Abstract
Cymothoid isopods (Crustacea, Isopoda) are considered as potential threat to the health of different fish species. In order to evaluate the prevalence and proximate analysis of Cymothoid isopods and its host, an investigation was carried out fish species belonging to families Hemiramphidae and Belonidae in the Palk Bay region, Southeastern India. A total of 1265 individuals of teleost fish belonging to family Hemiramphidae species, Hemiramphus far (462), H. archipelagicus (78), and H. lutkie (277) and another family Belonidae species, Tylosurus crocodilus (448), were examined for cymothoid ectoparasitic infestation. Prevalence in H. far was the highest (39%) for the cymothoid Mothocys plagulophora, while T. crocodilus was most infested (13%) with Mothocys renardi, H. far and H. lutkie were not infested by M. renardi while T. crocodilus was not infested by M. plagulophora. Proximate analysis showed reduced level of protein in parasite infested fish compared with non-infested individuals. However, carbohydrate and lipid concentrations were lower in infested fish than non-infested individuals. Proximate analysis values in the two parasites of Mothocys species were similar, and these values were comparable to those unaffected fish species indicating that parasites were well nourished. The proximate analysis of isopod parasite M. plagulophora showed 21.6 ± 7.7, 1.26 ± 0.05, 5.49 ± 1.06% of protein, carbohydrate and lipid respectively, and in M. renardi, 21.09 ± 6.6, 1.32 ± 0.12, 5.83 ± 0.72% of protein, carbohydrate and lipid respectively. Cadmium levels were similar between affected and non-affected fish individuals and among species. The Pb levels were comparable among all T. crocodilus individuals, but the levels of Cd not showed much variation between affected and unaffected individuals in all four fish species.
Keywords: Host parasite interaction, Proximate analysis, Mothocys plagulophora, M. renardi
Introduction
Cymothoid isopods are obligate fish parasites, occurring in all oceans with the exception of polar waters. It plays an important role in the food-web, in particular to removing the decaying materials from natural or altered environments. Cymothoids are largest parasites of fish and belonging to the genus Cymotha (Isopod: Cymothoidae). Cymothoids infest different parts of the host body such as buccal cavity, gill chamber, body surface, fins and some burrow inside the host and feed on the blood (Ravichandran et al. 2011). Sometimes, these parasites make large wounds and it leads to the death of the hosts (Lester and Roubal 2005). Considerable variations were noticed in the effect of isopod parasites on the marine fish Cheilodipterus quinquelineatus (Fogelman and Grutter 2008). Parasitic isopods are blood feeders, nutritionally dependent on their hosts and may affect points of infestation in the fish body. Damages include causing gill filament atrophy, removal of branchial arches, obstruction of the mouth cavity and destruction of the tongue, growth retardation, and weakened condition that often leads to death of the animal (Williams and Bunkley-Williams 1994; Bunkley-Williams and Williams 1998; Chavez-Lopez et al. 2005; Rhode 2005).
The cymothoids such as Mothocya plagulophora and M. renarde are highly host specific and occur in one or more host fish species (Poulin 2007). Studies on host–parasite interactions are the most important parasitological issues to fishery biologists. Inter-specific interactions among the parasites and fish are very common, but the information about interactions involving fish host and cymothoid parasitic isopods of the genus Mothocya are main scarce. In theory, cymothoid infestations involving blood and tissue consumption would decrease the nutritional quality of fish, and may lead to the retention of heavy metals in muscle tissues as normal metal excretion and detoxification mechanisms would have been impaired. The present study was aimed to determine the prevalence of parasitic cymothoid isopods in host fish species, and the proximate composition (including protein, lipid, carbohydrate and metals like cadmium and lead) of two cymothoids parasites and its four host fish from Kattumavadi coast, Southeasten India.
Materials and methods
Description of the study area
Kattumavadi is a significant coastal village (Lat. 10º4′N; Long. 79º12′E) in the Palk Bay region of the Pudukkottai District of Tamilnadu. The sea in this area appears very calm round the year with lesser tidal influence. There is a little discharges of water through a small river, Narasinga Cauvery only during the rainy season. Small scale fishing activities are carried out using wooden dugout canoes, rather than mechanized boats in the sampling area. The Kattumavadi coast is dominated by luxuriant growth of seagrasses, seaweeds and is devoid of rocks, corals or any other similar substrates (Fig. 1).
Fig. 1.
Map showing the sampling site of Kattumavadi coast, Southeast coast of India
Sample collection
Fish specimens from the families Hemiramphidae and Belonidae were collected from the Kattumavadi (Lat. 10o4′N; Long. 79o12′E) fish landing centre of Palk Bay region, Southeastern India during November 2013 to March 2014 (Fig. 1). The freshly dead fishes were collected and preserved using clean polythene bag containing water with broken pieces of ice and transported to the laboratory. Fish that could not be examined on the day of collection are placed in separate clean bags and kept in a deep freezer prior to examination of the next day. Individual fish was measured for total length (cm) and weight (g) using standard measuring scale and weighing balance, examined for ectoparasites and classified into parasite-affected and parasite-unaffected fishes. The host fish species were identified using FAO fish identification manuals and the FISHBASE database (www.fishbase.org). The external surface of the fishes such as the fins and skins were examined thoroughly using a hand lens. The eyes, skin, fins, gills, nostrils, and mouth cavity of each fish specimens were examined for the ectoparasites. The parasites recovered are washed free of debris in 95% Ethanol. The number of parasites recovered from the different parts of each fish was recorded. Parasitic isopod species were identified using the standard works (Haller 1880; Nair 1950; Bruce 1986; Trilles 1994; Gopalakrishnan et al. 2010; Rameshkumar et al. 2014).
Biochemical constituents
The parasite and host fish muscle tissues were dissected aseptically and homogenized manually. Then the samples were transferred to desiccators after the complete removal of moisture by using hot air oven for 24 h at 60 °C. Then the samples were weighed and grinded using mortar and pestle for further biochemical analysis using standard methods. The biochemical constituents, such as protein (mg/g), lipid (mg/g) and carbohydrate (mg/g) were estimated using the standard methods (Lowry et al. 1951; Folch et al. 1957; Dubois et al. 1956).
Determination of heavy metals
Fish samples were cleaned with sterile distilled water and then dissected aseptically. Two grams of fresh muscle tissue near the gill area of the fish were removed for the elemental analysis of cadmium and lead. For the estimation of Cd and Pb content, two grams of muscle tissue were placed in a 100-ml Borosil beaker, and were digested completely with 2 ml of HNO3 and 1 ml of HClO4 with the beaker heated on a hot plate at 100 ◦C. Complete digestion of organic matter by heated acids was ensured as trace of organic matter interferes metal analysis (Khandekar et al. 1984; Raghunath et al. 1997). A few drops of hydrochloric acid were added to the digested sample to further achieve complete digestion. At the end of digestion, samples were made up to 25 ml with metal free double distilled water and filtered through Whatman filter paper No. 42. The metals present in extracts of fish and parasites were analyzed using Flame Atomic Absorption Spectrometry (GBC HG 3000; Sens AA, Australia 2009). The working wave lengths for Cd and Pb were 228.8 and 283.31 nm respectively. Quality assurance testing depends on the control of blanks and yield for chemical procedure. For quality assurance, blanks, standardized reference materials and replicate samples were used during analysis. The standards of the metal samples were prepared by serial dilution using deionized water achieving of 0.5–7 ppm that was run to check the precision of the instrument throughout the analysis.
Data analysis
All the dataset was processed in SPSS for Windows version 11 software (SPSS Inc. 2002). One-way ANOVA was used to test the statistical difference of proximate analysis values among fish species and between affected and non-affected fish individuals at 0.05 levels.
Results and discussion
Prevalence
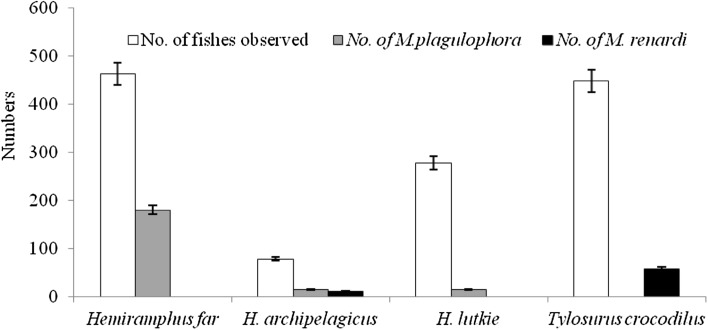
In the present study, 1265 specimens belonging to H. far (462), H. archipelagicus (78), H. lutkie (277) and T. crocodilus (448) were investigated for cymothoid parasitic infestation in 2 years. The results from this study depict that H. far was most infested with M. plagulophora (Fig. 2a), while T. crocodiles was most infested with M. renardi (Fig. 2b), and Table 1 showing differential infestation pattern. Interestingly, H. far and H. lutkie were not infested by M. renardi. In T. crocodilus, 58 specimens were infested by M. renardi out of 448 specimens examined. There is no M. plagulophora infestation on T. crocodilus (Fig. 3). The prevalence and intensity of M. plagulophora is more common in H. far compared to other host fish. A similar result was also reported (Rameshkumar et al. 2013). The prevalence of M. plagulophora infection in four different fishes like H. far, H. archipelagicus, H. lutkie and T. crocodilus were 39, 15, 14 and 0% whereas M. renardi showed 0, 14, 0 and 13%, respectively (Table 1). The main factors like size, sex, maturity, feeding and breeding behavior, life cycle and environmental conditions determining the fish parasite fauna as well as intensity and prevalence of infestation in marine environments were reported (Radhakrishnan and Nair 1983; Bharadhirajan et al. 2014).
Fig. 2.
a Hemiramphus archipelagicus is infested by isopode parasite Mothocys renardi. b Hemiramphus far is infested by isopode parasite Mothocys plagulophora
Table 1.
Prevalence of isopods parasites in marine fishes collected from Palk bay
| Name of the host | No. of examined fishes | Total length (cm) | Total weight (g) | No. of infected fishes by M. plagulophora | % of prevalence | No. of infected fishes by M. renardi | % of prevalence |
|---|---|---|---|---|---|---|---|
| Hemiramphus far | 462 | 75–80 | 250–300 | 180 | 39 | 0 | 0 |
| H. archipelagicus | 78 | 80–85 | 400–520 | 15 | 19 | 11 | 14 |
| H. lutkie | 277 | 60–70 | 100–160 | 14 | 5 | 0 | 0 |
| Tylosuruscrocodilus | 448 | 90–95 | 410–550 | 0 | 0 | 58 | 13 |
Fig. 3.
Abundance of cymothoid parasites and its host fishes
Proximate analysis
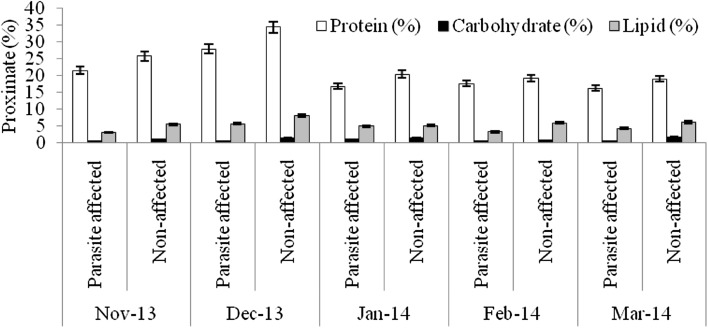
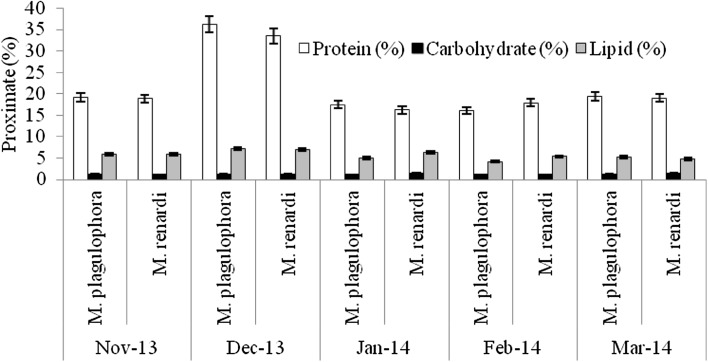
Proximate composition of parasites and parasite-infected and uninfected host fish were studied. Protein levels were reduced in affected fish but values were not significantly different from those in unaffected individuals (F = 1.57–2.85, p > 0.05 for all fish species). The results showed results of one-way ANOVA were shown in Table 2. In contrast, carbohydrate and lipid content in infested fish were significantly much reduced at 0.05 level (F = 6.60–22.70, p < 0.05 for all fish species) when compared to uninfected fish (Figs. 4, 5, 6, 7). The proximate analysis of isopod parasite M. plagulophora showed protein (21.66 ± 7.79%), carbohydrate (1.25 ± 0.05%) and lipid (5.49 ± 1.06%), and in M. renardi protein (21.09 ± 6.6%), carbohydrate (1.32 ± 0.12%) and lipid (5.83 ± 0.72%) respectively (Fig. 8). These values are similar to those in unaffected fish individuals of all four fish species indicating that parasites obtain proper nourishment from hosts. Parasites have been responsible for delay in fish growth and gain of weight by affecting the food ingestion (Barber et al. 2000; Barker et al. 2005). The results of the biochemical analyses revealed that the fishes were seriously affected by the parasite. The amounts of fundamental biomolecules in tissues of infested fish were reduced which coincides with the observation that parasites generally cause a decrease of organic constituents such as protein, carbohydrate and lipid (Love 1970).
Table 2.
Correlation co efficiency matrix of proximate levels among the affected and non-affected fishes
| Parameters | Cadmium (ppm) | Lead (ppm) | Protein (%) | Carbohydrate (%) | Lipid (%) |
|---|---|---|---|---|---|
| Affected | |||||
| Cadmium (ppm) | 1 | ||||
| Lead (ppm) | − 0.92 | 1 | |||
| Protein (%) | − 0.53 | 0.52 | 1 | ||
| Carbohydrate (%) | 0.66 | − 0.90 | − 0.47 | 1 | |
| Lipid (%) | 0.68 | − 0.80 | 0.09 | 0.75 | 1 |
| Non-affected | |||||
| Cadmium (ppm) | 1 | ||||
| Lead (ppm) | − 0.81 | 1 | |||
| Protein (%) | − 0.49 | 0.51 | 1 | ||
| Carbohydrate (%) | 0.56 | − 0.46 | 0.42 | 1 | |
| Lipid (%) | 0.19 | − 0.62 | 0.24 | 0.59 | 1 |
Fig. 4.
Proximate analysis of Hemiramphus far
Fig. 5.
Proximate analysis of Hemiramphus lutkie
Fig. 6.
Proximate analysis of Hemiramphus archipelagicus
Fig. 7.
Proximate analysis of Tylosurus crocodilus
Fig. 8.
Proximate analysis of fish parasites
Heavy metals concentration
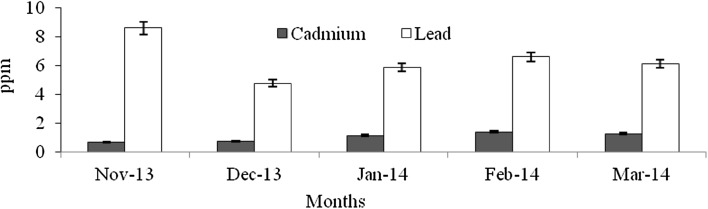
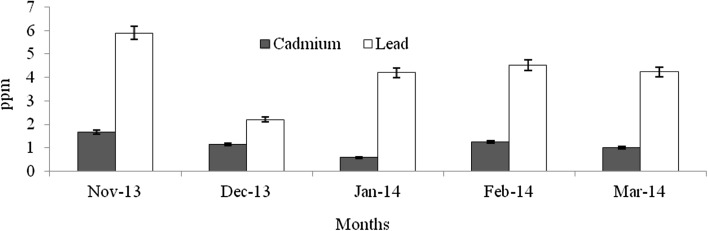
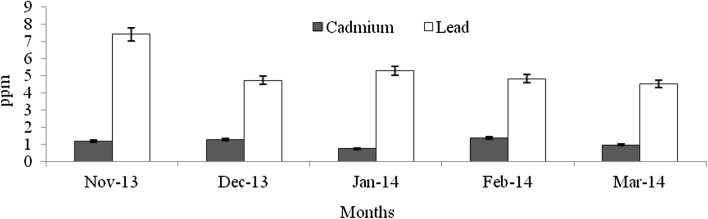
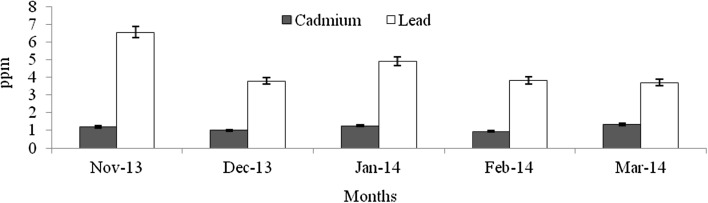
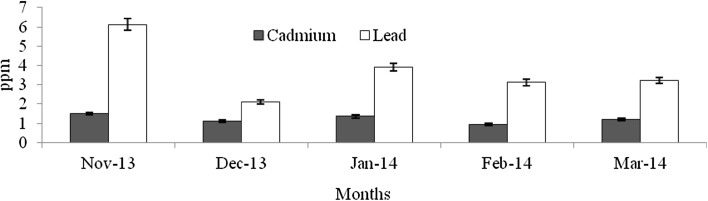
Heavy metals are natural trace components of the marine environment, but they constitute one of the most hazardous substances that could be accumulated in biota. Metals can be taken up by fish from water, food, sediment and suspended particulate material (Agusa et al. 2005). Pb and Cd belong to non-essential and toxic metals group, and Pb has no known function in biochemical processes (Ozparlak et al. 2012). These metals have a high potential for bioconcentration in fish (Wegner and Boman 2003). In the present study, the levels of Cd was not significantly varied among the infested and non-infested fish species (F = 0.004–0.28, p > 0.05) (Figs. 9, 10, 11, 12, 13, 14, 15, 16, 17) except T. crocodiles (F = 20.33; p = 0.002). The concentrations of Pb in all four species of host fishes were higher than those of Cd, but Pb levels were significantly higher in affected than in unaffected individuals for all three species (F = 0.09–6.75, p > 0.05) except H. far (F = 6.75; p = 0.03). Levels of Pb were similar in all individuals of T. crocodilus. The minimum and maximum accumulation of Pb in parasite infested T. crocodilus and H. far was 3.7 and 8.6 ppm, respectively. In contrast, the minimum and maximum accumulations of Pb in parasite non-infested fish were 2.12 and 6.34 ppm by T. crocodilus and H. archipelagicus, respectively. The bioconcentration of Cd in parasite-infested fish was 0.47 ppm (H. lutkie) to 1.4 ppm (H. far), but those in non-infested fishes were slightly elevated ranging from 0.58 to 1.68 ppm for H. lutkie. Higher Pb concentrations in parasite-infested fish compared to non-infested fish is due most likely to the health condition of the fish. Healthy non-infested fish detoxify the bioaccumulation of metals in tissue by normal metabolic pathways, active swimming, fast and easy exchange of gases through gills, and normal functioning of digestive and excretory systems (Dural et al. 2011). The correlation co-efficiency results showed that the inter relationships of proximate components among the different groups. Table 3 shows the strong negative relationship was observed between the levels of cadmium—lead (r = − 0.92), carbohydrate—lead (r = − 0.89) and lipid—lead (r = − 0.80) for affected fishes significant at the 0.05 level. But the kind of relationships were dramatically reduced for unaffected fishes, the near negative correlation was observed for cadmium—lead (r = − 0.80), carbohydrate—lead (r = − 0.46) and lipid—lead (r = − 0.61). These results cleary indicated that the parasite infections were influences the level of proximate concentrations of both affect and unaffected fishes. Infested fish may have impaired heavy metal detoxification capacities. Although, the accumulation of metals by the fish depends on the habit, feeding behaviour, trophic level, age, size, duration of exposure to metals and homeostatic regulation activities of fish (Peakall and Burger 2003). Levels of Cd and Pb were an order of magnitude lower than those in fish indicating healthy and unimpaired heavy metals excretion mechanisms.
Fig. 9.
Concentration of Cd and Pb in parasite affected H. far
Fig. 10.
Concentration of Cd and Pb in non-affected H. far
Fig. 11.
Concentration of Cd and Pb in parasite affected H. lutkie
Fig. 12.
Concentration of Cd and Pb in non-affected H. lutkie
Fig. 13.
Concentration of Cd and Pb in parasite affected H. archipelagicus
Fig. 14.
Concentration of Cd and Pb in non-affected H. archipelagicus
Fig. 15.
Concentration of Cd and Pb in parasite affected Tylosurus crocodilus
Fig. 16.
Concentration of Cd and Pb in parasite non-affected T. crocodilus
Fig. 17.
Concentration of Cd and Pb in parasites
Table 3.
One-way ANOVA results of proximate levels among the affect and non-affected fishes
| Proximate | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Protein | 2.15 | 0.173 | 2.4 | 0.152 | 1.57 | 0.239 | 2.85 | 0.122 |
| Carbohydrate | 11.98 | 0.006 | 18.64 | 0.002 | 22.55 | 0.001 | 6.60 | 0.028 |
| Lipid | 22.7 | 0.001 | 8.99 | 0.013 | 10.65 | 0.009 | 14.54 | 0.003 |
| Cadmium | 0.28 | 0.611 | 0.004 | 0.954 | 0.07 | 0.786 | 20.33 | 0.002 |
| Lead | 6.75 | 0.032 | 3.64 | 0.093 | 2.14 | 0.181 | 0.97 | 0.352 |
p = Significant difference; F = F statistic; Bolded values an indicatives of significance difference at 0.05 level
1 = Hemiramphus far; 2 = Hemiramphus lutkie; 3 = Hemiramphus archipelagicus; 4 = Tylosurus crocodiles
Conclusion
Both the parasite M. plagulophora and M. renardi infestation was found more on H. archipelagicus is due to high level of protein (20.07 ± 4.61%) and lipid (4.31 ± 1.02%) present in the tissue samples. The T. crocodilus was more infested by M. renardi is due to high amount of carbohydrate (0.83 ± 0.36%) reported in tissues. The parasite M. renardi was not found in H. far and H. lutkie and M. plagulophora was not infested in T. crocodilus. Although protein levels were reduced in affected fish individuals, values were still comparable to those in unaffected individuals. Carbohydrate and lipid were significantly reduced in parasitized individuals for all fish species. The concentration of Pb in parasite-infested fish was higher compared to those in non-infested fish in all three Hemiramphid species, but this was not observed in T. crocodilus. Levels of Cd did not differ between affected and unaffected individuals in all four fish species. Therefore, present study indicates that infestation of these two parasites can reduce the biochemical composition of fish species.
Acknowledgements
Authors are thankful to authorities of Bharathidasan University for the facilities provided and also grateful to Dr. S. Ravichandran, Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, India for identification of parasitic isopod species.
Author’s contribution
Conceived and designed the experiments: RR, SV. Performed the experiments: RR, RK and SV. Analyzed the data and wrote the paper: RR and EPM.
Compliance with ethical standards
Conflict of interest
All the authors declare that there is no conflict of interest regarding publication of the articles.
Ethical statements
The four species of marine food fishes viz. Hemiramphus far, H. archipelagicus, H. lutkie and Tylosurus crocodilus belonging to families Hemiramphidae and Belonidae are not governed by any law. Therefore, the organisms were not placed for Institutional Animal Ethical Committee (IAEC) approval. All the experiments were performed for best practice according to Bharathidasan University IAEC guidelines. Furthermore, we did not conduct any experimentation with human subjects.
References
- Agusa T, Kunitob T, Yasunaga G, Iwataa H, Subramanian A, et al. Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar Pollut Bull. 2005;51:896–911. doi: 10.1016/j.marpolbul.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Barber I, Hoare D, Krause J. Effects of parasites on fish behavior: a review and evolutionary perspective. Rev Fish Biol Fish. 2000;10:131–165. doi: 10.1023/A:1016658224470. [DOI] [Google Scholar]
- Barker DE, Cone DK, Burt MD. Trichodina murmanica (Ciliophora) and Gyrodactylus pleuronecti (Monogenea) parasitinzing haychery-reared winter flounder, Pseudopleuronectes americanus (Walbaum): effects on host growth and assessment of parasite interaction. J Fish Dis. 2005;25:81–89. doi: 10.1046/j.1365-2761.2002.00341.x. [DOI] [Google Scholar]
- Bharadhirajan P, Murugan S, Sakthivel A, Selvakumar P. Isopod parasites infection on commercial fish of Parangipettai waters, Southeast coast of India. Asian Pac J Trop Dis. 2014;4(1):S268–S272. doi: 10.1016/S2222-1808(14)60453-9. [DOI] [Google Scholar]
- Bruce NL. Revision of the isopod crustacean genus Mothocya Costa, in Hope, 1851, (Cymothoidae: Flabellifera), parasitic on marine fish. J Nat Hist. 1986;20:1089–1192. doi: 10.1080/00222938600770781. [DOI] [Google Scholar]
- Bunkley-Williams L, Williams EH., Jr Isopods associated with fish: a synopsis and corrections. J Parasitol. 1998;84:893–896. doi: 10.2307/3284615. [DOI] [PubMed] [Google Scholar]
- Chavez-Lopez R, Rocha-Ramirez A, Alvarez F, Wetzer R. Elthusa alvaradoensis Rocha-Ramirez, Chavez-Lopez & Bruce, 2005 (Isopoda; Cymothoidae) parasitizing the inshore lizard fish Synodus foetens (Linnaeus, 1766) on the continental shelf off central Vera Cruz, Mexico. Crustaceana. 2005;78:865–872. doi: 10.1163/156854005774445456. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smity F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Dural Mettem, Ercument Gene M, Sangum Kemal, Gunei Ozlem. Accumulation of some heavy metals in Hysterothylacium aduncum (Nematoda) and its host sea bream, Sparus aurata (Sparidae) from North–Eastern Mediterranean Sea (Iskenderun Bay) Environ Monit Asses. 2011;174:147–155. doi: 10.1007/s10661-010-1445-0. [DOI] [PubMed] [Google Scholar]
- Fogelman R, Grutter AS. Mancae of the parasitic isopod, Anilocra apogonae: early life history, host-specificity, and effect on growth and survival of preferred young cardinal fish. Coral Reefs. 2008;27(3):685–693. doi: 10.1007/s00338-008-0379-2. [DOI] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipid from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gopalakrishnan A, Rajkumar Sun Jun M, Trilles JP. Occurrence of double parasitism on balck-barred halfbeak fish from the southeast coast of India. Chin J Oceanol Limnol. 2010;28(4):832–835. doi: 10.1007/s00343-010-9919-z. [DOI] [Google Scholar]
- Haller G. Über einige neue Cymothoinen. Archiv fur Naturgeschichte, Berlin, Jahrsbuch. 1880;46:375–395. [Google Scholar]
- Khandekar RN, Mishra UC, Vohra KG. Environmental lead exposure of anurban Indian population. Sci Total Environ. 1984;40:269–278. doi: 10.1016/0048-9697(84)90356-5. [DOI] [PubMed] [Google Scholar]
- Lester RJG, Roubal FR. Isopod. In: Rohde K, editor. Marine parasitology. Collingwood: CSIRO Publishing; 2005. pp. 138–144. [Google Scholar]
- Love RM. The chemical biology of fish. London: Academic Press; 1970. pp. 17–35. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-Phenol reagents. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nair GS. Two new species of Irona (Isopoda) parasitic on Madras fish. J Madras Univ. 1950;20B:66–74. [Google Scholar]
- Ozparlak Haluk, Arslan Gulsin, Arslan Emine. Determination of some metal levels in muscle tissues of nine fish species from Beysehier Lake, Turkey. Turk J Fish Aquat Sci. 2012;12:761–770. doi: 10.4194/1303-2712-v12_4_04. [DOI] [Google Scholar]
- Peakall D, Burger J. Methodologies for assessing exposure to metals: speciation, bioavailability of metals & ecological host factors. Ecotoxicol Environ Saf. 2003;56:110–121. doi: 10.1016/S0147-6513(03)00055-1. [DOI] [PubMed] [Google Scholar]
- Poulin R. Evolutionary ecology of parasites. London: Chapman and Hall; 2007. [Google Scholar]
- Radhakrishnan S, Nair NB. Nature of crustacean infestation of fish along the south-west coast of India; distribution, mode of attachment to the host tissue and incidence and intensity of infestation. Acta Ichthyol Piscat. 1983;13(2):93–115. doi: 10.3750/AIP1983.13.2.06. [DOI] [Google Scholar]
- Raghunath R, Tripathi RM, Khandekar RN, Nambi KSV. Retention times of Pb, Cd, Cu and Zn in children’s blood. Sci Total Environ. 1997;207:133–139. doi: 10.1016/S0048-9697(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Rameshkumar G, Ramesh M, Ravichandran S, Trilles JP, Subbiah S. Host-parasite relationships: Mothocya plagulophora parasitizing Hemiramphus far in the Southeast coast of India. J Parasit Dis. 2014 doi: 10.1007/s12639-014-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshkumkar G, Ravichandran S, Sivasubramanian K. Invasion of parasitic isopods in marine fish. J Coast Life Med. 2013;1(2):88–94. [Google Scholar]
- Ravichandran S, Rameshkumar G, Trilles JP. New records of two parasitic cymothoids from Indian fish. J Parasit Dis. 2011;35(2):232–234. doi: 10.1007/s12639-011-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode K, editor. Marine parasitology. Victoria: CSIRO Publishing and CABI Publishing; 2005. p. 592. [Google Scholar]
- Trilles JP. Les Cymothoidae du Monde. Prodrome pour une Faune. Stud Mar. 1994;21/22:1–288. [Google Scholar]
- Wegner A, Boman J. Biomonitoring of trace elements in muscle and liver tissues of freshwater fish. Spectrochim Acta Part B. 2003;58:2215–2226. doi: 10.1016/j.sab.2003.05.003. [DOI] [Google Scholar]
- Williams EH, Jr, Bunkley-Williams L. Four cases of unusual crustacean–fish associations and comments on parasitic processes. J Aquat Anim Health. 1994;6:202–208. doi: 10.1577/1548-8667(1994)006<0202:FCOUCF>2.3.CO;2. [DOI] [Google Scholar]