Abstract
The aim of this study is to assess the antibacterial and anti-biofilm properties of the lipid extract from Mantidis ootheca against the gentamycin resistant Pseudomonas aeruginosa. The chemical composition of the lipid extract and its relative proportion were determined using the technique of gas chromatography coupled with mass spectrometry (GC-MS). Antibacterial susceptibility tests were performed using a disc diffusion assay and the minimum inhibition concentration (MIC) was determined by way of the agar dilution method. The anti-biofilm test was carried out with crystal violet staining and scanning electron microscopy (SEM). There were 16 compounds detected, and the most abundant components were sesquiterpenoids, monoterpenes, and trace aromatic compounds. The MIC for P. aeruginosa was 4 mg/ml and the eradication effect on preformed biofilms was established and compared with a ciprofloxacin control. The results of our study indicated that a lipid extract from M. ootheca could be used as a topical and antibacterial agent with anti-biofilm activity in the future.
Keywords: Antibacterial, Lipid extract, Mantidis ootheca, Pseudomonas aeruginosa
1. Introduction
Mantidis ootheca is the dry egg sheath of Tenodera sinensis Saussur, Statilia maculate, and Hierodula patellifera, which belongs to the insects of the Mantis family (Wen et al., 2013). M. ootheca is one of the Chinese traditional medicinal insects, which is often used as a diuretic along with other Chinese medicines in the clinical treatment of renal failure, spermatorrhea, pediatric enuresis, and leukorrhea with reddish discharge (Tan et al., 1997). Although M. ootheca is used traditionally to treat the above mentioned diseases, there is as yet no report on its effect in treating bacterial infections. The chemical components of the lipid extract from M. oothecal, its relative bioactivity, or its antibacterial and anti-biofilm effects against Pseudomonas aeruginosa have not been reported.
P. aeruginosa is one of the most relevant microorganisms that causes human opportunistic infections. It can infect a wide range of ecological niches including animals and plants in both aquatic and soil habitats because of its special metabolic plasticity and versatility (Silby et al., 2011; Gellatly and Hancock, 2013). P. aeruginosa is capable of causing acute nosocomial infections, and it particularly affects immunocompromised patients and patients in the intensive care unit (ICU). It is the most relevant pathogen causing ventilator-associated pneumonia and burn wound infections, which are related to a very high mortality rate (>30%) (Oliver et al., 2015). Additionally, P. aeruginosa is a common cause of chronic respiratory infections in patients suffering from cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and bronchiectasis (Oliver et al., 2015).
The usage of antibiotics has treated many devastating bacterial infections in humans. However, in recent years, the abuse of antibiotics has led to an increase in the emergence of multidrug resistant (MDR) bacteria, which makes it vital to look for novel effective drugs (O'Donnell et al., 2010). With the emerging resistance, researchers have gradually realized that the unique effects of natural products can provide abundant materials in which to find new antibacterial drugs for fighting MDR bacteria. The formation of biofilm protects the bacteria, which contributes significantly to several medical challenges such as symptomatic inflammation, antibiotic resistance, the recurrence and spread of infectious emboli, and also enables the bacteria to survive in severe conditions such as high temperatures and high concentrations of antibiotic treatments, all of which make it more challenging to treat biofilm-associated infection (BAI) (Costerton et al., 1999; Kim et al., 2012). BAI includes the infections on indwelling medical devices such as intravascular catheters, cardiac devices, and prosthetic joints, and it can also develop on indwelling medical devices independently such as in native valve endocarditis, open wounds, and dental plaque (Joo and Otto, 2012). Microbial biofilms pose global economic challenges in connection with equipment damage, product contamination, energy losses, and infections (Yang et al., 2012). In the USA, approximately 17 million people suffer from chronic infections each year and nearly 3% of these die as a result (Wolcott et al., 2010). Novel drugs that can treat both the planktonic bacteria and bacterial biofilm are therefore required.
This article focuses on the chemical composition, antibacterial activity, and anti-biofilm activity of the lipid extract from M. ootheca. The efficacy of the lipid extract was evaluated and compared with ciprofloxacin as a positive control against a P. aeruginosa strain that is resistant to gentamicin.
2. Materials and methods
2.1. Lipid extract preparation
Following the method of Li et al. (2009) with some modifications, M. ootheca was ground into a powder and 10 g of the dry powder was taken into a Soxhlet extractor. A volume of 220 ml of chloroform/methanol (2:1, v/v) was then added to the powder and heated at 60 °C in water for 2 h, before cooling the extracted liquid to room temperature, adding 30 g anhydrous sodium sulfate for 10 min, and then evaporating it to dryness at 40 °C by rotatory evaporator. The liquid was transferred into a separating funnel and extracted using diethyl ether. Finally, the lipid extract was obtained after drying the extracted liquid with a vacuum pump in a 40 °C water bath for 30 min, and was stored at 4 °C in amber glass vials before analysis.
2.2. Analytical conditions of the gas chromatography coupled with mass spectrometry (GC-MS)
According to the method developed by Sitaram et al. (2011) with some modifications, the gas chromatography conditions included the chromatography column DB1701 (30 m×320 μm×0.25 μm) elastic quartz capillary column. Temperature programming involved keeping the initial temperature at 50 °C for 3 min, then raising it to 280 °C for 20 min at a rate of 10 °C/min. The temperature at the sample-feeding gate was 280 °C and the split sampling ratio was 50:1 (v/v). The mass spectrometry conditions were as follows: the electron bombard was the electron impact (EI) ionization source, the ionizing energy was 70 eV, the temperature of the ionization source was 200 °C and the temperature of the connector was 250 °C. The mass spectra library used by the US National Institute of Standards and Technology (NIST, Gaithersburg, Maryland, USA) was used and the relative content of each component was calculated by their normalized peak area.
2.3. Bacterial strain and growth condition
The studied strain P. aeruginosa was isolated from patients suffering from CF in the Second Affiliated Hospital of Dalian Medical University (Dalian, China). The strain was determined by the TaKaRa company (Dalian, China) by the 16S rDNA amplification method, and the results were compared with data from the National Center for Biotechnology Information (NCBI). The sequences producing significant alignments were 100% identical and it was confirmed that the tested strain was a P. aeruginosa strain. Stock cultures of bacteria were prepared and stored with 8% glycerol at −80 °C. The strain was sub-cultured on Luria-Bertani (LB) broth agar plates containing tryptone (10 g/L), yeast extract (5 g/L), NaCl (10 g/L), 1 mol/L NaOH (0.2%, v/v), and agar (20 g/L) dissolved in 500 ml of tri-distilled water and stored in the fridge at 4 °C before any antibacterial testing. Single colonies were frequently replanted every 4 weeks. All broth and glassware, which were used for bacterial growth, were autoclaved at 121 °C for 30 min by high pressure steam sterilizer.
2.4. Disc diffusion assay
The antibacterial activity of the lipid extract from M. ootheca was initially analyzed using a disc diffusion assay according to the M2-A8 method as described by the Clinical Laboratory and Standards Institute (CLSI). Müeller-Hinton agar (0.042 g/ml) was dissolved in 200 ml of tri-distilled water and autoclaved at 121 °C for 30 min, cooled to 55 °C, and then poured onto glass plates and cooled to room temperature. The plates were stored in the fridge at 4 °C. One colony of the tested strain was transferred to LB broth and cultured at 37 °C, and the overnight culture was suspended in LB broth to a cell suspension of 0.5 McFarland turbidity prior to inoculation on the Müeller-Hinton agar plates. The discs (6 mm in diameter) were impregnated with 15 μl of the lipid extract (3 mg/disc) dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 200 mg/ml and placed on Müeller-Hinton agar plates containing bacterial inoculum. Discs impregnated with 15 μl DMSO were used as a control, while discs with 10 μg gentamycin or 5 μg ciprofloxacin were included according to CLSI. The diameters (mm) of the inhibition zones were measured after overnight incubation at 37 °C. The experiment was carried out in triplicate.
2.5. Determination of minimum inhibition concentration (MIC)
The agar dilution method was performed according to the M7-A6 protocol published by CLSI to determine the minimum inhibition concentration (MIC) of the lipid extract from M. ootheca against the bacteria strain tested. The Müeller-Hinton agar base (0.042 g/ml) was dissolved in 100 ml of tri-distilled water and autoclaved at 121 °C for 30 min before cooling to 55 °C. The lipid extract was then poured in and blended, and was poured onto plastic plates and cooled to room temperature. Plates with serial concentrations of the lipid extract were prepared. One colony of the tested strain was transferred to the LB broth and cultured at 37 °C, the overnight culture was measured and adjusted to 0.5 McFarland turbidity by diluting with LB broth. The assay was conducted by putting 2 μl of the bacterial cell suspension per spot onto the plates. The final quantity of bacteria was 1×105 CFU (colony-forming unit) per spot. The Müeller-Hinton agar without the lipid extract was used as the control, and a medium with DMSO was also prepared. The plates were incubated for 20 h at 37 °C. Each lipid extract concentration was tested against the strain three times. MIC was defined as the lowest concentration of the lipid extract that inhibited the growth of the bacteria by visual inspection.
The MIC determination of ciprofloxacin for the strain was performed by the broth dilution method according to the method by de Zoysa et al. (2015) with some modifications. In general, ciprofloxacin was dissolved in sterile water and a series of dilutions were made. The assay was conducted by putting 4 μl of each of the ciprofloxacin solutions at several concentrations and 96 μl of the bacterial culture into a 96-well culture plate. Each concentration was repeated five times. The lowest concentration that showed no bacterial growth after 24 h of incubation was defined as MIC, which was determined by both visual inspection and absorbance at 600 nm.
2.6. Effect of the lipid extract on preformed biofilms
The experiment for biofilm eradication was conducted according to the method described by de Zoysa et al. (2015) with some modifications. The ability of the lipid extract from M. ootheca to eradicate preformed biofilms of the strain was evaluated by crystal violet staining in 12-well plates. Preformed biofilms of the strain were prepared in 12-well plates. A bacterial cell suspension (1 ml) corresponding to 1×108 CFU/ml and 1 ml of LB broth was prepared in the plates. The final inoculum was 6×107 CFU/ml in each well. The plates were incubated at 37 °C for 24 h. After incubation, the supernatant was removed carefully without injuring the preformed biofilm architecture and gently washed twice with 2 ml of phosphate-buffered saline (PBS). Thereafter, 2 ml of LB broth with DMSO (negative control), 2 ml of the MIC of the lipid extract in sterile LB broth, and 2 ml of the MIC of ciprofloxacin in sterile LB broth (positive control) were added and incubated for a further 24 h at 37 °C. The supernatant was removed from each well after the second incubation and the wells were gently washed twice with PBS and stained with 1 ml of 0.1% crystal violet for 10 min. After staining, the crystal violet was removed and the wells were washed thrice with 1 ml of sterile water. The marked biofilms of each well were observed at 40×magnification under microscope. The procedure was done in triplicate.
2.7. Scanning electron microscopy
For the scanning electron microscopy (SEM) analysis, 12-well plates including plastic coverslips were prepared. After preparing the same double 24 h bacteria culture as described above, the plastic coverslips were washed with PBS buffer to remove the extra bacteria. Then each plastic coverslip was fixed in 2.5% glutaraldehyde for 30 min, then in 1% osmium tetraoxide for another 30 min. The samples were then dehydrated in a series of ethanol solutions which increased from 50% to 100% sequentially. Finally, samples were critical-point dried, then coated with 15 nm of gold, and examined by SEM (Murphy et al., 2014).
3. Results
3.1. Analytical results of the GC-MS
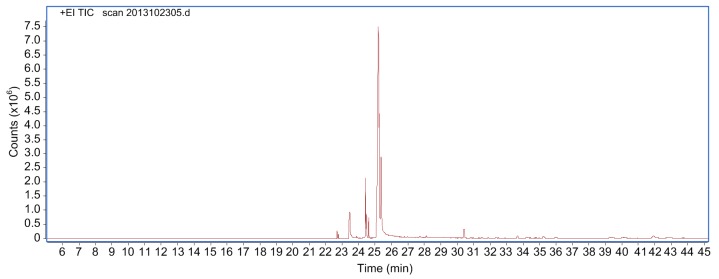
The lipid extract from M. ootheca was in the form of an oily liquid that had a faint yellow color and a rich fragrance. This lipid was extracted from M. ootheca by vapor distillation, with a fatty acid yield of 2.72%. Its chemical composition was quantified by GC-MS: the total ion flow chart of the lipid extract is shown in Fig. 1 and the identification and content of chemical components in the lipid extract are presented in Table 1.
Fig. 1.
GC-MS total ion current chromatogram of the lipid extract from Mantidis ootheca
Table 1.
Analytical results of chemical constituents of the lipid extract from Mantidis ootheca by GC-MS
| Component | RT (min) | Molecular formula | Relative content (%) |
| Ethyl-9-hexadecenoate | 22.701 | C18H34O2 | 0.69 |
| Hexadecanoic acid ethyl ester | 22.772 | C18H36O2 | 0.44 |
| Z-11-Hexadecenoic acid | 23.437 | C16H30O2 | 2.07 |
| n-Hexadecanoic acid | 23.457 | C16H32O2 | 3.32 |
| Z-9-Octadecenoic acid methyl ester | 23.880 | C19H36O2 | 0.24 |
| Z,Z-9,12-Octadecadienoic acid | 24.082 | C18H32O2 | 0.04 |
| Ethyl oleate | 24.427 | C20H38O2 | 6.01 |
| 9,12-Octadecadienoic acid ethyl ester | 24.476 | C20H36O2 | 2.37 |
| 9,12,15-Octadecatrienoic acid ethyl ester | 24.621 | C20H34O2 | 1.81 |
| Oleic acid | 25.201 | C18H34O2 | 51.22 |
| 9,12-Octadecadienoic acid | 25.241 | C18H32O2 | 14.26 |
| 9,12,15-Octadecatrienoic acid | 25.368 | C18H30O2 | 14.26 |
| Heptacosane | 28.124 | C27H56 | 0.27 |
| Octacosane | 30.361 | C28H58 | 1.48 |
| Hentriacontane | 33.656 | C31H64 | 0.52 |
| Tetratetracontane | 35.245 | C44H90 | 0.49 |
RT: retention time
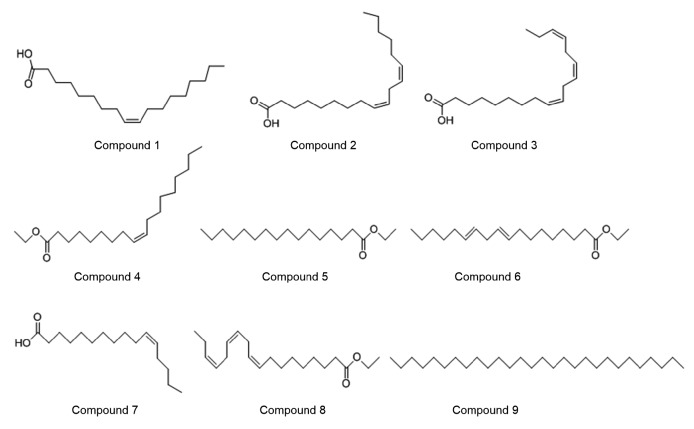
A total of 16 compounds were determined to be present in the extract, which accounted for 96.8% of the total peak area of the appraised components. The most abundant components detected in the extract were sesquiterpenoids and monoterpene in addition to the small-molecule aromatic components. The contents of the components in the lipid extract were found to be mainly: compound 1, oleic acid (51.22%); compound 2, 9,12-octadecadienoi acid (14.26%); compound 3, 9,12,15-octadecatrienoic acid (14.26%); compound 4, ethyl oleate (6.01%); compound 5, n-hexadecanoic acid ethyl ester (3.32%); compound 6, 9,12-octadecadienoic acid ethyl ester (2.37%); compound 7, Z-11-hexadecenoic acid (2.07%); compound 8, 9,12,15-octadecatrienoic acid ethyl ester (1.81%); compound 9, octacosane (1.48%). Their structures are presented in Fig. 2.
Fig. 2.
Representative compounds of the lipid extract from Mantidis ootheca
3.2. Antibacterial assay
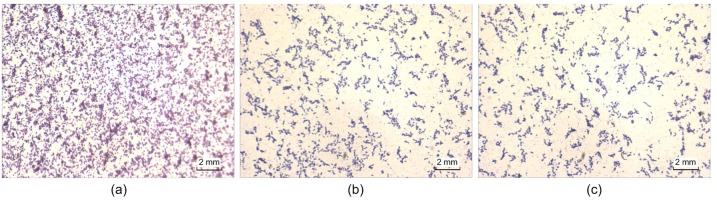
The ability of the lipid extract from M. ootheca to eradicate preformed biofilms of the strain tested was examined by the crystal violet staining method, and compared with ciprofloxacin. Microscopic images of biofilms, which were stained with crystal violet after 24 h of treatment with the MIC of the lipid extract on preformed biofilms (Fig. 3) clearly showed the disruption of the biofilm structure and the decrease of the adhesive bacteria.
Fig. 3.
Effect of treating preformed biofilms with the lipid extract from Mantidis ootheca at MIC for 24 h, imagined by microscopy with crystal violet staining
(a) DMSO control; (b) Lipid extract group; (c) Ciprofloxacin at 5 μg/ml
3.3. SEM
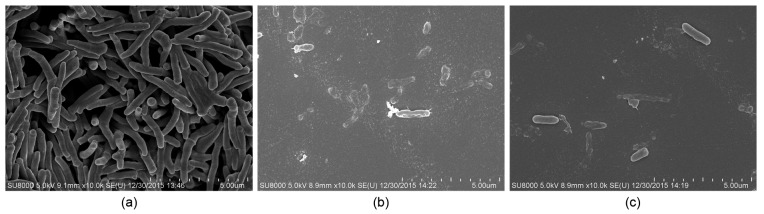
The effect of the lipid extract from M. ootheca on the morphology of the tested bacteria was studied using SEM (Fig. 4). Untreated bacterial cells were rod-shaped, with all cells presenting smooth intact surfaces. Treatment with the lipid extract at MIC and with the positive control led to severely disordered cell membranes, resulting in a corrugated and blistered appearance. Bacterial lysis was also detected with cellular remnants scattered across.
Fig. 4.
SEM images of the Pseudomonas aeruginosa
(a) DMSO control; (b) Lipid extract group; (c) Ciprofloxacin at 5 μg/ml
4. Discussion
P. aeruginosa is the main bacterium related to nosocomial infections, and has become more and more difficult to treat due to multiple drug resistance. It is also an opportunistic pathogen which can form biofilms and colonizes in the lung tissues of CF patients without good treatment (Lam et al., 1980).
Antibiotic resistance is a threat to human and animal health worldwide, and current antibacterial drugs cannot solve the problem with satisfaction (Berendonk et al., 2015). The formation of biofilm can provide the bacteria with a shield of a hydrated substance including polysaccharides and proteins (Sutherland, 2001), which makes treatment more difficult by increasing antibiotic resistance in bacteria. It has been demonstrated that about 80% of bacterial infections are related to biofilms, which offer bacteria protection and make treatment more difficult (Davies, 2003). It will be ideal to produce antibacterial drugs which can not only inhibit bacterial growth but also eradicate preformed biofilm to treat BAI in the future. The disadvantages of existing antibiotics and the demand for novel effective materials that have both antibacterial and anti-biofilm properties indicated that antibacterial drugs with a new mechanism of action should be urgently industrialized (Stewart and Costerton, 2001; Orhan et al., 2010).
So many new drugs originate from natural products dating back to ancient times because of their unique structural diversities (Baker et al., 2007). Studies on natural products for treating refractory infections have amassed significantly, resulting in the exploration of compounds with anti-biofilm ability (Chung and Toh, 2014). Previous data on natural products indicated vast potentials in treating BAI (Wang et al., 2015). For example, four compounds isolated from Krameria, Aesculus hippocastanum, and Chelidonium majus exhibited inhibition of biofilm formation in Staphylococcus aureus and Staphylococcus epidermidis strains; another two compounds that have displayed capacity for new drug manufacture (Artini et al., 2012). Tea tree oil, which was isolated from the leaves of Melaleuca alternifolia or tea tree, showed activity in eradicating the biofilms of S. aureus, including meticillin-resistant S. aureus (MRSA), by destroying the extracellular matrix and then removing the biofilm from the surface (Kwiecinski et al., 2009). Papa et al. (2015) found that some bioactive compounds, which derived from marine cold-adapted bacteria, showed anti-biofilm activity on Staphylococci and P. aeruginosa. In another research (Luis et al., 2014), Hakea sericea Schrader extracts displayed an obvious effect by inhibiting the biofilm formation of S. aureus including MRSA. The study of artemisinin (qinghaosu) by 2015 Nobel Prize winner Tu (2011) indicated the enormous potential of Chinese natural products.
In this study, the antibacterial and anti-biofilm properties of the lipid extract of M. ootheca against P. aeruginosa were evaluated and compared with ciprofloxacin. The lipid extract from M. ootheca consists of more than 16 compounds, and the proportions of oleic acid, 9,12-octadecadienoic acid, and 9,12,15-octadecatrienoic acid collectively comprise up to 79.74% of the extract. Some extracts from other natural products containing oleic acid, 9,12-octadecadienoic acid, or 9,12,15-octadecatrienoic acid were demonstrated to have antibacterial properties in previous studies (Clementi et al., 2013; Musa et al., 2015; Patra et al., 2015). However, whether the antibacterial function results from a single component or the synergistic action of several components has not yet been studied in detail. The purification and analysis of each compound need further investigation to understand the antibacterial and anti-biofilm properties. The MIC for the tested strain was 4 mg/ml. However, a standard MIC measurement was designed for planktonic bacteria which did not reveal the real antibiotic susceptibility of biofilms (Costerton et al., 1995). Therefore, an anti-biofilm assay was performed to evaluate the effect of the lipid extract on biofilm eradication. With the crystal violet staining, the lipid extract showed an obvious effect on the eradication of preformed biofilm compared with ciprofloxacin.
In the SEM assay, the results clearly indicated that the lipid extract caused severe damage to the cell membrane of the attached bacterial cells, and showed an obvious effect on biofilm, compared with the untreated bacteria. The lipid extract from M. ootheca, which was previously not reported to be related to antibacterial activities, showed an obvious effect not only on planktonic bacteria, but also on bacteria in biofilms. The results of the study indicated that the lipid extract from M. ootheca could be used as a topical and antibacterial agent with anti-biofilm activity in the future. However, further research is needed to determine the specific compound in M. ootheca that creates the observed effect.
Acknowledgments
We sincerely thank Prof. Jing WANG (Dalian University, Dalian, China) for providing technical assistance for this study.
Footnotes
Compliance with ethics guidelines: Wen-dong WANG, Nan-nan ZHANG, Warren CHANDA, Min LIU, Syed Riaz ud DIN, Yun-peng DIAO, Lei LIU, Jing CAO, Xiao-li WANG, Xing-yun LI, An-hong NING, Min HUANG, and Min-tao ZHONG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Artini M, Papa R, Barbato G, et al. Bacterial biofilm formation inhibitory activity revealed for plant derived natural compounds. Bioorg Med Chem. 2012;20(2):920–926. doi: 10.1016/j.bmc.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 2.Baker DD, Chu M, Oza U, et al. The value of natural products to future pharmaceutical discovery. Nat Prod Rep. 2007;24(6):1225–1244. doi: 10.1039/b602241n. [DOI] [PubMed] [Google Scholar]
- 3.Berendonk TU, Manaia CM, Merlin C, et al. Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol. 2015;13(5):310–317. doi: 10.1038/nrmicro3439. [DOI] [PubMed] [Google Scholar]
- 4.Chung PY, Toh YS. Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus . Pathog Dis. 2014;70(3):231–239. doi: 10.1111/2049-632X.12141. [DOI] [PubMed] [Google Scholar]
- 5.Clementi EA, Wilhelm KR, Schleucher J, et al. A complex of equine lysozyme and oleic acid with bactericidal activity against Streptococcus pneumoniae . PLoS ONE. 2013;8(11):e80649. doi: 10.1371/journal.pone.0080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Ann Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 7.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 8.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 9.de Zoysa GH, Cameron AJ, Hegde VV, et al. Antimicrobial peptides with potential for biofilm eradication: synthesis and structure activity relationship studies of battacin peptides. J Med Chem. 2015;58(2):625–639. doi: 10.1021/jm501084q. [DOI] [PubMed] [Google Scholar]
- 10.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 11.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19(12):1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Park HD, Chung S. Microfluidic approaches to bacterial biofilm formation. Molecules. 2012;17(8):9818–9834. doi: 10.3390/molecules17089818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiecinski J, Eick S, Wojcik K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int J Antimicrob Agents. 2009;33(4):343–347. doi: 10.1016/j.ijantimicag.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Lam J, Chan R, Lam K, et al. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Wu HQ, Liu ZG. Acaricidal activity of clove bud oil against Dermatophagoides farinae (Acari: Pyroglyphidae) Chin J Parasitol Parasit Dis. 2009;27(6):492–493. 492. (in Chinese) [PubMed] [Google Scholar]
- 16.Luis A, Breitenfeld L, Ferreira S, et al. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharmacogn Mag. 2014;10(Suppl 1):S6–S13. doi: 10.4103/0973-1296.127331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy K, Park AJ, Hao Y, et al. Influence of O polysaccharides on biofilm development and outer membrane vesicle biogenesis in Pseudomonas aeruginosa PAO1. J Bacteriol. 2014;196(7):1306–1317. doi: 10.1128/JB.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musa AM, Ibrahim MA, Aliyu AB, et al. Chemical composition and antimicrobial activity of hexane leaf extract of Anisopus mannii (Asclepiadaceae) J Intercult Ethnopharmacol. 2015;4(2):129–133. doi: 10.5455/jice.20150106124652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell F, Smyth TJP, Ramachandran VN, et al. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolones. Int J Antimicrob Agents. 2010;35(1):30–38. doi: 10.1016/j.ijantimicag.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Oliver A, Mulet X, Lopez-Causape C, et al. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat. 2015;21-22:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Orhan DD, Ozcelik B, Ozgen S, et al. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol Res. 2010;165(6):496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Papa R, Selan L, Parrilli E, et al. Anti-biofilm activities from marine cold adapted bacteria against staphylococci and Pseudomonas aeruginosa . Front Microbiol, 6:1333. 2015 doi: 10.3389/fmicb.2015.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patra JK, Das G, Baek KH. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules. 2015;20(7):12093–12113. doi: 10.3390/molecules200712093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silby MW, Winstanley C, Godfrey SA, et al. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 2011;35(4):652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 25.Sitaram C, Rupakula RB, Reddy BN, et al. Determination of alkyl methanesulfonates in doxazosin mesylate by gas chromatography-mass spectrometer. Indian J Pharm Sci. 2011;73(1):107–110. doi: 10.4103/0250-474X.89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland IW. The biofilm matrix–an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9(5):222–227. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 28.Tan Z, Lei Y, Zhang B, et al. Comparison of pharmacological studies on Ootheca Mantidis . China J Chin Mater Med. 1997;22(8):496–499. (in Chinese) [PubMed] [Google Scholar]
- 29.Tu YY. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17(10):1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Chanda W, Zhong M. The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol Lett. 2015;362(15):fnv117. doi: 10.1093/femsle/fnv117. [DOI] [PubMed] [Google Scholar]
- 31.Wen LL, Wan DG, Ren Y, et al. Corresponding relationship between Mantis and Mantidis oötheca (Sangpiaoxiao) China J Chin Mater Med. 2013;38(7):966–968. (in Chinese) [PubMed] [Google Scholar]
- 32.Wolcott RD, Rhoads DD, Bennett ME, et al. Chronic wounds and the medical biofilm paradigm. J Wound Care. 2010;19(2):45–46. doi: 10.12968/jowc.2010.19.2.46966. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Liu Y, Wu H, et al. Combating biofilms. FEMS Immunol Med Microbiol. 2012;65(2):146–157. doi: 10.1111/j.1574-695X.2011.00858.x. [DOI] [PubMed] [Google Scholar]