Abstract
Oxidative stress is a critical feature of several common neurologic disorders. The brain is well adapted to neutralize oxidative injury by maintaining a high steady-state concentration of small-molecule intracellular antioxidants including glutathione in astrocytes and ascorbic acid in neurons. Ascorbate-derived imaging probes for hyperpolarized 13C magnetic resonance spectroscopy and positron emission tomography have been used to study redox changes (antioxidant depletion and reactive oxygen species accumulation) in vivo. In this study, we applied these imaging probes to the normal rat brain and a rat model of glutathione depletion. We first studied hyperpolarized [1-13C]dehydroascorbate in the normal rat brain, demonstrating its robust conversion to [1-13C]vitamin C, consistent with rapid transport of the oxidized form across the blood-brain barrier. We next showed that the kinetic rate of this conversion decreased by nearly 50% after glutathione depletion by diethyl maleate treatment. Finally, we showed that dehydroascorbate labeled for positron emission tomography, namely [1-11C]dehydroascorbate, showed no change in brain signal accumulation after diethyl maleate treatment. These results suggest that hyperpolarized [1-13C]dehydroascorbate may be used to non-invasively detect oxidative stress in common disorders of the brain.
Introduction
Reactive oxygen species (ROS) are expected products of oxidative metabolism, and must be tightly regulated in the normal brain. Several pathways are required to maintain redox homeostasis, in particular the glutathione-glutaredoxin and thioredoxin-thioredoxin reductase systems1. These systems maintain high steady-state concentrations of small-molecule antioxidants including reduced glutathione (GSH) and vitamin C (VitC). Glial cells harbor a high concentration (in the mM range) of GSH, while neurons capture the majority of the VitC in the central neural system2,3. Although the reasons for this partitioning are only partially understood, these antioxidants are considered critical in both health and disease. Oxidative stress, marked by increased ROS production and GSH consumption, is implicated in various neurodegenerative and neuropsychiatric disorders such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and schizophrenia4,5. Therefore, non-invasive evaluation of brain antioxidants could provide critical insights into these disease processes.
The relationship between VitC and its oxidized counterpart dehydroascorbic acid (DHA) has been extensively studied. In contrast to VitC, which is slowly imported via sodium-dependent vitamin C transporter-2 (SVCT-2) and maintained at high steady-state concentrations in the brain6, DHA is a transient species with remarkably different transport properties despite its structural similarity to VitC. DHA readily crosses the blood-brain barrier (BBB), actively transported by glucose transporters (GLUTs) as shown in Fig. 1a7–9. Once transported into cells, DHA undergoes a GSH-dependent, two-electron reduction to VitC, catalyzed by redox enzymes including glutaredoxin, omega-class glutathione transferases and protein-disulfide isomerase10–13. This GSH-dependent conversion is one of the motivations for studying ascorbate-derived imaging agents, whose interconversion depends on both the concentration of GSH and the presence or absence of reactive oxygen species14,15. In particular, [1-13C]DHA, a 13C enriched version of dehydroascorbic acid, has been studied with hyperpolarized (HP) 13C magnetic resonance (MR) in preclinical models of cancer, diabetes, acute kidney injury, and fatty liver disease15–20. In addition, ascorbates labeled for positron emission tomography (PET), namely [1-11C]DHA and [1-11C]VitC, have recently been synthesized and studied in ROS-producing immune cells in vitro21. Together, these studies suggest that ascorbate-derived imaging methods may be able to image oxidative stress in vivo. In particular, DHA-derived imaging agents were designed to image intracellular redox capacity, typically defined as the concentration of intracellular reducing equivalents and the ability to regenerate them. We therefore compared these methodologies in an animal model of pharmacologic glutathione depletion, to determine if ascorbate-derived HP MR or PET probes could detect the modulation of redox capacity.
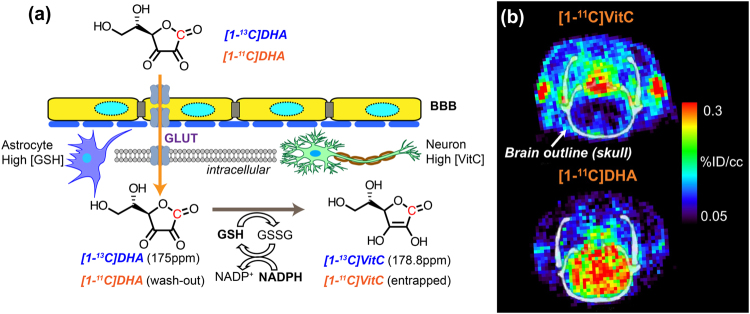
Figure 1.
Ascorbates as redox imaging probes. (a) Dehydroascorbate (DHA) can traverse the blood-brain-barrier, enter cells via glucose transporter (GLUT)1,3,4 and be rapidly reduced to ascorbic acid (vitamin C, VitC) by intracellular antioxidants including glutathione (GSH), which is coupled with the pentose phosphate pathway via NADPH. The conversion from hyperpolarized [1-13C]DHA to [1-13C]VitC can be monitored by MR spectroscopy, taking advantage of their unique chemical shifts. The C1 position of DHA and VitC can also be labelled with 11C for PET and biodistribution studies. (b) In these PET images (adapted from Carroll et al.21), the accumulation of [1-11C]DHA in the normal rat brain is compared to that of [1-11C]VitC in the normal rat brain. In normal rats, higher brain signal for [1-11C]DHA is expected since it readily crosses the blood-brain barrier and is transported into cells via GLUT transporters. In contrast, transport of VitC into the brain via sodium-dependent vitamin C transporter-2 (SVCT2) is a slower process. Unlike measuring the real-time conversion rate by hyperpolarized MR, PET and biodistribution with [11C]ascorbates measures the uptake and retention of 11C radiopharmaceuticals.
Although [1-13C]DHA and [1-11C]DHA are chemically and biochemically equivalent, the techniques used to study them in vivo are considerably different. HP 13C MR via dissolution dynamic nuclear polarization (dDNP) is an emerging imaging technology that allows direct visualization of metabolic conversion of 13C-enriched molecules, and has been applied extensively to study metabolism in cells, tissues, animal models, and patients22–24. dDNP transfers polarization from electron spins to 13C nuclei spins at extreme low temperature (0.8–1.3 °K) through microwave irradiation, providing more than 10,000-fold MR signal enhancement of 13C-labelled substrates25. The HP substrate can be subsequently injected into a living system and its conversion to other metabolites can be monitored by MR spectroscopy in a rapid, real-time manner. HP MR with [1-13C]DHA takes advantage of the chemical shift between [1-13C]DHA and [1-13C]VitC (3.8 ppm) and the drastic signal enhancement (~10% polarization, >104-fold at 3 Tesla) provided by dDNP to enable in vivo interrogation of redox capacity. Since the hyperpolarized signal lifetime is prescribed by the T1 of the substrate (approximately 57 s for [1-13C]DHA at 3 Tesla), HP 13C signal can be observed in vivo for approximately 1 to 2 minutes at most23. Therefore, the real-time detection of [1-13C]DHA to [1-13C]VitC in vivo is primarily a kinetic measurement that depends on substrate transport and the availability of intracellular reducing equivalents.
In contrast, PET is an established metabolic imaging technology whereby a positron-emitting isotope, such as 11C or 18F, is incorporated into a drug or metabolite of interest, and usually imaged following a significant delay depending on the half-life of the radioactive isotope. Therefore, the primary determinants of image contrast are the transport and retention/entrapment of the radiopharmaceutical, and the clearance of nonspecific signal. As shown in Fig. 1b, PET images of rat brains demonstrated the distinctive difference in accumulated signal between [1-11C]DHA and [1-11C]VitC, as previously reported, reflecting their differential transport across the blood-brain barrier and cellular membrane21. In the case of [1-11C]DHA, the radiopharmaceutical is expected to be entrapped inside the cell once it is reduced to [1-11C]VitC, a charged molecule at physiological pH, and unreacted [1-11C]DHA may be washed out. We hypothesized that retention of [1-11C]DHA would depend partially on intracellular reducing agents including GSH26, and therefore may also be modulated following glutathione-depletion.
In this study, we aimed to characterize ascorbate-derived HP 13C MR and 11C PET probes for brain imaging, and take advantage of their different capabilities to assess in vivo redox capacity of the rat brain. First, we studied the transport, compartmentalization, and biodistribution of both DHA and VitC in normal rat brain with HP 13C MR and 11C PET probes. Then we modulated GSH content in rats by pharmacological treatment and studied the changes in DHA to VitC conversion in the brain using both HP 13C MR and 11C PET probes. Specifically, four groups of Sprague-Dawley rats were studied using: (1) two-dimensional (2D) chemical shift imaging (CSI) with HP [1-13C]DHA and [1-13C]VitC separately to determine the transport and compartmentalization of ascorbates in the head region (n = 6 for DHA, n = 3 for VitC); (2) one-dimensional (1D) slice-selective dynamic spectroscopy with HP [1-13C]DHA, at 24 hours before and 2 hours after diethyl maleate (DEM) intraperitoneal injection (4.6 mmol/kg)27, to measure the real-time DHA to VitC conversion rate (n = 4); (3) biodistribution using [1-11C]DHA and [1-11C]VitC, respectively, to determine the accumulation of ascorbate-derived probes in the brain and other organs (n = 3 each); (4) biodistribution with [1-11C]DHA in rats with DEM treatment as in group (2) to measure 11C probe accumulation following glutathione depletion (n = 3). Additionally, 1D slice-selective dynamic spectroscopy with HP [1-13C]DHA at 60% concentration of group (2) was performed to demonstrate DHA to VitC reaction order (n = 3), and an in vitro GSH assay was performed on brain tissues collected from normal and DEM-treated rats to validate the redox modulation by DEM (n = 7 each).
Results
Behavior of HP 13C DHA and VitC in vivo is consistent with known features of blood-brain barrier penetration and transport
Both HP [1-13C]DHA and HP [1-13C]VitC were administered separately to normal rats (group 1). Following HP [1-13C]DHA injection, its reduction to [1-13C]VitC was observed in the brain, demonstrated by the presence of a new downfield resonance (approximate 3.8ppm) in the 13C MR spectrum (n = 6). For HP [1-13C]DHA study, the DHA resonance was observed in most tissue voxels including brain and its surrounding tissues (muscle and vasculature), while the VitC metabolite was only observed in the brain voxels (Fig. 2a) with VitC/(DHA + VitC) = 0.48 ± 0.15 (estimated from 18 brain voxels), consistent with penetration of DHA across the blood-brain barrier. In contrast, when HP VitC is injected, the 2D CSI (n = 3) found drastically lower VitC signal in the brain (mostly below quantification limit) than in surrounding tissues and no oxidation to DHA (Fig. 2b). These data are consistent with the slower transport of VitC across the blood-brain barrier, and the high reducing capacity of the normal brain8.
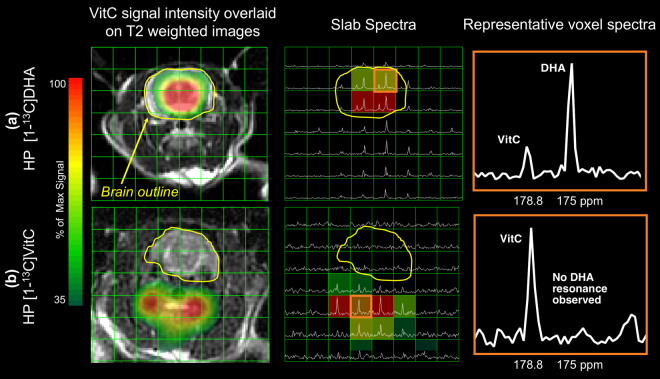
Figure 2.
2D Chemical Shift Imaging with hyperpolarized 13C ascorbates reveals different transport and compartmentalization of dehydroascorbate (DHA) and vitamin C (VitC). (a) Injection of hyperpolarized [1-13C]DHA, with conversion to VitC in the normal rat brain. The VitC resonance is only observed in voxels corresponding to the brain as shown in the VitC signal intensity map and slab spectra, while the DHA resonance is observed both in the brain and surrounding tissue. A representative voxel corresponding to brain tissue shows both a resonance corresponding to the introduced hyperpolarized [1-13C]DHA and its metabolite [1-13C]VitC. (b) Injection of hyperpolarized [1-13C]VitC in normal rats. Lower (in this case negligible) VitC signal is seen in voxels corresponding to brain as shown in the VitC signal intensity map and slab spectra, and no oxidation to DHA is observed. A representative voxel corresponding to tissues in the neck show a resonance corresponding to the introduced hyperpolarized [1-13C]VitC without evidence of metabolic conversion.
Glutathione depletion via diethyl maleate results in a lower rate of DHA-to-VitC conversion
Using a 1D dynamic HP [1-13C]DHA study, we investigated whether the DHA to VitC conversion rate is correlated with GSH content in the rat brain (group 2). We adopted a variable flip angle scheme to account for the non-renewable nature of hyperpolarized signal and to utilize signal-to-noise ratio (SNR) efficiently since the T1 of [1-13C]VitC is relatively short (29.1 s, in vitro at 3 T)16. Dynamic spectra of the rat brain showed that the [1-13C]VitC signal gradually increases during the acquisition window used (Fig. 3), suggesting VitC production rate is greater than signal decay rate mediated by T1 relaxation. Because VitC signal is mainly from in the brain while DHA signal is from both the brain and surrounding tissues in the 1D slab spectra, we focused our analysis on the kinetic rates of VitC production as opposed to including both DHA and VitC in the kinetic model or using ratiometric approach.
Figure 3.
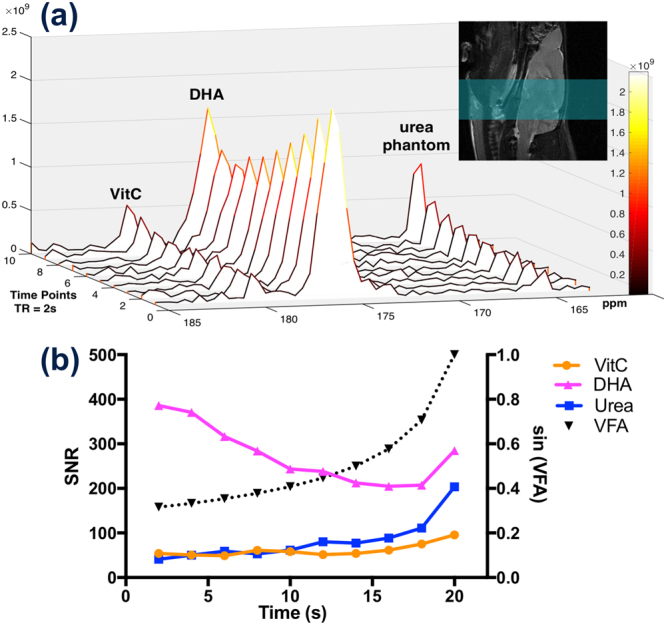
Dynamic 1D slice-selective spectroscopy reveals real-time hyperpolarized [1-13C]dehydroascorbate (DHA) to [1-13C]vitamin C (VitC) conversion. MR spectra of a normal rat brain is shown in (a), and signal-to-noise ratio quantification of DHA, VitC, and urea phantom (solid line), as well as variable flip angle (VFA) scheme (dash line), are plotted in (b). Hyperpolarized [1-13C]VitC signal in a normal rat brain gradually increases over time despite the rapid longitudinal relaxation (in vitro T1 ~ 29.2 s at 3 Tesla), and hyperpolarized [1-13C]DHA signal gradually decreases due to metabolic conversion and longitudinal relaxation (in vitro T1 ~ 56.5 s at 3 Tesla). Signal corresponding to the [13C]urea phantom (placed next to the rat head) also gradually increases, demonstrating the VFA scheme. A sagittal T2-weighted image demonstrates the imaging slab (green highlighted region).
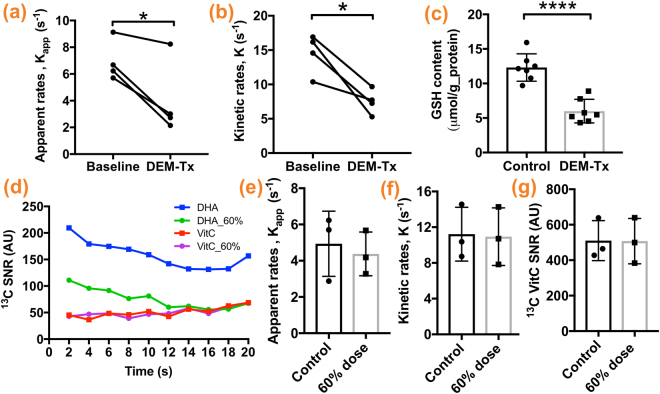
We found the apparent VitC production rate (kapp) decreased by nearly 40% from 6.94 ± 1.52 s−1 to 4.03 ± 2.83 s−1 (p = 0.0293) after DEM treatment (Fig. 4a). The apparent in vivo T1 of VitC was estimated to be 7.80 ± 1.28 s (n = 9) using a subset of data (3 datasets were excluded due to poor fitting quality), and we found kinetic rate (k) decreased by nearly 50% from 14.15 ± 2.93 s−1 to 7.48 ± 1.80 s−1 (p = 0.0218) after DEM treatment (Fig. 4b). Correspondingly, the in vitro GSH assay (methods reported in the supplementary information) showed that brain GSH content in the DEM-treated rats is significantly lower than in the control rats (5.99 ± 0.64 vs. 12.31 ± 0.75 μmol/g protein, n = 7, p < 0.0001) (Fig. 4c).
Figure 4.
Glutathione depletion via diethyl maleate (DEM) results in a significantly lower rate of [1-13C]dehydroascorbate (DHA) to [1-13C]vitamin C (VitC) conversion. Both apparent VitC production rate (kapp) and kinetic rate (k) of VitC production in the brain decreased significantly after DEM treatment (a,b), consistent with significantly lower brain GSH content in DEM-treated rats compared to control rats (c). To demonstrate DHA to VitC reaction order, 1D spectroscopy was performed on a separate cohort of rats with HP [1-13C]DHA at 60% of the usual dose. The average signal of [1-13C]DHA and [1-13C]VitC at each time point were plotted (d), and VitC signal appears unchanged with decreased DHA dose. The resulting kapp, k, and total 13C VitC SNR are not significantly different from the control group (e,f,g). (*p < 0.05, ****p < 0.0001).
To validate our kinetic modeling of the DHA to VitC conversion, dynamic 1D spectroscopy was performed on a separate cohort of rats (n = 3) with 18 μmoles HP DHA (approximately 60% dose of the control/baseline group). We found there was no statistically significant difference between the control/baseline group and lower dose group in kapp (4.94 ± 1.04 vs. 4.38 ± 0.70 s−1, p = 0.678), or k (11.22 ± 1.74 vs. 0.94 ± 1.86 s−1, p = 0.917) as well as the sum of VitC signal (510.3 ± 65.06 vs. 507.6 ± 73.94 A.U., p = 0.9792) (Fig. 4d–g), suggesting that DHA dose used in our experimental set-up was in excess and not a rate limiting factor for VitC production.
11C Biodistribution studies showed expected differences between [1-11C]DHA and [1-11C]VitC, but no effect of diethyl maleate treatment on [1-11C]DHA brain accumulation
The biodistribution of [1-11C]DHA or [1-11C]VitC was compared by ex vivo biodistribution analysis on harvested normal rat tissues 1 hour following administration (group 3, n = 3 each). In addition, [1-11C]DHA was administrated to DEM-treated rats (group 4, n = 3) to study the effect of GSH content on 11C radiopharmaceutical accumulation. Biodistribution of 11C radiopharmaceutical in three groups of rats are shown in Fig. 5. [1-11C]VitC showed lower uptake/retention in the brain than [1-11C]DHA (0.25 ± 0.012 vs. 0.49 ± 0.075% Injection Dose(ID)/g, p = 0.0330), consistent with our HP MR observations and our previously reported PET imaging study (Fig. 1b). However, lung and liver showed significantly higher uptake/retention for [1-11C]VitC than [1-11C]DHA (lungs: 2.10 ± 0.12 vs. 0.42 ± 0.066%ID/g, p = 0.0002; liver: 3.57 ± 0.30 vs. 2.12 ± 0.39%ID/g, p = 0.0408). For the glutathione modulation study using [1-11C]DHA, no significant difference was found in radiopharmaceutical accumulation for major organs between control and DEM-treated subjects (0.49 ± 0.075 vs. 0.62 ± 0.14%ID/g, p = 0.449).
Figure 5.
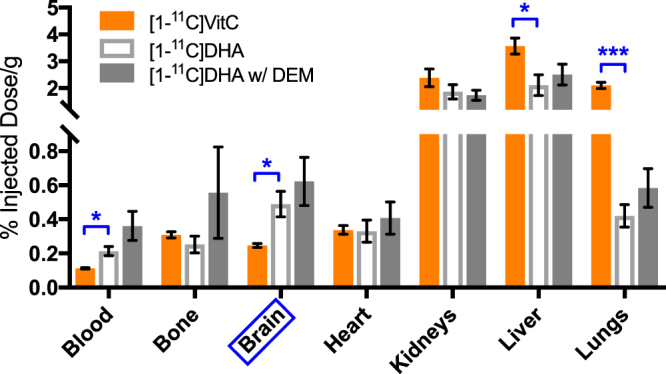
Biodistribution studies reveal different accumulation of [1-11C]dehydroascorbate (DHA) and [1-11C]vitamin C (VitC) in normal rat brain, but no effect of diethyl maleate (DEM) treatment. Gamma counting of harvested brains showed a significantly higher signal accumulation for [1-11C]DHA than [1-11C]VitC in the normal brain 1 hour following radiopharmaceutical administration. In addition, there was significantly higher uptake/retention for [1-11C]VitC versus [1-11C]DHA in the lungs and liver in normal rats. In the DEM-treated rats with [1-11C]DHA, no significant differences were found in 11C radiopharmaceutical uptake/retention between normal and DEM-treated rats in major organs, including the brain. (*p < 0.05, ***p < 0.001).
Discussion
Non-invasive in vivo evaluation of brain redox capacity and oxidative stress could provide insights into neurological disease progression and therapeutic response. In this study, we used ascorbate-based imaging probes to study brain redox capacity leveraging both HP-MR and PET modalities. We first demonstrated that HP [1-13C]DHA is readily converted to HP [1-13C]VitC in the normal brain, evidenced by 2D chemical shift imaging revealing both DHA and VitC resonances in voxels corresponding to brain tissues following [1-13C]DHA injection. This finding is consistent with the penetration of DHA through BBB and its rapid entry into cells. In contrast, when HP [1-13C]VitC was administered, lower hyperpolarized signal was seen in brain voxels and no oxidation to HP [1-13C]DHA was observed. These findings reflect the slow cellular transport process of VitC via SVCT2 against a concentration gradient, and the role of VitC as an antioxidant reservoir that is not easily oxidized. These features represent the major shortcomings of [1-13C]VitC as a HP MR probe for imaging the brain.
After characterizing the transport and compartmentalization of DHA and VitC, we modulated GSH content with DEM treatment which depletes GSH by direct conjugation27, and studied the changes in DHA to VitC kinetic rates using dynamic HP 13C MR spectroscopy. 2D CSI with radiometric quantification has lower reproducibility due to sensitivity to small differences in acquisition timing and substrate delivery, demonstrated by the variabilities in VitC/DHA ratios in 6 normal rats (reported in Supporting Information). Therefore, we adopted 1D slab dynamic acquisition approach with variable flip angle scheme for efficient SNR usage, which is important given the short T1 of [1-13C]VitC, to study the effect of redox modulation. In dynamic spectra of normal brain, there was observable real-time production of VitC; we also observed that DHA signal is about 5 times higher than VitC signal, suggesting that cellular transport and/or enzyme and cofactor turnover rate are likely rate-limiting factors for the observed VitC production. In other words, these data suggested that the DHA dose was in excess and not a rate-limiting factor in our experimental set-up. To demonstrate the reaction order, we performed dynamic spectroscopy with lower (60%) doses of [1-13C]DHA and found that the kinetic rates of VitC production and sum of VitC SNR are independent of DHA concentration, which validated the pseudo-zero-order kinetic model. To quantify in vivo reaction kinetics, we generated two kinetic parameters (reported in Methods section): apparent VitC production rate (kapp) is a straightforward parameter that reflects the rate of product accumulation, assuming T1 relaxation equally affects the control and DEM-treated groups; whereas kinetic rate (k) was generated by non-linear fitting and takes account of T1 relaxation. We found that DHA to VitC conversion rate correlated with the brain GSH content, as evidenced by significantly reduced kinetic parameters (kapp and k) after DEM treatment that depletes GSH by more than 50%.
There are several important considerations and limitations of our dynamic [1-13C]DHA MR study. First, each animal was subjected to two intravenous injections of [1-13C]DHA, an oxidant that could deplete GSH, 24 hours apart between baseline and post-DEM-treatment imaging12. However, Weber et al. have demonstrated that rat brain GSH content returns to normal 24 hours after DEM treatment, a prototypical GSH deleting agent that directly conjugates with GSH and is more potent than DHA27. Furthermore, depletion of GSH by DHA could induce increases in other intracellular reducing equivalents (such as thioredoxins) whose pool size would be expected to impact the rate of DHA to VitC reduction. Timm et al. have shown that DHA administration resulted in a rapid increase in GSSG/GSH ratio and pentose phosphate pathway (PPP) flux in cells and tumors20. It can be argued that the initial DHA administration for baseline imaging could upregulate PPP flux and other reducing equivalents, which would be a confounding factor for our study. Nevertheless, the DEM treatment seemed to offset the increase in PPP flux and other reducing equivalents as the VitC production rate decreased significantly. Second, the supraphysiologic concentration of administered HP [1-13C]DHA could saturate the transporter or relevant enzymes and create a rate-limiting condition, or result in substrate inhibition and decrease of enzymatic flux. Third, unlike the first-order reaction model, the kinetic parameters generated from pseudo-zero-order model are highly influenced by the numerical scale (i.e. SNR), which could potentially explain the inter-subject variability. For this reason, our dynamic acquisition and analysis scheme could only detect the relative changes in GSH content, but not the absolute concentration.
Another important consideration is the physiologic effect of DEM treatment in rats. We noted that rats became lethargic after DEM treatment. Numerous studies have explored the potential toxic effects of DEM in the heart and lung, and the dose we used was approximately 40% of the LD50 reported for oral administration to rats28–31. We also observed that the total 13C SNR was approximately 35% lower on average in DEM-treated than normal rats despite similar DNP solid state buildup, suggesting less HP 13C substrate was delivered to the imaging slab. Although we have demonstrated that the rate of VitC production is independent of DHA dose administered in our supraphysiologic dose range, it is still possible that lower DHA substrate delivery contributed to the lower VitC production rate.
In addition to ascorbate-derived probes designed for HP MR, we also leverage 11C radiopharmaceuticals, namely [1-11C]DHA and [1-11C]VitC, to study the uptake and biodistribution of ascorbates. [1-11C]VitC showed lower accumulation than [1-11C]DHA in the brain, consistent with HP 13C MR findings and previously reported PET imaging data21, but higher retention in the lung and liver 1 hour after administration, possibly due to high expression of sodium-depended vitamin C transporters (SVCT) in these tissues32–34. [1-11C]DHA reduction to [1-11C]VitC represents a potential trapping mechanism21,26, with unreduced [1-11C]DHA likely washed out of the cell. In the DEM redox modulation study using [1-11C]DHA, we initially hypothesized that the lower brain GSH content in DEM-treated cannot adequately reduce [1-11C]DHA to [1-11C]VitC, and un-reacted [1-11C]DHA would be washed out resulting less [1-11C]VitC retention (lower gamma counting). However, there was no difference in 11C radiopharmaceutical retention rate in major organs between the normal and DEM-treated groups.
There are several important differences between HP 13C MR and 11C biodistribution studies that could explain the inconsistency between their findings. As discussed in Methods section, 11C radiopharmaceuticals were administrated at a considerably lower pharmacologic dose compared to HP 13C probes (0.16μmol vs. 30μmol). It is possible that residual GSH in DEM-treated rats is adequate to reduce the lower pharmacologic dose of DHA used in 11C studies. More importantly, the two technologies differ significantly in their mechanisms of generating image contrast and timing, making direct comparison of data difficult. Biodistribution studies were performed at 1 hour after DHA administration as compared to the 1 minute kinetic analysis applied to HP MR studies. Hence, it appears that although [1-11C]VitC was likely generated at a slower rate in GSH-depleted animals, there was insufficient washout of unreacted [1-11C]DHA to yield observable differences in 11C accumulation. It is possible that dynamic PET imaging or biodistribution studies by gamma counting at earlier time points could reveal subtle differences in the radiopharmaceutical accumulation rate in the brain. Overall, our results suggested that the [1-11C]DHA was insensitive to changes in redox capacity in this pharmacologic GSH depletion model using our experiment set-up. This highlights the potential value in studying real-time substrate conversion by HP MR as opposed to probe retention rate by biodistribution of radiopharmaceutical or PET imaging analysis.
In conclusion, we demonstrated the transport and biodistribution features of DHA and VitC in the brain by both HP 13C MR and 11C PET probes, and found that the kinetic rates of [1-13C]DHA to [1-13C]VitC conversion correlated with brain GSH content. These studies suggest that hyperpolarized [1-13C]DHA can assess brain redox capacity non-invasively, and potentially allow visualization of oxidative stress in numerous brain pathologies.
Methods
Dynamic Nuclear Polarization (DNP)
Both [1-13C]DHA and [1-13C]VitC were prepared, polarized, and dissolved in aqueous media as previously described14. A 2.2 M solution of [1-13C]DHA dimer (Sigma Aldrich ISOTEC, Miamisburg, OH) in dimethyacetamide (DMA) containing 15 mM OX063 trityl radical (GE Healthcare, Menlo Park, CA) was polarized on a HyperSense DNP instrument (Oxford Instruments, Abingdon, UK) operating at 3.35 Tesla and 1.3°K, achieving approximately 10% polarization after 1 hour microwave irradiation (determined by separate NMR studies using the same material). The frozen sample was then rapidly dissolved in distilled water containing 0.3 mM ethylenediaminetetracetic acid (EDTA) with a final concentration of 15–22 mM and pH of 5.5. For all in vivo studies, approximately 30 μmoles of [1-13C]DHA (1.3–2.0 ml) were administered intravenously to rats. Similarly, a 2.2 M solution of [1-13C]VitC (Omicron Biochemicals, South Bend, IN) was prepared as a sodium salt in NaOH/water/DMSO, polarized, and dissolved in 100 mM phosphate buffer to a final pH of 7.0. For 2D CSI studies, approximately 100 μmoles of HP [1-13C]VitC were administered intravenously.
11C radiopharmaceutical synthesis
[1-11C]VitC were synthesized from L-xylosone and [11C]HCN precursors using a GE PETtrace cyclotron at our on-site radiopharmaceutical facility as previously described21 and reported in the supplementary information. Overall, [1-11C]VitC was obtained with a decay-corrected radiochemical yield of 35.8 ± 18% and a specific activity of 1.7 ± 0.4 mCi/μmol (n = 3). [1-11C]DHA was obtained by oxidation of [1-11C]VitC with a decay-corrected radiochemical yield of 25.8 ± 2.6% and a specific activity of 1.85 ± 0.5 mCi/μmol (n = 6).
MR studies
All animal experiments were approved by our Institutional Animal Care and Use Committee (IACUC) and performed in accordance with IACUC protocols. Rats were placed under 1.5–2% isoflurane anesthesia and administered 30 μmoles HP [1-13C]DHA or 100 μmoles HP [1-13C]VitC solution via tail-vein catheter for both 2D CSI studies and dynamic 1D spectroscopy. For group (1) rats (n = 6 for DHA and n = 3 for VitC), two-dimensional single time-point CSI was performed on a GE Signa 3 Tesla MR scanner using a dual-tuned 1H-13C coil with the following parameters: voxel size = 5 × 5 × 20 mm, TE/TR = 135/210 ms, flip angle = 20°, spectral bandwidth/resolution = 5000/9.77 Hz, and 25 s acquisition delay after the start of injection. For group (2) rats, one-dimensional slice-selective dynamic spectroscopy with HP [1-13C]DHA was performed using a similar set-up on a GE Signa 3 Telsa (n = 2 before and after DEM treatment) and a Bruker Biospec 3 Tesla MR scanner (n = 3 before DEM treatment, n = 2 after DEM treatment, n = 3 for 60% dose experiments), with the following parameters: spectral bandwidth/resolution = 25 kHz/12.2 Hz, slice thickness = 10 mm, TR = 2 s, 10 repetitions, RF-compensated variable flip angle scheme35,36, and 25 s acquisition delay after the start of injection. Axial and sagittal T2-weighted anatomic images were acquired with a fast spin echo sequence prior to HP 13C studies and used to prescribe the spectroscopy slab. Rats were fasted for 8 hours before HP 13C MR studies to reduce glucose competition with DHA for GLUTs as previously reported14,17,37.
11C biodistribution studies
Rats were placed under 1.5–2% isoflurane anesthesia and administered 304 ± 78 μCi [1-11C]DHA or 360 ± 180 μCi [1-11C]VitC via tail vein catheters (for group 3 and 4 rats). Animals were subsequently sacrificed at 1 hour after radiopharmaceutical injection, and internalized radioactivity was immediately measured on their harvested organs using a Hidex automatic gamma counter (Turku, Finland) (n = 3 each).
Data analysis
2D CSI data was reconstructed and overlaid on axial anatomic images using SIVIC38; the peak heights of DHA and VitC resonances were manually quantified on the magnitude spectrum. The metabolite ratio, VitC/(DHA + VitC), was calculated for each voxel. For 1D dynamic MR spectroscopy data, SNRs of DHA and VitC resonances for each time point were quantified based on the fit peak integral in MestreNova12 (Madrid, Spain), and were subsequently used to generate kinetic parameters in MATLAB 2017b (Natick, MA). We demonstrated that the DHA to VitC conversion in this experiment was a pseudo-zero-order reaction as shown in Results section, and generated two kinetic parameters based on a zero-order kinetic model assuming no back conversion from VitC to DHA: (1) apparent VitC production rate (kapp) via linear fitting of VitC signal and normalized to total VitC SNR, and (2) kinetic rate (k) via non-linear least square fitting of VitC signal with the Trust-Region algorithm to the following equations:
| 1 |
| 2 |
where Mz is longitudinal magnetization, Mxy is transverse magnetization (observed signal), n is the index of time points, and is the flip angle. Dynamic data was first fit into kinetic models with T1 as an unknown parameter to estimate apparent in vivo T1 of VitC, which was subsequently incorporated into the kinetic model as a fixed parameter to generate k assuming T1 is the same across the subjects. Apparent kinetic rate (kapp) reflects the rate of observed VitC signal change caused by both T1 relaxation and VitC production from DHA, while k reflects only the VitC production rate.
A paired Student’s t-test was used to compare kinetic parameters before and after DEM treatment. An unpaired Student’s t-test was used to compare the GSH content of rat brains with and without DEM treatment and biodistribution of [1-11C]ascorbates. All statistical analyses were performed using GraphPad Prism 7d (La Jolla, CA). All quantitative results are reported and graphed as mean ± standard deviation; p values < 0.05 were considered statistically significant, and our p values for biodistribution analysis were not adjusted for multiple comparisons.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work is supported by National Institute of Health [grant number: R01CA166766 (DMW), P41EB013598 (JK)], and UCSF Department of Radiology and Biomedical Imaging internal grants. We thank Robert Bok, MD, PhD, Romelyn Delos Santos, MD, Caroline Guglielmetti, PhD, and Tony Huynh, Hsin-Yu Chen, PhD for their assistance with experiments and helpful discussion.
Author Contributions
D.M.W. and J.K. proposed and supervised the overall project. H.Q., V.N.C., R.S., C.V.M., C.A.M., K.R.K. and D.M.W. performed M.R. imaging studies. V.N.C., R.R.F. and D.M.W. performed the radiochemistry and biodistribution studies. H.Q. performed biochemistry assays. H.Q., V.N.C., R.S., J.V.M., Z.J.W., R.R.F., K.R.K., J.K., and D.M.W. processed the data and wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26296-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oswald, M. C. W., Garnham, N., Sweeney, S. T. & Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 10.1002/1873-3468.12972 (2018). [DOI] [PMC free article] [PubMed]
- 2.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/S0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 3.Fornai F, et al. Localization of a glutathione-dependent dehydroascorbate reductase within the central nervous system of the rat. Neuroscience. 1999;94:937–948. doi: 10.1016/S0306-4522(99)00349-8. [DOI] [PubMed] [Google Scholar]
- 4.Gu F, Chauhan V, Chauhan A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:89–95. doi: 10.1097/MCO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 5.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/S0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 6.May JM. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012;56:85–103. doi: 10.1007/978-94-007-2199-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 8.Agus DB, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell CL, Yang J, Pardridge WM. GLUT-1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. J. Histochem. Cytochem. 1992;40:193–199. doi: 10.1177/40.2.1552163. [DOI] [PubMed] [Google Scholar]
- 10.Wells WW, Xu DP, Yang YF, Rocque PA. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J. Biol. Chem. 1990;265:15361–15364. [PubMed] [Google Scholar]
- 11.Washburn MP, Wells WW. Identification of the dehydroascorbic acid reductase and thioltransferase (Glutaredoxin) activities of bovine erythrocyte glutathione peroxidase. Biochem. Biophys. Res. Commun. 1999;257:567–571. doi: 10.1006/bbrc.1999.0508. [DOI] [PubMed] [Google Scholar]
- 12.May JM, Qu Z, Li X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem. Pharmacol. 2001;62:873–881. doi: 10.1016/S0006-2952(01)00736-5. [DOI] [PubMed] [Google Scholar]
- 13.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic. Biol. Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 14.Keshari KR, et al. Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proc. Natl. Acad. Sci. USA. 2011;108:18606–18611. doi: 10.1073/pnas.1106920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohndiek SE, et al. Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: vitamin C as a probe for imaging redox status in vivo. J. Am. Chem. Soc. 2011;133:11795–11801. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshari KR, et al. Hyperpolarized [1-13C]dehydroascorbate MR spectroscopy in a murine model of prostate cancer: comparison with 18F-FDG PET. J. Nucl. Med. 2013;54:922–928. doi: 10.2967/jnumed.112.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baligand, C. et al. Hyperpolarized (13) C magnetic resonance evaluation of renal ischemia reperfusion injury in a murine model. NMR Biomed. 10.1002/nbm.3765 (2017). [DOI] [PMC free article] [PubMed]
- 18.Wilson DM, et al. Hyperpolarized (13)C spectroscopic evaluation of oxidative stress in a rodent model of steatohepatitis. Sci. Rep. 2017;7:46014. doi: 10.1038/srep46014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keshari KR, et al. Noninvasive in vivo imaging of diabetes-induced renal oxidative stress and response to therapy using hyperpolarized 13C dehydroascorbate magnetic resonance. Diabetes. 2015;64:344–352. doi: 10.2337/db13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timm KN, et al. Assessing Oxidative Stress in Tumors by Measuring the Rate of Hyperpolarized [1-13C]Dehydroascorbic Acid Reduction Using 13C Magnetic Resonance Spectroscopy. J. Biol. Chem. 2017;292:1737–1748. doi: 10.1074/jbc.M116.761536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll VN, et al. [(11)C]Ascorbic and [(11)C]dehydroascorbic acid, an endogenous redox pair for sensing reactive oxygen species using positron emission tomography. Chem. Commun. (Camb) 2016;52:4888–4890. doi: 10.1039/C6CC00895J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brindle KM. Imaging metabolism with hyperpolarized (13)C-labeled cell substrates. J. Am. Chem. Soc. 2015;137:6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]
- 23.Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2014;43:1627–1659. doi: 10.1039/C3CS60124B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurhanewicz J, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ardenkjaer-Larsen JH, et al. Increase in signal-to-noise ratio of 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner ES, White W, Jennings M, Bennett K. The entrapment of [14C]ascorbic acid in human erythrocytes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1987;902:133–136. doi: 10.1016/0005-2736(87)90143-X. [DOI] [PubMed] [Google Scholar]
- 27.Weber CA, Duncan CA, Lyons MJ, Jenkinson SG. Depletion of tissue glutathione with diethyl maleate enhances hyperbaric oxygen toxicity. Am. J. Physiol. 1990;258:L308–12. doi: 10.1152/ajplung.1990.258.6.L308. [DOI] [PubMed] [Google Scholar]
- 28.Bassett DJ, Reichenbaugh SS. Lung mitochondrial function following oxygen exposure and diethyl maleate-induced depletion of glutathione. Toxicol. Appl. Pharmacol. 1992;115:161–167. doi: 10.1016/0041-008X(92)90319-N. [DOI] [PubMed] [Google Scholar]
- 29.Blaustein A, et al. Myocardial glutathione depletion impairs recovery after short periods of ischemia. Circulation. 1989;80:1449–1457. doi: 10.1161/01.CIR.80.5.1449. [DOI] [PubMed] [Google Scholar]
- 30.Kramer K, Dijkstra H, Bast A. Control of physical exercise of rats in a swimming basin. Physiol. Behav. 1993;53:271–276. doi: 10.1016/0031-9384(93)90204-S. [DOI] [PubMed] [Google Scholar]
- 31.Deneke SM, Lynch BA, Fanburg BL. Transient depletion of lung glutathione by diethylmaleate enhances oxygen toxicity. J. Appl. Physiol. 1985;58:571–574. doi: 10.1152/jappl.1985.58.2.571. [DOI] [PubMed] [Google Scholar]
- 32.Macias RIR, et al. Hepatic expression of sodium-dependent vitamin C transporters: ontogeny, subtissular distribution and effect of chronic liver diseases. Br. J. Nutr. 2011;106:1814–1825. doi: 10.1017/S0007114511002273. [DOI] [PubMed] [Google Scholar]
- 33.Jin SN, et al. Immunohistochemical study on the distribution of sodium-dependent vitamin C transporters in the respiratory system of adult rat. Microsc. Res. Tech. 2005;68:360–367. doi: 10.1002/jemt.20255. [DOI] [PubMed] [Google Scholar]
- 34.Tsukaguchi H, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 35.Nagashima K. Optimum pulse flip angles for multi-scan acquisition of hyperpolarized NMR and MRI. J. Magn. Reson. 2008;190:183–188. doi: 10.1016/j.jmr.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Koelsch BL, et al. Rapid in vivo apparent diffusion coefficient mapping of hyperpolarized (13) C metabolites. Magn. Reson. Med. 2015;74:622–633. doi: 10.1002/mrm.25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu H, et al. Low red blood cell vitamin C concentrations induce red blood cell fragility: A link to diabetes via glucose, glucose transporters, and dehydroascorbic acid. EBioMedicine. 2015;2:1735–1750. doi: 10.1016/j.ebiom.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crane JC, Olson MP, Nelson SJ. SIVIC: Open-Source, Standards-Based Software for DICOM MR Spectroscopy Workflows. Int J Biomed Imaging. 2013;2013:169526. doi: 10.1155/2013/169526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.