Abstract
Genistein (4′,5,7-trihydroxyisoflavone) is naturally present in plants of the soy family and is known to have various pharmacological activities, such as anti-cancer, anti-diabetic, anti-oxidant, etc. The phytoestrogen is one of the major isoflavones found in some medicinal plants having anthelmintic properties. This review describes the putative role of genistein as an anthelmintic, which has been tested on some helminth parasites in vitro. Genistein has been shown to cause paralysis and alterations in the tegument and tegumental enzymes (acid phosphatase, alkaline phosphatase, adenosine triphosphatase, and 5′-nucleotidase) of helminth parasites. Alterations in the activities of several enzymes associated with the coordination system (specifically non-specific esterases, acetylcholine esterase, and nitric oxide synthase), and changes in the concentration of nitric oxide, cGMP, free amino acid pool, and tissue ammonia are observed in helminth parasites treated with genistein. The phytoestrogen also affects the carbohydrate metabolism by altering the activities of key enzymes involved in glycogen- and glucose-metabolism of a cestode parasite. Considering the significance of phosphoenolpyruvate carboxykinase (PEPCK) in glycolysis of the cestode parasite, Ki of the phytoestrogen for PEPCK in the parasite has been determined, and molecular docking of genistein into the active site of the enzyme has also been described. The potential beneficial role of genistein as a natural alternative in management of helminth parasites needs to be further explored, particularly considering its in vivo efficacy and pharmacokinetics.
Keywords: Medicinal plants, Genistein, Anthelmintic, Helminths
Introduction
Since ancient times, traditional plant remedies have been the main source against several diseases. Plants or their products have potential to provide an alternative to current practices involving chemotherapy against helminths (Didier et al. 1988; Robinson et al. 1990; Tandon et al. 2011). Drug resistance against several diseases has surged in development of new topical and systemic medicines. Therefore, in view of the urgent need of developing new treatments, there are intensified efforts to search for novel drugs from plants or plant products, which could replace or to be used in conjunction with the existing ones (Duke 1983). The pharmaceutical industry has responded to this need by developing new systemic drugs and topical treatments. Globally, there is a patient-driven trend towards “natural” remedies. Some plants have been reported to have anthelmintic efficacy against helminth parasites in vitro (Table 1). According to recent studies, some plants or their parts have also been shown to have cidal activity against schistosomules of Schistosoma mansoni, metacestodes of Hymenolepis diminuta, Echinococcus multilocularis and E. granulosus, and larvae of filarid or other nematode parasites (Comely 1990; Satrija et al. 1995; Ghosh et al. 1996; Khunkitti et al. 2000; Sparg et al. 2000; Al-Qarawi et al. 2001; Mølgaard et al. 2001; Singh et al. 2001; Lyddiard et al. 2002; Marley et al. 2003; Naguleswaran et al. 2006). Anthelmintic efficacy of some plant-derived components has been found to be comparable or at par with commonly used broad-spectrum drugs like albendazole, piperazine and diethylcarbamazine (Kalyani et al. 1989; Koko et al. 2000; Enwerem et al. 2001; Onyeyili et al. 2001; Temjenmongla and Yadav 2003).
Table 1.
List of medicinal plants having anthelmintic efficacy against helminth parasites in vitro
| Medicinal plants | References |
|---|---|
| Acacia auriculiformis | Ghosh et al. (1996) |
| A. oxyphylla | Dasgupta et al. (2010, 2013b) and Roy et al. (2012c) |
| Acorus calamus | Nath and Yadav (2016) |
| Adhatoda vasica | Yadav and Tangpu (2008) |
| Albizia anthelmintica | Galal et al. (1991a, b) and Koko et al. (2000) |
| A. lebbek | Galal et al. (1991a) |
| Allium sativum | Soffar and Mokhtar (1991) |
| Alpinia nigra | Roy et al. (2009) |
| Artocarpus lakoocha | Charoenlarp et al. (1981, 1989) |
| Balanites aegyptiaca | Koko et al. (2000) |
| Buddleja asiatica | Garg and Dengre (1992) |
| Carex baccans | Challam et al. (2012), Roy et al. (2012a) and Giri et al. (2015) |
| Clerodendrum colebrookianum | Yadav and Temjenmongla (2012b) |
| Diospyros mollis | Maki et al. (1983) |
| Flemingia vestita | Yadav et al. (1992), Roy and Tandon (1996) and Tandon et al. (1997) |
| Lasia spinosa | Yadav and Temjenmongla (2012a) |
| Lysimachia ramosa | Challam et al. (2010) |
| Mallotus philippinensis | Gupta et al. (1984) and Akhtar and Ahmad (1992) |
| Matteuccia orientalis | Shiramizu et al. (1993) |
| Millettia thonningii | Lyddiard et al. (2002) |
| M. pachycarpa | Roy et al. (2008) |
| M. reticulate | Fang et al. (2010) |
| Oroxylum indicum | Deori and Yadav (2016) |
| Potentilla fulgens | Roy et al. (2010), Roy et al. (2012a, 2012b) and Giri et al. (2013) |
| Saussurea lappa | Akhtar and Riffat (1991) |
| Securinega virosa | Dasgupta et al. (2013a, b) |
| Solanum myriacanthum | Yadav and Tangpu (2012) |
| Stephania glabra | Tandon et al. (2004); Lyndem et al. (2008); Das et al. (2009) |
| Streblus asper | Chatterjee et al. (1992) |
| Strobilanthes discolor | Tangpu et al. (2006) |
| Teloxys graveolens | Del Rayo Camacho et al. (1991) |
| Terminalia catappa L. | Anuracpreeda et al. (2016, 2017) |
| Trichosanthes multiloba | Tandon et al. (2004) and Lyndem et al. (2008) |
| Uvaria narum | Hisham et al. (1992) |
There are few medicinal plants available in northeastern region of India (Rao 1981). One of them, Flemingia vestita Benth (Fabaceae), has usage as anthelmintic against intestinal worms in local traditional medicine. To get rid of worm infections, the natives in the region consume the edible tuberous root (unpeeled) of the plant. To validate the use of root tuber of F. vestita against helminths, the phytochemicals in the ethanolic extract of root tuber peel of F. vestita have been isolated and identified. The root tuber peel of F. vestita has rich flavone content; genistein (4′,5,7-trihydroxyisoflavone) (Fig. 1) is found to be the major active principle present in the root tuber peel, besides other isoflavones—formononetin, pseudobaptigenin and daidzein (Rao and Reddy 1991). Isoflavones are naturally occurring phytoestrogens, notably found in the soybean family. Besides F. vestita, genistein is also found in many other plants that belong to the same family or related family like Rutaceae family, Fortunella obovata Hort, Erythrina variegata, Millettia reticulata Benth, Tetracera scandens, Genista sessilifolia, and Amaryllidaceae (Puerariae radix) (Lapcík et al. 2004; Ha et al. 2006; Zhang et al. 2007; Koblovská et al. 2008; Lee et al. 2009; Fang et al. 2010; Bontempo et al. 2013; Mikšátková et al. 2014), and is shown to have various activities. The in vitro anthelmintic efficacy of genistein has been tested against few helminth parasites in order to authenticate traditional usage of F. vestita against intestinal worms (Tandon et al. 1997).
Fig. 1.
Structure of genistein (4′,5,7-trihydroxyisoflavone), isolated from ethanolic root peel extract of Flemingia vestita
The biological functions of isoflavones are well known; genistein, in particular, is an inhibitor of tyrosine protein kinases (Akiyama et al. 1987) and competitive inhibitor of several protein kinase reactions (O’Dell et al. 1991). The phytoestrogen is known to have various other pharmacological activities, such as anti-cancer, anti-diabetic, and anti-oxidant. Isoflavones, genistein and daidzein in particular, have been shown health-promoting benefits in different types of cancers (Barnes and Peterson 1995; Fotsis et al. 1995; Lamartiniere et al. 1995; Wiseman and Duffy 2001; Nagata et al. 2002; Duffy et al. 2007; Satih et al. 2008, 2010; Chemler et al. 2010). Studies on whether genistein has an effect on diabetes or not are very limited; however, animals and humans studies have shown that ingestion of soy protein moderates hyperglycemia (Lavigne et al. 2000; Jayagopal et al. 2002; Choi et al. 2008), suggesting its beneficial role in diabetes. Another important consideration is that genistein modulates pancreatic β-cell function via activation of the cAMP/PKA-dependent ERK½ signaling pathway (Fu et al. 2010). Additionally, Lee (2006) suggests that genistein is capable of reducing hyperglycemia and diabetic complication via minimization of islet cell loss in pancreas, which is also supported by Yang et al. (2011). Recently, biocomputational study demonstrates that genistein inhibits human cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) by mixed inhibition mechanism (Katiyar et al. 2015). In various cell lines, the modulatory effect of genistein on PEPCK-C expression has also been demonstrated (Seenappa et al. 2016). Dkhar et al. (2017) demonstrate that genistein represses human PEPCK-C in insulin-independent pathways in HepG2 cell line. Furthermore, it exerts anti-diabetic effect in type 2 diabetic conditions by enhancing the glucose and lipid metabolism (Park et al. 2006). There has been a surge of curiosity in exploring the antioxidant activities of isoflavones and their analogous metabolites, and genistein is found to be an antioxidant too (Arora et al. 1998; Mitchell et al. 1998; Liu et al. 2004; Rufer and Kulling 2006; Sienkiewicz et al. 2008; Ma et al. 2010). Mitchell et al. (1998) show that S-equol, a metabolite of genistein, is more effective antioxidant than genistein and daidzein. Besides, genistein is reported to have beneficial effects on atherosclerosis and chronic inflammatory diseases for its inhibitory effect on NO production (Sheu et al. 2001), and its possible role in cell cycle is also reported in Candida albicans (Lamartiniere et al. 1995; Yazdanyar et al. 2001).
The multifarious genistein as anthelmintic
Considering the multifarious role of genistein in various metabolic activities as stated above and its usage as a cure against worm infections in traditional medicine practices, its probable anthelmintic role has been tested on several commonly occurring helminth parasites. Raillietina spp., the cestodes of domestic fowl; E. multilocularis and E. granulosus, the cestodes of dog; Fasciolopsis buski, the giant intestinal flukes parasitizing pig/human; Artyfechinostomum sufrartyfex; Fasciola hepatica; Heterakis gallinarum and Ascaridia galli, gut nematodes from fowl; and Ascaris suum, the giant round worm from the intestine of pig, have been used for investigations in order to find out the role of genistein as an anthelmintic.
Parasites and paralysis
In vitro treatment of helminth parasites with genistein causes flaccid paralysis in them in a dose-dependent manner (Yadav et al. 1992; Roy and Tandon 1996; Tandon et al. 1997, 2004; Lyndem et al. 2008; Toner et al. 2008). The phytoestrogen inflicts complete immobilization/reversible flaccid paralysis in soft-bodied cestodes and trematodes (e.g. R. echinobothrida and F. buski) within short time; however, due to the presence of a collagen-rich fibrose surface interface, the nematodes take longer time for the onset of paralysis. These studies indicate that genistein acts transtegumentally on these parasites (Yadav et al. 1992; Roy and Tandon 1996; Tandon et al. 1997).
Effect of genistein on surface architecture and tegumental enzymes
Presuming that genistein may pass through the tegumental interface of the soft-bodied parasites, its effect on the tegumental architecture and tegumental enzymes in helminth parasites has been studied by several authors. Stereoscan- and transmission-electron microscopic observations reveal that there are tegumental alterations and deformity in trematode and cestode parasites (viz. A. sufrartyfex, F. buski, R. echinobothrida) when treated with genistein (Roy and Tandon 1996; Tandon et al. 1997; Pal and Tandon 1998a). Alterations in the contour of microtriches and disorganization of the tegumental region are also observed in the parasites exposed to genistein. The inner sub-tegumental region and muscle layers are the sites that are predominantly affected; the former shows pronounced vacuolization in the genistein treated parasites compared to the control parasite (Pal and Tandon 1998a). In trematodes, genistein causes sloughing off or deformation of the spines as well as deleterious effect on the tegumental surface, which may be associated with paralysis and subsequent death of the parasites (Roy and Tandon 1996). Genistein and its derivatives, Rm 6423 and Rm 6426, induce truncation of microtriches, nuclear pyknosis and vesiculations in E. granulosus and E. multilocularis, causing paralysis in the parasites (Naguleswaran et al. 2006). Rm 6423 also induces interruption of metacestode germinal layer in E. granulosus and E. multilocularis (Naguleswaran et al. 2006). The phytoestrogen causes morphological and neuromuscular disruption in F. hepatica as shown in in vitro; the surface changes comprised swelling and blebbing, especially in the posterior region of the fluke showing disruption to the spines, accompanied by some spine loss (Toner et al. 2008). At different concentrations of genistein (10, 100 and 1 mM), there occurs a significant increase in the frequency and/or amplitude of the somatic muscle strips isolated from F. hepatica (Toner et al. 2008), which might lead to paralysis.
The tegumental enzymes (acid phosphatase, alkaline phosphatase, adenosine triphosphatase, and 5′-nucleotidase) play an important role in the metabolism of cestodes and trematodes for their survival in internal milieu of the host’s intestine (Roy 1982). When the parasites were exposed to the phytoestrogen in vitro, a decline in activity of these enzymes in the genistein treated parasites was histochemically demonstrated (Pal and Tandon 1998b; Kar and Tandon 2004). These effects may contribute to paralysis and subsequent death of cestode and trematode parasites under genistein treatment.
Effect of genistein on the nervous coordination system
Esterases (non-specific esterase and acetyl cholinesterase) have significant role in nervous coordination of helminths. In order to find out the effect of the phytoestrogen on these components of helminth parasites, R. echinobothrida and F. buski were treated with genistein. Besides alterations and deformity in their tegumental architecture, the genistein treated helminth parasites exhibit changes in the activities of nonspecific esterases and acetylcholine esterase with respect to their respective controls (Pal and Tandon 1998c; Kar and Tandon 2000). Acetylcholine, in particular, is known to be involved in muscular coordination and anchoring function; therefore, the neuromuscular disruption caused in genistein exposed F. hepatica may explain the onset of paralysis in helminths (Toner et al. 2008).
The free amino acids [comprising of aspartate, threonine, serine, glutamic acid, glutamine, proline, glycine, alanine, valine, methionine, isoleucine, leucine, tyrosine, lysine, histidine, arginine, phosphoserine, taurine, citrulline, ornithine, beta-alanine, and gamma-amino butyric acid (GABA)] have been demonstrated in helminth parasites (Tandon et al. 1998; Kar et al. 2004). There was a change in the free amino acids level in the helminth parasites treated with genistein (Tandon et al. 1998; Kar et al. 2004). The helminth parasites treated with genistein were found to have an enhancement in GABA and citrulline levels (Tandon et al. 1998; Kar et al. 2004). The change in the citrulline level may be involved in nitric oxide (NO) release and resultant paralysis in F. buski, the giant intestinal trematode (Kar et al. 2004). An increased in ammonia concentration, released by the parasites, in the host microenvironment may prove to be lethal for survival of the intestinal parasites.
NO, a unique gaseous neural messenger, is known to have a dual role—protective and toxic in organisms—and it has been reported to have anthelmintic effects (Mahmoud and Habib 2003). In helminth parasites, nitric oxide synthase (NOS) activity, accountable for NO production, has been demonstrated in neural tissues by NADPH-diaphorase histochemical staining (Bascal et al. 1995; Gustafsson et al. 1996, 1998; Lindholm et al. 1998; Terenina et al. 1999; Gustafsson et al. 2001; Tandon et al. 2001). There was an increase in NO level in neural tissues of the trematode parasite F. buski treated with genistein, which may have assisted the onset of paralysis in the parasite (Kar et al. 2002). Not only in the trematode parasite, but also in the cestode parasite, R. echinobothrida, the phytoestrogen enhances NOS activity and cGMP concentration in the parasite tissue (Das et al. 2007, 2009).
Effect of genistein on Ca2+ homeostasis in cestode
Ca2+ plays a key role in muscle contraction (Nelson and Cox 2012) and possibly involves in paralysis in helminth parasites (Day et al. 1992; Redman et al. 1996); hence, it is imperative to understand the role of genistein on Ca2+ homeostasis in helminth parasites. It has been seen that a significant amount of Ca2+ was found in a cestode, R. echinobothrida, besides other metal ions such as magnesium, iron, zinc, lead and chromium (Das et al. 2006). Following the genistein treatment, the Ca2+ homeostasis is disturbed in the parasite, and the Ca2+ concentration is decreased (39–49%) in the parasite tissues at the paralysis time; while, there is an increase in Ca2+ efflux (91–160%) into the media by the parasite (Das et al. 2006). Changes in the Ca2+ concentration at onset of paralytic state may be correlated with rapid muscular contraction caused by genistein in the cestode parasite. This hypothesis was also postulated by Toner et al. (2008), who observed that genistein changed the amplitudes of somatic muscle strips in F. hepatica.
Effect of genistein on carbohydrate metabolism
Helminth parasites, especially trematodes and cestodes, mainly derive their energy from simple carbohydrate molecules (Smyth and McManus 1989). With respect to carbohydrate metabolism in cestode parasite, genistein was found to alter glycogen metabolism in R. echinobothrida by activating the active form of glycogen phosphorylase and inhibiting the active form of glycogen synthase, thus promoting utilization of glycogen (Tandon et al. 2003). Activities of key enzymes of glycolysis—hexokinase, phosphofructokinase, PEPCK, pyruvate kinase (PK), lactate dehydrogenase, malate dehydrogenase and malic enzyme—in the cestode parasite were also altered at the time of paralysis, suggesting genistein does not influence glucose utilization towards aerobic reactions in the parasite (Das et al. 2004a). With respect to gluconeogenic pathway in R. echinobothrida, there was a decline in glucose 6-phosphate dehydrogenase activity and pyruvate carboxylase and PEPCK activities were altered in the cestode parasite under genistein treatment, whereas, the fructose 1,6-bisphosphatase activity remained unchanged (Das et al. 2004b). The glucose level was declined and the malate content and lactate efflux was increased in the genistein treated cestode parasite, suggesting an increased energy demand in the cestode parasite due to genistein treatment (Das et al. 2004c). Change in carbohydrate metabolism by altering activities of key enzymes of glycogen metabolism and glycolysis could be a function of energy demand of the cestode parasite due to genistein treatment (Tandon and Das 2007), which is also evident in some trematode parasites (El-Ansary et al. 2007).
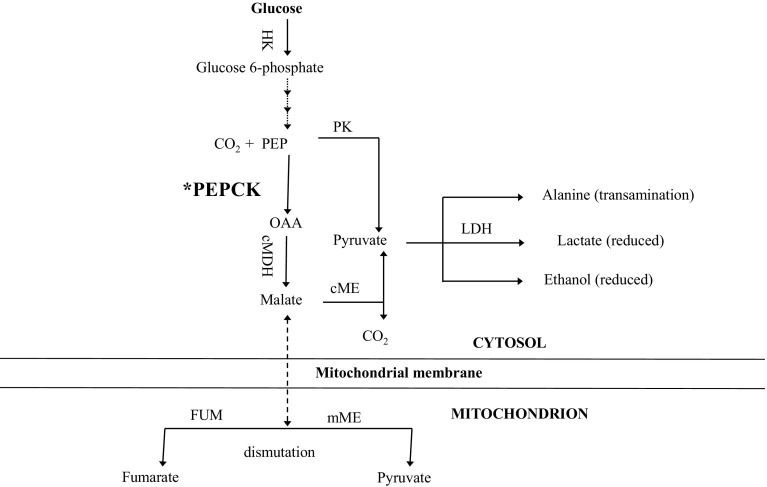
The PK/PEPCK branch point (as shown in Fig. 2) in glycolysis of cestode parasites has been considered as a plausible target for anthelmintic action (Reynolds 1980), since PEPCK plays different role in cestodes and their vertebrate hosts, and both the enzymes use phosphoenolpyruvate as their substrate (Smyth and McManus 1989). In view of understanding the functional differences between PK and PEPCK, the PK/PEPCK branch point has been studied in various helminths (Bueding and Saz 1968; Prichard 1976; Moon et al. 1977; El-Ansary et al. 2007).
Fig. 2.
Diagrammatic representation of possible role of phosphoenolpyruvate carboxykinase (PEPCK*) in glucose metabolism in cestodes.
(Excerpted from Smyth and McManus 1989)
The phytochemicals (including genistein) from F. vestita showed modulatory effect on the purified PEPCK from the cestode parasite, R. echinobothrida (Das et al. 2013). Genistein and daidzein showed non-competitive inhibition with respect to the substrate (phosphoenolpyruvate) for PK and PEPCK enzymes from the cestode, R. echinobothrida. Further, Ki of these compounds for PK and PEPCK from the cestode parasite has been determined. Ki for genistein is 0.26 and 0.17 μM for PK and PEPCK enzymes from the parasite, respectively; whereas, Ki for daidzein is 0.19 and 0.29 μM, respectively (Das et al. 2015).
Effect of genistein on PEPCK activities and their conformation
Considering differential activity of PEPCK in the cestode parasite and its host and in search for potential modulators for the parasite PEPCK, genistein and daidzein were tested on activity of rePEPCK (PEPCK from R. echinobothrida) and gdPEPCK (PEPCK from its host, Gallus domesticus). Genistein and daidzein inhibit the parasite PEPCK in a non-competitive manner; whereas, both the compounds inhibit the host PEPCK in a competitive manner (Ramnath et al. 2017). Ki of genistein and daidzein for the parasite PEPCK were found to be 0.15 and 0.26 µM, respectively (Fig. 3a); however, genistein and daidzein showed Ki of 0.20 and 0.35 µM (Fig. 3b), respectively, for host PEPCK carboxylation reaction, and 0.31 and 0.64 µM, respectively, for host PEPCK decarboxylation reaction, representing genistein is more inhibitory than daidzein (Ramnath et al. 2017). Using biocomputational approach, mixed inhibition of genistein for human PEPCK-C has been reported by Katiyar et al. (2015).
Fig. 3.
a Non-competitive inhibition of rePEPCK (PEPCK from R. echinobothrida) by genistein as determined by Lineweaver–Burk plot (LB plot). The plot was computed by taking different concentrations of PEP (0.01–8 mM) in presence of various concentrations of genistein {(filled star) control, (filled circle) 5 µM, (filled square) 10 µM, and (filled nabla) 20 µM}. Ki (0.15 µM) of genistein for rePEPCK was determined from the replot (inset) with R2 = 0.9972. b Competitive inhibition of gdPEPCK (PEPCK from Gallus domesticus) by genistein was calculated by LB plot, showing Ki of 0.20 µM for gdPEPCK with R2 = 0.9990 (inset). c Near-UV CD spectra (250–350 nm) of rePEPCK in absence/presence of genistein (20 µM)
The effect of genistein and daidzein on conformation of PEPCK has also been analyzed by Far-UV CD spectra analysis using CD spectrophotometer to understand their effect on the structure of PEPCK. The tertiary structure of PEPCK, both from the parasite and its host, remained unchanged in presence of these compounds (Fig. 3c). This suggests that genistein inhibits PEPCK without changing its conformation and may interact via different sites (Ramnath et al. 2017).
Differential binding of genistein with PEPCKs
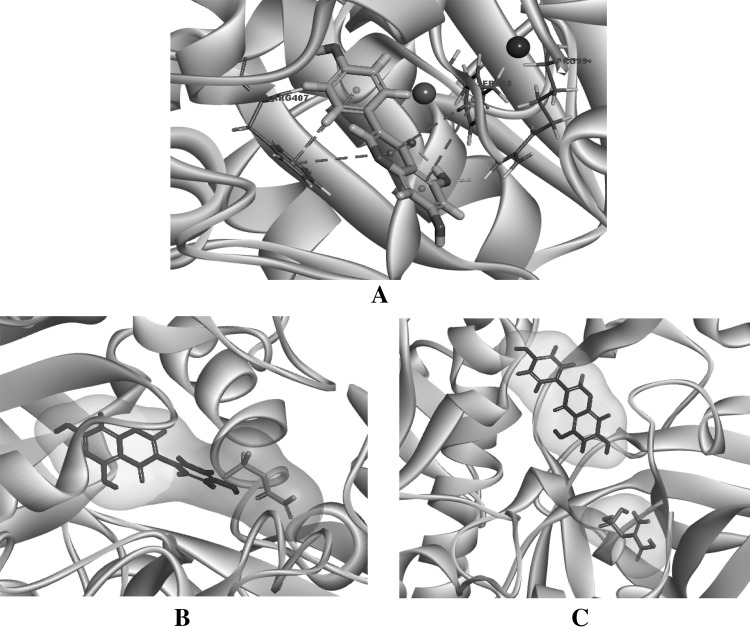
There is little structural information available for the parasite PEPCK, and recently, PEPCK gene (NCBI GenBank acc. no. KC252609.1) from R. echinobothrida has been isolated and analyzed by Dutta et al. (2016). In general, the PEPCK active site is large and encompasses a number of binding pockets, like PEP binding, GTP binding (Johnson and Holyoak 2012). In search of possible modulators for the parasite PEPCK, several plausible modulators (genistein, diadzein, etc.) have been tested on R. echinobothrida PEPCK model (Dutta et al. 2016). Out of which, genistein has been shown to have the lowest binding energy (CDocker energy) of 53.20 kcal/mol. From biocomputational study, it has also been revealed that in the host PEPCK, genistein binds adjoining to the substrate-binding site (Fig. 4a) by interacting with R89 through a hydrogen bond and with S288 and R407 through hydrophobic interactions. The role of R89 for optimal PEPCK activity in rat PEPCK-C has been explained by Johnson et al. (2016).
Fig. 4.
a Probable interactions of genistein with chicken mitochondrial PEPCK (mPEPCK). The interacting residues are shown as thin stick model, whereas genistein as thick stick model. Surface structure shows genistein and PEP compete for the same pocket of chicken mPEPCK (b), whereas different pocket in the case of rePEPCK (c)
In order to examine the differential binding of genistein to PEPCKs from the cestode parasite and its host, surface models were prepared using Discovery Studio 4.1 software (Fig. 4b, c) as described by Dutta et al. (2016). From the biocomputational studies, it has been observed that genistein overlaps with substrate binding site of the host PEPCK, indicating a competitive inhibitor for its substrate (phosphoenolpyruvate). However, in the case of parasite PEPCK, genistein overlaps with nucleotide-binding site of the enzyme, indicating a non-competitive inhibitor for its substrate, which holds true as in the enzyme kinetics studies by Ramnath et al. (2017). This differential binding affinity of genistein to the cestode parasite and its host PEPCKs need to be authenticated using X-ray crystallographic studies of the complexes.
Biological relevance of genistein in cestodes
To understand the biological significance of the lead modulator, genistein, predicted from biocomputational studies, the cestode parasite, R. echinobothrida, was exposed to various concentrations of genistein. It was observed that onset of paralysis in the genistein treated cestode parasite occurred within 8 h of incubation, depending upon the concentrations of genistein; whereas, the control parasite survived up to ~ 96 h (Ramnath et al. 2017). PEPCK activity in the genistein-treated cestode parasite was also decreased correspondingly to the dosage of genistein, signifying PEPCK plays an important role for the parasite’s survival (Ramnath et al. 2017).
Conclusion
From these studies, it appears that genistein primarily act transtegumentally as a vermifugal with several metabolic functions, e.g. amino acid metabolism, carbohydrate metabolism, in the helminth parasites being the plausible secondary targets in helminth parasites. The anthelmintic activity of the phytochemical from F. vestita thus provides a lead towards the development of a newer vermifuge, if not a vermicide. This review may form a basic platform for in vivo investigations of genistein to establish the natural phytoestrogen as an anthelmintic.
Acknowledgements
Authors would like to thank funding agencies (Govt. of India) like MoEF, DST-SAP, and DBT for their financial support. Thanks are also due to the Heads, Department of Zoology, and Coordinators, Bioinformatics Centre, NEHU, for providing infrastructural- and online-facilities for the work, respectively.
Author’s contribution
VT and BD prepared the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Akhtar MS, Ahmad I. Comparative efficacy of Mallotus philippinensis fruit (Kamala) or Nilzan® drug against gastrointestinal cestodes in Beetal goats. Small Rum Res. 1992;8:121–128. [Google Scholar]
- Akhtar MS, Riffat S. Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children. J Pak Med Assoc. 1991;41:185–187. [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh M, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Al-Qarawi A, Mahmoud A, Sobaih OM, Haroun EM, Adam SE. A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet Res Commun. 2001;25:61–70. doi: 10.1023/a:1026762002947. [DOI] [PubMed] [Google Scholar]
- Anuracpreeda P, Chankaew K, Puttarak P, Koedrith P, Chawengkirttikul R, Panyarachun B, Ngamniyom A, Chanchai S, Sobhon P. The anthelmintic effects of the ethanol extract of Terminalia catappa L. leaves against the ruminant gut parasite, Fischoederius cobboldi. Parasitology. 2016;143:421–433. doi: 10.1017/S0031182015001833. [DOI] [PubMed] [Google Scholar]
- Anuracpreeda P, Chawengkirttikul R, Ngamniyom A, Panyarachun B, Puttarak P, Koedrith P, Intaratat N. The in vitro anthelmintic activity of the ethanol leaf extracts of Terminalia catappa L. on Fasciola gigantica. Parasitology. 2017 doi: 10.1017/S0031182017001445. [DOI] [PubMed] [Google Scholar]
- Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. 1998;356:133–141. doi: 10.1006/abbi.1998.0783. [DOI] [PubMed] [Google Scholar]
- Barnes S, Peterson TG. Biochemical targets of the isoflavone genistein in tumor cell lines. Exp Biol Med. 1995;208:103–108. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- Bascal ZA, Montgommery A, Holden-dye L, Williams RG, Walker RJ. Histochemical mapping of NADPH-diaphorase in the nervous system of the parasitic nematode Ascaris suum. Parasitology. 1995;110:625–637. doi: 10.1017/s0031182000065343. [DOI] [PubMed] [Google Scholar]
- Bontempo P, Rigano D, Doto A, Formisano C, Conte M, Nebbioso A, Carafa V, Caserta G, Sica V, Molinari AM, Altucci L. Genista sessilifolia DC. extracts induce apoptosis across a range of cancer cell lines. Cell Prolif. 2013;46:183–192. doi: 10.1111/cpr.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E, Saz HJ. Pyruvate kinase and phosphoenolpyruvate carboxykinase activities of Ascaris muscle, Hymenolepis diminuta and Schistosoma mansoni. Comp Biochem Physiol B: Biochem Mol Biol. 1968;24:511–518. doi: 10.1016/0010-406x(68)91003-7. [DOI] [PubMed] [Google Scholar]
- Challam M, Roy B, Tandon V. Effect of Lysimachia ramosa (Primulaceae) on helminth parasites: motility, mortality and scanning electron microscopic observations on surface topography. Vet Parasitol. 2010;169:214–218. doi: 10.1016/j.vetpar.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Challam M, Roy B, Tandon V. In vitro anthelmintic efficacy of Carex baccans (Cyperaceae): ultrastructural, histochemical and biochemical alterations in the cestode, Raillietina echinobothrida. J Parasit Dis. 2012;36:81–86. doi: 10.1007/s12639-011-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenlarp P, Radomyos P, Harinasuta T. Treatment of taeniasis with Puag-Haad: a crude extract of Artocarpus lakoocha wood. Southeast Asian J Trop Med Public Health. 1981;12:568–570. [PubMed] [Google Scholar]
- Charoenlarp P, Radomyos P, Bunnag D. The optimum dose of Puag-Haad in the treatment of taeniasis. J Med Assoc Thai. 1989;72:71–73. [PubMed] [Google Scholar]
- Chatterjee RK, Fatma N, Murthy PK, Sinha P, Kulshrestha DK, Dhawan BN. Macrofilaricidal activity of the stembark of Streblus asper and its major active constituents. Drug Dev Res. 1992;26:67–68. [Google Scholar]
- Chemler JA, Lim CG, Daiss JL, Koffas MAG. A versatile microbial system for biosynthesis of novel polyphenols with altered estrogen receptor binding activity. Chem Biol. 2010;17:392–401. doi: 10.1016/j.chembiol.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Choi MS, Jung UJ, Yeo J, Kim MJ, Lee MK. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab Res Rev. 2008;24:74–81. doi: 10.1002/dmrr.780. [DOI] [PubMed] [Google Scholar]
- Comely JCW. New macrofilaricidal leads from plants? Trop Med Parasitol. 1990;41:1–9. [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Anthelmintic efficacy of Flemingia vestita (Fabaceae): alteration in the activities of some enzymes of glycolysis in the cestode, Raillietina echinobothrida. Parasitol Res. 2004;93:253–261. doi: 10.1007/s00436-004-1122-8. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Effects of phytochemicals of Flemingia vestita (Fabaceae) on glucose 6-phosphate dehydrogenase and enzymes of gluconeogenesis in a cestode (Raillietina echinobothrida) Comp Biochem Physiol C. 2004;139:141–146. doi: 10.1016/j.cca.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Anthelmintic efficacy of Flemingia vestita (Fabaceae): alterations in glucose metabolism of the cestode, Raillietina echinobothrida. Parasitol Int. 2004;53:345–350. doi: 10.1016/j.parint.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Effect of isoflavone from Flemingia vestita (Fabaceae) on the Ca2+ homeostasis in Raillietina echinobothrida, the cestode of domestic fowl. Parasitol Int. 2006;55:17–21. doi: 10.1016/j.parint.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Genistein from Flemingia vestita (Fabaceae) enhances NO and its mediator (cGMP) production in a cestode parasite, Raillietina echinobothrida. Parasitology. 2007;134:1457–1463. doi: 10.1017/S003118200700282X. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Lyndem LM, Gray AI, Ferro VA. Phytochemicals from Flemingia vestita (Fabaceae) and Stephania glabra (Menispermeaceae) alter cGMP concentration in the cestode Raillietina echinobothrida. Comp Biochem Physiol C. 2009;149:397–403. doi: 10.1016/j.cbpc.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saxena JK, Joshi S, Singh AK. Purification and characterization of phosphoenolpyruvate carboxykinase from Raillietina echinobothrida, a cestode parasite of the domestic fowl. Parasitology. 2013;140:136–146. doi: 10.1017/S0031182012001254. [DOI] [PubMed] [Google Scholar]
- Das B, Ramnath Dutta AK, Tandon V. Differential kinetics at PK/PEPCK branch point in the cestode, Raillietina echinobothrida. Exp Parasitol. 2015;153:151–159. doi: 10.1016/j.exppara.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Roy B, Tandon V. Ultrastructural alterations of the tegument of Raillietina echinobothrida treated with the stem bark of Acacia oxyphylla (Leguminosae) J Ethnopharmacol. 2010;127:568–571. doi: 10.1016/j.jep.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Giri BR, Roy B. Ultrastructural observations on Raillietina echinobothrida exposed to crude extract and active compound of Securinega virosa. Micron. 2013;50:62–67. doi: 10.1016/j.micron.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Roy B, Venkataswamy M, Giri BR. Effects of Acacia oxyphylla and Securinega virosa on functional characteristics of Raillietina echinobothrida (Phylum: Platyhelminthes; Class: Cestoidea), a poultry cestode parasite. J Parasit Dis. 2013;37:125–130. doi: 10.1007/s12639-012-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Bennett JL, Pax RA. Praziquantel: the enigmatic antiparasitic. Parasitol Today. 1992;8:342–344. doi: 10.1016/0169-4758(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Del Rayo Camacho M, Sanchez B, Quiroz H, Contreras JL, Mata R. Pinocembrine: a bioactive flavanone from Teloxys graveolens. J Ethnopharmacol. 1991;31:383–389. doi: 10.1016/0378-8741(91)90022-6. [DOI] [PubMed] [Google Scholar]
- Deori K, Yadav AK. Anthelmintic effects of Oroxylum indicum stem bark extract on juvenile and adult stages of Hymenolepis diminuta (Cestoda), an in vitro and in vivo study. Parasitol Res. 2016;115:1275–1285. doi: 10.1007/s00436-015-4864-6. [DOI] [PubMed] [Google Scholar]
- Didier JM, Bundy DAP, Mckenzie HI. Traditional treatment and community control of gastrointestinal helminthiases in ST. Lucia, West Indies. Trans R Soc Trop Med Hyg. 1988;82:303–304. doi: 10.1016/0035-9203(88)90454-3. [DOI] [PubMed] [Google Scholar]
- Dkhar B, Khongsti K, Thabah D, Syiem D, Satyamoorthy K, Das B. Genistein represses PEPCK-C expression in an insulin-independent manner in HepG2 cells and in alloxan-induced diabetic mice. J Cell Biochem. 2017 doi: 10.1002/jcb.26356. [DOI] [PubMed] [Google Scholar]
- Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J Clin. 2007;57:260–277. doi: 10.3322/CA.57.5.260. [DOI] [PubMed] [Google Scholar]
- Duke JA. Medicinal plants of the Bible. New York: Tradco-Medic Books, Conch Magazine, Ltd.; 1983. [Google Scholar]
- Dutta AK, Ramnath Tandon V, Das B. Biocomputational analysis of phosphoenolpyruvate carboxykinase from Raillietina echinobothrida, a cestode parasite, and its interaction with possible modulators. Parasitology. 2016;143:300–313. doi: 10.1017/S0031182015001742. [DOI] [PubMed] [Google Scholar]
- El-Ansary AK, Ahmed SA, Aly SA. Antischistosomal and liver protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J Exp Biol. 2007;45:791–801. [PubMed] [Google Scholar]
- Enwerem NM, Okogun JI, Wambebe CO, Okorie DA, Akah PA. Anthelmintic activity of the stem bark extracts of Berlina grandiflora and one of its active principles, Betulinic acid. Phytomedicine. 2001;8:112–114. doi: 10.1078/0944-7113-00023. [DOI] [PubMed] [Google Scholar]
- Fang SC, Hsu CL, Lin HT, Yen GC. Anticancer effects of flavonoid derivatives isolated from Millettia reticulata Benth in SK-Hep-1 human hepatocellular carcinoma cells. J Agric Food Chem. 2010;58:814–820. doi: 10.1021/jf903216r. [DOI] [PubMed] [Google Scholar]
- Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–797S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal M, Bashir AK, Salih AM, Adam SE. Activity of water extracts of Albizia anthelmintica and A. lebbek barks against experimental Hymenolepis diminuta infection in rats. J Ethnopharmacol. 1991;31:333–337. doi: 10.1016/0378-8741(91)90019-a. [DOI] [PubMed] [Google Scholar]
- Galal M, Bashir AK, Salih AM, Adam SE. Efficacy of aqueous and butanolic fractions of Albizia anthelmintica against experimental Hymenolepis diminuta infestation in rats. Vet Hum Toxicol. 1991;33:537–539. [PubMed] [Google Scholar]
- Garg SC, Dengre SL. Composition of the essential oil from the leaves of Buddleia asiatica Lour. Flavour Fragr J. 1992;7:25–127. [Google Scholar]
- Ghosh NK, Sinha Babu SP, Sukul NC, Ito A. Cestocidal activity of Acacia auriculiformis. J Helminthol. 1996;70:171–172. doi: 10.1017/s0022149x00015340. [DOI] [PubMed] [Google Scholar]
- Giri BR, Roy B, Sinha Babu SP. Evidence of apoptosis in Raillietina echinobothrida induced by methanolic extracts of three traditional medicinal plants of Northeast India. Exp Parasitol. 2013;134:466–473. doi: 10.1016/j.exppara.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Giri BR, Bharti RR, Roy B. In vivo anthelmintic activity of Carex baccans and its active principle resveratrol against Hymenolepis diminuta. Parasitol Res. 2015;114:785–788. doi: 10.1007/s00436-014-4293-y. [DOI] [PubMed] [Google Scholar]
- Gupta SS, Verma P, Hishikar K. Purgative and anthelmintic effects of Mallotus philippinensis in rats against tape worm. Indian J Physiol Pharmacol. 1984;28:63–66. [PubMed] [Google Scholar]
- Gustafsson MKS, Lindholm AM, Terenina NB, Reuter M. NO nerves in tapeworm. NADPH-diaphorase histochemistry in adult Hymenolepis diminuta. Parasitology. 1996;113:559–565. doi: 10.1017/s0031182000067603. [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Lindholm AM, Mäntylä K, Reuter M, Lundström CA, Terenina N. No news on the flatworm front! Nitric oxide synthase in parasitic and free living flatworms. Hydrobiologia. 1998;383:161–166. [Google Scholar]
- Gustafsson MKS, Terenina N, Kreshchenko ND, Reuter M, Maule AG, Halton DW. Comparative study of the spatial relationship between nicotinamide adenine dinucleotide phosphate diaphorase activity, serotonin immunoreactivity and the musculature of the adult liver fluke, Fasciola hepatica (Digenea, Fasciolidae) J Comp Neurol. 2001;429:71–79. doi: 10.1002/1096-9861(20000101)429:1<71::aid-cne6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee YS, Lee JH, Choi H, Kim C. High performance liquid chromatographic analysis of isoflavones in medicinal herbs. Arch Pharm Res. 2006;29:96–101. doi: 10.1007/BF02977475. [DOI] [PubMed] [Google Scholar]
- Hisham AK, Pieters L, Schepens P, Vlietinek AJ. The root bark essential oil of Uvaria narum. J Essent Oil Res. 1992;4:475–477. [Google Scholar]
- Jayagopal V, Albertazzi P, Kilpatrick ES, Howarthm EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Holyoak T. The Ω-loop lid domain of phosphoenolpyruvate carboxykinase is essential for catalytic function. Biochemistry. 2012;51:9547–9559. doi: 10.1021/bi301278t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Mcleod MJ, Holyoak T. Utilization of substrate intrinsic binding energy for conformational change and catalytic function in phosphoenolpyruvate carboxykinase. Biochemistry. 2016;55:575–587. doi: 10.1021/acs.biochem.5b01215. [DOI] [PubMed] [Google Scholar]
- Kalyani GA, Aithal KS, Srivastava KK. In vitro anthelmintic activity of essential oil from the fruits of Zanthoxylum limonella. Fitoterapia. 1989;60:160–162. [Google Scholar]
- Kar PK, Tandon V. Anthelmintic efficacy of Flemingia vestita (Fabaceae): genistein induced effect on the nervous components in two digenetic trematodes. J Parasit Dis. 2000;24:141–146. [Google Scholar]
- Kar PK, Tandon V. Anthelmintic efficacy of genistein, the active principle of Flemingia vestita (Fabaceae): Alterations in the activity of the enzymes associated with the tegumental and gastrodermal interfaces of the trematode, Fasciolopsis buski. J Parasit Dis. 2004;28:45–56. [Google Scholar]
- Kar PK, Tandon V, Saha N. Anthelmintic efficacy of Flemingia vestita: genistein-induced effect on the activity of nitric oxide synthase and nitric oxide in the trematode parasite, Fasciolopsis buski. Parasitol Int. 2002;51:249–257. doi: 10.1016/s1383-5769(02)00032-6. [DOI] [PubMed] [Google Scholar]
- Kar PK, Tandon V, Saha N. Anthelmintic efficacy of genistein, the active principle of Flemingia vestita (Fabaceae): alterations in the free amino acid pool and ammonia levels in the fluke, Fasciolopsis buski. Parasitol Int. 2004;53:287–291. doi: 10.1016/j.parint.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Katiyar SP, Jain A, Dhanjal JK, Sundar D. Mixed inhibition of cPEPCK by genistein, using an extended binding site located adjacent to its catalytic cleft. PLoS ONE. 2015;10:e0141987. doi: 10.1371/journal.pone.0141987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunkitti W, Fujimaki Y, Aoki Y. In vitro antifilarial activity of extracts of the medicinal plant Cardiospermum halicacabum against Brugia pahangi. J Helminthol. 2000;74:241–246. doi: 10.1017/s0022149x00000342. [DOI] [PubMed] [Google Scholar]
- Koblovská R, Macková Z, Vítková M, Kokoska L, Klejdus B, Lapcík O. Isoflavones in the Rutaceae family: twenty selected representatives of the genera Citrus, Fortunella, Poncirus, Ruta and Severinia. Phytochem Anal. 2008;19:64–70. doi: 10.1002/pca.1016. [DOI] [PubMed] [Google Scholar]
- Koko WS, Galal M, Khalid HS. Fasciolicidal efficacy of Albizia anthelmintica and Balanites aegyptiaca compared with albendazole. J Ethnopharmacol. 2000;71:247–252. doi: 10.1016/s0378-8741(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Moore J, Holland M, Barnes S. Neonatal genistein chemoprevents mammary cancer. Proc Soc Exp Biol Med. 1995;208:120–123. doi: 10.3181/00379727-208-43843. [DOI] [PubMed] [Google Scholar]
- Lapcík O, Klejdus B, Davidová M, Kokoska L, Kubán V, Moravcová J. Isoflavonoids in the Rutaceae family: 1. Fortunella obovata, Murraya paniculata and four Citrus species. Phytochem Anal. 2004;15:293–299. doi: 10.1002/pca.781. [DOI] [PubMed] [Google Scholar]
- Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006;79:1578–1584. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim CH, Hoang DM, Kim BY, Sohn CB, Kim MR, Ahn JS. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biol Pharm Bull Biol Pharm Bull. 2009;32:504–508. doi: 10.1248/bpb.32.504. [DOI] [PubMed] [Google Scholar]
- Lindholm AM, Reuter M, Gustafsson MKS. The NADPH-diaphorase staining reaction in relation to the aminergic and peptidergic nervous system and the musculature of adult Diphyllobothrium dendriticum. Parasitology. 1998;117:283–292. doi: 10.1017/s0031182098003047. [DOI] [PubMed] [Google Scholar]
- Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–5539. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- Lyddiard JR, Whitfield PJ, Bartlett A. Antischistosomal bioactivity of isoflavonoids from Millettia thonningii (Leguminosae) J Parasitol. 2002;88:163–170. doi: 10.1645/0022-3395(2002)088[0163:ABOIFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lyndem LM, Tandon V, Das B. Anthelmintic efficacy of medicinal plants from Northeast India against hookworms: an in vitro study on Ancylostoma ceylanicum. Pharmacologyonline. 2008;3:697–707. [Google Scholar]
- Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci. 2010;28:289–295. doi: 10.1016/j.ijdevneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Mahmoud MS, Habib FS. Role of nitric oxide in host defence against Hymenolepis nana infection. J Egypt Soc Parasitol. 2003;33:485–496. [PubMed] [Google Scholar]
- Maki J, Kondo A, Yanagisawa T. Effects of alcoholic extract from Ma-Klua (Diospyros mollis) on adults and larvae of the dwarf tapeworm, Hymenolepis nana in mice and on the infectivity of the eggs. Parasitology. 1983;87:103–111. doi: 10.1017/s0031182000052458. [DOI] [PubMed] [Google Scholar]
- Marley CL, Cook R, Keatinge R, Barrett J, Lampkin NH. The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vet Parasitol. 2003;112:147–155. doi: 10.1016/s0304-4017(02)00412-0. [DOI] [PubMed] [Google Scholar]
- Mikšátková P, Lanková P, Huml L, Lapčík O. Isoflavonoids in the Amaryllidaceae family. Nat Prod Res. 2014;28:690–697. doi: 10.1080/14786419.2013.873432. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Gardner PT, McPhail DB, Morrice PC, Collins AR, Duthie GG. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys. 1998;360:142–148. doi: 10.1006/abbi.1998.0951. [DOI] [PubMed] [Google Scholar]
- Mølgaard P, Nielsen SB, Rasmussen DE, Drummond RB, Makaza N, Andreassen J. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol. 2001;74:257–264. doi: 10.1016/s0378-8741(00)00377-9. [DOI] [PubMed] [Google Scholar]
- Moon TW, Mustafa T, Hulbert WC, Podesta RB, Mettrick DF. The phosphoenol-pyruvate branch point in adult Hymenolepis diminuta (Cestoda): a study of pyruvate kinase and phosphoenol-pyruvate carboxykinase. J Exp Zool. 1977;200:325–336. doi: 10.1002/jez.1402000303. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Kawakami N, Shimizu H. A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer. 2002;87:31–36. doi: 10.1038/sj.bjc.6600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, Hempill A. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother. 2006;50:3770–3778. doi: 10.1128/AAC.00578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath P, Yadav AK. Anthelmintic activity of a standardized extract from the rhizomes of Acorus calamus Linn. (Acoraceae) against experimentally induced cestodiasis in rats. J Intercult Ethnopharmacol. 2016;5:390–395. doi: 10.5455/jice.20160521124439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger principles of biochemistry. 6. New York: WH Freeman and Company; 2012. [Google Scholar]
- O’Dell TJ, Kandel ER, Grant SGN. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Onyeyili PA, Nwosu CO, Amin JD, Jibike JI. Anthelmintic activity of crude aqueous extract of Nauclea latifolia stems bark against ovine nematodes. Fitoterapia. 2001;72:12–21. doi: 10.1016/s0367-326x(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Pal P, Tandon V. Anthelmintic efficacy of Flemingia vestita (Leguminoceae): genistein-induced alterations in the ultrastructure of the tegument in the cestode, Raillietina echinobothrida. J Parasit Dis. 1998;22:104–109. [Google Scholar]
- Pal P, Tandon V. Anthelmintic efficacy of Flemingia vestita (Leguminoceae): Genistein-induced alterations in the activity of tegumental enzymes in the cestode, Raillietina echinobothrida. Parasitol Int. 1998;47:233–243. [Google Scholar]
- Pal P, Tandon V. Anthelmintic efficacy of Flemingia vestita (Fabaceae): Genistein-induced alterations in the esterase activity in the cestode, Raillietina echinobothrida. J Biosci. 1998;23:25–31. [Google Scholar]
- Park SH, Ko SK, Choi JG, Chung SH. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR Mice. Arch Pharm Res. 2006;29:256–264. doi: 10.1007/BF02969402. [DOI] [PubMed] [Google Scholar]
- Prichard RK. Regulation of pyruvate kinase and phosphoenolpyruvate carboxykinase activity in adult Fasciola hepatica (Trematoda) Int J Parasitol. 1976;6:227–233. doi: 10.1016/0020-7519(76)90039-4. [DOI] [PubMed] [Google Scholar]
- Ramnath Dutta AK, Dkhar B, Tandon V, Das B. Biological significance of phosphoenolpyruvate carboxykinase in a cestode parasite, Raillietina echinobothrida, and effect of phytoestrogens on the enzyme from the parasite and its host, Gallus domesticus. Parasitology. 2017;144:1264–1274. doi: 10.1017/S0031182017000518. [DOI] [PubMed] [Google Scholar]
- Rao RR. Ethnobotany of Meghalaya: medicinal plants used by Khasi and Garo tribes. Econ Bot. 1981;35:4–9. [Google Scholar]
- Rao HSP, Reddy KS. Isofavones from Flemingia vestita. Fitoterapia. 1991;63:458. [Google Scholar]
- Redman CA, Robertson A, Fallon PG, Modha J, Kusel JR, Doenhoff MJ, et al. Praziquantel: an urgent and exciting challenge. Parasitol Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- Reynolds CH. Phosphoenolpyruvate carboxykinase from the rat and from the tapeworm, Hymenolepis diminuta. Comp Biochem Physiol. 1980;65B:481–487. [Google Scholar]
- Robinson RD, Williams LAD, Linda JF, Terzy SI, Mansingh A. Inactivation of Strongyloides stercoralis larvae in vitro by six Jamaican plant extracts and three commercial anthelmintics. West Indian Med J. 1990;39:213–217. [PubMed] [Google Scholar]
- Roy TK. Hydrolytic enzymes and membrane digestion in parasitic platyhelminthes. J Sci Ind Res. 1982;41:439–454. [Google Scholar]
- Roy B, Tandon V. Effect of root tuber extract of Flemingia vestita, a leguminous plant, on Artyfechinostomum sufrartyfex and Fasciolopsis buski: a scanning electron microscopy study. Parasitol Res. 1996;82:248–252. doi: 10.1007/s004360050104. [DOI] [PubMed] [Google Scholar]
- Roy B, Dasgupta S, Tandon V. Ultrastructural observations on tegumental surface of Raillietina echinobothrida and its alterations caused by root-peel extract of Millettia pachycarpa. Microsc Res Tech. 2008;71:810–815. doi: 10.1002/jemt.20623. [DOI] [PubMed] [Google Scholar]
- Roy B, Dasgupta S, Tandon V. Ultrastructural observations on Fasciolopsis buski and its alterations caused by shoot extract of Alpinia nigra. Microsc Res Tech. 2009;72:61–66. doi: 10.1002/jemt.20643. [DOI] [PubMed] [Google Scholar]
- Roy B, Swargiary A, Syiem D, Tandon V. Potentilla fulgens (Family Rosaceae), a medicinal plant of north-east India: a natural anthelmintic? J Parasit Dis. 2010;34:83–88. doi: 10.1007/s12639-010-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Dasgupta S, Giri BR. Electron microscopic observations on the alterations of tegumental surface of Raillietina echinobothrida treated with root-peel extract of Potentilla fulgens. Microsc Res Tech. 2012;75:1000–1005. doi: 10.1002/jemt.20972. [DOI] [PubMed] [Google Scholar]
- Roy B, Dasgupta S, Manivel V, Parameswaran PS, Giri BR. Surface topographical and ultrastructural alterations of Raillietina echinobothrida and Ascaridia galli induced by a compound isolated from Acacia oxyphylla. Vet Parasitol. 2012;185:322–326. doi: 10.1016/j.vetpar.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Roy B, Giri BR, Chetia M, Swargiary A. Ultrastructural and biochemical alterations in rats exposed to crude extract of Carex baccans and Potentilla fulgens. Microsc Microanal. 2012;18:1067–1076. doi: 10.1017/S1431927612001456. [DOI] [PubMed] [Google Scholar]
- Rufer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54:2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- Satih S, Rabiau N, Bignon YJ, Bernard-Gallon DJ. Soy phytoestrogens and breast cancer chemoprevention: molecular mechanisms. Curr Nutr Food Sci. 2008;4:259–264. [Google Scholar]
- Satih S, Chalabi N, Rabiau N, Bosviel R, Fontana L, Yves-Jean Bignon, Bernard-Gallon DJ. Gene expression profiling of breast cancer cell lines in response to soy isoflavones using a pangenomic microarray approach. OMICS. 2010;14:231–238. doi: 10.1089/omi.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrija F, Nansen P, Murtini S, He S. Anthelmintic activity of papaya latex against patent Heligmosomoides polygyrus infections in mice. J Ethnopharmacol. 1995;48:161–164. doi: 10.1016/0378-8741(95)01298-r. [DOI] [PubMed] [Google Scholar]
- Seenappa V, Das B, Joshi BM, Satyamoorthy K. Context dependent regulation of PEPCK isoforms via promoter DNA methylation and RNA stability in human cells. J Cell Biochem. 2016;117:2506–2520. doi: 10.1002/jcb.25543. [DOI] [PubMed] [Google Scholar]
- Sheu F, Lai HH, Yen GC. Suppression effect of soy isoflavones on nitric oxide production in RAW 264.7 macrophages. J Agric Food Chem. 2001;49:1767–1772. doi: 10.1021/jf001198+. [DOI] [PubMed] [Google Scholar]
- Shiramizu K, Tsuchida T, Abu M. Anthelmintic effect of a crude preparation containing the root of Matteuccia orientalis on bovine fascioliasis. Nippon Juishikai Zasshi. 1993;46:561–564. [Google Scholar]
- Sienkiewicz P, Surazyński A, Pałka J, Miltyk W. Nutritional concentration of genistein protects human dermal fibroblasts from oxidative stress-induced collagen biosynthesis inhibition through IGF-I receptor-mediated signaling. Acta Pol Pharm. 2008;65:203–211. [PubMed] [Google Scholar]
- Singh SN, Vats P, Suri S, Shyam R, Kumria MML, Ranganathan S, Sridharan K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001;76:269–277. doi: 10.1016/s0378-8741(01)00254-9. [DOI] [PubMed] [Google Scholar]
- Smyth JD, McManus DP. The physiology and biochemistry of cestodes. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Soffar SA, Mokhtar GM. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in Hymenolepis nana and giardiasis. J Egypt Soc Parasitol. 1991;21:497–502. [PubMed] [Google Scholar]
- Sparg SG, Van Staden J, Jager AK. Efficiency of traditionally used South African plants against schistosomiasis. J Ethnopharmacol. 2000;73:209–214. doi: 10.1016/s0378-8741(00)00310-x. [DOI] [PubMed] [Google Scholar]
- Tandon V, Das B. In vitro testing of anthelmintic efficacy of Flemingia vestita (Fabaceae) on carbohydrate metabolism in Raillietina echinobothrida. Methods. 2007;42:330–338. doi: 10.1016/j.ymeth.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Tandon V, Pal P, Roy B, Rao HSP, Reddy KS. In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong. Parasitol Res. 1997;83:492–498. doi: 10.1007/s004360050286. [DOI] [PubMed] [Google Scholar]
- Tandon V, Pal P, Saha N. Anthelmintic efficacy of Flemingia vestita (Leguminoceae): genistein-induced alterations in the free amino acid pool of the cestode, Raillietina echinobothrida. J Parasit Dis. 1998;22:110–115. [Google Scholar]
- Tandon V, Kar PK, Saha N. NO nerves in trematodes, too! NADPH-diaphorase activity in adult Fasciolopsis buski. Parasitol Int. 2001;50:157–163. doi: 10.1016/s1383-5769(01)00074-5. [DOI] [PubMed] [Google Scholar]
- Tandon V, Das B, Saha N. Anthelmintic efficacy of Flemingia vestita (Fabaceae): effect of genistein on glycogen metabolism in the cestode, Raillietina echinobothrida. Parasitol Int. 2003;52:179–183. doi: 10.1016/s1383-5769(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Tandon V, Lyndem LM, Kar PK, Pal P, Das B, Rao HSP. Anthelmintic efficacy of rhizome extract of Stephania glabra and aerial root extract of Trichosanthes multiloba in vitro: two indigenous plants in Shillong, India. J Parasit Dis. 2004;28:37–44. [Google Scholar]
- Tandon V, Yadav AK, Roy B, Das B. Phytochemicals as cure of worm infections in traditional medicine systems. In: Srivastava UC, Kumar S, editors. Emerging trends in zoology. New Delhi: Narendra Publishing House; 2011. pp. 1–27. [Google Scholar]
- Tangpu V, Temjenmongla, Yadav AK. Anticestodal property of Strobilanthes discolor: an experimental study in Hymenolepis diminuta-rat model. J Ethnopharmacol. 2006;105:459–463. doi: 10.1016/j.jep.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Temjenmongla, Yadav AK. Larvicidal efficacy of some folklore medicinal plants against Setaria cervi (Nematoda: Filarioidea) Proc Zool Soc Calcutta. 2003;56:57–61. [Google Scholar]
- Terenina NB, Reuter M, Gustafsson MKS. An experimental, NADPH-diaphorase histochemical and immunocytochemical study of Mesocestoides vogae tetrathyridia. Int J Parasitol. 1999;29:787–793. doi: 10.1016/s0020-7519(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Toner E, McConvery F, Brennan GP, Meaney M, Fairweather I. A scanning electron microscope study on the route of entry of triclabendazole into the liver fluke, Fasciola hepatica. Parasitology. 2008;136:523–535. doi: 10.1017/S0031182009005642. [DOI] [PubMed] [Google Scholar]
- Wiseman H, Duffy R. New advances in the understanding of the role of steroids and steroid receptors in disease. Biochem Soc Trans. 2001;29:205–209. doi: 10.1042/0300-5127:0290205. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Tangpu V. Anticestodal activity of Adhatoda vasica extract against Hymenolepis diminuta infections in rats. J Ethnopharmacol. 2008;119:322–324. doi: 10.1016/j.jep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Tangpu V. Anthelmintic activity of ripe fruit extract of Solanum myriacanthum Dunal (Solanaceae) against experimentally induced Hymenolepis diminuta (Cestoda) infections in rats. Parasitol Res. 2012;110:1047–1053. doi: 10.1007/s00436-011-2596-9. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Temjenmongla Efficacy of Lasia spinosa leaf extract in treating mice infected with Trichinella spiralis. Parasitol Res. 2012;110:493–498. doi: 10.1007/s00436-011-2551-9. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Temjenmongla In vivo anthelmintic activity of Clerodendrum colebrookianum Walp., a traditionally used taenicidal plant in Northeast India. Parasitol Res. 2012;111:1841–1846. doi: 10.1007/s00436-012-2908-8. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Tandon V, Rao HSP. In vitro anthelmintic activity of fresh tuber extract of Flemingia vestita against Ascaris suum. Fitoterapia. 1992;63:395–398. [Google Scholar]
- Yang W, Wang S, Li L, Liang Z, Wang L. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas. 2011;40:396–402. doi: 10.1097/MPA.0b013e318204e74d. [DOI] [PubMed] [Google Scholar]
- Yazdanyar A, Essmann M, Larsen B. Genistein effect on growth and cell cycle of Candida albicans. J Biomed Sci. 2001;8:153–159. doi: 10.1007/BF02256407. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li XL, Lai WP, Chen B, Chow HK, Wu CF, Wang NL, Yao XS, Wong MS. Anti-osteoporotic effect of Erythrina variegata L. in ovariectomized rats. J Ethnopharmacol. 2007;109:165–169. doi: 10.1016/j.jep.2006.07.005. [DOI] [PubMed] [Google Scholar]