Abstract
Entomopathogenic nematodes form excellent tools to study insect immunity in response to during infection. Insects activate as several defense mechanisms, namely Phenoloxidase, haemocytes, detoxification and antioxidant enzymes. However little mechanistic information is available about the sublethal effects of entomopathogenic nematodes infection on detoxification and immune mechanisms in lepidopteran insects. In the present study, the effects of infection on antioxidant, detoxification and immune systems of Spodoptera litura larvae were studied. Results show a significant reduction in Total Haemocyte Count observed after 3 h of infection. A significant increase Superoxide dismutase, Catalase, Glutathione S-transferase, Glutathione Peroxidase and Acid phosphatase were observed 6 h after infection and, progressive decrease in Peroxidase, Alkaline phosphatase and Lipid peroxidation was also observed. This study shows that increased detoxification enzyme levels in response to nematode infection are a protective mechanism in insects. Nematode infection suppresses insect immune response, which is evident from low haemocyte count and Phenoloxidase levels to ultimately cause larval mortality.
Keywords: Heterorhabditis indica, Antioxidant enzymes, Phenoloxidase, Hemocyte count, Detoxification enzymes, Lipid peroxidation
Introduction
Agriculture today is facing a challenge due to insect pests (Oerke 2006). Insect pest control methods have mainly relied on systematic spraying of chemical insecticides in agricultural crops. This has resulted in environmental contamination and harmful effects on non-target organisms. Insecticides of biological origin are now being increasingly sought and are considered as promising alternatives to chemical insecticides (Mills 2014; Orr and Lahiri 2014). Biopesticide especially bacteria, nematodes and fungi are now increasingly being realized as important alternatives for chemical insecticides. Entomopathogenic Nematodes (EPN) parasitizing insects are classified into 23 families (Koppenhofer et al. 2007). EPNs are soil dwelling organisms and parasitize insect pests high target specificity and are considered environmentally safe (Burnell and Stock 2000). Soil dwelling EPNs, belong to Steinernematidae and Heterorhabditidae families are known for their biological control potential in agricultural crops (Shamseldean et al. 2013). Lepidopterans are susceptible to the Steinernematids and Heterorhabditids infections (Vashisth et al. 2013). EPNs infect insects by penetrating into insect cuticle and releasing of symbiotic bacteria in insect haemocoel and gut region the symbiotic bacteria secrete proteases and protoxins, other insecticidal substances complex that cause morbidity and mortality in insects (Toubarroa et al. 2009). Nematodes of Heterorhabditis genus have symbiotic bacteria belonging to Photorhabdus genus. Secondary metabolites produced by Photorhabdus have several bioactive properties which affect physiology and survival in several insect species. The secondary metabolites from bacteria also cause immune suppression in host and cause fatal septicemia within 48–72 h (Ullah et al. 2014; Grewal et al. 2005; Jung and Kim 2007). Insects have evolved a variety of defenses against nematode infection, these include morphological, and immunological defenses (Kunc et al. 2017).
Insects have cellular and humoral immune response for protection against microbial infections. Humoral immune responses involve various enzymes and antibacterial proteins produced in fat body and hemocytes target primarily bacterial infections (Wang and Zhang 2008). Pathogen infection in insects activates a host of immune activities, most prominent among them being phagocytosis and encapsulation by haemocytes (Hoffmann 1996). Cellular immune response is provided by circulating haemocytes, whose number increases in response to microbial pathogenesis. Haemocytes are responsible for formation of cell aggregates; nodulation, phagocytosis and encapsulation (Chapman 1998). Cuticular phenoloxidase (PO) is a melanizing enzyme that which is produced in response to infection by activated hemocytes (Pham and Schneider 2008; Castillo et al. 2011). PO activity leads to formation of melanin coat around the invading pathogen. PO defense involves the formation of short lived chemically reactive quinones (Castillo et al. 2011). In addition to the activity of Insect PO, secondary metabolites produced from symbiotic bacteria result in generation of reactive oxygen species (ROS). These free radicals are highly reactive and result in harmful effects on cells and tissues in organisms. Certain components of insect immune system also produce ROS as a measure to limit microbial growth. In insects several antioxidant and detoxification enzymes are in involved in scavenging of free radicals and also in biotransformation of toxic metabolites and xenobiotics some of the most important antioxidant enzymes present are Superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), reduced glutathione (GSH) and glutathione reductase (GR). SOD scavenges superoxide anions and detoxifies them by converting to hydrogen peroxide and oxygen. Hydrogen peroxide is then transformed to the water and oxygen by CAT, GPx, and (Nordberg and Arner 2001). GPX is a selenium dependent enzyme present in cytosol and mitochondria of the cell. Active site uses reduced glutathione (GSH) as a substrate to transfer electrons to H2O2, there by converting it into two molecules of water by H2O2 metabolizing enzyme is catalase (CAT) present in peroxisomes (Mates 2000). Glutathione S-transferase (GSTs) catalyse conjugation of glutathione to electrophilic center of lipophilic compounds, thereby making products more soluble and excretable from cells (Hemingway 2000). Lipid peroxidation (LPO) is a well known marker of cell membrane damage (Pavlick et al. 2002). Acid phosphatase (ACP) and alkaline phosphatase (ALP) are the hydrolytic enzymes, found in the intestinal epithelium cells of animals they hydrolyze phosphate monoesters. ACP is a marker enzyme for detection of lysosomes in cell fractions. Level of ACP differs in response to xenobiotic exposure to the host. ALP appearance in the plasma membrane, its activity is affected due to cell membrane damage, so ALP is also considered as a biomarker for cellular stress. In the present study we investigated the physiological changes occurring in immune, antioxidant and detoxification system of Spodoptera litura following infection with Heterorhabditis indica.
Materials methods
Insect culture
Spodoptera litura eggs were collected from Castor (Ricinus communis) agricultural field and were maintained in laboratory following hatching the first instar larvae were transferred to trays containing castor leaves and were maintained at 75% RH and 28 ± 1 °C in insectary till fifth instar. The fifth instar larvae were allowed to pupate in moist soil and following pupation, pupae were segregated in males and females based on the morphology of the abdominal terminal segments. Virgin adult males (1) and females (2) was kept together in multiwell Plastic cups (20 × 20 × 30 cm), for mating and fed with a 10% honey solution. The F1 generation third instar larvae were used for experiments.
Heterorhabditis indica (Poinar 1990)
An established culture of Heterorhabditis indica was obtained from Marine Biotechnology and Ecological Genomics Laboratory Department of Biotechnology, Periyar University, Tamilnadu, India. Infective juveniles (IJs) were harvested from Galleria mellonella after 48 h following infection with Heterorhabditis indica (KPR 8 strain) stored in distilled water at 15 °C in BOD incubator for further experiments.
Larval bioassay
Larval bioassays were performed using 24 multi-well tissue culture plates were each well filled with 0.5 g of sterilized air-dried sand, and 80 µl of distilled water containing 100 IJs of H. indica 1 h after acclimatization, a single 3rd instar S.litura larva was released into each well. 10 larvae were used for each treatment and each experiment was repeated thrice observations were taken after 3, 6, 9, 12 and 24 h after infection. Dead larvae were taken and washed with distilled water; dead larvae were transferred into white’s trap (Kaya 1988) after 24–48 h IJs emerging from cadaver were collected while larvae which were alive after treatment were used for collection of haemolymph and midgut tissues.
Collection of haemolymph
A drop of fresh insect haemolymph was collected by puncturing the abdominal prolegs using sterilized needle. The haemolymph was collected in clean eppendorf tubes or vials and stored at − 20 °C for further analysis.
Total haemocyte count
20 µl of fresh hemolymph collected in 200 µl of PBS. From this 100 µl haemolymph was placed in neubaeur chamber for total count. Haemocytes were observed under a phase contrast microscope at 40 × magnification and cells in four corne ruled squares in the slide were counted in neubaeur chamber the following formula was used to calculate the total haemocyte count:
Dilution factor = 20; Depth factor = 10; Number of squares counted = 4.
Phenoloxidase assay (PO)
PO activity measured as described by Ashida (1971) and Seed et al. (1978). Insect haemolymph was collected after 3, 6, 9, 12, and 24 h after H. indica infection, was chilled in 1.5 ml micro centrifuge tubes on ice and was diluted with 1:24 (V/V) ice cold PBS. Haemolymph was frozen for 48 h to lyse in the haemocytes and release the cell plasma. Then sample was thawed and centrifuged at 5000 rpm for 1 min to separate the plasma cell containing ProPO. 50 µl aliquot of samples, and DL-DOPA (DL-3,4dihydroxyphenlalanine) were added to 150 µl of 10 mM PBS (pH 7.0) and incubated in dark at RT for 15 min. Supernatant was used for PO assay and reading was taken at 490 nm every 3 min using UV–visible spectrophotometer (Systronics, India).
Total protein
Total Protein was measured following Lowry et al. (1951), using BSA as standard.
Enzyme preparation
Larval midgut tissue was homogenized in 2 ml buffer, and centrifuged at 4 °C, 10,000 rpm for 15 min, solid debris, cellular material were discarded while the supernatant was decanted into a clean eppendorf tube, placed on ice and used immediately for, enzyme assays.
Peroxidase assay (POD)
POD activity was measured as described by Reddy et al. (1995). 3 ml of pyrogallol solution and 0.1 ml of the larvae midgut tissue homogenates sample were mixed well with 0.5 ml of hydrogen peroxide. The change in absorbance was recorded every 30 s up to 3 min in a spectrophotometer. Peroxidase was measured at 660 nm with a UV–visible spectrophotometer.
Glutathione-S-transferase assay (GST)
GST assay was done following method of Habig et al. (1974). In clean test tubes, 50 µl of larval midgut tissue homogenates sample was added to 2.7 ml phosphate buffer, and 50 µl (dichloro-2, 4-dinitrobenzene (CDNB) and 150 µl of ethanol added to the above mixture. The content was mixed thoroughly incubated 2-3 min at 25 °C enzyme source record the increasing absorbance for 5 min at 340 nm by UV–Visible spectrophotometer (Systronics, India) against an enzyme blank.
Superoxide dismutase assay (SOD)
SOD activity was assayed using the method of Marklund and Marklund (1974). 50 μl of larvae midgut tissue homogenate was added to fresh test tubes. Final volume was adjusted by adding of 2.90 ml with 50 mM Tris and 10 mM EDTA, pH 8.2 followed by addition of 100 μl of pyrogallol solution and thorough mixing. The absorbance was recorded at 440 nm using UV–visible spectrophotometer.
Catalase assay (CAT)
CAT activity was assayed using the method of Wang et al. (2001). 2.9 ml of Solution-A (50 mM KPO4; pH 7.0) and Solution-B (0.036% H2O2, KPO4). 0.03 ml of H2O2 mixing with phosphate buffer were mixed with 0.1 ml of larvae midgut tissue homogenate and read against control cuvette containing H2O2 and phosphate buffer only. Absorbance was recorded at 240 nm.
Acid and alkaline phosphatase assays (ACP and ALP)
ACP and ALP levels were measured following the procedure of Asakura et al. (1978) with slight modifications. Acid phosphatase activity was estimated by mixing thoroughly 0.05 ml of as enzyme source and 2 ml alkaline buffer solution. Then each tube was incubated at 36 °C for 30 min. 2 ml of NaOH solution was added and mixed well. Then absorbance was recorded at 405 nm in a spectrophotometer. Instead of alkaline buffer, 50 mM sodium acetate buffer (pH; 4.6) was added in the alkaline phosphatase activity. For 20 μl larvae midgut tissue homogenates sample were made up to 500 μl with 50 mM Tris–HCl buffer (pH 8.0) and mixed with an equal volume of the respective buffer containing 12.5 mM p-nitrophenyl phosphate. After incubation for 15 min at 37 °C in water bath, the enzymatic reaction was stopped by adding 0.5 N NaOH solutions and centrifuged (4000×g; 5 min). The absorbance of the resulting clear supernatants was read at 405 nm.
Glutathione-peroxidase assay (GPx)
Glutathione peroxidase (GPx) was measured by the method described by Rotruck et al. (1973). The reaction mixture containing 0.2 ml of phosphate buffer (pH 7.0) and 0.1 ml sodium azide and 0.2 ml larvae midgut tissue homogenates sample in 0.2 ml reduced glutathione and 0.2 ml of hydrogen peroxide and 1.55 ml of distilled water were added. The contents were incubated for 10 min at 37 °C. 0.4 ml 10% TCA was added to stop the reaction and centrifuged at 10,000 rpm for 10 min. The supernatant was assayed for glutathione content using Ellman’s reagent. Colour formation was measured at 412 nm using UV–visible spectrophotometer.
Lipid peroxidation (LPO)
Lipid peroxidation was measured by the method of Ohkawa et al. (1979). 0.1 ml of the larvae midgut tissue homogenates sample was taken added 1.9 ml of 0.1 M sodium phosphate buffer (pH 7.4). Then the mixture was incubated at 37 °C for 1 h. After these mixtures was precipitated with 10% TCA and centrifuged at 5000 rpm for 15 min and the supernatant was collected. 1 ml of 1% TBA was added to the supernatant collected after centrifugation. The sample was boiled in a water bath for 15 min. After boiling, the supernatant was cooled and the absorbance was recorded at 532 nm UV–visible spectrophotometer.
Statistical analysis
All the treatment and enzyme assays described above were performed in three replicates. The data obtained from enzyme assays were subjected to Analysis of Variance, which Dunnett’s multiple comparison tests using PRISM 5 software (Graph Pad Software Inc, USA). P values < 0.05 were considered significant.
Results
Immune profile of Heterorhabditis indica infected S. litura larvae
Results show a significant decreased haemocyte count in all treatments when compared with control (F-88.39; df-5, 12; P < 0.001) (Fig. 1a). High PO activity was observed at 3 and 6 h when compared to the control (F-17.04; df-5, 12; P < 0.01) (Fig. 1b).
Fig. 1.
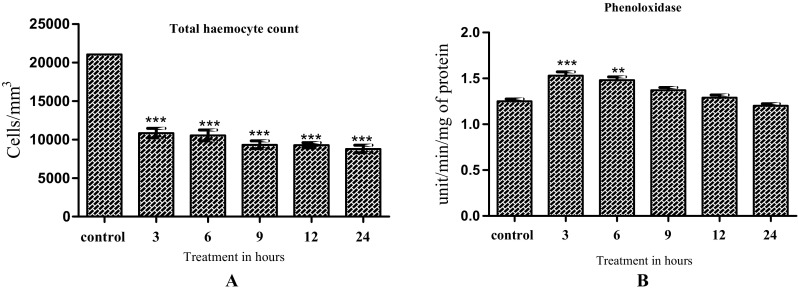
Activity change of cellular (a) and humoral (b) immune response in 3rd instar larvae hemolymph Spodoptera litura infected with (Heterorhabditis indica) larvae in different time intervals. The values are expressed in mean (± SD). One-way ANOVA was used to analyze the significant difference among groups. The symbol (**) (***) above the bar represents the significant difference (P < 0.01) (P < 0.001) with respect to control
Effect of Heterorhabditis indica on oxidative stress marker, antioxidant and detoxification enzymes of S. litura
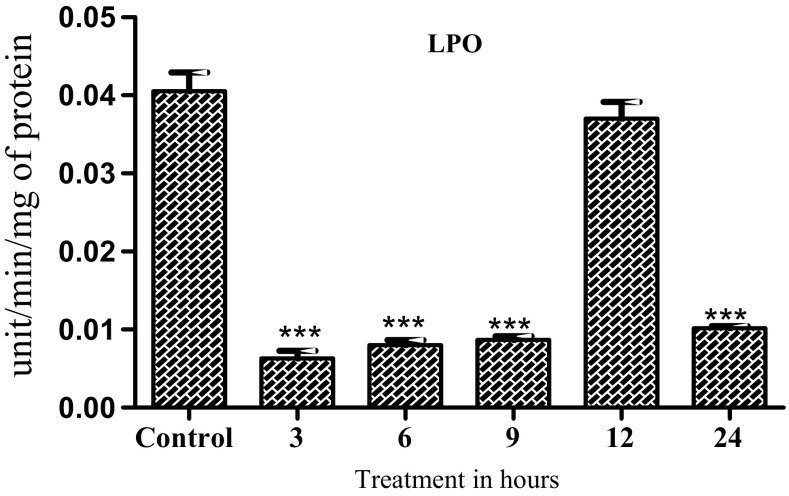
LPO levels showed significant decrease at 3, 6 and 9 h post treatment (F-123.2; df-5, 12; P < 0.01) (Fig. 2). SOD activity significantly decreased in 3, 12 and 24 h post treatment (f-42.17; df-5, 12; P < 0.01) (Fig. 3a). CAT activity showed a significant increase at 6 and 9 h post treatment (F-737.5; df-5, 12; P < 0.001) (Fig. 3b). POD activity was significantly reduced from 3 to 12 h post treatment (F-342.6; df-5, 12; P < 0.001 (Fig. 3c). ACP activity was reduced after 12 and 24 h of treatment (F-31.76; df-5, 12; P < 0.001; P < 0.01) (Fig. 3d). ALP activity showed a significant reduction after 24 h of H. indica exposure (F-314.1; df-5, 12; P < 0.01; P < 0.001) (Fig. 3e). GST activity showed an increase at 6 and 9 h (F-2.903; df-5, 12; (Fig. 3f). GPX enzyme activity shows significant increases at 6, 12 and 24 h as compared to control (F-958.9; df-5, 12; P < 0.001) (Fig. 3g).
Fig. 2.
Activity change of oxidative stress marker in 3rd instar larvae Spodoptera litura infected with (Heterorhabditis indica) larvae in different time intervals. The values are expressed in mean (± SD). One-way ANOVA was used to analyze the significant difference among groups. The symbol (***) above the bar represents the significant difference (P < 0.001) with respect to control
Fig. 3.
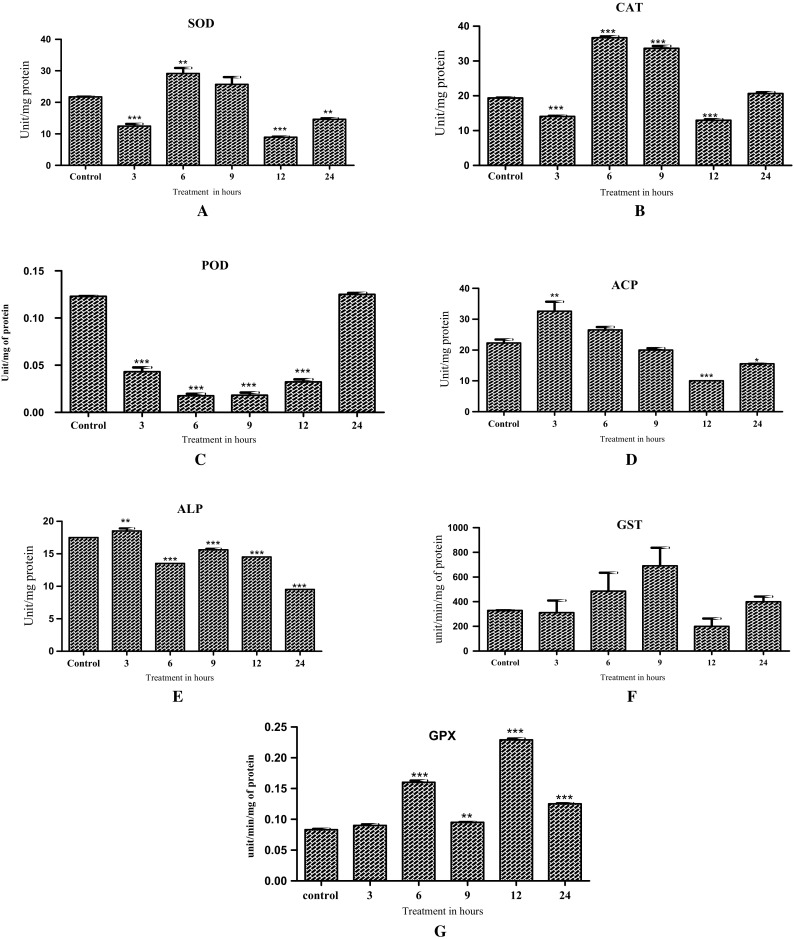
Activity change of antioxidant and detoxication related enzymes in 3rd instar larvae Spodoptera litura infected with (Heterorhabditis indica) larvae in different time intervals. The values are expressed in mean (± SD). One-way ANOVA was used to analyze the significant difference among groups. The symbol (*) (**) (***) above the bar represents the significant difference (P < 0.05) (P < 0.01) (P < 0.001) with respect to control
Discussion
EPN of Heterorhabditidae family are lethal endoparasites of insects. Their role in insect pathogenicity is aided by toxic secondary metabolites produced by symbiotic Photorhabdus bacterial species. H. indica is widely used in biological control of insect pest in agriculture (Brown et al. 2004, 2006; Ffrench-Constant et al. 2007). H. indica is also useful model system to study an insect immune mechanism against bacterial infection. Following infection in insect host H. indica releases symbiotic bacteria which produce and release several toxins complex resulting in activation of immune response or insect mortality, based on the nature and dose of toxin. In insect haemocytes, antimicrobial peptides, phenoloxidase systems and host of antioxidant and detoxification enzyme are activated in response to infection.
Hemocytes are known to be involved in intermediary metabolism such as protein synthesis, transport of nutrients, phenol metabolism and growth stimulation (Wigglesworth 1959). Insect larval stage is an actively growing state which requires high energy, which is provided by intermediary metabolic processes. Considering this insect larval haemocytes number should be naturally high. Following H.indica infection, symbiotic Photorhabdus species are released after 30 min (Wigglesworth 1959). Decrease in Total Hemocyte Count (THC) after 3 h indicates the damage caused by toxins produced by Photorhabdus spp (Fig. 1). Symbiotic bacterial toxins have been shown to cause actin polymerization, destabilizing the cytoskeleton architecture of haemocytes (Li et al. 2009). Studies have also shown that following infection of symbiotic bacteria H. bacteriophora and S. feltiae infection in Helicoverpa armigera larvae, a marked reduction in THC after 4 and 8 h is observed (Li et al. 2009). The present study also shows a marked reduction in THC levels suggesting a quick action of H. indica infection on larval THC levels.
Phenoloxidase (PO) activity is a measure of protective response against invading microbes, fungi, and parasitoids in insects (Freitak et al. 2007). PO participates in encapsulation, clotting of haemolymph, wound healing (Cerenius et al. 2008) and stimulates phagocytosis. Activation of PO is strongly dependent of the cell surface proteins on bacterial cell wall. There are reports suggesting that S. feltiae infection in G.mellonella suppresses PO activity by interfering with LPS-mediated ProPO activation pathway in Galleria mellonella larvae (Brivio et al. 2002), leading to reduced PO activation and encapsulation. However in our study a significant increase in PO levels were observed suggesting that H. indica cell wall components are recognized by ProPO and results in PO activation.
ROS are toxic species to all cellular and biochemical process in the cell. Oxidative stress in biological systems is a result of superoxide anion radicals (O·), hydroxyl radical (OH·), and H· which are generated during normal oxidative processes in cells and extracellular fluids. Participation of O·2 and other ROS in immunological defense is studied increasingly in insects significant increase in Superoxide dismutases (SOD) levels 6 h after infection were observed in the present study, while SOD levels increased, within 12 h of exposure to S. feltiae in G.mellonella (Krystyna et al. 2006). This suggests that a robust antioxidant system is present in S. litura as compared to G.mellonella.
Glutathione S- transferases (GST) play an in conversion of nonpolar xenobiotic toxins into water soluble extretable forms by conjugation reactions. It is one of the most important detoxification enzymes present in insects. Infection of G. mellonella and T. molitor larvae by H. beicherriana results in increase GST activity (Wu et al. 2013). In our study increased GST activity was observed in a time dependent manner in S. litura larvae after infection with H. indica.
Peroxidase (POD) protects cells from oxidative damage induced by xenobiotic and pathogenic infection in insects. Exposure to chemical pesticides usually result in remarkable increase in POD activity in Callosobrochus maculates (Kolawole and Kolawole 2014), Our result shows that POD activity was significantly low for 3, 6, 9 and 12 h following H. indica exposure. Our results suggest that POD activity does not change in response to H.indica infection.
Catalase (CAT) activity after exposure to pesticides signifies an enhanced removal of H2O2 and prevention of oxidative damage. CAT activity in the tissue surrounding the primary infection site is reported to have a close relation to programmed cell death and hypersensitive responses (Schenk et al. 2000). CAT activity decreased after exposure of microbes on G. mellonella larvae (Dubovskiy et al. 2008). In our study, a high catalase activity was observed at 6 h exposure of H. indica on S. litura larvae.
Lipid Peroxidation (LPO) is oxidative degradation of lipids present in the cell membrane and is a common marker of oxidative damage to cell membranes (Felton and Summers 1995). Following xenobiotic or pathogen exposure usually, high LPO levels are observed. In the present study, a significant increase in LPO was observed after 12 h which suggests extensive cellular damage as a result of H.indica infection. H2O2 generated in response to infection is reduced by selenium-dependent glutathione peroxidase (GPX). It plays an important role in detoxifying lipids damage and hydroperoxides, protecting cell membranes and other cellular components from oxidative damage (Arthur 2000; Liu et al. 2004). Our results shows in 6 and 12 h, there were significant increase in glutathione peroxidase levels. Acid phosphatase (ACP) enzymes belong to the wide group of non-specific esterase’s that catalyze hydrolytic cleavage of P-O bonds in many monoesters of phosphoric acid. ACP and Alkaline Phosphatase (ALP) enzymes are found in insect hemocytes. When the haemocyte interacts with nematodes, the phosphate group is removed and the insect haemocytes cells degraded (Cheng 1983). In response to Cypermethrin exposure, increased ACP levels were observed in beetles (Saleem and Shakoori 1996).Our result also shows that increased ACP and ALP enzyme activities at 3, 6, 9, and 6 h but a decreased activity at 12 h was observed which probably may be a result of low cell availability.
In conclusion, our study shows that a reduction in total haemocyte count lowers the insect’s immunity to H. indica infection. A transient increased PO activity shortly after infection might be a mechanism to defend against H. indica infection. The increase in activities of antioxidant enzymes may be the result of free radical generation due to immune function and damage of insect tissues caused by H. indica infection. The study shows that H. indica is very effective in lowering insect’s immunity and also leads to reduced antioxidant enzyme activity, which may leads to insect mortality. They are finding entomopathogenic nematodes used good biological control agent of insecticides.
Acknowledgements
We thank the Department of Biotechnology, Periyar University, Salem, Tamil Nadu, India for providing infrastructure facilities for carrying out this research work.
Authors contribution
Experiment design: MSS, PP. Performing the experiments: KL RK and GV. Data analyzed: KL GV. Writing of manuscript: KL, MSS.
References
- Arthur JR. The glutathione peroxidase. Cell Mol Life Sci. 2000;57(13):1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T, Adachi K, Schwartz E. Stabilizing effect of various organic solvents on protein. J Biol Chem. 1978;253:6423–6425. [PubMed] [Google Scholar]
- Ashida M. Purification and characterization of pre-phenoloxidase from haemolymph of the silkworm Bombyx mori. Arch Biochem Biophys. 1971;144:749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Brivio MF, Pagani M, Restelli S. Immune suppression of Galleria mellonella (Insecta, Lepidoptera) humoral defenses induced by Steinernema feltiae (Nematoda, Rhabditida): involvement of the parasite cuticle. Exp Parasitol. 2002;101:149–156. doi: 10.1016/S0014-4894(02)00111-X. [DOI] [PubMed] [Google Scholar]
- Brown SE, Cao AT, Hines ER, Akhurst RJ, East PD. Novel secreted protein toxin from the insect pathogenic bacterium Xenorhabdus nematophila. J Biol Chem. 2004;279:14595–14601. doi: 10.1074/jbc.M309859200. [DOI] [PubMed] [Google Scholar]
- Brown SE, Cao AT, Dobson P, Hines ER, Akhurst RJ, East PD. Txp40 a ubiquitous insecticidal toxin protein from Xenorhabdus and Photorhabdus bacteria. Appl Environ Microbiol. 2006;72:1653–1662. doi: 10.1128/AEM.72.2.1653-1662.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell AM, Stock P. Heterorhabditis, Steinernema and their bacterial symbionts: lethal pathogens of insects. Nematol. 2000;2:31–42. doi: 10.1163/156854100508872. [DOI] [Google Scholar]
- Castillo JC, Reynolds SE, Eleftherianos I. Insect immune responses to nematode parasites. Trends Parasitol. 2011;27:537–547. doi: 10.1016/j.pt.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Lee BL, Soderhall K. ProPOsystem: pros and cons for its role in invertebrate Immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Chapman RF. Insects structure and function. Cambridge: Cambridge University Press; 1998. pp. 94–127. [Google Scholar]
- Cheng TC. Role of lysosomes in mollusc inflammation. Am Zool. 1983;23:129–144. doi: 10.1093/icb/23.1.129. [DOI] [Google Scholar]
- Dubovskiy IM, Martemyanow VV, Vorontsova YL, Rantala MJ, Gryzanova EV, Glupov VV. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae) Comp Biochem Physiol C. 2008;148:1–5. doi: 10.1016/j.cbpc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Felton GW, Summers CB. Antioxidant systems in insects. Arch Insect Biochem Physiol. 1995;29:187–197. doi: 10.1002/arch.940290208. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant RH, Dowling A, Waterfield NR. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon. 2007;49:436–451. doi: 10.1016/j.toxicon.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Freitak D, Wheat CW, Heckel DG, Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal P, Ehlers RU, Shapiro-Ilan DI. Nematodes as biological control agents. Wallingford: CABI Publishing; 2005. [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hemingway J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem Mol Biol. 2000;30:1009–1015. doi: 10.1016/S0965-1748(00)00079-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. Innate immunity higher insects. Curr Opin Immunol. 1996;7:410. doi: 10.1016/s0952-7915(96)80098-7. [DOI] [PubMed] [Google Scholar]
- Jung SC, Kim YG. Potentiating effect of Bacillus thuringensis sub sp. Kurstaki on pathogenicity of entomopathogenic bacterium Xenorhabdus nematophila against diamond backmoth. J Econ Entomol. 2007;100(1):246–250. doi: 10.1093/jee/100.1.246. [DOI] [PubMed] [Google Scholar]
- Kaya HK. Steinernematidae and heterorhabditidae nematodes. A handbook of techniques. Fayetteville: Arkansas Agriculture Experimental Station; 1988. p. 30. [Google Scholar]
- Kolawole AO, Kolawole AN. Insecticides and bio-insecticides modulate the glutathione-related antioxidant defense system of cowpea storage Bruchid (Callosobruchus maculatus) Int J Insect Sci. 2014;6:79–88. doi: 10.4137/IJIS.S18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhofer AM, Grewal PS, Fuzy EM. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biol Control. 2007;38:397–404. doi: 10.1016/j.biocontrol.2005.12.013. [DOI] [Google Scholar]
- Krystyna Z, Grochla P, Biernat EL. Activity of superoxide dismutase in Galleria mellonella larvae infected with entomopathogenic nematodes Steinernema affinis and S. feltiae. Wisdom Parazytol. 2006;52(4):283–286. [PubMed] [Google Scholar]
- Kunc M, Badrul A, Pavel H, Ulrich T. Monitoring the effect of pathogenic nematodes on locomotion of Drosophila larvae. Fly. 2017;3:1–10. doi: 10.1080/19336934.2017.1297350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun Y, Wang G, Liu X. Affects of the mermithid nematode Ovomermis sinensis on the hemocytes of its host Helicoverpa armigera. J Insect Physiol. 2009;55:47–50. doi: 10.1016/j.jinsphys.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Zhang K, Ren XJ, Luo GM, Shen JC. Bio imprinted protein exhibits glutathione peroxidase activity. Anal Chim Acta. 2004;504(1):185–189. doi: 10.1016/S0003-2670(03)00763-3. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of superoxide anion radical in the autoxidation of pyrogallol convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–471. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicol. 2000;153(1):83–104. doi: 10.1016/S0300-483X(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Mills N. Plant health management. In: Van Alfen NK, editor. Biological control of insect pests. Encyclopedia of agriculture and food systems. Oxford: Academic Press; 2014. pp. 375–387. [Google Scholar]
- Nordberg J, Arner ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31–43. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annu Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Orr D, Lahiri S. Biological control of insect pests in crops. In: Abrol DP, editor. Integrated pest management. San Diego: Academic Press; 2014. pp. 531–548. [Google Scholar]
- Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–322. doi: 10.1016/S0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Pham LN, Schneider DS. Evidence for specificity and memory in the insect innate immune response. In: Beckage NE, editor. Insect immunology. San Diego: Academic Press; 2008. pp. 97–128. [Google Scholar]
- Poinar GO., Jr . Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Gauglar R, Kaya HK, editors. Entomopathogenic nemtaodes in biological. Boca Raton: CRC Press; 1990. pp. 23–61. [Google Scholar]
- Reddy KP, Subhani SM, Khan PA, Kumar KB. Effect of light and benzyl adenine on dark treated growing rice (Oryza sativa) leaves-changes in peroxidase activity. Plant Cell Physiol. 1995;26:987–994. doi: 10.1093/oxfordjournals.pcp.a077018. [DOI] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HL. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Saleem MA, Shakoori AR. Biochemical studies Tal cord 10EC. II. Effect on some enzyme activities macromolecules of adult beetles of Tribolium castaneum. Pak J Zool. 1996;28:151–162. [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed JL, Boff M, Bennett JL. Phenoloxidase activity: induction in female schistosomes by in vitro incubation. J Parasitol. 1978;64:283–289. doi: 10.2307/3279674. [DOI] [PubMed] [Google Scholar]
- Shamseldean MM, Sharaby AF, Gesraha MA, Montasser SA, Ibrahim SA. Utilization of entomopathogenic nematodes combined with plant extracts and plant essential oils against grass hoppers Heteracrir littoralis. J Basic Appl Sci Res. 2013;3:289–294. [Google Scholar]
- Toubarroa D, Lucena Roblesa M, Nascimentoa G, Costab G, Montiela R, Coelhob AV, Simoesan N. An apoptosis inducing serine protease secreted by the entomopathogenic nematode Steinernema carpocapsae. Int J Parasitol. 2009;39:1319–1330. doi: 10.1016/j.ijpara.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ullah I, Khan AL, Ali L, Khan AR, Waqaset M, Lee I, Shin J. An insecticidal compound produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase. Molecules. 2014;19:20913–20928. doi: 10.3390/molecules191220913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisth S, Chandel YS, Sharma PK. Entomopathogenic nematodes—a review. Agric Rev. 2013;34:163–175. doi: 10.5958/j.0976-0741.34.3.001. [DOI] [Google Scholar]
- Wang W, Zhang X. Comparison of antiviral efficiency of immune responses in shrimp. Fish Shellfish Immunol. 2008;25:522–527. doi: 10.1016/j.fsi.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oberley LW, Murhammer DW. Evidence of oxidative stress following the viral infection of two Lepidopteran insect cell lines. Free Radic Biol Med. 2001;31:1448–1455. doi: 10.1016/S0891-5849(01)00728-6. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. Insect blood cells. Annu Rev Entomol. 1959;4:1–16. doi: 10.1146/annurev.en.04.010159.000245. [DOI] [Google Scholar]
- Wu H, Liu Q, Li X, Wang Y, Zhang H. Activities of four enzymes in Galleria mellonella larvae infected with entomopathogenic nematode Heterorhabditis beicherrianan sp. Afr J Agric Res. 2013;8:3245–3250. [Google Scholar]