Abstract
Neddylation is a ubiquitination-like pathway. It has been reported that neddylation inhibition with the pharmacological agent MLN4924 potently uppresses lipopolysaccharide (LPS)-induced proinflammatory cytokine production, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, by preventing the degradation of phosphorylated inhibitor of κB (p-IκB) in macrophages. However, whether neddylation serves a similar role in neutrophils remains unknown. In the present study MLN4924 treatment led to the accumulation of P-IκBα in neutrophils as well as the decreased production of TNF-α, IL-6 and IL-1β in response to LPS, in a dose-dependent manner. The viability of neutrophils was only marginally affected in the same conditions, without statistical significance. Furthermore, the nuclear factor (NF)-κB inhibitor JSH-23 mimicked the effects of MLN4924 in neutrophils, and the inhibitory effects of MLN4924 on LPS-induced proinflammatory cytokine production diminished in the presence of JSH-23. Thus, the results of the present study suggest that neddylation inhibition suppresses neutrophil function by suppressing the NF-κB signaling pathway.
Keywords: neddylation, MLN4924, neutrophils, nuclear factor κB, proinflammatory cytokine
Introduction
Neutrophils serve crucial roles in the anti-bacterial and anti-fungal innate immune response and produce cytokines to initiate inflammatory responses; however, their inappropriate or excessive activation contributes to tissue damage during autoimmune and inflammatory diseases (1). Furthermore, the activity of neutrophils is associated with tumor progression, as demonstrated in colitis-associated cancer and glioblastoma (2,3).
Previous studies have revealed that in order to protect the human body from invading pathogens and endogenous damage-associated molecules, neutrophils sense these threats through innate immune receptors, such as toll-like receptors (TLRs) and other pattern recognition receptors, to upregulate the secretion of inflammatory cytokines (4,5). The recognition of lipopolysaccharide (LPS) from gram-negative bacteria by TLR4 is a well-characterized example with the initiation of proinflammatory gene transcription through multiple signaling pathways, including nuclear factor (NF)-κB) (6,7). The exposure of neutrophils to bacterial LPS may contribute to tissue damage, under certain conditions, including endotoxic shock and infection-induced adult respiratory distress syndrome (8).
Neddylation is a novel-type protein post-translational modification, a multistep enzymatic process that adds the ubiquitin-like molecule neural precursor cell expressed developmentally downregulated protein 8 (Nedd8) to target proteins in an ATP-dependent manner. Neddylation is vital for a range of processes, including cell viability, growth and development (9–12). To the best of our knowledge, the only known E1 for neddylation is a heterodimer comprised of the amyloid precursor protein-binding protein and ubiquitin-like modifier activating enzyme 3 (9–12). A previous study identified the ubiquitin-conjugating enzymes Ube2M and Ube2F as Nedd8 E2s (13). Several targets for Nedd8 have been identified, however the well-characterized substrates of the neddylation system are Cullins and key components of Cullin-RING E3 ligases (CRLs) (14,15). Neddylation activates CRLs, which mediate the ubiquitination and subsequent degradation of various cellular proteins, including inhibitor of κB (IκB), that sequester NF-κB to the cytoplasm (16–18). Based on previous studies demonstrating that it suppressed the growth of diverse cancer cell lines, MLN4924, a small-molecule inhibitor of Nedd8 E1, may be a novel anticancer therapeutic agent (16–18). Additionally, previous studies also revealed that MLN4924 suppresses proinflammatory cytokine production in macrophages and dendritic cells by preventing the degradation of IκB proteins. Consequently, MLN4924 treatment led to alleviated mucosal inflammation in a colitis mouse model, indicating that neddylation may be involved in immune regulation (6,19,20).
Under certain conditions, neutrophils behave distinctly to macrophages in response to LPS (21). Nevertheless, the association between neddylation and LPS-induced proinflammatory cytokine production in neutrophils remains unclear. The results of the present study revealed that neddylation inhibition by MLN4924 led to the suppression of LPS-induced proinflammatory cytokine production in neutrophils in a dose-dependent manner by disrupting the NF-κB signaling pathway; this suggests that MLN4924 may serve a role as a potential chemotherapeutic agent for neutrophil-mediated autoimmune or inflammatory diseases.
Materials and methods
Mice
Female C57BL/6 mice at the age of 6–8 weeks were purchased from Beijing Vital River Laboratory Animal, Inc. (Beijing, China). All mice were maintained under specific pathogen-free conditions. The care, use and treatment of mice in the present study strictly followed the guidelines set by the Institute of Basic Medical Sciences. The Institute of Basic Medical Sciences (Beijing, China), from whom ethical approval was granted, approved the present study.
Reagents
Antibodies against phosphorylated (p)-IκBα (catalog no. #2859), p-IκB kinase (IKK)α/β (catalog no. #2697) and Nedd8 (catalog no. #2745) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against IκBα (catalog no. sc-7877), IKKα/β (catalog no. sc-7606) and β-actin (catalog no. sc-58673) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). MLN4924, dissolved in dimethyl sulfoxide, was purchased from Active Biochemku Ltd. (Maplewood, NJ, USA). The ATPlite 1 Step kit was purchased from Perkin Elmer, Inc. (Waltham, MA, USA). LPS (serotype, Escherichia coli) and thioglycolate were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Flow cytometry analysis
Mice received 2 ml 3% thioglycolate by intraperitoneal injection (Sigma-Aldrich; Merck KGaA). After 4 h, the mice were anaesthetized by administrating pentobarbital (50 mg/kg) and were subjected to euthanasia by cervical dislocation. The peritoneal cells were washed in 6 ml of 3 mM EDTA in PBS. The cells were washed with fluorescence-activated cell sorting (FACS) washing buffer [2% fetal bovine serum (Amresco, LLC, Solon, OH, USA) 0.1% NaN3 in PBS] twice prior to incubation with fluorescein isothiocyanate-conjugated antibodies [anti-F4/80 (catalog no. 565409), anti-γδ T cell receptor (TCR; catalog no. 561486) or anti-cluster of differentiation (CD)19 (catalog no. 561738)], phycoerythrin (PE)-conjugated antibodies [anti-Gr1 (catalog no. 560601), anti-natural killer cell (NK)1.1 (catalog no. 553164)], or allophycocyanin-conjugated anti-CD3 (catalog no. 563123) (1 µg/1×106 cells; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) for 30 min on ice in the presence of 2.4G2 monoclonal antibody (catalog no. 562896; 1 µg/1×106 cells; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) to block Fcγ receptor binding. Isotype antibodies [FITC-anti-Mouse IgG1 (catalog no. 550616), PE-anti-Mouse IgG1 (catalog no. 562027), APC-anti-Mouse IgG1 (catalog no. 550874)] were included as the negative control. Following washing with FACS buffer, the cells were fixed with 1% (w/v) paraformaldehyde in PBS and preserved at 4°C. Flow cytometry 6 was carried out with a Becton Dickinson FACSCalibur™ (BD Biosciences) and analyzed using FlowJo software (version 10; FlowJo LLC, Ashland, OR, USA).
Purification of neutrophils
Peritoneal cells were stained with PE-conjugated anti-Gr1 antibody as above. Other cells were then magnetically separated using anti-PE antibody-coated mircobeads (Miltenyi Biotec, Inc., Auburn, CA, USA), according to the manufacturer's protocol. Cell purity was verified to be >90% by flow cytometry.
Western blot
The purified neutrophils were seeded into 24-well plates at a density of 1×106 cells/well. Neutrophils were treated by MLN4924 with various concentrations (0, 0.1, 0.5 and 2.5 µM) for 30 min at 37°C and then washed with PBS and harvested using ice-cold lysis buffer (0.5% Tert-NP-40, 20 mM Tris-Cl, pH 7.6, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 10 mM PNPP, 10 mg/ml aprotinin). Protein concentration was determined by bicinchoninic acid assay (cat. no. SK3021; Bio Basic Inc., Ontario, Canada) according to the manufacturer's protocol. Cell lysates were resolved by SDS-PAGE (10% gel; 20 µg protein/lane) prior to being transferred to nitrocellulose membranes. Nitrocellulose membranes were then incubated with 5% (w/v) nonfat dry milk in washing buffer (20 mM Tris-Cl, pH 7.6, 150 mM NaCl, and 0.1% Tween 20) for 1 h at 37°C to block nonspecific protein binding. Primary antibodies against Nedd8, (p)-IκBα, IκBα, IKKα/β, (p)-IKKα/β and β-actin were diluted in PBS (1:1,000) containing 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) and applied to the membranes overnight at 4°C. Following extensive washing, the membranes were incubated with goat anti-rabbit IgG-horseradish peroxidase (HRP) or anti-mouse IgG-HRP (1:2,500 in washing buffer containing 5% (w/v) nonfat dry milk) antibodies for 1 h at room temperature. Following washing, immunoreactive bands were visualized using an ECL™ Western Blotting Detection Reagents (GE Healthcare, Chicago, IL, USA).
ELISA
The purified neutrophils were seeded into 24-well plates at a density of 1×106 cells/well. The supernatants were collected at 0 and 4 h after LPS treatment. The expression levels of TNF-α (catalog no. 70-EK2822/2), IL-6 (catalog no. 70-EK2062/2), IL-10 (catalog no. 70-EK2102/2) and IL-1β (catalog no. 70-EK201B2/2) were measured using ELISA kits purchased from eBioscience (Thermo Fisher Scientific, Inc.) following the manufacturer's protocols.
ATPlite assays
The purified cells were seeded into 96-well plates (2.5×105 cells/well). Following pretreatment with distinct doses (0, 0.1, 0.5 and 2.5 µM) of MLN4924 for 30 min at 37°C, cells were stimulated with 100 ng/ml LPS for 4 h. Then, cell viability was assessed using an ATPlite 1 step Single Addition Luminescence ATP Detection Assay System, according to the manufacturer's protocol (Perkin Elmer, Waltham, MA, USA).
Statistical analysis
The data are presented as the mean ± standard deviation using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). The data are presented as the mean ± standard deviation. The Student's t-test was employed to determine significant differences between two groups (paired or unpaired), and one-way analysis of variance (Please refer to cover letter) was used to determine significant differences among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
MLN4924 suppresses the neddylation of Cullins in neutrophils in a dose-dependent manner
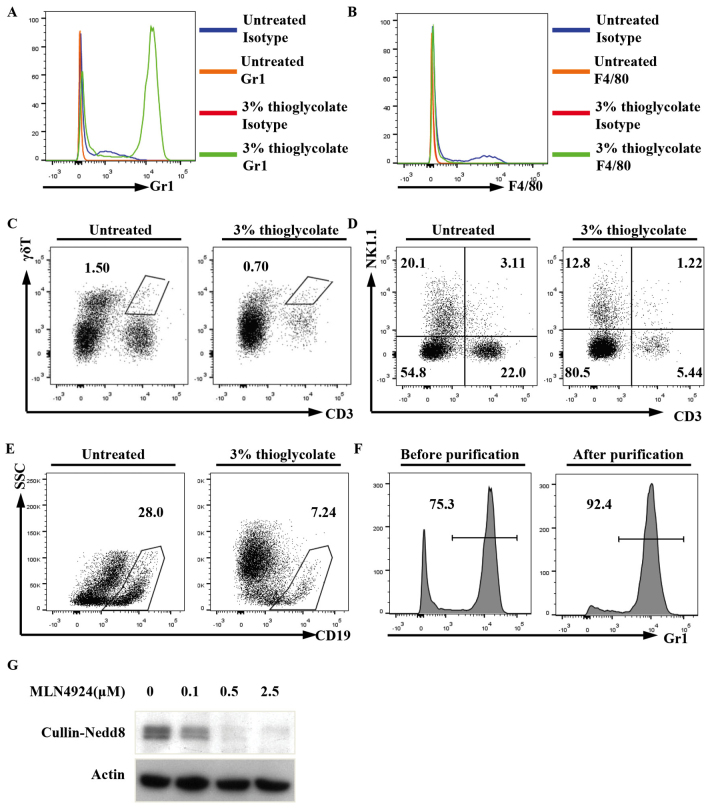
To examine the possible role of neddylation in neutrophils, peritoneal neutrophils were induced by a 3% thioglycolate intraperitoneal injection. Flow cytometry revealed that the percentage of peritoneal neutrophils (Gr1+) in the treated mice markedly increased after 4 h, whereas the percentages of macrophages (F4/80+), γδT cells (CD3+γδTCR+), T cells (CD3+NK1.1-), NK cells (CD3-NK1.1+), NKT cells (CD3+NK1.1+) and B cells (CD19+) decreased in the treated mice, to varying extents, as compared with in the non-treated mice (Fig. 1A-E). Peritoneal neutrophils were then purified with magnetic microbeads (Fig. 1F).
Figure 1.
MLN4924 suppressed the neddylation of Cullins in neutrophils in a dose-dependent manner. Mice received an intraperitoneal injection of 3% thioglycolate or were left untreated. After 4 h, peritoneal cells were subjected to flow cytometry to examine the percentages of (A) neutrophils, (B) macrophages, (C) γδT cells, (D) NKT cells and (E) B cells. (F) The purity of Gr1+ cells was >90% as confirmed by flow cytometry. (G) The purified peritoneal neutrophils were seeded into 24-well plates at a density of 1×106 cells/well. Following treatment with various doses of MLN4924, ranging between 0 and 2.5 µM for 30 min, the cell lysates were prepared and used for western blot analysis with antibodies against Nedd8 and β-actin (representative data are presented; n>3). Nedd8, neural precursor cell expressed developmentally downregulated protein 8; CD, cluster of differentiation; SSC, side scattering; NK, natural killer.
To explore whether MLN4924 suppresses the neddylation of Cullins in neutrophils, immunoblotting analysis with an antibody against Nedd8 was used. A major band between 90 and 100 kDA is usually recognized as neddylated Cullins (Nedd8-Cullin) and was examined in the present study (22–27). As predicted, the major band was detected in the neutrophils, and MLN4924 treatment for 30 min led to diminished Nedd8-Cullin in a dose-dependent manner (Fig. 1G). The results suggest that neddylation is active in neutrophils and that MLN4924 inhibits the neddylation of Cullins in neutrophils.
MLN4924 inhibits LPS-induced proinflammatory cytokine production in neutrophils in a dose-dependent manner
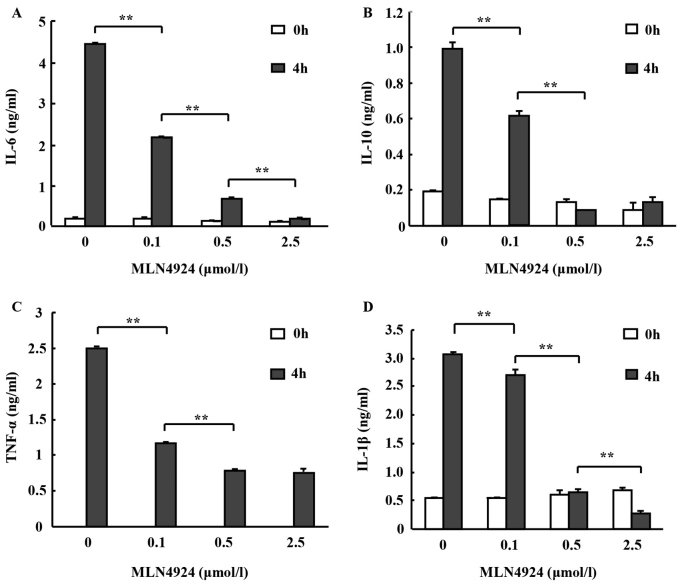
To investigate whether MLN4924 affects inflammatory cytokine secretion from neutrophils, peritoneal neutrophils were pretreated with various doses of MLN4924 for 30 min followed by stimulation with or without LPS for 4 h. Culture supernatants of the neutrophils were collected and the levels of TNF-α, IL-6 and IL-10 were determined using an ELISA. MLN4924 inhibited LPS-induced IL-6, IL-10 (observed in low concentrations in neutrophils) and TNF-α production in a dose-dependent manner (Fig. 2A-C; *P<0.05, **P<0.01). The decrease of the inflammatory cytokine secretion from neutrophils is associated with Cullins neddylation. The results indicate that MLN4924 may suppress the neutrophil-mediated inflammatory response.
Figure 2.
MLN4924 inhibited LPS-induced proinflammatory cytokine production in neutrophils in a dose-dependent manner. The purified peritoneal neutrophils were seeded into 24-well plates at a density of 1×106 cells/well. Following pretreatment with various doses of MLN4924 ranged between 0 and 2.5 µM for 30 min, cells were stimulated with or without 100 ng/ml LPS for 4 h. The culture supernatants were harvested and the levels of proinflammatory cytokine were investigated using ELISA: (A) IL-6, (B) IL-10, (C) TNF-α and (D) IL-1β. Data represent ≥3 independent experiments performed in triplicate and are represented as mean ± standard deviation **P<0.01. LPS, lipopolysaccharide; IL, interleukin; TNF, tumor necrosis factor.
Nedd8 silencing, or treatment with MLN4924, led to diminished caspase-1 processing and decreased IL-1β maturation in macrophages and epithelial cells following inflammasome activation (28,29). Aberrant IL-1β production may lead to various diseases, including pneumonia, diabetes, atherosclerosis, obesity, cancer, Alzheimer's and arthritis (30,31). Therefore, mature IL-1β was detected in the culture media in the presence of ATP and it was observed that MLN4924 inhibited LPS-induced production of mature IL-1β in neutrophils in a dose-dependent manner, as revealed by ELISA (Fig. 2D).
MLN4924 pretreatment followed by LPS stimulation for 4 h revealed marginal effects on the viability of neutrophils
The neddylation inhibitor MLN4924 has been demonstrated to suppress the oncogenic growth of various hematological malignancies, including myeloma, B-cell lymphoma and acute myeloid leukemia via cell cycle arrest and apoptosis (22,32,33). Furthermore, prolonged and complete (1.0 µM for 24 or 48 h) inhibition of neddylation led to decreased macrophage viability (34). To detect whether MLN4924 inhibited LPS-induced inflammatory cytokine production in neutrophils as a result of neutrophil apoptosis, the viability of neutrophils was examined using ATPlite assays. As presented in Fig. 3, MLN4924 pretreatment for 30 min followed by LPS stimulation for 4 h demonstrated only marginal effects on the viability of neutrophils and no statistically significant differences were identified.
Figure 3.
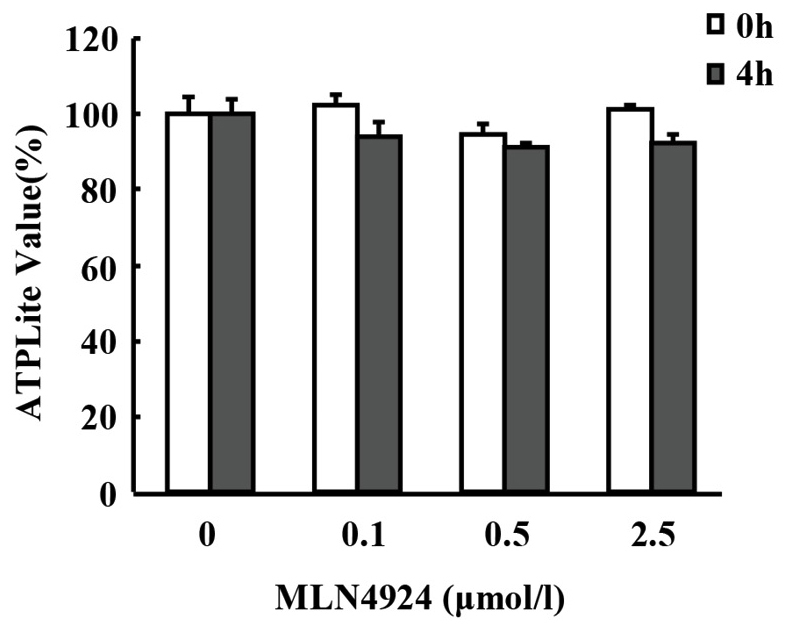
MLN4924 pretreatment followed by lipopolysaccharide stimulation for 4 h revealed only marginal effects on the viability of neutrophils using ATPlite assays. No statistically significant differences were identified.
MLN4924 inhibits LPS-induced proinflammatory cytokine production in neutrophils by suppressing NF-κB
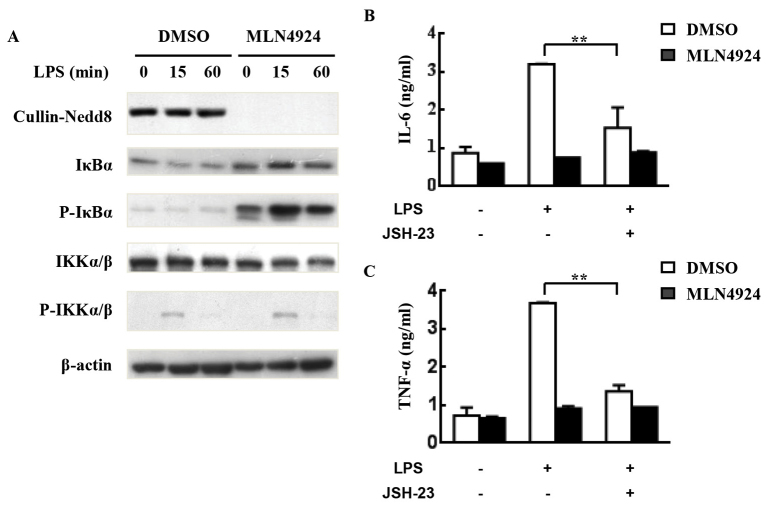
LPS can induce proinflammatory cytokine production in neutrophils through activating the IKK/NF-κB signaling pathway (27). The NF-κB signaling pathway is also associated with the production of pro-IL-1β (35). Neddylation has been demonstrated to serve a key role in the degradation of IκB proteins and subsequent NF-κB activation (6,27). The present study investigated whether a similar molecular mechanism occurred in neutrophils; as expected, MLN4924 suppressed the degradation and resynthesis of IκBα, which is associated with accumulated P-IκBα. However, MLN4924 treatment exhibited no effect on the phosphorylation of IKK (Fig. 4A). The results suggest that the inhibition of NF-κB signaling may be involved in the inhibitory effects of MLN4924.
Figure 4.
MLN4924 inhibits LPS-induced proinflammatory cytokine production from neutrophils by suppressing NF-κB. (A) The purified peritoneal neutrophils were seeded into 24-well plates at a density of 5×106 cells/well. Following pretreatment with 0.5 µM MLN4924 for 30 min, cells were stimulated with 100 ng/ml LPS for 0, 15 or 60 min. Cell lysates were then prepared to analyze the levels of neddylated Cullins, P-IKKα/β, P-IκBα, IKKα/β, IκBα and β-actin. Neutrophils were pretreated with 0.5 µM MLN4924 and/or 80 µM JSH-23 or an equal volume of DMSO for 30 min. Then neutrophils were treated with LPS for the indicated periods of time. The concentration of (B) IL-6 and (C) TNF-α in the supernatants was measured with ELISA. **P<0.01. LPS, lipopolysaccharide; NF-κB, nuclear factor κB; IκB, inhibitor of κB; P-IKK, phosphorylated IκB kinase; DMSO, dimethyl sulfoxide; IL, interleukin; TNF, tumor necrosis factor.
To investigate the role NF-κB has in the inhibitory effects of MLN4924 on LPS-induced proinflammatory cytokine production, neutrophils were pretreated with JSH-23, a specific inhibitor of NF-κB nuclear translocation and transcriptional activity (36), prior to LPS treatment for 4 h in the presence or absence of MLN4924. ELISA revealed that JSH-23 treatment alone resulted in marked inhibition of LPS-induced IL-6/TNF-α production (Fig. 4B and C). The inhibitory effects of MLN4924 on LPS-induced proinflammatory cytokine production diminished in the presence of the NF-κB inhibitor JSH-23 (Fig. 4B and C). Thus, the results of the present study suggest that neddylation inhibition may suppress neutrophil functions through, at least partially, suppression of the NF-κB signaling pathway.
Discussion
The results of the present study demonstrated that the neddylation inhibitor MLN4924 suppressed the proinflammatory cytokine production in neutrophils in a dose-dependent manner, which is consistent with that observed in mononuclear cells (6,19,20,28,37). In order to purify peritoneal neutrophils induced by 3% thioglycolate intraperitoneal injection, peritoneal cells were stained with PE-conjugated anti-mouse Gr1, followed by isolation with anti-PE antibody-coated magnetic microbeads (38). The purity of neutrophils was verified to be >90% by flow cytometry.
Previous studies had demonstrated that MLN4924 may be used as an anticancer treatment, being tested in Phase I clinical trials for both hematological and non-hematological tumors (39,40). Previous studies have also revealed that MLN4924 markedly suppressed inflammatory responses mediated by innate immune cells, including macrophages and dendritic cells (19,20,34). The results of the present study suggest that the same suppressed inflammatory response is experienced in neutrophils. As neutrophils promote tumor progression under certain circumstance (2,3), neddlylation may contribute to tumor growth partially through enhancing neutrophil-mediated inflammation.
However, the mechanism by which neddlylation promotes inflammation remains unclear. NF-κB proteins are a family of dimeric transcription factors that mediate the expression of inflammatory cytokines (41); transcriptional mediators are pre-existing in the cytoplasm and are able to be rapidly activated by various extracellular stimuli, including microbial components and inflammatory cytokines, prior to entry into the nucleus to mediate an inflammatory response (42). The degradation of IκB proteins is the key to NF-κB activity (42). Impaired degradation of IκBα upon neddylation inhibition has been attributed to the inhibitory effects of MLN4924 on cytokine production in macrophages and dendritic cells (6,19,20,37). Hence, MLN4924 blocks neddylation, thereby suppressing production of inflammatory cytokines by inhibiting the NF-κB signaling pathway. However, mechanisms other than the NF-κB signaling pathway may also be involved; therefore, further studies are required to investigate this issue.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Futosi K, Fodor S, Mocsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang K, Han GC, Wang RX, Xiao H, Hou CM, Guo RF, Dou Y, Shen BF, Li Y, Chen GJ. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014;7:1106–1115. doi: 10.1038/mi.2013.126. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar A, Cederarv M, Wolmer-Solberg N, Tammik C, Stragliotto G, Peredo I, Fornara O, Xu X, Dzabic M, Taher C, et al. Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. Oncoimmunology. 2015;5:e1075693. doi: 10.1080/2162402X.2015.1075693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Chang FM, Reyna SM, Granados JC, Wei SJ, Innis-Whitehouse W, Maffi SK, Rodriguez E, Slaga TJ, Short JD. Inhibition of neddylation represses lipopolysaccharide-induced proinflammatory cytokine production in macrophage cells. J Biol Chem. 2012;287:35756–35767. doi: 10.1074/jbc.M112.397703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabroe I, Parker LC, Wilson AG, Whyte MK, Dower SK. Toll-like receptors: Their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy. 2002;32:984–989. doi: 10.1046/j.1365-2745.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie LA, McPhail LC, Henson PM, Johnston RB., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus SJ, Liu G, Carr SM, Munro S, La Thangue NB. NEDDylation regulates E2F-1-dependent transcription. EMBO Rep. 2012;13:811–818. doi: 10.1038/embor.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36:802–806. doi: 10.1042/BST0360802. [DOI] [PubMed] [Google Scholar]
- 11.Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, Zhang T, Lee HW, Jeong LS, Chen Y, et al. C-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor. Mol Cell. 2013;49:499–510. doi: 10.1016/j.molcel.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Huang DT, Ayrault O, Hunt HW, Taherbhoy AM, Duda DM, Scott DC, Borg LA, Neale G, Murray PJ, Roussel MF, Schulman BA. E2-RING expansion of the NEDD8 cascade confers specificity to cullin modification. Mol Cell. 2009;33:483–495. doi: 10.1016/j.molcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liakopoulos D, Doenges G, Matuschewski K, Jentsch S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998;17:2208–2214. doi: 10.1093/emboj/17.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osaka F, Kawasaki H, Aida N, Saeki M, Chiba T, Kawashima S, Tanaka K, Kato S. A new NEDD8-ligating system for cullin-4A. Genes Dev. 1998;12:2263–2268. doi: 10.1101/gad.12.15.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L, et al. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 17.Zhu T, Wang J, Pei Y, Wang Q, Wu Y, Qiu G, Zhang D, Lv M, Li W, Zhang J. Neddylation controls basal MKK7 kinase activity in breast cancer cells. Oncogene. 2016;35:2624–2633. doi: 10.1038/onc.2015.323. [DOI] [PubMed] [Google Scholar]
- 18.Gao F, Cheng J, Shi T, Yeh ET. Neddylation of a breast cancer-associated protein recruits a class III histone deacetylase that represses NFkappaB-dependent transcription. Nat Cell Biol. 2006;8:1171–1177. doi: 10.1038/ncb1483. [DOI] [PubMed] [Google Scholar]
- 19.Mathewson N, Toubai T, Kapeles S, Sun Y, Oravecz-Wilson K, Tamaki H, Wang Y, Hou G, Sun Y, Reddy P. Neddylation plays an important role in the regulation of murine and human dendritic cell function. Blood. 2013;122:2062–2073. doi: 10.1182/blood-2013-02-486373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng M, Hu S, Wang Z, Pei Y, Fan R, Liu X, Wang L, Zhou J, Zheng S, Zhang T, et al. Inhibition of neddylation regulates dendritic cell functions via Deptor accumulation driven mTOR inactivation. Oncotarget. 2016;7:35643–35654. doi: 10.18632/oncotarget.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D, Su B, et al. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity. 2012;36:43–54. doi: 10.1016/j.immuni.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, Oberheu K, Padmanabhan S, O'Dwyer M, Nawrocki ST, et al. Inhibition of NEDD8-activating enzyme: A novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 23.Hjerpe R, Thomas Y, Chen J, Zemla A, Curran S, Shpiro N, Dick LR, Kurz T. Changes in the ratio of free NEDD8 to ubiquitin triggers NEDDylation by ubiquitin enzymes. Biochem J. 2012;441:927–936. doi: 10.1042/BJ20111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guihard S, Ramolu L, Macabre C, Wasylyk B, Noël G, Abecassis J, Jung AC. The NEDD8 conjugation pathway regulates p53 transcriptional activity and head and neck cancer cell sensitivity to ionizing radiation. Int J Oncol. 2012;41:1531–1540. doi: 10.3892/ijo.2012.1584. [DOI] [PubMed] [Google Scholar]
- 26.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: Bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 2005;175:4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 27.Jubelin G, Taieb F, Duda DM, Hsu Y, Samba-Louaka A, Nobe R, Penary M, Watrin C, Nougayrède JP, Schulman BA, et al. Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways. PLoS Pathog. 2010;6:e1001128. doi: 10.1371/journal.ppat.1001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segovia JA, Tsai SY, Chang TH, Shil NK, Weintraub ST, Short JD, Bose S. Nedd8 regulates inflammasome-dependent caspase-1 activation. Mol Cell Biol. 2015;35:582–597. doi: 10.1128/MCB.00775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73:2349–2367. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMillin DW, Jacobs HM, Delmore JE, Buon L, Hunter ZR, Monrose V, Yu J, Smith PG, Richardson PG, Anderson KC, et al. Molecular and cellular effects of NEDD8-activating enzyme inhibition in myeloma. Mol Cancer Ther. 2012;11:942–951. doi: 10.1158/1535-7163.MCT-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: Rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Liu B, Dong T, Lee HW, Yu J, Zheng Y, Gao H, Zhang Y, Chu Y, Liu G, et al. Neddylation pathway regulates the proliferation and survival of macrophages. Biochem Biophys Res Commun. 2013;432:494–498. doi: 10.1016/j.bbrc.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638–650. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ, Hong JT, Min KR, Kim Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett. 2004;571:50–54. doi: 10.1016/j.febslet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Ye Z, Pei Y, Qiu G, Wang Q, Xu Y, Shen B, Zhang J. Neddylation is required for herpes simplex virus type I (HSV-1)-induced early phase interferon-beta production. Cell Mol Immunol. 2016;13:578–83. doi: 10.1038/cmi.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Medeiros BC, Erba HP, DeAngelo DJ, Giles FJ, Swords RT. Targeting protein neddylation: A novel therapeutic strategy for the treatment of cancer. Expert Opin Ther Targets. 2011;15:253–264. doi: 10.1517/14728222.2011.550877. [DOI] [PubMed] [Google Scholar]
- 40.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 41.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem Res Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]