Abstract
Liver diseases are one of the fatal syndromes due to the vital role of the liver. Most of the effective treatment of liver conditions are of natural origin. Silymarin (SI) is the standard drug used for treatment of impaired liver functions. Two natural compounds possessing promising liver protection and with different chemical structures namely; the bioflavonoid hinokiflavone (HF) isolated from Junipers phoenicea family Cupressaceae and the sweet saponin Glycyrrhizin (GL) present in Glycyrrhiza glabra (liquorice) were selected for the current study. Since the two compounds are of different nature, they may act by different mechanisms and express synergistic effect. Combination of the two compounds using to dose levels were challenged with single doses of HF, GL and SI as well. The comparison was monitored via measuring serum biochemical parameters including, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltranspeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin, tissue parameters such as MDA, NP-SH and TP, histopathological study using light and electron microscope. Protective effect on kidney was also monitored histopathologically and biochemically through observing the levels of LDH, creatinine, creatinine-kinase, urea and uric acid. The combinations of HF and GL showed protective effect more than the used single doses of HF and GL alone. However, SI was superior to the used combination in the two used doses in all the measured parameters. The liver and kidney cells appearance under normal and electron microscope showed that SI treated groups showed almost normal cells with slight toxic signs. Cells from group treated with the higher doses of the combination of HF and GL showed slight signs of intoxication under light and electron microscope indicating good level of protection. Although the combination of HF and GL expressed good protection in the higher dose, however, the combination did not exceed the protective effect of SI.
Keywords: Hepatoprotective, Hinokiflavone, Glycyrrhizin, Electron microscopy, CCl4, Rats
1. Introduction
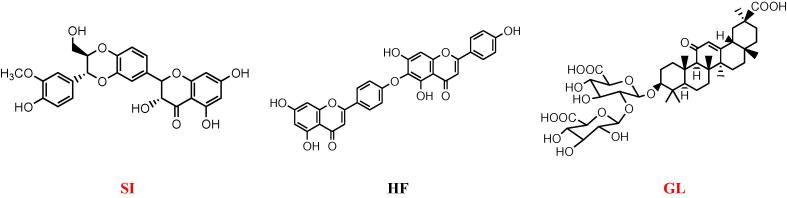
Liver diseases are ranked as the second leading cause of mortality in the US (Everhart and Ruhl, 2009) and the fifth according to National statistics in the UK (UK National Statistics, 2013). In Saudi Arabia 31.8% of the patient suffering from liver problems are using herbs in addition to the prescribed drugs (Al-Zahim et al., 2013). Our previous studies on the hepatoprotactive effect of Junipers species showed promising results (Alqasoumi et al., 2009, Abdel-Kader et al., 2009, Abdel-Kader et al., 2017). Both J. phoenicea and J. procera were subjected to detailed phytochemical study directed by hepatoprotective activity resulted in the identification of the bioflavonoid hinokiflavone (HF) (Fig. 1), 4-epi-abietol and sugiol as the most active components (Alqasoumi and Abdel-Kader, 2012a, Alqasoumi et al., 2013). In addition, HF reported to express potent inhibitory activity on dengue 2 NS5 polymerase (Coulerie et al., 2012). HF displayed HIV-1 reverse transcriptase (RT), influenza A and B virus sialidase inhibitory activity (Lin et al., 1997, Miki et al., 2008). HF potentiated the antibiotic activity of ciprofloxacin against Staphylococcus aureus by targeting RecA (Cottarel et al., 2007). Among 65 flavonoids, HF was the most active to inhibit the procoagulant activity of adherent human monocytes stimulated by endotoxin and interleukin-1b in vitro (Lale et al., 1996).
Fig. 1.
Structures of SI, HF and GL.
The most common use of the saponin Glycyrrhizin (GL)(Fig. 1), the chief sweet-tasting constituent of G. glabra (liquorice), is in the treatment of liver disease (Lin et al., 2008). GL containing preparation prevents hepatic steatosis possibly by protecting mitochondria against oxidative stress induced by HCV proteins and iron overload (Korenaga et al., 2011). GL was found to inhibit the replication of the SARS-associated virus (Cinatl et al., 2003). In the treatment of HCV (hepatitis C virus) infection, GL can inhibit HCV full-length viral particles and HCV core gene expression or function in a dose-dependent manner and have a synergistic effect with interferon (Ashfaq et al., 2011). GL and omega-3 fatty acids combination protected the liver from TAA hepatotoxic effects as indicated from biochemical and histopathological parameters. GL and omega-3 fatty acids combination showed potent anti-inflammatory, anti-oxidant and anti-fibrotic effects (Abo El-Magd et al., 2015).
In the present study, single doses of HF, GL and two doses of combination between HF and GL were evaluated for protective effect against CCl4 induced liver toxicity in rats in comparison with the standard drug SI.
2. Material and methods
2.1. Chemicals
Silymarin (SI), glycyrrhizin (GL), ethylenediamine tetraacetic acid (EDTA), Folin reagent, Mayer hematoxylin, eosinphloxine, trichloroacetic acid, 5, 5 -dithio-bis-(2-nitrobenzoic acid), 2-thiobarbituric acid and paraffin were obtained from Sigma-Aldrich, St. Louis, MO, USA. All solvents used were of analytical grade. Hinokiflavone (HF) was isolated and identified as described earlier from J. phoenicea (Alqasoumi et al., 2013).
2.2. Animals
Male Wistar albino rats (150–200 g) within the same age range (8–10 weeks) were obtained from the Experimental Animal Care Center at the College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA. Animals were housed under controlled laboratory conditions: temperature (22 ± 2 °C), humidity (55%) and light/dark conditions (12/12 h) and provided with Purina chow (LabDiet, St. Louis, USA) with free access to drinking water ad libitum (Abdel-Kader et al., 2017). The experimental protocol of the current study was approved by the Ethical Committee of the College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA.
2.3. Hepatoprotective and nephroprotective activity
Male Wistar rats were divided into seven groups’ five animals each. Group I served as control and received normal saline (1 mL, p.o.). Groups II- VII received a single dose of CCl4 (1.25 mL/kg body weight). The negative control group II received CCl4 without any other treatment. The positive control group III was treated with SI at a dose of 10 mg/kg p.o. (20.7 µmole/ kg). Groups IV treated with 12 mg/kg body weight of HF and group V was treated with GL at dose of 84/kg body weight. The last two groups were treated with combinations of HF and GL. Group VI was treated with 6 mg and 42 mg/kg body weight of HF and GL, respectively, while group VII received 12 mg and 84 mg/kg body weight of HF and GL respectively. Treatment with the protective agent continued for six days, CCl4 administration preformed in day six. Animals were sacrificed using ether anesthesia after 24 h, following CCl4 administration. Blood samples were collected by heart puncture and the serum was separated for evaluating the biochemical parameters.
2.4. Determination of liver and kidney serium paramaters
The biochemical serium parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltranspeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin were estimated by reported methods (Edwards and Bouchier, 1991). The enzyme activities were measured using Reflotron® diagnostic strips and were read using Reflotron® Plus instrument (Roche, Basel, Switzerland). Serum creatinine and blood urea were determined using Randox Diagnostic kits (Randox Laboratories Ltd., Crumlin, U.K.) applying the reported method (Varley and Alan, 1984). Creatinine kinase and uric acid were estimated by Reflotron, Roche kit and LDH was estimated using Human diagnostic kit with UV–VIS Spectrophotometer (Shimadzu, Japan) (Braun et al., 1987, Merdes et al., 1985).
2.5. Determination of tissue parameters
The livers and kidneys samples were separately cooled using ice bath. The tissues were homogenized in 0.02 M ethylenediamine tetraacetic acid (EDTA) in a Potter-Elvehjem type C homogenizer (Sigma-Aldrich, St. Louis, MO, USA). Homogenate equivalent to 100 mg tissues were used for the measurements.
For the determination of Non-protein sulfhydryl groups (NP-SH) tissue homogenate was mixed with 4 mL distilled water and 1 mL of 50% trichloroacetic acid (TCA). The mixtures were shaken for 10–15 min and then subjected to centrifugation for 15 min at 3000 rpm. Two mL of the supernatant were mixed with 0.1 mL of 0.01 M DTNB [5, 5′-dithiobis(2-nitrobenzoic acid)] and 4 mL of 0.4 M Tris buffer pH 8.9 and the resulted solutions were measured spectrophotometrically at 412 nm (Sedlak and Lindsay, 1968).
For the level of MDA, aliquots of homogenate were incubated at 37 °C for 3 h with shaking in a metabolic shaker and then mixed with 1 mL of 10% aqueous TCA and centrifuged at 800 rpm for 10 min. 1 mL of the supernatants were mixed with 1 mL aqueous solution of 0.67% 2-thiobarbituric and heated in a boiling water bath for 10 min. After cooling the mixtures were diluted with 1 mL distilled water and the absorbance's were measured at 535 nm. The content of MDA (nmol/g wet tissue) were calculated from the standard curve of MDA solution (Utley et al., 1967).
To determine the TP parts of the homogenate were treated with 0.7 mL of Lowry’s solution, mixed and incubated for 20 min in dark at room temperature followed by 0.1 mL of diluted Folin's reagent and samples were again incubated at room temperature for 30 min away from light. The absorbance of the resulted solutions were then measured at 750 nm (Lowry et al., 1951).
2.6. Histopathology
The liver and kidney samples were fixed, placed in cassettes and mounted into automated vacuum tissue processor (ASP 300 S, Leica Biosystems, Nussloch, Germany). The samples were embedded and blocked in paraffin wax, and thin sections (3 μm) were made using microtome (TBS SHUR/cut 4500, Triangle Biomedical Sciences, Durham, North Carolina, USA). Sections were stained with Mayer’s hematoxylin solution and counterstained in eosinphloxine solution (Prophet et al., 1994). Slides were examined using Slide Scanner (SCN 400F Leica Microsystems, Wetzlar, Germany) for slide observation and imaging on the magnification of 10x and 40x objectives.
2.7. Electron microscopy
Specimen rotator (Product 15920D, Thermo Fisher Scientific, Carlsbad, CA, USA) was used during fixation, dehydration and infiltration. Tissue samples of 2–3 mm thick were fixed in 4% glutaraldehyde (Product 16210, Electron Microscopy Sciences (EMS), Hatfield, PA, USA) for 2 h followed by 1% osmium tetroxide (Product 19100, EMS) for 1 h. Then, samples were dehydrated using 70% ethanol for 10 min, 100% ethanol for 10 min, and 100% ethanol for 15 min, 100% propylene oxide (Product 8.07027.1001, Merck KGaA, Darmstadt, Germany) for 15 min and another 100% propylene Oxide for 15 min. Infiltration was performed using mixture of EMbed 812 one-step single mix formula composed of 20 mL of EMbed 812 (Product 14900, EMS), 16 mL of Dodecenyl Succinic Anhydride (DDSA) (Product 13710, EMS), 8 mL of Methyl-5-Norbornene-2,3-Dicarboxylic Anhydride (NMA) (Product 19000, EMS) and 0.66–0.88 mL of 2,4,6-Tri(dimethylaminomethyl) phenol (DMP-30) (Product 13600, EMS). The tissues were drained of most of the propylene oxide, leaving a little so the tissue did not dry out. Then tissues were soaked in 1:1 solution of propylene oxide: embedding medium for 1 h at room temperature (RT) followed by 2:1 embedding medium to propylene oxide at RT overnight. Finally, the mixture was replaced with 100% embedding medium for 2 h at RT. Embedding was accomplished by transferring tissues in EMS embedding capsules (Product 69910-05, EMS) then filled with the embedding medium. Capsules were incubated in oven at 60 °C for 24 h to make blocks. After cooling to RT the blocks were trimmed manually and ultra thin section of 100–200 nm were produced using ultramicrotome (Product PT-PC #75,840, RMC Boeckeler Instruments, Inc., Tucson, AZ, USA). Sections were loaded on grid (Product G200-Cu, EMS) and stained manually in 1% uranyl acetate (Product 93-2840, STREM CHEMICALS, Newburyport, MA, USA) for 15 min in dark conditions, rinsed 6 times with normal saline followed by 0.5% lead citrate (Product 17810, EMS) beside several pellets of sodium hydroxide then rinsed in distilled water (DW). After drying, tissues were examined using transmission electron microscope (TEM) (Product FEI TECNAI 12, Thermo Fisher Scientific, Hillsboro, Oregon, USA) (Luft, 1961, Woods and Stirling, 2013).
2.8. Statistical analysis
Results are expressed as mean ± standard error (SE) of mean. Statistical analysis was performed, using one-way analysis of variance (ANOVA). In case of statistically significant (P < .05) when the F-value was found, further comparisons among groups were conducted using Dunnett’s multiple comparisons test. All statistical analyses were performed using SPSS software 17.0 (Released Aug. 23, 2008), Chicago, USA.
3. Results and discussion
Carbon tetrachloride is one of the commonly used agents to induce hepatotoxicity in experimental animals. In the endoplasmic reticulum CCl4 is converted to •CCl3 and •Cl3COO resulted in lipid peroxidation (Snyder and Andrews, 1996). Lipid peroxidation increase endoplasmic reticulum and other membranes permeability to Ca2+ resulting in a severe disturbances of calcium homeostasis and consequently necrotic cell death (Weber et al., 2003). Significant increase in the levels of transaminases (AST and ALT) alkaline phosphatase (ALP) and serum bilirubin were observed in the control group treated with CCl4 (Table 1). Treatment with10 mg/kg (20.7 μmol/kg) of SI prior to the administration of CCl4 resulted in a significant decrease (p < .001) in the elevated AST, ALT, GGT, ALP and bilirubin levels in rats (Table 1). SI have scavenging ability against prooxidant free radicals. In addition, it increases the intracellular concentration of GSH. SI restores the cellular membrane permeability and increases its stability against xenobiotics injury; increases the synthesis of ribosomal RNA and stimulates protein synthesis to regenerate liver cells (Saller et al., 2007, Dehmlow et al., 1996). The hepatoprotective and nephroprotective effect of HF at 12 mg, GL at 84 mg/kg body weight as well as combination of the two compounds at 6 mg + 42 mg/kg body weight and 12 mg + 84 mg /kg body weight of HF and GL, respectively, was explored in comparison with SI. The protective effect was monitored via serum parameters, tissue parameters as well as histopathological study using light and electron microscope.
Table 1.
Effect of HF and GL on serum marker enzymes of control and experimental rats.
| Treatment | Dose (mg/kg) | AST (U/L) |
ALT (U/L) |
GGT (U/L) |
ALP (U/L) |
Bilirubin (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % C | ||
| Control | – | 100.71 ± 4.47 | 26.66 ± 1.32 | 3.88 ± 0.33 | 421.66 ± 13.27 | 0.54 ± 0.01 | |||||
| CCl4a | 1.25 mL/kg | 320.33 ± 8.82*** | 310.33 ± 6.28*** | 18.38 ± 1.06*** | 705.83 ± 21.23*** | 3.55 ± 0.23*** | |||||
| SI + CCl4b | 10 | 122.50 ± 4.87*** | 62 | 100.78 ± 4.97*** | 67 | 8.01 ± 0.43*** | 56 | 432.33 ± 9.19*** | 39 | 1.10 ± 0.07*** | 69 |
| HFb | 12 | 177.16 ± 6.11*** | 46 | 169.33 ± 6.99*** | 45 | 11.48 ± 0.40*** | 38 | 502.33 ± 14.24*** | 29 | 1.14 ± 0.03*** | 68 |
| GLb | 84 | 266.83 ± 15.60* | 17 | 213.50 ± 6.24*** | 31 | 13.68 ± 0.61** | 26 | 551.00 ± 11.15*** | 22 | 1.44 ± 0.06*** | 59 |
| HF + GLb | 6 + 42 | 150.83 ± 5.82*** | 53 | 143.83 ± 4.07*** | 54 | 10.13 ± 0.43*** | 45 | 472.33 ± 13.88*** | 33 | 1.39 ± 0.04*** | 60 |
| HF + GLb | 12 + 84 | 138.16 ± 3.85*** | 57 | 118.60 ± 4.46*** | 62 | 8.88 ± 0.20*** | 52 | 444.83 ± 8.32*** | 37 | 1.13 ± 0.07*** | 68 |
All values represent mean ± SEM.
As compared with Control group.
As compared with CCl4 only group.
p < .05;
p < .01;
p < .001; ANOVA, followed by Dunnett's multiple comparison test.
Serum liver parameters (Table 1) indicated that GL alone offers the least reduction in the elevated levels of the studied enzymes. SI at 10 mg /kg body weight was still the best in improving the level of liver serum parameters. SI reduced the AST by 62%, ALT by 67%, GGT by 56%, ALP by 39% and bilirubin by 69%. Following SI the combination of HF and GL at 12 and 84 mg /kg body weight reduced significantly (p < .001), the elevated levels of AST by 57%, ALT by 62%, GGT by 52%, ALP by 37% and bilirubin by 68%.
Table 2 represents the effect of the studied compounds on the levels of LDH, creatinine kinase, serum creatinine, urea and uric acid. The same pattern was observed where SI provide the best protection followed by the HF and GL at 12 and 84 mg/kg combination except for the effect of GL at 84 mg/kg body weight where the level of LDH was reduced by 20% following SI that offered 23% reduction.
Table 2.
Effect of HF and GL on LDH, Creatinine-Kinase, Urea, Uric acid and Creatinin of control and experimental rats.
| Treatment | Dose (mg/kg) | LDH |
Creatinine-Kinase |
Creatinine (mg/dl) |
Urea (nmol/l) |
Uric acid (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % | Mean ± S.E | % | ||
| Control | – | 113.32 ± 4.70 | 183.66 ± 5.01 | 0.91 ± 0.0 | 36.90 ± 1.79 | 1.92 ± 0.04 | |||||
| CCl4a | 1.25 mL/kg | 198.31 ± 7.12*** | 325.16 ± 11.00*** | 3.10 ± 0.14*** | 192.33 ± 8.34*** | 6.44 ± 0.21*** | |||||
| SI + CCl4b | 10 | 152.80 ± 11.32** | 23 | 195.50 ± 8.64*** | 39 | 1.84 ± 0.05*** | 40 | 126.66 ± 6.53*** | 34 | 2.83 ± 0.1*** | 56 |
| HFb | 12 | 210.66 ± 7.93 | 6 | 266.33 ± 8.19** | 18 | 2.65 ± 0.13 | 14 | 161.00 ± 715* | 16 | 5.40 ± 0.26* | 16 |
| GLb | 84 | 157.92 ± 11.96* | 20 | 270.83 ± 7.75** | 17 | 3.09 ± 0.07 | – | 160.66 ± 7.97* | 16 | 5.11 ± 0.27** | 21 |
| HF + GLb | 6 + 42 | 168.47 ± 9.74* | 15 | 212.16 ± 7.43*** | 35 | 2.11 ± 0.07*** | 32 | 148.66 ± 7.49** | 23 | 4.55 ± 0.28*** | 29 |
| HF + GLb | 12 + 84 | 162.44 ± 4.41** | 18 | 203.333 ± 9.76*** | 37 | 2.05 ± 0.04*** | 34 | 143.00 ± 6.26*** | 23 | 3.16 ± 0.24*** | 51 |
All values represent mean ± SEM.
LDH: Lactate dehydrogenase.
As compared with Control group.
As compared with CCl4 only group.
p < .05;
p < .01;
p < .001; ANOVA, followed by Dunnett's multiple comparison test.
Malonaldehyde (MDA) is the main end-product of polyunsaturated fatty acid peroxidation that cause toxic stress in cells. The increase in MDA levels induced by toxic agents leads to tissue damage (Alqasoumi and Abdel-Kader, 2012b). SI reduced the levels of MDA to 0.92 ± 0.07 and 1.00 ± 0.06 nmol/g in both liver and kidney tissues after the elevation caused by CCl4 to 4.79 ± 0.18 and 3.92 ± 0.24 nmol/g respectively (Table 3). Best results from the tested compounds were observed from the HF and GL combination at 12 and 84 mg/kg where the levels of MDA reduced to 2.07 ± 0.13 and 1.52 ± 0.09 in both liver and kidney tissues respectively. Treatment of animals with CCl4 lead also to reduction in the non-protein sulfhydryl moiety (NP-SH) (Alqasoumi and Abdel-Kader, 2012b). SI restored the levels to 4.24 ± 0.21 and 4.41 ± 0.21 followed by HF and GL combination at 12 and 84 mg/kg where measured levels were 4.18 ± 0.22 and 3.47 ± 0.11 nmol/g in both liver and kidney tissues respectively. One of the liver damage circumstances is the disruption and disassociation of polyribosomes on endoplasmic reticulum leading to reduction in the biosynthesis of protein expressed as decrease in TP. Restoring the normal levels of TP is an important indicator for liver recovery (Alqasoumi and Abdel-Kader, 2012b). The levels of TP reduced from 105.38 ± 3.76 and 150.44 ± 2.82 to 52.68 ± 2.31 and 70.25 ± 4.81 g/l following CCl4 intoxication in liver and kidney tissues. SI restored the TP levels to 97.40 ± 3.74 and 119.35 ± 2.58 while the HF and GL combination at 12 and 84 mg/kg came in the second position by increasing levels to 72.65 ± 2.36 and 110.97 ± 3.19 g/l in both liver and kidney tissues respectively.
Table 3.
Effect of HF and GL on MDA, NP-SH and Total protein in liver and Kidney tissues.
| Treatment | Dose (mg/kg) | MDA (nmol/g) |
NP-SH (nmol/g) |
Total protein (g/l) |
|||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | Liver | Kidney | ||
| Control | – | 0.36 ± 0.02 | 0.29 ± 0.03 | 6.75 ± 0.40 | 4.58 ± 0.25 | 105.38 ± 3.76 | 150.44 ± 2.82 |
| CCl4a | 1.25 mL/kg | 4.79 ± 0.18*** | 3.92 ± 0.24*** | 2.76 ± 0.33*** | 2.71 ± 0.15*** | 52.68 ± 2.31*** | 70.25 ± 4.81*** |
| SI + CCl4b | 10 | 0.92 ± 0.07*** | 1.00 ± 0.06*** | 4.24 ± 0.21** | 4.41 ± 0.21*** | 97.40 ± 3.74*** | 119.35 ± 2.58*** |
| HFb | 12 | 4.02 ± 0.29* | 3.09 ± 0.09* | 2.68 ± 0.30 | 2.87 ± 0.15 | 56.28 ± 1.83 | 80.23 ± 3.84 |
| GLb | 84 | 4.17 ± 0.36 | 2.79 ± 0.14** | 2.94 ± 0.26 | 3.24 ± 0.22 | 55.08 ± 2.31 | 83.82 ± 2.62* |
| HF + GLb | 6 + 42 | 2.79 ± 0.17*** | 2.10 ± 0.11*** | 3.96 ± 0.36* | 3.45 ± 0.14** | 65.86 ± 2.81** | 96.20 ± 3.11*** |
| HF + GLb | 12 + 84 | 2.07 ± 0.13*** | 1.52 ± 0.09*** | 4.18 ± 0.22** | 3.47 ± 0.11** | 72.65 ± 2.36*** | 110.97 ± 3.19*** |
All values represent mean ± SEM of n = 5.
NP-SH: Non-protein sulfhydryl groups; MDA: malondialdehyde.
As compared with Control group (administered with a solution of 0.9% sodium chloride).
As compared with CCl4 only group.
p < .05;
p < .01;
p < .001; ANOVA, followed by Dunnett's multiple comparison test.
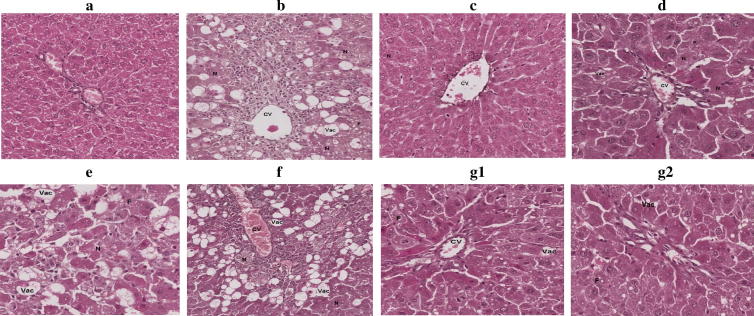
Histopathological study for liver (Fig. 2) and kidney (Fig. 3) tissues were supportive for the biochemical parameters results. Liver of rats treated with CCl4 showed multiple areas of necrosis, severe vacuolization, fatty changes, parenchymal architecture disruption, deposition of collagen fibers, decrease in glycogen content, dilatation of sinusoids, central vein congestion and inflammatory infiltration. Treatment with SI showing slight necrosis, vacuolization, portal triage with slight aggregation of nuclei towards endothelial of portal vein. Liver cells of rats treated with CCl4 and combination of 12 mg HF, 84 mg GL/kg body weight showed central vein area and triode area with slight to moderate fatty changes and vacuolization. Other than this, it is almost look like normal. Other treatment did not show good protection for the hepatocytes.
Fig. 2.
Histopathological appearance of liver cells. (A) normal cells; (B) Liver cells of rats treated with CCl4 showing multiple areas of necrosis, severe vacuolization of hepatocytes, fatty changes, parenchymal architecture disruption, deposition of collagen fibers, decrease in glycogen content, dilatation of sinusoids, central vein congestion and inflammatory infiltration. (C) Liver cells of rats treated with CCl4 and SI showing slight necrosis and vacuolization of hepatocytes, portal triage with slight aggregation of nuclei towards endothelial of portal vein. (D) Liver cells of rats treated with CCl4 and 12 mg/kg body weight of HF with multiple areas of necrosis, vacuolization of hepatocytes, fatty changes and absence of central vein congestion. (E) Liver cells of rats treated with CCl4 and 84 mg/kg body weight of GL showing sever necrosis, vacuolization and fatty change. (F) Liver cells of rats treated with CCl4 and 6 mg of HF, 42 mg of GL/kg body weight showing severely congested central vein accompanied by inflammatory infiltration, severe vacuolization and necrosis. (G1&2) Liver cells of rats treated with CCl4 and 12 mg of HF, 84 mg of GL/kg body weight G1showing central vein area with slight to moderate fatty changes and vacuolization, while G2 showing triode area with slight to moderate fatty changes and vacuolization. Other than this, it is almost look like normal. Magnification 20×, 100 μm. CV = Central vein; N = Necrosis: dead cells or group of cells within living tissue; Vac = Vacuolization: large empty space within the tis-sue; F = Fatty change: accumulation of fat in form of small droplets within cells.
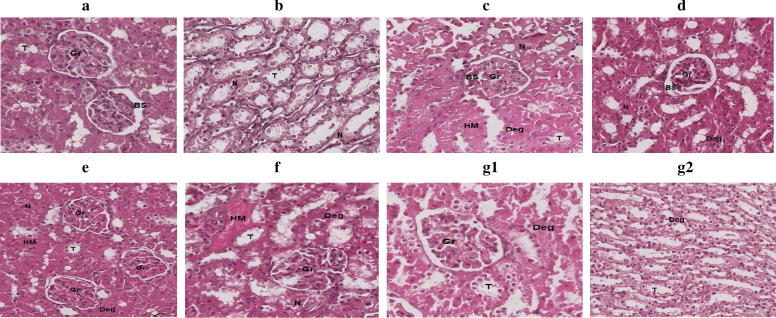
Fig. 3.
Histopathological appearance of kidney cells. (A) normal cells; (B) kidney cells of rats treated with CCl4 showing necrotic tubule walls accompanied by intertubular hyaline material. (C) kidney cells of rats treated with CCl4 and SI showing necrosis in glomerulus and tubules along with degeneration and accumulation of hyaline material. (D) kidney cells of rats treated with CCl4 and 12 mg/kg body weight of HF showing few necrotic and degenerative areas, otherwise normal parenchyma. (E) kidney cells of rats treated with CCl4 and 84 mg/kg body weight of GL showing slight to moderate necrosis and degeneration accompanied by presence of hyaline material. (F) kidney cells of rats treated with CCl4 and 6 mg of HF, 42 mg of GL/kg body weight showing severe necrosis and degeneration along with presence of hyaline material and deposition of collagen. (G1&2) kidney cells of rats treated with CCl4 and 12 mg of HF, 84 mg of GL/kg body weight G1 showing glomerulus area with slight degenerative changes, while G2 showing tubules area with very slight degenerative changes. Magnification 20×, 100 μm.
Kidney cells of rats treated with CCl4 showed necrotic tubule walls accompanied by intertubular hyaline material, while those treated with CCl4 and SI showed slight necrosis in glomerulus and tubules along with degeneration and accumulation of hyaline material. Kidney cells of rats treated with CCl4 and combination of 12 mg HF, 84 mg GL/kg body weight showed glomerulus and tubules area with very slight degenerative changes.
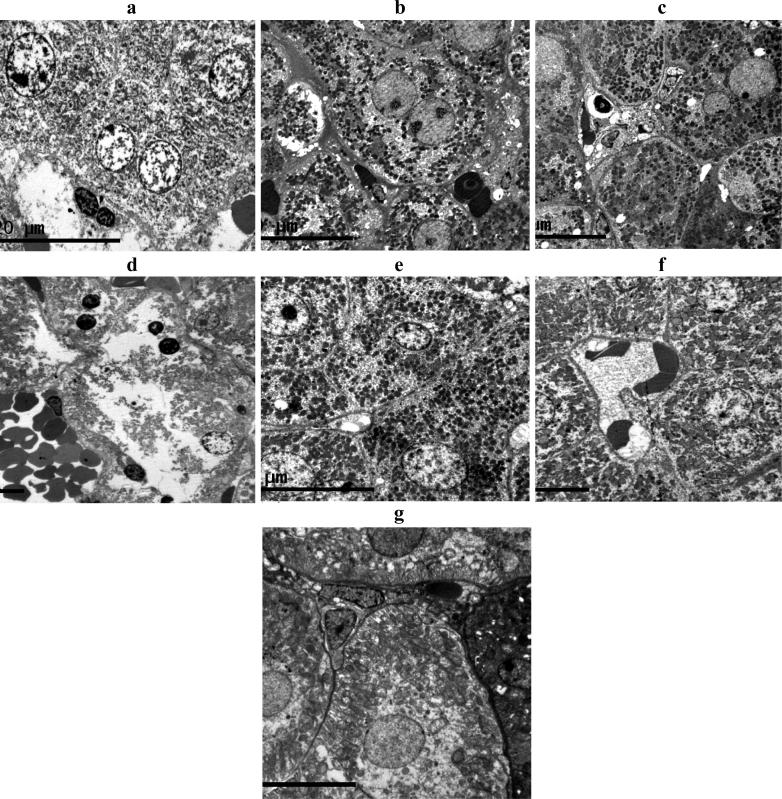
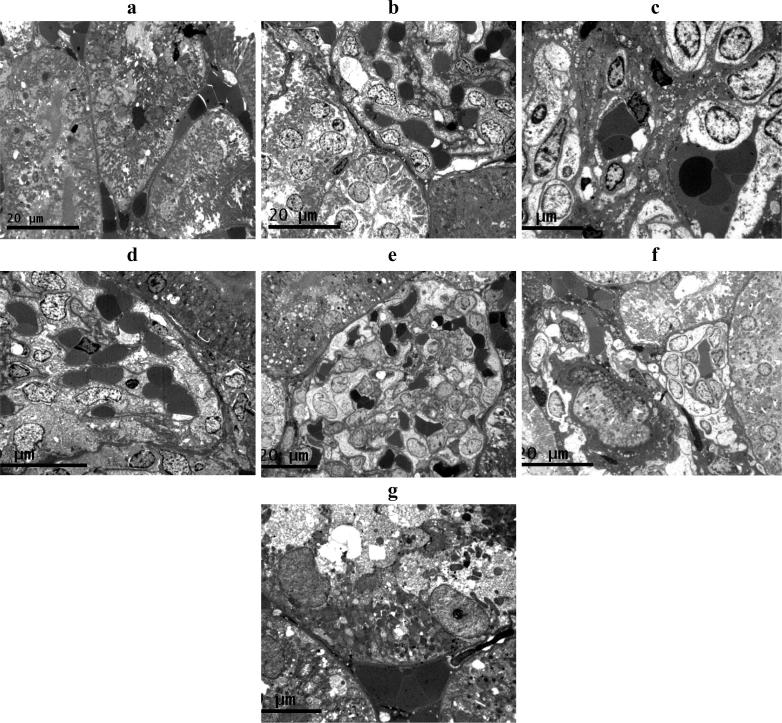
The liver and kidney tissues obtained from each group were also prepared for electron microscopic study (Fig. 4, Fig. 5). Liver cells of rats treated with CCl4 showing nuclear and cellular deterioration and accumulation of cytoplasmic electro-dense material. Treatment with SI prior to CCl4 showed almost normal appearance with the least cellular deterioration and accumulation of electro-dense materials. Liver cells of group treated with CCl4 and combination of 12 mg HF, 84 mg GL/kg body weight showed almost normal appearance with minimal cellular deterioration. Other treatment showed less protective effect for the liver cells from CCl4 toxicity.
Fig. 4.
Electron Microscopy appearance of liver cells. (A) normal cells; (B) Liver cells of rats treated with CCl4 showing nuclear and cellular deterioration and accumulation of cytoplasmic electro-dense material. (C) Liver cells of rats treated with CCl4 and SI showing almost normal appearance with the least cellular deterioration and accumulation of electro-dense materials. (D) Liver cells of rats treated with CCl4 and 12 mg/kg body weight of HF with severe nuclear and cytoplasmic deterioration. (E) Liver cells of rats treated with CCl4 and 84 mg/kg body weight of GL showing minimum if no cellular deterioration but much accumulation of electro-dense materials. (F) Liver cells of rats treated with CCl4 and 6 mg of HF, 42 mg of GL/kg body weight showing intracellular deterioration and accumulation of electro - less dens materials. (G) Liver cells of rats treated with CCl4 and 12 mg of HF, 84 mg of GL/kg body weight showing almost normal appearance with minimal cellular deterioration.
Fig. 5.
Histopathological appearance of kidney cells. (A) normal cells; (B) kidney cells of rats treated with CCl4 showing intracellular deterioration and accumulation of electro-dense materials. (C) kidney cells of rats treated with CCl4 and SI showing almost normal appearance with the least cellular deterioration and accumulation of electro-dense materials. (D) kidney cells of rats treated with CCl4 and 12 mg/kg body weight of HF showing severe nuclear and cytoplasmic deterioration along with presence of electro-dense materials. (E) kidney cells of rats treated with CCl4 and 84 mg/kg body weight of GL showing intracellular deterioration and accumulation of electro-dense materials. (F) kidney cells of rats treated with CCl4 and 6 mg of HF, 42 mg of GL/kg body weight showing intracellular deterioration and accumulation of electro-dense materials. (G) kidney cells of rats treated with CCl4 and 12 mg of HF, 84 mg of GL/kg body weight G1 showing less intracellular deterioration and accumulation of electro-dense materials.
Treated with CCl4 resulted in sever intracellular deterioration and accumulation of electro-dense materials in the kidney cells. Group treated with CCl4 and SI showed kidney cells with almost normal appearance with the least cellular deterioration and accumulation of electro-dense materials. Best protection from the tested compounds for the kidney cells was observed in the group treated with CCl4 and combination of 12 mg HF, 84 mg GL/kg body weight where cells looks like normal with slight intracellular deterioration and accumulation of electro-dense materials was observed.
4. Conclusion
In the current study testing combination of HF and GL in two doses to explore their protective effect comparing with the standard drug SI. The comparison based on serum biochemical parameters, tissue parameters, histological study using light and electron microscope. The combinations were more effective than HF and GL alone. However, SI was superior to the used combination in the two used doses in all the measured parameters. The liver and kidney cells appearance under normal and electron microscope showed that SI treated groups showed almost normal cells with slight toxic signs. Cells from group treated with combination of 12 mg HF, 84 mg GL/kg body weight indicated resemblance to the normal group pictures of both kidney and liver under light microscope. Electron microscope showed little cellular deterioration with some accumulation of small electro-dense materials. Although the combination of HF and GL expressed good protection in the higher dose, however, the combination did not exceed the protective effect of SI.
Acknowledgment
This project was supported by Grant No. 2015/03/4597, Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-kharj, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Kader M.S., Alanazi M.T., Bin Saeedan A.S., Al-Saikhan F.I., Abubaker M., Hamad A.M. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. J. Pharm. Pharmacogn. Res. 2017;5(1):29–39. [Google Scholar]
- Abdel-Kader M.S., Asiri Y.A., Al-Sheikh A.M., Alqasoumi S.I. Evaluation of the claimed hepatoprotective effect of Juniperus procera, Terminalia chebula and Anagallis arvensis from Saudi folk medicine against experimentally induced liver injury in rats. Alex. J. Pharm. Sci. 2009;23:7–10. [Google Scholar]
- Abo El-Magd N.F., El-Karef A., El-Shishtawy M.M., Eissa L.A. Hepatoprotective effects of glycyrrhizin and omega-3 fatty acids on nuclear factor-kappa B pathway in thioacetamide-induced fibrosis in rats. Egyptian J. Basic Appl. Sci. 2015;2(2):65–74. [Google Scholar]
- Alqasoumi S.I., Abdel-Kader M.S. Terpenoids from Juniperus procera with hepatoprotective activity. Pak. J. Pharm. Sci. 2012;25(2):315–322. [PubMed] [Google Scholar]
- Alqasoumi, S.I., Abdel-Kader, M.S., 2012b. Screening of Some Traditionally Used Plants for their Hepatoprotective Effect. INTECH Open Access Publisher.
- Alqasoumi S.I., El Tahir K.E.H., AlSheikh A.M., Abdel-Kader M.S. Hepatoprotective Effect and Safety Studies of Juniperus phoenicea. Alex. J. Pharm. Sci. 2009;23:81–88. [Google Scholar]
- Alqasoumi S.I., Farraj A.I., Abdel-Kader M.S. Study of the hepatoprotective effect of Juniperus phoenicea constituents. Pak. J. Pharm. Sci. 2013;26(5):999–1008. [PubMed] [Google Scholar]
- Al-Zahim A.A., Al-Malki N.Y., Al-Abdulkarim F.M., Al-Sofayan S.A., Abunab H.A., Abdo A.A. Use of alternative medicine by Saudi liver disease patients attending a tertiary care center: prevalence and attitudes. Saudi J. Gastroenterol. 2013;19(2):75–80. doi: 10.4103/1319-3767.108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq U.A., Masoud M.S., Nawaz Z., Riazuddin S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J. Transl. Med. 2011;9(1):112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun H.-P., Deneke U., Rittersdorf W. Analytical performance of reflotron creatin kinase reagent carriers compared with the CK NAC-method. Clin. Chem. 1987;33:988. [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottarel G., Wierzbowski J., Pal K., Kohanski M., Dwyer D., Collins J., Almstetter M., Thormann M., Treml A. Reca inhibitors with antibiotic activity, compositions and methods of use PCT Int. Appl. WO. 2007;2007097940(A2):20070830. [Google Scholar]
- Coulerie P., Eydoux C., Hnawia E., Stuhl L., Maciuk A., Lebouvier N., Canard B., Figadère B., Guillemot J.C., Nour M. Biflavonoids of Dacrydium balansae with potent inhibitory activity on dengue 2 NS5 polymerase. Planta Med. 2012;78(7):672–677. doi: 10.1055/s-0031-1298355. [DOI] [PubMed] [Google Scholar]
- Dehmlow C., Murawski N., de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;52:1591–1600. doi: 10.1016/0024-3205(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Edwards C.R.W., Bouchier I.A.D. Churchill Livingstone Press; UK: 1991. Davidson’s Principles and Practice Medicine. [Google Scholar]
- Everhart J.E., Ruhl C.E. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Korenaga M., Hidaka I., Nishina S., Sakai A., Shinozaki A., Gondo T., Furutani T., Kawano H., Sakaida I., Hino K. A glycyrrhizin-containing preparation reduces hepatic steatosis induced by hepatitis C virus protein and iron in mice. Liver Int. 2011;31(4):552–560. doi: 10.1111/j.1478-3231.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- Lale A., Herbert J.M., Augereau J.M., Billon M., Leconte M., Gleye J. Ability of different flavonoids to inhibit the procoagulant activity of adherent human monocytes. J. Nat. Prod. 1996;59(3):273–276. doi: 10.1021/np960057s. [DOI] [PubMed] [Google Scholar]
- Lin A., Liu A.Y., Huang Y., Sun J., Wu Z., Zhang X., Ping Q. Glycyrrhizin surface-modified chitosan nanoparticles for hepatocyte-targeted delivery. Int. J. Pharm. 2008;359(1–2):247–253. doi: 10.1016/j.ijpharm.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Lin Y.-M., Anderson H., Flavin M.T., Pai Y.-H., Mata-Greenwood E., Pengsuparp T., Pezzuto J.M., Schinazi R.F., Hughes S.H., Chen F.-C. In Vitro Anti-HIV activity of biflavanoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997;60(9):884–888. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luft J.H. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 1961;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes H., Rittersdorf W., Werner W. Reflotron uric acid: Evaluation of new dry chemistry test for reflotron. Clin. Chem. 1985;31:921. [Google Scholar]
- Miki K., Nagai T., Nakamura T., Tuji M., Koyama K., Kinoshita K., Furuhata K., Yamada H., Takahashi K. Synthesis and evaluation of influenza virus sialidase inhibitory activity of hinokiflavone-sialic acid Conjugates. Heterocycles. 2008;75(4):879–885. [Google Scholar]
- Prophet E.P., Mills B., Arrington J.B., Sobin L.H. second ed. American Registry of Pathology; Washington DC: 1994. Laboratory Methods in Histology. [Google Scholar]
- Saller R., Melzer J., Rechling J., Brignoli R., Meier R. An updated systematic review of the pharmacology of silymarin. Forsch Komplementarmed. 2007;14:70–80. doi: 10.1159/000100581. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Snyder R., Andrews L.S. Toxic effects of solvents and vapors. In: Klassen C.D., editor. Toxicology: the Basic Science of Poisons. McGraw-Hill; New York: 1996. [Google Scholar]
- Utley H.G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- UK National Statistics, <http://www.statistics.gov.uk/> (Consult-ed February 8, 2013).
- Varley., H., Alan, H.G., 1984. Tests in renal disease. In: Practical Clinical Biochemistry vol. 1123 London: William Heinemann Medical Book Ltd.
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. CRC Cr. Rev. Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Woods A.E., Stirling J.W. Transmission electron microscopy. In: Bancroft J.D., Layton C., Suvarna S.K., editors. Theory and practice of histological techniques. 7th ed. Churchill; Livingstone: 2013. [Google Scholar]