Abstract
Objective: To investigate the effect of tea polyphenols on cardiac function in rats with diabetic cardiomyopathy, and the mechanism by which tea polyphenols regulate autophagy in diabetic cardiomyopathy. Methods: Sixty Sprague-Dawley (SD) rats were randomly divided into six groups: a normal control group (NC), an obesity group (OB), a diabetic cardiomyopathy group (DCM), a tea polyphenol group (TP), an obesity tea polyphenol treatment group (OB-TP), and a diabetic cardiomyopathy tea polyphenol treatment group (DCM-TP). After successful modeling, serum glucose, cholesterol, and triglyceride levels were determined; cardiac structure and function were inspected by ultrasonic cardiography; myocardial pathology was examined by staining with hematoxylin-eosin; transmission electron microscopy was used to observe the morphology and quantity of autophagosomes; and expression levels of autophagy-related proteins LC3-II, SQSTM1/p62, and Beclin-1 were determined by Western blotting. Results: Compared to the NC group, the OB group had normal blood glucose and a high level of blood lipids; both blood glucose and lipids were increased in the DCM group; ultrasonic cardiograms showed that the fraction shortening was reduced in the DCM group. However, these were improved significantly in the DCM-TP group. Hematoxylin-eosin staining showed disordered cardiomyocytes and hypertrophy in the DCM group; however, no differences were found among the remaining groups. Transmission electron microscopy revealed that the numbers of autophagosomes in the DCM and OB-TP groups were obviously increased compared to the NC and OB groups; the number of autophagosomes in the DCM-TP group was reduced. Western blotting showed that the expression of LC3-II/I and Beclin-1 increased obviously, whereas the expression of SQSTM1/p62 was decreased in the DCM and OB-TP groups (P<0.05). Conclusions: Tea polyphenols had an effect on diabetic cardiomyopathy in rat cardiac function and may alter the levels of autophagy to improve glucose and lipid metabolism in diabetes.
Keywords: Tea polyphenol, Autophagy, Diabetic cardiomyopathy, Obesity, Lipid metabolism disorder
1. Introduction
Diabetic cardiomyopathy is distinguished by diastolic relaxation abnormalities in the early stage and by clinical heart failure later without coronary artery disease, hypertension, or other potential etiological conditions (Chung et al., 2017). With changes in lifestyle and diet, the rate of incidence has become higher and higher. Although the pathophysiology of diabetic cardiomyopathy has been widely researched, it is still only partly understood (Ding S et al., 2017). Alterations in energy metabolism, lipid metabolism disorder, glucotoxicity with intervention of advanced glycation end products, increased oxidative stress, and insulin resistance all lead to damaged myocardial function in diabetics (Kanamori et al., 2015). Autophagy always begins with the engulfment of lipid droplets, ribosomes, soluble proteins, or organelles in a double membrane, and when combined with lysosomes, continued enzymolysis occurs (Wang et al., 2009). The products of autophagy, basic new nutrients, such as lipids, amino acids, and sugars, are then transported into the cytoplasm, where they may be used as a source of energy (Zhang et al., 2016). Enhanced autophagy plays a relatively protective role in the heart function of diabetic cardiomyopathy under the circumstances of energy metabolism disorder (Fahie and Zachara, 2016; Sun et al., 2016).
Polyphenolic compounds extracted from tea plants (Camellia sinensis) have the advantage of a curative effect against various types of cancers, cardiovascular problems, and atherosclerosis (Shin et al., 2016). Several studies (Nazio and Cecconi, 2017; Ruiz et al., 2017) have indicated that tea polyphenols regulate insulin signal transduction pathways and the AMP-activated protein kinase (AMPK) pathway of multiple molecularly targeted phosphorylation levels, intervening in mRNA and protein expression of adiponectin to improve myocardial glycolipid energy metabolism (Lin et al., 2017). Further research also showed that tea polyphenols can regulate autophagy levels via the mammalian target of the rapamycin (mTOR) pathway (Trivedi et al., 2016) or mediate levels via the protein kinase B (PKB) signaling pathway (Velázquez et al., 2016). As the active ingredient, gallic catechin gallic acid ester can induce autophagy in ovarian cancer SKOV3 cells (Yang et al., 2017).
In this study, we aimed, through establishment of a diabetic cardiomyopathy model, to explore the role of tea polyphenols in the regulation of autophagy in diabetic cardiomyopathy, providing a new method for treatment. The possible underlying mechanisms are also discussed.
2. Materials and methods
2.1. Tea polyphenol preparation
At room temperature, a total of 1000 g of tea leaves were extracted with 8 L of 75% ethanol for 3 h. The ethanol was evaporated in a rotary evaporator at 40 °C. The dried extract was extracted with dichloromethane to remove caffeine. It was then transferred to an XAD-16 resin column, rinsed with five bed volumes of water, and then eluted with pure ethanol. Finally, the extract was dried using the rotary evaporator.
2.2. Reagents
Monoclonal rabbit antibodies, including anti-SQSTM1/p62 and Beclin-1, were obtained from Cell Signalling Technology (MA, USA). The dilution ratio was 1:1000 (5 μl/5 ml). A polyclonal rabbit anti-LC3 antibody was obtained from Sigma (St. Louis, MO, USA). The dilution ratio was 1:3000 (1.7 μl/5.1 ml). A horseradish peroxidase (HRP)-marked anti-GAPDH antibody was purchased from Kangchen (Shanghai, China). The dilution ratio was 1:1000 (5 μl/5 ml).
2.3. Rat grouping, modeling, and treatment
Six-week-old male Sprague-Dawley (SD) rats (weight, (200±20) g) were purchased from the Animal Experimental Center of Zhejiang Chinese Medical University and housed at room temperature.
Sixty male SD rats were housed under specific pathogen-free (SPF) conditions and divided into six groups. Ten rats were fed a normal laboratory diet as control group (NC). Ten rats were fed a high-fat diet (every 100 g contained 20 g whole milk powder, 10 g lard, and 40 g egg yolk) for eight weeks. Rats weighing 20% more than those on the normal diet were included in the obese rat model group (OB group). Based on the obese rat model, one week after intraperitoneal injection with streptozotocin (STZ, 30 mg/kg, Sigma, USA), rats with a blood glucose level of 16.7 mmol/L or higher and an ejection fraction detected by echocardiography lower than 40% were assigned to the diabetic cardiomyopathy group (DCM group). Ten rats fed ordinary food for one week and then large doses of tea polyphenols (400 mg/(kg∙d)) for eight weeks were included in the tea polyphenol group (TP group). This tea polyphenol group modeling was applied to OB and DCM modeled rats, to produce the OB tea polyphenol intervention group (OB-TP group) and the DCM tea polyphenol intervention group (DCM-TP group), respectively. Body weight was recorded weekly.
2.4. Serum biochemical detection
Blood glucose was measured by tail vein puncture to a glucometer purchased from Roche Group (Basle, Switzerland). Serum biochemical indicators, such as triglyceride (TG), total cholesterol (TC), and low-and high-density lipoprotein-cholesterol (LDL-C/HDL-C), were tested by a routine diagnostic analyzer (Hitachi 7600, Japan) using enzymatic colorimetric assays before the rats were sacrificed.
2.5. Measurements of cardiac and hemodynamic functions in vivo
According to previously described techniques (Lai et al., 2017), transthoracic echocardiography was performed using a Vivid Sonic 2100 echocardiography imaging system (GE, USA) equipped with a 21-MHz probe.
The animals were anesthetized with isoflurane. Left ventricular (LV) systolic function was compared by the left ventricular fraction shortening (FS):
FS=(LVEDD−LVESD)/LVEDD×100%,
where LVEDD is the left ventricular end-diastolic diameter and LVESD is the left ventricular end-systolic diameter.
2.6. Hematoxylin-eosin staining
The heart of each rat was excised, washed three times using saline solution, fixed in 10% neutral-buffered formalin, then embedded in paraffin for cutting into 4-μm sections. The sections were stained with hematoxylin-eosin for histochemistry. All histopathological characteristics, such as intercellular spaces, inflammatory cell infiltration, and edema of cardiomyocytes, were assessed using a microscope in a blinded fashion (Fujifilm, Tokyo, Japan).
2.7. Electron microscopy
The myocardial tissue was cut into 1-mm3 pieces, and fixed with 2.5% glutaraldehyde overnight at 4 °C. The tissues were then immersed in 1% osmium tetroxide for 2 h, dehydrated in graded ethanol, and then embedded in epoxy resin. An ultramicrotome was used to incise the tissues into ultrathin sections (60–70 nm). The sections were post-stained with uranyl acetate and lead citrate for electron microscopic analysis.
2.8. Western blot analysis
After each rat heart was excised, the left ventricle of the heart tissues was homogenized with phosphatase inhibitors and protease inhibitors, and then lysed on ice for solubilizing in lysis buffer for 30 min. The total protein concentration was determined using a bicinchoninic acid (BCA) Protein Assay kit (Biyuntian, Hangzhou, China). Equal sample concentrations of denatured total protein were separated by 15% (w/v) and 10% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in a Tris-buffered solution (10 mmol/L Tris-HCl (pH 7.6), 100 mmol/L NaCl, and 0.1% Tween 20) for 2 h at room temperature. The membranes were incubated with primary antibody overnight at 4 °C. After being washed three times, the membranes were incubated with horseradish peroxidase (HPR)-conjugated secondary antibodies. Finally, the membranes were detected by enhanced chemiluminescence (ECL) reagents (Amersham, Haemek, Israel) and scanned by Image Quant LAS (Bioscience, Buchinghamshire, UK). The grayscale value was determined using imaging software J2x (Rawak Software, Germany).
2.9. Statistical analysis
Data from at least three independent experiments are expressed as the mean±standard error of the mean (SEM). Statistical comparisons among different groups were performed by one-way analysis of variance (ANOVA). The level of statistical significance was set at P<0.05.
3. Results
3.1. Changes of body weight in each group
There were no differences in initial weight among groups, because all rats were assigned randomly. After twelve weeks, mean body weights began to diverge, and rats in the OB group were significantly heavier than those in the other groups. The mean body weight of the DCM-TP group decreased between 12 and 16 weeks. At the end of study, the OB-TP group showed a much smaller increase in weight than the OB group, while the DCM group weighed the least. The mean body weights of the NC, TP, and DCM-TP groups were much the same (Fig. 1).
Fig. 1.
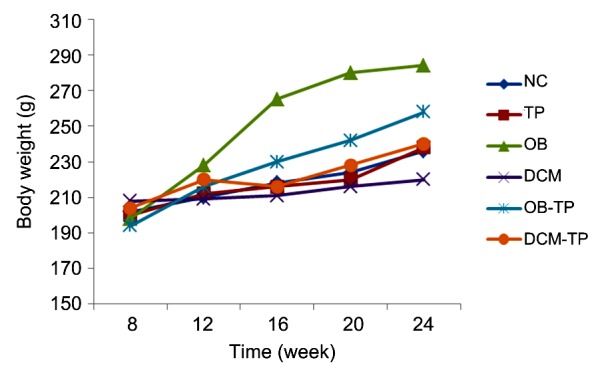
Mean body weight in each group over time
NC: normal control group; TP: tea polyphenol group; OB: obesity group; DCM: diabetic cardiomyopathy group; OB-TP: obesity tea polyphenol treatment group; DCM-TP: diabetic cardiomyopathy tea polyphenol treatment group. Values are expressed as mean±standard error (SE) (n=6–10)
3.2. Effects of tea polyphenols on blood glucose and lipid levels in diabetic cardiomyopathy rats
Comparisons of serum biochemical markers among the groups showed that, compared to the NC group, both the OB group and the DCM group had high levels of TG, TC, and LDL-C; glucose increased significantly in the DCM group compared to the NC group (P<0.05). Compared to the OB group, the OB-TP group had low levels of TG, TC, and LDL-C. This indicated that tea polyphenols had the effect of reducing blood lipids. The levels of glucose, TG, TC, and LDL-C in the DCM-TP group were significantly lower than those in the DCM group, indicating that tea polyphenols had a dual effect of lowering glucose and lowering blood lipids (P<0.05) in this group (Table 1).
Table 1.
Contents of glucose, TC, TG, LDL-C, and HDL-C in each group
| Group | Concentration (mmol/L) |
||||
| Glucose | TC | TG | LDL-C | HDL-C | |
| NC | 6.32±0.29 | 1.59±0.23 | 0.80±0.08 | 1.30±0.31 | 0.68±0.11 |
| OB | 18.85±0.49* | 4.72±0.35* | 12.53±0.95* | 23.74±1.17* | 1.63±0.10* |
| DCM | 18.15±1.03* | 4.65±0.50* | 15.56±0.23* | 26.98±1.34* | 1.89±0.11* |
| OB-TP | 7.48±0.55# | 2.11±0.21# | 7.11±0.89# | 15.40±0.75# | 3.60±0.20# |
| DCM-TP | 14.35±0.86▲ | 2.79±0.18▲ | 9.11±0.19▲ | 16.31±0.82▲ | 3.07±0.12▲ |
Values are expressed as the mean±SEM (n=6).
P<0.05 compared to the NC group,
P<0.05 compared to the OB group,
P<0.05 compared to the DCM group. NC: normal control group; OB: obesity group; DCM: diabetic cardiomyopathy group; OB-TP: obesity tea polyphenol treatment group; DCM-TP: diabetic cardiomyopathy tea polyphenol treatment group; TC: total cholesterol; TG: triglyceride; LDL-C/HDL-C: low and high density lipoprotein-cholesterol
3.3. Effects of tea polyphenols on myocardial structure and function in diabetic cardiomyopathy
Our pathology experiment results showed that the architecture of the myocardial fibers in the NC group and TP group rats was normal, with cardiomyocytes arranged closely and regularly, and the nuclei were clearly visible. However, in the DCM group, cardiomyocytes presented extensive edema, enlarged intercellular spaces, and the nuclei were not clear or were even fused. Clearly, after treatment with tea polyphenols, the changes in the myocardium were markedly relieved, and cardiomyocytes presented mild edema and showed a more regular arrangement (Fig. 2).
Fig. 2.
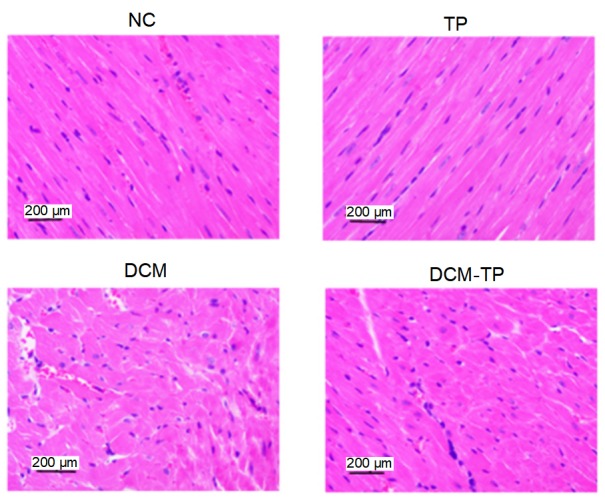
Effects of tea polyphenols on histology shown by hematoxylin-eosin staining
NC: normal control group; TP: tea polyphenol group; DCM: diabetic cardiomyopathy group; DCM-TP: diabetic cardiomyopathy tea polyphenol treatment group
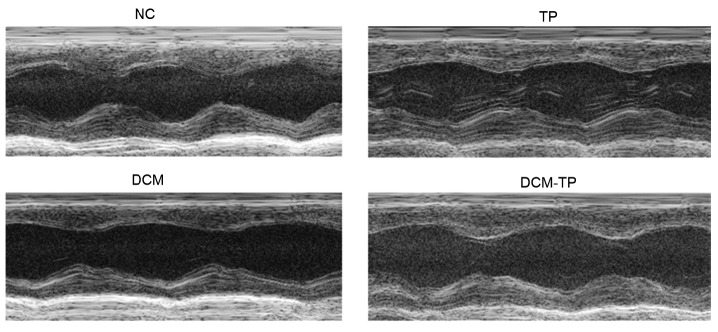
Our analysis of cardiac function at the endpoint, via echocardiography, indicated that compared with the NC group, the DCM group rats showed decreases in FS indicative of significant cardiac dysfunction. In the TP group, these indicators were unchanged. However, compared to the DCM group, FS was improved. Thus, tea polyphenols can improve the heart function of diabetic cardiomyopathy rats (Fig. 3).
Fig. 3.
Echocardiography results for each group
NC: normal control group; TP: tea polyphenol group; DCM: diabetic cardiomyopathy group; DCM-TP: diabetic cardiomyopathy tea polyphenol treatment group
3.4. Effect of tea polyphenols on autophagy levels in rats in a high-fat state
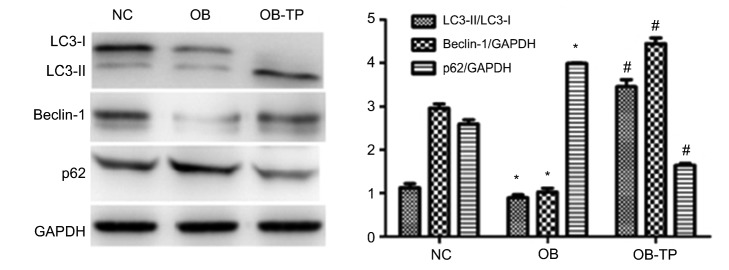
To determine whether tea polyphenols can affect the level of autophagy in a high-fat state, we used antibodies to detect protein expression. Western blotting showed that compared to the NC group, the expression of autophagy-related proteins, LC3-II/LC3-I and Beclin-1, was notably decreased, whereas the expression of SQSTM1/p62 was increased in the OB group. This indicated that autophagy can be inhibited under a high-fat state. Furthermore, compared to the OB group, we found that the level of Beclin-1 and the ratio of LC3-II to LC3-I in the OB-TP group were increased, and the level of SQSTM1/p62 was decreased. These results showed that tea polyphenols can induce autophagy in rats in a high-fat state (Fig. 4).
Fig. 4.
Autophagy induced by tea polyphenols in rats in a high-fat state
Values are expressed as the mean±SEM (n=6). * P<0.05 compared to the NC group; # P<0.05 compared to the OB group
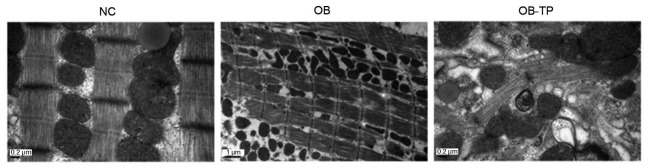
To visualize autophagy directly, we used transmission electron microscopy to examine autophagosomes. LV tissues from NC group rats showed normal structure with proper mitochondria distribution (Fig. 5). The OB group rats showed myofilaments and mitochondria in disarray. Compared to the OB group rats, the OB-TP group rats had an almost normal myofilament arrangement and mitochondrial distribution, and some autophagosome formation (Fig. 5).
Fig. 5.
Morphological effects of tea polyphenols on autophagy in rats in a high-fat state
NC: normal control group; OB: obesity group; OB-TP: obesity tea polyphenol treatment group
3.5. Effect of tea polyphenols on autophagy in diabetic cardiomyopathy
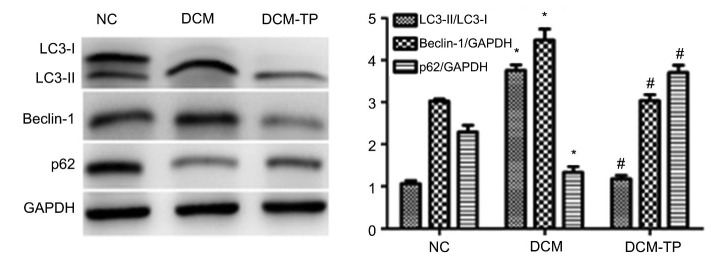
We also investigated the level of autophagy in the DCM and DCM-TP groups. Western blotting showed that, compared to the NC group, the expression of Beclin-1 and the ratio of LC3-II to LC3-I were notably increased, whereas the expression of SQSTM1/p62 was decreased in the DCM group. This demonstrated that autophagy could be induced in diabetic cardiomyopathy. Compared to the DCM group, we found that the level of Beclin-1 and the ratio of LC3-II to LC3-I in the DCM-TP treatment group decreased, and the level of SQSTM1/p62 was increased accordingly. These results showed that tea polyphenols can inhibit the level of high blood glucose-induced autophagy (Fig. 6).
Fig. 6.
Polyphenols inhibit autophagy in diabetic cardiomyopathy
Values are expressed as the mean±SEM (n=6). * P<0.05 compared to the NC group; # P<0.05 compared to the DCM group
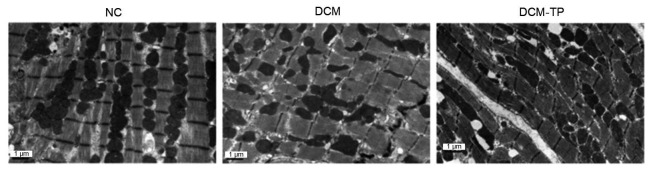
Similarly, LV tissues from the NC group showed neat muscle fibers, horizontal grain clarity, normal mitochondrial structure, and aligned crest lines. The DCM group showed a disordered arrangement of myocardial fibers, unevenly arranged mitochondria, crest gap expansion, and some mitochondrial swelling, despite a double or multilayer membrane structure of autophagosomes (Fig. 7). In contrast, the DCM-TP group showed a nearly normal structure of myocardial muscle fibrils, neat mitochondrial distribution, and hardly any autophagosome formation (Fig. 7).
Fig. 7.
Tea polyphenols inhibit formation of autophagosomes in diabetic cardiomyopathy
NC: normal control group; DCM: diabetic cardiomyopathy group; DCM-TP: diabetic cardiomyopathy tea polyphenol treatment group
4. Discussion
It is well-known that a long time spent in a diabetic state can readily induce molecular changes. These changes occur independently of other cardiac risk factors leading to diabetic cardiomyopathy, which is a specific form of heart disease (Chen et al., 2017). Previous studies have described streptozotocin-induced diabetic mice in which diastolic function is impaired, though autophagic activity is significantly increased (Ding ML et al., 2017). Gu et al. (2017a, 2017b) showed that breviscapine prevented cardiac hypertrophy in diabetic rats by inhibiting the expression of protein kinase C, which may have a protective effect in the pathogenesis of diabetic cardiomyopathy (Li et al., 2015). Nonetheless, the mechanism of diabetic cardiomyopathy is complex, and still only partly understood. Currently, there is no consensus about the clinical diagnosis of diabetic cardiomyopathy, or no effective treatment. Autophagy aims to reduce the accumulation of macromolecular aggregates and dysfunctional organelles and to preserve cellular homeostasis, maintaining intracellular renewal. It plays an important role in maintaining cardiac function, but also contributes to the pathogenesis of heart disease. Therefore, we have attempted to intervene in the progression of diabetic cardiomyopathy by regulating autophagy.
The polyphenol hydroxyphenol extracted from tea leaves is regarded as an effective active substance (Yanagi et al., 2011). In recent years, it has been found that tea polyphenols can reduce glucose and lipid levels in diabetic patients (Zhuo et al., 2017). There is mounting evidence that green tea polyphenol treatment can alleviate inhibition of autophagy induced by high glucose (Zhao et al., 2017) and attenuate atherosclerosis by up-regulating autophagy (Zhang L et al., 2016; Zhang PW et al., 2016). It is unknown whether green tea polyphenols could balance glucolipid metabolism to improve diabetic cardiomyopathy by regulating the level of autophagy. There is complex crosstalk between glucolipid metabolism and autophagy (Kubli and Gustafsson, 2015; Wieczór et al., 2016). Nevertheless, studies have yielded conflicting results. There is no conclusive evidence on these aspects at present (Ding S et al., 2017; Fan et al., 2017).
In accordance with previous findings, our results demonstrated that tea polyphenols effectively reduced hyperglycemia and hyperlipidemia in a rat model and protected heart structure and function in diabetic cardiomyopathy. In contrast, comparison of results from the OB and NC groups showed that tea polyphenols induced autophagy in rats under a high-fat state. This indicates that an increased level of autophagy may play a protective role in a high-fat state. The potential mechanism might be that tea polyphenols may stimulate AMPK activity via a Ca2+/CaMKK/AMPK-mediated signaling pathway thereby increasing autophagy. Lipid metabolism may then be accelerated, avoiding excessive beta-oxidation and the production of toxic substances that lead to myocardial damage (Yu et al., 2015; Liu et al., 2017; Xie et al., 2017). Autophagy in the DCM group increased significantly. By means of autophagy, there is excessive apoptosis of cardiomyocytes and cardiac function is impaired, so autophagy may have a negative effect. However, the intervention of tea polyphenols can restrain this phenomenon, avoiding excessive myocardial apoptosis or necrosis, meanwhile adjusting glucolipid metabolism, protecting myocardial cells from dying, and improving heart function (Su et al., 2016). This may be mediated through the mammalian target of rapamycin complex 1 (mTORC1) signal pathway, at the same time inhibiting autophagy (Singh et al., 2009; Efeyan et al., 2015; Jaishy and Abel, 2016). However, whether it can reverse the whole process of diabetic cardiomyopathy is unclear, and there is a need for more research and experiments.
In summary, our studies demonstrated that tea polyphenols have an effect on diabetic cardiomyopathy in rat cardiac function and may be able to adjust the level of cardiomyocyte autophagy, thereby improving glucose and lipid metabolic disorders under diabetes. Further studies are needed to dissect the signaling pathway between tea polyphenols and autophagy in diabetic cardiomyopathy rat models, and to explore possible drug-targeting methods to regulate this pathway.
Acknowledgments
We are thankful to Ms. Qi WANG and Xiao-xia LIN, the lab administrator in Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (Hangzhou, China) for their help. Most of research was performed in the Biomedical Research Centre.
Footnotes
Project supported by the Scientific and Technological Projects for Medicine and Health of Zhejiang Province (No. 2015128660) and the Major Research and Development Projects for the Zhejiang Science and Technology Agency (No. 2017C03034), China
Compliance with ethics guidelines: Hui ZHOU, Yan CHEN, Shu-wei HUANG, Peng-fei HU, and Li-jiang TANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Chen C, Yang S, Li H, et al. Mir30c is involved in diabetic cardiomyopathy through regulation of cardiac autophagy via BECN1. Mol Ther Nucleic Acids. 2017;7:127–139. doi: 10.1016/j.omtn.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung YR, Park SJ, Moon KY, et al. Diabetic retinopathy is associated with diastolic dysfunction in type 2 diabetic patients with non-ischemic dilated cardiomyopathy. Cardiovasc Diabetol. 2017;16(1):82. doi: 10.1186/s12933-017-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding ML, Ma H, Man YG, et al. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can J Physiol Pharmacol. 2017;95(12):1396–1405. doi: 10.1139/cjpp-2016-0333. [DOI] [PubMed] [Google Scholar]
- 4.Ding S, Jiang J, Yu P. Green tea polyphenol treatment attenuates atherosclerosis in high-fat diet-fed apolipoprotein E-knockout mice via alleviating dyslipidemia and up-regulating autophagy. PLoS ONE. 2017;12(8):e0181666. doi: 10.1371/journal.pone.0181666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efeyan A, Comb WC, Sabatini DM, et al. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahie K, Zachara NE. Molecular functions of glycoconjugates in autophagy. J Mol Bol. 2016;428(16):3305–3324. doi: 10.1016/j.jmb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan DM, Fan K, Yu CP, et al. Tea polyphenols dominate the short-term tea (Camellia sinensis) leaf litter decomposition. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(2):99–108. doi: 10.1631/jzus.B1600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu W, Lin Y, Gou X, et al. Tea polyphenol inhibits autophagy to sensitize epirubicin-induced apoptosis in human bladder cancer cells. Neoplasma. 2017;64(05):674–680. doi: 10.4149/neo_2017_504. [DOI] [PubMed] [Google Scholar]
- 9.Gu W, Yin H, Liu Y, et al. The mechanism underlying the effects of tea polyphenol on epirubicin-induced autophagy and apoptosis in T24 bladder cancer cells. Chin J Cell Mol Immunol. 2017;33(6):772–777. (in Chinese) [PubMed] [Google Scholar]
- 10.Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res. 2016;57(9):1619–1635. doi: 10.1194/jlr.R067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori H, Takemura G, Goto K. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11(7):1146–1160. doi: 10.1080/15548627.2015.1051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubli DA, Gustafsson ÅB. Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy. Ann Surg Oncol. 2015;22(17):1527–1544. doi: 10.1089/ars.2015.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai D, Gao J, Bi X, et al. The Rho kinase inhibitor, fasudil, ameliorates diabetes-induced cardiac dysfunction by improving calcium clearance and actin remodeling. J Mol Med. 2017;95(2):155–165. doi: 10.1007/s00109-016-1469-1. [DOI] [PubMed] [Google Scholar]
- 14.Li HL, Li ZJ, Wei ZS, et al. Long-term effects of oral tea polyphenols and Lactobacillus brevis M8 on biochemical parameters, digestive enzymes, and cytokines expression in broilers. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(12):1019–1026. doi: 10.1631/jzus.B1500160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C, Zhang M, Zhang Y, et al. Helix B surface peptide attenuates diabetic cardiomyopathy via AMPK-dependent autophagy. Biochem Biophys Res Commun. 2017;482(4):665–671. doi: 10.1016/j.bbrc.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 16.Liu SM, Ou SY, Huang HH. Green tea polyphenols induce cell death in breast cancer MCF-7 cells through induction of cell cycle arrest and mitochondrial-mediated apoptosis. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(2):89–98. doi: 10.1631/jzus.B1600022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazio F, Cecconi F. Autophagy up and down by outsmarting the incredible ULK. Autophagy. 2017;13(5):967–968. doi: 10.1080/15548627.2017.1285473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz M, Coderre L, Allen BG, et al. Protecting the heart through MK2 modulation, toward a role in diabetic cardiomyopathy and lipid metabolism. Biochim Biophys Acta. 2017;39(17):30241–30247. doi: 10.1016/j.bbadis.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Shin HR, Kim H, Kim KI. Epigenetic and transcriptional regulation of autophagy. Autophagy. 2016;12(11):2248–2249. doi: 10.1080/15548627.2016.1214780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su HM, Feng LN, Zheng XD, et al. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(6):437–446. doi: 10.1631/jzus.B1600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun XH, Yin MS, Mu YL. Alterations of mTOR pathway and autophagy in early type 2 diabetic cardiomyopathy in rats. Chin J Pathol. 2016;45(10):707–710. doi: 10.3760/cma.j.issn.0529-5807.2016.10.008. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 23.Trivedi PC, Bartlett JJ, Perez LJ, et al. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim Biophys Acta. 2016;1861(12):1893–1910. doi: 10.1016/j.bbalip.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Velázquez AP, Graef M. Autophagy regulation depends on ER homeostasis controlled by lipid droplets. Autophagy. 2016;12(8):1409–1410. doi: 10.1080/15548627.2016.1190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Zhang WB, Zhu JH. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009;30(8):1081–1091. doi: 10.1038/aps.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieczór R, Wieczór AM, Gadomska G, et al. Overweight and obesity versus concentrations of VEGF-A, sVEGFR-1, and sVEGFR-2 in plasma of patients with lower limb chronic ischemia. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(11):842–849. doi: 10.1631/jzus.B1600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie X, Yi W, Zhang P. Green tea polyphenols, mimicking the effects of dietary restriction, ameliorate high-fat diet-induced kidney injury via regulating autophagy flux. Nutrients. 2017;9(5):497. doi: 10.3390/nu9050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagi S, Matsumura K, Marui A, et al. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia-reperfusion injury in an isolated rat heart model. J Thorac Cardiovasc Surg. 2011;141(2):511–517. doi: 10.1016/j.jtcvs.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Rong X, Lv X, et al. Inhibition of mevalonate pathway prevents ischemia-induced cardiac dysfunction in rats via RhoA-independent signaling pathway. Cardiovasc Ther. 2017;35(5):e12285. doi: 10.1111/1755-5922.12285. [DOI] [PubMed] [Google Scholar]
- 30.Yu HT, Zhen J, Pang B, et al. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(5):344–354. doi: 10.1631/jzus.B1400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Ding WY, Wang ZH, et al. Early administration of trimetazidine attenuates diabetic cardiomyopathy in rats by alleviating fibrosis, reducing apoptosis and enhancing autophagy. J Transl Med. 2016;14(1):109. doi: 10.1186/s12967-016-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang PW, Tian C, Xu FY, et al. Green tea polyphenols alleviate autophagy inhibition induced by high glucose in endothelial cells. Biomed Environ Sci. 2016;29(7):524–528. doi: 10.3967/bes2016.069. [DOI] [PubMed] [Google Scholar]
- 33.Zhao P, Kuai J, Gao J, et al. Delta opioid receptor agonist attenuates lipopolysaccharide-induced myocardial injury by regulating autophagy. Biochem Biophys Res Commun. 2017;492(1):140–146. doi: 10.1016/j.bbrc.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo C, Jiang R, Lin X, et al. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget. 2017;8(1):1429–1437. doi: 10.18632/oncotarget.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]