Abstract
In aged patients, acute kidney injury (AKI) is a common clinical complication after percutaneous coronary intervention (PCI), highlighting the need for timely and certain diagnosis of this disease. A single centre, nested case-control study was conducted, which assessed the usefulness of urinary liver-type fatty acid-binding protein (uL-FABP), neutrophil gelatinase-associated lipocalin (uNGAL), and kidney injury molecule-1 (uKIM-1) for early detection of AKI. One hundred and thirty-two patients at or over 60 years old undergoing PCI were included. Serum creatinine (SCr) was measured before PCI, 24 and 48 h after PCI; uL-FABP, uNGAL, and uKIM-1 were measured before PCI, 6, 24, and 48 h after PCI. We identified 16 AKI patients and selected 32 control patients matched by admission time (<1 week), age (±5 years), and gender. In the receiver operating characteristic (ROC) curve analysis, the areas under the curve (AUCs) for the relative measurements of uL-FABP, uNGAL, and uKIM-1 were 0.809, 0.867, and 0.512 at 6 h after PCI, and 0.888, 0.840, and 0.676 at 24 h after PCI, respectively. AUC for the combination of uL-FABP and uNGAL was 0.899 at 6 h after PCI, and 0.917 at 24 h after PCI. Thus, measurement of uL-FABP and uNGAL levels at 6 and 24 h after PCI may be useful in detecting AKI in aged patients. Measurement of uKIM-1 levels provides inferior predictive power for early diagnosis of AKI.
Keywords: Liver-type fatty acid-binding protein, Neutrophil gelatinase-associated lipocalin, Kidney injury molecule-1, Percutaneous coronary intervention, Acute kidney injury
In aged patients, acute kidney injury (AKI) is a common clinical complication after percutaneous coronary intervention (PCI), highlighting the need for timely and certain diagnosis of this disease. A single centre, nested case-control study was conducted, which assessed the usefulness of urinary liver-type fatty acid-binding protein (uL-FABP), neutrophil gelatinase-associated lipocalin (uNGAL), and kidney injury molecule-1 (uKIM-1) for early detection of AKI. One hundred and thirty-two patients at or over 60 years old undergoing PCI were included. Serum creatinine (SCr) was measured before PCI, 24 and 48 h after PCI; uL-FABP, uNGAL, and uKIM-1 were measured before PCI, 6, 24, and 48 h after PCI. We identified 16 AKI patients and selected 32 control patients matched by admission time (<1 week), age (±5 years), and gender. In the receiver operating characteristic (ROC) curve analysis, the areas under the curve (AUCs) for the relative measurements of uL-FABP, uNGAL, and uKIM-1 were 0.809, 0.867, and 0.512 at 6 h after PCI, and 0.888, 0.840, and 0.676 at 24 h after PCI, respectively. AUC for the combination of uL-FABP and uNGAL was 0.899 at 6 h after PCI, and 0.917 at 24 h after PCI. Thus, measurement of uL-FABP and uNGAL levels at 6 and 24 h after PCI may be useful in detecting AKI in aged patients. Measurement of uKIM-1 levels provides inferior predictive power for early diagnosis of AKI.
AKI is a recognized complication of PCI, accounting for 11% of hospital-acquired renal insufficiency (Nash et al., 2002). This complication is associated with prolonged hospitalization, long-term mortality, and development of end stage renal disease (James et al., 2013).
Biomarkers are used effectively in the diagnosis of AKI, with the assay of SCr being the gold standard. On the other hand, McCullough and Sandberg (2003) reported that SCr typically peaks at 3‒5 d postcontrast from the baseline. Also a single 24-h determination of SCr would have missed 58.2% of contrast-induced nephropathy patients who were detected by the 48-h determination, which really underlines the need for identifying reliable biomarkers to facilitate early determination of AKI (Reddan et al., 2009).
Furthermore, SCr is a marker reflective of renal function change, and is not specific for tissue injury. Recent research efforts have identified several new potential biomarker proteins, which derive from the injured renal tubule cells of patients with AKI; these new compounds might detect AKI at early stages (Schrezenmeier et al., 2017). Currently, uL-FABP, uNGAL, and uKIM-1 are three of the most promising biomarkers (Luo et al., 2013; Torregrosa et al., 2015). However, it remains unknown whether changes in these three biomarkers could predict early AKI in aged patients subject to PCI. Thus, we performed a prospective trial to evaluate the diagnostic performance of these novel biomarkers.
A total of 132 in-hospital patients at Ningbo No. 2 Hospital, Medical School of Ningbo University, Ningbo, China, were eligible for enrollment during the period between July 2015 and August 2016 (Table 1). AKI was identified with an elevation from the preoperative value in SCr level of 0.3 mg/dl (26.5 μmol/L) within 48 h after the procedure (Stacul et al., 2011). In our study, 16 patients developed AKI. Controls (n=32) were selected from the remainder. They were individually matched to cases by admission time (<1 week), age (±5 years), and gender. This study was approved by the Ethics Committee of Ningbo No. 2 Hospital (PJ-NBEY-KY-2015-020-01). We registered this study at the Chinese Clinical Trial Registry (ChiCTR-IPD-17010596).
Table 1.
Background of total patients in this study
| Parameter | Value |
| N | 132 |
| Age (year) | 74.42±7.40 |
| Female | 42 (31.8%) |
| Body mass index (kg/m2) | 23.58±3.28 |
| Diabetes mellitus | 40 (30.3%) |
| Hypertension | 96 (72.7%) |
| Diseased vessels (branch) | |
| Average | 2.20 |
| 1 | 31 |
| 2 | 44 |
| 3 | 57 |
| ACEI | 32 (24.2%) |
| ARB | 56 (42.4%) |
| Diuretics | 58 (43.9%) |
| Statin | 132 (100.0%) |
| eGFR (ml/(min·1.73 m2)) | 77.22±21.05 |
| Hemoglobin (g/L) | 126.89±16.71 |
| C-reactive protein (mg/L) | 3.26 (1.51‒9.79) |
| Albumin (g/L) | 38.54±4.79 |
| Uric acid (μmol/L) | 352.12±103.35 |
| Total cholesterol (mmol/L) | 4.01±1.06 |
| LDL (mmol/L) | 2.20±0.84 |
| Triglycerides (mmol/L) | 1.29 (0.93‒1.72) |
| NT-proBNP (pg/ml) | 682 (348‒2332) |
| LVEF (%) | 62.03±8.17 |
| Contrast medium (ml) | 169.00 (148.25‒193.00) |
Data are presented as number, the mean±standard deviation (SD), number (percentage) of patients, or medians with interquartile ranges (25%–75%). N, total number of patients; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction
Detailed materials, methods, and declarations are described in Data S1.
There was a significantly higher level of N-terminal pro-brain natriuretic peptide (NT-proBNP) in the AKI group (P=0.01; Table 2).
Table 2.
Background of AKI and non-AKI groups
| Parameter | Patients with AKI | Patients without AKI | P-value |
| N | 16 | 32 | |
| Age (year) | 75.06±8.31 | 74.34±6.08 | 0.74 |
| Female | 4 (25.0%) | 8 (25.0%) | 1.00 |
| Body mass index (kg/m2) | 24.40±3.19 | 23.84±2.71 | 0.54 |
| Diabetes mellitus | 5 (31.3%) | 10 (31.3%) | 1.00 |
| Hypertension | 14 (87.5%) | 23 (71.9%) | 0.40 |
| Diseased vessels (branch) | |||
| Average | 2.19 | 2.00 | 0.42 |
| 1 | 4 | 9 | |
| 2 | 5 | 14 | |
| 3 | 7 | 9 | |
| ACEI | 2 (12.5%) | 10 (31.3%) | 0.29 |
| ARB | 7 (43.8%) | 16 (50.0%) | 0.68 |
| Diuretics | 9 (56.3%) | 16 (50.0%) | 0.68 |
| Statin | 16 (100.0%) | 32 (100.0%) | 1.00 |
| eGFR (ml/(min·1.73 m2)) | 62.46 (51.01–92.35) | 79.05 (66.43–95.12) | 0.36 |
| Hemoglobin (g/L) | 121.5±12.71 | 124.69±18.45 | 0.54 |
| C-reactive protein (mg/L) | 7.57 (2.54–12.43) | 3.59 (1.51–10.52) | 0.26 |
| Albumin (g/L) | 36.44±4.24 | 38.31±4.13 | 0.15 |
| Uric acid (μmol/L) | 374.57±123.84 | 378.27±105.67 | 0.91 |
| Total cholesterol (mmol/L) | 3.66±0.65 | 3.96±0.95 | 0.26 |
| LDL (mmol/L) | 2.03±0.61 | 2.16±0.75 | 0.54 |
| Triglycerides (mmol/L) | 1.24 (1.02–1.67) | 1.32 (1.02–1.71) | 0.65 |
| NT-proBNP (pg/ml) | 3592 (496–6258) | 562 (300–1889) | 0.01 |
| LVEF (%) | 57.00 (53.25–66.50) | 64.00 (59.00–68.00) | 0.16 |
| Contrast medium (ml) | 203.5 (148.5–233.5) | 170.5 (149.0–189.0) | 0.16 |
Data are presented as number, the mean±standard deviation (SD), number (percentage) of patients, or medians with interquartile ranges (25%–75%). N, total number of patients; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; AKI: acute kidney injury
For both groups, the patients displayed significant increases in SCr from baseline at the 48 h time point (P<0.01 for AKI group, P<0.05 for non-AKI group). The SCr did not significantly change at the 24 h time point in the two groups, but the relative SCr levels at 24 h after PCI were significantly higher in patients with AKI (P<0.01; Table 3).
Table 3.
Serial changes of serum and urinary markers
| Parameter | Patients with AKI | Patients without AKI | P-value |
| SCr (μmol/L) | |||
| Pre PCI | 87.19±29.88 | 84.82±29.55 | |
| 24 h after PCI | 110.33±38.94 | 87.90±27.28 | |
| 48 h after PCI | 135.79±53.99** | 90.02±29.74* | |
| A relative measurement at 24 h after PCI | 1.30±0.26 | 1.07±0.19 | 0.001 |
| uL-FABP (μg/g Cr) | |||
| Pre PCI | 1.92 (1.23–3.22) | 3.93 (1.48–5.55) | |
| 6 h after PCI | 4.31 (2.22–6.75)** | 4.62 (1.96–6.96)** | |
| 24 h after PCI | 6.64 (5.92–9.40)** | 4.63 (2.22–8.19)** | |
| 48 h after PCI | 5.85 (2.75–8.65)** | 4.78 (2.01–7.42)* | |
| A relative measurement at 6 h after PCI | 1.95 (1.69–2.21) | 1.18 (1.01–1.75) | 0.001 |
| A relative measurement at 24 h after PCI | 3.13 (2.64–5.48) | 1.39 (1.10–1.89) | <0.001 |
| uNGAL (μg/g Cr) | |||
| Pre PCI | 25.20 (11.25–55.62) | 59.69 (39.81–85.98) | |
| 6 h after PCI | 88.05 (45.22–248.75)** | 56.32 (37.63–122.26) | |
| 24 h after PCI | 104.05 (50.08–162.82)** | 60.69 (33.86–112.10) | |
| 48 h after PCI | 103.15 (40.83–178.27)** | 61.86 (35.41–118.14) | |
| A relative measurement at 6 h after PCI | 4.30 (2.02–4.93) | 1.12 (0.66–1.80) | <0.001 |
| A relative measurement at 24 h after PCI | 3.22 (1.72–5.53) | 1.09 (0.60–2.03) | <0.001 |
| uKIM-1 (μg/g Cr) | |||
| Pre PCI | 1.24 (0.46–2.64) | 1.10 (0.70–1.57) | |
| 6 h after PCI | 1.21 (0.50–2.66) | 1.18 (0.66–1.57) | |
| 24 h after PCI | 1.72 (1.16–7.08) | 1.21 (0.64–1.68) | |
| 48 h after PCI | 2.08 (1.18–7.74)* | 1.07 (0.78–1.76) | |
| A relative measurement at 6 h after PCI | 1.08 (0.71–1.53) | 0.98 (0.81–1.47) | 0.896 |
| A relative measurement at 24 h after PCI | 2.91 (0.86–7.13) | 1.11 (0.75–1.53) | 0.049 |
SCr: serum creatinine; Cr: creatinine; uL-FABP: urinary liver-type fatty acid-binding protein; uNGAL: urinary neutrophil gelatinase-associated lipocalin; uKIM-1: urinary kidney injury molecule-1; PCI: percutaneous coronary intervention; AKI: acute kidney injury. Data are presented as the mean±SD or medians with interquartile ranges (25%–75%). Values that are significantly different between pre-and post-PCI are indicated by
P<0.05,
P<0.01
Compared to baseline levels, patients with AKI showed a significant increase in uL-FABP and uNGAL at 6, 24, and 48 h after PCI, but uKIM-1 levels were significantly increased only at 48 h after PCI. Patients without AKI displayed statistically insignificant changes in uNGAL and uKIM-1 from baseline at 6, 24, and 48 h after PCI, but significant increases in uL-FABP from baseline at 6, 24, and 48 h after PCI (Table 3). At 6 and 24 h after PCI, there were significant differences in the relative measurements of uL-FABP and uNGAL between patients developing AKI and those without AKI. However, the relative measurement of uKIM-1 was able to discriminate between the two patient groups only at the 24 h time point (Table 3).
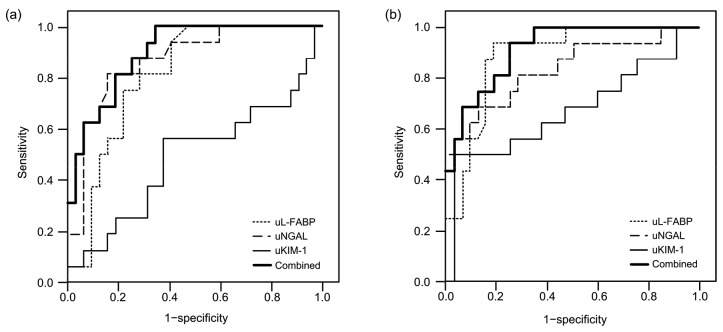
We performed ROC curve analysis to assess the ability of the biomarkers in predicting AKI. As early as 6 h after PCI, the AUCs of uL-FABP, uNGAL, and uKIM-1 were 0.809 (95% CI 0.669–0.908; P<0.001), 0.867 (95% CI 0.738–0.948; P<0.001), and 0.512 (95% CI 0.363–0.659; P=0.90), respectively (Fig. 1a). Based on the logistic model, combined predictors of uL-FABP and uNGAL were gained, with an AUC of 0.899 (95% CI 0.778–0.967; P<0.001).
Fig. 1.
ROC curves for the different markers analyzed for early detection of AKI
(a) A relative measurement at 6 h after PCI (AUC for uL-FABP: 0.809; AUC for uNGAL: 0.867; AUC for uKIM-1: 0.512; AUC for combined predictors: 0.899). (b) A relative measurement at 24 h after PCI (AUC for uL-FABP: 0.888; AUC for uNGAL: 0.840; AUC for uKIM-1: 0.676; AUC for combined predictors: 0.917). uL-FABP: urinary liver-type fatty acid-binding protein; uNGAL: urinary neutrophil gelatinase-associated lipocalin; uKIM-1: urinary kidney injury molecule-1; Combined: combined predictors of uL-FABP and uNGAL
At the 24 h time point after PCI, the AUCs of uL-FABP, uNGAL, and uKIM-1 were 0.888 (95% CI 0.763–0.960; P<0.001), 0.840 (95% CI 0.705–0.930; P<0.001), and 0.676 (95% CI 0.525–0.804; P=0.049), respectively (Fig. 1b). Combined predictors of uL-FABP and uNGAL were gained, with an AUC of 0.917 (95% CI 0.801–0.977; P<0.001).
In this study, we evaluated the usefulness of uL-FABP, uNGAL, and uKIM-1 determinations for the early (6 and 24 h following PCI) detection of AKI in a group of aged patients undergoing PCI. This prospective pilot study found that AKI occurred in 12.12% patients within 48 h after intervention. A relative increase in uL-FABP or uNGAL was useful for the diagnosis of AKI as early as 6 h after PCI.
In the human kidney, L-FABP is expressed predominantly in the proximal tubules and plays a key role in fatty acid metabolism. L-FABP binds to lipid peroxidation products and is excreted from the cytoplasm of ischemic proximal tubule cells. Urinary excretion of L-FABP is elevated in kidney injury, reflecting tubulointerstitial damage (Kamijo et al., 2004). In our study, compared with the levels before PCI, uL-FABP levels were significantly higher at the 6 h time point in patients both who experienced AKI and who did not. However, the relative measurement was significantly higher in the AKI group. In a report by Torregrosa et al. (2015), uL-FABP has also been shown to increase significantly at 12 h after coronary angiography in patients without AKI. We think uL-FABP might reflect unrecognized tubular damage or nonspecific expression, and the relative measurement was probably more suitable for the diagnosis of AKI. In addition, uL-FABP has been reported as a promising indicator for the occurrence of AKI in patients undergoing cardiac surgery or coronary angiography (Katagiri et al., 2012; Manabe et al., 2012). Although these cohorts are heterogeneous because the enrolled patients had undergone different cardiac therapy, it can be concluded that uL-FABP is an early biomarker for AKI, and the relative measurement at 6 h after PCI could detect AKI with an AUC value of 0.809 in our report.
NGAL is normally reabsorbed by megalin-facilitated endocytosis in the proximal tubules. Once the proximal tubular damage occurs, uNGAL concentration will increase (Singer et al., 2013). Moreover, NGAL may be produced in the damaged renal tubules and participate in renal repair (Mishra et al., 2003). In the research by Liebetrau et al. (2014), uNGAL at 24 h after contrast medium application is predictive of AKI in patients undergoing PCI, but the uNGAL levels before PCI were significantly higher in the AKI group and this may influence the subsequent uNGAL levels. In a meta-analysis, uNGAL appeared to be a promising biomarker of AKI within 6 h after cardiac surgery, especially in neonates/children (Zhou et al., 2016). In our study, uNGAL increased significantly at 6 h after PCI in aged patients with AKI, and remained at high levels at the 24 and 48 h time points. However, it changed little in those who did not experience AKI. Furthermore, the AUCs of uNGAL relative measurement were 0.867 at the 6 h time point and 0.840 at the 24 h time point in this report.
KIM-1 is a transmembrane tubular protein that is induced after kidney injury, and the US Food and Drug Administration approved KIM-1 as a biomarker for preclinical trials (Dieterle et al., 2010). Urinary KIM-1 is the ectodomain of KIM-1, and reflects tissue KIM-1; it is a non-invasive biomarker associated with inflammation and renal proximal tubular damage (van Timmeren et al., 2007). A systematic review had reported that uKIM-1 was a specific predictor for early AKI in patients undergoing cardiopulmonary bypass surgery (Shao et al., 2014). In addition, uKIM-1 has also been shown to be a moderately useful predictor for AKI 12 h after coronary angiography (AUC=0.713) and 24 h after PCI (AUC=0.850) (Luo et al., 2013; Torregrosa et al., 2015). In this study, compared to the non-AKI group, the relative measurement of uKIM-1 was significantly higher at 24 h after intervention, showing relatively low predictive power for early diagnosis of AKI (AUC=0.676, P=0.049). However, uKIM-1 levels significantly increased only at the 48 h time point, and in a report by Parikh et al. (2013), uKIM-1 peaked 2 d after cardiac surgery and remained elevated for several days. A variety of reasons might explain the differences: definitions of AKI adopted in the individual studies varied, and age, population settings, and time of urine collections may also contribute to the heterogeneity in results.
Because of the differential expression of urinary biomarkers, combining these biomarkers may enhance their own predictive value, and it might be a reasonable strategy to combine the markers with different sensitivity and specificity (Katagiri et al., 2012). Luo et al. (2013) described the advantages of combining the biomarker levels (uKIM-1, uNGAL, and urinary interleukin-18 (uIL-18)) to improve their performance, but we considered that the biomarker levels were probably unsuitable for patients with abnormal baseline levels. In our study, we combined the relative levels and found that combinations of uL-FABP and uNGAL at the 6 and 24 h time points were able to increase the AUC for AKI up to 0.899 and 0.917, respectively. However, optimal combinations of urinary biomarkers still need further investigation.
Several limitations might affect the results obtained in our study. First, we studied aged patients at a single hospital. Evaluation should be conducted at multicenter hospitals to confirm the findings. Second, patients with AKI had relatively low estimated glomerular filtration rate and left ventricular ejection fraction on admission, and were more likely to receive higher contrast medium volume during the operation. These factors had been shown to be independent predictors of AKI (Andò et al., 2014, 2015). Our study population is small and therefore in a larger population it is likely that the differences would be significant between the AKI and non-AKI groups. Third, although we recruited a relatively homogeneous cohort, there was a significant difference in NT-proBNP between the two groups, and NT-proBNP is associated with heart failure and poor renal function. In addition, owing to the possible fundamental expression, uL-FABP and uNGAL baseline levels in AKI patients were lower than those in patients without AKI. Based on this condition, a relative increase in urinary biomarker seems better than the level for the assessment of biomarker performance. Finally, AKI was diagnosed using only SCr. Another criterion based on urine output was not used, and therefore renal injury might be underestimated. Also this definition may not reflect tubular injury, so it might appear inaccurate for evaluation of the diagnostic performance of urinary biomarkers. Stricter criteria to diagnose AKI still require further studies.
Urinary L-FABP and NGAL are predictive of AKI in aged patients at 6 and 24 h after PCI, and a combination of urinary biomarkers (including uL-FABP and uNGAL) shows better predictive capacity. However, uKIM-1 provides inferior predictive power for early diagnosis of AKI.
List of electronic supplementary materials
Materials, methods, and declarations
Footnotes
Project supported by the Medical Science and Technology in Zhejiang Province Plan (No. 2015KYA200), China
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1700427) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Hong-hua YE, Gen SHEN, Qun LUO, Fang-fang ZHOU, Xiao-ling XIE, Chun-yan WANG, and Li-na HAN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
References
- 1.Andò G, de Gregorio C, Morabito G, et al. Renal function-adjusted contrast volume redefines the baseline estimation of contrast-induced acute kidney injury risk in patients undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(4):465–472. doi: 10.1161/CIRCINTERVENTIONS.114.001545. [DOI] [PubMed] [Google Scholar]
- 2.Andò G, Cortese B, Frigoli E, et al. Acute kidney injury after percutaneous coronary intervention: rationale of the AKI-MATRIX (acute kidney injury-minimizing adverse hemorrhagic events by TRansradial access site and systemic implementation of angioX) sub-study. Catheter Cardiovasc Interv. 2015;86(5):950–957. doi: 10.1002/ccd.25932. [DOI] [PubMed] [Google Scholar]
- 3.Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 4.James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6:37–43. doi: 10.1161/CIRCINTERVENTIONS.112.974493. [DOI] [PubMed] [Google Scholar]
- 5.Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165(4):1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katagiri D, Doi K, Honda K, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93(2):577–583. doi: 10.1016/j.athoracsur.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Liebetrau C, Gaede L, Doerr O, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest. 2014;74(2):81–88. doi: 10.3109/00365513.2013.860615. [DOI] [PubMed] [Google Scholar]
- 8.Luo Q, Zhou F, Dong H, et al. Implication of combined urinary biomarkers in early diagnosis of acute kidney injury following percutaneous coronary intervention. Clin Nephrol. 2013;79:85–92. doi: 10.5414/CN106852. [DOI] [PubMed] [Google Scholar]
- 9.Manabe K, Kamihata H, Motohiro M, et al. Urinary liver-type fatty acid-binding protein level as a predictive biomarker of contrast-induced acute kidney injury. Eur J Clin Invest. 2012;42(5):557–563. doi: 10.1111/j.1365-2362.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4(Suppl 5):S3–S9. [PubMed] [Google Scholar]
- 11.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 12.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddan D, Laville M, Garovic VD. Contrast-induced nephropathy and its prevention: what do we really know from evidence-based findings? J Nephrol. 2009;22(3):333–351. [PubMed] [Google Scholar]
- 15.Schrezenmeier EV, Barasch J, Budde K, et al. Biomarkers in acute kidney injury–pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219(3):554–574. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS ONE. 2014;9(1):e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207(4):663–672. doi: 10.1111/apha.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 19.Torregrosa I, Montoliu C, Urios A, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels. 2015;30(6):703–711. doi: 10.1007/s00380-014-0538-z. [DOI] [PubMed] [Google Scholar]
- 20.van Timmeren MM, van den Heuvel MC, Bailly V, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212(2):209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Luo Q, Wang L, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardio-thorac Surg. 2016;49(3):746–755. doi: 10.1093/ejcts/ezv199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials, methods, and declarations