Abstract
Neurotrophins have been implicated in the pathophysiology of many neuropsychiatric diseases. Brain-derived neurotrophic factor (BDNF) is the most abundant and widely distributed neurotrophin in the brain. Its Val66Met polymorphism (refSNP Cluster Report: rs6265) is a common and functional single-nucleotide polymorphism (SNP) affecting the activity-dependent release of BDNF. BDNF Val66Met transgenic mice have been generated, which may provide further insight into the functional impact of this polymorphism in the brain. Considering the important role of BDNF in brain function, more than 1,100 genetic studies have investigated this polymorphism in the past 15 years. Although these studies have reported some encouraging positive findings initially, most of the findings cannot be replicated in following studies. These inconsistencies in BDNF Val66Met genetic studies may be attributed to many factors such as age, sex, environmental factors, ethnicity, genetic model used for analysis, and gene–gene interaction, which are discussed in this review. We also discuss the results of recent studies that have reported the novel functions of this polymorphism. Because many BDNF polymorphisms and non-genetic factors have been implicated in the complex traits of neuropsychiatric diseases, the conventional genetic association-based method is limited to address these complex interactions. Future studies should apply data mining and machine learning techniques to determine the genetic role of BDNF in neuropsychiatric diseases.
Keywords: brain-derived neurotrophic factor, Val66Met polymorphism, transgenic mice, genetic study, age, sex, environmental factors, ethnicity
Introduction
Brain-derived neurotrophic factor (BDNF), a major member of the neurotrophin family, is widely expressed in the mammalian brain (Hofer et al., 1990). The highest level of BDNF is found in the hippocampus and the cerebral cortex, which are regions of the brain that are involved in many neuropsychiatric diseases (Hofer et al., 1990). BDNF is critical to the growth, survival, and differentiation of the developing nervous system through its binding to a high affinity tyrosine kinase receptor B (TrkB) and/or the p75 neurotrophin receptor. Mutant mice lacking BDNF exhibit developmental brain abnormalities and die soon after birth (Ernfors et al., 1994). In addition, BDNF can modulate synaptic transmission and activity-dependent plasticity, and it can promote long-term potentiation (LTP) (Xu et al., 2000; Bramham and Messaoudi, 2005).
The human BDNF gene is located on chromosome 11p13 and has 11 exons and 9 functional promoters that are brain region- and tissue-specific (Pruunsild et al., 2007). In this gene, a non-synonymous polymorphism (refSNP Cluster Report: rs6265; also called Val66Met or G196A polymorphism) is common; this polymorphism causes a valine (Val) to methionine (Met) change at position 66 of the proBDNF protein. The replacement of Val by Met impairs the neuronal activity-dependent secretion of BDNF (Egan et al., 2003). The first two genetic studies investigating the BDNF Val66Met polymorphism were published in 2002 (Momose et al., 2002; Ventriglia et al., 2002). Considering the important role of BDNF in the brain, over the past 15 years, many genetic studies have investigated the effects of this BDNF polymorphism on brain function and behavior in health, as well as in diseases, particularly neuropsychiatric diseases (Hong et al., 2011; Notaras et al., 2015b) (Table 1). A search with the keywords “(bdnf val66met) OR rs6265 OR (bdnf g196a) OR (bdnf 196g/a) OR (bdnf 196a/g) OR (bdnf 196 a/g)” performed in the PubMed database up to February 14, 2018 found 1,176 reports on this polymorphism (Figure 1). Although many reports have demonstrated the possible genetic effects of this BDNF polymorphism in diseases or brain function, other reports have failed to replicate the findings. The inconsistent findings of BDNF Val66Met genetic studies may result from many factors such as age, sex, environmental factors, ethnicity, genetic model used for analysis, and gene–gene interaction. In this review, we discuss these issues in genetic studies of the BDNF Val66Met polymorphism. We also discuss some findings for the novel function of this polymorphism.
Table 1.
Meta-analyses of studies of the BDNF Val66Met polymorphism in neuropsychiatric diseases.
| Number of | ||||
|---|---|---|---|---|
| Disease/phenotype | Studies | studies | Participants | Result |
| Major depressive disorder | Verhagen et al., 2010 | 14 | 2,812 cases; 10,843 controls | Met increased risk for depression in men but not in women. |
| Pei et al., 2012 | 5 | 523 cases; 1,220 controls | Met increased risk for geriatric depression. | |
| Gyekis et al., 2013 | 26 | 4,582 cases; 12,995 controls | Lack of association. | |
| Hosang et al., 2014 | 22 | 14,233 participants | Val66Met polymorphism significantly moderated the relationship between life stress and depression. | |
| Zhao M. et al., 2017 | 31 | 21,060 participants | Life stress interacted with the Met in depression risk. | |
| Response to antidepressant | Zou et al., 2010 | 8 | 1,115 cases | Val66Met heterozygous patients had a better response rate in comparison to Val homozygous patients, especially in Asian population. |
| Yan et al., 2014 | 16 | Met carriers had a better response rate than Val/Val carriers in Asians. | ||
| Suicide behaviors | Zai et al., 2012 | 12 | 1,202 cases; 2,150 controls | Met carriers and Met allele conferred risk for suicide. |
| Gonzalez-Castro et al., 2017 | 23 | 4,532 cases; 5,364 controls | Met is the risk allele in Caucasian; Val is the risk allele in Asian. | |
| Bipolar disorder | Kanazawa et al., 2007 | 11 | 3,143 cases; 6,347 controls | Lack of association. |
| Gonzalez-Castro et al., 2015 | 22 | 9,349 cases; 7,437 controls | Lack of association. | |
| Wang et al., 2014 | 21 | 7,219 cases; 9,832 controls | Lack of association. | |
| Schizophrenia | Zintzaras, 2007 | 9 | 1,404 cases; 1597 controls | Lack of association. |
| Xu et al., 2007 | 11 | 3,032 cases; 4,080 controls | Lack of association. | |
| Naoe et al., 2007 | 8 | 2,059 cases; 2,765 controls | Lack of association. | |
| Gratacos et al., 2007 | 12 | 3,338 cases; 4,635 controls | Met/Met increased the risk of schizophrenia. | |
| Qian et al., 2007 | 16 | 2,991 cases; 3,962 controls | Lack of association. | |
| Kanazawa et al., 2007 | 13 | 2,955 cases; 4,035 controls | Lack of association. | |
| Kawashima et al., 2009 | 22 | 6,568 cases; 8,824 controls | Lack of association. | |
| Kheirollahi et al., 2016 | 39 | Met/Met increased the risk of schizophrenia in Asian and European populations. | ||
| Zhao et al., 2015 | 44 | 11,480 cases; 13,490 controls | Lack of association. | |
| Response to antipsychotics | Cargnin et al., 2016 | 9 | 2,461 antipsychotic-treated patients | Lack of association. |
| Antipsychotic-induced tardive dyskinesia | Miura et al., 2014 | 6 | 1,740 antipsychotic-treated patients | Lack of association. |
| Generalized Anxiety Disorder | Frustaci et al., 2008 | 7 | 1,092 cases; 8,394 controls | Lack of association. |
| Neuroticism | Frustaci et al., 2008 | 5 | 1,633 participants | Met carriers had lower Neuroticism score. |
| Posttraumatic stress disorder (PTSD) | Wang, 2015 | 6 | 696 cases; 1,726 controls | Lack of association. |
| Bruenig et al., 2016 | 9 | 1,066 cases; 2,559 were controls | Met carriers had increased risk of PTSD. | |
| Panic disorder | Chen et al., 2017 | 6 | A significant association in recessive model. | |
| Obsessive-compulsive disorder | Wang et al., 2015 | 8 | 1,632 cases; 2,417 controls | Lack of association. |
| Attention-deficit hyperactivity disorder | Sanchez-Mora et al., 2010 | 4 | 1,445 adulthood patients; 2,247;controls | Lack of association. |
| Eating disorder | Gratacos et al., 2007 | 5 | 1,733cases; 1,811 controls | Met increased the risk of eating disorder. |
| Brandys et al., 2013 | 9 | 2,767 cases; 3,322 controls | Lack of association. | |
| Cognition | Kambeitz et al., 2012 | 32 | 5,922 participants | Met carriers performed worse than the Val homozygotes in memory. |
| Mandelman and Grigorenko, 2012 | 23 | 7,095 participants | Lack of association. | |
| Hippocampal volume | Hajek et al., 2012 | 7 | 399 participants | Met carriers had smaller hippocampal volumes than Val homozygotes. |
| Harrisberger et al., 2014 | 27 | 5,298 participants | Met carriers had slightly smaller hippocampal volumes than Val homozygotes. | |
| Harrisberger et al., 2015 | 18 | 1,695 neuropsychiatric patients | Lack of association. | |
| Alcohol dependence | Forero et al., 2015 | 9 | 2,553 cases; 2,709 controls | Lack of association. |
| Substance abuse | Gratacos et al., 2007 | 6 | 1,361 cases; 1,164 controls | Val homozygotes conferred risk for substance abuse. |
| Haerian, 2013 | 20 | 4,665 cases; 4,754 controls | Val increased the risk of methamphetamine dependence in south Asian participants and the risk of heroin dependence in Chinese participants. | |
| Adult-onset dystonia | Gomez-Garre et al., 2014 | 7 | 1,936 cases; 2,519 controls | Lack of association. |
| Migraine | Terrazzino et al., 2017 | 5 | 1,442 cases; 1,880 controls | Met increased the risk of migraine. |
| Cai et al., 2017 | 4 | 1,598 cases; 1,585 controls | Met increased the risk of migraine. | |
| Parkinson’s disease | Zintzaras and Hadjigeorgiou, 2005 | 6 | 1,419 cases; 1,406 controls. | Lack of association. |
| Dai et al., 2013 | 13 | 3,333 cases; 3,418 controls | Lack of association. | |
| Mariani et al., 2015 | 15 | 3,754 cases; 4,026 controls | Lack of association. | |
| Alzheimer’s disease (AD) | Fukumoto et al., 2010 | 16 | 4,711 cases; 4,537 controls | Met increased the risk of AD in women, but not in men. |
| Lin et al., 2014 | 29 | 7,548 cases; 7,334 controls | Met increased the risk of AD in Caucasian females. | |
| Ji et al., 2015 | 23 | 6,504 cases; 6,636 controls | Lack of association. | |
FIGURE 1.
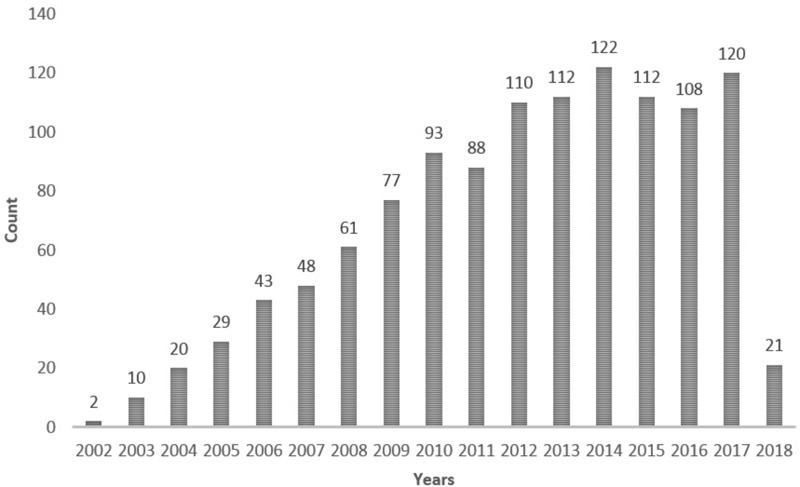
A search for reports on the BDNF Val66Met polymorphism with the keywords “(bdnf val66met) OR rs6265 OR (bdnf g196a) OR (bdnf 196g/a) OR (bdnf 196a/g) OR (bdnf 196 a/g)” performed in the PubMed database up to February 14, 2018.
BDNF Val66Met Polymorphism and Ethnicity
Meta-analysis, which is a statistical tool for combining the results of different studies investigating the same topic, can provide convincing and reliable evidence relevant to genetic studies with differing results. Several meta-analyses of BDNF Val66Met polymorphism studies have demonstrated that the positive association findings of this polymorphism are dependent on ethnicity (Table 1). For example, converging evidence suggests that BDNF is implicated in the pathogenesis of bipolar disorder. In 2003, two research groups reported a significant association between the BDNF Val66Met polymorphism and bipolar disorder (Neves-Pereira et al., 2002; Sklar et al., 2002). However, most of the other studies cannot replicate this association (Hong et al., 2003a; Nakata et al., 2003). In 2016, due to the lack of reproducibility, Li et al. (2016) performed a systematic meta-analysis of reports evaluating diverse ethnic groups. They found that the BDNF Val66Met polymorphism is significantly associated with bipolar disorder in Europeans, but not in Asians.
Brain-derived neurotrophic factor is characterized by survival-promoting activity in various brain neurons, including midbrain dopaminergic variants. Postmortem brain studies have suggested that BDNF is involved in the pathogenesis of Parkinson’s disease (PD) (Joyce et al., 2002; Hong et al., 2003b). Therefore, genetic studies have tested the association of this polymorphism with PD risk but have reported inconsistent results. A meta-analysis of 12 studies showed no association between PD and this BDNF polymorphism in all study subjects (Lee and Song, 2014). However, an ethnicity-specific meta-analysis showed that Met carriers have an increased susceptibility to PD in Europeans, but not in Asians.
Strong evidence suggests genetic predisposition to suicidal behaviors (Tsai et al., 2011). To determine the genetic effect of the BDNF Val66Met polymorphism on suicidal behaviors, a meta-analysis evaluated 23 studies, including 4,532 patients and 5,364 controls, but found no evidence of an association between this polymorphism and suicidal behaviors (Gonzalez-Castro et al., 2017). However, a significantly increased risk was found in a subgroup analysis by ethnicity in Asian populations (Val homozygotes vs. Met carriers: odds ratio [OR]: 1.36; 95% confidence interval [CI], 1.04–1.78) and in Caucasian populations (Met homozygotes vs. Val carriers: OR: 1.96; 95% CI, 1.58–2.43).
The disparate associations among ethnic groups may be attributed to several reasons. First, considerable BDNF allele and haplotype diversity is present among populations globally, and the frequency of the Met allele considerably ranges from 0 to 72% across populations (Petryshen et al., 2010). The low prevalence of the risk allele in some populations may lead to an inadequate population size in studies validating associations found to be significant in low-powered studies. Second, the Met allele is present in different population-specific haplotypes in Caucasians and Asians (Petryshen et al., 2010). If the BDNF Val66Met polymorphism is not the true risk variant but links to the probable true functional loci with differing strengths among populations, different associations with the Val66Met polymorphism may be found due to different haplotypic backgrounds. Third, different interactions may occur between the BDNF Val66Met polymorphism with other genetic or environmental features that vary among ethnic groups.
Genetic Model for Analysis of BDNF Val66Met Polymorphism
The genetic model for the analysis of an single-nucleotide polymorphism (SNP), such as the BDNF Val66Met polymorphism, may be dominant (Met carriers vs. Val/Val), codominant (Met/Met vs. Val/Met vs. Val/Val), or recessive (Met/Met vs. Val carriers). The BDNF Met allelic frequency is often reported to be high in Asian populations but low in Caucasian, Central and South American, and African populations (Tsai et al., 2010; Hong et al., 2011; Gonzalez-Castro et al., 2017). Many studies in non-Asian populations have grouped carriers of BDNF Val/Met and Met/Met genotypes together as Met carriers because of the small number of Met homozygotes. However, whether the Met allele is dominant, codominant, or recessive is unclear. Furthermore, stratifying the BDNF Val66Met polymorphism into two genotypic groups may ignore the molecular heterosis effect. For example, a meta-analysis suggested that Val/Met heterozygotes show higher antidepressant therapeutic effects than Val or Met homozygotes, particularly Asian patients (Verhagen et al., 2010; Zou et al., 2010; Yan et al., 2014). This is referred to as the positive molecular heterosis effect, in which subjects heterozygous for a specific genetic polymorphism show a greater effect (Tsai et al., 2003; Liu et al., 2014). This observation is consistent with the findings an animal study showing that although BDNF exerts an antidepressant effect, very high BDNF expression may have an unfavorable effect on mood (Govindarajan et al., 2006).
The association between the BDNF Val66Met polymorphism and panic disorder is inconclusive given the mixed findings (Lam et al., 2004; Chen and Tsai, 2016). A meta-analysis of six studies found no association between the polymorphism and panic disorder in the dominant model (Chen et al., 2017). However, in the recessive model, a significant association was found between the BDNF Val66Met polymorphism and panic disorder.
BDNF Val66Met Polymorphism and Sex
There are sex differences in brain BDNF and its receptor expression. Animal study illustrated that male mice have higher BDNF in the frontal cortex, hippocampus and brain stem (Szapacs et al., 2004). The distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stages that phosphorylated TrkB were more abundant in high-estradiol states (proestrus females) than low-estradiol states (estrus and diestrus females and males) (Spencer-Segal et al., 2011). In human, postmortem study found that there is no significant difference in hippocampal BDNF levels between the two genders but female subjects have higher BDNF in the prefrontal cortex (Hayley et al., 2015). Sex differences in the level of BDNF and its receptor in different brain regions could potentially explain some of the disorder-specific sex differences in the association of BDNF Val66Met polymorphism.
In the brain, sex hormones and BDNF have mutual effects. The first linkage between BDNF and sex steroids was indicated in a study showing co-localization of BDNF and its receptor in the estrogen receptor (ER) mRNA-containing neurons during forebrain development (Toran-Allerand et al., 1992). Evidence from animal studies suggested that estrogen modulates BDNF expressions through at least four different mechanisms (Gibbs, 1998; Chan and Ye, 2017). First of all, estrogen can directly induce BDNF expression by activating ER. Second, estrogen modifies the activity of BDNF promoter epigenetically. Third, the ER regulates the activity of CREB, a major transcription factor that controls BDNF expression in neurons, through non-genomic activities. Lastly, estrogen affects BDNF expression indirectly via inter-neuronal activity. In contrast, evidence suggests that some estrogen actions are mediated by BDNF. For example, BDNF was reported to modulate estradiol-induced dendritic spine formation in rat hippocampal neurons (Murphy et al., 1998).
Within the hippocampus, estrogen and BDNF both interact with a number of common receptors, enzymes and proteins such as MAP kinase, ERKs, PI3 kinase, CaMKII, CREB, and Src/Fyn (Luine and Frankfurt, 2013). The interactions between BDNF and estrogen affect hippocampal neurons during development and in adulthood, and these interactions play an important role in the normal brain as well as in diseases (Harte-Hargrove et al., 2013).
When compared with estrogen, the effect of androgen on BDNF expression is less studied. Study in mice demonstrated that gonadectomy induced a significant decrease in the BDNF levels in the hippocampal CA1 area, which were prevented by replacement of testosterone, the major component of androgens (Li et al., 2012). Androgens are crucial for the development of male-specific behaviors and for physiological functioning. Animal studies have demonstrated that BDNF and androgens may work cooperatively to influence neuronal plasticity and modulate hippocampal function (Ottem et al., 2013; Atwi et al., 2016).
An animal study demonstrated the effect of sex hormones on BDNF; female BDNFMet/Met transgenic mice exhibited significant fluctuations in anxiety-like behaviors over the estrous cycle; specifically, these mice exhibited increased anxiety-like behaviors during the estrus phase (Bath et al., 2012a). A human study found that during the menstrual cycle, plasma BDNF levels were significantly higher in the luteal phase than in the follicular phase (Begliuomini et al., 2007). A recent multimodal imaging study in 39 healthy women found an ovarian hormone-by-BDNF interaction on working memory-related hippocampal function, suggesting that differential hippocampal recruitment occurs in Met carriers but only in the presence of estradiol (Wei et al., 2017).
Studies from the fields of genetic epidemiology, clinical psychiatry, behavioral neuroscience and neuroimaging suggest that the BDNF Val66Met polymorphism may not be a major risk allele for the development of schizophrenia per se, but the polymorphism modulates a range of clinical features of the illness, including age of onset, symptoms, therapeutic responsiveness, neurocognitive function and brain morphology (Notaras et al., 2015a). Findings from clinical and animal studies of schizophrenia showed that estrogen may provide a protective effect in schizophrenia, including through mediating BDNF expression and activity (Wu et al., 2013). This posited estrogen-BDNF interaction could play a key role in sex differences in clinical aspects of schizophrenia.
Because sex hormones may affect BDNF function, sex may contribute to the discrepancy in the findings of BDNF Val66Met genetic studies. For example, BDNF plays a critical role in neuronal survival, synaptic plasticity, and memory (Tsai, 2003b; Huang et al., 2014; Lin et al., 2016). Therefore, BDNF is a favorable candidate for Alzheimer’s disease (AD) genetic studies. The first genetic association study of the BDNF Val66Met polymorphism and AD demonstrated that Val is the risk allele for AD (Ventriglia et al., 2002). Studies attempting to replicate this finding have obtained inconsistent results (Tsai et al., 2004a, 2006). To establish the true effect of the BDNF polymorphism on AD, Fukumoto et al. (2010) performed a meta-analysis of studies investigating the effects of the BDNF Val66Met polymorphism on AD. The results revealed a clear sex difference in the allelic association; the Met allele confers susceptibility to AD in women (P = 0.002), but not in men. This finding suggests that the BDNF Val66Met polymorphism has a sexually dimorphic effect on susceptibility to AD. This result is consistent with the finding that the BDNF Val66Met polymorphism has a sex-specific role (in women, but not in men) in cognitive function during normal cognitive aging (Laing et al., 2012). Similarly, a meta-analysis of studies evaluating the effect of the BDNF Val66Met polymorphism on major depressive disorder showed that, in the total sample, the BDNF Val66Met polymorphism is not significantly associated with depression; however, sex-stratified allelic and genotypic analyses revealed significant effects in men (Verhagen et al., 2010).
Sex-specific associations of the BDNF Val66Met polymorphism with cortisol responses to mental stress (Jiang et al., 2017), neurocognitive function in schizophrenia (Kim et al., 2016), sympathetic tone (Chang et al., 2014), HPA axis reactivity to psychological stress (Shalev et al., 2009), and attention-deficit/hyperactivity disorder (ADHD) (Cho et al., 2010) have also been reported.
In addition to BDNF Val66Met genetic studies in neuropsychiatric diseases, studies of serum BDNF levels in neuropsychiatric diseases have shown a sex effect. For example, BDNF has been implicated in the pathogenesis of ADHD (Tsai, 2003a, 2017a; Tzang et al., 2013). In a recent meta-analysis of studies examining peripheral BDNF levels in ADHD, although no significant difference was found in peripheral BDNF levels between ADHD patients and normal controls, overall, BDNF levels were significantly higher in male ADHD subjects than in male controls (Zhang et al., 2017).
BDNF Val66Met Polymorphism and Age
The tissue expression of BDNF varies across the life span. The human serum BDNF concentration increases in the first several years of life and then slightly decreases in adulthood (Katoh-Semba et al., 2007). Another study found that plasma BDNF levels decrease significantly with age, whereas platelet levels do not, suggesting the age effect on BDNF levels is tissue-specific (Lommatzsch et al., 2005). Age not only affects BDNF expression but also affects the conversion of proBDNF to mature BDNF. A study examining BDNF expression in mouse hippocampal lysates showed that the expression of both pro- and mature BDNF was low on postnatal day 0 (Yang et al., 2014). The expression of proBDNF peaked on postnatal day 15 and declined in later stages. The expression of mature BDNF peaked on postnatal day 21 and plateaued in adulthood (Yang et al., 2014).
Brain-derived neurotrophic factor is involved in pruning and shaping the adolescent brain and has been implicated in the pathogenesis of neurodevelopmental disorders. Study in male mice found significant changes in BDNF expressions in the forebrain regions during weeks 7–10 (Hill et al., 2012). Castration and testosterone replacement experiments demonstrated an androgen receptor-dependent effect on BDNF-TrkB signaling in the forebrain and hippocampal regions during adolescence. Female mice showed changes in BDNF-TrkB signaling at a much earlier time point (weeks 4–8) in the forebrain and hippocampal regions (Hill et al., 2012). During adolescence, the incidence of mental illnesses such as schizophrenia and depression increases substantially. Accordingly, altered synthesis and/or activity of BDNF, which are key regulators of many mental disorders, may contribute to the development of these mental diseases in adolescence.
Studies examining the (mRNA and protein) expression of BDNF and its receptors in the hippocampus and hypothalamus throughout the life span of rats have found that receptors, rather than BDNF itself, are impaired with aging (Silhol et al., 2005; Rage et al., 2007). These findings suggest that age also affects BDNF signaling through changes in its receptor.
Based on the aforementioned findings, age may mediate the effect of the BDNF Val66Met polymorphism on disease susceptibility. In our studies of the BDNF Val66Met polymorphism and major depression, we found that Met carriers have an increased risk of geriatric depression, but not non-geriatric depression (Hong et al., 2003a; Tsai et al., 2003; Hwang et al., 2006). This finding was further confirmed by a meta-analysis of five studies including 523 patients with geriatric depression and 1,220 psychiatrically healthy controls (Pei et al., 2012). Similarly, a recent study showed a complex relationship between the BDNF Val66Met polymorphism and mortality for traumatic brain injury, and that study demonstrated that this polymorphism interacts with age to influence survival predictions beyond clinical variables alone (Failla et al., 2015).
BDNF Val66Met Polymorphism and Gene–Gene Interaction
Brain-derived neurotrophic factor exerts its trophic action mainly by signaling through the trkB receptor (encoded by the NTRK2 gene). The trkB signaling pathway involves many proteins that also possibly affect BDNF function. In addition, the proteolytic cleavage of proBDNF (a BDNF precursor with effects opposite to those of BDNF) to BDNF by plasmin determines the direction of BDNF action (Lu et al., 2005; Tsai, 2017b). Therefore, polymorphisms in the genes encoding proteins involved in the trkB or plasmin signaling pathway may interact with the BDNF Val66Met polymorphism to affect disease susceptibility (Tsai, 2004a, 2007b; Hwang et al., 2006). For example, using a generalized multifactor dimensionality reduction method, we found the BDNF Val66Met polymorphism interacts with NTRK2 genetic polymorphisms (rs1187323 and rs1778929) to affect susceptibility to geriatric depression (Lin et al., 2009).
The BDNF Val66Met polymorphism has also been reported to interact with the 𝜀4 allele of apolipoprotein E (APOE), thereby affecting AD susceptibility in women (Zhao Q. et al., 2017). Another study found that the BDNF Val66Met polymorphism interacts with the serotonin transporter gene polymorphism to influence neuroticism-related personality traits (Terracciano et al., 2010). Recently, Prats et al. (2017) demonstrated an interaction between the rs1475157 polymorphism of NRN1 (a neurotrophic factor involved in synaptic plasticity) and the BDNF Val66Met polymorphism; this interaction modulated depressive symptoms in 410 non-clinical participants (Prats et al., 2017).
To analyze interactions in genetic data, many statistical methods have been suggested, with most of them relying on statistical regression models. Given the known limitations of classical methods, approaches with the machine-learning have also become favorable. Among them, the multifactor dimensionality reduction (MDR), a powerful statistical tool for detecting and modeling epistasis, has been widely applied (Ritchie et al., 2001). Polygenic risk score is another approach to summarize the additive trait variance captured by a set of genetic markers that do not individually achieve significance in a large-scale association study (Baker et al., 2018).
Interaction Between BDNF Val66Met Polymorphism and Environmental Factors
Evidence suggests that interactions between genes and the environment influence brain development and the risk of neuropsychiatric diseases (Keverne, 2014; Booij et al., 2015; Lin et al., 2017; Misiak et al., 2017). Many environmental factors (such as prenatal adverse environments, childhood trauma, weather and life stress) have been found to play an important role in the causality of brain diseases.
The BDNF Val66Met polymorphism has been reported to interact with early life stress; thus, Val carriers with childhood trauma are more susceptible to the occurrence of subclinical psychotic experiences (de Castro-Catala et al., 2016). Another study in subjects with the schizophrenia spectrum or bipolar disorder demonstrated that Met carriers with high levels of childhood trauma have significantly low levels of blood BDNF mRNA and decreased CA2/3 and CA4 subfield areas in the dentate gyrus (Aas et al., 2014).
The BDNF Val66Met polymorphism has been long considered an important candidate for reducing depression risk; however, inconsistent findings have been obtained. A meta-analysis with a pooled total of 14,233 participants found that the Met allele significantly moderates the link between life stress and depression risk (Hosang et al., 2014). When stratified by the type of environmental stressor, the interaction between the BDNF Val66Met polymorphism and life stress in depression became stronger for stressful life events rather than for childhood adversity. The findings were replicated by a recent meta-analysis of 31 studies, involving of 21,060 participants, providing further evidence for an interaction between the BDNF Val66Met polymorphism and life stress in depression (Zhao M. et al., 2017).
Epigenetic studies have suggested that histone modifications, DNA methylation, and hydroxymethylation are possible mediators linking individual response to environmental factors and brain diseases (McEwen et al., 2015). These mediators may change the pattern of gene expression, influencing protein levels and ultimately shaping phenotypes during the life span. A study evaluating BDNF Val66Met polymorphism methylation in the peripheral blood of healthy subjects demonstrated that the increased methylation was associated with hypoxia-related early life events and impaired working memory in Val/Val individuals, and the opposite was true for Val/Met individuals (Ursini et al., 2016).
The interplay of genetic, epigenetic, and environmental factors may influence cognitive function. A study in normal subjects and subjects with amnestic mild cognitive impairment (aMCI) demonstrated that the increased BDNF promoter methylation status was associated with aMCI and its progression to AD (Xie et al., 2017). The interaction between DNA methylation and Met homozygosity increased the risk of aMCI and its progression to AD.
An epigenetic study of anxiety/depression in older women found higher BDNF DNA methylation in subjects with anxiety/depression than in controls, and this difference was more pronounced in BDNF Val66Met heterozygotes than in Val homozygotes (Chagnon et al., 2015).
It should be noted that, in terms of the two-hit hypothesis, there are studies which show that a second hit actually led to improvements, and some genetic polymorphisms, including BDNF Val66Met polymorphism, may actually increase resilience. For example, a recent study showed that BDNFMet/Met transgenic mice had spatial and fear-associated memory deficits, but corticosterone treatment recovered this phenotype (Notaras et al., 2017).
BDNFMet/Met Transgenic Mice
Chen et al. (2006) generated an inbred genetic knock-in mouse (BDNFMet/Met) that recapitulates the phenotypic hallmarks of human carriers with the Met allele. BDNFMet/Met mice represent a potential model to study the biological mechanism of this polymorphism in the brain.
BDNFMet/Met mice had decreased basal BDNF protein levels in the hippocampus, which could not be normalized by antidepressant (fluoxetine) administration (Bath et al., 2012b). BDNFMet/Met mice also showed impaired survival of newly generated cells and LTP in the dentate gyrus (Bath et al., 2012b). A recent study demonstrated that BDNFMet/Met mice exhibited diminished development of serotonergic fibers projecting particularly to the prefrontal cortex compared with wild-type mice; this diminished development was rescued by fluoxetine administration during peri-adolescence (Dincheva et al., 2017).
Compared with wild-type mice, significant decreases of 13.7% ± 0.7% and 14.4% ± 0.7% were observed in the hippocampal volume of BDNF+/Met and BDNFMet/Met mice, respectively (Chen et al., 2006). The transgenic mice showed increased depression and anxiety-like behaviors in stressful settings, and the behaviors were not normalized by antidepressant (fluoxetine) administration (Chen et al., 2006; Yu et al., 2012). In addition, the variant mice showed impaired learning of cues that signal safety (Soliman et al., 2010). These findings provide an example of a human genetic variant that has been modeled in transgenic mice can produce similar phenotypic hallmarks observed in some clinical studies.
The aforementioned findings should be interpreted with caution because not all findings demonstrated in BDNFMet/Met mice have been consistently found in human studies. For example, BDNFMet/Met mice had a decreased hippocampal volume compared with that of wild-type mice (Chen et al., 2006). An earlier report also showed that human Met carriers had reduced hippocampal gray matter volume compared with that of Val homozygotes (Pezawas et al., 2004). However, following imaging genetic studies have shown controversial results regarding the genetic effect of BDNF Val66Met on hippocampal volumes in normal subjects (Harrisberger et al., 2014; Liu et al., 2014). A meta-analysis including 5,298 healthy subjects revealed no significant BDNF genotype effect on hippocampal volume (Harrisberger et al., 2014).
Another example is the genetic association studies of the BDNF Val66Met polymorphism and cognitive function, which has been the focus of several clinical studies. Cognitive impairment has been reported in a mouse model of the BDNF Met allele (Chen et al., 2006; Dincheva et al., 2012). Conflicting findings have been obtained for the genetic effect of BDNF Val66Met on human cognitive function (Tsai et al., 2004b, 2008a; Hong et al., 2011). A meta-analysis including 7,095 individuals failed to support significant genetic associations between the Val66Met polymorphism and any of the cognitive phenotypes (Mandelman and Grigorenko, 2012).
Brain-derived neurotrophic factor has been implicated in the pathogenesis of major depression (Duman et al., 1997; Tsai et al., 2008b). Animal studies have demonstrated that BDNFMet/Met mice exhibited depression-like behaviors in stressful situations (Chen et al., 2006; Yu et al., 2012). However, in clinical studies, we found the Met allele is not associated with depression in either psychiatric outpatients or inpatients (Hong et al., 2003a; Tsai et al., 2003).
Finally, it should be noted that the knock-in mouse model developed by the Lee group simply replaced the valine (which in rodents is in position 68, not 66) with a methionine (Chen et al., 2006). Recently the Ron research team generated another transgenic mice carrying the mouse homolog of the human BDNF Met allele (Met68BDNF) (Warnault et al., 2016). Using this model, they demonstrated that Met allele increases the risk of compulsive alcohol drinking which can be reversed by directly activating the TrkB receptor (Warnault et al., 2016).
It is not known if and how this slight difference with the human BDNF Val66Met polymorphism affects the validity of these mouse models. A more precise transgenic model was developed by the Gogos group, where the mice were ‘humanized’ by inserting a small stretch of human sequence, including Val/Met at position 66 (Cao et al., 2007). This genetic manipulation generated knock-in alleles that express human BDNF genes controlled by endogenous mouse Bdnf regulatory elements. This one has now been used by several other investigators. For example, recent studies using this hBDNFV al66Met knock-in mice, van den Buuse et al. (2017) showed that the BDNF Val66Met Val/Met and Met/Met genotypes are more sensitive than the Val/Val genotype to the effect of apomorphine on prepulse inhibition. A history of stress, modeled by long-term treatment with corticosterone in young adults, increases the effects of apomorphine in Val/Val mice (van den Buuse et al., 2017).
Findings of the Novel Function of BDNF Val66Met Polymorphism
The first study investigating the function of this polymorphism demonstrated that BDNF Val66Met polymorphism affects activity-dependent BDNF release (Egan et al., 2003). In addition to this genetic effect, recent studies have found more functional effects for this polymorphism.
Brain-derived neurotrophic factor is initially synthesized as the precursor protein proBDNF, which is then cleaved by intracellular (furin/PC1) or extracellular peptidase enzymes (tPA/plasmin/MMP) into bioactive mature BDNF and pro-peptide (or pro-domain) (Pang et al., 2004). The Val66Met substitution is present in the BDNF pro-peptide region. The BDNF pro-peptide is detected in the hippocampus, and the application of the Met-type, but not Val-type, BDNF pro-peptide can induce acute growth cone retraction, suggesting that the Met-type pro-peptide is a new active ligand that can modulate neuronal morphology (Anastasia et al., 2013).
The BDNF pro-peptide functions as a modulator of synaptic plasticity by enhancing hippocampal long-term depression (LTD) (Mizui et al., 2015). Mizui et al. found that the Val-type BDNF pro-peptide facilitates low-frequency stimulation–induced hippocampal LTD, whereas the Met-type pro-peptide attenuates LTD (Mizui et al., 2015).
The BDNF pro-peptide can bind to mature BDNF with high affinity, and compared with the complex with the Val-type pro-peptide, the complex with the Met-type pro-peptide is more stable, suggesting that the BDNF Val66Met polymorphism affects the stability of the complex formed between BDNF and its pro-peptide (Uegaki et al., 2017).
The BDNF Val66Met polymorphism may affect the protein or mRNA expression of BDNF. The effect of the Val66Met polymorphism on the constitutive expression of BDNF was tested in HEK293T cells transiently transfected with recombinant plasmids to induce overexpression of either the Val or Met variant (Jin et al., 2015). A significant decrease in secreted BDNF protein levels in the culture supernatants of cells overexpressing the Met variant was found. In the same study, Met carriers had increased blood BDNF mRNA and protein levels. A higher circulating BDNF concentration associated with the Met allele was also found in a large cohort (Kaess et al., 2015), but a negative association was also found (Jiang et al., 2009). In a meta-analysis, no association was found between serum BDNF levels and the Val66Met polymorphism (Terracciano et al., 2013).
Is Met or Val the Risk Allele?
The BDNF Val66Met polymorphism has been reported to be associated with psychiatric disorders, including obsessive-compulsive disorder, schizophrenia, psychosis, major depression, anxiety, and eating disorders (Hong et al., 2011; Notaras et al., 2015b). Most positive association studies have reported that the Met allele is the risk allele for psychiatric disease given that Met carriers exhibit reduced activity-dependent secretion of BDNF (Table 1). However, the higher activity BDNF Val allele is associated with bipolar disorder (Neves-Pereira et al., 2002; Sklar et al., 2002) and substance use disorder (Cheng et al., 2005; Liu et al., 2005; Sim et al., 2010). In the Mexican–American population, it has been found that individuals homozygous for the Val allele have an increased chance of depression (Ribeiro et al., 2007). These findings suggest that this BDNF polymorphism has pleiotropic effects on multiple phenotypes; thus, this polymorphism imparts separate advantageous traits and disadvantageous traits in the same organism.
The different effects of this polymorphism in different disorders here could be due to the differential expression of BDNF and its receptor in different regions of the brain. For example, over or under activity-dependent secretion of BDNF will have varying effects on amygdala related behaviors (e.g., fear/anxiety) when compared with cognition (hippocampal-dependent) (Andero et al., 2014; Ilchibaeva et al., 2018).
Furthermore, evidence suggests that increased BDNF activity has a deleterious effect and may be implicated in the pathogenesis of some diseases (Tsai, 2005, 2006, 2007a,c). For example, increased BDNF activity in the ventral tegmental area-nucleus accumbens (VTA-NAc) pathway may be implicated in the pathogenesis of major depression (Eisch et al., 2003). Evidence also suggests that BDNF overactivity in the brain may be implicated in the pathogenesis of bipolar disorder (Tsai, 2004b), substance abuse (Tsai, 2007a), and autism (Tsai, 2005). Moreover, the genetic overexpression of the BDNF mature isoform in female mice impaired working memory functions, reduced breeding efficiency, increased anxiety-like behaviors, impaired prepulse inhibition, and elicited higher susceptibility to seizures (Govindarajan et al., 2006; Papaleo et al., 2011). Thus, the Val allele, which is associated with the increased activity-dependent secretion of BDNF, may be the risk allele for some neuropsychiatric diseases.
Other BDNF Polymorphisms
Investigating a single BDNF polymorphism (i.e., the Val66Met polymorphism) might only reveal some of the BDNF genetic variability and result in the overlooking of some information from other BDNF SNPs (Tsai et al., 2010; Yeh et al., 2015). Furthermore, the use of a haplotype constructed by several tag BDNF SNPs can improve genotyping efficiency by reducing the number of polymorphisms to be genotyped, and the haplotype itself may also tag other genetic variants that affect gene function.
Genetic studies of other BDNF polymorphisms have been conducted. For example, Proschel et al. (1992) identified a dinucleotide repeat polymorphism (GT) that maps 1,040 bp upstream from the transcription start site (Proschel et al., 1992). The BDNF GT repeat polymorphism is associated with age at onset, therapeutic response, susceptibility, and chlorpromazine-induced extrapyramidal syndrome in schizophrenia (Krebs et al., 2000; Xu et al., 2008).
Another common BDNF SNP, namely the C270T polymorphism (rs56164415) in the BDNF 5′ non-coding region, has been identified and reported to be associated with AD (Kunugi et al., 2001).
By sequencing the entire BDNF gene and the 5-kb flanking region, Licinio et al. (2009) demonstrated that six BDNF SNPs (rs12273539, rs11030103, rs6265, rs28722151, rs41282918, and rs11030101) are significantly associated with MDD.
Conclusion
Considering the important role of BDNF in the brain and the functional effect of the common BDNF Val66Met polymorphism, this polymorphism is one of the most studied polymorphisms in neuropsychiatric diseases. However, following studies have been unable to replicate most positive findings in initial genetic studies. In this review, we highlighted critical issues in BDNF Val66Met studies, which may affect the findings of these studies. Most neuropsychiatric diseases are complex diseases that are dependent on many genetic and environmental factors that cannot be analyzed by conventional genetic association studies. Future studies should analyze various BDNF polymorphisms and these related factors by using machine learning techniques to accurately understand the genetic effect of BDNF on disease pathogenesis.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grant MOST 107-2634-F-075-002 and MOST 104-2745-B-075-002 from Taiwan Ministry of Science and Technology, and grants V105E17-002-MY2-1, V105D17-002-MY2-2, and VGHUST103-G1-4-1 from the Taipei Veterans General Hospital.
References
- Aas M., Haukvik U. K., Djurovic S., Tesli M., Athanasiu L., Bjella T., et al. (2014). Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. 59 14–21. 10.1016/j.jpsychires.2014.08.011 [DOI] [PubMed] [Google Scholar]
- Anastasia A., Deinhardt K., Chao M. V., Will N. E., Irmady K., Lee F. S., et al. (2013). Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. 4:2490. 10.1038/ncomms3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R., Choi D. C., Ressler K. J. (2014). BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. 122 169–192. 10.1016/B978-0-12-420170-5.00006-4 [DOI] [PubMed] [Google Scholar]
- Atwi S., Mcmahon D., Scharfman H., Maclusky N. J. (2016). Androgen modulation of hippocampal structure and function. 22 46–60. 10.1177/1073858414558065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E., Schmidt K. M., Sims R., O’donovan M. C., Williams J., Holmans P., et al. (2018). POLARIS: polygenic LD-adjusted risk score approach for set-based analysis of GWAS data. 10.1002/gepi.22117 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K. G., Chuang J., Spencer-Segal J. L., Amso D., Altemus M., Mcewen B. S., et al. (2012a). Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. 72 499–504. 10.1016/j.biopsych.2012.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K. G., Jing D. Q., Dincheva I., Neeb C. C., Pattwell S. S., Chao M. V., et al. (2012b). BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. 37 1297–1304. 10.1038/npp.2011.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begliuomini S., Casarosa E., Pluchino N., Lenzi E., Centofanti M., Freschi L., et al. (2007). Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. 22 995–1002. 10.1093/humrep/del479 [DOI] [PubMed] [Google Scholar]
- Booij L., Tremblay R. E., Szyf M., Benkelfat C. (2015). Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. 40 5–18. 10.1503/jpn.140099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C. R., Messaoudi E. (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. 76 99–125. 10.1016/j.pneurobio.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Brandys M. K., Kas M. J., Van Elburg A. A., Ophoff R., Slof-Op’t Landt M. C., Middeldorp C. M., et al. (2013). The Val66Met polymorphism of the BDNF gene in anorexia nervosa: new data and a meta-analysis. 14 441–451. 10.3109/15622975.2011.605470 [DOI] [PubMed] [Google Scholar]
- Bruenig D., Lurie J., Morris C. P., Harvey W., Lawford B., Young R. M., et al. (2016). A case-control study and meta-analysis reveal BDNF Val66Met is a possible risk factor for PTSD. 2016:6979435. 10.1155/2016/6979435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Shi X., Zhang X., Zhang A., Zheng M., Fang Y. (2017). The association between brain-derived neurotrophic factor gene polymorphism and migraine: a meta-analysis. 18:13. 10.1186/s10194-017-0725-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Dhilla A., Mukai J., Blazeski R., Lodovichi C., Mason C. A., et al. (2007). Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. 17 911–921. 10.1016/j.cub.2007.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnin S., Massarotti A., Terrazzino S. (2016). BDNF Val66Met and clinical response to antipsychotic drugs: a systematic review and meta-analysis. 33 45–53. 10.1016/j.eurpsy.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Chagnon Y. C., Potvin O., Hudon C., Preville M. (2015). DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. 6:230. 10.3389/fgene.2015.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. B., Ye K. (2017). Sex differences in brain-derived neurotrophic factor signaling and functions. 95 328–335. 10.1002/jnr.23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chang H. A., Chen T. Y., Fang W. H., Huang S. Y. (2014). Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism affects sympathetic tone in a gender-specific way. 47 17–25. 10.1016/j.psyneuen.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Chen K., Wang N., Zhang J., Hong X., Xu H., Zhao X., et al. (2017). Is the Val66Met polymorphism of the brain-derived neurotrophic factor gene associated with panic disorder? A meta-analysis. 9:e12228. 10.1111/appy.12228 [DOI] [PubMed] [Google Scholar]
- Chen M. H., Tsai S. J. (2016). Treatment-resistant panic disorder: clinical significance, concept and management. 70 219–226. 10.1016/j.pnpbp.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Jing D., Bath K. G., Ieraci A., Khan T., Siao C. J., et al. (2006). Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. 314 140–143. 10.1126/science.1129663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Hong C. J., Yu Y. W., Chen T. J., Wu H. C., Tsai S. J. (2005). Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. 140 86–90. 10.1016/j.molbrainres.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Cho S. C., Kim H. W., Kim B. N., Kim J. W., Shin M. S., Chung S., et al. (2010). Gender-specific association of the brain-derived neurotrophic factor gene with attention-deficit/hyperactivity disorder. 7 285–290. 10.4306/pi.2010.7.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Wang D., Meng H., Zhang K., Fu L., Wu Y., et al. (2013). Association between the BDNF G196A and C270T polymorphisms and Parkinson’s disease: a meta-analysis. 123 675–683. 10.3109/00207454.2013.798784 [DOI] [PubMed] [Google Scholar]
- de Castro-Catala M., Van Nierop M., Barrantes-Vidal N., Cristobal-Narvaez P., Sheinbaum T., Kwapil T. R., et al. (2016). Childhood trauma, BDNF Val66Met and subclinical psychotic experiences. Attempt at replication in two independent samples. 83 121–129. 10.1016/j.jpsychires.2016.08.014 [DOI] [PubMed] [Google Scholar]
- Dincheva I., Glatt C. E., Lee F. S. (2012). Impact of the BDNF Val66Met polymorphism on cognition: implications for behavioral genetics. 18 439–451. 10.1177/1073858411431646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I., Yang J., Li A., Marinic T., Freilingsdorf H., Huang C., et al. (2017). Effect of early-life fluoxetine on anxiety-like behaviors in BDNF Val66Met Mice. 174 1203–1213. 10.1176/appi.ajp.2017.15121592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R. S., Heninger G. R., Nestler E. J. (1997). A molecular and cellular theory of depression. 54 597–606. 10.1001/archpsyc.1997.01830190015002 [DOI] [PubMed] [Google Scholar]
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. 112 257–269. 10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- Eisch A. J., Bolanos C. A., De Wit J., Simonak R. D., Pudiak C. M., Barrot M., et al. (2003). Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. 54 994–1005. 10.1016/j.biopsych.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee K. F., Jaenisch R. (1994). Mice lacking brain-derived neurotrophic factor develop with sensory deficits. 368 147–150. 10.1038/368147a0 [DOI] [PubMed] [Google Scholar]
- Failla M. D., Kumar R. G., Peitzman A. B., Conley Y. P., Ferrell R. E., Wagner A. K. (2015). Variation in the BDNF gene interacts with age to predict mortality in a prospective, longitudinal cohort with severe TBI. 29 234–246. 10.1177/1545968314542617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero D. A., Lopez-Leon S., Shin H. D., Park B. L., Kim D. J. (2015). Meta-analysis of six genes (BDNF, DRD1, DRD3, DRD4, GRIN2B and MAOA) involved in neuroplasticity and the risk for alcohol dependence. 149 259–263. 10.1016/j.drugalcdep.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Frustaci A., Pozzi G., Gianfagna F., Manzoli L., Boccia S. (2008). Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. 58 163–170. 10.1159/000182892 [DOI] [PubMed] [Google Scholar]
- Fukumoto N., Fujii T., Combarros O., Kamboh M. I., Tsai S. J., Matsushita S., et al. (2010). Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: new data and meta-analysis. 153B 235–242. 10.1002/ajmg.b.30986 [DOI] [PubMed] [Google Scholar]
- Gibbs R. B. (1998). Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. 787 259–268. 10.1016/S0006-8993(97)01511-4 [DOI] [PubMed] [Google Scholar]
- Gomez-Garre P., Huertas-Fernandez I., Caceres-Redondo M. T., Alonso-Canovas A., Bernal-Bernal I., Blanco-Ollero A., et al. (2014). BDNF Val66Met polymorphism in primary adult-onset dystonia: a case-control study and meta-analysis. 29 1083–1086. 10.1002/mds.25938 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castro T. B., Nicolini H., Lanzagorta N., Lopez-Narvaez L., Genis A., Pool Garcia S., et al. (2015). The role of brain-derived neurotrophic factor (BDNF) Val66Met genetic polymorphism in bipolar disorder: a case-control study, comorbidities, and meta-analysis of 16,786 subjects. 17 27–38. 10.1111/bdi.12227 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castro T. B., Salas-Magana M., Juarez-Rojop I. E., Lopez-Narvaez M. L., Tovilla-Zarate C. A., Hernandez-Diaz Y. (2017). Exploring the association between BDNF Val66Met polymorphism and suicidal behavior: meta-analysis and systematic review. 94 208–217. 10.1016/j.jpsychires.2017.07.020 [DOI] [PubMed] [Google Scholar]
- Govindarajan A., Rao B. S., Nair D., Trinh M., Mawjee N., Tonegawa S., et al. (2006). Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. 103 13208–13213. 10.1073/pnas.0605180103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacos M., Gonzalez J. R., Mercader J. M., De Cid R., Urretavizcaya M., Estivill X. (2007). Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. 61 911–922. 10.1016/j.biopsych.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Gyekis J. P., Yu W., Dong S., Wang H., Qian J., Kota P., et al. (2013). No association of genetic variants in BDNF with major depression: a meta- and gene-based analysis. 162B 61–70. 10.1002/ajmg.b.32122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerian B. S. (2013). BDNF rs6265 polymorphism and drug addiction: a systematic review and meta-analysis. 14 2055–2065. 10.2217/pgs.13.217 [DOI] [PubMed] [Google Scholar]
- Hajek T., Kopecek M., Hoschl C. (2012). Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: meta-analysis. 13 178–187. 10.3109/15622975.2011.580005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisberger F., Smieskova R., Schmidt A., Lenz C., Walter A., Wittfeld K., et al. (2015). BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: a systematic review and meta-analysis. 55 107–118. 10.1016/j.neubiorev.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Harrisberger F., Spalek K., Smieskova R., Schmidt A., Coynel D., Milnik A., et al. (2014). The association of the BDNF Val66Met polymorphism and the hippocampal volumes in healthy humans: a joint meta-analysis of published and new data. 42 267–278. 10.1016/j.neubiorev.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove L. C., Maclusky N. J., Scharfman H. E. (2013). Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. 239 46–66. 10.1016/j.neuroscience.2012.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S., Du L., Litteljohn D., Palkovits M., Faludi G., Merali Z., et al. (2015). Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. 600 12–16. 10.1016/j.neulet.2015.05.052 [DOI] [PubMed] [Google Scholar]
- Hill R. A., Wu Y. W., Kwek P., Van Den Buuse M. (2012). Modulatory effects of sex steroid hormones on brain-derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. 24 774–788. 10.1111/j.1365-2826.2012.02277.x [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. (1990). Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. 9 2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. J., Huo S. J., Yen F. C., Tung C. L., Pan G. M., Tsai S. J. (2003a). Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. 48 186–189. 10.1159/000074636 [DOI] [PubMed] [Google Scholar]
- Hong C. J., Liou Y. J., Tsai S. J. (2011). Effects of BDNF polymorphisms on brain function and behavior in health and disease. 86 287–297. 10.1016/j.brainresbull.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Hong C. J., Liu H. C., Liu T. Y., Lin C. H., Cheng C. Y., Tsai S. J. (2003b). Brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms in Parkinson’s disease and age of onset. 353 75–77. [DOI] [PubMed] [Google Scholar]
- Hosang G. M., Shiles C., Tansey K. E., Mcguffin P., Uher R. (2014). Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. 12:7. 10.1186/1741-7015-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Liu M. E., Chou K. H., Yang A. C., Hung C. C., Hong C. J., et al. (2014). Effect of BDNF Val66Met polymorphism on regional white matter hyperintensities and cognitive function in elderly males without dementia. 39 94–103. 10.1016/j.psyneuen.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Hwang J. P., Tsai S. J., Hong C. J., Yang C. H., Lirng J. F., Yang Y. M. (2006). The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. 27 1834–1837. 10.1016/j.neurobiolaging.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Ilchibaeva T. V., Tsybko A. S., Kozhemyakina R. V., Kondaurova E. M., Popova N. K., Naumenko V. S. (2018). Genetically defined fear-induced aggression: focus on BDNF and its receptors. 343 102–110. 10.1016/j.bbr.2018.01.034 [DOI] [PubMed] [Google Scholar]
- Ji H., Dai D., Wang Y., Jiang D., Zhou X., Lin P., et al. (2015). Association of BDNF and BCHE with Alzheimer’s disease: meta-analysis based on 56 genetic case-control studies of 12,563 cases and 12,622 controls. 9 1831–1840. 10.3892/etm.2015.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang R., Liu Y., Zhang Y., Chen Z. Y. (2009). BDNF Val66Met polymorphism is associated with unstable angina. 400 3–7. 10.1016/j.cca.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Jiang R., Babyak M. A., Brummett B. H., Siegler I. C., Kuhn C. M., Williams R. B. (2017). Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism interacts with gender to influence cortisol responses to mental stress. 79 13–19. 10.1016/j.psyneuen.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Andiappan A. K., Quek J. M., Lee B., Au B., Sio Y. Y., et al. (2015). A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. 135 1486.e8–1493.e8. 10.1016/j.jaci.2014.12.1870 [DOI] [PubMed] [Google Scholar]
- Joyce J. N., Ryoo H. L., Beach T. B., Caviness J. N., Stacy M., Gurevich E. V., et al. (2002). Loss of response to levodopa in Parkinson’s disease and co-occurrence with dementia: role of D3 and not D2 receptors. 955 138–152. 10.1016/S0006-8993(02)03396-6 [DOI] [PubMed] [Google Scholar]
- Kaess B. M., Preis S. R., Lieb W., Beiser A. S., Yang Q., Chen T. C., et al. (2015). Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. 4:e001544. 10.1161/JAHA.114.001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J. P., Bhattacharyya S., Kambeitz-Ilankovic L. M., Valli I., Collier D. A., Mcguire P. (2012). Effect of BDNF val(66)met polymorphism on declarative memory and its neural substrate: a meta-analysis. 36 2165–2177. 10.1016/j.neubiorev.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Glatt S. J., Kia-Keating B., Yoneda H., Tsuang M. T. (2007). Meta-analysis reveals no association of the Val66Met polymorphism of brain-derived neurotrophic factor with either schizophrenia or bipolar disorder. 17 165–170. 10.1097/YPG.0b013e32801da2e2 [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Wakako R., Komori T., Shigemi H., Miyazaki N., Ito H., et al. (2007). Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. 25 367–372. 10.1016/j.ijdevneu.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Kawashima K., Ikeda M., Kishi T., Kitajima T., Yamanouchi Y., Kinoshita Y., et al. (2009). BDNF is not associated with schizophrenia: data from a Japanese population study and meta-analysis. 112 72–79. 10.1016/j.schres.2009.03.040 [DOI] [PubMed] [Google Scholar]
- Keverne E. B. (2014). Significance of epigenetics for understanding brain development, brain evolution and behaviour. 264 207–217. 10.1016/j.neuroscience.2012.11.030 [DOI] [PubMed] [Google Scholar]
- Kheirollahi M., Kazemi E., Ashouri S. (2016). Brain-derived neurotrophic factor gene Val66Met polymorphism and risk of schizophrenia: a meta-analysis of case-control studies. 36 1–10. 10.1007/s10571-015-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., Lee J. Y., Kang H. J., Kim S. Y., Bae K. Y., Kim J. M., et al. (2016). Gender-specific associations of the brain-derived neurotrophic factor Val66Met polymorphism with neurocognitive and clinical features in schizophrenia. 14 270–278. 10.9758/cpn.2016.14.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M. O., Guillin O., Bourdell M. C., Schwartz J. C., Olie J. P., Poirier M. F., et al. (2000). Brain derived neurotrophic factor (BDNF) gene variants association with age at onset and therapeutic response in schizophrenia. 5 558–562. 10.1038/sj.mp.4000749 [DOI] [PubMed] [Google Scholar]
- Kunugi H., Ueki A., Otsuka M., Isse K., Hirasawa H., Kato N., et al. (2001). A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer’s disease. 6 83–86. 10.1038/sj.mp.4000792 [DOI] [PubMed] [Google Scholar]
- Laing K. R., Mitchell D., Wersching H., Czira M. E., Berger K., Baune B. T. (2012). Brain-derived neurotrophic factor (BDNF) gene: a gender-specific role in cognitive function during normal cognitive aging of the MEMO-Study? 34 1011–1022. 10.1007/s11357-011-9275-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P., Cheng C. Y., Hong C. J., Tsai S. J. (2004). Association study of a brain-derived neurotrophic factor (Val66Met) genetic polymorphism and panic disorder. 49 178–181. 10.1159/000077362 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Song G. G. (2014). BDNF 196 G/A and 270 C/T polymorphisms and susceptibility to Parkinson’s disease: a meta-analysis. 46 59–66. 10.1080/00222895.2013.862199 [DOI] [PubMed] [Google Scholar]
- Li M., Chang H., Xiao X. (2016). BDNF Val66Met polymorphism and bipolar disorder in European populations: a risk association in case-control, family-based and GWAS studies. 68 218–233. 10.1016/j.neubiorev.2016.05.031 [DOI] [PubMed] [Google Scholar]
- Li M., Masugi-Tokita M., Takanami K., Yamada S., Kawata M. (2012). Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. 1484 76–84. 10.1016/j.brainres.2012.09.028 [DOI] [PubMed] [Google Scholar]
- Licinio J., Dong C., Wong M. L. (2009). Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. 66 488–497. 10.1001/archgenpsychiatry.2009.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Hong C. J., Hwang J. P., Liou Y. J., Yang C. H., Cheng D., et al. (2009). Gene-gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. 12 387–393. 10.1089/rej.2009.0871 [DOI] [PubMed] [Google Scholar]
- Lin E., Kuo P. H., Liu Y. L., Yang A. C., Kao C. F., Tsai S. J. (2017). Effects of circadian clock genes and environmental factors on cognitive aging in old adults in a Taiwanese population. 8 24088–24098. 10.18632/oncotarget.15493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. H., Tsai S. J., Huang C. W., Mu-En L., Hsu S. W., Lee C. C., et al. (2016). Dose-dependent genotype effects of BDNF Val66Met polymorphism on default mode network in early stage Alzheimer’s disease. 7 54200–54214. 10.18632/oncotarget.11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Cheng S., Xie Z., Zhang D. (2014). Association of rs6265 and rs2030324 polymorphisms in brain-derived neurotrophic factor gene with Alzheimer’s disease: a meta-analysis. 9:e94961. 10.1371/journal.pone.0094961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. E., Huang C. C., Chen M. H., Yang A. C., Tu P. C., Yeh H. L., et al. (2014). Effect of the BDNF Val66Met polymorphism on regional gray matter volumes and cognitive function in the Chinese population. 16 127–136. 10.1007/s12017-013-8265-7 [DOI] [PubMed] [Google Scholar]
- Liu Q. R., Walther D., Drgon T., Polesskaya O., Lesnick T. G., Strain K. J., et al. (2005). Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s disease. 134B 93–103. 10.1002/ajmg.b.30109 [DOI] [PubMed] [Google Scholar]
- Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., et al. (2005). The impact of age, weight and gender on BDNF levels in human platelets and plasma. 26 115–123. 10.1016/j.neurobiolaging.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Lu B., Pang P. T., Woo N. H. (2005). The yin and yang of neurotrophin action. 6 603–614. 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- Luine V., Frankfurt M. (2013). Interactions between estradiol, BDNF and dendritic spines in promoting memory. 239 34–45. 10.1016/j.neuroscience.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelman S. D., Grigorenko E. L. (2012). BDNF Val66Met and cognition: all, none, or some? A meta-analysis of the genetic association. 11 127–136. 10.1111/j.1601-183X.2011.00738.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani S., Ventriglia M., Simonelli I., Bucossi S., Siotto M., R R. S. (2015). Meta-analysis study on the role of bone-derived neurotrophic factor Val66Met polymorphism in Parkinson’s disease. 18 40–47. 10.1089/rej.2014.1612 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Bowles N. P., Gray J. D., Hill M. N., Hunter R. G., Karatsoreos I. N., et al. (2015). Mechanisms of stress in the brain. 18 1353–1363. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B., Stramecki F., Gaweda L., Prochwicz K., Sasiadek M. M., Moustafa A. A., et al. (2017). Interactions between variation in candidate genes and environmental factors in the etiology of schizophrenia and bipolar disorder: a systematic review. 10.1007/s12035-017-0708-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura I., Zhang J. P., Nitta M., Lencz T., Kane J. M., Malhotra A. K., et al. (2014). BDNF Val66Met polymorphism and antipsychotic-induced tardive dyskinesia occurrence and severity: a meta-analysis. 152 365–372. 10.1016/j.schres.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizui T., Ishikawa Y., Kumanogoh H., Lume M., Matsumoto T., Hara T., et al. (2015). BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. 112 E3067–E3074. 10.1073/pnas.1422336112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose Y., Murata M., Kobayashi K., Tachikawa M., Nakabayashi Y., Kanazawa I., et al. (2002). Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. 51 133–136. 10.1002/ana.10079 [DOI] [PubMed] [Google Scholar]
- Murphy D. D., Cole N. B., Segal M. (1998). Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. 95 11412–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Ujike H., Sakai A., Uchida N., Nomura A., Imamura T., et al. (2003). Association study of the brain-derived neurotrophic factor (BDNF) gene with bipolar disorder. 337 17–20. 10.1016/S0304-3940(02)01292-2 [DOI] [PubMed] [Google Scholar]
- Naoe Y., Shinkai T., Hori H., Fukunaka Y., Utsunomiya K., Sakata S., et al. (2007). No association between the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and schizophrenia in Asian populations: evidence from a case-control study and meta-analysis. 415 108–112. 10.1016/j.neulet.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M., Mundo E., Muglia P., King N., Macciardi F., Kennedy J. L. (2002). The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. 71 651–655. 10.1086/342288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M., Du X., Gogos J., Van Den Buuse M., Hill R. A. (2017). The BDNF Val66Met polymorphism regulates glucocorticoid-induced corticohippocampal remodeling and behavioral despair. 7:e1233. 10.1038/tp.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M., Hill R., Van Den Buuse M. (2015a). A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. 51 15–30. 10.1016/j.neubiorev.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Notaras M., Hill R., Van Den Buuse M. (2015b). The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. 20 916–930. 10.1038/mp.2015.27 [DOI] [PubMed] [Google Scholar]
- Ottem E. N., Bailey D. J., Jordan C. L., Breedlove S. M. (2013). With a little help from my friends: androgens tap BDNF signaling pathways to alter neural circuits. 239 124–138. 10.1016/j.neuroscience.2012.12.019 [DOI] [PubMed] [Google Scholar]
- Pang P. T., Teng H. K., Zaitsev E., Woo N. T., Sakata K., Zhen S., et al. (2004). Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. 306 487–491. 10.1126/science.1100135 [DOI] [PubMed] [Google Scholar]
- Papaleo F., Silverman J. L., Aney J., Tian Q., Barkan C. L., Chadman K. K., et al. (2011). Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. 18 534–544. 10.1101/lm.2213711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Smith A. K., Wang Y., Pan Y., Yang J., Chen Q., et al. (2012). The brain-derived neurotrophic-factor (BDNF) val66met polymorphism is associated with geriatric depression: a meta-analysis. 159B 560–566. 10.1002/ajmg.b.32062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen T. L., Sabeti P. C., Aldinger K. A., Fry B., Fan J. B., Schaffner S. F., et al. (2010). Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. 15 810–815. 10.1038/mp.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Verchinski B. A., Mattay V. S., Callicott J. H., Kolachana B. S., Straub R. E., et al. (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. 24 10099–10102. 10.1523/JNEUROSCI.2680-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats C., Arias B., Ortet G., Ibanez M. I., Moya J., Pomarol-Clotet E., et al. (2017). Neurotrophins role in depressive symptoms and executive function performance: association analysis of NRN1 gene and its interaction with BDNF gene in a non-clinical sample. 211 92–98. 10.1016/j.jad.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Proschel M., Saunders A., Roses A. D., Muller C. R. (1992). Dinucleotide repeat polymorphism at the human gene for the brain-derived neurotrophic factor (BDNF). 1:353 10.1093/hmg/1.5.353-a [DOI] [PubMed] [Google Scholar]
- Pruunsild P., Kazantseva A., Aid T., Palm K., Timmusk T. (2007). Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. 90 397–406. 10.1016/j.ygeno.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Zhao J., Shi Y., Zhao X., Feng G., Xu F., et al. (2007). Brain-derived neurotrophic factor and risk of schizophrenia: an association study and meta-analysis. 353 738–743. 10.1016/j.bbrc.2006.12.121 [DOI] [PubMed] [Google Scholar]
- Rage F., Silhol M., Biname F., Arancibia S., Tapia-Arancibia L. (2007). Effect of aging on the expression of BDNF and TrkB isoforms in rat pituitary. 28 1088–1098. 10.1016/j.neurobiolaging.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Ribeiro L., Busnello J. V., Cantor R. M., Whelan F., Whittaker P., Deloukas P., et al. (2007). The brain-derived neurotrophic factor rs6265 (Val66Met) polymorphism and depression in Mexican-Americans. 18 1291–1293. 10.1097/WNR.0b013e328273bcb0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. D., Hahn L. W., Roodi N., Bailey L. R., Dupont W. D., Parl F. F., et al. (2001). Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. 69 138–147. 10.1086/321276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mora C., Ribases M., Ramos-Quiroga J. A., Casas M., Bosch R., Boreatti-Hummer A., et al. (2010). Meta-analysis of brain-derived neurotrophic factor p.Val66Met in adult ADHD in four European populations. 153B 512–523. 10.1002/ajmg.b.31008 [DOI] [PubMed] [Google Scholar]
- Shalev I., Lerer E., Israel S., Uzefovsky F., Gritsenko I., Mankuta D., et al. (2009). BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. 34 382–388. 10.1016/j.psyneuen.2008.09.017 [DOI] [PubMed] [Google Scholar]
- Silhol M., Bonnichon V., Rage F., Tapia-Arancibia L. (2005). Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. 132 613–624. 10.1016/j.neuroscience.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Sim M. S., Mohamed Z., Hatim A., Rajagopal V. L., Habil M. H. (2010). Association of brain-derived neurotrophic factor (Val66Met) genetic polymorphism with methamphetamine dependence in a Malaysian population. 1357 91–96. 10.1016/j.brainres.2010.08.053 [DOI] [PubMed] [Google Scholar]
- Sklar P., Gabriel S. B., Mcinnis M. G., Bennett P., Lim Y., Tsan G., et al. (2002). Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. 7 579–593. 10.1038/sj.mp.4001058 [DOI] [PubMed] [Google Scholar]
- Soliman F., Glatt C. E., Bath K. G., Levita L., Jones R. M., Pattwell S. S., et al. (2010). A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. 327 863–866. 10.1126/science.1181886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal J. L., Waters E. M., Bath K. G., Chao M. V., Mcewen B. S., Milner T. A. (2011). Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. 31 6780–6790. 10.1523/JNEUROSCI.0910-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapacs M. E., Mathews T. A., Tessarollo L., Ernest Lyons W., Mamounas L. A., Andrews A. M. (2004). Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. 140 81–92. 10.1016/j.jneumeth.2004.03.026 [DOI] [PubMed] [Google Scholar]
- Terracciano A., Piras M. G., Lobina M., Mulas A., Meirelles O., Sutin A. R., et al. (2013). Genetics of serum BDNF: meta-analysis of the Val66Met and genome-wide association study. 14 583–589. 10.3109/15622975.2011.616533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A., Tanaka T., Sutin A. R., Deiana B., Balaci L., Sanna S., et al. (2010). BDNF Val66Met is associated with introversion and interacts with 5-HTTLPR to influence neuroticism. 35 1083–1089. 10.1038/npp.2009.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazzino S., Cargnin S., Viana M., Sances G., Tassorelli C. (2017). Brain-derived neurotrophic factor val66met gene polymorphism impacts on migraine susceptibility: a meta-analysis of case-control studies. 8:159. 10.3389/fneur.2017.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand C. D., Miranda R. C., Bentham W. D., Sohrabji F., Brown T. J., Hochberg R. B., et al. (1992). Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. 89 4668–4672. 10.1073/pnas.89.10.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. J. (2003a). Attention-deficit hyperactivity disorder and brain-derived neurotrophic factor: a speculative hypothesis. 60 849–851. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2003b). Brain-derived neurotrophic factor: a bridge between major depression and Alzheimer’s disease? 61 110–113. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2004a). Down-regulation of the Trk-B signal pathway: the possible pathogenesis of major depression. 62 215–218. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2004b). Is mania caused by overactivity of central brain-derived neurotrophic factor? 62 19–22. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2005). Is autism caused by early hyperactivity of brain-derived neurotrophic factor? 65 79–82. 10.1016/j.mehy.2005.01.034 [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2006). TrkB partial agonists: potential treatment strategy for epilepsy, mania, and autism. 66 173–175. 10.1016/j.mehy.2005.05.033 [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2007a). Increased central brain-derived neurotrophic factor activity could be a risk factor for substance abuse: implications for treatment. 68 410–414. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2007b). The P11, tPA/plasminogen system and brain-derived neurotrophic factor: implications for the pathogenesis of major depression and the therapeutic mechanism of antidepressants. 68 180–183. [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2007c). TrkB partial agonists: potential treatment strategy for major depression. 68 674–676. 10.1016/j.mehy.2006.06.019 [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2017a). Role of neurotrophic factors in attention deficit hyperactivity disorder. 34 35–41. 10.1016/j.cytogfr.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Tsai S. J. (2017b). Role of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in psychological stress and depression. 8 113258–113268. 10.18632/oncotarget.19935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. J., Cheng C. Y., Yu Y. W., Chen T. J., Hong C. J. (2003). Association study of a brain-derived neurotrophic-factor genetic polymorphism and major depressive disorders, symptomatology, and antidepressant response. 123B 19–22. 10.1002/ajmg.b.20026 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Gau Y. T., Liu M. E., Hsieh C. H., Liou Y. J., Hong C. J. (2008a). Association study of brain-derived neurotrophic factor and apolipoprotein E polymorphisms and cognitive function in aged males without dementia. 433 158–162. 10.1016/j.neulet.2007.12.057 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Liou Y. J. (2008b). Brain-derived neurotrophic factor and antidepressant action: another piece of evidence from pharmacogenetics. 9 1353–1358. 10.2217/14622416.9.9.1353 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Liou Y. J. (2010). Effects of BDNF polymorphisms on antidepressant action. 7 236–242. 10.4306/pi.2010.7.4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Liou Y. J. (2011). Recent molecular genetic studies and methodological issues in suicide research. 35 809–817. 10.1016/j.pnpbp.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Liu H. C., Liu T. Y., Hsu L. E., Lin C. H. (2004a). Association analysis of brain-derived neurotrophic factor Val66Met polymorphisms with Alzheimer’s disease and age of onset. 49 10–12. 10.1159/000075332 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Liu H. C., Liu T. Y., Liou Y. J. (2006). The brain-derived neurotrophic factor gene as a possible susceptibility candidate for Alzheimer’s disease in a Chinese population. 21 139–143. 10.1159/000090673 [DOI] [PubMed] [Google Scholar]
- Tsai S. J., Hong C. J., Yu Y. W., Chen T. J. (2004b). Association study of a brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and personality trait and intelligence in healthy young females. 49 13–16. [DOI] [PubMed] [Google Scholar]
- Tzang R. F., Hsu C. D., Liou Y. J., Hong C. J., Tsai S. J. (2013). Family-based association of the brain-derived neurotrophic factor gene in attention-deficit hyperactivity disorder. 23 177–178. 10.1097/YPG.0b013e328360c8a9 [DOI] [PubMed] [Google Scholar]
- Uegaki K., Kumanogoh H., Mizui T., Hirokawa T., Ishikawa Y., Kojima M. (2017). BDNF binds its pro-peptide with high affinity and the common Val66Met polymorphism attenuates the interaction. 18:E1042. 10.3390/ijms18051042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini G., Cavalleri T., Fazio L., Angrisano T., Iacovelli L., Porcelli A., et al. (2016). BDNF rs6265 methylation and genotype interact on risk for schizophrenia. 11 11–23. 10.1080/15592294.2015.1117736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M., Lee J. J. W., Jaehne E. J. (2017). Interaction of brain-derived neurotrophic factor Val66Met genotype and history of stress in regulation of prepulse inhibition in mice. 10.1016/j.schres.2017.08.019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ventriglia M., Bocchio Chiavetto L., Benussi L., Binetti G., Zanetti O., Riva M. A., et al. (2002). Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer’s disease. 7 136–137. 10.1038/sj.mp.4000952 [DOI] [PubMed] [Google Scholar]
- Verhagen M., Van Der Meij A., Van Deurzen P. A., Janzing J. G., Arias-Vasquez A., Buitelaar J. K., et al. (2010). Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. 15 260–271. 10.1038/mp.2008.109 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang F., Zhu W., Liu Y., Zhou Z. (2015). Meta-analysis of the association of brain-derived neurotrophic factor Val66Met polymorphism with obsessive-compulsive disorder. 27 327–335. 10.1017/neu.2015.38 [DOI] [PubMed] [Google Scholar]
- Wang T. (2015). Does BDNF Val66Met polymorphism confer risk for posttraumatic stress disorder? 71 149–153. 10.1159/000381352 [DOI] [PubMed] [Google Scholar]
- Wang Z., Li Z., Gao K., Fang Y. (2014). Association between brain-derived neurotrophic factor genetic polymorphism Val66Met and susceptibility to bipolar disorder: a meta-analysis. 14:366. 10.1186/s12888-014-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V., Darcq E., Morisot N., Phamluong K., Wilbrecht L., Massa S. M., et al. (2016). The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. 79 463–473. 10.1016/j.biopsych.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S. M., Baller E. B., Kohn P. D., Kippenhan J. S., Kolachana B., Soldin S. J., et al. (2017). Brain-derived neurotrophic factor Val66Met genotype and ovarian steroids interactively modulate working memory-related hippocampal function in women: a multimodal neuroimaging study. 23 1066–1075. 10.1038/mp.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. C., Hill R. A., Gogos A., Van Den Buuse M. (2013). Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. 239 67–83. 10.1016/j.neuroscience.2012.10.024 [DOI] [PubMed] [Google Scholar]
- Xie B., Liu Z., Liu W., Jiang L., Zhang R., Cui D., et al. (2017). DNA methylation and tag SNPs of the BDNF gene in conversion of amnestic mild cognitive impairment into Alzheimer’s disease: a cross-sectional cohort study. 58 263–274. 10.3233/JAD-170007 [DOI] [PubMed] [Google Scholar]
- Xu B., Gottschalk W., Chow A., Wilson R. I., Schnell E., Zang K., et al. (2000). The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. 20 6888–6897. 10.1523/JNEUROSCI.20-18-06888.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Q., St Clair D., Feng G. Y., Lin Z. G., He G., Li X., et al. (2008). BDNF gene is a genetic risk factor for schizophrenia and is related to the chlorpromazine-induced extrapyramidal syndrome in the Chinese population. 18 449–457. 10.1097/FPC.0b013e3282f85e26 [DOI] [PubMed] [Google Scholar]
- Xu M. Q., St Clair D., Ott J., Feng G. Y., He L. (2007). Brain-derived neurotrophic factor gene C-270T and Val66Met functional polymorphisms and risk of schizophrenia: a moderate-scale population-based study and meta-analysis. 91 6–13. 10.1016/j.schres.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Yan T., Wang L., Kuang W., Xu J., Li S., Chen J., et al. (2014). Brain-derived neurotrophic factor Val66Met polymorphism association with antidepressant efficacy: a systematic review and meta-analysis. 6 241–251. 10.1111/appy.12148 [DOI] [PubMed] [Google Scholar]
- Yang J., Harte-Hargrove L. C., Siao C. J., Marinic T., Clarke R., Ma Q., et al. (2014). proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. 7 796–806. 10.1016/j.celrep.2014.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F. C., Kao C. F., Kuo P. H. (2015). Explore the features of brain-derived neurotrophic factor in mood disorders. 10:e0128605. 10.1371/journal.pone.0128605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Wang D. D., Wang Y., Liu T., Lee F. S., Chen Z. Y. (2012). Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. 32 4092–4101. 10.1523/JNEUROSCI.5048-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai C. C., Manchia M., De Luca V., Tiwari A. K., Chowdhury N. I., Zai G. C., et al. (2012). The brain-derived neurotrophic factor gene in suicidal behaviour: a meta-analysis. 15 1037–1042. 10.1017/S1461145711001313 [DOI] [PubMed] [Google Scholar]
- Zhang J., Luo W., Li Q., Xu R., Wang Q., Huang Q. (2017). Peripheral brain-derived neurotrophic factor in attention-deficit/hyperactivity disorder: a comprehensive systematic review and meta-analysis. 227 298–304. 10.1016/j.jad.2017.11.012 [DOI] [PubMed] [Google Scholar]
- Zhao M., Chen L., Yang J., Han D., Fang D., Qiu X., et al. (2017). BDNF Val66Met polymorphism, life stress and depression: a meta-analysis of gene-environment interaction. 227 226–235. 10.1016/j.jad.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Shen Y., Zhao Y., Si L., Jiang S., Qiu Y. (2017). Val66Met polymorphism in BDNF has no sexual and APOE epsilon4 status-based dimorphic effects on susceptibility to Alzheimer’s disease: evidence from an updated meta-analysis of case-control studies and high-throughput genotyping cohorts. 33 55–63. 10.1177/1533317517733037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Huang Y., Chen K., Li D., Han C., Kan Q. (2015). The brain-derived neurotrophic factor Val66Met polymorphism is not associated with schizophrenia: an updated meta-analysis of 11,480 schizophrenia cases and 13,490 controls. 225 217–220. 10.1016/j.psychres.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Zintzaras E. (2007). Brain-derived neurotrophic factor gene polymorphisms and schizophrenia: a meta-analysis. 17 69–75. 10.1097/YPG.0b013e32801119da [DOI] [PubMed] [Google Scholar]
- Zintzaras E., Hadjigeorgiou G. M. (2005). The role of G196A polymorphism in the brain-derived neurotrophic factor gene in the cause of Parkinson’s disease: a meta-analysis. 50 560–566. 10.1007/s10038-005-0295-z [DOI] [PubMed] [Google Scholar]
- Zou Y. F., Ye D. Q., Feng X. L., Su H., Pan F. M., Liao F. F. (2010). Meta-analysis of BDNF Val66Met polymorphism association with treatment response in patients with major depressive disorder. 20 535–544. 10.1016/j.euroneuro.2009.12.005 [DOI] [PubMed] [Google Scholar]


