Abstract
Nasopharyngeal myiasis is an important high incidence disease among camels in the Middle East and North of Africa caused by Cephalopina titillator (C. titillator) that results in sever economic losses in many camel breeding areas around the world. The current study was conducted to evaluate the efficacy of three essential oils; camphor, ginger and cinnamon oils and their histopathological effects on the 3rd larval instar of C. titillator, with special regard to the prevalence percentage of C. titillator infestation in slaughtered camels at Egyptian abattoirs in addition to investigate histopathological alterations of the infested animal’s tissue. This study fulfilled that the prevalence of C. titillator infestation was 35.2% among slaughtered camels during summer season. The three tested essential oils were caused a significant mortality of C. tittilator; however, camphor oil was exhibited greater and quicker insecticidal effect than ginger and cinnamon oils at the same concentration in terms of mortality of the 3rd instar C. tittilator larvae. There was a concentration-dependent effect on the larvae among the tested essential oils. The tested essential oils were caused remarkable histopathological alterations on the treated larval cuticle. The main salient lesions of the examined infested camel’s tissue were necrotic and inflammatory alterations associated with cystic dilation of submucosal glands.
Keywords: Cephalopina titillator, Camels, Histopathological changes, Essential oils, Insecticidal effect, Prevalence
Introduction
Nasopharengeal myiasis has been reported as a health hazard disease that adversely affects camel industry in many producing regions (Oryan et al. 2008). Camels consider very important species of the livestock in arid and semiarid environment. The causative agent of the disease is the larvae of C. titillator; oestrid fly or the nasal botfly which has been identified as an obligate parasite of camel (Higgins 1985). The female fly deposits larvae in the nostrils of animal. They reach up to the nasopharynx and once in a while the paranasal sinuses and molt twice while attached to the mucus membrane they nourish and cause sever irritation and damage of tissues (Musa et al. 1989; Zayed et al. 1994; Shakerian et al. 2011). The infested animal mostly suffered from respiratory manifestations such as nasal mucofibrinous discharge, difficulty in breathing, recurrent sneezing; expel the larvae from their nostrils, snoring; due to blockage of the nasopharynx by larvae additionally anxiety and anorexia (Khater et al. 2013). The mechanical injuries for example, penetrating the ethmoid bone by the larvae may help in the access of bacterial and viral infection to the cerebrospinal canal (Zumpt 1965). These damages may lead to secondary complications in the form of neurological disorders and finally death which was reported as a consequence of meningitis (Zumpt 1965; Musa et al. 1989; Al-Ani et al. 1991). Infestations induce reduction of host physiological functions; destroy host tissues (El Bassiony et al. 2005) and causing economical losses through decrease body weight, interfering with fertility, causing abortion, bad hide quality, declining milk production and a debilitation of the host’s immune system (Hall and Wall 1995; Otranto 2001). Studies from Egypt and surrounding countries revealed that the fly is a prevalent parasite of camels which indicate that has the ability of flourishing in faltering natural conditions (Ashmawy et al. 1985; Fatani and Hilali 1994; Alahmed 2002; Al-Ani and Amr 2016). Treatment of nasal myiasis has depended on systemic parasiticides as macrocyclic lactones (Seddiek et al. 2013). Currently bio-insecticides, especially those of plant origin extracts have been progressively assessed in controlling medicinally important insect populations (Wins-Purdy et al. 2009; Kumar et al. 2013; Bedini et al. 2017) for examples camphor oil (Cinnamomum camphora), which is an excellent disinfectant, insecticide and germicide. It can be eradicate lice or other small parasites of bugs from on the body. Also, it has been utilized as a disinfectant, antispasmodic, carminative, heart stimulant, respiratory guide, and anthelmintic. Medicinal plants could be utilized efficiently and safely to control endo and ecto parasite (Khater and Khater 2009). EOs and products based on them deem predominately non-toxic and safe to mammals (Rajendran and Sriranjini 2008). Application of camphor oil proved to be safe and has no any side effects in the treatment of parasitic infection (Khater et al. 2009, 2013). Also, Zingiber officinale Roscoe, commonly called ginger, has insecticidal oviposition, growth regulating, reducing fertility, development modifying properties and repellent activity against many tested insects (Abdurrahman et al. 2008). Cinnamon oil (Cinnamonium zeylanicum) possesses anti-parasitic properties (Zenner et al. 2003 and Abu El Ezz et al. 2011), it has insecticidal activity against Coptotermes formosanus, also in controlling house-dust mites and arthropod pests (Kim et al. 2008).
The present study aimed to evaluate the insecticidal effect of three essential oils; camphor, ginger and cinnamon on C. titillator 3rd larvae for implementation of safe control method. In addition to study histopathological effects of the tested oils on C. titillator larvae, determine C. titillator infestation incidence among camels at Cairo and Giza governorates and investigate histopathological alterations of infested camel’s tissue.
Materials and methods
Prevalence percentage
Over summer season (June–August 2017) two hundred and fifty random slaughtered male camels were examined at Elbasatin and Monieb abattoirs in Cairo and Giza governorates, Egypt. Gross examination was applied on the nasal cavity, frontal sinuses, turbinate bones and nasopharyngeal area. Different larval instars were detected and the larvae were identified as stated by Zumpt (1965).
Collection of larvae
Different larval instars of C. titillator were collected from nasopharyngeal cavities of naturally infested camel heads after slaughtering at abattoir. The collected larvae were quickly washed several times with water, then with 10 mM phosphate buffer saline, pH 7.2. Morphological identification of 2nd and 3rd C. titillator larvae was performed according to (Zumpt 1965).
In vitro treatments
Dipping technique
The impact of the three tested essential camphor, ginger and cinnamon oils (obtained from El-Captain Co., Al-Oboor city, Cairo, Egypt, approved for human use from the Egyptian Ministry of Health) on the 3rd C. titillator larvae were tested as stated by Khater et al. (2013). Three freshly prepared concentrations (10, 30 and 50%) in distilled water were used. Few drops of Tween 80 were included as an emulsifier to essential oils. In each test, five larvae of C. tittilator were utilized per replicate. Each concentration was checked in 10 replicates (i.e., 50 larvae for each concentration). Each group of larvae was put in a mesh cloth place and larval dipping was done for just 60 s in a 100-ml of each oil solution. The control negative group was placed in distilled water and few drops of Tween 80 for the same period. The immersed larvae were reserved in petri-dishes having filter papers (Whatman No. 1) at 27 ± 2 °C and 80 ± 5% relative humidity (RH). The larval mortality was observed after different time intervals (3, 6, 12, 24 and 48 h). Mortality percentages for each concentration were calculated. The larvae were photographed before and after the treatment with the selected oils (Fig. 1a, b).
Fig. 1.
a The appearance of pre treated normal C. titillator larvae, b C. titillator larvae post treated 48 h with 50% essential oils; the larvae turn uniformly dark black after death
Light microscopic observations
Histopathological effect of the tested oils on 3rd instars C. tittilator larvae
Samples were selected from the larvae subjected to 50% concentration of the three tested oils compared to those of the control negative group. The larvae were fixed in 10% buffered formalin saline. Sections were prepared in paraffin blocks and stained with hematoxylin and eosin (H&E) stain as mentioned by Bancroft et al. (1996). The body wall of larvae was inspected and photographed using Olympus CX41 microscope.
Histopathological alterations of tissues infested with C. tittilator larvae
Representative specimens from the nasal and pharyngeal cavities were collected after slaughtering from infested and un infested camels. Fixation of these tissue specimens were done in 10% neutral buffered formalin. The specimens were then dehydrated, cleaned in xylol and embedded in paraffin blocks. Preparation of paraffin sections of 5 μm thickness was performed; the samples were then stained with H&E stain and examined microscopically to determine histopathological alterations according to (Bancroft et al. 1996).
Statistical analysis
The obtained data analyzed for the mean and standard deviation (SD). Statistical comparison between the means of different treatments was made by one way ANOVA with SPSS program version 10. P value of < 0.05 was assumed for statistical significance.
Results
Prevalence percentage of C. titillator infestation
Examination of nasopharyngeal cavity of two hundred and fifty random slaughtered male camels at Elbasatin and Monieb abattoirs in Cairo and Giza governorates during summer season (Jun to August 2017) revealed that 88 (35.2%) camels were infested by C. titillator larvae. The highest infestation percentage was 43.7% during June while the lowest one was 28.4% during August (Table 1).
Table 1.
Prevalence of C. titillator infestation during Summer season among the examined slaughtered camels
| Month | No. of examined animals | No. of infested animals | Monthly Infestation Percentage |
|---|---|---|---|
| June | 80 | 35 | 43.7 |
| July | 75 | 28 | 37.3 |
| August | 95 | 25 | 28.4 |
| Total | 250 | 88 | 35.2 |
The insecticidal efficacy of the tested essential oils
Data of the current study revealed the in vitro efficacy of the tested oils against the 3rd instar C. titillator larvae (Table 2). All the tested essential oils confirmed insecticidal effects on C. tittilator compared with negative control. No mortality was observed in the negative control group. Three tested essential oils caused a significant level of death of C. tittilator; however, camphor oil was exhibited greater and quicker insecticidal activity than ginger and cinnamon oils at the same concentration in terms of mortality of the 3rd instar instar C. tittilator larvae. Mortality of all larvae (100%) achieved 12 h post treatment with 50% concentration of camphor and ginger oils. At the meantime, 42% larval mortality was detected with cinnamon oil. These three tested essential oils were effective in concentration 50 mg/ml compared to the concentrations at 10 and 30 mg/ml. There was a concentration-dependent effect on the larvae among the tested essential oils.
Table 2.
Mortality percentages of 3rd instar C. titillator larvae after treatment with different concentrations of camphor, ginger and cinnamon oils
| Conc. (%) | % Mortality of larva | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interval time/h | |||||||||||||||
| 3 h | 6 h | 12 h | 24 h | 48 h | |||||||||||
| 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | |
| Camphor | 6 ± 4 b | 8 ± 2 b | 24 ± 6.7 a | 34 ± 6.7 a | 42 ± 5.8 a | 56 ± 9.2 a | 88 ± 2 b | 90 ± 3.1 b | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| Ginger | 12 ± 2 a | 12 ± 3.7 a | 18 ± 3.7 a | 20 ± 4.4 a | 20 ± 3.1 a | 26 ± 5 a | 48 ± 5.8 c | 64 ± 5 b | 100 ± 0 a | 66 ± 5 b | 100 ± 0 a | 100 ± 0 a | 96 ± 2.4 a | 100 ± 0 a | 100 ± 0 a |
| Cinnamon | 4 ± 2.5 b | 14 ± 2.4 a | 12 ± 2 a | 4 ± 2.4 b | 18 ± 2 a | 18 ± 3.7 a | 24 ± 5 a | 32 ± 5.8 a | 42 ± 7.3 a | 62 ± 7.3 a | 64 ± 5 a | 60 ± 5.4 a | 92 ± 4.8 a | 92 ± 3.7 a | 94 ± 4 a |
Fifty larvae were treated for each concentration. SE: Standard Error of means (n = 5). Values with different letters (a, b) are significantly (P < 0.05) different within treatments (based on the non-overlapping confidence limits) according to Litchfield and Wilcoxon (1949). No mortalities were observed in the negative control group
Light microscopic observations
Histopathological effect of the tested oils on 3rd instars C. tittilator
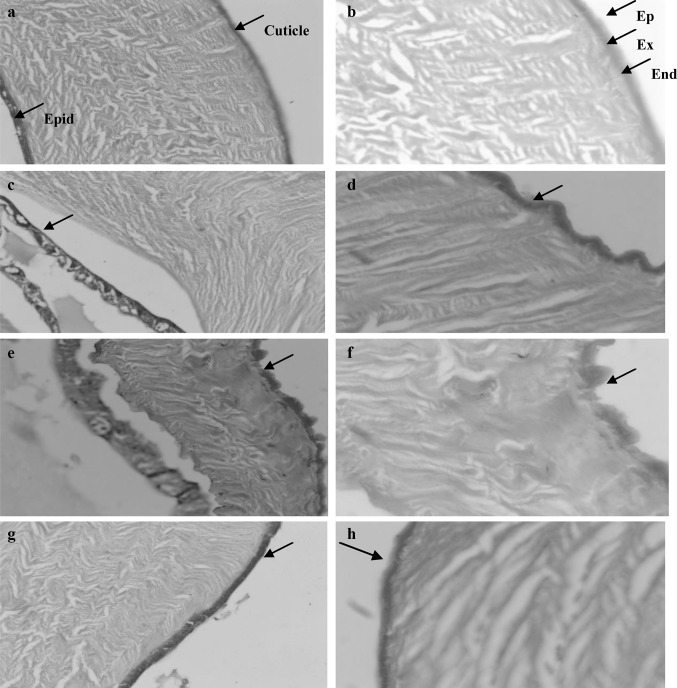
The integument in normal insects is comprised of one layer of cells and cuticle. In the synthesis of the cuticle, the epithelium secretes the cuticulin and then the lamellate endocuticle (Fig. 2a, b). The exocuticle is formed later by quinine tanning of the external lamellae of the endocuticle. The normal histological structure of cuticle of 3rd instars C. tittilator is consisted of outer electron-dense layer of epicuticle attached to lamelleted procuticle consisting of exocuticle and endocuticle and inner cellular layer of epidermal cells (Fig. 2a, b).
Fig. 2.
Light microscopy of the cuticle cross section of the third instar larvae. a, b Normal control larvae. c, d Following 24 h post treatment with 50 mg/ml concentration of camphor oil. e, f Following 24 h post treatment with 50 mg/ml concentration of ginger oil. g, h Following 24 h post treatment with 50 mg/ml concentration of cinnamon oil at ×20 and ×40, respectively. Note disruption of inner cellular layer of epidermal cells. Ep: epicuticle, Ex: exocuticle, End: endocuticle, Epid: epidermal cell
Dipping C. tittilator larvae in 50% concentration of camphor oil after 24 h post treatment caused thickening and corrugated cuticular surface with splitting of inner layer of epidermal cells (Fig. 2c, d). Also the larvae were dipped in ginger oil 50% concentration which induced damage in the form of swollen cuticle and disrupted inner cellular layer of epidermal cells (Fig. 2e, f). The results showed that cinnamon oil was less effective than camphor and ginger at the same concentration (Fig. 2g, h); it induced thinning of the epicuticle layer.
Histopathological alterations of tissue infested with C. tittilator
Larvae were detected mostly attached to the mucosa of the nasopharynx, whereas a few were found in the nasal cavity. Congestion of the nasal cavities and sinuses were noticed and, red nodules in nasopharyngeal mucosa were observed at the site of the larval attachment. Pharynx of a non- infested control camel was within normal histologic structure, normal stratified squamous epithelium with predominant submucosal glands (Fig. 3a). However, the main microscopic lesions in C. titillator-infested camels were the necrosis and desquamation of mucosal epithelium, as well as, there were mononuclear inflammatory cells infiltration and some acini were cystically dilated (Fig. 3b).
Fig. 3.
a Pharynx of anon –infested control camel normal stratified squamous epithelium (E) with predominant submucosal glands (G). b Pharynx of a C. titillator-infested camel. Some acini were cystically dilated (Black arrows) with presence of mononuclear inflammatory cells (Blue arrows). HE. ×100
Discussion
The present study revealed that C. titillator considers a common and an important parasite among camel in the surveyed governorates during summer season. In this study 35% of the investigated camels were infested by C. titillator and that may be coincided with the results obtained by Morsy et al. (1998) who showed that prevalence of C. titillator larvae in Egypt was 25%, and they reported that autumn was the highest prevalence period. Also, Oryan et al. (2008) detected that C. titillator infestation percentage of camels was significantly more severe in winter (69.8%) than in the summer (36.2%). In addition to Abd El-Rahman (2010) found that the rate of infestation of camels with C. titillator in El-Zawia abattoir, Libya was significantly greater in winter (68.8%) compared to summer (31%). However Khater (2013) observed that the prevalence of C. titillator infestation was 41.67%. While Al-Ani and Amr (2016) recorded that the highest infestation rate of the larvae of C. titillator infesting camel was (46%) at Ramtha slaughter house in Jordan in January and the lowest between May and July. Also Hussein et al. (1983) found that 67.6% of indigenous Saudi camels examined at Riyadh abattoir infested by nasal flies. These variations may be attributed to the different localities, climatic conditions and host susceptibilities. This study clearly proved that the three tested essential oils showed an insecticidal effect with dose higher than 10% against 3rd instar C. titillator and these results exhibited high toxic effect at different concentrations and intervals time. Camphor oil showed potent larval deterrent at 12 h post-treatment followed by ginger and cinnamon oils which caused 100% mortalities after 24 and 48 h. This result may be a line with that made by Khater et al. (2013) found that all treated 2nd instar C. tililator (L.) died (100% mortality) 18 h post treatment with 0.003% doramectin (Dectomax®, Pfizer Inc. packed by Pfizer Egypt) and 50% lavender (Lavandula angustifolia) obtained from El-Captain Co., Al-Oboor city, Cairo, Egypt, approved for human use from the Egyptian Ministry of Health, while 3rd instar larvae was 100% mortality after 24 and 30 h post treatment with lavender and dormactin. Moreover the results are agreed with the study of Khater (2014) mentioned that essential oils (obtained from El-Captain Co., Al-Oboor city, Cairo, Egypt, approved for human use from the Egyptian Ministry of Health) of 2% pumpkin (Cucurbita maxima), 30%lupinus (Lupinus luteus), 7.5% garlic (Allium sativum) and 7.5% peppermint (Mentha piperita) can be killed C. titillator larvae 24 h post treatment.
No adverse effects were recorded as a result of expositing to Eos (Khater et al. 2009). Eos are chemically related to the plants derived from so they analyzed by common microbes in the most of soils into harmless compounds (Rajendran and Sriranjini 2008; Khater 2013). Plenty of the EOs commercial products are on the Generally Recognized as Safe list fully approved by the Food and Drug Administration and the Environmental Protection Agency in the USA for food and beverage consumption (Khater 2013). So it is advisable to consider EOs that have insecticidal effects as the alternative treatment of choice particularly against insects resistant to synthetic pesticides.
The results of the light microscopy in the larvae treated with the essential oils showed a remarkable effect on cuticle and morphological features of 3rd C. titillator larvae that treated with the three used essential oils. These changes occurred in response to oil and were consisted of swelling which became pronounced and so severe with wrinkled and irregular curricular surface. This is consistent with Stadler et al. (1996) and Najar-Rodríguez et al. (2007) who observed integument alterations post exposure with sub-lethal doses of oils including cell membrane disruption and darkening. Also, Najar-Rodríguez et al. (2007) revealed that essential oils could penetrate the cell membranes, accumulated inside the cytoplasm causing cell dehydration and DNA condensation inside the nucleus. Damage to the structural integrity of curricular waxes by submersion of insect in organic solvents (Hurst 1940; Wigglesworth 1941) might be led to dehydration. Whereas some non-polar hydrocarbons have caused a complete removal of the cuticle wax layer, as well as stiffening of the cuticle (Hayes and Smith 1994; Barbakadze 2005). Mineral and vegetable oils could make a competing equilibrium with some of the components of the insect cuticle wax layer and soften the cuticle, this was confirmed by Stadler and Buteler (2009) who observed that mineral oils resulted in a softening of the cuticle in adult cotton boll weevils. Light microscopical findings could be utilized to determine the penetrating ability of the tested oils to larval cuticle (Abdel-shafy et al. 2009). It could be suggested that the main route of the oils entry into the C. titillator larvae might be via the transcuticular uptake of this applied oils; this may explain the observed rapid and tense larval mortality.
Cephalopina titillator infestation has strong influences on respiratory function, health and productivity of infested camels which are all not fully understood, so it is necessary to study the other features of this disease (Abd El-Rahman 2010). Although the high incidence of camel nasal botfly, little information is known about its histopathological effects (Oryan et al. 2008). The histopathologic necrotic and inflammatory alterations associated with C. titillator larvae in camels might be due to the mechanical injuries induced by the parasite and heavy infestation with the parasite (Shakerian et al. 2011). Cystically dilated submucosal glands indicated hypersecretory activity of these glands. The noticeable histopathologic changes were mentioned previously by Oryan et al. (2008) and Shakerian et al. (2011).These changes might occur as a result of the continued irritation and feeding behaviors of the larvae (Oryan et al. 2008).
The present study provides that nasal myiasis is considered a common and an important disease among camels in the undergoing localities of the experiment in Egypt. It was concluded that the three tested essential oils had insecticidal effect against 3rd instar C. titillator larvae. Camphor oil exhibited the greater effect followed by ginger and cinnamon oils. Additionally, the study indicated alteration and dysfunction of the affected tissue in camel. These tested essential oils could be deemed safe, potent and easy to reach alternative source of treatment. However, further studies are recommended to establish a method of application of these essential oils in vivo trial in form of gel or capsules to be easy use and applicable that may increase the efficacy of the treatment.
Author contributions
Nadia M. T. Abu El Ezz: 1. Designed, supervised and provided the steering for the experiment. 2. Collected C. tittilator larvae samples. 3. Performed in vitro treatment of larvae; the insecticidal efficacy of the tested essential oils. 4. Carried out writing and revising the manuscript. Noha M. F. Hassan: 1. Collected C. tittilator larvae samples. 2. Proceeded in vitro treatment of larvae; the insecticidal efficacy of the tested essential oils. 3. Determined prevalence percentage of C. titillator infestation. 4. Recorded light microscopic observations; histopathological effect of the tested oils on 3rd instars C. tittilator larvae. 5. Implemented statistical analysis. 6. Carried outwriting, interpertation and revising the manuscript. Amira H. El Namaky: 1. Collected C. tittilator larvae samples. 2. Continued in vitro treatment of larvae; the insecticidal efficacy of the tested essential oils. 3. Described light microscopic observations; histopathological effect of the tested oils on 3rd instars C. tittilator larvae. 4. Carried out writing and revising the manuscript. Faten A. M. Abo-Aziza: 1. Collected tissue samples. 2. Mentioned light microscopic observations; histopathological alterations of tissues infested by C. C. tittilator larvae. 3. Carried out writing the manuscript. All authors read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Nadia M. T. Abu El Ezz, Email: nadia_talaat60@yahoo.com
Noha M. F. Hassan, Phone: +202-37211968, Email: nohamhassan555@yahoo.com
Amira H. El Namaky, Email: amiraelnamaky@gmail.com
Faten Abo-Aziza, Email: faten.aboaziza@gmail.com.
References
- Abd El-Rahman S. Prevalence and pathology of nasal myiasis in camels slaughtered in El-Zawia Province-Western Libya: with a reference to thyroid alteration and renal lipidosis. Glob Vet. 2010;4(2):190–197. [Google Scholar]
- Abdel-Shafy S, El-Khateeb RM, Soliman MM, Abdel-Aziz MM. The efficacy of some wild medicinal plant extracts on the survival and development of third instar larvae of Chrysomyia albiceps (Weid) (Diptera: Calliphoridae) Trop Anim Health Prod. 2009;41:1741–1753. doi: 10.1007/s11250-009-9373-0. [DOI] [PubMed] [Google Scholar]
- Abdurrahman A, Osman S, Salih K, Ismet O. Insecticidal activity of the essential oils from different plants against three stored-product insect. J Ins Sci. 2008;10(21):1–13. doi: 10.1673/031.010.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu El Ezz NMT, Khalil FAM, Shaapan RM. Therapeutic effect of onion (Allium cepa) and Cinnamon (Cinnamomum zeylanicum) oils on Cryptosporidiosis in experimentally infected mice. Glob Vet. 2011;7:179–183. [Google Scholar]
- Alahmed AM. Seasonal prevalence of Cephalopina titillator larvae in camels in Riyadh region, Saudi Arabia, Arab Gulf. J Sci Res. 2002;20(3):161–164. [Google Scholar]
- Al-Ani F, Amr Z. Seasonal prevalence of the larvae of the nasal fly (Cephalopina titillator) in camels in Jordan. Rev Elev Med Vet Pays Trop. 2016;69(3):125–127. doi: 10.19182/remvt.31196. [DOI] [Google Scholar]
- Al-Ani FK, Khamas WA, Zenad KH. Camel nasal myiasis: clinical epidemiological and pathological studies in Iraq. Ind J Anim Sci. 1991;61:576–578. [Google Scholar]
- Ashmawy KI, Fahmy MM, Hilali M (1985) Incidence and seasonal variations of the larvae of Cephalopina titillator infesting camels (Camelus dromedarius) in Egypt. In: Proceedings of the 12th symposium, pp 43–44. Scandinavian Society of Parasitology, Tromsy, Norway, 17–19 June, Abo Akademi, Finland
- Bancroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4. New York: Churchill Livingstone; 1996. [Google Scholar]
- Barbakadze N (2005) Micro/nanomechanical measurements on insect and plant cuticles. PhD Thesis, Dissertation an der Universität Stuttgart, Bericht, vol 172, p 128
- Bedini S, Flamini G, Cosci F, Ascrizzi R, Echeverria MC, Guidi L, Landi M, Lucchi A, Conti B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors. 2017;10:80. doi: 10.1186/s13071-017-2006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bassiony GM, Al Sagair OA, El Dally ES, El Nady AM. Alterations in the pituitary–thyroid axis in camel (Camelus dromedarius) infected by larvae of nasal bot fly Cephalopina titillator. J Anim Vet Adv. 2005;4(3):345–348. [Google Scholar]
- Fatani A, Hilali M. Prevalence and monthly variations of the second and third instars of Cephalopina titllator (Diptera: Oestridae) infesting camels (Camelus dromedarius) in the Eastern Province of Saudi Arabia. Vet Parasit. 1994;53(1/2):145–151. doi: 10.1016/0304-4017(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Hall MJR, Wall R. Myiasis of humans and domestic animals. Adv Parasitol. 1995;35:257–334. doi: 10.1016/S0065-308X(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Hayes JW, Smith JW. Diflubenzuron plus cottonseed oil: effects on boll weevil (Coleoptera: Curculionidae) cuticle hardness, mating and flight. J Econ Entomol. 1994;87:339–344. doi: 10.1093/jee/87.6.1586. [DOI] [Google Scholar]
- Higgins AJ. The camel in health and disease. 4. Common ectoparasites of the camel and their control. Br Vet J. 1985;141:197–216. doi: 10.1016/0007-1935(85)90153-8. [DOI] [PubMed] [Google Scholar]
- Hurst H. Permeability of insect cuticle. Nature. 1940;145:462–463. doi: 10.1038/145462b0. [DOI] [Google Scholar]
- Hussein MF, Hassan HAR, Bilal HK, Basmae’il SM, Younis TM, Al-Motlaq AAR, Al-Sheikh MA. Cephalopina titillator infection in Saudi Arabian camels. J Vet Med. 1983;30:553–558. doi: 10.1111/j.1439-0450.1983.tb01882.x. [DOI] [PubMed] [Google Scholar]
- Khater HF. Bioactivity of essential oils as green biopesticides: recent global scenario. In: Govil JN, Bhattacharya S, editors. Essentials oils II. Recent progress in medicinal plants. Houston: Studium Press; 2013. pp. 151–218. [Google Scholar]
- Khater HF. Bioactivities of some essential oils against the camel nasal botfly, Cephalopina titillator. Parasitol Res. 2014;113(2):593–605. doi: 10.1007/s00436-013-3688-5. [DOI] [PubMed] [Google Scholar]
- Khater HF, Khater DH. The insecticidal activity of four medicinal plants against the blowfly Lucilia sericata (Diptera: Calliphoridae) Int J Dermatol. 2009;48(5):492–497. doi: 10.1111/j.1365-4632.2009.03937.x. [DOI] [PubMed] [Google Scholar]
- Khater HF, Ramadan MY, El-Madawy RS. The lousicidal, ovicidal, and repellent efficacy of some essential oils against lice and flies infesting water buffaloes in Egypt. Vet Parasitol. 2009;164:257–266. doi: 10.1016/j.vetpar.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Khater HF, Ramadan MY, Abdel Mageid AD. In vitro control of the camel nasal botfly, Cephalopina titillator, with doramectin, lavender, camphor, and onion oils. Parasitol Res. 2013;112(7):2503–2510. doi: 10.1007/s00436-013-3415-2. [DOI] [PubMed] [Google Scholar]
- Kim HK, Yun YK, Ahn YJ. Fumigant toxicity of cassia bark and cassia and cinnamon oil compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae) Exp Appl Acarol. 2008;44(1):1–9. doi: 10.1007/s10493-008-9129-y. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole) Parasitol Res. 2013;112(1):69–76. doi: 10.1007/s00436-012-3105-5. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon FA. Simplified method of evaluating dose–effect experiments. J Pharmacol Exper Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Morsy TA, Aziz AS, Mazyad SA, Al Sharif KO. Myiasis caused by Cephalopina titillator (Clark) in slaughtered camels in Al Arish abattoir, North Sinai governorate, Egypt. J Egypt Soc Parasitol. 1998;28(1):67–73. [PubMed] [Google Scholar]
- Musa MT, Harrison M, Ibrahim AM, Taha TO. Observation on Sudanese camel nasal myiasis caused by the larvae of Cephalopina titillator. Rev Elev Med Vet Pays Trop. 1989;42(1):27–31. [PubMed] [Google Scholar]
- Najar-Rodríguez AJ, Walter GH, Mensah RK. The efficacy of a petroleum spray oil against Aphis gossypii glover on cotton. Part 1: Mortality rates and sources of variation. Pest Manag Sci. 2007;63:586–589. doi: 10.1002/ps.1385. [DOI] [PubMed] [Google Scholar]
- Oryan A, Valinezhad A, Moraveji M. Prevalence and pathology of camel nasal myiasis in eastern areas of Iran. Trop Biomed. 2008;25(1):30–36. [PubMed] [Google Scholar]
- Otranto D. The immunology of myiasis: parasite survival and host defense strategies. Trends Parasitol. 2001;17(4):176–182. doi: 10.1016/S1471-4922(00)01943-7. [DOI] [PubMed] [Google Scholar]
- Rajendran S, Sriranjini V. Plant products as fumigants for stored product insect control. J Stored Prod Res. 2008;44(2):126–135. doi: 10.1016/j.jspr.2007.08.003. [DOI] [Google Scholar]
- Seddiek SA, Khater HF, El-Shorbagy MM, Ali MM. The acaricidal efficacy of aqueous neem extract and ivermectin against Sarcoptes scabiei var. cuniculi in experimentally infested rabbits. Parasitol Res. 2013;112(6):2319–2330. doi: 10.1007/s00436-013-3395-2. [DOI] [PubMed] [Google Scholar]
- Shakerian A, Hosseini SR, Abbasi A. Prevalence of Cephalopina tittilator (Diptera: Oestridae) larvae in one-humped camel (Camelus dromedarius) In Najaf-Abad, Iran. Glob Vet. 2011;6(3):320–323. [Google Scholar]
- Stadler T, Buteler M. Modes of entry of petroleum distilled spray-oils into insects: a review. Bull Insectol. 2009;62:169–177. [Google Scholar]
- Stadler T, Schang MM, Zerba E. Caracterización fisicoquímica y toxicológica de algunos aceites minerales de uso fitosanitario. Rev Invest Agrop. 1996;27:67–80. [Google Scholar]
- Wigglesworth VB. Oils aiding loss of water from the cuticle. Nature. 1941;147:116. doi: 10.1038/147116a0. [DOI] [Google Scholar]
- Wins-Purdy AH, Whitehouse C, Judd GJR, Evenden ML. Effect of horticultural oil on oviposition behavior and egg survival in the oblique banded leaf roller (Lepidoptera: Tortricidae) Canad Entomol. 2009;141:86–94. doi: 10.4039/n08-042. [DOI] [Google Scholar]
- Zayed AA, Abdel-Meguid A, Madbouly MH, EI-Moursy AA, EL-Khateeb RM. Incidence and monthly prevalence of Cephalopina titillator (Diptera: Oestridae) larvae infesting dromedary camels slaughtered at Egyptian abattoir. J Egypt Vet Med Ass. 1994;54:13–22. [Google Scholar]
- Zenner L, Callait MP, Granier C, Chauve C. In vitro effect of essential oils from Cinnamomum aromaticum, Citrus limon and Allium sativum on two intestinal flagellates of poultry, Tetratrichomonas gallinarum and Histomonas meleagridis. Parasite. 2003;10:153–157. doi: 10.1051/parasite/2003102153. [DOI] [PubMed] [Google Scholar]
- Zumpt F. Myiasis in man and animals in the Old World. A text book for physicians, veterinarians and zoologists. London: Butterworth’s; 1965. [Google Scholar]