance | PRB-159P | 1:4000 |
| Mouse monoclonal anti-BrdU | 85-2C8 | 5-Bromodeoxyuridine (BrdU), an analog of thymidine | Novocastra | NCL-BrdU | 1.0 μg/ml |
NA not available
Plasmids
The hKGF cDNA for the cording region was kindly provided by Dr. Jeffrey Rubin from the National Cancer Institute (Bethesda, MD). The 3X FLAG hKGF vector (Matsumoto et al. 2009) was constructed by inserting the cDNA to p3XFLAG–CMV14 vector (Sigma Chemical Co.).
Specific Methods
Western Blot Analysis of KGF
The expression of KGF proteins after vector transfection in the ear tissues was examined by Western blot analysis as previously described with primary antibodies against KGF (0.1 μg/ml; Sigma) and secondary antibody against goat (1:10,000 dilution; Sigma) (Yamamoto-Fukuda et al. 2015). As a control, actin protein was detected with rabbit polyclonal anti-Actin antibody (H-196; 1:1000 dilution; Santa Cruz Biotechnology, CA, USA) and a secondary antibody against rabbit (1:10,000 dilution; Sigma).
Immunohistochemistry
For the detection of FLAG, KGF, KGFR, p63, PCNA, CK14, CK10, BrdU, pp63, and p-ERK, an enzyme or fluorescence immunohistochemistry was performed on the paraffin sections of skin tissue, as described previously (Yamamoto-Fukuda et al. 2014, 2015, 2010; Akiyama et al. 2014; Miyata et al. 2008; Ulziibat et al. 2006). In the case of FLAG detection, each section was pretreated with proteinase K dissolved in PBS at 10 μg/ml at 37 °C for 15 min. For the detection of KGFR, CK14, and CK10, the sections were immersed with 0.2 % TritonX-100. For the detection of p63, the sections were autoclaved in a 0.01-M citrate buffer (pH 6.0) at 120 °C to retrieve the antigen for 10 min. For the detection of BrdU, the section was incubated with proteinase K at 100 μg/ml at 37 °C for 15 min and immersed with 2 N HCl for 30 min. Pretreatment was omitted in the immunohistochemistry for the detection of KGF, PCNA, pp63, and p-ERK. For the enzyme immunohistochemistry after the inactivation of endogenous peroxidase with 0.3 % H2O2 in methanol for 15 min, the slides were preincubated with 500 μg/ml normal goat IgG in 1 % BSA in PBS for 1 h to block a nonspecific reaction. The sections were then reacted overnight with the first antibody in 1 % BSA in PBS. For the detection of phosphorylated protein, 0.05 M tris-buffered saline (TBS) was used instead of PBS in the above steps. After reaction with the HRP-conjugated second antibody, the sites of HRP were visualized with DAB and H2O2, or in the presence of nickel and cobalt ions. For the fluorescence immunohistochemistry, after immersion with the single or mixed first antibody, the sections were incubated with the second antibodies (Alexa Fluor 488-azide, Alexa Fluor 546-goat anti-mouse IgG and Alexa Fluor 647-goat anti-rabbit IgG) for 1 h. After washing three times with 0.075 % Brij 35 in PBS, the sections were counterstained with DAPI. For every experimental run, negative control samples were prepared by reacting the sections with normal mouse IgG or normal rabbit IgG instead of the specific first antibody.
EdU staining was performed according to the manufacturer’s protocol (Click-iT EdU Imaging Kits).
TUNEL Staining
To identify apoptotic cells, TUNEL was performed as described previously (Yamamoto-Fukuda et al. 2000). The signals were detected immunohistochemically with HRP-conjugated goat anti-biotin antibody, and the HRP sites were visualized with DAB and H2O2 in the presence of nickel and cobalt ions, as described above.
Detection of the Phosphorylated Level of p63
To detect the phosphorylated level of p63 in each mouse, we performed double immunofluorescence staining. After de-paraffinization, the slides were reacted with 20 μM Phos-tag BTL-111 and mouse monoclonal anti-p63 antibody overnight at RT. After washing four times with 0.075 % Brij 35 in 0.05 M TBS (pH 7.5), the sections were reacted with FITC-labeled goat anti-biotin and Alexa Fluor 546-goat anti-mouse IgG as secondary antibodies for 1 h. The nuclei were stained with DAPI and the sections analyzed using fluorescence confocal laser scanning microscopy (LSM 510 PASCAL, LSM 710 ELYRA PS.1, Zeiss). High-resolution images were obtained using SR-SIM (ELYRA PS.1 system, Zeiss). All images were acquired and processed with the Zeiss Zen 2012 imaging software. The nuclei were defined using the DAPI channel, and the number of the color pixels of pp63 in one nucleus was values to the total number of p63 pixel values.
Microacopy, Image Analysis, and Cell Count
For each time point, at least three independent specimens were used. Images of the H&E stainings and immnostainings were captured using an Axio Cam camera and the AxioVision software (Carl Zeiss) under light microscopy. Epidermal thickness was measured at three locations (central and at each extreme) using the ImageJ software from the National Institutes of Health (n = 4, each time point). The immunohistochemistry results were graded as positive or negative and compared to the negative control. For each section, the number of cell nuclei was counted at more than 1000 nuclei in three equal epithelial regions at × 400 magnification. Per specimen of immunofluorostaining, three 10,000-μm2 areas (100 × 100 μm squares) for the equal epithelial regions were assumed with confocal laser scanning microscopy (LSM 510 PASCAL, Carl Zeiss, LSM 710 ELYRA PS.1 system, Zeiss, Gottingen, Germany) and the Zeiss acquisition analysis software (Zen Black), and the number of positive cell nuclei were counted. DAPI labeling was used to obtain the total cell number. The labeling index (LI) (mean ± SD) represented the percentage of positive cell nuclei per the total number of counted nuclei.
Statistical Analysis
All data were expressed as mean ± SEM. Differences between the groups were examined for statistical significance using the two-way analysis of variance (ANOVA) test followed by Tukey’s post hoc tests for normally distributed data. A p value of less than 0.05 denoted the presence of a statistically significant difference. All analyses were performed using a statistical software package (JMP version 13; SAS Institute Japan, Tokyo, Japan). Precise values for p are given in the results for all significant differences, as stipulated in the guidelines for JARO publications.
Results
p63, pp63, KGF, and KGFR Expression in Cholesteatoma Tissue
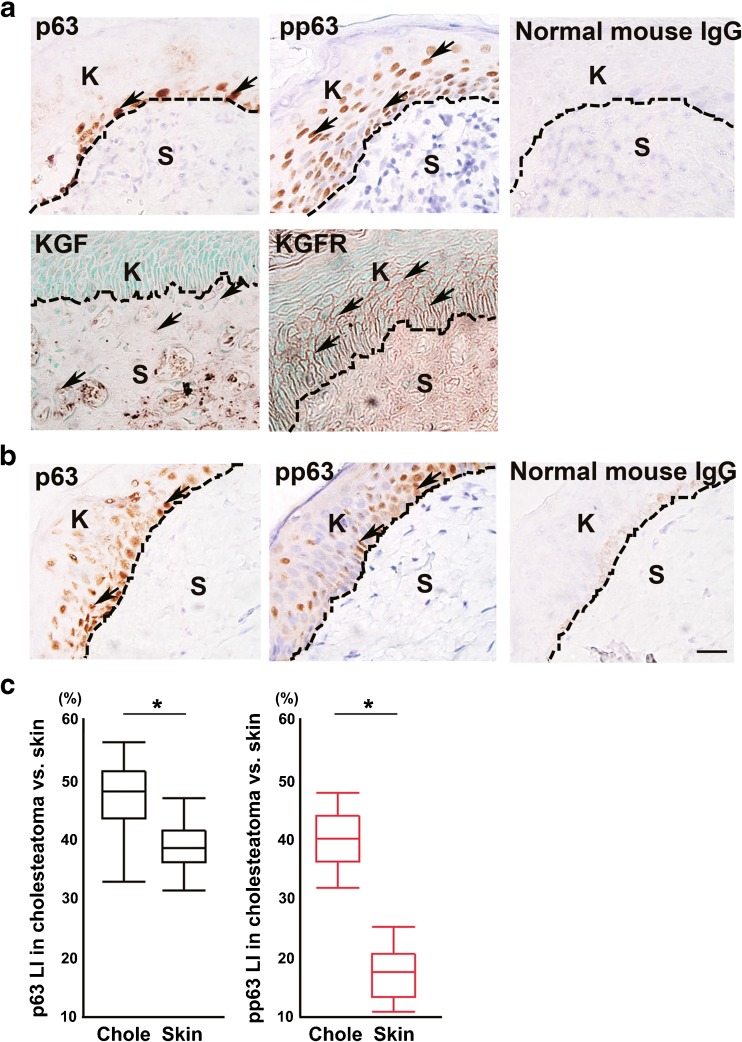
In the cholesteatoma tissue, most of the p63-positive cells were detected in the basal layer (Fig. 1a). Many pp63-positive cells were detected in the basal layer and in the upper layer of the thickened wall of the cholesteatoma matrix (Fig. 1a). KGF expression was detected in some stromal cells (Fig. 1a). KGFR-positive cells were detected in the basal layer and in the upper layer of the thickened wall of the cholesteatoma matrix (Fig. 1a). The expression level of p63 was almost the same as in normal skin tissue (Fig. 1b). On the other hand, the expression level of pp63 was slightly weaker in normal skin tissue (Fig. 1b). We previously indicated that KGF and KGFR were scarcely detected in normal skin tissue by immunohistochemical analysis (Yamamoto-Fukuda et al. 2003). p63 LI in middle ear cholesteatoma (47.6 ± 5.6 %) was almost the same as that of normal skin (39.2 ± 4.0 %; one-way ANOVA F(1, 49) = 36.22, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 1c). pp63 LI in middle ear cholesteatoma (40.1 ± 4.8 %) was significantly higher than that of normal skin (17.5 ± 4.3 %; one-way ANOVA F(1, 48) = 294.20, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 1c).
Fig. 1.
Immunohistochemical analysis of KGF, KGFR, p63, and pp63 in human specimens. a p63-positive cells were detected mainly in the basal layer; pp63-positive cells were detected in the basal and upper layers of the cholesteatoma matrix (upper panels). KGF expression was detected in some stromal cells, and KGFR-positive cells were detected in the basal and upper layers of the cholesteatoma matrix (lower panels). Normal mouse IgG was used instead of first antibody as a negative control. b p63-positive cells and pp63-positive cells were detected in the basal and upper layers and the expression level was slightly weak in the control. Normal mouse IgG was used instead of first antibody as a negative control. Arrows indicate positive cells. Dashed lines indicate the basement membrane. K indicates keratinizing squamous epithelium. S indicates the subepithelial region. c Box plot showing the labeling index (LI) of p63 (black boxes) in cholesteatoma (n = 29) vs. skin (n = 22) and pp63 (red boxes) in cholesteatoma (n = 29) vs. skin (n = 21). (p63 LI: *p < 0.0001 as determined by a one-way ANOVA F(1, 49) = 36.22 with a Tukey’s post hoc test, pp63 LI: *p < 0.0001 as determined by a one-way ANOVA F(1, 48) =294.20)
Morphological Changes in the Mouse Epidermis after KGF Gene Transfection
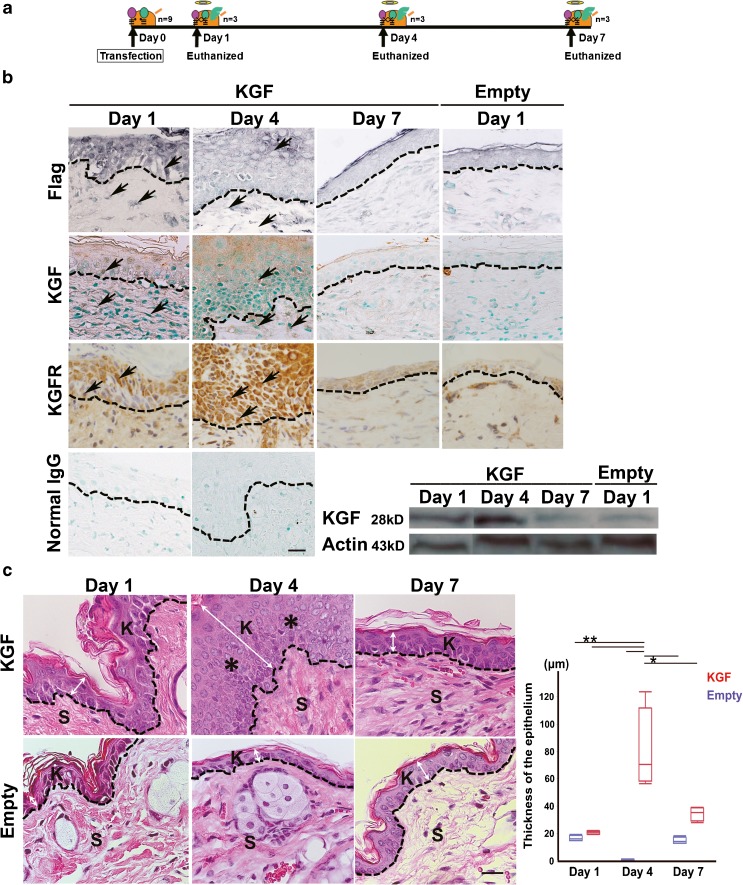
The FLAG-hKGF expression vector was successfully transfected in vivo and induced KGF and KGFR expression until day 4 (Fig. 2b). A Western blot analysis using anti-KGF antibody demonstrated a 28-kDa band in the hKGF gene-transfected ears at days 1, 4, and 7 (Fig. 2b). The human KGF protein level was highest at day 4 and scarcely detected at day 7, which was almost the same level as that of day 1 in the vector alone-transfected ears (Fig. 2b). H&E staining of the mouse ear skin tissues revealed that KGF gene transfection induced a marked thickening of the epithelium, as compared to the vector alone-transfected ear skin tissue (Fig. 2c). However, the effect of KGF transfection was transient. The thickness of the epithelium reached a maximum at day 4 (80.3 ± 29.9 μm; compared to 17.6 ± 2.1 μm vector alone at day1, 15.9 ± 2.5 μm vector alone at day 4, 15.3 ± 5.0 μm vector alone at day 7, or 21.2 ± 1.2 μm KGF at day 1; two-way ANOVA F(5, 18) = 19.74, p < 0.0001 with Tukey’s multiple comparison test, compared to 34.7 ± 5.0 μm KGF at day 7; two-way ANOVA F(5, 18) = 19.74, p = 0.0008 with Tukey’s multiple comparison test) and then declined to the control level at day 7 (34.7 ± 5.0 μm compared to 15.9 ± 2.5 μm vector alone at day 7; two-way ANOVA F(5, 18) = 19.74, p = 0.2787 with Tukey’s multiple comparison test) (Fig. 2c, Table 2).
Fig. 2.
Morphological changes in the mouse epidermis after KGF gene transfection. a Schematic description of the method for electroporated transfection of KGF vector in mouse ear skin. The animals were euthanized using an intraperitoneal injection of 200 mg/kg pentobarbital and their ear skin tissue was removed 1, 4, and 7 days after vector transfection. b Immunohistochemical analysis using the anti-FLAG M2 and KGF antibody in sections of KGF gene-transfected ear skin tissue (KGF) (day 1, day 4, and day 7) and the vector alone-transfected ears (Empty) (day 1). Intense staining for FLAG and KGF was detected abundantly in epithelial cells and in some stromal cells of KGF gene-transfected ear skin tissue at day 1 and day 4. FLAG-hKGF expression vector was successfully transfected in vivo and induced KGF expression until day 4. KGFR expression was detected until day 4. No staining of Flag, KGF, and KGFR was observed in the vector alone-transfected ears. No staining was observed with normal mouse IgG and rabbit IgG instead of first antibody. The results of a Western blot analysis of KGF in transfected tissue. KGF: lysate of the skin of the hKGF gene-transfected ears. Empty: lysate of skin of the vector alone-transfected ears. Each lysate was obtained after vector transfection at day 1, day 4, and day 7. The total applied volume was 50 μg per lane. Each lane was reacted with 0.2 μg/ml anti-KGF antibody. An intense band was detected in the hKGF-transfected ears at day 4. c H&E staining of mouse ear skin tissue after vector transfection. KGF gene-transfected ear (KGF) (upper panels, day 1, day 4, and day 7). Vector alone-transfected ear (Empty) (lower panels, day 1, day 4, and day 7). Single transfection of KGF-induced keratin accumulation and thickened squamous epithelium (asterisk) of ear skin tissue at day 4. Dashed lines indicate the basement membrane. K indicates keratinizing squamous epithelium. S indicates the subepithelial region. Scale bar, 20 μm. Arrows indicate positive cells. Double arrow indicates the thickness of the epithelium. Scale bars, 20 μm. Box plot showing the thickness of the epithelium (μm) in vector alone-transfected ear skin (blue boxes) and KGF gene-transfected ears (red boxes). (*p < 0.001, **p < 0.0001 as determined by a two-way ANOVA F(5, 18) = 19.74 with a Tukey’s post hoc test)
Table 2.
Epithelial thickness and the LI of CK14 and CK10 PCNA LI, TUNEL LI, and pp63/p63 ratio after vector-alone transfection or KGF-gene transfection
| Control (n = 5) | Day 1 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|
| Epithelial thickness | ||||||
| Empty (n = 4) | – | 17.06 + 2.06 | 15.94 + 2.47 | – | – | 15.27 + 2.51 |
| KGF (n = 4) | – | 21.15 + 1.16 | 80.30 + 29.85 | – | – | 34.75 + 4.96 |
| PCNA | ||||||
| Empty (7, 4, 4) | 6.33 ± 5.13 | 19.02 ± 11.22 | 4.38 ± 0.60 | – | – | 5.27 ± 2.64 |
| KGF (6, 7, 10) | 35.49 ± 20.75 | 47.44 ± 7.19 | – | – | 19.13 ± 10.90 | |
| TUNEL | ||||||
| Empty (n = 3) | 4.66 + 1.40 | 10.63 + 0.976 | 1.29 + 0.87 | 3.61 + 0.71 | 1.80 + 0.41 | 3.57 + 0.30 |
| KGF (n = 3) | 9.74 + 1.40 | 1.31 + 1.14 | 2.76 + 0.06 | 1.67 + 0.19 | 4.21 + 0.77 | |
| CK14 | ||||||
| Empty (n = 5) | 34.52 + 4.29 | 36.59 + 6.34 | – | – | 38.70 + 5.84 | |
| KGF (n = 5) | 62.37 + 6.31 | 80.34 + 8.23 | – | – | 40.83 + 4.55 | |
| CK10 | ||||||
| Empty (n = 5) | 23.63 + 5.23 | 21.47 + 4.15 | – | – | 24.41 + 9.30 | |
| KGF (n = 5) | 24.81 + 4.66 | 0.53 + 0.12 | – | – | 22.88 + 7.37 | |
| pp63/p63 | ||||||
| Empty (n = 3) | 0 ± 0 | 0.34 ± 0.28 | – | – | 0.02 ± 0.04 | |
| KGF (n = 3) | 0.03 ± 0.03 | 24.03 ± 11.78 | 30.58 ± 2.56 | – | – | 0 ± 0 |
Epithelial Cell Proliferation in Mouse Ear Skin Tissue after KGF Gene Transfection
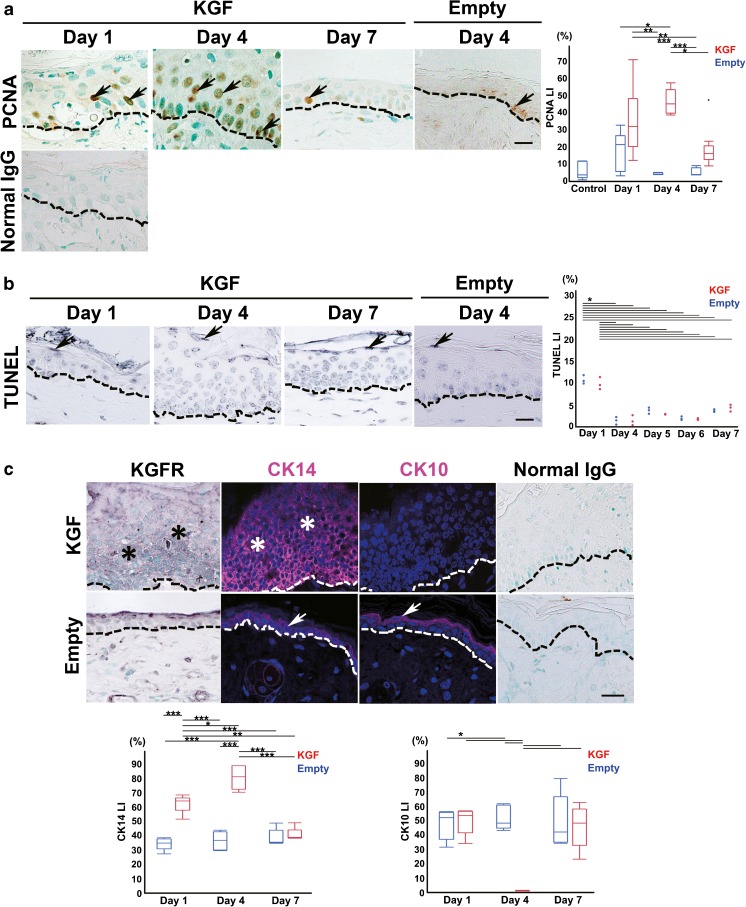
An immunohistochemical analysis for PCNA was performed to evaluate the effects of KGF-gene transfection on the proliferative activity of epithelial cells. In the vector alone-transfected ears, PCNA-positive cells were found only in the basal layer, the same as in normal epithelium (Fig. 3a). In contrast, PCNA-positive cells were widely distributed throughout the upper layer of the epithelium in the KGF gene-transfected ears (Fig. 3a). PCNA LI in the KGF gene-transfected ears at day 4 (47.4 ± 7.2 %) was significantly higher than in the vector alone-transfected ears at day 1 (19.7 ± 10.6 %; two-way ANOVA F(5, 32) = 11.86, p = 0.0009 with Tukey’s multiple comparison test), day 4 (4.4 ± 0.6 %; two-way ANOVA F(5, 32) = 11.87, p < 0.0001 with Tukey’s multiple comparison test), and day 7 (5.3 ± 2.6 %; two-way ANOVA F(5, 32) = 11.87, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 3a, Table 2). Highest PCNA LI was observed at day 4 after transfection (47.4 ± 7.2 % KGF at day 4 compared to 19.1 ± 10.9 % KGF at day 7; two-way ANOVA F(5, 32) = 11.87, p = 0.0003 with Tukey’s multiple comparison test) (Fig. 3a, Table 2).
Fig. 3.
KGF gene transfection induced epithelial cell proliferation in mouse ear skin tissue. a Immunohistochemical detection of PCNA (a marker of late G1 to S) in sections of KGF gene-transfected ear skin tissue (KGF; day 1, day 4, and day 7) and vector alone-transfected ear skin tissue (Empty; day 4). PCNA-positive cells (arrows) were detected in the basal layer at day 1, basal and upper layers at day 4. No staining was observed with normal mouse IgG instead of first antibody. Dashed lines indicate basement membrane. Scale bar, 20 μm. Box plot showing the PCNA LI of the epithelium in vector alone-transfected ear skin (day 1, n = 7, day 4, n = 4, day 7, n = 4) (blue boxes) and the KGF gene-transfected ears (day 1, n = 6, day 4, n = 7, day 7, n = 10) (red boxes). (*p < 0.001, **p < 0.005, ***p < 0.0001 as determined by a two-way ANOVA F(5, 32) = 11.87 with a Tukey’s post hoc test). b TUNEL-positive epithelial cells in mouse ear skin tissue after vector transfection. TUNEL staining in sections of KGF gene-transfected ear skin tissue (KGF; day 1, day 4, and day 7) and vector alone-transfected ear skin tissue (Empty) (day 4). A few epithelial cells in the cornified layer were TUNEL-positive (arrows). Dashed lines indicate basement membrane. Scale bar, 20 μm. Dots plot showing the TUNEL LI of the epithelium in vector alone-transfected ear skin (n = 3 for each) (blue boxes) and KGF gene-transfected ears (n = 3 for each) (red boxes). (*p < 0.0001 as determined by a two-way ANOVA F(9, 20) = 54.17 with a Tukey’s post hoc test). c Immunohistochemical detection of KGFR, and immunofluorescence detection of CK14 (undifferentiated cell marker) and CK10 (differentiated cell marker) in the section of KGF gene-transfected (KGF, day 4) and vector alone-transfected rat ear skin tissue (Empty, day 4). The expression of KGFR was detected in the basal and upper layers of the KGF gene-transfected epithelium at day 4 but scarcely detected in vector alone-transfected epithelium. CK14 positive cells (magenta) were detected in all layers but CK10-positive cells (magenta) were not detected in any layer at day 4 after KGF gene transfection. In vector alone-transfected epithelium, CK14 was detected in the basal layer and CK10 was detected in the upper layer. As a negative control, sections were reacted with normal rabbit IgG instead of specific antibodies. Arrows: positive cells. Asterisks: positive region. Dashed lines: basement membrane. Scale bars, 20 μm. Nuclei stained with DAPI (4′, 6′- diamidino-2-phenylindole) (blue). Box plot showing the CK14 LI or CK10 LI of the epithelium in vector alone-transfected ear skin (n = 5 for each) (blue boxes) and KGF gene-transfected ears (n = 5 for each) (red boxes). (CK14: *p < 0.05, **p < 0.001, ***p < 0.0001 as determined by a two-way ANOVA F(5, 24) = 46.09 with a Tukey’s post hoc test, CK10: *p < 0.0001 as determined by a two-way ANOVA F(5, 24) = 13.81 with a Tukey’s post hoc test)
Frequency of TUNEL-Positive Cells in Mouse Ear Tissue after KGF Gene Transfection
To evaluate the effects of KGF gene transfection on epithelial cell death, we conducted TUNEL staining. TUNEL-positive cells were found only in the corneal layer, as in the control specimens (Fig. 3b). The frequency of these cells in the KGF gene-transfected skin tissue was almost the same as that of the control tissue (Fig. 3b, Table 2). TUNEL LI in the KGF gene-transfected ears at day 1 (9.7 ± 1.4 %) was significantly highest in the KGF gene transfection group (compared to 1.3 ± 1.1 % KGF at day 4, 2.7 ± 0.1 % KGF at day 5, 1.7 ± 0.2 % KGF at day 6, 4.2 ± 0.7 % KGF at day 7; two-way ANOVA F(9, 20) = 54.17, p < 0.0001 with Tukey’s multiple comparison test). In the vector alone-transfection group, a significantly higher level of TUNEL LI was detected at day 1 (10.6 ± 10.0 % compared to 1.3 ± 0.9 % vector alone at day 4, 3.6 ± 0.7 % vector alone at day 5, 1.8 ± 0.4 % vector alone at day 6, 3.5 ± 0.3 % vector alone at day 7; two-way ANOVA F(9, 20) = 54.17, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 3b, Table 2). However, TUNEL LI in the KGF gene-transfected ears at day 1 (9.7 ± 1.4 %) was almost the same as that in the vector alone-transfection ears at day 1 (10.6 ± 10.0 %; two-way ANOVA F(9, 20) = 54.17, p = 0.9254 with Tukey’s multiple comparison test) (Fig. 3b, Table 2).
Detection of KGFR, CK14, and CK10 Proteins in Proliferative Mouse Ear Skin Tissue after KGF Gene Transfection
To evaluate the effects of KGF through paracrine action on epithelial cell differentiation in the thickened epithelium at day 4, we analyzed the expression of KGFR, CK14 (an undifferentiated epithelial cell marker), and CK10 (a differentiated epithelial cell marker) by immunohistochemistry. As shown in Fig. 3c, KGFR protein was detected in the basal and upper layers of the epithelium in the KGF-transfected ears at day 4. In contrast, KGFR-positive cells were not found in the KGF gene-transfected ears at day 7 (data not shown) or in the vector alone-transfected ears (Fig. 3c). Staining for the undifferentiated cell marker CK14 was detected in the basal and upper layers but the differentiated cell marker CK10 was not detected in all layers of the epithelium at day 4 after KGF gene transfection (Fig. 3c). In the control ears, CK14 was detected in the basal layer and CK10 was detected in the upper layers (Fig. 3c). No staining was detected with normal rabbit IgG instead of the first antibody (Fig. 3c). CK14 LI in the KGF gene-transfected ears at day 4 (80.3 ± 8.2 %) was significantly highest in all of the groups (compared to 62.4 ± 6.3 % KGF at day 1; two-way ANOVA F(5, 24) = 46.09, p = 0.0012 with Tukey’s multiple comparison test, compared to 40.8 ± 4.6 % KGF at day 7, 34.5 ± 4.3 % empty at day 1, 36.6 ± 6.3 % empty at day 4 or 38.7 ± 5.8 % empty at day 7; two-way ANOVA F(5, 24) = 46.09, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 3c, Table 2). CK10 LI in KGF gene-transfected ears at day 4 (0.5 ± 0.1 %) was significantly lowest in all of the groups (compared to 24.8 ± 4.7 % KGF at day 1, 23.6 ± 5.2 % empty at day 1, 21.4 ± 4.1 % empty at day 4, 22.9 ± 7.4 % KGF at day 7 or 24.4 ± 9.3 % empty at day 7; two-way ANOVA F(5, 24) = 13.81, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 3c, Table 2).
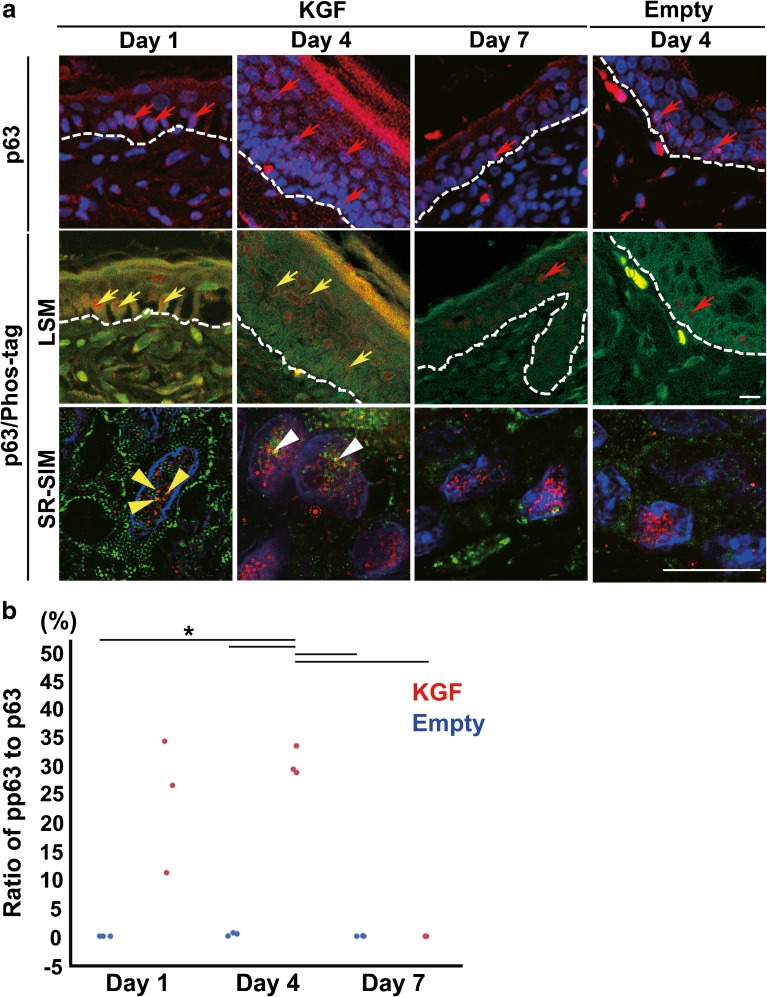
Localization of p63 and pp63-Positive Cells in Mouse Ear Epithelium and the Phosphorylation Level of p63 in Epithelial Cells after KGF Transfection
When we assessed the effects of KGF on the proliferative potential of stem and/or progenitor cells in the thickened epithelium, we analyzed the expression pattern of p63, a stem cell marker of stratified squamous epithelium, and pp63, a marker for progenitor cells, by immunohistochemistry. p63 was detected in many basal cells in the epithelium at day 1 after KGF gene transfection (Fig. 4a). We also noted many cells in the upper layer were also positive for p63 in the KGF gene-transfected ears at day 4 (Fig. 4a). In contrast, p63-positive cells were reduced and found mainly in the basal layer of the KGF gene-transfected ears at day 7 and in the vector alone-transfected ears at any day (Fig. 4a). We performed double staining for p63 and Phos-tag to assess pp63. (Suzuki and Senoo 2013; Candi et al. 2008) The results indicated that pp63 expression was highly variable within the basal layer of the KGF gene-transfected ears at day 1 (Fig. 4a). Consistent with the KGF gene-transfected ears at day 4, pp63-positive cells were localized in the upper layer and the number of these cells increased (Fig. 4a). SR-SIM images revealed that three yellow dots depicting pp63 were detected in the nuclei of the p63-positive basal cells at day 1 and p63-positive suprabasal cells at day 4 after KGF gene transfection (Fig. 4a). The ratio of pp63 spots to p63 spots (pp63/p63 ratio) at day 4 after KGF gene transfection (30.6 ± 2.6 %) was significantly higher than in the vector alone-transfected ears (compared to 0.0 ± 0.0 % empty at day 1, 0.3 ± 0.3 % empty at day 4, or 0.0 ± 0.0 % empty at day 7; two-way ANOVA F(5, 12) = 24.96, p < 0.0001 with Tukey’s multiple comparison test) and at day 7 after KGF gene transfection (compared to 0.0 ± 0.0 %; two-way ANOVA F(5, 12) = 24.96, p < 0.0001 with Tukey’s multiple comparison test) (Fig. 4b, Table 2). The pp63/p63 ratio at day 7 after KGF gene transfection (0.0 ± 0.0 %) was almost same as in the vector alone-transfected ears (compared to 0.0 ± 0.0 %; two-way ANOVA F(5, 12) = 24.96, p = 1.00, with Tukey’s multiple comparison test) (Fig. 4b, Table 2).
Fig. 4.
KGF gene transfection activated the phosphorylation level of p63 in epithelial cells. a Immunofluorescence detection of p63 (red) in the section of KGF gene-transfected (KGF, day 1, day 4, and day 7) and vector alone-transfected mouse ear skin tissue (Empty, day 4) (upper panels). DAPI (4′, 6′- diamidino-2-phenylindole) was used to stain the nuclei (blue). The expression of p63 (red arrows), a reliable stem cell marker of stratified epithelia, was detected in many basal cells and in some upper basal cells at day 1 and in many upper basal cells at day 4 after KGF gene transfection. Double immunofluorescence detection of p63 and Phos-tag in the section of KGF gene-transfected (day 1, day 4, and day 7) (middle panels) and vector alone-transfected (day 4) mouse ear skin tissue (middle panels). Merged LSM images and SR-SIM images show p63 (red) and Phos-tag (green) and nuclei stained with DAPI (blue). In KGF gene-transfected ear skin tissue, high pp63 expression (yellow arrows) within the nuclei was detected in the basal and upper layers at day 1 and in the upper layer at day 4. SR-SIM images (lower panels) show three yellow dots depicting the pp63 (yellow arrow heads) detected in the nuclei of p63-positive basal cells at day 1 and pp63 spots (white arrow heads) in upper-layer cells at day 4 after KGF gene transfection. Dashed lines: basement membrane. Scale bars, 10 μm. b Dots plot showing the ratio of pp63/p63 in a nuclear of the epithelium in vector alone-transfected ear skin (n = 3 for each) (blue dots) and KGF gene-transfected ear (n = 3 for each) (red dots). (*p < 0.0001 as determined by a two-way ANOVA F(5, 12) = 24.96, with a Tukey’s post hoc test)
Detection of Stem and Progenitor Cells by Cell Tracing with BrdU-, EdU-, and CK14-Positive Cells in Mouse Ear Skin Tissue after KGF Transfection
We labeled DNA with EdU and BrdU, two analogues of thymidine, to investigate the stem and progenitor cells before and after KGF gene transfection (Fig. 5a). As shown in Fig. 5b, BrdU(+)EdU(−) cells were detected in the basal layer of the normal ears, KGF gene-transfected ears, and vector alone-transfected ears. In contrast to the results of the vector alone-transfected specimens, a higher number of BrdU(−)EdU(+) cells were detected in the CK14(+)CK10(−) basal layer at day 1 after KGF gene transfection and LI (9.7 ± 6.8 %) was significantly higher than that of the vector alone-transfected ears (2.9 ± 1.4 % at day 1; two-way ANOVA F(5, 24) = 5.41, p = 0.0471 with Tukey’s multiple comparison test, 0.4 ± 0.5 % at day 4; two-way ANOVA F(5, 24) = 5.41, p = 0.0030 with Tukey’s multiple comparison test, 0.7 ± 0.6 % at day 7; two-way ANOVA F(5, 24) = 5.41, p = 0.0070 with Tukey’s multiple comparison test) and decreased at day 7 (1.3 ± 1.2 % compared to 9.7 ± 6.8 % KGF at day 4; two-way ANOVA F(5, 24) = 5.41, p = 0.0056 with Tukey’s multiple comparison test)(Fig. 5b, c, d, Table 3). Some BrdU(+)EdU(+) cells were detected in the CK14(+)CK10(−) upper layer of the thickened CK14-positive epithelium at day 4 after KGF gene transfection (Fig. 5b, c). BrdU(+)EdU(+) LI at day 4 in the KGF-transfected ears (17.6 ± 2.7 %) was significantly higher (compared to 1.8 ± 0.3 % KGF at day 1, 1.3 ± 1.2 % KGF at day 7, 0.7 ± 0.5 % vector alone at day 1, 0.9 ± 0.3 % vector alone at day 4, or 0.7 ± 0.6 % vector alone at day 7; two-way ANOVA F(5, 24) = 139.39, p < 0.0001 with Tukey’s multiple comparison test)(Fig. 5d, Table 3).
Table 3.
LI of BrdUEdU-positive cells of ear epithelium after vector-alone transfection or KGF-gene transfection
| BrdU/EdU (+/−) | BrdU/EdU (−/+) | BrdU/EdU (+/+) | |
|---|---|---|---|
| Day 1 | |||
| KGF (n = 5) | 2.00 ± 1.13 | 9.67 ± 6.82 | 1.81 ± 0.34 |
| Empty (n = 5) | 2.91 ± 0.72 | 2.93 ± 1.38 | 0.72 ± 0.53 |
| Day 4 | |||
| KGF (n = 5) | 0.93 ± 0.50 | 5.21 ± 4.54 | 17.62 ± 2.72 |
| Empty (n = 5) | 2.37 ± 1.56 | 0.38 ± 0.52 | 0.92 ± 0.56 |
| Day 7 | |||
| KGF (n = 5) | 2.47 ± 2.26 | 0.94 ± 0.74 | 1.30 ± 1.16 |
| Empty (n = 5) | 2.51 ± 0.92 | 1.14 ± 0.35 | 0.68 ± 0.63 |
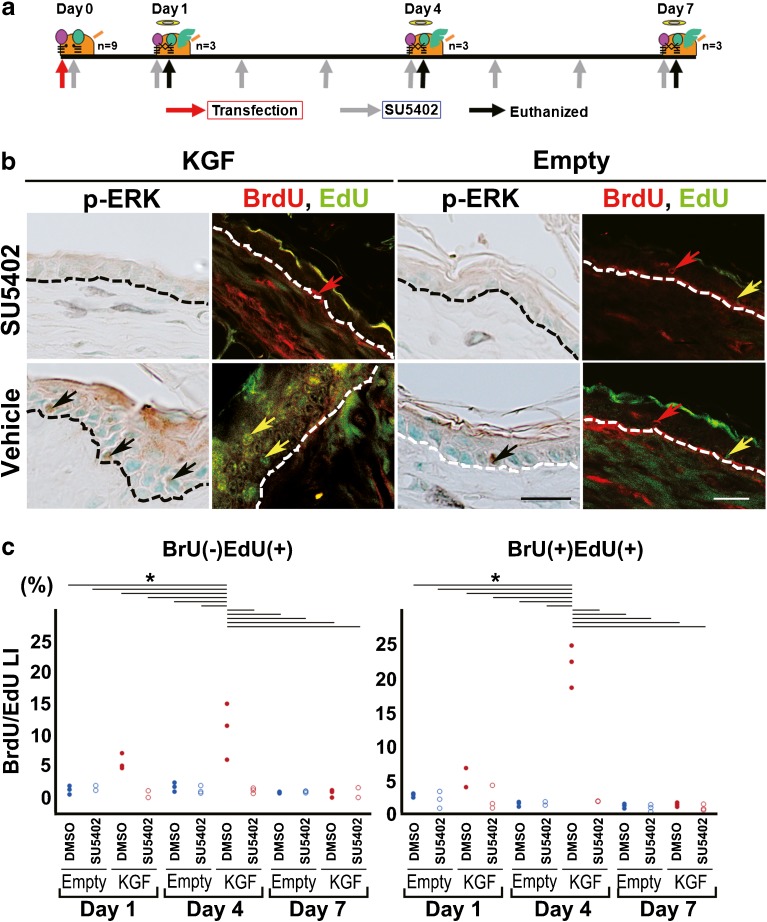
Detection of BrdU- and EdU-Positive Cells of KGF-Transfected Mouse Ear Skin Tissue after KGFR Tyrosine Kinase Inhibitor Treatment
To examine the effects of KGF against the proliferative activation of epithelial stem and/or progenitor cells that expressed KGFR, KGFR tyrosine kinase inhibitor administration was performed after KGF-gene transfection in vivo (Fig. 6a). The number of p-ERK positive cells was dramatically lower at day 1 and almost no positive epithelial cells were found at days 4 and 7 in the KGF gene-transfected ear skin tissue treated with 2 mM SU5402 (Fig. 6b). However, intensely stained epithelial cells were detected in KGF gene-transfected ear skin tissue treated with 1 % DMSO in PBS (Fig. 6b). According to the results of an immunohistochemical analysis of p-ERK, SU5402 inhibits ERK activation mediated by a constitutively activated KGFR induced by KGF-gene transfection. We labeled DNA synthetic activity with BrdU and EdU, two analogues of thymidine, by successive intraperitoneal injections in mice. In contrast to the vehicle, BrdU(+)EdU(+) cells in the upper layer at day 4 were scarcely detected (Fig. 6b). At day 4 after KGF transfection, BrdU(+)EdU(+) LI of the SU5402-treated ears (2.3 ± 1.8 %) was significantly lower than that of the vehicle-treated ears (compared to 5.0 ± 1.6 %; two-way ANOVA F(11, 24) = 68.55, p < 0.0001 with Tukey’s multiple comparison test)(Fig. 6c, Table 4). At day 1 after KGF transfection, BrdU(−)EdU(+) LI of the SU5402-treated ears (0.4 ± 0.6 %) was significantly lower than that of the vehicle-treated ears (compared to 5.6 ± 1.3 %; two-way ANOVA F(11, 24) = 13.23, p = 0.0076 with Tukey’s multiple comparisons test)(Fig. 6c, Table 4). BrdU(+)EdU(−) LI were almost same in all of the KGF gene-transfected ears and vector alone-transfected ears with or without SU5402 treatment (Table 4).
Fig. 6.
Under the overexpression of KGF, stimulation of progenitor cell proliferation was inhibited by SU5402, an inhibitor for tyrosine kinase of KGFR. a Schematic description of the method for administering an inhibitor for tyrosine kinase of KGFR (SU5402) in vivo and the injection of two thymidine analogues in KGF-transfected mouse ear skin. BrdU was injected intraperitoneally at 24 h prior to vector transfection and EdU was injected intraperitoneally at 2 h prior to each euthanization. Two millimolars SU5402 was administered via eardrops each day every 24 h from the day of vector transfection to the day before euthanization. b Immunohistochemical detection of p-ERK and double immunofluorescence detection of BrdU (red)/EdU (green) in sections of ear skin tissue treated with SU5402 or 2 % DMSO in PBS (vehicle) after KGF gene transfection (KGF, day 4) or vector-alone transfection (Empty, day 4). The ear tissue treated with SU5402 shows no staining with the anti-p-ERK antibody. In the section of KGF gene-transfected ear tissue treated with a vehicle, many p-ERK-positive cells were stained brown (black arrows). Large numbers of BrdU(+)EdU(+) cells (yellow arrows) were detected in the thickened epithelium of KGF gene-transfected ears treated with a vehicle but not detected in the SU5402-treated specimen. Red arrows: BrdU(+)EdU(−) cells. Scale bars: 20 μm. c Dots plot showing the LI of BrdU(−) EdU(+) positive cells or BrdU(+)EdU(+) positive cells of the ear epithelium treated with SU5402 or 2 % DMSO in PBS (vehicle) after vector-alone transfection (n = 3 for each) or KGF gene transfection (n = 3 for each). BrdU(−) EdU(+) LI: *p < 0.0001 as determined by a two-way ANOVA F(11, 24) = 13.23 with a Tukey’s post hoc test. BrdU(+)EdU(+) LI: *p < 0.0001 as determined by a two-way ANOVA F(11, 24) = 68.55 with a Tukey’s post hoc test
Table 4.
LI of BrdUEdU-positive cells of ear epithelium treated with SU5402 or 2 % DMSO in PBS (vehicle) after vector-alone transfection or KGF-gene transfection
| Day 1 | Day 4 | Day 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BrdU/EdU (+/−) | BrdU/EdU (−/+) | BrdU/EdU (+/+) | BrdU/EdU (+/−) | BrdU/EdU (−/+) | BrdU/EdU (+/+) | BrdU/EdU (+/−) | BrdU/EdU (−/+) | BrdU/EdU (+/+) | |
| KGF + SU5402 (n = 3) | 0.93 ± 1.00 | 0.36 ± 0.62 | 2.31 ± 1.76 | 0.95 ± 0.28 | 1.12 ± 0.43 | 1.98 ± 0.03 | 3.98 + 0.56 | 1.56 + 0.90 | 3.11 + 0.46 |
| KGF + DMSO (n = 3) | 1.26 ± 0.44 | 5.61 ± 1.28 | 4.97 ± 1.62 | 0 ± 0 | 10.81 ± 4.50 | 21.92 ± 3.11 | 1.18 + 0.29 | 0.69 + 0.61 | 1.47 + 0.31 |
| Empty + SU5402 (n = 3) | 1.77 ± 0.58 | 1.40 ± 0.44 | 2.21 ± 1.23 | 1.10 ± 0.26 | 1.18 ± 0.64 | 1.72 ± 0.28 | 3.19 + 0.33 | 2.72 + 0.15 | 2.99 + 0.51 |
| Empty + DMSO (n = 3) | 1.00 ± 0.44 | 1.21 ± 0.69 | 2.86 ± 0.30 | 1.40 ± 0.86 | 1.67 ± 0.72 | 1.59 ± 0.35 | 1.06 + 0.48 | 0.80 + 0.12 | 1.29 + 0.34 |
Discussion
Many pp63-Positive Epithelial Cells Were Detected in the Human Cholesteatoma Specimens
It is well known that middle ear cholesteatoma is characterized by the presence of a keratinizing epithelium that is believed to have hyper-proliferative properties (Sudhoff and Tos 2000). Our understanding of the molecular mechanism underlying the pathogenesis of cholesteatoma is limited, but an active proliferation of epithelial cells is thought to be irreversible. In this study, we clearly demonstrated that many pp63-positive epithelial cells were detected in human cholesteatoma specimens (Fig. 1). The transcription factor p63 plays an essential role in epithelial development (Mills et al. 1999). As previously reported, assessing the p63 level alone is not sufficient to identify stem cells and progenitor cells. A previous study introduced a second marker, pp63, and showed that relative pp63 levels to total p63 expression can distinguish stem cells from progenitor cells, which have more limited proliferative capacity (Linardi et al. 2015). As expected, pp63 was strongly and diffusely expressed in the entire cholesteatoma group, indicating the possibility that cholesteatomas are derived from undifferentiated cells—the progenitor cell—in the basal layer of the epidermis.
KGF Enhanced Proliferative Activity and Prevented Differentiation of Epithelial Cells in Mouse Ear Skin Tissue
In the previous study, we indicated that KGF and KGFR enhanced epithelial cell proliferative activity and correlated to the recurrence of human middle ear cholesteatoma (Yamamoto-Fukuda et al. 2003). In addition, the repetitive electroporatic transfection of the KGF gene expression vector induced middle ear cholesteatoma formation in vivo (Yamamoto-Fukuda et al. 2015). Based upon these observations, we analyzed the cell kinetics of epithelial cells in our in vivo model.
The transfection of the hKGF expression vector by electroporation increased the thickness and keratinization of the epithelial portion of ear skin tissue. In the previous study, it was indicated that KGF has an anti-apoptotic role against some kinds of cells (Tamaru et al. 2004), so we hypothesized that thickened epithelium might be induced under the anti-apoptotic role of KGF. However, we found no changes in apoptosis as assessed by TUNEL staining after KGF gene transfection, compared with vector-alone transfection (Fig. 3).
We also hypothesized that KGF could activate epithelial cell proliferation in ear skin. Regarding proliferation, the LI of PCNA-positive cells in KGF gene-transfected ear skin tissue had increased until day 4 (Fig. 3). When we assessed the markers of differentiation, we noted the appearance of undifferentiated marker CK14 in all of the layers of the epithelium at day 4 after KGF gene transfection (Fig. 3). It has been reported that KGF signals induce proliferation and prevents the terminal differentiation of keratinocyte in vitro (Andreadis et al. 2001), which supports our results.
KGF Induces p63 Gene Expression and pp63-Positive Progenitor Cell Proliferation
According to the results of the immunohistochemical analysis, p63-positive cells increased in the thickened epithelium after KGF transfection (Fig. 4). It has recently been shown that highly expressed KGF induced p63 expression and as a result, hyper-proliferative epithelium developed in transgenic mice (Chikama et al. 2008). Another study showed p63 strongly expressed in the epithelial cells of a KGF-treated limbal explant culture (Cheng et al. 2009). The p63 gene, a homolog of the tumor suppressor p53, is highly expressed in the basal or progenitor layers of many epithelial tissues and it is likely that p63 preserves the self-renewal capacity of progenitor cells (Yang et al. 1998). Taken together, our results indicate the possibility that p63 is critical for maintaining the progenitor cell populations that are necessary to sustain epithelial hyper-proliferation and morphogenesis of middle ear cholesteatoma.
Furthermore, another study showed that KGF affects stem and/or progenitor cell proliferation (Greco et al. 2009). Based upon these observations, we analyzed progenitor cell proliferation using a cell tracing system in our vivo model. According to the results of our cell tracing analysis, many progenitor cells (BrdU(+)EdU(+) cells) were detected in the upper layer of the epithelium at day 4 after KGF gene expression (Fig. 5). The unbalanced proliferation of basal stem and/or progenitor cells induces devastating diseases such as skin cancer and epidermal disease (Mancuso et al. 2006). In our in vivo model, KGF overexpression increased the proliferative activity of undifferentiated epithelial cells such as stem and/or progenitor cells, resulting in epithelial hyperplasia and stratification. Theoretically, disturbances in the regulation of basal stem cells could lead to a hypertrophic epithelium, which is characterized by excess basal stem cells and stratified squamous metaplasia (O’Koren et al. 2013; Rock et al. 2010). However, skin cancer could not be detected in KGF gene-transfected ear skin and the thickness of the epithelium was transient in our in vivo model. After a reduction of KGF in the KGF gene-transfected epithelium, epithelial cell differentiation was induced and hyper-keratinization of the outer layers was shown during epithelial homeostasis. One possibility is that KGF increased progenitor cells, but these cells have a limited proliferative potential. Indeed, progenitor cells (BrdU(+)EdU(+) cells) were scarcely found in the epithelium at day 7 after KGF gene expression (Fig. 5). The previous study indicated that during the wound healing process, the strong induction of KGF-expressing stem cells becomes active, and contributes substantially to the repair and production of more progenitor cells for tissue recovery (Vorotnikova et al. 2010; Auf demKeller et al. 2004; Finch and Rubin 2004). Also, in the epithelium of the esophagus, progenitors have been shown to transition to a proliferative state and effect repair following injury (Doupé et al. 2012).
In a recent study, it was shown that pp63 could be induced to differentiate basal stem cells to upper progenitor cells and prevent continued proliferation and a transformation to malignancy (Suzuki and Senoo 2013). We also detected a high phosphorylation level of p63 in the transient KGF gene-activated keratinocytes of the upper layer under high-resolution microscopy (Fig. 4). In fact, a high level of pp63 expression is associated with certain skin disorders, such as dermatitis (Keyes et al. 2011). These observations support that KGF might stimulate stem cells to produce more progenitor cells, which in turn could prevent a transformation to malignancy.
Treatment with SU5402, a KGFR-Tyrosine Kinase Inhibitor, Suppressed Progenitor Cell Proliferation, Resulting in the Suppression of Hyper-proliferation and Incomplete Differentiation of the Epithelium
The action of KGF depends upon KGFR, which is a transmembrane tyrosine kinase receptor with an alternatively spliced variant of the FGF receptor-2/bek gene (Miki et al. 1992). By binding to KGFR, KGF activates various MAPKs, including p-ERK, and induces epithelial cell growth (Bao et al. 2005; Sharma et al. 2003). In our study, KGFR expression was consistently upregulated and detected in all layers of the epithelium and many progenitor cells were induced at day 4 after KGF gene transfection (Fig. 3). In recent research, p63 has been shown to regulate the transcription of FGFR2 (Zhao et al. 2014; Ramsey et al. 2013; Candi et al. 2007). Indeed, a previous study observed an increase in KGFR expression when deltaNp63 was overexpressed by keratinocytes (Yang et al. 1999). Very recently, Fgfr2b expression was shown to be reduced in the developing epidermis of p63−/− mice (Candi et al. 2007; Laurikkala et al. 2006) and affected by mutant p63 and p63 knockdown through a global gene expression analysis (Ferone et al. 2012). According to the results of the expression pattern of p63 in KGF-induced hyper-proliferative epithelium, it is assumed that the transcriptional activation of KGFR might be induced by p63 under KGF gene transfection. However, an investigation of the direct evidence that argues the regulatory elements in the promotion of p63 should be the subject of future research. Furthermore, by using a tyrosine kinase inhibitor of KGFR, SU5402, in vivo, the number of progenitor cells was decreased and thickening of the epithelium reduced (Fig. 6). These results strongly indicated that stem-cell-to-progenitor-cell production might be regulated under the paracrine action of KGF and KGFR.
In conclusion, KGF might accelerate the proliferative activity of stem and/or progenitor cells and inhibit the differentiation of progenitor cells in the suprabasal layer through the induction of pp63 expression, resulting in epithelial hyperplasia and stratification. The KGF and KGFR-signaling pathway is a potentially suitable therapeutic target for the treatment of chronic proliferating skin diseases in the future.
Acknowledgements
We would like to thank Dr. Jeffrey S. Rubin with the National Cancer Institute/CCR/LCMB for providing the human KGF cDNA construct. We thank Miss Sayumi Harakawa and Daisuke Endo (Department of Histology and Cell Biology, Nagasaki University Graduate School of Biomedical Sciences,) for their excellent technical assistance in this work. We also thank Prof. Takehiko Koji (Department of Histology and Cell Biology, Nagasaki University Graduate School of Biomedical Sciences,) for his comments in this work.
Compliance with Ethical Standards
All experiments were conducted according to the principles and procedures outlined in the guidelines for animal experimentation of Nagasaki University with the approval of the Institutional Animal Care and Use Committee (Nos. 1209241015-2 and 1404011269). This study protocol was approved by the Human Ethics Review Committee of Jikei University School of Medicine, and signed informed consent was obtained from all the patients or their guardians for this study (approval number is 27-344 8229).
Conflict of Interest
This study is supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotor of Science (JSPS) (no. 25462647 and JP16K11186 to T. Yamamoto-Fukuda, no. JP26462608 to N. Akiyama).
References
- Akiyama N, Yamamoto-Fukuda T, Takahashi H. Influence of continuous negative pressure in the rat middle ear. Laryngoscope. 2014;124(10):2404–2410. doi: 10.1002/lary.24767. [DOI] [PubMed] [Google Scholar]
- Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 2001;15(6):898–906. doi: 10.1096/fj.00-0324com. [DOI] [PubMed] [Google Scholar]
- Auf demKeller U, Krampert M, Kümin A, Braun S, Werner S. Keratinocyte growth factor: effects on keratinocytes and mechanisms of action. Eur J Cell Biol. 2004;83(11–12):607–612. doi: 10.1078/0171-9335-00389. [DOI] [PubMed] [Google Scholar]
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, Knoell DL (2005) Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 288(1):L36–42. doi:0.1152/ajplung.00309.2003 [DOI] [PubMed]
- Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nat Rev Mol Cell Biol. 2013;14(8):489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- Candi E, Cipollone R, Rivetti di Val Cervo P, Gonfloni S, Melino G, Knight R. p63 in epithelial development. Cell Mol Life Sci. 2008;65(20):3126–3133. doi: 10.1007/s00018-008-8119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Rufini A, Terrinoni A, Giamboi-Miraglia A, Lena AM, Mantovani R, Knight R, Melino G. DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc Natl Acad Sci U S A. 2007;104(29):11999–12004. doi: 10.1073/pnas.0703458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122(Pt 24):4473–4480. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- Chikama T, Liu CY, Meij JT, Hayashi Y, Wang IJ, Yang L, Nishida T, Kao WW. Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. Am J Pathol. 2008;172(3):638–649. doi: 10.2353/ajpath.2008.070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupé DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, Jones PH. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337(6098):1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone G, Thomason HA, Antonini D, De Rosa L, Hu B, Gemei M, Zhou H, Ambrosio R, Rice DP, Acampora D, van Bokhoven H, Del Vecchio L, Koster MI, Tadini G, Spencer-Dene B, Dixon M, Dixon J, Missero C. Mutant p63 causes defective expansion of ectodermal progenitor cells and impaired FGF signalling in AEC syndrome. EMBO Mol Med. 2012;4(3):192–205. doi: 10.1002/emmm.201100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203(2):223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- Finch PW, Murphy F, Cardinale I, Krueger JG. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am J Pathol. 1997;151(6):1619–1628. [PMC free article] [PubMed] [Google Scholar]
- Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4(2):155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121(6):1440–1446. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20(8):847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W, Vogel H, Guo X, Garcia EL, Michurina TV, Enikolopov G, Muthuswamy SK, Mills AA. ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell. 2011;8(2):164–176. doi: 10.1016/j.stem.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E, Takahashi M, Takeda H, Shiro M, Koike T. Recognition of phosphate monoester dianion by an alkoxide-bridged dinuclear zinc (II) complex. Dalton Trans. 2004;21(8):1189–1193. doi: 10.1039/b400269e. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133(8):1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- Li M, Firth JD, Putnins EE. An in vitro analysis of mechanical wounding-induced ligand-independent KGFR activation. J Dermatol Sci. 2009;53(3):182–191. doi: 10.1016/j.jdermsci.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Linardi RL, Megee SO, Mainardi SR, Senoo M, Galantino-Homer HL. Expression and localization of epithelial stem cell and differentiation markers in equine skin, eye and hoof. Vet Dermatol. 2015;26(4):213–e47. doi: 10.1111/vde.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Leonardi S, Tanori M, Pasquali E, Pierdomenico M, Rebessi S, Di Majo V, Covelli V, Pazzaglia S, Saran A. Hair cycle-dependent basal cell carcinoma tumorigenesis in Ptc1neo67/+ mice exposed to radiation. Cancer Res. 2006;66(13):6606–6614. doi: 10.1158/0008-5472.CAN-05-3690. [DOI] [PubMed] [Google Scholar]
- Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489(7415):257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nagayasu T, Hishikawa Y, Tagawa T, Yamayoshi T, Abo T, Tobinaga S, Furukawa K, Koji T. Keratinocyte growth factor accelerates compensatory growth in the remaining lung after trilobectomy in rats. J Thorac Cardiovasc Surg. 2009;137(6):1499–1507. doi: 10.1016/j.jtcvs.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Miyata T, Minai Y, Haga M. Impaired growth of small intestinal epithelium by adrenalectomy in weaning rats. Acta Histochem Cytochem. 2008;41(4):83–88. doi: 10.1267/ahc.08004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276(5314):955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Ogawa E, Okuyama R, Egawa T, Nagoshi H, Obinata M, Tagami H, Ikawa S, Aiba S. p63/p51-induced onset of keratinocyte differentiation via the c-Jun N-terminal kinase pathway is counteracted by keratinocyte growth factor. J Biol Chem. 2008;283(49):34241–34249. doi: 10.1074/jbc.M804101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49(5):788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR-2) Dev Biol. 1993;158(2):475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Portnoy J, Curran-Everett D, Mason RJ. Keratinocyte growth factor stimulates alveolar type II cell proliferation through the extracellular signal-regulated kinase and phosphatidylinositol 3-OH kinase pathways. Am J Respir Cell Mol Biol. 2004;30(6):901–907. doi: 10.1165/rcmb.2003-0406OC. [DOI] [PubMed] [Google Scholar]
- Potten CS, Saffhill R, Maibach HI. Measurement of the transit time for cells through the epidermis and stratum corneum of the mouse and guinea-pig. Cell Tissue Kinet. 1987;20(5):461–472. doi: 10.1111/j.1365-2184.1987.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Raffa S, Leone L, Scrofani C, Monini S, Torrisi MR, Barbara M. Cholesteatoma-associated fibroblasts modulate epithelial growth and differentiation through KGF/FGF7 secretion. Histochem Cell Biol. 2012;138(2):251–269. doi: 10.1007/s00418-012-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MR, Wilson C, Ory B, Rothenberg SM, Faquin W, Mills AA, Ellisen LW. FGFR2 signaling underlies p63 oncogenic function in squamous cell carcinoma. J Clin Invest. 2013;123(8):3525–3538. doi: 10.1172/JCI68899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3(9–10):545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105(7):2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129(3):523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278(24):21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- Sudhoff H, Tos M. Pathogenesis of attic cholesteatoma: clinical and immunohistochemical support for combination of retraction theory and proliferation theory. Am J Otol. 2000;21(6):786–792. [PubMed] [Google Scholar]
- Suzuki D, Senoo M. Expansion of epidermal progenitors with high p63 phosphorylation during wound healing of mouse epidermis. Exp Dermatol. 2013;22(5):374–376. doi: 10.1111/exd.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru N, Hishikawa Y, Ejima K, Nagasue N, Inoue S, Muramatsu M, Hayashi T, Koji T. Estrogen receptor-associated expression of keratinocyte growth factor and its possible role in the inhibition of apoptosis in human breast cancer. Lab Invest. 2004;84(11):1460–1471. doi: 10.1038/labinvest.3700166. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kojima H, Miyazaki H, Koga T, Moriyama H. Roles of cytokines and cell cycle regulating substances in proliferation of cholesteatoma epithelium. Laryngoscope. 1999;109(7 Pt 1):1102–1107. doi: 10.1097/00005537-199907000-00017. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Ulziibat S, Ejima K, Shibata Y, Hishikawa Y, Kitajima M, Fujishita A, Ishimaru T, Koji T. Identification of estrogen receptor beta-positive intraepithelial lymphocytes and their possible roles in normal and tubal pregnancy oviducts. Hum Reprod. 2006;21(9):2281–2289. doi: 10.1093/humrep/del176. [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, Badylak SF, Braunhut SJ. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29(8):690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Werner S, Munz B. Suppression of keratin 15 expression by transforming growth factor beta in vitro and by cutaneous injury in vivo. Exp Cell Res. 2000;254(1):80–90. doi: 10.1006/excr.1999.4726. [DOI] [PubMed] [Google Scholar]
- Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Akiyama N, Shibata Y, Takahashi H, Ikeda T, Kohno M, Koji T. KGFR as a possible therapeutic target in middle ear cholesteatoma. Acta Otolaryngol. 2014;134(11):1121–1127. doi: 10.3109/00016489.2014.907501. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Akiyama N, Shibata Y, Takahashi H, Ikeda T, Koji T. In vivo over-expression of KGF mimic human middle ear cholesteatoma. Eur Arch Otorhinolaryngol. 2015;272(10):2689–2696. doi: 10.1007/s00405-014-3237-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Aoki D, Hishikawa Y, Takahashi H, Kobayashi T, Koji T. Possible involvement of keratinocyte growth factor and its receptor in enhanced epithelial-cell proliferation and acquired recurrence of middle-ear cholesteatoma. Lab Invest. 2003;83(1):123–136. doi: 10.1097/01.LAB.0000050763.64145.CB. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Shibata Y, Hishikawa Y, Shin M, Yamaguchi A, Kobayashi T, Koji T. Effects of various decalcification protocols on detection of DNA strand breaks by terminal dUTP nick end labelling. Histochem J. 2000;32(11):697–702. doi: 10.1023/A:1004171517639. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Takahashi H, Terakado M, Hishikawa Y, Koji T. Expression of keratinocyte growth factor and its receptor in noncholesteatomatous and cholesteatomatous chronic otitis media. Otol Neurotol. 2010;31(5):745–751. doi: 10.1097/MAO.0b013e3181dd15ef. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2(3):305–316. doi: 10.1016/S1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yennek S, Tajbakhsh S. DNA asymmetry and cell fate regulation in stem cells. Semin Cell Dev Biol. 2013;24(8–9):627–642. doi: 10.1016/j.semcdb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30(2):151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]